Abstract
Introduction: This consensus statement of Australian clinicians provides new recommendations for the pharmacological management of heart failure based on studies reported since the publication of the 2018 Australian heart failure guidelines.
Main recommendations:
-
▪
Use of sodium–glucose cotransporter 2 (SGLT2) inhibitors to prevent hospitalisation for heart failure in type 2 diabetes mellitus can be extended to patients with multiple cardiovascular risk factors, albuminuric chronic kidney disease, or atherosclerotic cardiovascular disease.
-
▪
New evidence supports the use of a mineralocorticoid receptor antagonist (finerenone) to prevent heart failure in type 2 diabetes mellitus associated with albuminuric chronic kidney disease.
-
▪
In addition to renin angiotensin system inhibitors (angiotensin receptor neprilysin inhibitor preferred), beta blockers and mineralocorticoid receptor antagonists, an SGLT2 inhibitor (dapagliflozin or empagliflozin) is recommended in all patients with heart failure with reduced left ventricular ejection fraction (LVEF ≤ 40%) (HFrEF). Lower quality evidence supports these therapies in patients with heart failure with mildly reduced LVEF (41‐49%) (HFmrEF).
-
▪
A soluble guanylate cyclase stimulator (vericiguat), selective cardiac myosin activator (omecamtiv mecarbil) and, if iron deficient, intravenous iron (ferric carboxymaltose) provide additional benefits in persistent HFrEF.
-
▪
An SGLT2 inhibitor (empagliflozin) should be considered in patients with heart failure with preserved LVEF (≥ 50%) (HFpEF).
Key changes in management from this statement: This document broadens the scope of angiotensin receptor neprilysin inhibitor use in patients with HFrEF and HFmrEF. SGLT2 inhibitor use expands to become a cornerstone therapy in HFrEF, with increasing evidence to support its use in HFmrEF and HFpEF.
Keywords: Heart failure, Cardiomyopathies, Guidelines as topic
There have been a number of clinical trials evaluating novel therapies in patients with heart failure that have led to recent updates of international heart failure guidelines. 1 , 2 Given that the 2018 National Heart Foundation of Australia (NHFA) and Cardiac Society of Australia and New Zealand (CSANZ) heart failure guidelines 3 , 4 were not scheduled for review and the high level of clinician interest, an academic group (Evidence to Practice) facilitated a working group comprising clinicians with expertise in the diagnosis and management of heart failure in Australia to develop a consensus statement focusing on studies evaluating new and established drugs to prevent or treat heart failure, published since the 2018 guidelines (Box 1).
Box 1. Methodology for developing consensus statement.
-
▪
Evidence to Practice, an academic group closely linked to the South Australian Health and Medical Research Institute and Monash University, selected a working group comprising ten clinicians with expertise in the diagnosis and management of adult patients with heart failure in Australia.
-
▪
Evidence to Practice facilitated virtual roundtables comprising all working group members, with the initial roundtable conducted in August 2021 to discuss the scope and development process of the consensus statement.
-
▪
It was agreed that the consensus statement would focus on randomised controlled trials (including post hoc analyses) or meta‐analyses of randomised controlled trials evaluating the safety and efficacy of pharmacological agents to prevent or manage heart failure published since the 2018 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand heart failure guidelines.3
-
▪
A review of the evidence (including published studies up to January 2022) was undertaken by three working group members (AS, CDeP, JA) and a draft manuscript was written.
-
▪
Recommendations were drafted, accompanied by a Grading of Recommendations Assessment, Development and Evaluation (GRADE) strength of recommendation and quality of evidence. The strength of recommendation for or against an intervention (graded as strong or weak) was guided by the quality of the evidence (graded as high, moderate, low or very low), balance between benefits and harms, preferences and values, and resource considerations.5
-
▪
All working group members reviewed and edited the manuscript, with further discussion via virtual roundtable. All authors approved the final version of the manuscript.
The categorisation of heart failure according to left ventricular ejection fraction (LVEF) is unchanged, although minor alterations to the acronyms will allow for consistency with the recent universal definition. 6 Heart failure with a moderate to severely reduced LVEF (≤ 40%) will be referred to as HFrEF; heart failure with a mildly reduced LVEF (41–49%) will be referred to as HFmrEF; and heart failure with a preserved LVEF (≥ 50%) will be referred to as HFpEF.
Prevention of heart failure
The 2018 NHFA/CSANZ heart failure guidelines gave strong recommendations for blood pressure lowering and lipid lowering to prevent heart failure. 3 , 4 Strong recommendations were also given for angiotensin‐converting enzyme (ACE) inhibitors and beta blockers in patients with left ventricular systolic dysfunction, ACE inhibitors in patients with cardiovascular disease, and sodium–glucose cotransporter 2 (SGLT2) inhibitors in patients with type 2 diabetes mellitus associated with cardiovascular disease. 3 , 4 Further trials evaluating the efficacy of SGLT2 inhibitors have reported consistent reductions in heart failure hospitalisation, such that this indication may be extended to patients with type 2 diabetes mellitus at increased cardiovascular risk either because of multiple cardiovascular risk factors or macroalbuminuric chronic kidney disease (estimated glomerular filtration rate [eGFR] > 30 mL/minute/1.73 m2 body surface area) 7 , 8 , 9 , 10 (Box 2).
Box 2. New recommendations to prevent heart failure.
-
▪
A sodium–glucose cotransporter 2 inhibitor is recommended in patients with type 2 diabetes mellitus who are at high cardiovascular risk due to associated atherosclerotic cardiovascular disease, multiple cardiovascular risk factors or macroalbuminuric chronic kidney disease to decrease the risk of developing heart failure (strong recommendation for; high quality of evidence).
-
▪
A mineralocorticoid receptor antagonist (finerenone) may be considered in patients with type 2 diabetes mellitus associated with albuminuric chronic kidney disease who are taking a renin angiotensin system inhibitor, to reduce the risk of developing heart failure (weak recommendation for; moderate quality of evidence).
Two trials evaluated a selective non‐steroidal mineralocorticoid receptor antagonist (MRA), finerenone, in patients with type 2 diabetes mellitus with albuminuric chronic kidney disease (eGFR > 25 mL/minute/1.73 m2). 11 , 12 The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO‐DKD) trial achieved its primary composite renal endpoint and secondary composite cardiovascular endpoint, the latter including a trend to decreased heart failure hospitalisation. 11 The Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO‐DKD) trial demonstrated a reduction in its primary composite cardiovascular endpoint, driven almost exclusively by decreased heart failure hospitalisation, 12 including decreased new‐onset heart failure 13 (Box 2).
Heart failure with reduced LVEF (HFrEF)
The 2018 NHFA/CSANZ heart failure guidelines strongly recommend an ACE inhibitor (or angiotensin receptor blocker [ARB] if ACE inhibitors are contraindicated or not tolerated), beta blocker and MRA in all patients with HFrEF to decrease mortality and hospitalisation, and an angiotensin receptor neprilysin inhibitor (ARNI), sinus node inhibitor (ivabradine), diuretic and intravenous iron in selected patients. 3 , 4 In this section, we will review new evidence regarding the use of an ARNI (sacubitril–valsartan), intravenous iron (ferric carboxymaltose), SGLT2 inhibitors (dapagliflozin, empagliflozin), a guanylate cyclase stimulator (vericiguat) and a selective cardiac myosin activator (omecamtiv mecarbil).
Angiotensin receptor neprilysin inhibitor
The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) trial demonstrated the additional benefit of neprilysin inhibition in addition to a renin angiotensin system inhibitor. 14 This led to the 2018 NHFA/CSANZ heart failure guidelines strongly recommending an ARNI, sacubitril–valsartan, as a replacement for an ACE inhibitor or ARB in patients with HFrEF despite maximally tolerated or target doses of an ACE inhibitor (or ARB) and beta blocker, to decrease mortality and hospitalisation. 3 , 4
Post hoc analyses of PARADIGM‐HF identified benefit within 30 days of sacubitril–valsartan versus enalapril in reducing heart failure hospitalisation, supporting its earlier introduction in the treatment pathway. 15 , 16 However, PARADIGM‐HF was done in patients with ambulatory heart failure with an active run‐in phase requiring participants to demonstrate tolerability to target doses of both drugs. 14 , 17
The PIONEER‐HF trial in patients stabilised from an acute heart failure episode showed sacubitril–valsartan was superior to enalapril for the primary efficacy endpoint of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) reduction. The study recorded no adverse safety signals in patients following acute decompensated HFrEF with a systolic blood pressure of at least 100 mmHg, accompanied by a reduction in the exploratory endpoint of rehospitalisation for heart failure at 8 weeks. 18 The TRANSITION study, an open‐label study following acute decompensated HFrEF, demonstrated comparable safety and tolerability in participants randomised to receive sacubitril–valsartan either before discharge or within 14 days of discharge from hospital. 19 These two studies also reported no difference in safety or efficacy in the subgroups of patients with de novo HFrEF or who were ACE inhibitor or ARB naïve. 18 , 19
A small study comparing sacubitril–valsartan with valsartan alone in patients with advanced HFrEF associated with severe symptoms reported no difference in the primary endpoint of change in NT‐proBNP through to 24 weeks. While under‐powered for clinical outcomes, there was also no beneficial effect on the secondary efficacy endpoint of days alive out‐of‐hospital and free from heart failure events. 20
Given the superiority of sacubitril–valsartan over an ACE inhibitor and the reported early benefits, sacubitril–valsartan should be considered a first line HFrEF therapy, provided it does not compromise the commencement of the other first line HFrEF therapies. Alternatively, an ACE inhibitor may be prescribed and switched to sacubitril–valsartan following commencement and up‐titration of other HFrEF therapies. The latter approach may be preferred in patients with hypotension or severely symptomatic, advanced heart failure (Box 3).
Box 3. New recommendations to treat heart failure with reduced ejection fraction (LVEF ≤ 40%).
-
▪
Either an ARNI (sacubitril–valsartan) or ACE inhibitor (ARNI preferred) is recommended in patients with HFrEF (including newly diagnosed) to decrease mortality and decrease hospitalisation for heart failure (strong recommendation for; high quality of evidence).
-
▪
An ARNI (sacubitril–valsartan) is recommended as a replacement for an ACE inhibitor (with at least a 36‐hour washout window) or ARB in patients with HFrEF despite receiving an ACE inhibitor (or ARB) and a beta blocker to decrease mortality and decrease hospitalisation for heart failure (strong recommendation for; high quality of evidence).
-
▪
An SGLT2 inhibitor (dapagliflozin or empagliflozin) is recommended in patients with HFrEF to decrease mortality and decrease hospitalisation for heart failure (strong recommendation for; high quality of evidence).
-
▪
A soluble guanylate cyclase stimulator (vericiguat) may be considered in patients with persistent HFrEF and recent worsening heart failure despite receiving maximally tolerated or target doses of a renin angiotensin system inhibitor, beta blocker and MRA to decrease cardiovascular death or hospitalisation for heart failure (weak recommendation for; moderate quality of evidence).
-
▪
A selective cardiac myosin activator (omecamtiv mecarbil) may be considered in patients with persistent HFrEF and an LVEF ≤ 35% despite receiving maximally tolerated or target doses of a renin angiotensin system inhibitor, beta blocker and MRA to decrease cardiovascular death or hospitalisation for heart failure (weak recommendation for; moderate quality of evidence).
-
▪
In patients with HFrEF associated with persistent symptoms despite optimised therapy, if the patient is iron deficient (ie, ferritin < 100 mg/L, or ferritin 100–299 mg/L with transferrin saturation < 20%), intravenous iron (ferric carboxymaltose) should be considered to improve symptoms and quality of life and decrease hospitalisation for heart failure (strong recommendation for; moderate quality of evidence).
ACE = angiotensin‐converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; SGLT2 = sodium–glucose cotransporter 2.
Sodium–glucose cotransporter 2 inhibitor
The SGLT2 inhibitors dapagliflozin and empagliflozin have been evaluated in placebo‐controlled, randomised trials enrolling patients with HFrEF. 21 , 22 A study evaluating the efficacy of dapagliflozin in treating HFrEF (DAPA‐HF) reported the group randomised to receive dapagliflozin had a significant reduction in the composite primary endpoint of cardiovascular death or worsening heart failure, driven by significant reductions in both heart failure hospitalisation and cardiovascular mortality. 21 The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced) reported that use of empagliflozin was associated with a significant reduction in the composite primary endpoint of cardiovascular death or heart failure hospitalisation, driven by a significant reduction in heart failure hospitalisation and a non‐significant reduction in cardiovascular mortality. 22 A meta‐analysis combining these studies reported SGLT2 inhibitor use was associated with significant relative risk reductions in all‐cause mortality (13%), cardiovascular mortality (14%), first hospitalisation for heart failure (31%) and first kidney composite event (38%), with no significant heterogeneity for treatment effect. 23
The drugs were well tolerated with a favourable adverse event profile, and the benefits were independent of background therapy and similar in patients with and without diabetes. 21 , 22 , 23 The benefits were also statistically significant within 30 days of commencing therapy. 24 , 25 The combined SGLT2 and SGLT1 inhibitor sotagliflozin has been shown in the SOLOIST‐WHF trial to decrease the composite primary endpoint of total number of cardiovascular deaths and hospitalisations and urgent visits for heart failure in patients with type 2 diabetes mellitus who were recently hospitalised for worsening heart failure. 26 Although published after the cut‐off date for our evidence review, a trial of empagliflozin in patients hospitalised for acute heart failure who had been stabilised (EMPULSE) reported a significant improvement in a hierarchical endpoint comprising mortality, heart failure events and quality of life within 90 days. 27 Collectively, these findings support the use of SGLT2 inhibitors early in the HFrEF treatment pathway (Box 3).
Guanylate cyclase stimulator
The oral soluble guanylate cyclase stimulator vericiguat was shown in a placebo‐controlled, randomised trial to improve clinical outcomes in patients with HFrEF with evidence of worsening heart failure. 28 The overall treatment effect was modest, with a 10% relative risk reduction in the primary endpoint, cardiovascular death or first hospitalisation for heart failure. While the study aimed to enrol patients with more severe heart failure, patients with an NT‐proBNP level in the highest quartile appeared not to benefit. 28 A subsequent analysis reported that the benefit of vericiguat extended to NT‐proBNP levels up to 8000 pg/mL. 29 This suggests that selected patients with progressive heart failure despite optimal medical therapy may benefit from the addition of vericiguat; however, patients with very advanced heart failure are less likely to benefit (Box 3).
Cardiac myosin activator
The selective cardiac myosin activator omecamtiv mecarbil was shown in a placebo‐controlled, randomised trial to decrease the composite endpoint of cardiovascular death and heart failure hospitalisation in patients with HFrEF. 30 The overall treatment effect was modest, with an 8% relative risk reduction in the primary endpoint. Prespecified subgroup analyses identified that patients with an LVEF above the median value of 28% and patients in atrial fibrillation or flutter may be less likely to benefit. 30 A recent analysis confirmed that the LVEF was the strongest modifier of treatment effect among the prespecified subgroups. 31 While this should be interpreted with caution, these interactions are biologically plausible given one might expect a cardiac myosin activator to have greater benefit in patients with a more severely reduced LVEF who remain in sinus rhythm. Furthermore, a post hoc analysis reported greater clinical benefit in patients with severe heart failure with an LVEF ≤ 30% and heart failure hospitalisation within 6 months. 32 This suggests that selected patients with persistent heart failure and a severely reduced LVEF despite optimal medical therapy may benefit from the addition of omecamtiv mecarbil (Box 3).
Intravenous iron
The 2018 NHFA/CSANZ heart failure guidelines recommended that intravenous iron should be considered in patients with HFrEF associated with iron deficiency and persistent symptoms despite optimised therapy to improve symptoms and quality of life. 3 , 4 Intravenous ferric carboxymaltose was recently evaluated in a placebo‐controlled, randomised trial in patients hospitalised with acute heart failure (HFrEF or HFmrEF) with iron deficiency (ferritin < 100 μg/L, or ferritin 100–299 μg/L with transferrin saturation below 20%). 33 While the study did not show a statistically significant difference between groups for the primary endpoint, there was a trend in favour of intravenous ferric carboxymaltose with a 21% relative risk reduction in the composite endpoint of cardiovascular death and total heart failure hospitalisations, driven by a nominally significant 26% relative risk reduction in total heart failure hospitalisations. 33 Trial recruitment was affected by the COVID‐19 pandemic, with the study showing that intravenous ferric carboxymaltose reduced the incidence of the primary endpoint in a prespecified, pre‐COVID‐19 sensitivity analysis. This study demonstrated that intravenous ferric carboxymaltose can be safely administered in patients hospitalised with acute heart failure and appears to decrease recurrent heart failure hospitalisation (Box 3). The effect of intravenous iron on cardiovascular mortality is being evaluated in ongoing clinical trials (NCT03037931, NCT03036462 and NCT02642562).
HFrEF treatment algorithm
The HFrEF treatment algorithm in the 2018 NHFA/CSANZ heart failure guidelines encouraged a step‐wise approach after starting an ACE inhibitor, beta blocker and MRA in patients with HFrEF. 3 , 4 While this was guided by the available clinical trial evidence, this approach might delay the commencement of highly effective therapies that have been shown to have similar safety and efficacy regardless of background treatment. 23 , 34 Furthermore, the benefits of ARNIs and SGLT2 inhibitors are seen early, making a strong case for commencing these treatments upfront before full titration of the individual medications, which may include starting more than one drug simultaneously. 15 , 16 , 24 , 25 We therefore recommend that all patients with HFrEF should be commenced on an ARNI (or ACE inhibitor), beta blocker, MRA and SGLT2 inhibitor. Preference should be given to either an ARNI or ACE inhibitor over an ARB, because none of the heart failure ARB studies demonstrated a reduction in all‐cause mortality. A suggested treatment algorithm adapted from the 2018 NHFA/CSANZ heart failure guidelines is provided in (Box 4).
Box 4. Heart failure with reduced ejection fraction management algorithm, with one of several possible drug initiation regimens based on presence or absence of clinical congestion.
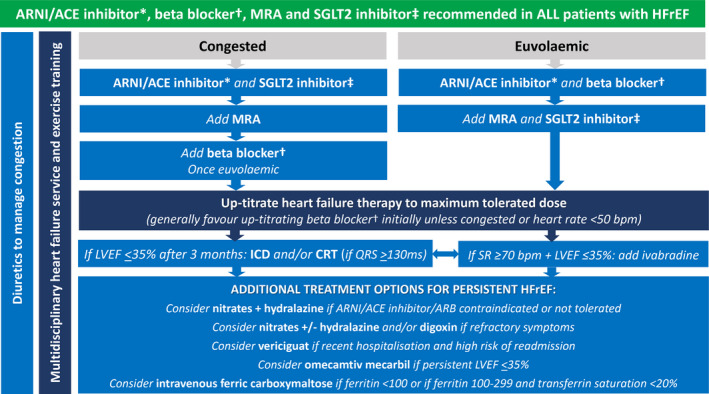
ACE = angiotensin‐converting enzyme; ARNI = angiotensin receptor neprilysin inhibitor; CRT = cardiac resynchronisation therapy; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter defibrillator; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; SGLT2 = sodium–glucose cotransporter 2; SR = sinus rhythm.
The key overarching theme (green background) is to commence all patients on the four destination therapies of ARNI/ACE inhibitor*, beta blocker†, MRA and SGLT2 inhibitor‡ as soon as clinically possible, given their early morbidity and mortality benefit.
* ARNI preferred. ACE inhibitor can be considered as an alternative if problematic hypotension, and consider switching to ARNI later. † Use beta blocker with outcome trial proven HFrEF efficacy (ie, carvedilol, bisoprolol, metoprolol succinate or nebivolol). ‡ Use SGLT2 inhibitor with outcome trial proven HFrEF efficacy (ie, dapagliflozin or empagliflozin).
Heart failure with mildly reduced LVEF (HFmrEF)
The 2018 NHFA/CSANZ heart failure guidelines stated that an ACE inhibitor (or ARB), beta blocker and MRA may be considered in patients with HFmrEF based on post hoc analyses from randomised controlled trials that included patients with HFmrEF. 4 Post hoc and subgroup analyses from recent studies (described below) provide evidence to support the consideration of additional therapies in patients with HFmrEF.
Angiotensin receptor neprilysin inhibitor
The PARADIGM‐HF trial (evaluating sacubitril–valsartan versus enalapril in HFrEF) and the PARAGON‐HF trial (evaluating sacubitril–valsartan versus valsartan in heart failure with LVEF ≥ 45%) were combined to observe the impact of sacubitril–valsartan across the spectrum of LVEF. 14 , 35 , 36 This analysis supported an extended benefit of sacubitril–valsartan to patients with HFmrEF (Box 5).
Box 5. New recommendations to treat heart failure with mildly reduced ejection fraction (LVEF 41–49%).
-
▪
Either an ACE inhibitor, ARNI (sacubitril–valsartan) or ARB may be considered in patients with HFmrEF to decrease cardiovascular mortality or hospitalisation for heart failure (weak recommendation for; low quality of evidence).
-
▪
An SGLT2 inhibitor (empagliflozin) should be considered in patients with HFmrEF to decrease cardiovascular mortality or hospitalisation for heart failure (strong recommendation for; moderate quality of evidence).
-
▪
In patients with HFmrEF associated with persistent symptoms despite optimised therapy, if the patient is iron deficient (ie, ferritin < 100 mg/L, or ferritin 100–299 mg/L with transferrin saturation < 20%), intravenous iron (ferric carboxymaltose) may be considered to improve symptoms and quality of life and decrease hospitalisation for heart failure (weak recommendation for; low quality of evidence).
ACE = angiotensin‐converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; HFmrEF = heart failure with mildly reduced ejection fraction; LVEF = left ventricular ejection fraction; SGLT2 = sodium–glucose cotransporter 2.
Sodium–glucose cotransporter 2 inhibitor
The EMPEROR‐Preserved study included patients with HFmrEF. The reduction in the composite primary endpoint was similar across the prespecified LVEF subgroups and was nominally statistically significant in patients with HFmrEF. 37 It is therefore reasonable to extend the HFrEF recommendation for SGLT2 inhibitors to patients with HFmrEF (Box 5).
Intravenous iron
The AFFIRM study, which evaluated intravenous ferric carboxymaltose in acute heart failure included patients with HFmrEF. 33 While the trial was not powered for this subgroup, there was no significant heterogeneity according to LVEF. It is therefore reasonable to consider intravenous iron in such patients (Box 5).
Heart failure with preserved LVEF (HFpEF)
The 2018 NHFA/CSANZ heart failure guidelines did not give specific treatment recommendations for patients with HFpEF, given that none of the major HFpEF randomised controlled trials had demonstrated a significant benefit of trialled interventions for the primary study endpoints. 4
Angiotensin receptor neprilysin inhibitor
The PARAGON‐HF study was done in patients with HFpEF. and did not show a significant difference between sacubitril–valsartan and valsartan alone for the primary endpoint, with the addition of sacubitril leading to a 13% relative risk reduction in total heart failure hospitalisations and cardiovascular mortality (P = 0.06). The trend towards a benefit of sacubitril–valsartan over its active albeit unproven comparator, valsartan, was driven by reduced heart failure hospitalisation. 35 Based on current evidence, despite the impression of a positive impact on heart failure hospitalisation from the addition of sacubitril to valsartan, no recommendation can be given to support its use in HFpEF.
Sodium–glucose cotransporter 2 inhibitor
The EMPEROR‐Preserved study, which enrolled patients with HFpEF, demonstrated that empagliflozin led to a significant reduction in the primary endpoint of cardiovascular death or heart failure hospitalisation, mainly driven by a reduction in heart failure hospitalisation. 37 There was a non‐significant 9% relative risk reduction in cardiovascular mortality, with no change in overall mortality. 37 These findings support the use of SGLT2 inhibitors in patients with HFpEF. While the benefits of sotagliflozin were similar in patients with a reduced or preserved LVEF in the SOLOIST‐WHF study, and dapagliflozin has been reported to improve quality of life in patients with HFpEF, the evidence for efficacy in this population is strongest for empagliflozin 26 , 38 (Box 6).
Box 6. New recommendation to treat heart failure with preserved ejection fraction (LVEF ≥ 50%).
-
▪
An SGLT2 inhibitor (empagliflozin) should be considered in patients with HFpEF to decrease cardiovascular mortality or hospitalisation for heart failure (strong recommendation for; moderate quality of evidence).
HFpEF = heart failure with preserved ejection fraction; LVEF = left ventricular ejection fraction; SGLT2 = sodium–glucose cotransporter 2.
Conclusion
Heart failure prevention remains a major health priority, with recent studies reporting that SGLT2 inhibitors and MRAs can prevent or delay the development of heart failure in patients with diabetic kidney disease. In all patients with established HFrEF, there is now strong evidence to support combining either an ARNI or ACE inhibitor with a beta blocker, MRA and SGLT2 inhibitor. Post hoc analyses support a similar approach in patients with HFmrEF. Furthermore, the benefits of SGLT2 inhibitors extend to patients with HFpEF, with this being the first treatment to meet its primary endpoint in an HFpEF randomised controlled trial powered for major clinical outcomes. Additional therapies that may be considered in selected patients with HFrEF include vericiguat, omecamtiv mecarbil, and intravenous iron. Evidence gaps remain, including the need for additional therapies in patients with HFpEF, persistent HFrEF despite optimised therapy, and advanced heart failure.
Open access
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Competing interests
All authors were supported by the South Australian Health and Medical Research Institute for the initial heart failure consensus statement conference. We were offered an honorarium to attend the virtual meeting by the Evidence to Practice Group. No other funding was provided. Andrew Sindone reports consultancy fees, speaking honoraria or research support from Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol Myers Squibb, Menarini, Merck Sharp and Dohm, Mylan, Novartis, Otsuka, Pfizer, Sanofi, Servier, and Vifor. Carmine De Pasquale reports consultancy fees, speaking honoraria or research support from AstraZeneca, Novartis, Vifor, Boehringer Ingelheim, Bayer, Lily, Roche Diagnostics, and American Regent. John Amerena reports consultancy fees, speaking honoraria, conference and research support from AstraZeneca, Novartis, Vifor, Boehringer Ingelheim, Bayer, Lilly, and Amgen. Christine Burdeniuk reports consultancy fees and speaking honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, and Novartis. Alicia Chan reports speaking honoraria or research support from Novartis, AstraZeneca, Boehringer Ingelheim, Vifor, Medtronic, Biotronik, Abbott, and Boston Scientific. Andrew Coats reports consultancy fees, speaking honoraria or research support from AstraZeneca, Boehringer Ingelheim, Menarini, Novartis, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Impulse Dynamics, Respicardia, and Viatris. David Hare reports research grants, consultancy fees and speaking honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lundbeck, Menarini, Merck, Novartis, Pfizer, Sanofi, Servier, and Vifor. Peter Macdonald reports peer‐reviewed research funding from the National Health and Medical Research Council and NSW Health, industry‐supported research funding to his institution from Amgen and Novartis, and consultancy fees paid to him from AstraZeneca, Boehringer Ingelheim, and Novartis. Aaron Sverdlov is supported by National Heart Foundation of Australia Future Leader Fellowships (Award IDs 101918 and 106025) and reports research grants from the NSW Health, Hunter Medical Research Institute, Biotronik, RACE Oncology, Bristol Myer Squibb, Roche Diagnostics, and Vifor; and consultancy fees and speaking honoraria from Novartis, Bayer, Bristol Myer Squibb, AstraZeneca, and Boehringer Ingelheim. John Atherton reports consultancy fees, speaking honoraria or research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, and Novartis.
Provenance
Not commissioned; externally peer reviewed.
Acknowledgements
We thank Philip Aylward and Stephen Nicholls, and the Evidence to Practice team (Julie Butters and Georgia Rejack) for their administration and logistic support in convening the group and preparing this document.
References
- 1. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599‐3726. [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022; 79: e263‐e421. [DOI] [PubMed] [Google Scholar]
- 3. Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of heart failure 2018. Med J Aust 2018; 209: 363‐369. https://www.mja.com.au/journal/2018/209/8/national‐heart‐foundation‐australia‐and‐cardiac‐society‐australia‐and‐new‐0 [DOI] [PubMed] [Google Scholar]
- 4. Atherton JJ, Sindone A, De Pasquale CG, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ 2018; 27: 1123‐1208. [DOI] [PubMed] [Google Scholar]
- 5. Schünemann HBJ, Guyatt G, Oxman A, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. https://gdt.gradepro.org/app/handbook/handbook.html (viewed Apr 2022).
- 6. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021; 23: 352‐380. [DOI] [PubMed] [Google Scholar]
- 7. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295‐2306. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347‐357. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Pratley R, Dagogo‐Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425‐1435. [DOI] [PubMed] [Google Scholar]
- 10. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol 2021; 6: 148‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219‐2229. [DOI] [PubMed] [Google Scholar]
- 12. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385: 2252‐2263. [DOI] [PubMed] [Google Scholar]
- 13. Filippatos G, Anker SD, Agarwal R, et al. Finerenone reduces risk of incident heart failure in patients with chronic kidney disease and type 2 diabetes: analyses from the FIGARO‐DKD Trial. Circulation 2022; 145: 437‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMurray JJ, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993‐1004. [DOI] [PubMed] [Google Scholar]
- 15. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131: 54‐61. [DOI] [PubMed] [Google Scholar]
- 16. Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30‐day readmission after heart failure hospitalization. J Am Coll Cardiol 2016; 68: 241‐248. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013; 15: 1062‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019; 380: 539‐548. [DOI] [PubMed] [Google Scholar]
- 19. Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019; 21: 998‐1007. [DOI] [PubMed] [Google Scholar]
- 20. Mann DL, Givertz MM, Vader JM, et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol 2022; 7: 17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995‐2008. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413‐1424. [DOI] [PubMed] [Google Scholar]
- 23. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020; 396: 819‐829. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR‐Reduced trial. Circulation 2021; 143: 326‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berg DD, Jhund PS, Docherty KF, et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2021; 6: 499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117‐128. [DOI] [PubMed] [Google Scholar]
- 27. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022; 28: 568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883‐1893. [DOI] [PubMed] [Google Scholar]
- 29. Ezekowitz JA, O’Connor CM, Troughton RW, et al. N‐terminal pro‐B‐type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail 2020; 8: 931‐039. [DOI] [PubMed] [Google Scholar]
- 30. Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021; 384: 105‐116. [DOI] [PubMed] [Google Scholar]
- 31. Teerlink JR, Diaz R, Felker GM, et al. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC‐HF. J Am Coll Cardiol 2021; 78: 97‐108. [DOI] [PubMed] [Google Scholar]
- 32. Felker GM, Solomon SD, Claggett B, et al. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALACTIC‐HF randomized clinical trial. JAMA Cardiol 2022; 7: 26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ponikowski P, Kirwan BA, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020; 396: 1895‐1904. [DOI] [PubMed] [Google Scholar]
- 34. Okumura N, Jhund PS, Gong J, et al. Effects of sacubitril/valsartan in the PARADIGM‐HF trial (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) according to background therapy. Circ Heart Fail 2016; 9: e003212. [DOI] [PubMed] [Google Scholar]
- 35. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609‐1620. [DOI] [PubMed] [Google Scholar]
- 36. Solomon SD, Vaduganathan M, Claggett BL, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020; 141: 352‐361. [DOI] [PubMed] [Google Scholar]
- 37. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021; 385: 1451‐1461. [DOI] [PubMed] [Google Scholar]
- 38. Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med 2021; 27: 1954‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


