Abstract
Background and Aims
Mulibrey nanism (MUL) is a multiorgan disease caused by recessive mutations in the TRIM37 gene. Chronic heart failure and hepatopathy are major determinants of prognosis in MUL patients, which prompted us to study liver biochemistry and pathology in a national cohort of MUL patients.
Methods
Clinical, laboratory and imaging data were collected in a cross‐sectional survey and retrospectively from hospital records. Liver histology and immunohistochemistry for 10 biomarkers were assessed.
Results
Twenty‐one MUL patients (age 1–51 years) with tumour suspicion showed moderate congestion, steatosis and fibrosis in liver biopsies and marginally elevated levels of serum GGT, AST, ALT and AST to platelet ratio index (APRI) in 20%–66%. Similarly, GGT, AST, ALT and APRI levels were moderately elevated in 12%–69% of 17 MUL patients prior to pericardiectomy. In a cross‐sectional evaluation of 36 MUL outpatients, GGT, total bilirubin and galactose half‐life (Gal½) correlated with age (r = 0.45, p = .017; r = 0.512, p = .007; r = 0.44, p = .03 respectively). The frequency of clearly abnormal serum values of 15 parameters analysed, however, was low even in patients with signs of restrictive cardiomyopathy. Transient elastography (TE) of the liver revealed elevated levels in 50% of patients with signs of heart failure and TE levels correlated with several biochemistry parameters. Biomarkers of fibrosis, sinusoidal capillarization and hepatocyte metaplasia showed increased expression in autopsy liver samples from 15 MUL patients.
Conclusion
Liver disease in MUL patients was characterized by sinusoidal dilatation, steatosis and fibrosis with individual progression to cirrhosis and moderate association of histology with cardiac function, liver biochemistry and elastography.
Keywords: congestive hepatopathy, elasticity imaging techniques, immunohistochemistry, liver cirrhosis, MUL, TRIM37
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- APRI
AST to platelet ratio index
- AST
aspartate aminotransferase
- CK7
cytokeratin 7
- EpCAM
epithelial cellular adhesion molecule
- FV
coagulation factor V
- Gal½
galactose half‐life
- GGT
gamma‐glutamyltransferase
- HSC
hepatic stellate cells
- MUL
Mulibrey nanism
- NYHA
New York Heart Association
- proBNP
pro‐B‐type natriuretic peptide
- PSGL‐1
P‐selectin glycoprotein ligand‐1
- SMA
alpha smooth muscle actin
- TE
transient elastography
- TT
partial thromboplastin time
Lay Summary
Patients with Mulibrey nanism showed variable degree of liver disease, which mostly seems to be caused by heart dysfunction. Laboratory tests and measurement of liver stiffness could only partially reveal the liver changes.
1. INTRODUCTION
Mulibrey nanism (MUL) is an autosomal recessive disease caused by mutations in the TRIM37 gene encoding the peroxisomal TRIM37 protein with ubiquitin ligase activity. 1 , 2 MUL typically causes growth restriction of prenatal onset, typical craniofacial features, infertility and multiple organ manifestations, but no major neurological defects. 3 , 4 The patients are thin and gracile in childhood, but from puberty, a tendency towards abdominal obesity, insulin resistance, metabolic syndrome and type 2 diabetes is observed. 5 Moreover, an increased risk for tumours in several organs has been reported. 6 , 7 The majority of known MUL cases have been reported from Finland. The Finnish patients are genotypically homogenous as over 90% of the patients carry the founder mutation (Fin‐major, c. 493‐2A > G) in a homozygous form. 1 , 3
Cardiac abnormalities are major determinants of prognosis in MUL disease. Variable signs of pericardial constriction and myocardial restriction may lead to congestive heart failure and right heart disease already at young age. 8 Some patients may benefit from pericardiectomy, but congestive heart failure nevertheless progresses in one‐third. 9 Thus, these patients are at a risk for developing liver failure. Clinical experience shows that some MUL patients have hepatomegaly already in early childhood and at the time of diagnosis, 45% of patients display liver enlargement. 3 Moreover, steatosis, fibrosis and blood‐filled cysts (peliosis) have been described in MUL livers. 6
Laboratory tests and non‐invasive methods, such as liver stiffness measurement by elastography, give conflicting results of the liver status in patients with chronic heart disease and especially those with congestive heart failure. 10 , 11 , 12 Also, little is known how the absence of TRIM37 protein affects liver pathology in general. These questions prompted us to study systematically hepatic findings in MUL patients, using microscopy and immunohistochemistry of liver biopsy and autopsy samples, blood biochemistry and transient elastography (TE). The liver findings were associated with the cardiac status of the patients.
2. PATIENTS AND METHODS
2.1. Patients
The study comprises four overlapping groups of the Finnish MUL patients. The first group includes 21 stable MUL patients (age range 0.9–59 years), who underwent liver biopsy owing to suspicious ultrasound findings (mostly tumorous lesions). These biopsy findings were retrospectively compared with contemporary liver biochemistry values. The second group comprises 17 patients who underwent pericardiectomy owing to constrictive pericarditis (age range 0.5–38 years). Preoperative liver biochemistry in these patients was retrospectively recorded. The third group is a cross‐sectional cohort of 36 MUL patients (age range 0.21–51.1 years), who were clinically stable and had a routine visit at the outpatient clinics of the Helsinki University Hospital. The studies of these patients included blood biochemistry, galactose half‐life measurement, liver ultrasound and transient elastography as well as cardiac evaluation. The fourth group includes 22 deceased patients (age at death 0.65–48.7 years), of whom original autopsy reports were available. In addition, 15 of these patients had liver tissue samples available for histological and immunohistochemical analyses.
The inclusion criteria comprised adequate clinical findings and TRIM37 gene mutations. One patient had limited clinical data and was excluded from the analyses. In clinical studies, all except one patient were Fin major (c.493‐2A > G) homozygotes. One patient had Fin minor (c.2212delG)/Fin‐major genotype. Of the deceased patients, 17 were Fin‐major homozygotes, one had Fin‐minor/Fin‐major genotype, three sisters had c.1166A > G/Fin‐major mutations and one had c.227 T > C/Fin‐major mutations. All mutations led to truncated TRIM37 protein.
2.2. Clinical evaluation
MUL patients were clinically followed by experienced paediatricians and paediatric and adult cardiologists, as previously described. 13 , 14 The patients were mostly treated and followed at the Helsinki University Hospital, forming the biggest MUL cohort worldwide. Liver ultrasound and transient elastography (TE) (FibroScan®; Echosens) were performed according to standard methods. 15 TE measurement values over 7 kPa were regarded as increased in all age groups. Cardiac evaluation was performed by an experienced cardiologist (EJ). The assessment of heart function was based on clinical signs (hepatomegaly and filling level of the jugular veins), impaired ejection fraction or signs of restrictive physiology on echocardiography. These signs included impaired collapsing of inferior vena cava, retrograde flow of hepatic veins and breathing variation of tricuspid E wave exceeding 30%. Besides echocardiography findings, the plasma proBNP levels were used in the assessment of cardiac function (Table 4). Values over 178 ng/L (patients under 20 years), 80 ng/L (males) and 150 ng/L (females) were regarded as abnormal. 16
TABLE 4.
Laboratory values and TE compared with signs of heart disease in 36 MUL patients
| Signs of restrictive physiology in echocardiography a | ProBNP above normal | |||||
|---|---|---|---|---|---|---|
| Present (n = 18) | Absent (n = 12) | p | Yes (n = 12) | No (n = 15) | p | |
| TE, kPa | 6.9 (4.0–26.1) | 5.4 (3.8–21.4) | .28 | 7.1 (4.5–25.3) | 6.6 (4.0–26.1) | .92 |
| Galactose t½, min | 9.5 (6.5–12.0) | 9.8 (8.0–12.5) | .44 | 9.5 (6.5–12.0) | 9.5 (7.5–12.5) | .85 |
| APRI | 0.3 (0.1–0.6) | 0.3 (0.2–0.6) | .67 | 0.3 (0.2–0.6) | 0.3 (0.1–0.6) | .92 |
| TT, % | 97 (55–136) | 126 (96–155) | .047 | 103 (65–116) | 113.5 (55–156) | .20 |
| Fibrinogen, g/L | 2.9 (2.2–5.0) | 2.4 (1.9–3.7) | .14 | 3.0 (1.9–4.5) | 2.65 (2.2–5.0) | .37 |
| ALT, U/L | 29 (13–57) | 29 (18–130) | .48 | 28.5 (13–57) | 28 (14–130) | .67 |
| AST, U/L | 35 (20–60) | 34.5 (29–53) | .64 | 39 (31–60) | 35 (20–53) | .39 |
| GGT, U/L | 24 (9–109) | 26 (12–128) | 1.00 | 24 (11–109) | 21.5 (9–128) | .88 |
| Bilirubin, μmol/L | 8 (3–31) | 10 (7–17) | .19 | 7 (3–21) | 8 (4–31) | .43 |
| Albumin, g/L | 41.2 (36.5–44.8) | 41.4 (37.4–46.4) | .37 | 41.4 (37.4–44.8) | 39.7 (36.5–46.4) | .60 |
| Factor V,% | 99 (66–130) | 112 (78–143) | .64 | 101 (83–130) | 113 (66–143) | .64 |
| Bile acids μmol/L | 3.4 (0.0–47.6) | 3.7 (2.6–6.6) | .54 | 3.4 (0.0–47.6) | 3.4 (0.0–11.1) | .80 |
| Prealbumin, mg/L | 219 (134–261) | 263 (190–429) | .07 | 190.5 (134–261) | 240.5 (190–429) | .031 |
| Haemoglobin, g/L | 131 (118–170) | 139 (94–172) | .34 | 144 (118–170) | 136 (123–172) | .91 |
| Leucocytes, E9/L | 5.6 (4.1–15.0) | 5.6 (4.5–11.0) | .49 | 5.6 (4.3–15.0) | 6.0 (4.1–11.0) | .73 |
| Thrombocytes, E9/L | 252 (152–526) | 236 (198–350) | 1.00 | 236 (152–526) | 254 (183–361) | .92 |
Note: Data presented as median (range). Significant p values are bolded.
Signs of restrictive physiology include impaired collapsing of inferior vena cava, retrograde flow of hepatic veins and breathing variation of tricuspid E wave exceeding 30%.
2.3. Liver biochemistry
Liver biochemistry included blood values of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyl transferase (GGT), total bilirubin, conjugated bilirubin, albumin, fibrinogen, partial thromboplastin time (TT), coagulation factor V (FV), prealbumin, haemoglobin, leucocytes and thrombocytes. AST to platelet ratio index (APRI) was calculated using a standard formula with a cut‐off value of 0.5. 17 Galactose half‐life test (Gal½) was performed as previously reported, with a cut‐off value of 12 min. 18 The normal range for each parameter was defined by the HUS Clinical Laboratory, Helsinki University Hospital. The cut‐off values were adjusted for age and sex when needed.
2.4. Liver histology and immunohistochemistry
Liver specimens from MUL patients were obtained by percutaneous core‐needle biopsies on clinical indications (21 patients) as well as from autopsies (15 patients). For immunohistochemistry, six samples from liver transplant donors were used as normal controls. These specimens were taken as wedge biopsies at the operation.
Haematoxylin‐eosin and Masson’s trichrome stainings were used for histological analysis. Immunohistochemical stainings on formalin‐fixed autopsy samples were performed as previously reported. 19 Immunohistochemical grading was done semiquantitatively by the first and last authors on a scale of 0–3 for each marker. At least 20 visual fields per sample were analysed. Central, lobular (sinusoidal) and portal areas were separately scored, except in the staining for CK7, where bile ducts and parenchyma (hepatocytes) were scored separately. Also, search for malignant lesions was performed using EpCAM and glypican‐3 stainings in 15 MUL autopsy livers (and 5 controls). The primary antibodies used for stainings were as follows: alpha smooth muscle actin (SMA) (1:400, Agilent Dako), vimentin (1:100, Agilent), collagen 1 (1:1000, Abnova), CD34 (1:100, Agilent), CD68 (1:400, Cell Signalling Technology), P‐selectin glycoprotein ligand‐1 (PSGL‐1) (1:500, Santa Cruz Biotechnology), cytokeratin 7 (CK7) (1:100, DakoCytomation Norden A/S), EpCAM (BER‐EP4, 1:100, Dako) and glypican‐3 (1:100, Invitrogen, Thermo Fisher). Microscopy was performed with Zeiss microscope (Carl Zeiss AG) using 10–40× magnification.
2.5. Statistics
Statistical analyses were performed by IBM SPSS Statistics 25 (IBM). The normality of the distribution among different groups was assessed with Kolmogorov–Smirnov and Shapiro–Wilk tests. For comparisons, T‐test was used for normally distributed parameters and Mann–Whitney U or independent samples Kruskal–Wallis test for not normally distributed parameters. Correlations were calculated with Spearman’s rho. Two‐tailed p‐values <.05 were considered statistically significant.
2.6. Ethical issues
This study was approved by the Institutional Ethical Review Board at the University of Helsinki (approval numbers 420/E7/2001 and HUS/1538/2016§38). Informed consent was obtained from all patients or their guardians. The procedures involving human subjects were in accordance with the Helsinki Declaration of 1975 as revised in 1983 or comparable ethical standards.
3. RESULTS
3.1. Liver pathology and biochemistry in patients undergoing liver biopsy
Twenty‐one clinically stable MUL patients underwent liver biopsy owing to abnormal ultrasound findings in the clinical follow‐up. The indications included suspicion of tumour (n = 13), hepatomegaly (n = 5), abnormal blood biochemistry (n = 1) and suspicion of cirrhosis (n = 1). Eight of the patients were < 10 years of age, five between 10 and 20 years and eight over 20 years.
Moderate fibrosis (62%) and steatosis (43%) were observed in all age groups. Two adults (ages 41 and 48 years) had advanced cirrhosis. Congestion or sinus dilatation (38%), biliary tract proliferation (24%) and cholestasis (14%) were present in a minority of the samples. Neoplasia or dysplasia was not found in any of the biopsies, indicating that ultrasound revealed vascular lesions which are common in MUL. The benign nature of the lesions was supported by the fact that none of the patients undergoing biopsy developed liver carcinoma during the follow‐up of 2–26 years.
Data on ALT, AST, GGT, total bilirubin, APRI and TT levels were available in 16 patients (Table 1). Half of the patients with fibrosis had moderately elevated levels of GGT, ALT and AST (up to 411, 63 and 71 U/L respectively). All patients with congestion had slightly increased AST levels (up to 71 U/L). APRI score was slightly increased (0.5–0.9) in half of the patients with steatosis.
TABLE 1.
Liver parameters in MUL patients undergoing liver biopsy or pericardiectomy (see the text)
| ALT, U/L | AST, U/L | GGT, U/L | Bilirubin, μmol/L | TT, % | APRI | |
|---|---|---|---|---|---|---|
| Biopsy finding | ||||||
| Fibrosis | 40.5 (13–63) a | 40 (22–71) | 59.5 (10–411) | 11 (2–18) | 87.5 (52–118) | 0.4 (0.3–1.2) |
| 5/12 (42) b | 6/10 (60) | 5/10 (50) | 0/9 (0) | 4/12 (33) | 4/9 (44) | |
| Congestion | 26 (17–43) | 60.5 (38–71) | 159 (21–411) | 8 (3–16) | 99 (59–118) | 0.45 (0.3–0.7) |
| 1/5 (20) | 4/4 (100) | 2/3 (66) | 0/5 (0) | 1/5 (20) | 2/4 (50) | |
| Steatosis | 43 (25–58) | 40 (33–71) | 28 (10–159) | 6.5 (2–8) | 90.5 (52–101) | 0.5 (0.3–0.9) |
| 4/8 (50) | 3/7 (42) | 2/7 (29) | 0/4 (0) | 2/8 (25) | 4/7 (57) | |
| Pericardiectomy | ||||||
| Preoperative value | 32 (12–50) | 49.5 (13–84) | 62 (31–411) | 10.5 (8–35) | 62 (46–129) | 0.4 (0.1–1.4) |
| 4/17 (24) | 9/16 (56) | 9/13 (69) | 2/10 (20) | 9/15 (60) | 2/15 (13) | |
| Postoperative value | 27 (11–91) | 39 (23–47) | 55.5 (42–69) | 14 (9–19) | 65 (46–78) | 0.25 (0.1–0.4) |
| 2/7 (29) | 1/6 (17) | 2/2 (100) | 0/2 (0) | 3/4 (75) | 0/4 (0) | |
Median value, range.
Number of patients with abnormal value/number of patients in the group (percentage).
3.2. Liver biochemistry in patients before and after pericardiectomy
Seventeen MUL patients underwent pericardiectomy owing to severe congestive heart failure at a median age of 8 years (0.5–38 years; five patients <2 years). The GGT level was moderately increased (up to 411 U/L) in 9/13 (69%) patients before the operation. Similarly, AST levels were abnormal (up to 84 U/L) in 9/16 (56%) patients. On the other hand, ALT values were marginally elevated (up to 50 U/L) in only 4/17 (24%) patients. APRI was abnormal (0.8 and 1.4) in two (12%) MUL children (Table 1). Data on the liver parameters after pericardiectomy were limited (Table 1). Before and after the operation, the median values of ALT (32 vs 27 U/L) and AST (49.5 vs. 39 U/L) were quite similar with a maximal individual decrease of 10 and 31 U/L respectively.
3.3. Blood biochemistry in a cross‐sectional survey of clinically stable MUL patients
Liver function of 36 stable MUL patients was evaluated in a cross‐sectional analysis. Ten of the patients were under 10 years of age, 12 were 10–20 years and 14 were over 20 years. Liver values in the three age groups were mostly normal or only marginally elevated as shown in Tables 2 and 3. Overall, transaminase (ALT and AST) levels were elevated above the normal range in 10–36% of the patients, cholestatic markers (GGT, bilirubin and bile acids) in 0%–27%, synthetic markers (prealbumin, albumin, TT and fibrinogen) in 0%–29% and APRI‐score (0.5–1.2) in 17% of the patients (Table 3). Values exceeding two times the normal range were, however, recorded in only seven measurements: ALT 108 and 130 U/L; GGT 109 and 128 U/L; bile acids 47.6 and 16.8 μmol/L and TT 25%.
TABLE 2.
Liver biochemistry and transient elastography (TE) in 36 clinically stable MUL patients
| Normal value | 0–10 years (n = 10) | 11–20 years (n = 12) | ≥21 years (n = 14) | p | |
|---|---|---|---|---|---|
| TE, kPa | <7 | 6.0 (3.8–25.3) | 6.8 (4.0–26.1) | 9.7 (4.7–26.3) | .30 |
| Galactose t1/2, min | <12 | 9.5 (6.5–10.5) | 9.5 (7.5–12.5) | 10.0 (8.0–12.5) | .33 |
| APRI | <0.5 | 0.3 (0.2–0.6) | 0.3 (0.1–0.6) | 0.3 (0.2–1.2) | .74 |
| ALT, U/L | <35–50 a | 28 (18–43) | 32 (14–130) | 29.5 (13–67) | .65 |
| AST, U/L | <36–50 a | 44.5 (30–60) | 34 (20–53) | 33.5 (26–46) | .10 |
| GGT, U/L | <40–60 a | 16 (9–80) | 36 (12–128) | 33 (19–93) | .14 |
| Bilirubin, μmol/L | <20 | 7 (3–9) | 9.5 (5–31) | 10 (5–22) | .049 † |
| Bile acids, μmol/L | <6.0 | 4.4 (0.0–11.1) | 3.3 (0.0–6.6) | 3.7 (0.0–47.6) | .15 |
| TT, % | ≥70 | 104.5 (65–155) | 97 (55–136) | 108 (25–156) | .97 |
| Fibrinogen, g/L | ≥2 | 2.6 (1.9–4.5) | 3.0 (2.2–5.0) | 2.6 (2.2–3.3) | .41 |
| FV,% | ≥65 | 95 (83–143) | 109 (66–126) | 96.5 (47–128) | .61 |
| Albumin, g/L | ≥35–37 a | 40.9 (37.8–44.8) | 39.45 (36.5–44.7) | 41.55 (36.3–46.4) | .65 |
| Prealbumin, mg/L | ≥95–200 a | 190 (134–261) | 240.5 (167–429) | 233 (127–388) | .30 |
| Haemoglobin, g/L | ≥94–134 a | 123.5 (94–146) | 139.5 (124–172) | 152 (137–170) | .001 * |
| Leucocytes, E9/L | ≥3.4–6.0 a | 5.45 (4.3–15.0) | 6.15 (4.1–12.1) | 5.6 (4.0–6.6) | .80 |
| Thrombocytes, E9/L | ≥150–200 a | 261.5 (176–526) | 249 (183–361) | 232 (106–280) | .34 |
Note: Values are in median (range). Significant p values (<.05) are bolded.
Depending on age and/or gender.
Between first and third age group p = .001.
Between first and third age groups p = .040, between first and second groups p = .026.
TABLE 3.
Frequency of abnormal values and TE (%) in MUL patients
| ALT | AST | GGT | Bilirubin | Bile acids | Albumin | Prealbumin | TT | FV | Gal½ | APRI | TE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||||
| 0–10 | 2/8 (25) | 3/8 (38) | 2/8 (25) | −/8 | 3/7 (43) | −/8 | 1/7 (14) | −/8 | −/7 | −/5 | 2/7 (29) | 3/9 (33) |
| 11–20 | 4/11 (36) | 1/10 (10) | 3/11 (27) | 2/10 (20) | 1/11 (9) | −/12 | 1/10 (10) | 1/11 (9) | −/11 | −/11 | 1/10 (10) | 5/10 (50) |
| >20 | 4/12 (33) | 1/8 (13) | 1/8 (13) | 1/8 (13) | 2/7 (29) | −/10 | 2/7 (29) | −/9 | 1/8 (13) | −/8 | 1/8 (13) | 6/10 (60) |
| Echocardiography | ||||||||||||
| Restriction a | 5/17 (29) | 3/16 (19) | 4/16 (25) | 2/15 (13) | 4/15 (27) | 1/17 (6) | 3/14 (21) | 2/16 (13) | −/14 | −/14 | 2/16 (13) | 8/16 (50) |
| No restriction | 3/8 (38) | 1/6 (17) | 1/7 (14) | −/7 | 1/7 (14) | −/9 | 0/7 | −/7 | −/7 | −/6 | 1/5 (20) | 3/9 (33) |
| ProBNP | ||||||||||||
| Elevated | 4/12 (33) | 2/9 (22) | 3/9 (33) | 1/9 (11) | 3/8 (34) | −/9 | 3/8 (34) | 1/9 (11) | −/7 | −/8 | 2/9 (22) | 5/10 (50) |
| Normal b | 4/14 (29) | 2/13 (15) | 2/14 (14) | 1/13 (8) | 2/13 (15) | 1/15 (7) | −/12 | 1/14 (7) | −/14 | −/12 | 1/12 (8) | 5/11 (45) |
| TE | ||||||||||||
| ≥7 kPa | 4/13 (31) | 2/11 (18) | 4/11 (36) | 3/11 (27) | 3/10 (30) | 1/12 (8) | 2/11 (18) | 4/12 (33) | 1/12 (8) | −/10 | 3/10 (30) | 14/14 |
| <7 kPa | 2/12 (17) | 2/12 (17) | 1/12 (8) | −/11 | 3/12 (25) | −/12 | 2/10 (20) | −/12 | −/10 | −/10 | −/12 | −/15 |
Signs of restrictive physiology in echocardiography.
Children and adolescents >178, adult males >50, adult females >180.
In Spearman’s bivariate analysis, GGT and total bilirubin values increased with age (r = 0.45, p = .017; r = 0.512, p = .007 respectively), which was not seen with the other markers (Figure 1). Gal½ showed an association with ALT (r = 0.53, p = .008), GGT (r = 0.63, p = .001) and APRI (r = 0.65, p = .002) in the Spearman analysis.
FIGURE 1.
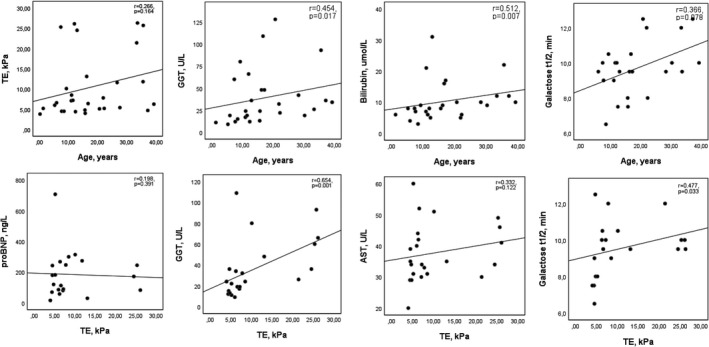
Association of transient elastography, age and some biochemical parameters in clinically stable MUL patients. AST, aspartate aminotransferase; GGT, gamma‐glutamyltransferase; proBNP, pro‐B‐type natriuretic peptide; TE, transient elastography
3.4. Liver biochemistry and cardiac function in clinically stable patients
Cardiac evaluation was performed in 26 MUL patients concurrently with liver biochemistry analyses. Echocardiography showed signs of restrictive physiology in 11/22 (50%) patients, and proBNP levels were above the normal range in 16/26 (62%) patients. The liver values in these patient groups were not very different (Tables 3 and 4). GGT levels were more often above the normal range in patients with restriction (25%) or elevated proBNP level (33%) as compared to those with normal heart findings (14% in both). The TT value was slightly lower in patients with signs of heart disease (p = .047, median 97% vs. 126%). Similarly, median prealbumin value was marginally decreased (190 vs. 240 mg/L; p = .031) in patients with elevated proBNP levels. The Spearman analysis showed a negative correlation between proBNP and prealbumin levels (r = −0.63, p = .003), but no association with other parameters was found.
3.5. Transient elastography (liver stiffness) in comparison with hepatic and cardiac functions
Transient elastography (TE) of the liver showed elevated values (>7 kPa) in 14/29 (58%) patients who underwent a cross‐sectional survey of liver and heart functions. Six patients (20%) had a highly elevated TE value (21.4–26.3 kPa). TE levels did not correlate significantly with the age of the patients (r = 0.266, p = .164) (Figure 1). However, they are associated with several biochemical parameters. In Spearman’s bivariate analysis, positive association was strong with ALT (r = 0.65, p < .001), GT (r = 0.65, p = .001) and moderate with Galt1/2 (r = 0.48, p = .033), FV (r = 0.52, p = .012) and APRI (r = 0.58, p = .005). Thrombocytes and TT showed a strong negative correlation with TE (r = −0.64, p = .001 and r = −0.68, p < .001 respectively).
Patients with and without signs of heart disease as indicated by restrictive physiology on echocardiography (Table 4) had a median TE value of 6.9 kPa (4.0–26.1 kPa) and 5.4 (3.8–21.4 kPa) (p = .28) respectively. TE levels were above 7 kPa in 8/16 (50%) and 3/9 (33%) in the two groups. Patients with and without an elevated proBNP level had an increased TE value in 5/10 (50%) and 5/11 (45%) patients respectively. TE values did not associate with proBNP levels in the Spearman analysis (r = 0.198, p = .391).
3.6. Liver pathology and biomarker expression in autopsy samples
Original autopsy reports on liver pathology were available from 22 MUL patients who deceased between the years 1970 and 2020. The major finding in 10 children (0.65–14.6 years) was congestion with variable amount of fibrosis, but no cirrhosis. In seven young adults (22.3–39.3 years), advanced fibrosis was reported in all samples with cirrhosis in three (youngest 24 years). Five deceased patients were over 40 years (41.7–48.7 years), and all had severe cirrhosis.
Liver specimens from 15 deceased MUL patients (age 0.65 to 48.7 years) were available for in‐depth histological and immunohistochemical assessments. The major cause of death was heart failure in 11 patients and pneumonia in 4 cases. All liver specimens showed congestion and sinus dilatation. Fibrosis was abundant and fibrotic bridging (porto‐portal and porto‐central) was present in 13/15 samples. Nodularity, hepatocyte atrophy and steatosis were observed in 60%, 73% and 60% respectively (Table 5). Five of the six patients, who were compound heterozygotes, had died at the age of 0.65–31.1 years and in autopsy samples, their liver histology was not significantly different from that of Fin‐major homozygotes (Table 5).
TABLE 5.
Liver histology in 15 autopsy samples of MUL patients deceased at the age of 0.65–48.7 years
| Patient | Age | Genotype | Steatosis | Congestion | Sinus dilatation | Hepatocyte atrophy | Bridging fibrosis | Nodularity |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.65 | Fin‐maj/c.1166A > G | − | + | + | − | − | − |
| 2 | 1.1 | Fin‐maj/c.1166A > G | + | +++ | ++ | +++ | + | − |
| 3 | 1.3 | Fin‐maj/Fin‐maj | − | +++ | ++ | ++ | ++ | − |
| 4 | 2 | Fin‐maj/Fin‐maj | + | +++ | ++ | ++ | +++ | + |
| 5 | 2.1 | Fin‐maj/c.1166A > G | − | ++ | − | − | − | − |
| 6 | 2.6 | Fin‐maj/Fin‐maj | + | ++ | + | − | ++ | − |
| 7 | 3.5 | Fin‐maj/c.227 T > C | − | ++ | ++ | + | ++ | + |
| 8 | 12.6 | Fin‐maj/Fin‐maj | − | ++ | ++ | + | + | − |
| 9 | 13.9 | Fin‐maj/Fin‐maj | + | +++ | ++ | + | +++ | + |
| 10 | 14.5 | Fin‐maj/Fin‐maj | + | ++ | ++ | + | ++ | + |
| 11 | 31.1 | Fin‐maj/Fin‐min | + | +++ | + | ++ | + | + |
| 12 | 31.6 | Fin‐maj/Fin‐maj | + | + | − | + | ++ | + |
| 13 | 39 | Fin‐maj/Fin‐maj | +++ | ++ | − | − | +++ | ++ |
| 14 | 41.3 | Fin‐maj/Fin‐maj | − | + | + | + | +++ | +++ |
| 15 | 48.7 | Fin‐maj/Fin‐maj | ++ | ++ | ++ | +++ | +++ | +++ |
Note: Semi‐quantitative scoring from − to +++. Fin‐major mutation = c.493‐2A > G; Fin‐minor mutation = c.2212delG. Staining, haematoxylin‐eosin.
The amount of fibrosis was scored (0–3) using Masson’s trichrome staining in MUL and control livers (Table 6). The staining, indicating fibrosis, was significantly increased in central (p < .001), sinusoidal (p < .001) and portal (p < .001) areas of MUL livers. The immunohistochemical expression of type 1 collagen, the main marker for fibrosis, was clearly elevated in the portal and sinusoidal areas (p < .001, .05), and SMA, the marker for activated HSC, was increased in portal areas (p = .019). Scores for endothelial cell marker CD34 were higher in the portal and sinusoidal areas of MUL livers as compared to controls (p < .001 and p = .004). Scores for epithelial‐mesenchymal transformation marker vimentin, Kupffer cell (activation) marker CD68 and inflammation marker PSGL‐1 were not increased in MUL livers as compared to controls. Finally, the levels of cytokeratin CK7 in bile ducts did not differ in MUL and control livers, whereas the aberrant expression of CK7 in hepatocytes was clearly increased in MUL livers (1.08 ± 0.65 vs. 0.11 ± 0.12; p < .001) speaking for metaplastic changes in hepatocytes. In search for malignant lesions, EpCAM staining was strongly positive in the portal tracts but not in liver parenchyma of 5 control or 15 MUL livers. Two small clusters of EpCAM positive hepatocytes were observed in two liver noduli of a MUL patient with cirrhosis. Glypican‐3 stainings remained negative for both control and MUL livers and did not reveal malignant lesions.
TABLE 6.
Scoring of Masson trichrome staining and 7 immunohistochemical biomarkers in autopsy liver samples of 14 MUL patients and 6 control specimens
| Marker | Central vein region | p | Portal space | p | Sinusoids | p | |||
|---|---|---|---|---|---|---|---|---|---|
| MUL | Control | MUL | Control | MUL | Control | ||||
| Trichrome | 1.45 ± 0.57 | 0.31 ± 0.32 | <.001 | 2.16 ± 0.53 | 1.07 ± 0.10 | <.001 | 1.50 ± 0.66 | 0.28 ± 0.20 | <.001 |
| SMA | 1.23 ± 0.38 | 1.02 ± 0.04 | ns | 1.43 ± 0.40 | 1.02 ± 0.04 | .019 | 1.29 ± 0.43 | 1.14 ± 0.30 | ns |
| Vimentin | 0.37 ± 0.37 | 0.52 ± 0.26 | ns | 1.61 ± 0.81 | 1.06 ± 0.06 | ns | 1.25 ± 0.32 | 1.01 ± 0.03 | ns |
| Collagen I | 0.89 ± 0.41 | 1.00 ± 0.37 | ns | 1.62 ± 0.48 | 1.00 ± 0.00 | .001 | 1.22 ± 0.51 | 0.54 ± 0.58 | .033 |
| CD34 | 0.60 ± 0.36 | 0.90 ± 0.09 | .008 | 1.25 ± 0.29 | 1.01 ± 0.02 | .004 | 1.21 ± 0.67 | 0.20 ± 0.15 | <.001 |
| CD68 | 0.49 ± 0.93 | 0.11 ± 0.11 | ns | 1.05 ± 0.82 | 1.06 ± 0.18 | ns | 1.53 ± 0.62 | 1.05 ± 0.07 | ns |
| PSGL‐1 | 0.24 ± 0.16 | 0.72 ± 0.33 | .014 | 1.12 ± 0.43 | 1.20 ± 0.13 | ns | 1.11 ± 0.17 | 1.00 ± 0.00 | ns |
| Ductules | p | Parenchyma | p | |||
|---|---|---|---|---|---|---|
| MUL | Control | MUL | Control | |||
| CK7 | 1.17 ± 0.26 | 1.03 ± 0.05 | ns | 1.08 ± 0.65 | 0.11 ± 0.12 | <.001 |
Note: Scoring range 0–3, over 20 visual fields per sample analysed. Values are in mean ± SD.
Abbreviations: CK7, cytokeratin 7; PSGL‐1, p‐selectin glycoprotein ligand‐1; SMA, alpha smooth muscle actin;
4. DISCUSSION
Mulibrey nanism (MUL) is a rare genetic disorder with prenatal onset growth failure and multiorgan manifestations, including perimyocardial heart disease, primary hypogonadism, insulin resistance and a susceptibility for tumours. In this work, we characterized liver pathology and assessed liver biochemistry in MUL patients in relation to cardiac status. The results indicate that MUL patients develop sinusoidal dilatation, congestion, nodularity, steatosis and increased fibrosis in periportal, sinusoidal and central vein areas of liver lobuli. While these lesions can be seen in early childhood, the development of frank cirrhosis is often delayed until middle age. Non‐invasive assessment of liver status by transient elastography (TE) and blood biochemistry revealed moderately abnormal values in up to half of the MUL children and adults. The tests, however, were insensitive in revealing the congestive and fibrotic liver processes.
Congestive heart failure is a major determinant of prognosis in MUL patients. 9 It is caused by pericardial constriction and myocardial restriction‐related diastolic dysfunction. Pericardiectomy may provide clinical benefit, but heart failure progresses in at least a third of patients following pericardiectomy. In general, right heart failure leads to congestive hepatopathy owing to increased hepatic venous pressure, decreased hepatic blood flow and decreased arterial oxygen saturation as observed in other forms of right heart disease. 20 , 21
Centrilobular congestion and sinusoidal dilatation, atrophy of liver cell plates and fibrosis are common in congestive heart failure. In later stages, central‐to‐central and central‐to‐portal bridging fibrosis with nodularity as well as frank cirrhosis may develop. 11 , 22 In our study, autopsy liver samples showed congestion, sinus dilatation, hepatocyte atrophy and fibrosis already in young MUL children who had died of heart failure. Interestingly, early fibrosis was evident not only in the central vein area but also in periportal and sinusoidal areas which are regarded as signs of more advanced right heart failure. 11 , 23 , 24 Importantly, both signs were evident already in MUL infants who died, suggesting that fibrogenesis in MUL‐related heart disease may evolve rapidly. On the other hand, the liver biopsy samples from clinically stable MUL patients in the present study showed moderate fibrotic and other histological changes (congestion, sinus dilatation and steatosis) suggesting that considerable increase in right‐sided pressure is required to generate microscopic changes.
Non‐invasive assessment of liver status, especially the amount fibrosis and congestion, is based on radiology, liver biochemistry and fibrosis indexes. Measurement of liver stiffness by TE can reveal advanced fibrosis in many forms of liver diseases. 12 The accuracy of TE is, however, limited in congestive hepatopathy as increased blood volume within the liver results in elevated liver stiffness measures. These may, however, predict the outcome in patients with heart failure. In a recent study, liver stiffness values over 6.9 kPa were associated with advanced NYHA class, low haemoglobin and haematocrit levels, high serum bilirubin level and a higher mortality rate compared to patients having lower liver stiffness scores. 25 Another study showed increased liver stiffness values in Fontan patients and in patients with right heart failure. 26 In our study, TE showed elevated levels (>7 kPa) in half of the patients studied, with six patients having a value over 20 kPa. TE levels did not correlate with patient age, nor with the cardiac findings or proBNP levels. Of the liver biochemistry values, TT, FV, ALT, GT and APRI showed statistically significant correlations with TE.
Liver biochemistry values in patients with chronic heart disease and especially those with congestive heart failure are conflicting. In a study of 2679 patients with symptomatic chronic heart failure, moderate elevation of serum ALT was seen in 3.1% of patients, AST in 4.1%, ALP in 14%, total bilirubin in 13% and low albumin in 18.3%. 10 Several studies have shown that GGT and bilirubin are prognostic factors in predicting mortality in patients with chronic heart failure. 10 , 27 , 28 A cholestatic liver chemistry profile is commonly encountered also in patients with hepatic venous outflow obstruction owing to right heart failure. 29 , 30 On the other hand, liver tests, including serum AST, ALT, ALP, GGT and total and direct bilirubin, were not significantly different in congestive patients with various amount of hepatic fibrosis. 11
In the present study, liver values were available in 17 MUL patients with significant pericardial constriction referred for pericardiectomy. Although many patients had life‐threatening congestion, the liver values were mostly near the normal range. Elevated GGT values were observed in 67% of the patients and in four cases, the values were significantly elevated (116–420 U/L). The other markers were moderately abnormal (<2 times of normal) in 12–56% of the patients. Our results do not support the findings that abnormal liver values are associated with the degree of congestion rather than the liver changes. 31
Liver and cardiac assessment of clinically stable MUL patients (age range 0.2–51 years) visiting the outpatient clinic was performed cross sectionally. Over 400 values of 15 parameters were obtained from 36 patients. Marginally abnormal values were common, but clinically relevant results were rare. The situation was similar in patient groups divided by age, heart function (restriction in echocardiography and serum proBNP levels) and TE findings. The results indicate that conventional liver tests as well as Gal½ and APRI are insensitive in the follow‐up of patients with MUL.
Hepatic stellate cells (HSC) play a critical role in liver fibrosis. The actin network protein alpha‐SMA and the intermediate filament protein, vimentin, are typically overexpressed in activated HSCs undergoing cytoskeleton remodelling. 32 , 33 In the autopsy samples of MUL patients, immunohistochemical staining of both HSC markers showed a tendency for increased expression, especially in the sinusoidal and portal areas and less so around central veins. The same was seen with the fibrosis marker collagen type I. These results emphasize that the fibrotic process in MUL‐related heart failure affects the whole liver lobule and not only the centrilobular zone. Inflammation precedes fibrosis in most liver diseases, but this does not seem to be the case among MUL patients with heart failure. Inflammatory cells were not abundant in MUL livers and the immunohistochemistry for Kupffer cells (CD68) showed marginally increased staining in sinusoidal and central vein areas. Also, the expression of inflammatory lectin, PSGL‐1, located on white cells and endothelial cells, was comparable in MUL and control livers.
Sinusoidal dilatation (peliosis) and congestion are typical in congestive heart failure and are also commonly seen in MUL livers. Not surprisingly, the immunohistochemical score of CD34 was highly increased in the sinusoids of MUL livers, as compared to controls, which speaks for a reactive capillarization. Recently, increased endothelial CD34 positivity was reported especially in ‘centrizonal scars’ of livers with hepatic venous outflow obstruction. 34 In MUL livers, the expression of CD34 pericentrally was reduced rather than increased, which suggests a more diffuse pressure effect on liver endothelium. As peliosis is seen also in other organs in MUL, the CD34 expression may reflect other types of pathophysiology related to TRIM37 function. 6 , 35 Besides heart failure, MUL patients suffer from insulin resistance and metabolic syndrome, which are typically associated with fatty liver. Somewhat surprisingly, steatosis was clearly a minor finding in the liver biopsy samples and a major finding in only two of the 22 original autopsy reports. Also, the amount of inflammation was low, which was evident, especially in immunohistochemistry with normal expression of CD68 and PSLG‐1, and speaks against metabolic steatohepatitis.
Bile ductule proliferation often occurs in patients with congestive heart failure which can be visualized by staining for CK7, normally expressed only in biliary cells. 30 , 36 However, in many liver disorders, the expression of CK7 is detected also in hepatocytes. 22 Interestingly, the ductular proliferation was modest in autopsy samples of MUL livers as verified by the CK7 staining. On the other hand, hepatocyte CK7 staining was abundant in MUL livers indicating cellular metaplasia of these cells.
No hepatic neoplasia was found in the 22 autopsy samples or 21 biopsies taken from MUL patients with tumour suspicion. This was supported by the finding that biomarkers of liver carcinoma, EpCAM and glypican‐3, did not reveal malignant lesions in fibrotic or cirrhotic MUL livers (with the exception of two small hepatocyte clusters positive for EpCAM in one patient). This is interesting since chronic hepatopathy may lead to hepatocellular carcinomas, and benign and malignant tumours have been reported in many non‐hepatic organs in MUL patients. 6 , 7 The Finnish MUL patients have truncating TRIM37 mutations with total loss of TRIM37 protein expression. In vitro, knockdown of TRIM37 inhibits tumorigenesis in cellular models. 37 , 38 On the other hand, TRIM37 has profound effects in the cell cycle in various cell types (e.g. chondrocytes), 39 , 40 and over‐expression of TRIM37 has been reported in the progression of hepatic, colorectal and pancreatic carcinomas. 41 , 42 , 43 The explanation for these somewhat conflicting observations is not clear.
Owing to the rarity of MUL, the main limitation of this study is the small number of patients and samples. In addition, liver biopsies were only obtained for clinical indications. Therefore, we assessed four overlapping patient groups to get maximal information. In the immunohistochemical assessment, the autopsy samples of MUL patients varied more in quality than those taken from normal control livers, which brought some challenges to the analyses.
The lack of TRIM37 protein causes a multiorgan syndrome and according to our previous studies and clinical findings, hepatopathy of different stages is one of the main findings in MUL. Our study suggests that the typical changes in MUL liver disease include congestion, fibrosis at different stages and steatosis. Despite the histological changes, most MUL patients do not seem to suffer from marked liver dysfunction at young age. However, liver cirrhosis is common in MUL patients after 40 years of age. No malignant liver tumours were found in biopsies. The histological and macroscopical changes speak for congestive hepatopathy. However, it cannot be excluded that the lack of TRIM37 protein modifies the liver pathology also independently of heart disease in these patients.
CONFLICT OF INTEREST
None of the authors have to declare any conflict of interest.
ACKNOWLEDGEMENTS
We thank Tuike Helmiö of her technical assistance with the immunohistochemistry. We also thank our patients and their families.
Sivunen J, Karlberg S, Kivisaari R, et al. Liver pathology and biochemistry in patients with mutations in TRIM37 gene (Mulibrey nanism). Liver Int. 2022;42:1369–1378. doi: 10.1111/liv.15213
Handling Editor: Luca Valenti
Funding informationThis study was financially supported by the Foundation for Pediatric Research, Sigrid Jusélius Foundation, Finska Läkaresällskapet and Helsinki University Hospital Research Funds.
REFERENCES
- 1. Avela K, Lipsanen‐Nyman M, Idanheimo N, et al. Gene encoding a new RING‐B‐box‐coiled‐coil protein is mutated in mulibrey nanism. Nat Genet. 2000;25:298‐301. [DOI] [PubMed] [Google Scholar]
- 2. Kallijarvi J, Avela K, Lipsanen‐Nyman M, Ulmanen I, Lehesjoki AE. The TRIM37 gene encodes a peroxisomal RING‐B‐box‐coiled‐coil protein: classification of mulibrey nanism as a new peroxisomal disorder. Am J Hum Genet. 2002;70:1215‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karlberg N, Jalanko H, Perheentupa J, Lipsanen‐Nyman M. Mulibrey nanism: clinical features and diagnostic criteria. J Med Genet. 2004;41:92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karlberg S, Tiitinen A, Lipsanen‐Nyman M. Failure of sexual maturation in Mulibrey nanism. N Engl J Med. 2004;351:2559‐2560. [DOI] [PubMed] [Google Scholar]
- 5. Karlberg N, Jalanko H, Kallijarvi J, Lehesjoki AE, Lipsanen‐Nyman M. Insulin resistance syndrome in subjects with mutated RING finger protein TRIM37. Diabetes. 2005;54:3577‐3581. [DOI] [PubMed] [Google Scholar]
- 6. Karlberg N, Karlberg S, Karikoski R, Mikkola S, Lipsanen‐Nyman M, Jalanko H. High frequency of tumours in Mulibrey nanism. J Pathol. 2009;218:163‐171. [DOI] [PubMed] [Google Scholar]
- 7. Karlberg S, Lipsanen‐Nyman M, Lassus H, Kallijarvi J, Lehesjoki AE, Butzow R. Gynecological tumors in Mulibrey nanism and role for RING finger protein TRIM37 in the pathogenesis of ovarian fibrothecomas. Mod Pathol. 2009;22:570‐578. [DOI] [PubMed] [Google Scholar]
- 8. Sarkola T, Lipsanen‐Nyman M, Jalanko H, Jokinen E. Pericardial constriction and myocardial restriction in pediatric Mulibrey nanism—a complex disease with diastolic dysfunction. CJC Open. 2021;4(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipsanen‐Nyman M, Perheentupa J, Rapola J, Sovijarvi A, Kupari M. Mulibrey heart disease: clinical manifestations, long‐term course, and results of pericardiectomy in a series of 49 patients born before 1985. Circulation. 2003;107:2810‐2815. [DOI] [PubMed] [Google Scholar]
- 10. Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Eur J Heart Fail. 2009;11:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai DF, Swanson PE, Krieger EV, Liou IW, Carithers RL, Yeh MM. Congestive hepatic fibrosis score: a novel histologic assessment of clinical severity. Mod Pathol. 2014;27:1552‐1558. [DOI] [PubMed] [Google Scholar]
- 12. Ferraioli G, Barr RG. Ultrasound liver elastography beyond liver fibrosis assessment. World J Gastroenterol. 2020;26:3413‐3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eerola A, Pihkala JI, Karlberg N, Lipsanen‐Nyman M, Jokinen E. Cardiac dysfunction in children with mulibrey nanism. Pediatr Cardiol. 2007;28:155‐162. [DOI] [PubMed] [Google Scholar]
- 14. Sivunen J, Piirila P, Karlberg S, et al. Restriction of lung volumes but normal function of pulmonary tissue in mulibrey nanism. Pediatr Pulmonol. 2020;55:122‐129. [DOI] [PubMed] [Google Scholar]
- 15. Fitzpatrick E, Quaglia A, Vimalesvaran S, Basso MS, Dhawan A. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J Pediatr Gastroenterol Nutr. 2013;56:72‐76. [DOI] [PubMed] [Google Scholar]
- 16. Lam E, Higgins V, Zhang L, et al. Normative values of high‐sensitivity cardiac troponin T and N‐terminal pro‐B‐type natriuretic peptide in children and adolescents: a study from the CALIPER cohort. J Appl Lab Med. 2021;6:344‐353. [DOI] [PubMed] [Google Scholar]
- 17. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 18. Lampela H, Kosola S, Jalanko H, Pakarinen MP. Galactose half‐life is a useful tool in assessing prognosis of chronic liver disease in children. Transpl Int. 2012;25:1041‐1049. [DOI] [PubMed] [Google Scholar]
- 19. Voutilainen SH, Kosola SK, Lohi J, et al. Expression of 6 biomarkers in liver grafts after pediatric liver transplantation: correlations with histology, biochemistry, and outcome. Ann Transplant. 2020;25:e925980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011;20:135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397‐2405. [DOI] [PubMed] [Google Scholar]
- 22. Koehne de Gonzalez AK, Lefkowitch JH. Heart disease and the liver: pathologic evaluation. Gastroenterol Clin North Am. 2017;46:421‐435. [DOI] [PubMed] [Google Scholar]
- 23. Bosch DE, Koro K, Richards E, et al. Validation of a congestive hepatic fibrosis scoring system. Am J Surg Pathol. 2019;43:766‐772. [DOI] [PubMed] [Google Scholar]
- 24. Lemmer A, VanWagner LB, Ganger D. Assessment of advanced liver fibrosis and the risk for hepatic Decompensation in patients with congestive hepatopathy. Hepatology. 2018;68:1633‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taniguchi T, Ohtani T, Kioka H, et al. Liver stiffness reflecting right‐sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging. 2019;12:955‐964. [DOI] [PubMed] [Google Scholar]
- 26. Yoo BW, Choi JY, Eun LY, Park HK, Park YH, Kim SU. Congestive hepatopathy after Fontan operation and related factors assessed by transient elastography. J Thorac Cardiovasc Surg. 2014;148:1498‐1505. [DOI] [PubMed] [Google Scholar]
- 27. Ruttmann E, Brant LJ, Concin H, et al. Gamma‐glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130‐2137. [DOI] [PubMed] [Google Scholar]
- 28. Poelzl G, Eberl C, Achrainer H, et al. Prevalence and prognostic significance of elevated gamma‐glutamyltransferase in chronic heart failure. Circ Heart Fail. 2009;2:294‐302. [DOI] [PubMed] [Google Scholar]
- 29. Poelzl G, Ess M, Mussner‐Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest. 2012;42:153‐163. [DOI] [PubMed] [Google Scholar]
- 30. Pai RK, Hart JA. Aberrant expression of cytokeratin 7 in perivenular hepatocytes correlates with a cholestatic chemistry profile in patients with heart failure. Mod Pathol. 2010;23:1650‐1656. [DOI] [PubMed] [Google Scholar]
- 31. Fortea JI, Puente A, Cuadrado A, et al. Congestive hepatopathy. Int J Mol Sci. 2020;21:9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512‐10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang PW, Wu TH, Lin TY, Chen MH, Yeh CT, Pan TL. Characterization of the roles of Vimentin in regulating the proliferation and migration of HSCs during hepatic Fibrogenesis. Cell. 2019;8(10):1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsukuma S, Takeo H, Utsumi Y, Sato K. In hepatic venous outflow obstruction, alcoholic liver disease, and nonalcoholic fatty liver disease, centrilobular scars, CD34+ vessels, and keratin 7+ hepatocytes are in close proximity. Virchows Arch. 2017;470:411‐420. [DOI] [PubMed] [Google Scholar]
- 35. Crocetti D, Palmieri A, Pedulla G, Pasta V, D’Orazi V, Grazi GL. Peliosis hepatis: personal experience and literature review. World J Gastroenterol. 2015;21:13188‐13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kakar S, Kamath PS, Burgart LJ. Sinusoidal dilatation and congestion in liver biopsy: is it always due to venous outflow impairment? Arch Pathol Lab Med. 2004;128:901‐904. [DOI] [PubMed] [Google Scholar]
- 37. Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX, Wang XJ. Knockdown of tripartite motif‐containing protein 37 (TRIM37) inhibits the proliferation and tumorigenesis in colorectal cancer cells. Oncol Res. 2017;25:115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang SL, Gao YL, Wen‐Zhong H. Knockdown of TRIM37 suppresses the proliferation, migration and invasion of glioma cells through the inactivation of PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;99:59‐64. [DOI] [PubMed] [Google Scholar]
- 39. Meitinger F, Ohta M, Lee KY, et al. TRIM37 controls cancer‐specific vulnerability to PLK4 inhibition. Nature. 2020;585:440‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brigant B, Demont Y, Ouled‐Haddou H, et al. TRIM37 is highly expressed during mitosis in CHON‐002 chondrocytes cell line and is regulated by miR‐223. Bone. 2020;137:115393. [DOI] [PubMed] [Google Scholar]
- 41. Jiang J, Yu C, Chen M, Tian S, Sun C. Over‐expression of TRIM37 promotes cell migration and metastasis in hepatocellular carcinoma by activating Wnt/beta‐catenin signaling. Biochem Biophys Res Commun. 2015;464:1120‐1127. [DOI] [PubMed] [Google Scholar]
- 42. Hu CE, Gan J. TRIM37 promotes epithelialmesenchymal transition in colorectal cancer. Mol Med Rep. 2017;15:1057‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brigant B, Metzinger‐Le Meuth V, Rochette J, Metzinger L. TRIMming down to TRIM37: relevance to inflammation, cardiovascular disorders, and cancer in MULIBREY Nanism. Int J Mol Sci. 2018;20(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]


