Abstract
Background and aims
There are few longitudinal studies assessing the association of cannabis use and subsequent onset of bipolar disorder. We aimed to measure the association between early cannabis exposure and subsequent bipolar disorder.
Design, Setting and Participants
Observational study linking a sample from the northern Finland birth cohort 1986 (n = 6325) to nation‐wide register data to examine the association of life‐time cannabis exposure at age 15/16 years and subsequent bipolar disorder until age 33 (until the end of 2018); 6325 individuals (48.8% males) were included in the analysis.
Measurements
Cannabis exposure was measured via self‐report. Bipolar disorder was measured via bipolar disorder‐related diagnostic codes (ICD‐10: F30.xx, F31.xx) collected from the Care Register for Health Care 2001–18, the Register of Primary Health Care Visits 2011–18, the medication reimbursement register of the Social Insurance Institution of Finland 2001–05 and the disability pensions of the Finnish Center for Pensions 2001–16. Potential confounders included demographic characteristics, parental psychiatric disorders, emotional and behavioral problems and other substance use.
Findings
Three hundred and fifty‐two adolescents (5.6%) reported any cannabis use until the age of 15–16 years. Of the whole sample, 66 (1.0%) were diagnosed with bipolar disorder. Adolescent cannabis use was associated with bipolar disorder [hazard ratio (HR) = 3.46; 95% confidence interval (CI) = 1.81–6.61]. This association remained statistically significant after adjusting for sex, family structure and parental psychiatric disorders (HR = 3.00; 95% CI = 1.47–6.13) and after further adjusting for adolescent emotional and behavioral problems (HR = 2.34; 95% CI = 1.11–4.94). Further adjustments for frequent alcohol intoxications, daily smoking and lifetime illicit drug use attenuated the associations to statistically non‐significant.
Conclusions
In Finland, the positive association between early cannabis exposure and subsequent development of bipolar disorder appears to be confounded by other substance use.
Keywords: Adolescent, bipolar disorders, birth cohort, cannabis, mood disorders, substance use
INTRODUCTION
Bipolar disorder is a severe mental disorder which frequently first presents in early adulthood [1], and is associated with significant morbidity [2] and reduced life expectancy [3]. The endocannabinoid system has been implicated in mood control functions [4], and acute tetrahydrocannabinol (THC) ingestion has been demonstrated to alter mood in several clinical trials [5, 6]. Thus, it is plausible that perturbations of the endocannabinoid system caused by cannabis use might contribute to the onset of bipolar disorder. However, the few existing prospective longitudinal studies examining the association between cannabis use and the onset of bipolar disorder have had mixed findings. Five such studies have utilized an adult sample [7, 8, 9, 10, 11] and four examined the association with adolescent cannabis use [12, 13, 14, 15]. Moreover, the only meta‐analysis published thus far on this subject [16] included only two studies [9, 15], further underscoring the paucity of the evidence base.
Studying the impact of cannabis exposure in adolescence in relation to bipolar disorder is of paramount importance, as adolescence is a time‐period when the brain is most vulnerable and the risk of future mental disorders arising from cannabis use is greatest at this developmental phase [17, 18]. Of the four prospective longitudinal studies focusing upon adolescent cannabis use and onset of bipolar disorder‐related outcomes, three [12, 13, 15] reported a significant positive association and one small study reported a negative finding [14]. Those studies have been conducted with small, highly selective samples [12, 14] or they have utilized symptom scales as proxy measures for bipolar disorder‐related outcomes [13, 15]. Furthermore, follow‐up times have been modest, ranging from 1 year [14] to 8.3 years [15]. In addition to this, although polysubstance use is common in adolescence and thus presents as a potential source of confounding [19], only one of these studies adjusted for other substance use [13]. Lastly, only one study analyzing data from the Avon Longitudinal Study of Parents and Children (ALSPAC) [13] has utilized a birth cohort‐based sample.
The northern Finland birth cohort (NFBC) 1986 is a prospective general population‐based study where rich phenotypical data are linked to national health‐care and medication registers for clinician‐rated ICD‐10 diagnoses for bipolar disorder. The study includes detailed information on socio‐demographic factors, mental health problems and adolescent substance use. This enables the examination of association between adolescent cannabis use and bipolar disorder in a robust analytical framework accounting for potential confounding factors.
The aim of our study was to examine the association between cannabis exposure prior to age 15/16 years and the onset of bipolar disorder. To our knowledge, this is the first study utilizing a register‐based clinical bipolar disorder diagnosis as an outcome measure. This methodological approach ensures minimal attrition, thus enhancing the generalizability of the results. Furthermore, to our knowledge, this is also the largest study in terms of sample size and only the second one to utilize a birth cohort‐based sample with prospective data. In addition, the 18‐year follow‐up in our study is more than twofold longer than any previous study conducted thus far covering the age range where diagnosis of bipolar disorder is most likely.
METHODS
Participants and data collection
The NFBC 1986 is an ongoing follow‐up study including 99% of all births in the two northernmost provinces in Finland between 1 July 1985 and 30 June 1986 [20]. The original sample included 9432 live‐born children. A multi‐disciplinary follow‐up study was conducted in 2001–02, when study members were aged 15–16 years. Initially, self‐report postal questionnaires were sent to the adolescents (n = 9215) in which they answered questions concerning their health and wellbeing (n = 7344). Thereafter, all participants were invited to take part in a clinical study where they completed self‐report questionnaires including questions on substance use. Participants who provided informed consent and answered questions on cannabis were included in the present study. To account for the effect of prior mental health disorders to study variables, we excluded those who had been diagnosed with any prior psychiatric disorder before the age of 16. The final sample totaled 6325 individuals (67.1% of original sample) (Figure 1). The 15–16‐year follow‐up study was approved by the Ethics committee of the Northern Ostrobothnia Hospital District in Finland (14 June 1999). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 and having complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies [21] The STROBE checklist is included in the Supporting information. As the primary research question and analysis plan were not pre‐registered on a publicly available platform, the results should be considered exploratory.
FIGURE 1.
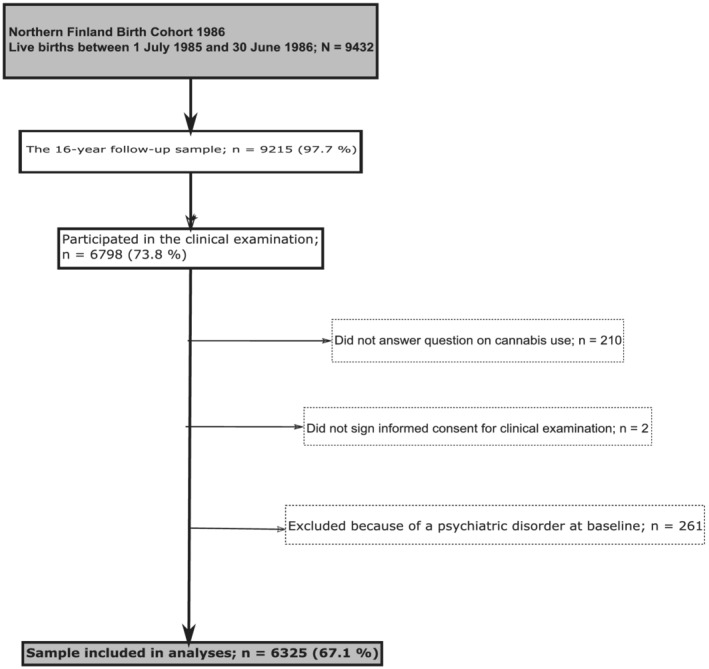
Flow‐chart of the current study from the northern Finland birth cohort 1986
Exposure variable: cannabis use
Data on life‐time adolescent cannabis use were collected during the clinical study when participants were aged 15–16 years. They were asked: ‘Have you ever used marihuana or hashish?’ with options ‘never, once, 2–4 times, 5 times or more, or I use regularly’. For the main multivariable analysis, cannabis use was studied as the dichotomized (no/yes). In addition to this, univariable analyses were conducted utilizing a three‐class cannabis variable (never, one to four times, five times or more).
Outcome variable: bipolar disorder
Data on bipolar disorder‐related diagnostic codes (ICD‐10: F30.xx, F31.xx) were collected cumulatively from the participants’ age of 16 years until the end of 2018, when participants were aged 33 years. Data on these diagnostic codes were obtained from the Care Register for Health Care 2001–18, the Register of Primary Health Care Visits 2011–18, the medication reimbursement register of the Social Insurance Institution of Finland 2001–05 and the disability pensions of the Finnish Center for Pensions 2001–16. The Care Register contains information on patients discharged from inpatient care, and since 1998 also on specialized outpatient care. The Register of Primary Health Care Visits includes all outpatient primary health care delivered in Finland. Detailed information concerning these registers is provided in previous studies [22, 23, 24]. Information on deaths and times of emigration, which were used as censoring points in our analyses, were obtained from the Population Register data and the Registry for causes of death.
Adolescent behavioral and emotional problems
Information on adolescent mental health was collected at age 15–16 years using the Youth Self Report (YSR) [25]. The YSR consists of 118 items measuring symptoms of emotional and behavioral problems in adolescents aged 11–18 years. Responses to YSR items are scored on a three‐point scale, with statements being not true (0); somewhat/sometimes true (1); or very true (2) in terms of reflecting how the young person has felt within the past 6 months. The sum of scores can be operationalized as YSR total score, YSR internalizing or externalizing scores or eight subscales [25]. If there were three or fewer missing values in a subscale, they were replaced by the mean value of items. If more than three items were missing from any subscale, the total score of the YSR was classified as missing [26]. For this study, the YSR item ‘I use alcohol or drugs for non‐medical purposes’ was removed from the YSR total sum score.
Alcohol use, daily smoking and other illicit substance use
Data on life‐time illicit substance, daily smoking and frequent alcohol intoxications use were collected at age 15–16 years using a questionnaire during the clinical study. The participants were asked: ‘Have you used ecstasy, heroin, cocaine, amphetamine, LSD or other similar intoxicating drugs?’. A person was categorized to the ‘yes’ group if they had used any of these substances at least once. Frequent alcohol intoxications were questioned as: ‘Have you been drunk during the past year? (0, 1–2, 3–5, 6–9, 10–19, 20–39 or 40 times or more)’, and this dichotomized (no/yes) to those who had been ‘drunk’ 10 times or more during the past year. Information on regular cigarette smoking was collected from postal questionnaires: adolescents were asked if they currently smoked cigarettes daily (at least one cigarette/day, no/yes).
Family structure, parental education and parental psychiatric disorders
Information on family structure and education level were ascertained in the parents’ questionnaire when participants were aged 15–16 years. Family structure was defined dichotomously as (a) both parents living with the participant continuously and (b) other families.
The highest educational level achieved by either parent was dichotomized as less than 12 years or 12 years or more of schooling.
Data on life‐time parental psychiatric diagnoses were obtained from the nation‐wide Registers of Health Care during 1972–2018 (including inpatient care and visits to specialized outpatient health care since 1998 and primary health care since 2011) and the Finnish Center for Pensions until 2016. The variable was classified dichotomously as whether or not either parent had been diagnosed with an ICD‐10 mental disorder.
Statistical analyses
We used cross‐tabulation and χ2 or Fisher's exact test or the Mann–Whitney U‐test, as appropriate, to assess the relationship of life‐time cannabis use and onset of bipolar disorder. Linear regression and multicollinearity diagnostics with variance inflation factor (VIF) scores were used to detect correlation between multiple covariates. VIF > 5 was used as an indicator of multicollinearity. Univariable analyses were also conducted utilizing a three‐class cannabis variable (never, one to four times, five times or more). Dose–response was studied with a trend test using a categorical cannabis use variable (never, one to four times, at least five times) as continuous in a logistic regression model with odds ratios (OR) and confidence intervals.
We applied Cox regression analysis with hazard ratios (HR) and 95% confidence intervals (CI) to study the association of life‐time adolescent cannabis use with subsequent bipolar disorder. Times at emigration (n = 256) and death (n = 50) were used as censoring points in the survival analyses. The models are as follows: model 1: sex, family structure and any parental psychiatric disorder; model 2: sex, family structure, any parental psychiatric disorder and YSR total; and model 3: sex, family structure, any parental psychiatric disorder, YSR total, frequent alcohol intoxications past 12 months, daily smoking and the use of other illicit drugs than cannabis. The Cox proportional hazard assumption was examined by using hazard logarithms and scaled Schoenfeld residuals.
To assess the effects of missing data, multiple imputations were conducted using fully conditional specifications with 10 data sets. A post‐hoc analysis using Fisher's exact test was conducted on the subpopulation of subjects diagnosed with unipolar depression to study the relationship between life‐time cannabis use and transitioning to bipolar disorder. Those subjects for whom a diagnosis of unipolar depression was recorded after a diagnosis of bipolar disorder had already been established (n = 11) were excluded from this analysis. Also, the mean ages of onset of bipolar disorder in relation to cannabis exposure at baseline were examined with the Mann–Whitney U‐test.
Previous attrition analyses have shown that males (64 versus 71%; P < 0.001), individuals living in urban areas (66 versus 71%, P < 0.001) and individuals with parental psychiatric disorder (58 versus 69%, P < 0.001) were less likely to participate in the 15–16‐year follow‐up study [26]. Thus, inverse probability weighting [27] was used to weight the sample data by sex, parental psychiatric disorder and urbanicity. Both the weighted and unweighted data were analyzed with logistic regression analysis and ORs. Statistical significance was retained in the weighted analyses of cannabis exposure and onset of bipolar disorder for all those associations that were statistically significant in the unweighted analyses, and the strength of the associations were of similar magnitude (data available from the authors).
All statistical analyses were performed using SPSS statistical software (IBM SPSS Statistics, version 25; IBM Co., Armonk, NY, USA), except for examination of the Cox proportional hazard assumption that was analyzed using R (R Foundation for Statistical Computing, version 3.6.0; R Core Team, Vienna, Austria).
RESULTS
The variables under study and their relation to cannabis use and bipolar disorder during the follow‐up are presented in Table 1. Of 6325 participants, 352 adolescents (5.6%) reported any cannabis use until the age of 15–16 years; 291 (4.6%) adolescents reported having tried cannabis one to four times and 61 (1.0%) five times or more frequently. In all, 54.5% of females and 45.5% of males reported any cannabis use (P = 0.191). Significant multicollinearity was not seen between any variables included in the final multivariable model (all VIFs < 1.3). Based on register information 1.0% (n = 66/6325, 23% male) had been diagnosed with bipolar disorder by the end of the follow‐up.
TABLE 1.
Association of covariates and cannabis use and bipolar disorder in northern Finland birth cohort 1986
| Cannabis use frequency | Bipolar disorder | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n | n = 6325 | No cannabis use n = 5973 | Once or more n = 352 | P‐value | No bipolar disorder n = 6259 | Bipolar disorder n = 66 | P‐value | ||||||
| n | % | n | % | n | % | n | % | n | % | ||||
| Sex | 6325 | ||||||||||||
| Male | 3089 | 48.8 | 2929 | 49.0 | 160 | 45.5 | 0.191 | 3074 | 49.1 | 15 | 22.7 | < 0.001 | |
| Female | 3236 | 51.2 | 3044 | 51.0 | 192 | 54.5 | 3185 | 50.9 | 51 | 77.3 | |||
| Family structure | 5413 | ||||||||||||
| Divorced | 1102 | 20.4 | 1004 | 19.6 | 98 | 33.4 | < 0.001 | 1085 | 20.3 | 17 | 29.3 | 0.089 | |
| Intact | 4311 | 79.6 | 4116 | 80.4 | 195 | 66.6 | 4270 | 79.7 | 41 | 70.7 | |||
| Parental education | 5504 | ||||||||||||
| Less than 12 years | 3382 | 61.4 | 3207 | 61.6 | 175 | 58.3 | 0.255 | 3349 | 61.5 | 33 | 57.9 | 0.580 | |
| 12 years or more | 2122 | 38.6 | 1997 | 38.4 | 125 | 41.7 | 2098 | 38.5 | 24 | 42.1 | |||
| Cannabis use | 6325 | ||||||||||||
| No | 5973 | 94.4 | 5973 | 100.0 | 0 | 0.0 | – | 5918 | 94.6 | 55 | 83.3 | 0.001 a | |
| Yes | 352 | 5.6 | 0.0 | 352 | 100.0 | 341 | 5.4 | 11 | 16.7 | ||||
| Other illicit drug use | 6299 | ||||||||||||
| No | 6268 | 99.5 | 5942 | 99.9 | 326 | 92.9 | < 0.001 a | 6204 | 99.5 | 64 | 97.0 | 0.041 a | |
| Yes | 31 | 0.5 | 6 | 0.1 | 25 | 7.1 | 29 | 0.5 | 2 | 3.0 | |||
| Alcohol intoxication 10 ≥ times past year | 6167 | ||||||||||||
| No | 5024 | 81.5 | 4910 | 84.4 | 114 | 32.9 | < 0.001 | 4986 | 81.7 | 38 | 59.4 | < 0.001 | |
| Yes | 1143 | 18.5 | 910 | 15.6 | 233 | 67.1 | 1117 | 18.3 | 26 | 40.6 | |||
| Parental psychiatric disorder | 6325 | ||||||||||||
| No | 4046 | 64.0 | 3836 | 64.2 | 210 | 59.7 | 0.083 | 4017 | 64.2 | 29 | 43.9 | 0.001 | |
| Yes | 2279 | 36.0 | 2137 | 35.8 | 142 | 40.3 | 2242 | 35.8 | 37 | 56.1 | |||
| Daily smoking | 5853 | ||||||||||||
| No | 5134 | 87.7 | 4960 | 89.7 | 174 | 54.2 | < 0.001 | 5089 | 87.9 | 45 | 70.3 | < 0.001 | |
| Yes | 719 | 12.3 | 572 | 10.3 | 147 | 45.8 | 700 | 12.1 | 19 | 29.7 | |||
| YSR total | Mean | SD | Mean | SD | Mean | SD | < 0.001 b | Mean | SD | Mean | SD | < 0.001 b | |
| 26.5 | 15.6 | 25.9 | 15.2 | 36.2 | 19.0 | 26.3 | 15.5 | 37.2 | 17.6 | ||||
Fisher's exact test;
Mann–Whitney U‐test. YSR = Youth Self Report.
In the univariable analyses (Figure 2), an association between cannabis use frequency and onset of bipolar disorder onset was seen as follows: one to four times (HR = 3.03; 95% CI = 1.44–6.36), five times or more (HR = 5.55; 95% CI = 1.74–17.73). A dose–response was seen with the trend test (OR = 2.57; 95% CI = 1.61–24.12).
FIGURE 2.
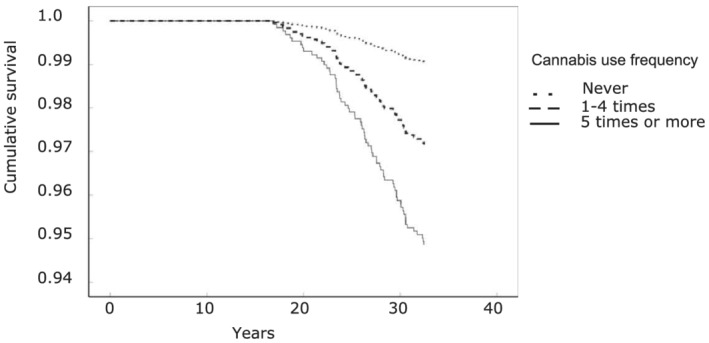
Crude association of cannabis exposure at baseline and onset of bipolar disorder
The results of the multivariable analyses are summarized in Table 2. Violations of the Cox proportional hazard assumption were not detected. A crude association of adolescent cannabis use and risk of bipolar disorder was seen (HR = 3.46; 95% CI = 1.81–6.61). When adjusted for sex, family structure and parental psychiatric disorders, the association between cannabis use and bipolar disorder diagnosis attenuated but remained statistically significant (HR = 3.00; 95% CI = 1.47–6.13). Statistical significance persisted even after further adjusting for the YSR total score (HR = 2.34; 95% CI = 1.11–4.94). However, further adjustments for frequent alcohol intoxications, daily smoking and lifetime illicit drug use attenuated the associations to statistically non‐significant. The results in the complete case sample were similar to those in the imputed sample (model 3, data available from the authors).
TABLE 2.
Hazard ratios (HR) for the risk of bipolar disorder in northern Finland birth cohort 1986 in different groups of cannabis use
| Frequency of cannabis use | Sample size | No cannabis use | Once or more | |||
|---|---|---|---|---|---|---|
| Cases | Cases | HR | 95% CI | |||
| Bipolar disorder | Crude | 6325 | 55 | 11 | 3.46 | 1.81–6.61 |
| Model 1 | 5413 | 49 | 9 | 3.00 | 1.47–6.13 | |
| Model 2 | 5190 | 49 | 9 | 2.34 | 1.11–4.94 | |
| Model 3 | 4992 | 47 | 9 | 1.70 | 0.73–3.98 | |
Model 1: sex, family structure, parental psychiatric disorder; model 2: sex, family structure, parental psychiatric disorder, YSR total; model 3: sex, family structure, parental psychiatric disorder, YSR total, frequent alcohol intoxications past year, daily smoking, other illicit substance use. Statistically significant results in bold type. CI = confidence interval; YSR = Youth Self Report.
In the subsample of subjects diagnosed with unipolar major depression (n = 572), cannabis use was not associated with transitioning from unipolar depression to bipolar disorder [8.8% (n = 6) versus 5.6% (n = 28), P = 0.271]. Furthermore, the mean of age of onset of bipolar disorder was similar in participants with or without cannabis exposure at baseline [25.0 years (n = 11) versus 25.9 years (n = 55), P = 0.810).
DISCUSSION
In this birth cohort study, we report a crude association between adolescent cannabis use and subsequent onset of bipolar disorder. This association remained significant after adjusting for sex, family structure, parental psychiatric disorders and baseline adolescent emotional and behavioral problems. However, further adjustments for frequent alcohol intoxications, daily smoking and other illicit drug use attenuated association to statistically non‐significant. Early cannabis use was thus not found to be associated with onset of bipolar disorder after extensive confounder control including adolescent substance use other than cannabis. However, while an independent association between cannabis use and bipolar disorder was not seen due to confounding by other substance use, the data still suggest early cannabis exposure to be an adverse clinical marker for onset of bipolar disorder. Moreover, participants in this cohort who smoked cannabis before age 16 years have been shown to be at increased risk of other adverse mental health outcomes, including psychosis [28] and self‐harm [29]. Therefore, reducing cannabis use in adolescents is an important public health priority.
The previously published prospective longitudinal studies on adolescent cannabis use and bipolar disorder have been heterogeneous in terms of outcome measures, sample sizes and characteristics, lengths of follow‐up and substance use‐related covariates controlled for (Supporting information, Table S2). To the best of our knowledge, there are no prospective longitudinal community studies that have examined the association between adolescent cannabis use and bipolar disorder‐related outcomes with register‐based data on bipolar disorder diagnoses made in clinical practice. In two studies, small high‐risk samples were utilized: a sample comprised of 211 probands of individuals with Type 1 bipolar disorder [12] and a clinical at‐risk sample of 52 individuals [14]. These studies used DSM‐IV diagnoses as their primary outcome measure. However, neither controlled for other substance use. The two general‐population‐based studies had sample sizes of 3370 [13] and 543 [15], and the former controlled for both hazardous alcohol use and other drug use in the final multivariable model. Hypomania symptoms assessed by the 32‐item version of the Hypomania Checklist (HCL‐32) [13] and mania symptoms assessed by the Composite International Diagnostic Interview (CIDI) [15] were used for the main outcome measures. The duration of follow‐up ranged from 1 year [14] to 8 years [15] raising concerns regarding reverse causality. Also, studies in adult populations have utilized either the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) [7, 8, 11] or Netherlands Mental Health Survey and Incidence Study (NEMESIS) [9, 10] cohorts, each with a follow‐up time of only 3 years and both lacking information concerning early‐onset exposure. This underscores the limitations of the evidence base concerning a possible independent association between cannabis use and onset of bipolar disorder.
The cumulative incidence of bipolar disorder (1.0%) observed in our study is similar to reported life‐time prevalence figures in the literature [30, 31]. In contrast, studies utilizing self‐report measures of mania symptoms [13] or high‐risk samples [12] have reported several‐fold higher incidences of their respective outcomes, but the aforementioned methodological choices introduce limitations regarding outcome validity [13] and generalizability [12]. Conversely, in our study, only 23% of the subjects who were diagnosed having bipolar disorder were male. Although higher prevalence rates have been reported in Type 2 bipolar disorder for females [32], bipolar disorder is thought to be equally prevalent in both sexes [33]. However, register‐based psychiatric outcomes, as utilized in our study, reflect help‐seeking behavior, with females more likely to obtain treatment for bipolar disorder [34].
The strengths of this study are as follows: the 18‐year follow‐up time is many‐folds longer than in any study conducted on the subject thus far, and captures the greatest risk period for diagnosis of bipolar disorder. We find the mean age of bipolar affective disorder register diagnosis to be, on average, 10 years after cannabis exposure, which reduces the likelihood of reverse causality. Several substance use‐related covariates were controlled for, as polysubstance use among adolescent cannabis use is common [19] and thus introduces a significant potential source of confounding. Importantly, cigarette smoking was included in the multivariable models, as it frequently co‐occurs with both bipolar disorder [35, 36] and cannabis use [19, 37, 38] and has also been associated with subsequent onset of bipolar disorder in prospective studies [39, 40, 41]. Parental psychiatric disorders were also controlled for, which is to be regarded as a strength, as genetic diathesis contributes significantly to the risk of bipolar disorder [42]. Given that an early age of onset is typical for bipolar disorder with an average age of onset of 22 years [43], the study design is well poised to study hypotheses concerning disease onset. The NFBC1986 birth cohort provides data on a large community sample with high ethnic and genetic homogeneity. To our knowledge, no other studies have examined the association of adolescent cannabis use and bipolar disorder‐related outcomes by utilizing a linkage to nation‐wide registers. Also, clinician‐made bipolar diagnoses entered in Finnish national registries have been found to be accurate after reassessment of medical records using DSM‐IV criteria [44]. Additionally, there is almost complete participant retention, with only a very small proportion of cohort members deceased or emigrated during the follow‐up.
However, there are also limitations. Power issues prevented the usage of a multi‐class cannabis variable in the multivariable models. This is the most significant limitation of the study, as cannabis exposure may vary from one episode of cannabis experimentation to heavy use and it is not biologically plausible that a single exposure would lead to bipolar disorder years later, and the use and compression of cannabis exposure information in the form of a categorical dichotomous variable may introduce an unwanted bias [45]. Also, information on the potency of the cannabis products consumed and mode of consumption was unavailable for analysis. Furthermore, information on life‐time cannabis use at age 15–16 years was collected using self‐reports and at one time‐point, which may result in an underestimation of true association. However, focusing on early‐onset exposure at age 15/16 years is crucial, as there is evidence that the age of initiation of cannabis use is associated with increased risk of other adverse sequelae such as psychosis [46, 47, 48]. Also, in this sample only 5.6% of the participants reported exposure to cannabis at age 15/16 years introducing power issues and increasing the likelihood of Type II error. In the European School Survey Project on Alcohol and Other Drugs (ESPAD) 2003 survey, the life‐time prevalence of cannabis use at the age of 15/16 in Finland was 11%, which suggests that under‐reporting might be an issue with our data [49]. However, this source of bias would more probably weaken the observed associations, rather than inflate them. As stated previously, probably due to gender differences in treatment‐seeking behavior, a disproportionate number of females with bipolar disorder were captured utilizing our register‐based outcome variable. Lastly, complementary sources of information on substance use such as biometrical assay data were unavailable for analysis.
CONCLUSIONS
In this study, the association between exposure to cannabis at age 15/16 years and subsequent onset of bipolar disorder was not significant after adjusting for other substance use. Future studies need to consider other substance use when evaluating the association of cannabis use with bipolar disorder. More prospective studies with multiple time‐points of cannabis use assessment, adequate sample sizes and lengths of follow‐up are required to inform whether the association of early exposure to cannabis and bipolar disorder is independent, as well as to eliminate the possibility of reverse causality.
DECLARATION OF INTERESTS
The NFBC study is funded by EU QLG1‐CT‐2000‐01643 (EUROBLCS) Grant no. E51560, NorFA Grant no. 731, 20056, 30167, USA/NIH 2000 G DF682 Grant no. 50945. A.D. has received funding from Juho Vainio Foundation and Yrjö Jahnsson Foundation. S.N. has received funding from Päivikki and Sakari Sohlberg Foundation and Juho Vainio Foundation. J.M. has received funding from Juho Vainio Foundation and Yrjö Jahnsson Foundation. A.M. has received grants from The Finnish Foundation for Alcohol Studies, Emil Aaltonen Foundation, Juho Vainio Foundation, Olvi Foundation and the Finnish Cultural Foundation.
AUTHOR CONTRIBUTIONS
Alexander Denissoff: Conceptualization; formal analysis. Antti Mustonen: Conceptualization; formal analysis. Anni‐Emilia Alakokkare: Formal analysis. James Scott: Formal analysis. Musa Sami: Formal analysis. Jouko Miettunen: Formal analysis. Solja Niemelä: Conceptualization; formal analysis.
Supporting information
Table S1. Frequencies and percentages of covariates of within different cannabis use categories
Table S2 a): Prospective longitudinal studies assessing the association between adolescent cannabis use and bipolar disorder
Table S2 b): Prospective longitudinal studies assessing association between adult cannabis use and onset of bipolar disorder or mania
Data S1. Supporting information
ACKNOWLEDGEMENTS
We wish to thank Hannu Vähänikkilä PhD for statistical support. We thank all cohort members and researchers who have participated in the study. We also wish to acknowledge the work of the NFBC project center. The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Denissoff A, Mustonen A, Alakokkare A‐E, Scott JG, Sami MB, Miettunen J, et al. Is early exposure to cannabis associated with bipolar disorder? Results from a Finnish birth cohort study. Addiction. 2022;117:2264–2272. 10.1111/add.15881
Funding information Juho Vainio Foundation; Yrjö Jahnsson Foundation; Finnish Cultural Foundation; Olvi Foundation; Emil Aaltonen Foundation; Päivikki and Sakari Sohlberg Foundation; EU, Grant/Award Number: QLG1‐CT‐2000‐01643
REFERENCES
- 1. Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large‐scale meta‐analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–95. 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382:1575–86. [DOI] [PubMed] [Google Scholar]
- 3. Hayes JF, Miles J, Walters K, King M, Osborn DPJ. A systematic review and meta‐analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand. 2015;131:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arjmand S, Behzadi M, Kohlmeier KA, Mazhari S, Sabahi A, Shabani M. Bipolar disorder and the endocannabinoid system. Acta Neuropsychiatr. 2019;31:193–201. [DOI] [PubMed] [Google Scholar]
- 5. D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta‐9‐tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. [DOI] [PubMed] [Google Scholar]
- 6. Hindley G, Beck K, Borgan F, Ginestet CE, McCutcheon R, Kleinloog D, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta‐analysis. Lancet Psychiatry. 2020;7:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feingold D, Weiser M, Rehm J, Lev‐Ran S. The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord. 2015;172:211–8. [DOI] [PubMed] [Google Scholar]
- 8. Gilman SE, Dupuy JM, Perlis RH. Risks for the transition from major depressive disorder to bipolar disorder in the National Epidemiologic Survey on alcohol and related conditions. J Clin Psychiatry. 2012;73:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henquet C, Krabbendam L, de Graaf R, ten Have M, van Os J. Cannabis use and expression of mania in the general population. J Affect Disord. 2006;95:103–10. [DOI] [PubMed] [Google Scholar]
- 10. Van Laar M, Van Dorsselaer S, Monshouwer K, De Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102:1251–60. [DOI] [PubMed] [Google Scholar]
- 11. Cougle JR, Hakes JK, Macatee RJ, Chavarria J, Zvolensky MJ. Quality of life and risk of psychiatric disorders among regular users of alcohol, nicotine, and cannabis: an analysis of the National Epidemiological Survey on alcohol and related conditions (NESARC). J Psychiatr Res. 2015;66–67:135–41. [DOI] [PubMed] [Google Scholar]
- 12. Duffy A, Horrocks J, Milin R, Doucette S, Persson G, Grof P. Adolescent substance use disorder during the early stages of bipolar disorder: a prospective high‐risk study. J Affect Disord. 2012;142:57–64. [DOI] [PubMed] [Google Scholar]
- 13. Marwaha S, Winsper C, Bebbington P, Smith D. Cannabis use and hypomania in young people: a prospective analysis. Schizophr Bull. 2018;44:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratheesh A, Cotton SM, Betts JK, Chanen A, Nelson B, Davey CG, et al. Prospective progression from high‐prevalence disorders to bipolar disorder: exploring characteristics of pre‐illness stages. J Affect Disord. 2015;183:45–8. [DOI] [PubMed] [Google Scholar]
- 15. Tijssen MJA, Van Os J, Wittchen HU, Lieb R, Beesdo K, Wichers M. Risk factors predicting onset and persistence of subthreshold expression of bipolar psychopathology among youth from the community. Acta Psychiatr Scand. 2010;122:255–66. [DOI] [PubMed] [Google Scholar]
- 16. Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: a systematic review and meta‐analysis. J Affect Disord. 2015;171:39–47. [DOI] [PubMed] [Google Scholar]
- 17. Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the risks and consequences of adolescent cannabis exposure. J Am Acad Child Adolesc Psychiatry. 2017;56:214–25. [DOI] [PubMed] [Google Scholar]
- 18. Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. The neuropsychopharmacology of cannabis: a review of human imaging studies. Pharmacol Ther. 2019;195:132–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halladay J, Woock R, El‐Khechen H, Munn C, MacKillop J, Amlung M, et al. Patterns of substance use among adolescents: a systematic review. Drug Alcohol Depend. 2020;216;108222. [DOI] [PubMed] [Google Scholar]
- 20. University of Oulu . Northern Finland Birth Cohort 1986 [internet]. University of Oulu. 1986. Available at: http://urn.fi/urn:nbn:fi:att:f5c10eef-3d25-4bd0-beb8-f2d59df95b8e. Accessed 1 Aug 2020
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening The Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.25046131 [Google Scholar]
- 22. Haukka J. Finnish health and social welfare registers in epidemiological research. Nor Epidemiol. 2009;14:113–20. [Google Scholar]
- 23. Miettunen J, Suvisaari J, Haukka J, Isohanni M. Use of register data for psychiatric epidemiology in the Nordic countries. In: Tsuang MT, Tohen M, Jones P, editors.Textbook of Psychiatric Epidemiology. 3rd ed. Chichester, UK: John Wiley & Sons Ltd; 2011. p. 117–31. [Google Scholar]
- 24. Filatova S, Marttila R, Koivumaa‐Honkanen H, Nordström T, Veijola J, Mäki P, et al. A comparison of the cumulative incidence and early risk factors for psychotic disorder in young adults in the northern Finland birth cohorts 1966 and 1986. Epidemiol Psychiatr Sci. 2017;26:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Achenbach T, Rescorla L. Achenbach system of empirically based assessment. In: Volkmar FR, editor. ncyclopedia of Autism Spectrum Disorders. New York, NY: Springer; 2013. p. 31–9. [Google Scholar]
- 26. Miettunen J, Murray GK, Jones PB, Mäki P, Ebeling H, Taanila A, et al. Longitudinal associations between childhood and adulthood externalizing and internalizing psychopathology and adolescent substance use. Psychol Med. 2014;44:1727–38. [DOI] [PubMed] [Google Scholar]
- 27. Haukoos JS, Newgard CD. Advanced statistics: Missing data in clinical research‐part 1: an introduction and conceptual framework. Acad Emerg Med. 2007;14:662–8. [DOI] [PubMed] [Google Scholar]
- 28. Mustonen A, Niemelä S, Nordström T, Murray GK, Mäki P, Jääskeläinen E, et al. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br J Psychiatry. 2018;212:227–333. [DOI] [PubMed] [Google Scholar]
- 29. Denissoff A, Niemelä S, Scott JG, Salom CL, Hielscher E, Miettunen J, et al. Does cannabis use in adolescence predict self‐harm or suicide? Results from a Finnish birth cohort study. Acta Psychiatr Scand. 2021;145:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suvisaari J, Aalto‐Setälä T, Tuulio‐Henriksson A, Härkänen T, Saarni SI, Perälä J, et al. Mental disorders in young adulthood. Psychol Med. 2009;39:287–99. [DOI] [PubMed] [Google Scholar]
- 31. Moreira ALR, Van Meter A, Genzlinger J, Youngstrom EA. Review and meta‐analysis of epidemiologic studies of adult bipolar disorder. J Clin Psychiatry. 2017;78:e1259–69. [DOI] [PubMed] [Google Scholar]
- 32. Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsuchiya KJ, Byrne M, Mortensen PB. Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord. 2003;5:231–42. [DOI] [PubMed] [Google Scholar]
- 34. Humpston CS, Bebbington P, Marwaha S. Bipolar disorder: prevalence, help‐seeking and use of mental health care in England. Findings from the 2014 adult psychiatric morbidity survey. J Affect Disord. 2020;282:426–33. [DOI] [PubMed] [Google Scholar]
- 35. Heffner JL, Strawn JR, Delbello MP, Strakowski SM, Anthenelli RM. The co‐occurrence of cigarette smoking and bipolar disorder: phenomenology and treatment considerations. Bipolar Disord. 2011;13:439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson JG, Diaz FJ, Lopez L, de Leon J. A combined analysis of worldwide studies demonstrates an association between bipolar disorder and tobacco smoking behaviors in adults. Bipolar Disord. 2015;17:575–97. [DOI] [PubMed] [Google Scholar]
- 37. Agrawal A, Budney AJ, Lynskey MT. The co‐occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107:1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fix BV, Smith D, O'Connor R, Heckman BW, Willemsen MC, Cummings M, et al. Cannabis use among a nationally representative cross‐sectional sample of smokers and non‐smokers in the Netherlands: results from the 2015 ITC Netherlands gold magic survey. BMJ Open. 2019;9:E024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Lima Bach S, de Azevedo Cardoso T, Moreira FP, Mondin TC, Simjanoski M, Kapczinski FP, et al. Risk factors for new‐onset bipolar disorder in a community cohort: a five‐year follow up study. Psychiatry Res. 2021;303:114109. [DOI] [PubMed] [Google Scholar]
- 40. Martínez‐Ortega JM, Goldstein BI, Gutiérrez‐Rojas L, Sala R, Wang S, Blanco C. Temporal sequencing of nicotine dependence and bipolar disorder in the National Epidemiologic Survey on alcohol and related conditions (NESARC). J Psychiatr Res. 2013;47:858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mustonen A, Ahokas T, Nordström T, Murray GK, Mäki P, Jääskeläinen E, et al. Smokin’ hot: adolescent smoking and the risk of psychosis. Acta Psychiatr Scand. 2018;138:5–14. [DOI] [PubMed] [Google Scholar]
- 42. Kieseppä T, Partonen T, Haukka J, Kaprio J, Lönnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–21. [DOI] [PubMed] [Google Scholar]
- 43. Suominen K, Mantere O, Valtonen H, Arvilommi P, Leppämäki S, Paunio T, et al. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment seeking. Bipolar Disord. 2007;9:698–705. [DOI] [PubMed] [Google Scholar]
- 44. Kieseppä T, Partonen T, Kaprio J, Lönnqvist J. Accuracy of register‐ and record‐based bipolar I disorder diagnoses in Finland; a study of twins. Acta Neuropsychiatr. 2000;12:106–9. [DOI] [PubMed] [Google Scholar]
- 45. Sauerbrei W, Perperoglou A, Schmid M, Abrahamowicz M, Becher H, Binder H, et al. State of the art in selection of variables and functional forms in multivariable analysis—outstanding issues. Diagnostic Progn Res. 2020;4(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stefanis NC, Dragovic M, Power BD, Jablensky A, Castle D, Morgan VA. Age at initiation of cannabis use predicts age at onset of psychosis: the 7‐to 8‐year trend. Schizophr Bull. 2013;39:251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta‐analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42:1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. European Monitoring Centre fo Drugs and Drug Addiction (EMCDDA) . Summary of the 2003 Findings [internet]. EMCDDA. 2003. Available at: http://www.espad.org/files/The_2003_ESPAD_report.pdf. Accessed 7 Sep 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Frequencies and percentages of covariates of within different cannabis use categories
Table S2 a): Prospective longitudinal studies assessing the association between adolescent cannabis use and bipolar disorder
Table S2 b): Prospective longitudinal studies assessing association between adult cannabis use and onset of bipolar disorder or mania
Data S1. Supporting information


