Abstract
In the Escherichia coli genome, five putative open reading frame (ORF) clusters, mdlAB, ybjYZ, yddA, yojHI, and yhiH, have been assumed to be possible genes for ABC drug efflux transporters (I. T. Paulsen, M. K. Sliwinski, and M. H. Saier, Jr., J. Mol. Biol. 277:573–592, 1998). We cloned all of these ORFs in multicopy plasmids and investigated the drug resistance of drug-supersensitive host cells lacking constitutive multidrug efflux transporter genes acrAB. Among them, only ybjYZ gave significant erythromycin resistance and significantly decreased the accumulation of [14C]erythromycin. Therefore, ybjYZ was renamed macAB (macrolide-specific ABC-type efflux carrier). Plasmids carrying both the macA and -B genes conferred resistance against macrolides composed of 14- and 15-membered lactones but no or weak resistance against 16-membered ones. Neither of the two genes produced resistance alone. The DNA sequence suggests that MacB is an integral membrane protein with four transmembrane segments and one nucleotide-binding domain, while MacA belongs to a membrane fusion protein (MFP) family with a signal-like sequence at its N terminus. The expression of the histidine-tagged proteins confirmed that MacB is an integral membrane protein and MacA is a peripheral membrane protein. In addition, MacAB required TolC for its function in a way similar to that of most of the MFP-dependent transporters in E. coli. MacB is thus a novel ABC-type macrolide efflux transporter which functions by cooperating with the MFP MacA and the multifunctional outer membrane channel TolC. This is the first case of an experimentally identified ABC antibiotic efflux transporter in gram-negative organisms.
ATP-binding cassette (ABC) transporters are the major drug efflux transporters in mammalian cells that cause multidrug resistance of cancer cells (5). On the other hand, ABC transporters are involved mainly in the uptake of a wide range of molecules (13), protein export (13), and the efflux of toxic metal ions, such as an arsenite (23), in bacteria. LmrA in a gram-positive bacterium, Lactococcus lactis (30), has been the only example of an experimentally identified bacterial ABC multidrug exporter. A small number of ABC single-drug exporters for macrolides (22) and daunomycin (21) are also known in gram-positive bacteria, whereas no functional ABC drug exporter has been definitely established to exist in gram-negative bacteria yet (32). The efflux-based multidrug resistance of gram-negative bacteria is most often conferred by RND (resistance-nodulation-cell division) family transporters (9, 15), in addition to some SMR (small multidrug resistance) transporters (18). Efflux-based single-drug resistance of gram-negative bacteria is usually due to plasmid-encoded MFS (major facilitator superfamily) transporters such as tetracycline-H+ antiporters (11, 31).
Complete genomic DNA sequences have been determined for various microorganisms, including Escherichia coli (2). On the basis of these genomic DNA sequences, all of the possible open reading frames (ORFs) can be determined (25), and possible drug exporter genes have been determined (19). As a result, it was found that the E. coli chromosome contains five ORF clusters for possible ABC drug efflux genes (19), i.e., mdlAB, ybjYZ, yddA, yojHI, and yhiH. Recently, we cloned all of these putative ABC transporter genes in multicopy plasmids (14a), and their drug resistance was investigated in drug-supersensitive strain E. coli KAM3 (12), which lacks constitutive multidrug efflux transporter genes acrAB (13). We found that only the ybjYZ gene products confer macrolide-specific drug resistance, and the genes were renamed macAB. In this study, we investigated the macAB gene products and revealed that they comprise a macrolide efflux transporter system. This is the first case of an ABC drug efflux transporter being experimentally established in a gram-negative bacterium.
MATERIALS AND METHODS
Materials and bacterial strains.
[14C]erythromycin (1.85 GBq/mmol) was purchased from NEN Life Science Products, Inc. Erythromycin, clarithromycin, rokitamycin, azithromycin, oleandomycin, josamycin, and leucomycin were kindly provided by Takeshi Nishino (Kyoto Pharmaceutical University, Kyoto, Japan). All other materials were of reagent grade and were obtained from commercial sources. E. coli AcrAB-deficient strain KAM3 (12) and TolC-deficient strain ZK796 (33) were kindly provided by Tomofusa Tsuchiya and Yuji Morita (Okayama University, Okayama, Japan) and by Hiroshi Nikaido (University of California, Berkeley), respectively. All of the bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant phenotype or genotype | Reference or origin |
|---|---|---|
| E. coli strains | ||
| W3104 | Wild type | Laboratory collection |
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 gyrA96 thi-1 recA1 | Takara Shuzo Co. |
| KAM3 | Derivative of TG1 that lacks a restriction system and AcrAB | 13 |
| ZK796 | Tetr; same as MC4100 but tolC::Tn10 | 33 |
| Plasmids | ||
| pUC119 | Apr | Takara Shuzo Co. |
| pQE70 | Apr | Qiagen |
| pUCmdlAB | Apr (pUC119); 4.3-kb SphI-EcoRI fragment containing mdlA and mdlB | 14a |
| pUCmacAB | Apr (pUC119); 3.7-kb PstI-SalI fragment containing macA (ybjY) and macB (ybjZ) | 14a |
| pUCyddA | Apr (pUC119); 2.3-kb SalI-SacI fragment containing yddA | 14a |
| pUCyojIH | Apr (pUC119); 4.0-kb SphI-SalI fragment containing yojI and yojH | 14a |
| pUCyhiH | Apr (pUC119); 5.8-kb SphI-BamHI fragment containing yhiI, yhiH, and yhiJ | 14a |
| pUCmacA | Apr (pUC119); 2.6-kb SmaI-SmaI fragment containing macA (ybjY) | This study |
| pUCmacB | Apr (pUC119); 2.6-kb PstI-SalI fragment containing mac promoter and macB (ybjZ); macA gene deleted by crossover PCR method described in Materials and Methods | This study |
| pQEmacA | Apr (pQE70); 1.1-kb fragment containing macA (ybjY) | This study |
| pQEmacB | Apr (pQE70); 2.0-kb fragment containing macB (ybjZ) | This study |
Cloning and expression of ORFs encoding putative ABC transporters.
Chromosomal DNA was isolated from E. coli W3104 cells as previously described (14). The DNA fragments including putative ABC transporter ORFs and their upstream regions containing the natural promoters were amplified by the PCR method using primers containing the restriction enzyme site that exists in the multicloning sites of pUC119. The digested DNA fragments containing ORFs were ligated into the multicloning sites of pUC119 in the same direction as the lac promoter (Table 1), and then E. coli KAM3 cells were transformed with these recombinant DNAs.
pUCmacAB carries the 502-bp upstream region containing the native promoter, in addition to the entire macA and macB ORFs. The macA and macB ORFs were individually subcloned into pUC119 as follows. pUCmacA was constructed by subcloning the SmaI fragment of pUCmacAB, in which about one-half of the macB ORF was removed while the 500-bp upstream region and the entire macA ORF were left. For pUCmacB, the macA ORF was removed from pUCmacAB by means of crossover PCR; as a result, the 502-bp upstream region was directly connected to the macB ORF. In order to investigate the intracellular localization of MacA and MacB, the macA and macB genes were also individually subcloned into plasmid pQE70 (Table 1). Plasmid pQE70 carries an optimized promoter-operator element consisting of the phage T5 promoter, which is recognized by E. coli RNA polymerase, and two lac operator sequences. In addition, a six-His tag coding sequence was attached at the 3′ terminus of the cloned gene.
Drug resistance determination.
The drug resistance of cells was determined by means of a sequential twofold dilution method on yeast extract-Bacto Tryptone agar plates as previously described (14) without induction, and the results were expressed as MICs. E. coli KAM3 cells carry the lacIq gene; thus, the cloned genes are not expressed from the lac promoter without induction. Expression of the putative ABC transporter genes was expected from the native promoters.
Assay of [14C]erythromycin accumulation in E. coli cells.
E. coli KAM3/pUCmacAB and its host cells were grown in 30 ml of Luria-Bertani broth at 37°C until they reached an optical density at 600 nm of 0.6. The cells were then harvested and washed twice with 50 mM potassium phosphate buffer (pH 7.0). The cells were then finally suspended in 7.2 ml of the same phosphate buffer. The cell suspension (500 μl) was preincubated with 25 mM glucose at 37°C for 1 min. Erythromycin uptake was started by addition of 5 μl of [14C]erythromycin (1.53-μg/ml solution). At the indicated times, aliquots (250 μl) were withdrawn and filtered through Millipore filters (pore size, 0.45 μm). The filters were washed twice with 700 μl of potassium phosphate buffer, and then the radioactivity was measured with a liquid scintillation counter.
RESULTS
Drug resistance phenotypes produced by ORFs encoding putative ABC drug efflux transporters.
Five putative ABC transporter genes, mdlAB, macAB, yddA, yojHI, and yhiH, were cloned into pUC119 under the control of the native promoters as described in Materials and Methods. Drug-supersensitive strain E. coli KAM3 (12), which lacks constitutive multidrug efflux transporter AcrAB, was transformed with these plasmids. The resistance phenotypes of the cells carrying these plasmids against 22 different drugs and toxic compounds were determined without induction. The MICs (in parentheses in micrograms per milliliter) of the following 21 compounds were unaltered in KAM3 containing mdlAB, macAB, yddA, yojHI, and yhiH: chloramphenicol (0.39), tetracycline (0.39), minocycline (0.20), nalidixic acid (0.78), norfloxacin (0.025), enoxacin (0.05), vancomycin (200), fosfomycin (1.56), doxorubicin (3.13), novobiocin (1.56), rifampin (6.25), trimethoprim (3.13), acriflavine (12.5), crystal violet (1.56), ethidium bromide (12.5), methylviologen (100), tetraphenylphosphonium bromide; (6.25), carbonyl cyanide m-chlorophenylhydrazone (6.25), benzalkonium (3.13), sodium dodecyl sulfate (50), and deoxycholate (1,250). For cells carrying the macAB genes, the MIC of erythromycin was 25 μg/ml, which was eight times higher than the MIC for the host cells (3.13 μg/ml). Plasmids carrying the other four ORF clusters produced no alteration in the MIC of erythromycin.
Amino acid sequences of MacA and MacB.
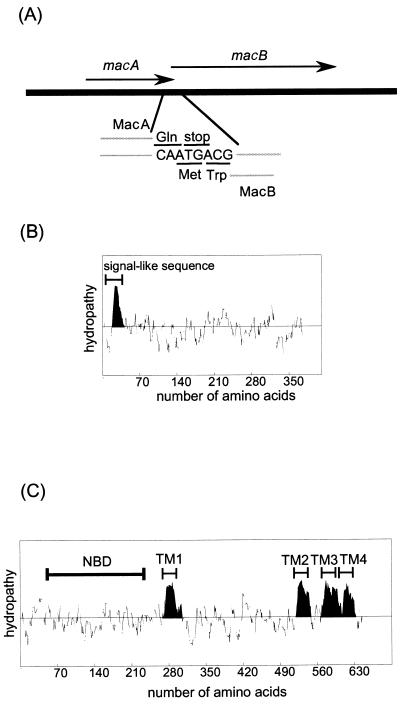
The DNA sequences of the macAB genes were determined by means of the dideoxy sequencing method with an ABI PRISM310 Genetic Analyzer. The ORFs of macA and macB partially overlap; i.e., the sequence ATGA at the end of macA contains the stop codon TGA for macA and the initiation codon ATG for macB (Fig. 1A). There is only one promoter sequence in the upstream region of macA, indicating that macA and macB form one operon. The DNA sequences of the macAB region cloned in this study are the same as those in the reported genome sequence of E. coli (2). MacA has a signal-like sequence composed of 40 amino acid residues at its N terminus, which is rich in positively charged residues in the N-terminal half and hydrophobic residues in the C-terminal half. There is no hydrophobic cluster in the MacA sequence except in this region (Fig. 1B). On the other hand, MacB contains four putative transmembrane (TM) regions in its C-terminal half (Fig. 1C). The N-terminal half of MacB includes a nucleotide-binding cassette domain containing Walker A and B and signature motifs.
FIG. 1.
(A) ORFs of macA and macB, which partially overlap. (B) Hydropathy analysis and prediction of the TM region of MacA. (C) Hydropathy analysis and prediction of the TM region of MacB. NBD, nucleotide-binding domain.
Contributions of MacA, MacB, and the multifunctional outer membrane channel TolC to resistance against macrolide antibiotics.
In order to examine the contributions of MacA and MacB to drug resistance, the macA and macB genes were separately subcloned in the pUC119 vector under the control of the natural promoter. The resistance of cells carrying pUCmacAB, pUCmacA, and pUCmacB against various macrolide antibiotics is shown in Table 2. Compared with the MIC for the host KAM3 cells, neither cells carrying pUCmacA nor those carrying pUCmacB showed an increase in resistance.
TABLE 2.
Resistance of E. coli KAM3 (ΔacrAB) and ZK796 (ΔtolC) cells harboring a plasmid carrying both the macA and -B genes or one of these genes against macrolide derivatives
| Ring size and compound | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| KAM3 (host) | KAM3 harboring multicopy plasmid:
|
ZK796 (host) | ZK796 (pUCmacAB) | |||
| pUCmacAB | pUCmacA | pUCmacB | ||||
| 14 members | ||||||
| Erythromycin | 3.13 | 25 | 3.13 | 3.13 | 3.13 | 3.13 |
| Clarithromycin | 1.56 | 12.5 | 1.56 | 1.56 | 1.56 | 1.56 |
| Oleandomycin | 50 | 400 | 50 | 50 | 25 | 25 |
| 15 members azithromycin | 1.56 | 12.5 | 1.56 | 1.56 | 1.56 | 1.56 |
| 16 members | ||||||
| Rokitamycin | 6.25 | 12.5 | 3.13 | 3.13 | 12.5 | 12.5 |
| Josamycin | 3.13 | 6.25 | 3.13 | 3.13 | 6.25 | 6.25 |
| Leucomycin | 6.25 | 6.25 | 3.13 | 3.13 | NDb | ND |
MICs were measured without induction. The mac genes were expressed from the natural promoters. Values in boldface are significantly different from the control values.
ND, not done.
On the other hand, cells carrying pUCmacAB exhibited increased resistance against the macrolide antibiotics with 14- and 15-membered rings that were examined. The MICs of erythromycin, clarithromycin, azithromycin, and oleandomycin were eight times as high as those for the host cells. On the other hand, the MICs of 16-membered lactones were increased only twofold (rokitamycin and josamycin) or not changed (leucomycin).
In order to investigate the role of the multifunctional outer membrane channel TolC in the MacAB system, TolC-deficient E. coli ZK796 was transformed with pUCmacAB. The resulting transformed cells showed no increase in resistance (Table 2), indicating that the MacAB system depends on TolC.
Intracellular localization of MacA and MacB.
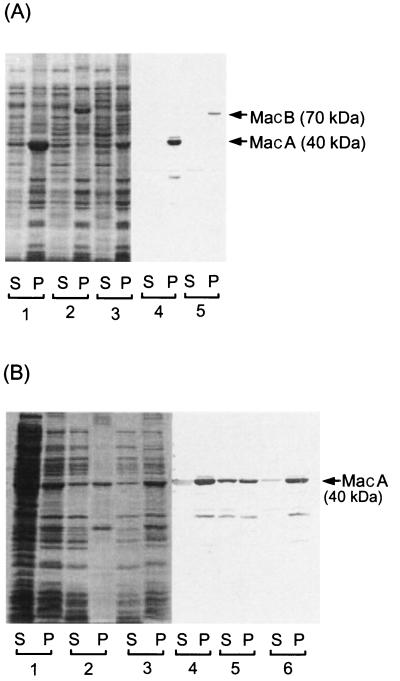
In order to reveal the intracellular localization of MacA and MacB, the macA and macB genes were separately subcloned into vector pQE70 to attach polyhistidine tags at their C termini. The expression of MacA and MacB was induced with isopropyl-β-d-thiogalactopyranoside (IPTG). Cells carrying pUCmacAB, pQEmacA, and pQEmacB were disrupted by brief sonication, and then supernatants (S) and membrane fractions (P) were obtained by ultracentrifugation. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, total proteins were visualized by Coomassie brilliant blue (CBB) staining and MacA and MacB were visualized by Western blotting with anti-polyhistidine serum (Fig. 2A). Both MacA (40 kDa) and MacB (70 kDa) bands were observed only for the membrane fractions (Fig. 2A, lanes 4 and 5). Both bands were also detected for the membrane fraction of cells carrying pUCmacAB on CBB staining (Fig. 2A, lane 3).
FIG. 2.
Expression and localization of MacA and MacB. (A) Expression of MacA and MacB under the control of the native promoter (pUC plasmids) and the T5 promoter (pQE plasmids) with a polyhistidine tag at the N-terminal end. In the case of pQE plasmids, expression was induced with IPTG. Cells were disrupted by brief sonication, and supernatant (S) and precipitated (membrane) (P) fractions were obtained by ultracentrifugation after removal of cell debris by brief low-speed centrifugation. Lanes 1, 2, and 3 were stained with CBB, and lanes 4 and 5 were visualized by Western blotting. Lanes: 1, cells carrying pQEmacA; 2, pQEmacB; 3, pUCmacAB; 4, pQEmacA; 5, pQEmacB. (B) Fractionation of MacA with 4.5 M urea washing or potassium phosphate buffer washing as a control. Lanes 1, 2, and 3 were stained with CBB, and lanes 4, 5, and 6 were visualized by Western blotting. S and P indicate the supernatants and precipitates obtained on ultracentrifugation, respectively. Lanes: 1 and 3, briefly sonicated cells carrying pQEmacA; 2 and 5, after 4.5 M urea washing of the precipitate (lane 1, P); 3 and 6, after potassium phosphate buffer washing of the precipitate (lane 1, P).
The membrane fraction of cells expressing MacA was treated with 4.5 M urea, and a supernatant and a precipitate were obtained by ultracentrifugation. A significant amount of MacA was released from the membrane fraction and detected in the supernatant fraction by Western blotting (Fig. 2B, lane 5). In contrast, when the membrane was treated with potassium phosphate buffer, MacA was not released from it (Fig. 2B, lane 6). These observations suggest that MacA is a peripheral membrane protein.
Uptake of [14C]erythromycin by cells carrying pUCmacAB.
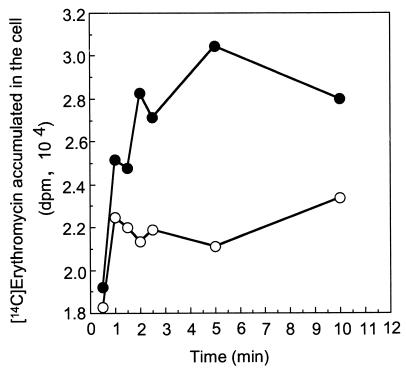
In order to determine whether or not macrolide resistance is based on the active efflux of drugs, we compared the uptake of [14C]erythromycin by E. coli KAM3 cells with and without the pUCmacAB plasmid (Fig. 3). [14C]erythromycin was taken up by the host KAM3 cells. In cells carrying pUCmacAB, uptake was significantly inhibited, indicating active efflux of this drug out of the cells.
FIG. 3.
Accumulation of [14C]erythromycin in cells carrying pUCmacAB and pUC119. Cells were incubated with 25 mM glucose and 1.53 μg of [14C]erythromycin per ml as described in Materials and Methods. Aliquots were then filtered through Millipore filters. After washing of the filters, radioactivity was measured with a liquid scintillation counter. Radioactivity is indicated as disintegrations per minute. Open symbols, cells carrying pUCmacAB; closed symbols, cells carrying pUC119.
DISCUSSION
In this study, we showed that the MacAB complex confers TolC-dependent macrolide resistance via active drug efflux. We examined the resistance pattern against 22 different drugs and toxic compounds which contain the typical compounds exported by multidrug transporters in bacteria and mammals. As a result, MacAB was revealed to confer macrolide-specific resistance. As shown in Fig. 4, MacB is a half-type ABC protein having four putative TM segments and one nucleotide-binding cassette. There is a large hydrophilic loop region between TM1 and TM2, which is probably located on the periplasmic surface and may interact with MacA. MacA is a peripheral membrane protein that belongs to the membrane fusion protein (MFP) family (4). According to the sequence characteristics of the signal-like sequence and the positive-inside rule of protein topogenesis (20), most of the MacA molecule seems to be secreted and attached on the periplasmic surface (Fig. 4).
FIG. 4.
Schematic model of the molecular construction of the novel ABC-type macrolide-specific drug exporter MacA-MacB system complexed with TolC in E. coli. The dotted line indicates the region having a signal-like sequence. NBD indicates a possible nucleotide-binding domain having Walker A and B and signature motifs.
A BLAST search revealed that there are homologues of macAB in gram-negative organisms. Putative ABC transporter proteins NMA0729 (16) and NMB0548 (29) in Neisseria meningitidis, PA2389 in Pseudomonas aeruginosa (26), and CAB75243.1 in Campylobacter jejuni (17) exhibit sequence identity with MacB, i.e., 48, 48, 43, and 41%, respectively. They show sequence similarity not only in the ATP-binding domains but also in the TM domains. In contrast, the sequence similarity between MacB and putative ABC transporters such as MsrA (24) in gram-positive organisms is restricted to the ATP-binding cassette region. The putative ABC transporter genes in the gram-negative organisms mentioned above include genes for MacA homologues in the same operon. Therefore, these genes can be classified into the same subfamily of ABC transporters in gram-negative organisms.
In eukaryotes, most ABC transporters are involved in multidrug resistance. However, in bacteria, the majority of drug exporters are drug-proton antiporters (8), including MFS-, SMR-, and RND-type transporters, while ABC transporters are usually involved in the uptake of a wide range of molecules (6). On the basis of the results of genome analysis, a number of putative ABC drug exporters are predicted (19), while the only ABC multidrug exporter experimentally identified in a bacterium is LmrA in Lactococcus lactis (30). In gram-positive organisms, some macrolide resistance genes code for ABC-type efflux transporter proteins (22, 24). Among them, MsrAB in Staphylococcus species has two nucleotide-binding domains and probably acts as an efflux system in cooperating with some other integral membrane proteins (10), while its characteristics as a drug exporter have not been fully revealed. As for macrolide exporters, some major facilitator-type exporters are known in gram-positive organisms (3, 28), such as MefA, which, similarly to MacAB, confers resistance against macrolides with 14- and 15-membered lactones.
A half-type ABC transporter is usually expected to have six TM segments (1, 30). MacB appears to have four TM segments. In addition, the N-terminal ABC on an ABC transporter is also unusual. Among the ABC transporters hitherto reported, only the ABCG subfamily in mammalian cells shows an N-terminal ABC (7). MacB requires the MFP MacA and the outer membrane channel TolC for drug efflux function. Although some bacterial ABC transporters such as HlyB (34) accompany an MFP and TolC, it is quite interesting that ABC-type drug efflux transporters depend on an MFP and TolC, as seen with RND-type multidrug efflux transporters (9, 13). In short, MacAB represents a quite unique and novel bacterial subfamily of ABC transporters.
Very recently, Sulavik et al. (27) constructed 22 E. coli strains with deletions of putative drug efflux transporters and outer membrane channels. The strain with a deletion of the macAB (ybjYZ) genes showed no change in drug susceptibility. However, since the macAB deletion strain carried the acrAB genes, the effect of macAB deletion might have been masked. The tolerance of E. coli cells to macrolide antibiotics is conferred mainly by AcrAB (35). Expression cloning of an individual gene into an AcrAB-deficient strain may be necessary to discover a potential drug efflux transporter gene.
ACKNOWLEDGMENTS
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. K. Nishino is supported by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists.
K. Nishino and N. Kobayashi contributed equally to this work, and both should be considered first authors.
REFERENCES
- 1.Abele R, Tampe R. Function of the transport complex TAP in cellular immune recognition. Biochim Biophys Acta. 1999;1461:405–419. doi: 10.1016/s0005-2736(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 2.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 3.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 4.Dinh T, Paulsen I T, Saier M H. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman M M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 6.Holland I B, Blight M A. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol. 1999;293:381–399. doi: 10.1006/jmbi.1999.2993. [DOI] [PubMed] [Google Scholar]
- 7.Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta. 1999;1461:237–262. doi: 10.1016/s0005-2736(99)00161-3. [DOI] [PubMed] [Google Scholar]
- 8.Levy S B. Active efflux mechanisms for antimicrobial resistance. Antimicrob Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka M, Endou K, Kobayashi H, Inoue M, Nakajima Y. A plasmid that encodes three genes for resistance to macrolide antibiotics in Staphylococcus aureus. FEMS Microbiol Lett. 1998;167:221–227. doi: 10.1111/j.1574-6968.1998.tb13232.x. [DOI] [PubMed] [Google Scholar]
- 11.McMurry L, Petrucci R E, Jr, Levy S B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci USA. 1980;77:3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido H, Hall J A. Overview of bacterial ABC transporters. Methods Enzymol. 1998;292:3–20. doi: 10.1016/s0076-6879(98)92003-1. [DOI] [PubMed] [Google Scholar]
- 14.Nishino K, Yamaguchi A. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J Bacteriol. 2001;183:1455–1458. doi: 10.1128/JB.183.4.1455-1458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Nishino, K., and A. Yamaguchi. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 15.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 17.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M-A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 18.Paulsen I T, Skurray R A, Tam R, Saier M H, Jr, Turner R J, Weiner J H, Goldberg E B, Grinius L L. The SMR family: a novel family of multidrug efflux proteins involved with the efflux of lipophilic drugs. Mol Microbiol. 1996;19:1167–1175. doi: 10.1111/j.1365-2958.1996.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 20.Persson B, Argos P. Topology prediction of membrane proteins. Protein Sci. 1996;5:363–371. doi: 10.1002/pro.5560050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reizer J, Reizer A, Saier M H., Jr A new subfamily of bacterial ABC-type transport systems catalyzing export of drugs and carbohydrates. Protein Sci. 1992;1:1326–1332. doi: 10.1002/pro.5560011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen B P. Families of arsenic transporters. Trends Microbiol. 1999;7:207–212. doi: 10.1016/s0966-842x(99)01494-8. [DOI] [PubMed] [Google Scholar]
- 24.Ross J I, Eady E A, Cove J H, Cunliffe W J, Baumberg S, Wootton J C. Inducible erythromycin resistance in Staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 25.Rudd K E. Linkage map of Escherichia coli K-12, edition 10: the physical map. Microbiol Mol Biol Rev. 1998;62:985–1019. doi: 10.1128/mmbr.62.3.985-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G K, Wu Z, Paulsen I T, Reizer J, Saier M H, Hancock R E W, Lory S, Olson M V. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 27.Sulavik M C, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, Didomenico B, Shaw K J, Miller G H, Hare R, Shimer G. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother. 2001;45:1126–1136. doi: 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tettelin H, Saunders N J, Heidelberg J, Jeffries A C, Nelson K E, Eisen J A, Ketchum K A, Hood D W, Peden J F, Dodson R J, Nelson W C, Gwinn M L, DeBoy R, Peterson J D, Hickey E K, Haft D H, Salzberg S L, White O, Fleischmann R D, Dougherty B A, Mason T, Ciecko A, Parksey D S, Blair E, Cittone H, Clark E B, Cotton M D, Utterback T R, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith H O, Fraser C M, Moxon E R, Rappuoli R, Venter J C. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 30.van Veen H W, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen A J, Konings W N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi A, Udagawa T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]
- 32.Young J, Holland I B. ABC transporters: bacterial exporters-revisited five years on. Biochim Biophys Acta. 1999;1461:177–200. doi: 10.1016/s0005-2736(99)00158-3. [DOI] [PubMed] [Google Scholar]
- 33.Zgurskaya H I, Nikaido H. Cross-linked complex between oligomeric periplasmic lipoprotein AcrA and the inner-membrane-associated multidrug efflux pump AcrB from Escherichia coli. J Bacteriol. 2000;182:4264–4267. doi: 10.1128/jb.182.15.4264-4267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Sheps J A, Ling V. Complementation of transport-deficient mutants of Escherichia coli alpha-hemolysin by second-site mutations in the transporter hemolysin B. J Biol Chem. 1993;268:19889–19895. [PubMed] [Google Scholar]
- 35.Zhong P, Shortridge V D. The role of efflux in macrolide resistance. Drug Resist Updates. 2000;3:325–329. doi: 10.1054/drup.2000.0175. [DOI] [PubMed] [Google Scholar]