Abstract
Background
Age‐related morbidities and frailty are associated with impaired blood pressure (BP) and heart rate (HR) recovery after standing. Here we investigate how multimorbidity affects cerebral and peripheral hemodynamics during standing in a large sample of older patients.
Methods
Patients were recruited from a national Falls and Syncope Unit. They underwent an active stand test (5–10 min lying +3 min standing) with monitoring of continuous BP, HR, total peripheral resistance (TPR), stroke volume (SV), and a near‐infrared spectroscopy (NIRS) derived cerebral tissue saturation index (TSI). A multimorbidity count was derived from a 26‐item list of conditions. Features derived from the signals included: nadir, overshoot, value at 30 s, steady‐state and recovery rate. Robust linear regression was used to assess the association between multimorbidity, TSI and peripheral hemodynamics while correcting for covariates. A p‐value <0.05 was considered statistically significant.
Results
Multimorbidity was associated with poorer recovery of TSI at 30 s after standing (β: −0.15, CI:[−0.25–0.06], p = 0.009) independent of all peripheral hemodynamics. Impaired diastolic BP (DBP) recovery at 30s (β:−1.34, CI:[−2.29–0.40], p = 0.032), DBP steady‐state (β:−1.18, CI:[−2.04–0.32], p = 0.032), TPR overshoot‐to‐nadir difference (β:−0.041, CI:[−0.070–0.013], p = 0.045), and SV at 30s (β:1.30, CI:[0.45 2.15], p = 0.027) were also associated with increasing multimorbidity. After sex stratification, only females demonstrated impaired TSI with multimorbidity at overshoot (β: −0.19, CI: [−0.32 ‐0.07], p = 0.009), 30 s (β: −0.22 [−0.35–0.10], p = 0.005) and steady‐state (β: −0.20, CI:[−0.35–0.04], p = 0.023), independent of peripheral hemodynamics.
Conclusions
Transient cerebral oxygenation and peripheral hemodynamic responses are impaired with multimorbidity (frailty) in older patients, particularly in females. This study demonstrates the feasibility of using NIRS in this clinical context and may inform the development of clinical management strategies targeting both cerebral oxygenation and blood pressure impairments in patients with faints and falls.
Keywords: active standing, cerebral oxygenation, multimorbidity, near‐infrared spectroscopy, orthostatic hypotension
Key points
Multimorbidity is associated with impaired cerebral oxygenation recovery after standing independent of peripheral hemodynamics
Multimorbidity is also associated with impaired blood pressure recovery from an initial nadir which can only be assessed with continuous beat‐to‐beat technology
NIRS is a convenient, easy to use clinical tool, with potential future applications in the assessment and management of frail older adults with multimorbidity, falls and faints
Why does this paper matter?
Cerebral oxygenation and blood pressure measurement may help us understand and manage multimorbidity and how it leads to poor health outcomes such as faints and falls.
INTRODUCTION
Age‐related conditions commonly experienced by older populations including falls, 1 hypertension, 2 cognitive impairment, 3 and depression, 4 are associated with impaired orthostatic peripheral hemodynamic such as delayed blood pressure (BP), impaired heart rate (HR) recovery, and orthostatic hypotension (OH). Frailty, often defined as a count of morbidities, has been consistently associated with neurocardiovascular dysregulation, characterized by blunted compensatory HR and BP responses after active standing. 5 , 6 Current hypotheses suggest that hypoperfusion related deficits in cerebral oxygenation play a pivotal role in the etiology of age‐related conditions. However, to date, little is known about transient cerebrovascular hemodynamics during standing, particularly in frailer older patients with multimorbidity.
Near‐infrared spectroscopy (NIRS) measures of cerebral oxygenation have recently emerged as objective surrogates of cerebral perfusion, suitable for clinical testing of dynamic cerebrovascular hemodynamics during standing. 7 , 8 While impairments in cerebral oxygenation recovery have been associated with orthostatic hypotension (OH), 9 syncope, 10 depression, 11 and postural instability 12 in older adults, the relationship between cerebral oxygenation responses and multimorbidity remains largely unexplored, 13 especially in clinical cohorts.
During dynamic BP transient stressors such as standing, cerebral oxygenation is expected to decrease and then recover due to contributions of peripheral BP and stroke volume (SV) recovery, and the active regulation of cerebral autoregulation (CA), among other factors. 14 Despite the strong influence of BP, whether impairments in cerebral oxygenation reported in age related conditions are independent of BP, SV and total peripheral resistance (TPR) remains largely unknown. Furthermore, while peripheral hemodynamic impairments, 15 multimorbidity, 16 frailty, 17 and orthostatic intolerance 18 are known to be increased in older females, no studies have examined the interaction between multimorbidity, sex, and cerebrovascular responses in clinical cohorts.
Given the recognized associations between age‐related conditions and impairments in both peripheral and cerebral responses to standing, we hypothesize that multimorbidity is accompanied by impairments in both of these responses during orthostatic challenges. Therefore, this study addresses the following research questions in a clinical cohort of older patients presenting with syncope and falls: (1) is multimorbidity associated with impaired peripheral hemodynamics during an active stand (AS) test? (2) is multimorbidity associated with impaired cerebral oxygenation responses during an AS test independent of peripheral hemodynamics? and (3) does sex modulate these relationships?
METHODS
Setting and patients
Consecutive patients were recruited prospectively from a national Falls and Syncope Unit (FASU) based at St. James's Hospital in Dublin, Ireland, with patients referred to the clinic from the emergency department and general practitioner services for investigation of episodes of dizziness, falls or syncope. The study had ethical approval from the Tallaght University Hospital/St. James's Hospital Joint Research Ethics Committee (REC:2018‐08,CA,16).
Inclusion and exclusion criteria
Patients aged 50 years and over attending the FASU were included in this study. Patients were excluded if they were unable to provide informed consent, had a previous diagnosis of cognitive impairment, or had any contraindication to perform an AS (e.g., mobility issues, severe pain).
Multimorbidity
Multimorbidity was measured as a count of self‐reported morbidities within a 26‐item scale collected from a comprehensive medical history, which included the presence or absence of the following: cardiovascular conditions (transient ischemic attack, stroke, carotid stenosis, hypertension, ischemic heart disease, angina, myocardial infarction, atrial fibrillation, other rhythm abnormalities, heart failure, diabetes, high cholesterol, other cardiovascular diseases), neurological disorders (mild cognitive impairment, Parkinson's disease, other neurodegenerative diseases, migraine, epilepsy), psychological disorders (depression or anxiety, other psychological diseases), and other disorders (benign paroxysmal positional vertigo or other vestibular disorders, obstructive sleep apnea, chronic obstructive airway disease, and endocrine diseases). A recent (in last year) history of all‐cause falls and syncope was also included.
Active stand testing
Protocol
All patients underwent an AS test as per standard international protocol. 19 Following a 5–10‐min signal calibration and stabilization period, patients were instructed to stand up quickly and remain standing quietly for 3 min. Assistance was provided during the test as required, and the test was terminated early if patients developed symptoms of severe orthostatic intolerance.
Equipment
Cerebral oxygenation was measured using a PortaLite NIRS monitor, (PortaLite, Artinis Medical Systems B.V., Elst, The Netherlands), which provides continuous values of tissue saturation index (TSI) (units of percentage, %). 20 The probe was placed on the left side of the forehead, that is, centered at 3 cm lateral and 3.5 cm above the nasion, 21 and secured with an opaque bandage. A Finometer NOVA (Finapres Medical Systems, Amsterdam, The Netherlands) was used concurrently to measure continuous beat‐to‐beat systolic BP (SBP), diastolic BP (DBP), and HR, and Modelflow™ derived TPR and SV. A finger cuff was placed on the middle or proximal phalanx of a finger (as per manufacturer instructions) on the left hand. Calibration was performed using brachial artery BP measurements and Physiocal™, while hydrostatic pressures differences were corrected for using the system's height correction unit.
Covariates
A comprehensive medical history was recorded which included age (years), sex, weight (kg), height (m), and body mass index (BMI, kg/m2). Behavioral health including smoking status (non‐smoker, current smoker, or ex‐smoker) and history of excessive alcohol intake (>14 units/week for females, >21 units/week for males) was also captured. A detailed medication record was also taken (using the TCD classification system) at the time of testing, including key cardioactive medications: antihypertensives (C0, C0C‐CC, C07), and antidepressants (N06A). Finally, medical conditions encompassing the multimorbidity count (see above) were also recorded.
Data analysis
Data quality
Patients with incomplete clinical information were excluded from analyses. BP, HR, and TSI signals were visually inspected and screened for signal quality following a pre‐established set of criteria, with poor signals not included in further analyses.
Signal processing
SBP, DBP, HR, TPR, SV, and TSI signals were analyzed with custom software developed in MATLAB R2019a (The Math Works, Inc., MATLAB, Version 2019a, Natick, MA) applying a standardized analytical process. 19 , 22 The Finometer height correction signal was used to derive the time of stand, standing speed, 23 and the supine rest duration was estimated as the duration of the recording before the stand.
Features extracted from the AS stand responses are detailed in Figure 1 (see Text S1 and Table S1 for more specific details). A resting baseline (average [−60, −30] s before standing), value at 30 s and steady‐state (average from [60,120] s) values were obtained. 19 For TSI, BP, and TPR signals, nadir and overshoot values were defined, while for HR and SV the maximum and subsequent minimum were extracted. 22 The average recovery rate from nadir to overshoot (or maximum and minimum for HR/SV) was also derived for all signals. Values at nadir (maximum for HR/SV), overshoot (minimum for HR/SV), 30 s, and steady‐state are reported as absolute changes from baseline, unless otherwise indicated. Absolute values for TPR, SV, and TSI are considered less accurate and therefore are not reported here. 24 , 25 Additional features were derived and are reported in the Supplemental Material.
FIGURE 1.

Features extracted from cerebral (TSI) and peripheral (SBP, DBP, HR) responses to an active standing test in a selected patient. Features extracted from the signals have been indicated. DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; TSI, tissue saturation index. Note that time is in seconds, and both TSI/SBP/DBP nadir and HR maximum occur within 15 s of standing (t = 0).
Statistical analysis
RStudio was used to perform all statistical analyses (RStudio Team, 2016, RStudio: Integrated Development for R. RStudio, Inc., Boston, MA). Histograms, Q‐Q plots, and Shapiro–Wilk tests were implemented to assess normality of the data. Differences in patients characteristics were tested using t‐tests for normally distributed variables (mean, standard deviation [SD]) and Mann–Whitney U tests for non‐normal distributions (median, interquartile range [IQR]). A Chi‐squared test was used for binary and categorical variables.
Multimorbidity was analyzed as a continuous variable (please note groups with ≤2 and ≥5 morbidities were used for the purposes of displaying the data only). Robust linear regression was employed to evaluate unadjusted and fully adjusted effects of multimorbidity on SBP, DBP, HR, TPR, SV, and TSI as independent variables. Models were adjusted for age, sex, BMI, baseline SBP, baseline HR, standing speed, supine rest duration, smoking status (non‐smoker, previous smoker, nonsmoker), alcohol excess, and antihypertensives and antidepressants use. TSI models were further corrected by simultaneously measured peripheral hemodynamic features (e.g., when analyzing the TSI nadir, the model included the SBP/DBP/mean arterial pressure [MAP]/TPR/SV nadir feature as a covariate). Sensitivity analyses were performed in separate models to examine the effect of controlling for OH (drop from baseline >20 mmHg in SBP and/or > 10 mmHg in DBP) at 30 s and sustained OH. Analyses were repeated after sex stratification. A false discovery rate (FDR) procedure was used to correct for multiple testing effects. A p‐value <0.05 was considered significant.
RESULTS
Patients' characteristics
A total of 351 patients were recruited. After screening for signal quality, 303 patients remained, aged 71 (15) years (57% females), with a BMI of 27 (6) kg/m2 (Table 1). Cardiovascular diseases, neurological and psychological disorders were present in 76%, 17%, and 25% of patients, respectively. Almost half of the patients had experienced syncope in the last year, with 34% reporting a fall. Polypharmacy was common, with patients prescribed 5 (4) medications; 52% of patients were taking antihypertensives, and 24% antidepressants.
TABLE 1.
Patients' characteristics
| Characteristic | All (n = 303) | Females (n = 173) | Males (n = 130) | p‐value a |
|---|---|---|---|---|
| Age, years (median, IQR) | 71 (15) | 71 (15) | 71 (12) | 0.830 |
| Weight, kg (median, IQR) | 74 (23) | 67 (20) | 84 (19) | <0.001*** |
| Height, m (mean, SD) | 166 (10) | 160 (7) | 174 (7) | <0.001*** |
| Body Mass Index, kg/m2 (median, IQR) | 27 (6) | 26 (8) | 27 (5) | 0.135 |
| Cardiovascular diseases, % (n) | 76 (231) | 75(129) | 79 (102) | 0.430 |
| Neurological disorders % (n) | 17 (51) | 16 (28) | 18 (23) | 0.729 |
| Psychological disorders, % (n) | 25 (75) | 27 (47) | 22 (28) | 0.261 |
| Vestibular disorders, % (n) | 8 (23) | 9 (16) | 5 (7) | 0.209 |
| Other conditions, % (n) | 30 (92) | 34 (59) | 25 (33) | 0.102 |
| Syncope, % (n) | 47 (142) | 45 (77) | 50 (65) | 0.343 |
| Falls, % (n) | 34 (104) | 40 (69) | 27 (35) | 0.019* |
| Multimorbidity (count of morbidities), n (median (IQR) | 3 (3) | 3 (2) | 3 (3) | 0.139 |
| Smoking status (n = 11 missing) | ||||
| Non‐smoker, % (n) | 57 (168) | 70 (118) | 40 (50) | <0.001*** |
| Current smoker, % (n) | 31 (90) | 21 (36) | 43 (54) | <0.001*** |
| Ex‐smoker, % (n) | 12 (35) | 8 (14) | 17 (21) | 0.027* |
| Excess of alcohol intake, % (n) (n = 1 missing) | 14 (42) | 3 (5) | 29 (37) | <0.001*** |
| Medication | ||||
| Antihypertensives, % (n) | 52 (157) | 51 (89) | 52 (68) | 0.882 |
| Antidepressants, % (n) | 24 (72) | 29 (50) | 17 (22) | 0.015* |
| Total number of medications, median (IQR) | 5(4) | 5 (5) | 5(5) | 0.817 |
| Baseline features | ||||
| Baseline SBP, mmHg (median, IQR) | 144 (27) | 145 (29) | 144 (27) | 0.714 |
| Baseline DBP, mmHg (median, IQR) | 82 (13) | 81 (14) | 83 (11) | 0.222 |
| Baseline HR, bpm (median, IQR) | 71 (16) | 72 (15) | 70 (17) | 0.045* |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; IQR, interquartile range; SBP, systolic blood pressure.
p‐values represent differences between males and females.
p < 0.05,
p < 0.001.
After sex stratification, a higher proportion of females reported a recent fall (f:40% vs. m:27%, p = 0.020) (Table 1). More males were either current (f:21% vs. m:43%, p < 0.001) or past smokers (f:8% vs. m:17%, p = 0.027), with males also reporting higher rates of alcohol excess (f:3% vs. m:29%, p < 0.001).
Figure 2 shows the group averages for TSI, SBP, DBP, and HR across 2 levels of accumulated morbidities (groups with ≤2 morbidities (lowest tertile) and ≥5 morbidities (highest tertile), were used only for the purpose of displaying the data, multimorbidity was analyzed as a continuous variable). Females had a significantly smaller TSI drop at nadir (f:−1.71(1.67) vs. m:−2.41(2.07), p = 0.015) and a slower recovery rate (f:0.19(0.16) vs. m:0.23(0.19), p = 0.005) (Figure 2, Table 2, Table S2). Females recovered more quickly to higher SBP and DBP values (Figure 2, Table 2, Table S2). No significant sex differences were observed in HR responses. Furthermore, females recovered to higher TPR values while they had lower SV recovery values (Figure S1, Table 2, Table S2). Regarding absolute values, only a higher SBP steady‐state value in females was identified to be different between both sexes (Figure S2 and Table S3).
FIGURE 2.

Absolute drops from baseline for cerebrovascular (TSI) and cardiovascular (SBP, DBP, HR) haemodynamics for all patients (left), females (middle) and males (right) during the active standing test for patients accumulating 2 or less morbidities (orange) and 5 or more morbidities (purple) (note that these categories were used only for the purpose of displaying the data, multimorbidity was analyzed as a continuous variable). Multimorbidity was associated with impaired TSI and DBP recovery, and an attenuated HR response in all patients and in the females cohort, while no associations were observed in males (after covariates adjustment). The start of the test has been indicated with a vertical line at t = 0. Error bars represent the confidence intervals of the mean at t = −30, 30, 60, 90, 120 and 150 s after standing. TSI, tissue saturation index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; morb, morbidities.
TABLE 2.
Average values for TSI, SBP, DBP and HR features analyzed for all patients, females and males, comparing differences between sex.
| All patients (n = 303) | Females (n = 173) | Males (n = 130) | FDR p‐value a | |
|---|---|---|---|---|
| TSI (%) | ||||
| TSI nadir, med (IQR) | −1.91 (1.67) | −1.71 (1.67) | −2.41 (2.07) | 0.015* |
| TSI overshoot, med (IQR) | 0.12 (1.3) | 0.09 (1.3) | 0.14 (2.22) | 0.379 |
| TSI recovery rate, med (IQR) b | 0.21 (0.16) | 0.19 (0.16) | 0.23 (0.19) | 0.005** |
| TSI at 30 at seconds, med (IQR) | −0.76 (1.52) | −0.82 (1.52) | −0.72 (1.55) | 0.357 |
| TSI steady‐state, med (IQR) | −1.09 (1.79) | −1.19 (1.79) | −1.0 (2.12) | 0.104 |
| SBP (mmHg) | ||||
| SBP nadir, med (IQR) | −31.94 (21.64) | −31.94 (21.64) | −31.96 (24.63) | 0.839 |
| SBP overshoot, m (SD) | 1.38 (25.81) | 1.42 (25.81) | 1.24 (34.1) | 0.839 |
| SBP recovery rate, med (IQR) b | 2.91 (2.00) | 2.9 (2.00) | 2.94 (2.19) | 0.839 |
| SBP at 30 s, m (SD) | −2.02 (27.35) | −0.96 (27.35) | −4.08 (26.92) | 0.130 |
| SBP steady‐state, m (SD) | 4.81 (21.78) | 6.54 (21.78) | 1.46 (17.83) | 0.027* |
| DBP (mmHg) | ||||
| DBP nadir, med (IQR) | −17.38 (16.15) | −15.27 (16.15) | −21.23 (17.12) | 0.007** |
| DBP overshoot, m (SD) | 2.51 (17.95) | 4.50 (17.95) | 0.60 (18.08) | 0.009** |
| DBP recovery rate, med (IQR) b | 1.77 (1.19) | 1.80 (1.19) | 1.74 (1.12) | 0.371 |
| DBP at 30 s, m (SD) | 1.62 (16.87) | 3.72 (16.87) | −2.11 (15.14) | <0.001*** |
| DBP steady‐state, m (SD) | 6.07 (15.03) | 8.72 (15.03) | 2.11 (13.32) | <0.001*** |
| HR (bpm) | ||||
| HR maximum, med (IQR) | 14.65 (9.1) | 15.24 (9.1) | 13.18 (10.52) | 0.272 |
| HR minimum, med (IQR) | 5.8 (7.9) | 6.29 (7.9) | 5.19 (9.64) | 0.479 |
| HR recovery rate, med (IQR) b | −1.11 (1.55) | −1.21 (1.55) | −0.99 (2.52) | 0.643 |
| HR at 30 s, m (SD) | 6.35 (7.45) | 6.24 (7.45) | 6.46 (8.68) | 0.389 |
| HR steady‐state, med (IQR) | 7.05 (7.41) | 6.9 (7.41) | 7.36 (8.16) | 0.920 |
| TPR (mmHg·min/L) | ||||
| TPR nadir, med (IQR) | −0.52 (0.52) | −0.53 (0.52) | −0.5 (0.37) | 0.562 |
| TPR overshoot, med (IQR) | 0.13 (0.54) | 0.24 (0.54) | 0.01 (0.41) | <0.001*** |
| TPR recovery rate, med (IQR) b | 0.05 (0.04) | 0.05 (0.04) | 0.04 (0.03) | <0.001*** |
| TPR at 30 s, med (IQR) | 0.11 (0.49) | 0.20 (0.49) | −0.06 (0.38) | <0.001*** |
| TPR steady‐state, med (IQR) | 0.15 (0.49) | 0.23 (0.49) | 0.06 (0.34) | <0.001*** |
| Stroke volume (ml) | ||||
| SV maximum, med (IQR) | 7.80 (20.63) | 4.44 (20.63) | 13.63 (26.20) | <0.001*** |
| SV minimum, med (IQR) | −7.90 (11.73) | −8.62 (11.73) | −5.25 (20.11) | 0.033* |
| SV recovery rate, med (IQR) b | −2.03 (1.67) | −1.86 (1.67) | −2.46 (2.29) | <0.001*** |
| SV at 30 s, med (IQR) | −8.80 (11.01) | −9.28 (11.01) | −7.34 (16.23) | 0.070 |
| SV steady‐state, med (IQR) | −9.62 (12.14) | −10.27 (12.14) | −7.70 (17.29) | 0.046* |
Note: Nadir, maximum, overshoot, minimum, value at 30 s and steady‐state values represent changes from baseline.
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; m (SD), mean (standard deviation); med (IQR), median (interquartile range); SBP, systolic blood pressure; SV, stroke volume; TPR, total peripheral resistance; TSI, tissue saturation index.
p‐values resulting from t‐test or Mann Whitney U test (for normal and non‐normal distributions, respectively) comparing females versus males populations.
Recovery rates are expressed in units of the signal/seconds. False discovery rate (FDR) corrected p‐values are reported.
p < 0.05,
p < 0.01,
p < 0.001.
All patients
Multimorbidity was associated with impaired cerebral oxygenation recovery at 30 s (β:−0.15, CI:[−0.25,−0.06], p = 0.009) (Figure 3, Table S4) even after peripheral hemodynamics adjustment (Tables S5‐S9).
FIGURE 3.
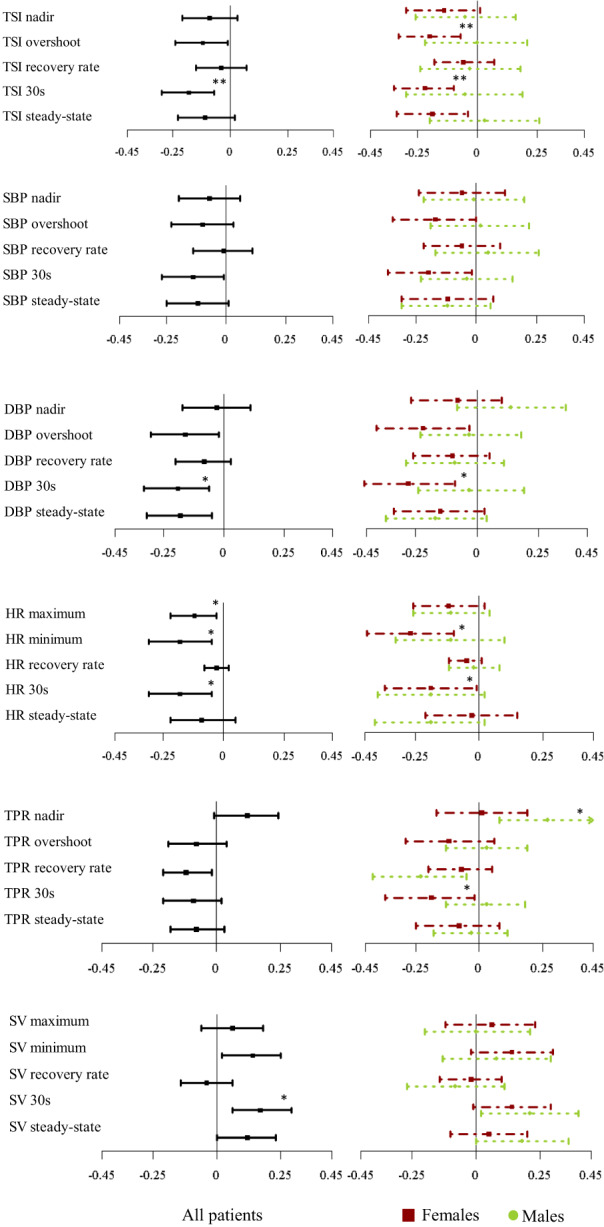
Forest plot representing standardized β coefficients and confidence intervals for the association of multimorbidity and the different features derived from TSI, SBP, DBP, HR, TPR and SV for all patients (left), females (right, red squares) and males (right, green circles) in the multivariate model adjusting for: age + sex + BMI + baseline SBP + baseline HR + speed of standing + supine rest duration + alcohol excess + smoking status + antihypertensives + antidepressants. DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure; TSI, tissue saturation index. *indicates p‐value <0.05 and **p‐value <0.01 after multiple comparison correction.
Multimorbidity was also associated with delayed DBP recovery at 30 s (β:−1.34, CI:[−2.29,−0.40], p = 0.032) and a lower DBP steady‐state (β:−1.18, CI:[−2.04,−0.32], p = 0.032) (Figure 3, Table S4). While a similar pattern was observed in SBP, none of these effects remained after correcting for covariates.
Multimorbidity was associated, after adjustment, with attenuated HR responses, characterized by a lower maximum HR (β:−0.70, CI:[−1.25,−0.15], p = 0.029), lower HR minimum (β:−0.75, CI:[−1.28,−0.22], p = 0.027) and a lower HR at 30 s (β: −0.63, CI:[−1.10,–0.16], p = 0.027) after standing (Figure 3, Table S4). Multimorbidity was also associated with a smaller TPR overshoot‐to‐nadir difference (β:−0.041, CI:[−0.070,−0.013], p = 0.045) and a higher value in SV at 30 s (β:1.30, CI:[0.45,2.15], p = 0.027) (Figure 3, Figure S1 and Table S4).
Finally, when examining absolute values, multimorbidity was associated with deficits in DBP only (Figure S2, Table S12).
Sex differences
Females
In females, multimorbidity was associated with lower TSI at overshoot (β:−0.19, CI: [−0.32,−0.07], p = 0.009), 30 s (β:−0.22 [−0.35,−0.10], p = 0.005) and during steady‐state (β: −0.20, CI:[−0.35,–0.04], p = 0.023) (Figure 3, Table S10), independent of SBP, DBP, MAP, TPR, and SV (Tables S5–9). Multimorbidity was also associated with impaired DBP recovery at 30 s (β: −2.04, CI:[−3.41,−0.68], p = 0.027) after standing after adjustment, with similar effects noted in SBP recovery patterns at a univariate level only (Figure 3, Table S10).
HR features were also significantly impaired in multimorbidity, with females demonstrating lower HR minima with higher multimorbidity (β:−1.14, CI:[−1.86,−0.42], p = 0.018) (Figure 3, Table S10). Last, while univariate associations were observed between multimorbidity and TPR and SV, they did not remain significant after adjusting for covariates (Figure 3, Figure S1 and Table S10).
In females, multimorbidity was not associated with differences in absolute values for any of the signals analyzed (Figure S2, Table S13).
Males
Cerebral oxygenation indices were not significantly associated with multimorbidity in males. Multimorbidity was associated with delayed DBP recovery at a univariate level only while it was not associated with any SBP features in males (Figure 3, Table S11). While a blunted HR response was also noted in males with higher multimorbidity (lower HR maximum), none of these effects remained after correction. However, with increasing multimorbidity, men demonstrated a smaller TPR drop at nadir (β:0.046, CI:[0.014,0.078], p = 0.034), as well as a smaller overshoot‐to‐nadir difference (β:−0.040, CI:[−0.072,−0.008], p = 0.034) (Figure 3, Figure S1 and Table S11).
Multimorbidity was associated with lower absolute DBP steady‐state values (Figure S2, Table S14).
DISCUSSION
This study presents a number of novel and clinically relevant findings: multimorbidity is associated with impairments in the initial recovery of TSI and peripheral hemodynamic responses to active standing in older patients, with effects most evident in females.
Our main result supports the hypothesis that impairments in cerebral perfusion accompany multimorbidity. Here we noted that cerebral oxygenation recovery at 30 s after standing was impaired in adults with more morbidities (β:‐0.15%/morbidity), translating into a TSI change of 1.1% in adults accumulating 7 morbidities (~25% of the total count). This change is clinically relevant, given that a 5%–10% TSI drop is associated with presyncope or loss of consciousness. 26 Maguire et al. similarly reported impaired cerebral oxygenation recovery with increasing levels of frailty (measured as an index of morbidities and ADL deficits) at 20–27 s after standing in a population sample of community‐dwelling adults (mean(SD): 64.4(7.7) years). 13 In a large study of patients undergoing head‐up tilting (median(IQR): 40 (35) years), Jedrzejczyk‐Spaho et al. also demonstrated that having multiple comorbidities was associated with larger TSI drops. 27
On initial inspection, impaired cerebral oxygenation may suggest that CA is impaired in multimorbidity. However, with a CA impairment one would expect TSI to follow BP drops, while intact CA would counteract BP drops. If multimorbidity resulted in impaired CA, we would expect that correcting for the effects of BP would attenuate or even eliminate the associations found between TSI and multimorbidity. This was not the case here, with TSI impairments remaining unattenuated after BP adjustment (Table S5, S6 and S7). Thus, our results indicate that the deficits in TSI recovery may not be attributed to impaired CA. CA is a complex, nonlinear and multifactorial mechanism and thus more advanced models are likely necessary to better understand the relationship between multimorbidity and CA. Other mechanisms of cerebral oxygenation regulation must therefore be considered, for example, decreases in PaCO2, which could be induced due to postural hyperventilation and lead to a reduced cerebral perfusion and oxygenation. 28 , 29 We also tested the hypothesis that SV might be driving the cerebral oxygenation impairments observed, 30 but adjusting for SV did not alter the results.
In this study, we identified for the first time, to our knowledge, significant impairments in TPR and SV mostly univariately (especially in males), which may underlie the impairments observed in BP. Moloney et al. demonstrated a univariate association between different BP recovery groups and the prevalence of multimorbidity in a population of community‐dwellers (mean(SD) age: 61.0(8.8) years). 31 Conversely, Lagro et al. reported an equivalent level of multimorbidity between three SBP recovery groups in 238 falls patients (mean(SD) age: 78.4(7.8) years), 32 although their multimorbidity scale was more exhaustive than ours. Capturing a broader range of morbidities may have masked underlying differences in cardiovascular multimorbidity, which may ultimately be the driver of the observed BP differences. Last, our finding of impaired BP and HR recovery with multimorbidity agrees with previous studies investigating frailty indices, 5 , 13 , 33 , 34 , 35 suggesting that our multimorbidity scale captures key frailty factors contributing toward peripheral circulation impairment, and is likely an appropriate surrogate for frailty in the context of falls and syncope patients. Furthermore, we have found the strongest associations during the recovery period (30 s), which encourages the use of newly developed definitions such as delayed BP recovery 1 over more classic definitions such as initial OH or classical OH.
After sex stratification, an association between increased multimorbidity and lower TSI independent of peripheral hemodynamics was identified in females at 30 s after standing, suggesting that cerebral oxygenation is not passively following BP. In males, similar but weaker trends may support a similar hypothesis, but the alternative hypothesis may also apply. Maguire et al. identified differences in TSI between males and females during the first 30 s after standing, 13 and Jedrzejczyk‐Spaho et al. found lower values of TSI during a HUT in females than in males in their cohort of patients with suspected syncope. 27 Interestingly, resting cerebral perfusion is known to be higher in healthy females, 36 with CA often preserved in this cohort. 37 However, studies investigating these differences have not considered the modulating effect of multimorbidity. The reasons why cerebrovascular responses to orthostasis are more affected by multimorbidity in females than in males remain unclear. However, these sex differences are in line with a well described lower tolerance to orthostatic challenges in females. 38 It is also noteworthy to highlight the higher proportion of patients with a history of falls in females (42% in females vs. 29% in males, p = 0.016). These results might suggest that frailer females are more prone to the effects of cerebral hypoperfusion and consequently syncope and falls, although further research is needed to confirm the causality of this relationship.
A number of hypotheses may explain our results. The impairments identified here in the cerebral circulation may result from the cumulative effect of sub‐components of multimorbidity such as atrial fibrillation, 39 heart failure, 40 stroke, 41 small vessel disease, 42 hypertension, 43 depression, 11 and falls, 12 which are all associated with impaired cerebral perfusion. Conversely, transient deficits in perfusion may lead to brain and end organ damage, contributing to the development of new morbidities. Previous work has shown that cerebral hypoperfusion is associated with hypoxia‐induced cerebral tissue damage, 44 which may accelerate progression of depression, cognitive impairment, or dementia through hypoperfusion related neurodegenerative changes. Regarding peripheral hemodynamics, baroreflex impairment, 15 increased arterial stiffnes, 45 or reduced muscle pump 28 resulting from the cumulative effect of coexisting morbidities may mechanistically underlie the observed TPR, SV, and HR impairments. This may ultimately reduce BP and cerebral oxygenation and contribute to orthostatic intolerance symptoms and postural instability, 12 increasing the likelihood of negative outcomes such as falls and syncope. In our study, adjusting the cerebral oxygenation features by concurrent peripheral signals did not eliminate TSI differences, suggesting that the impairments in cerebral oxygenation observed are partially independent of peripheral circulation. Correction was performed using separate analysis considering either the concurrent BP values or for the presence of delayed BP recovery and OH (not reported). Similar results were obtained in both analyses.
Limitations
A number of limitations need to be considered. First, we studied a population of patients attending a FASU. Second, the accumulation of morbidities in our population was skewed, with only 31 participants (10%) accumulating more than 25% of the morbidities recorded, but this is comparable to community‐dwelling populations. 46 The moderate levels of multimorbidity observed here were sufficient to highlight cerebral oxygenation impairments, although increasing the number of patients with high levels of multimorbidity may increase the study power to detect changes in SPB, TPR, and SV. In addition, our multimorbidity scale included 26 deficits. Covering more morbidities could enhance our ability to capture the multi‐system aspects of multimorbidity, especially given that our scale has a cardiovascular bias (~60%). The lack of PaCO2 measurements is also an important limitation, given its strong vasoactive action on the cerebrovasculature, 47 therefore limiting our ability to fully understand the pathophysiology underlying our results. Finally, the interpretation of NIRS derived signals is challenging, with the venous and arterial contribution to the signal remaining unclear. 8 A number of factors influence the interpretation of absolute TSI values, including extracerebral tissue contamination, motion artifacts, underlying tissue heterogeneity, and skull and cerebrospinal fluid thickness. 48 , 49 , 50 Here we only interpreted relative changes in TSI given the challenges of interpreting absolute values of NIRS. Furthermore, NIRS only captures a small region of the prefrontal cortex circulation, thus not accounting for differences in blood flow redistribution throughout the cerebral tissue.
Strengths
To our knowledge, this is the first clinical study to investigate the role of multimorbidity on the cerebral oxygenation responses to standing as measured by NIRS in a population of older patients with a wide range of ages (50–95 years). We have identified novel biomarkers of multimorbidity during the initial transient part of the cerebral oxygenation response to standing. In addition, we have separated our population by sex, which has allowed us to identify major differences in their cardiovascular and cerebrovascular responses to standing and how they are associated with multimorbidity. A further novel contribution is the adjustment for BP and other peripheral signals, which demonstrated that the deficits in cerebral oxygenation found are independent of such signals.
CONCLUSION
NIRS derived cerebral oxygenation recovery during standing is impaired with multimorbidity (frailty) (particularly in females), independent of BP, TPR, and SV. TSI responses may represent new biomarkers for identifying multimorbidity in clinical populations. Clinical interventions targeting improvements in both peripheral and cerebral hemodynamics, and the use of readily applicable systems like NIRS may prove valuable in the future management of older adults with multimorbidity, potentially reducing poor health outcomes like faints and falls, and improving their quality of life. Similarly, interventions to reverse or prevent multimorbidity may prevent the development of cerebral hypoperfusion and its associated negative health outcomes.
AUTHOR CONTRIBUTIONS
Laura Pérez‐Denia conceived and designed the study, collected the data, processed and interpreted the data, performed the statistical analysis and drafted the article and approved its final version. Paul Claffey, Lisa Byrne, and Ciara Rice assisted with design of the study, data collection and critical review and approval of the manuscript. Rose Anne Kenny and Ciarán Finucane conceived and designed the study, interpreted the results, and critically reviewed and approved the final version of the article.
SPONSOR'S ROLE
The authors would like to acknowledge sources of funding: LPD is supported by the Irish Research Council: Government of Ireland Postgraduate Scholarship Programme 2018 (Grant No: GOIPG/2018/134), Dublin, Ireland and the Fundació Universitària Agustí Pedro i Pons, Universitat de Barcelona, Barcelona, Spain; CF holds Science Foundation Ireland and Irish Research Council grants; Grant No: 19/IFA/7409 and Grant No: GOIPG/2018/134, Dublin, Ireland, and is currently working on industry partnership with Endotronix Inc.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Editor's Note .
This is another in a series of studies with very important and underpublicized clinical implications conducted by Drs. Ciaran Finucane and Rose Anne Kenny and their colleagues at the national Falls and Syncope Unit at St. James Hospital and Trinity University in Dublin, Ireland. For the past several years they have been investigating the physiology of postural hemodynamic changes and their potential relationship to falls and syncopal events in older adults. Their findings shed new light on some of the most common and devastating conditions in the patient population served by geriatrics health professionals, and should guide us in rethinking how we approach them.
They have previously shown in elegant studies, at least one of which published in JAGS, that blood pressure drops precipitously within a few seconds of standing and recovery is impaired in some older people. This finding has critical implications for diagnosing and managing falls, near syncope, and syncope. Among them is the potential importance of beat to beat monitoring of blood pressure to detect this phenomenon. The typical strategy of taking blood pressure and heart rate one, three, and sometimes 5 min after standing will not detect these rapid drops in blood pressure, and thus potentially miss the underlying cause of some falls and syncopal episodes.
In the current study they demonstrate additional findings. First, patients with multiple comorbidities, especially women, appear to be more prone to have impaired blood pressure recovery after an initial drop on standing. Second, multimorbidity is associated with impaired cerebral oxygenation recovery independent of the peripheral hemodynamics. And third, near‐infrared spectroscopy may be a useful clinical tool in diagnosing older patients with unexplained falls and syncopal events.
I applaud these investigators for their innovative work, and hope others will join them in studying what may be a vastly under‐diagnosed phenomenon underlying many serious injurious events in the patient population we serve.
‐Joseph G. Ouslander, MD
Supporting information
Text S1 Description of all features derivation
Table S1: Summary of features derivation
Table S2: Average and variability values for the TSI, SBP, DBP, HR, TPR, and SV features analyzed for all patients, females and males, comparing differences between sex groups.
Table S3: Average and variability absolute values for the SBP, DBP and HR features analyzed for all patients, females and males, comparing differences between sex.
Table S4: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR, SV, TPR) and cerebrovascular (TSI) features in all patients.
Table S5: Effects of multimorbidity on TSI when correcting for covariates and concurrent SBP features.
Table S6: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent DBP features
Table S7: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent MAP features
Table S8: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent TPR features
Table S9: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent SV features
Table S10: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in females.
Table S11: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in males.
Table S12: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features in all patients.
Table S13: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in females.
Table S14: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in males.
Figure S1: Absolute drops from baseline in TPR and SV responses for all patients, females and males during the active standing test for two levels of multimorbidity.
Figure S2: Absolute cardiovascular (SBP, DBP, HR) hemodynamic responses for all patients, females and males during the active standing test for two levels of multimorbidity.
ACKNOWLEDGMENTS
The authors would like to acknowledge the participation of all the individuals involved in this study, and the contribution of the healthcare professionals at the Falls and Syncope Unit and the Medical Physics and Bioengineering department at St. James's Hospital, Dublin, Ireland. The authors thank Ms. Maria Delgado‐Ortet, Department of Radiology, University of Cambridge, UK, for her role in processing the clinical information of the patients involved in this study. Open access funding provided by IReL.
Pérez‐Denia L, Claffey P, Byrne L, Rice C, Kenny RA, Finucane C. Increased multimorbidity is associated with impaired cerebral and peripheral hemodynamic stabilization during active standing. J Am Geriatr Soc. 2022;70(7):1973‐1986. doi: 10.1111/jgs.17810
The study reported in this article has been accepted for presentation the European Society of Cardiology Congress (August 2021).
Funding information Fundació Universitària Agustí Pedro i Pons, Universitat de Barcelona, Barcelona, Spain; Irish Research Council: Government of Ireland Postgraduate Scholarship Programme 2018, Grant/Award Number: GOIPG/2018/134; Science Foundation Ireland, Grant/Award Number: 19/IFA/7409
Contributor Information
Laura Pérez‐Denia, Email: prezdenl@tcd.ie, @LauraPerezDenia.
Ciarán Finucane, Email: cfinucane@stjames.ie.
REFERENCES
- 1. Finucane C, O'Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc. 2017;65(3):474‐482. doi: 10.1111/jgs.14563 [DOI] [PubMed] [Google Scholar]
- 2. Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567‐1582. doi: 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNicholas T, Tobin K, Carey D, O'Callaghan S, Kenny RA. Is baseline orthostatic hypotension associated with a decline in global cognitive performance at 4‐year follow‐up? Data from TILDA (the Irish longitudinal study on ageing). J Am Heart Assoc. 2018;7(19):e008976. doi: 10.1161/JAHA.118.008976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briggs R, Carey D, Kennelly SP, Kenny RA. Longitudinal association between orthostatic hypotension at 30 seconds post‐standing and late‐life depression. Hypertension. 2018;71(5):946‐954. doi: 10.1161/hypertensionaha.117.10542 [DOI] [PubMed] [Google Scholar]
- 5. O'Connell MD, Savva GM, Finucane C, Romero‐Ortuno R, Fan CW, Kenny RA. Impairments in hemodynamic responses to orthostasis associated with frailty: results from the Irish longitudinal study on ageing (TILDA). J Am Geriatr Soc. 2018;66(8):1475‐1483. doi: 10.1111/jgs.15327 [DOI] [PubMed] [Google Scholar]
- 6. Rockwood MRH, Howlett SE, Rockwood K. Orthostatic hypotension (OH) and mortality in relation to age, blood pressure and frailty. Arch Gerontol Geriatr. 2012;54(3):e255‐e260. doi: 10.1016/j.archger.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 7. Szufladowicz E, Maniewski R, Kozluk E, Zbiec A, Nosek A, Walczak F. Near‐infrared spectroscopy in evaluation of cerebral oxygenation during vasovagal syncope. Physiol Meas. 2004;25(4):823‐836. doi: 10.1088/0967-3334/25/4/004 [DOI] [PubMed] [Google Scholar]
- 8. Lieshout JJV, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94(3):833‐848. doi: 10.1152/japplphysiol.00260.2002 [DOI] [PubMed] [Google Scholar]
- 9. Kharraziha I, Holm H, Bachus E, et al. Cerebral oximetry in syncope and syndromes of orthostatic intolerance. Front Cardiovasc Med. 2019;6:171. doi: 10.3389/fcvm.2019.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bachus E, Holm H, Hamrefors V, et al. Monitoring of cerebral oximetry during head‐up tilt test in adults with history of syncope and orthostatic intolerance. Europace. 2018;20(9):1535‐1542. doi: 10.1093/europace/eux298 [DOI] [PubMed] [Google Scholar]
- 11. Briggs R, Carey D, Claffey P, et al. The association between frontal lobe perfusion and depressive symptoms in later life. Br J Psychiatry. 2019;214(4):230‐236. doi: 10.1192/bjp.2018.288 [DOI] [PubMed] [Google Scholar]
- 12. Fitzgibbon‐Collins LK, Heckman GA, Bains I, Noguchi M, McIlroy WE, Hughson RL. Older adults' drop in cerebral oxygenation on standing correlates with postural instability and may improve with sitting prior to standing. J Gerontol A Biol Sci Med Sci. 2020;76:1124‐1133. doi: 10.1093/gerona/glaa194 [DOI] [PubMed] [Google Scholar]
- 13. Maguire F, Romero‐Ortuno R, O'Connor JD, Reilly RB, Knight SP, Kenny RA. One‐dimensional statistical parametric mapping identifies impaired orthostatic cerebrovascular and cardiovascular response in frailty index. J Gerontol A Biol Sci Med Sci. 2020;76:885‐892. doi: 10.1093/gerona/glaa315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487‐1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finucane C, O'Connell MD, Fan CW, et al. Age‐related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130(20):1780‐1789. doi: 10.1161/circulationaha.114.009831 [DOI] [PubMed] [Google Scholar]
- 16. Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42(2):189‐214. doi: 10.1353/dem.2005.0011 [DOI] [PubMed] [Google Scholar]
- 17. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta‐analysis. Exp Gerontol. 2017;89:30‐40. doi: 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 18. Romme JJ, van Dijk N, Boer KR, et al. Influence of age and gender on the occurrence and presentation of reflex syncope. Clin Auton Res. 2008;18(3):127‐133. doi: 10.1007/s10286-008-0465-0 [DOI] [PubMed] [Google Scholar]
- 19. Finucane C, van Wijnen VK, Fan CW, et al. A practical guide to active stand testing and analysis using continuous beat‐to‐beat non‐invasive blood pressure monitoring. Clin Auton Res. 2019;29(4):427‐441. doi: 10.1007/s10286-019-00606-y [DOI] [PubMed] [Google Scholar]
- 20. Suzuki S, Takasaki S, Ozaki T, Kobayashi Y. Tissue oxygenation monitor using NIR spatially resolved spectroscopy. vol 3597. BiOS '99 International Biomedical Optics Symposium. SPIE; 1999.
- 21. Klem GH, Lüders HO, Jasper HH, Elger C. The ten‐twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3‐6. [PubMed] [Google Scholar]
- 22. Soraghan CJ, Fan CW, Hayakawa T, et al. TILDA Signal Processing Framework (SPF) for the analysis of BP responses to standing in epidemiological and clinical studies. IEEE‐EMBS International Conference on Biomedical and Health Informatics (BHI). 2014:793–796.
- 23. O'Connor JD, O'Connell MDL, Nolan H, Newman L, Knight SP, Kenny RA. Impact of standing speed on the peripheral and central hemodynamic response to orthostasis. Hypertension. 2020;75(2):524‐531. doi: 10.1161/HYPERTENSIONAHA.119.14040 [DOI] [PubMed] [Google Scholar]
- 24. Remmen JJ, Aengevaeren WR, Verheugt FW, et al. Finapres arterial pulse wave analysis with Modelflow is not a reliable non‐invasive method for assessment of cardiac output. Clin Sci. 2002;103(2):143‐149. doi: 10.1042/cs1030143 [DOI] [PubMed] [Google Scholar]
- 25. la Cour A, Greisen G, Hyttel‐Sorensen S. In vivo validation of cerebral near‐infrared spectroscopy: a review. Neurophotonics. 2018;5(4):40901. doi: 10.1117/1.NPh.5.4.040901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madsen P, Lyck F, Pedersen M, Olesen HL, Nielsen HB, Secher NH. Brain and muscle oxygen saturation during head‐up‐tilt‐induced central hypovolemia in humans. Clin Physiol. 1995;15(5):523‐533. doi: 10.1111/j.1475-097X.1995.tb00541.x [DOI] [PubMed] [Google Scholar]
- 27. Jedrzejczyk‐Spaho J, Pietrucha AZ, Bzukala I, Konduracka E, Nessler J. P6630Changes of cerebral oxygen saturation measured by near infra‐red spectroscopy in patients with vasovagal syncope during head‐up tilt test, considering their age, gender and concomitant diseases. Eur Heart J. 2018;39(suppl_1):6630. doi: 10.1093/eurheartj/ehy566.P66302018 [DOI] [Google Scholar]
- 28. Lieshout JJ, Pott F, Madsen PL, Goudoever J, Secher NH. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32(7):1546‐1551. doi: 10.1161/01.STR.32.7.1546 [DOI] [PubMed] [Google Scholar]
- 29. Immink RV, Pott FC, Secher NH, van Lieshout JJ. Hyperventilation, cerebral perfusion, and syncope. J Appl Physiol. 2014;116(7):844‐851. doi: 10.1152/japplphysiol.00637.2013 [DOI] [PubMed] [Google Scholar]
- 30. Lankford J, Numan M, Hashmi SS, Gourishankar A, Butler IJ. Cerebral blood flow during HUTT in young patients with orthostatic intolerance. Clin Auton Res. 2015;25(5):277‐284. doi: 10.1007/s10286-015-0295-9 [DOI] [PubMed] [Google Scholar]
- 31. Moloney D, O'Connor J, Newman L, et al. Clinical clustering of eight orthostatic haemodynamic patterns in The Irish Longitudinal Study on Ageing (TILDA). Age Ageing. 2021;50(3):854‐860. doi: 10.1093/ageing/afaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lagro J, Schoon Y, Heerts I, et al. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J Gerontol A Biol Sci Med Sci. 2014;69(4):471‐478. doi: 10.1093/gerona/glt111 [DOI] [PubMed] [Google Scholar]
- 33. Romero‐Ortuno R, Cogan L, O'Shea D, Lawlor BA, Kenny RA. Orthostatic haemodynamics may be impaired in frailty. Age Ageing. 2011;40(5):576‐583. doi: 10.1093/ageing/afr076 [DOI] [PubMed] [Google Scholar]
- 34. Romero‐Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc. 2011;59(4):655‐665. doi: 10.1111/j.1532-5415.2011.03352.x [DOI] [PubMed] [Google Scholar]
- 35. Shaw BH, Borrel D, Sabbaghan K, et al. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC Geriatr. 2019;19(1):80. doi: 10.1186/s12877-019-1082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leidhin CN, McMorrow J, Carey D, et al. Age‐related normative changes in cerebral perfusion: data from The Irish Longitudinal Study on Ageing (TILDA). Neuroimage. 2021;229:117741. doi: 10.1016/j.neuroimage.2021.117741 [DOI] [PubMed] [Google Scholar]
- 37. Deegan BM, Sorond FA, Galica A, Lipsitz LA, O'Laighin G, Serrador JM. Elderly women regulate brain blood flow better than men do. Stroke. 2011;42(7):1988‐1993. doi: 10.1161/strokeaha.110.605618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng Y‐C, Vyas A, Perlmuter LC, Hymen E. Gender differences in orthostatic hypotension. Am J Med Sci. 2011;342(3):221‐225. doi: 10.1097/MAJ.0b013e318208752b [DOI] [PubMed] [Google Scholar]
- 39. McNicholas T, Tobin K, Newman L, Claffey P, Briggs R, Kenny RA. P3835Cerebral perfusion in atrial fibrillation. Eur Heart J. 2018;39(Suppl_1):817. doi: 10.1093/eurheartj/ehy563.P3835 28025192 [DOI] [Google Scholar]
- 40. Fraser KS, Heckman GA, McKelvie RS, Harkness K, Middleton LE, Hughson RL. Cerebral hypoperfusion is exaggerated with an upright posture in heart failure: impact of depressed cardiac output. JACC: Heart Fail. 2015;3(2):168‐175. doi: 10.1016/j.jchf.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 41. Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep. 2018;20(8):37. doi: 10.1007/s11883-018-0739-5 [DOI] [PubMed] [Google Scholar]
- 42. Guo ZN, Xing Y, Wang S, Ma H, Liu J, Yang Y. Characteristics of dynamic cerebral autoregulation in cerebral small vessel disease: diffuse and sustained. Sci Rep. 2015;5:15269. doi: 10.1038/srep15269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid Distensibility in hypertensive elderly subjects. Hypertension. 2005;45(2):216‐221. doi: 10.1161/01.HYP.0000153094.09615.11 [DOI] [PubMed] [Google Scholar]
- 44. Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172(2):117‐120. doi: 10.1016/j.pscychresns.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mattace‐Raso FU, van der Cammen TJ, Knetsch AM, et al. Arterial stiffness as the candidate underlying mechanism for postural blood pressure changes and orthostatic hypotension in older adults: the Rotterdam study. J Hypertens. 2006;24(2):339‐344. doi: 10.1097/01.hjh.0000202816.25706.64 [DOI] [PubMed] [Google Scholar]
- 46. Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle‐aged and older Europeans. Age Ageing. 2013;42(5):614‐619. doi: 10.1093/ageing/aft010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas KN, Cotter JD, Galvin SD, Williams MJA, Willie CK, Ainslie PN. Initial orthostatic hypotension is unrelated to orthostatic tolerance in healthy young subjects. J Appl Physiol. 2009;107(2):506‐517. doi: 10.1152/japplphysiol.91650.2008 [DOI] [PubMed] [Google Scholar]
- 48. Chen W‐L, Wagner J, Heugel N, et al. Functional near‐infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Review. Front Neurosci. 2020;14(724). doi: 10.3389/fnins.2020.00724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoshitani K, Kawaguchi M, Miura N, et al. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near‐infrared spectroscopy measurements. Anesthesiology. 2007;106(3):458‐462. [DOI] [PubMed] [Google Scholar]
- 50. Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116(4):834‐840. doi: 10.1097/ALN.0b013e31824c00d7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1 Description of all features derivation
Table S1: Summary of features derivation
Table S2: Average and variability values for the TSI, SBP, DBP, HR, TPR, and SV features analyzed for all patients, females and males, comparing differences between sex groups.
Table S3: Average and variability absolute values for the SBP, DBP and HR features analyzed for all patients, females and males, comparing differences between sex.
Table S4: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR, SV, TPR) and cerebrovascular (TSI) features in all patients.
Table S5: Effects of multimorbidity on TSI when correcting for covariates and concurrent SBP features.
Table S6: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent DBP features
Table S7: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent MAP features
Table S8: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent TPR features
Table S9: Effects of multimorbidity on tissue saturation index when correcting for covariates and concurrent SV features
Table S10: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in females.
Table S11: Effects of multimorbidity on the full set of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in males.
Table S12: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features in all patients.
Table S13: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in females.
Table S14: Effects of multimorbidity on absolute values of cardiovascular (SBP, DBP, HR) and cerebrovascular (TSI) features investigated in males.
Figure S1: Absolute drops from baseline in TPR and SV responses for all patients, females and males during the active standing test for two levels of multimorbidity.
Figure S2: Absolute cardiovascular (SBP, DBP, HR) hemodynamic responses for all patients, females and males during the active standing test for two levels of multimorbidity.


