Summary
Background
Topical dexamethasone and prednisolone are currently the mainstay treatment for equine ophthalmic inflammatory diseases, such as equine recurrent uveitis. Comparative pharmacokinetic studies in horses are lacking and current guidelines are mainly based on empirical data and extrapolation from other species.
Objectives
To investigate the penetration and local concentrations of topically applied dexamethasone and prednisolone in normal equine ocular fluids and serum.
Study design
Prospective randomised experimental pharmacokinetic study.
Methods
Twenty‐one Shetland ponies without ophthalmic disease were treated bilaterally topically every 2 hours during 24 hours to obtain steady state drug concentrations. One eye was treated with 0.15 mg of dexamethasone disodium phosphate (0.1%), and the other eye was simultaneously treated with 1.5 mg of prednisolone acetate (1%). Serum samples were taken prior to the induction of general anaesthesia. Aqueous and vitreous humour samples were taken during euthanasia at time points after administration of the last dose (t = 5 min, t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min, t = 180 min). Each pony was randomly assigned to one time point, and three ponies were sampled per time point. Dexamethasone and prednisolone concentrations were measured by liquid chromatography‐mass spectrometry.
Results
The mean dexamethasone concentration in aqueous humour was 32.4 ng/mL (standard deviation [SD] 10.9) and the mean prednisolone concentration was 321.6 ng/mL (SD 96.0). In the vitreous and in serum samples concentrations of both corticosteroids were below the limit of detection (LOD 2.5 ng/mL).
Main limitations
The study group was limited to subjects without evidence of current ophthalmic disease. A limited number of time points were measured.
Conclusions
Potentially effective dexamethasone and prednisolone concentrations were measured in the anterior chamber, but vitreal concentrations were negligible. Systemic uptake was low. Therefore, treatment with only topically administered corticosteroids is deemed insufficient in horses in cases of posterior uveitis. Further studies evaluating other routes of administration are warranted.
Keywords: corticosteroids, drug dosing interval, horse, liquid chromatography‐mass spectrometry, ophthalmic, topical administration
1. INTRODUCTION
Equine recurrent uveitis (ERU) is one of the most common and debilitating ophthalmic diseases in horses. 1 , 2 It is an immune‐mediated disease with a reported prevalence of 7%‐10% in Europe. 1 , 2 Treatment of ERU remains a challenge and is aimed at eliminating inflammation, preventing sequelae and recurrence to maintain vision. Blindness is often the end stage of the disease, despite intensive medical and surgical treatment. 1 , 2 , 3 , 4
Medical treatment of ERU frequently consists of topical corticosteroids in combination with cycloplegics (atropine) and systemic non‐steroidal anti‐inflammatory drugs (NSAIDs). 1 , 3 , 5 Corticosteroids are the most important drugs to decrease the intraocular inflammation. 1 , 2 Depending on the veterinarian's personal preference, topical corticosteroids are used alone or in combination with systemic corticosteroids. 1 , 2 , 3 Local therapy is often preferred over systemic administration because of potentially undesirable side effects of systemic corticosteroids such as laminitis. 6 There are several different commercially available corticosteroid preparations, including dexamethasone disodium phosphate solution 0.1% and prednisolone acetate suspension 1% (both approved human formulations). Choice of preparation may be based on clinical evidence regarding glucocorticoid potency, suggested differences in ocular penetration, or personal preference of the clinician. 1 , 7
Previous studies in humans, rabbits, dogs and cats have shown poor penetration of topically applied corticosteroids into the posterior segment of the eye. 8 , 9 , 10 , 11 , 12 , 13 The same was found for topical dexamethasone ointment in the equine eye. 14 To achieve higher concentrations in the posterior segment of the globe, alternative routes of administration, such as subconjunctival, peribulbar injection or systemic (oral) administration, are used in human medicine. 15 , 16 , 17 , 18 Scientific evidence on the penetration and distribution of corticosteroids in equine ocular fluids is very limited, and guidelines for their use are largely based on studies in other species or clinical experience. 9 , 10 , 11 , 12 , 14 One report in horses showed the concentration of dexamethasone in cornea, aqueous humour, iris, lens, vitreous body and choroid/retina after topical administration of a dexamethasone containing ophthalmic ointment formulation; however, the frequency of application and the concentration of the preparation used was lower than currently used clinically. 14 There are no studies that directly compare dexamethasone disodium phosphate and prednisolone acetate, and the studies that are available are difficult to compare due to the use of different dosage regimens. 7 , 9 , 12 , 13 , 14 , 16 , 19 , 20
The aim of the current study was to determine the penetration of both dexamethasone (DEX) and prednisolone (PRED) into the aqueous humour, vitreous humour and systemic circulation after repeated topical administration in ponies, using commercially available dexamethasone disodium phosphate (0.1%) and prednisolone acetate (1%). We hypothesised that, after adjustment for differences in dose, there would be no difference in the concentration of DEX and PRED in the aqueous and vitreous humour as well as the blood. We also hypothesised that concentrations of both corticosteroids would be lower in the vitreous humour compared to the aqueous humour.
2. MATERIALS AND METHODS
2.1. Animals
Twenty‐one healthy Shetland pony mares (mean bodyweight 181 kg, standard deviation [SD] 32.8 kg) with a median age of 7.5 years (range: 4‐14 years) were used in this study (Table S1). These ponies were used in a large terminal orthopaedic study and were to be euthanised regardless of participation in this study. Prior to initiation of the study, all ponies underwent a thorough ophthalmic examination performed by a board‐certified (ECVO) veterinary ophthalmologist (MB), and/or a third‐year resident in veterinary ophthalmology (IS) and included neuro‐ophthalmic testing, hand‐held slit lamp biomicroscopy (SL‐15, Kowa Optimed, Inc) and direct ophthalmoscopy (Pneumatic Otoscope, Welch Allyn). Only ponies without any signs of ophthalmic inflammation were included in the study.
2.2. Study design and medication administration
All ponies were treated topically bilaterally every 2 hours for 24 hours to mimic a typical aggressive dosage regimen for treating acute ERU in an equine clinic and to reach a steady state concentration. It was anticipated that steady state concentrations would be achieved by 24 hours. 9 , 19 The left eye (OS) was randomly assigned (using a simple lottery system and taking equal distribution into account) to one of the following treatments: 0.1% dexamethasone disodium phosphate (Teva Nederland) or 1% prednisolone acetate (Allergan Pharmaceuticals Ireland). The right eye (OD) received the other treatment. Dexamethasone disodium phosphate was given 10 times to OS and 11 times to OD (Table S1). One eye was treated with 0.15 mg (0.15 mL) of dexamethasone disodium phosphate and the other eye was simultaneously treated with 1.5 mg (0.15 mL) of prednisolone acetate using a 1‐mL syringe at each time point. All syringes were pre‐filled. The bottles of prednisolone acetate were tilted at least 10 times before drawing up the syringes to ensure even distribution of the formulation over all syringes, and the syringes were adequately shaken before topical application. Animal handlers not involved in further study execution applied the medication into the lower conjunctival sac. The total cumulative dose administered to each pony was 1.95 mg of dexamethasone disodium phosphate and 19.5 mg of prednisolone acetate.
2.3. Sampling and sample preparation
To characterise the absorption and elimination phases of the time–concentration curve over the dosing interval, one sample from each eye per pony was taken at an exact time interval after administration of the last treatment: t = 5 min, t = 15 min, t = 30 min, t = 60 min, t = 90 min, t = 120 min and t = 180 min. Three ponies were needed per time point as this is the minimum needed to calculate a mean and standard deviation, and only twenty‐one ponies were available. These three ponies were randomly assigned to be sampled at each time point.
Prior to sampling, the ponies were sedated with detomidine hydrochloride (Domosedan, Orion Corporation) 0.01 mg/kg bwt IV. An intravenous catheter was placed in the right or left jugular vein. Blood samples were taken from the jugular catheter prior to anaesthetic induction. General anaesthesia was induced with ketamine (Anaestamine, AST Farma BV) 2 mg/kg bwt IV and midazolam (Midazolam, Actavis Group PTC ehf.) 0.06 mg/kg bwt IV. The ponies were placed in dorsal recumbency. Prior to sampling, the conjunctival sac was rinsed with saline (0.9%, B. Braun Melsungen AG). The ponies were humanely euthanised using sodium pentobarbital (Euthanimal, Alfasan Diergeneesmiddelen BV) 50mg/kg IV. During euthanasia a paracentesis of the anterior chamber and vitreal body was performed using a needle coupled to a 5‐mL syringe. For paracentesis of the anterior chamber a 21‐G needle was directed through the limbal cornea anterior and parallel to the iris and aqueous humour was withdrawn. 21 Vitreous paracentesis was performed by placing an 18‐G needle approximately 10‐12 mm from the dorsolateral limbus with the needle directed posterior. 21 Mean amount of aqueous humour samples was 2.30 mL (range: 1.5‐3 mL) and vitreous humour 3.26 mL (range: 1.5‐7 mL) (total of 42 eyes). The blood samples were centrifuged to obtain serum. All samples were immediately transferred to plain micro tubes (1 mL/tube, PCT‐PT Micro Tubes, Sarstedt AG & Co. KG), and stored at −80°C until further analysis.
2.4. Liquid chromatography‐mass spectrometry analysis
DEX and PRED concentrations were measured by liquid chromatography‐mass spectrometry (LC/MS/MS, AB Sciex Netherlands BV and PerkinElmer) by a technician independent of the study (LN). All measurements were performed in duplicate, or triplicate if possible with the sample volume. A sample's concentration was calculated as the mean of the measurements. Each sample was tested for both analytes (DEX and PRED).
2.4.1. Standards and solutions
Reference standards (DEX and PRED) were purchased from Sigma Aldrich (Sigma‐Aldrich Chemie NV) and the internal standard (triamcinolone) was made by BUFA (Spruyt hillen). These analytes were dissolved in 100% methanol to obtain a solution of 1 mg/mL, which was then further diluted to obtain a stock solution of 100 ng/mL. From these stock solutions, a working solution with 250 ng/mL of triamcinolone was prepared and calibrators were obtained by spiking serum, aqueous and vitreous humour from equine pooled samples with PRED and DEX to obtain concentrations of 0.5, 1, 2.5, 5, 7.5, 10, 20, 40, 50, 60, 80 and 100 ng/mL.
2.4.2. Ocular fluid sample preparation
The aqueous and vitreous humour samples were prepared by adding 5 μL of formic acid (2%) to denature and precipitate proteins and 5 μL of the triamcinolone working solution to 490μl of the sample fluid. The sample was then centrifuged for 10 min at 4000 g, and 100 μL of the supernatant was transferred to a sample vial so that 10μl could be injected into the LC/MS/MS system. Sample vials were kept in an autosampler at 4°C.
2.4.3. Serum sample preparation
Serum samples were prepared by adding 1000 μL of methanol and 5 μL of triamcinolone working solution to 495 μL serum. This mixture was centrifuged for 10 min at 4000 g, and the supernatant was transferred to glass tubes to which 7.5 mL of dichloromethane was added. The tube was then centrifuged for 10 minutes at 4000 g, after which the dichloromethane was evaporated. The residue was dissolved in 100 μL of 50% methanol, and 100 μL of this solution was transferred to a sample vial and 10 μL was injected into the LC/MS/MS system. Sample vials were kept in an autosampler at 4°C.
2.4.4. LC/MS/MS analysis
The concentrations of DEX and PRED were measured using an AB Sciex Triple Quadrupole Mass Spectrometer (AB Sciex Netherlands BV) coupled with a PerkinElmer chromatography system (PerkinElmer). The mass spectrometer used negative heated electrospray ionisation at 400°C with a spray voltage of 3500 V and nebuliser gas flow of 50 arbitrary units of dry nitrogen.
Chromatography used a GraceSmart RP18, 150 × 2.1 mm, 3 μm column with a guard column and two mobile phases (Pump A: 20 mL of 5 mmol/L ammonium acetate with 180 mL of water, and Pump B: 20 mL of 5 mmol/L ammonium acetate with 180 mL of methanol) at a flow rate of 0.2 mL/min and pressure of 132 bar. The gradient started with 80% A and 20% B, switched to 90% A and 10% B from 10 until 13 minutes and then returned to 80% A and 20% B from 13 until 18 minutes.
Detection and quantification were conducted using multiple reaction monitoring with two or three adducts (mass‐to charge ration [m/z]) per analyte.
The peak area ratios analyte/internal standard versus the corresponding concentrations of the corticosteroids in spiked samples were plotted to obtain a calibration curve, which was linear over 5‐100 ng/mL with a correlation coefficient of 0.99. PRED samples were diluted to fall within this range. The limit of detection (LOD) was defined as a signal that was at least three times higher than the background. A variability of less than 20% was accepted for all standards and samples at a concentration that was considered as the limit of quantification (LOQ). The LOQ of DEX and PRED in aqueous and vitreous humour was 5 ng/mL, and the LOD was 2.5 ng/mL. In the serum samples, the LOQ was 10 ng/mL and the LOD 5 ng/mL. For accuracy and precision, duplicates of quality control samples at three different concentrations (20, 40 and 60 ng/mL) were prepared and analysed on two different days.
2.5. Data analysis
Statistical analysis was performed by a statistician (JV) using freely available software (R) (R version 4.0.2 Patched [R Core Team]). 22 For each fluid, the mean DEX and PRED concentration, range (min‐max), and standard deviation (SD) were calculated using Microsoft Excel (Excel for Windows for Mac 2011, version 14.4.7, Microsoft).
The measured concentration of PRED in the aqueous and vitreous humour was divided by 10 to correct for the differences in dose between PRED and DEX. This concentration is referred to as the dose‐adjusted PRED. A linear mixed effects regression model 23 was used to analyse the association between the outcome of DEX and PRED concentration in the eye fluid. Treatment (DEX/PRED), time, eye treated (OS/OD) and interaction between treatment and time were explanatory variables. Horse was added to the model as a random effect to take the correlation between repeated observations within a horse into account. Visual inspection of the residuals of the full model showed no abbreviations of the model assumptions. The AIC was used in a backward selection approach to select the best model. Consecutively eye side (OS/OD), the interaction between treatment and time and time were removed from the model. Treatment remained in the model as this provided the answer to the research question. The mean difference in concentration between DEX and PRED with 95% confidence interval was reported.
To test for differences in actual PRED and DEX concentrations, first the mean PRED and DEX concentrations were calculated for each time point within a horse, and second, the ratio of the mean of dose‐adjusted PRED divided by mean of DEX was calculated. The ratio was analysed by a linear regression model with normal distribution with time as explanatory variable. Time was removed from the model based in the AIC selection criterion, leaving an intercept only model. The intercept as estimated mean ratio with 95% confidence interval was reported for the potency. The residuals of the full model were used to check the model assumptions, and no abbreviations were observed. For both linear regression models, the first two measurements of DEX/PRED concentrations were taken (Table S1).
3. RESULTS
3.1. Ophthalmic examination
Sixteen (76.2%) ponies did not have ophthalmic abnormalities. One pony had an old laceration of the upper eyelid of the left eye causing slight focal corneal oedema (fluorescein negative). Four (19.0%) ponies had incipient cataract (three unilateral focal axial anterior cortical cataract and one bilateral focal axial posterior cortical cataract). The ponies with cataract were evenly distributed over the different sampling time points. During the period of drug administration, 95.2% (20/21) of the ponies showed hyperaemic conjunctiva (14.2% severe and 81.0% mild hyperaemic conjunctiva), associated with minor ocular discomfort, particularly in the eyes treated with PRED.
3.2. Concentrations
By treating the eye topically every 2 hours for 24 hours a steady state concentration was reached in the aqueous humour. Concentrations remained constant even at 180 minutes after administration (Figure 1).
FIGURE 1.
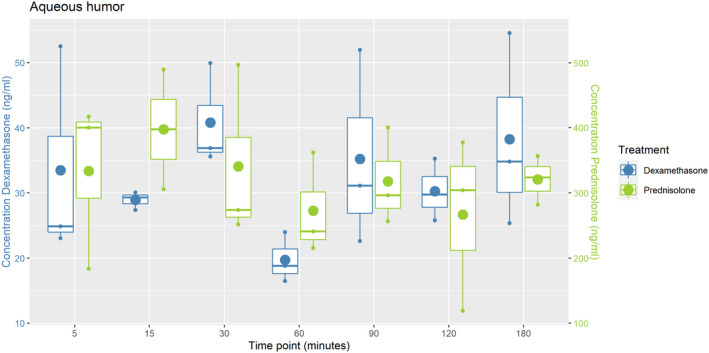
Plot of the DEX (blue) and PRED (green) concentrations in aqueous humour after repeated topical administration of dexamethasone disodium phosphate (0.1%) and prednisolone acetate (1%) every 2 h for 24 h. Three ponies were randomly assigned to each time point (t = 5, t = 15, t = 30, t = 60, t = 90, t = 120, t = 180 min); The box indicates the standard deviation; the small dots the three concentrations measured per time point; the big dot the mean concentration of the three samples; the horizontal line indicates the median
The mean DEX concentration of all time points in aqueous humour was found to be 32.4 ng/mL (range 15.7‐58.3 ng/mL, SD 10.9) (Table S1), while the mean PRED concentration of all time points was 321.6 ng/mL (range 119.0‐605.3 ng/mL, SD 96.0) (Table S1).
In the vitreous humour and serum samples, DEX and PRED concentrations were both below the detection limit of the assay at all time points.
The estimated mean difference between DEX and dose‐adjusted PRED over time is 0 [95% CI: −4.1; 4.1]. The estimated mean PRED/DEX ratio was 10.7 [95% CI 8.8; 12.7].
4. DISCUSSION
Our study was performed to investigate and compare the penetration of both dexamethasone and prednisolone into the equine ocular fluids and systemic circulation after repeated topical administration, using commercially available dexamethasone disodium phosphate (0.1%) and prednisolone acetate (1%). According to the current literature, prednisolone acetate has a better ocular penetration than dexamethasone phosphate solutions. 1 , 5 , 13 , 24 This was not supported by the results of our study. In our study, the penetration of DEX and PRED were not statistically different.
The study was designed to mimic current clinical practice of frequent dosing every 2 hours for 24 hours without nocturnal intermission to achieve a steady state. 1 , 3 , 5 Previous studies related to topical eye treatment in dogs, cats and horses have shown a detectable concentration of DEX mainly in the aqueous humour. 10 , 11 , 14 Similarly, in our study detectable concentrations of DEX and PRED were only found in aqueous humour samples, the concentrations of DEX and PRED in vitreous humour and in serum were below the detection limit in all ponies at all time points. Therefore, treatment with only topically administered corticosteroids is deemed inadvisable in cases of equine posterior uveitis.
Steady state concentrations were reached in our study. The fact that the concentrations plateaued up to 3 hours post administration suggests that dosing every 2 hours is not necessary as elimination is slow, and in a clinical situation, dosing could be tapered after 24 hours.
The mean DEX concentration found in aqueous humour in the current study was almost identical to the mean DEX concentration found in human aqueous humour after frequently repeated topical administration of dexamethasone disodium phosphate (0.1%): 30.5 ng/mL. 9 In this study, a steady state concentration was reached as well by treating patients with 0.05 mg of dexamethasone disodium phosphate every 1.5 hours starting 1 day before pars plana vitrectomy surgery, and the mean DEX concentration of all time points (14‐120 minutes) was calculated. 9 In studies in cats, dogs and horses, aqueous humour concentrations of DEX of, respectively, 8, 4 and 2.5 ng/mL were measured. 10 , 11 , 14 The results from the latter studies cannot be easily compared with our study as other treatment schedules and different formulations of DEX were used.
The mean PRED concentration in aqueous humour in our study was 321.6 ng/mL. Two studies in human medicine have shown mean peak concentrations of PRED in aqueous humour of 669.9 and 1130 µg/l within two hours of topical administration of prednisolone acetate (1%). 20 , 25 However, we determined the concentration in a steady state situation after repeated topical administration of prednisolone acetate (1%), rather than the mean peak. It could be speculated that the lower concentration of PRED in our study could infer a difference in ocular penetration of prednisolone acetate (1%) between species; however, direct comparison between species is inadvisable without further evidence.
Previous studies in humans have found that prednisolone acetate 1% had a better corneal penetration than dexamethasone disodium phosphate 0.1%. 9 , 13 , 20 , 24 , 25 , 26 Prednisolone acetate has a lipid‐soluble character, which facilitates passage through the lipid‐rich corneal epithelium. 24 , 27 , 28 Dexamethasone disodium phosphate is a water‐soluble preparation. However, dexamethasone disodium phosphate will be hydrolysed by enzymes in the tear film and cornea and will be partly converted to dexamethasone, which is lipid‐soluble. 9 The concentration of PRED detected in the aqueous humour in our study was about 10 times higher than the DEX concentration (321.6 ng/mL vs 32.4 ng/mL). Based on our results, it can be concluded that the penetration of the cornea of both steroid preparations is similar in equines, and the difference in concentrations found in this study can be solely explained by the ten times higher concentration of the prednisolone preparation (1% vs 0.1%).
Dexamethasone is about seven times more potent than prednisolone. 28 , 29 Purely based on the relative potency we could predict that concentrations of PRED need to be seven times higher than DEX concentrations to achieve the same effect. With the formulations in this study having a 1:10 (DEX:PRED) difference in concentration, a slight preference of topical use of PRED can be supported from a drug potency point of view. However, from a clinical point of view and prednisolone acetate (1%) has the disadvantage that it is a suspension containing drug particles so it can cause ocular discomfort. 27 , 30 In our study we observed minor discomfort in the eyes treated with prednisolone acetate. Inappropriate use of prednisolone acetate (1%) might also provide less consistent dosages with each administration, as vigorous shaking of the suspension before use is needed for adequate drug dispersion in the suspension. 31 , 32 In short, it is our opinion that there is no significant evidence advocating the preference of either PRED or DEX in equine clinical practice.
The optimal corticosteroid concentration required for treating different inflammatory ophthalmic conditions has not been established yet and no information is available concerning the penetration of corticosteroids in the inflamed eye. Theoretically, achieved concentrations may be anticipated to be higher than in the current study due to possible disturbance of the blood‐aqueous barrier in eyes following inflammation, leading to an increase in drug distribution into the ocular fluids; however, at the current time, there is no literature that supports this statement. 27 , 33 , 34 In vitro studies have shown that a minimum concentration of dexamethasone of 20‐25 ng/mL is needed for anti‐inflammatory effects. 7 , 34 No studies have looked at the minimum concentration of prednisolone needed for anti‐inflammatory effects. There are no studies reported in the scientific literature investigating the impact of ocular inflammation on effective intraocular concentrations of DEX and PRED.
In our study, concentrations of DEX ranged from 15.7 to 58.3 ng/mL and PRED concentrations from 119.0 to 605.3 ng/mL despite steady state having been achieved. This inter‐individual variation in measured concentrations could be ascribed to yet unidentified anatomical or functional variations, differences in the actual dose administered or underlying eye disease. 9 , 27 It is known that anatomical/functional variation in the lacrimal drainage system may lead to different amounts of drugs in the tear film; however, this is likely to be negligible as only Shetland ponies in the same age range were used in this study. Differences in administered dose could have influenced the concentrations measured. We have standardised the drug administration protocol by using a 1‐mL syringe for all treatment time points, but some leakage of drug occurred in all ponies, for example, due to tearing. The cumulative administered dose was high compared with other studies (1.95 mg of dexamethasone disodium phosphate and 19.5 mg of prednisolone acetate, respectively). 9 , 11 However, as the bioavailability of topical ophthalmic drugs is low (around 5%), the influence of differences in administered dose could be considered to be minimal. 35 An underlying ophthalmic disease such as corneal epithelial defects or breakdown of the blood‐aqueous barrier, for example in cases of ERU, may lead to increased corticosteroid concentrations in ocular fluids. In our study, one pony had unilateral focal corneal oedema (fluorescein negative) in the eye treated with PRED. Excluding the eye of this pony from the results did not significantly alter the mean PRED concentration in the aqueous humour (321.6 ng/mL vs 322.4 ng/mL). No ponies had signs of active uveitis at ophthalmic examination. The corticosteroid concentrations in serum were below the LOD in all ponies, so drug redistribution from the systemic circulation was also unlikely to add to the concentrations in the aqueous humour. Hence, due to reasonable elimination of other explanations it is likely that the range of the measured DEX and PRED concentrations are related to interindividual variation, which is often encountered in studying biochemical processes.
Systemic concentrations of corticosteroids after topical administration have been measured in humans and rabbits. 9 , 12 , 36 In our study, the systemic concentrations of DEX and PRED were below the LOD in all ponies (5 ng/mL). Spiess et al. determined DEX concentrations in serum of horses after continued topical treatment with 0.1% dexamethasone ophthalmic ointment for 8 consecutive days (every 5‐9 hours). 37 They found serum DEX concentrations between 0.10 and 0.49 ng/mL, which is probably related with a lower LOD (0.06 ng/mL). 37 The results of Spiess et al. 37 and those from our own study suggest that after topical administration of dexamethasone and prednisolone systemic absorption is very minor.
Nineteen percent of the Shetland ponies included in our study had unilateral or bilateral cataracts. It is estimated that between 5% and 7% of horses have some form of cataract in otherwise clinically normal eyes. 38 It is our clinical experience that Shetland ponies have a higher prevalence of cataracts. Since the ponies with cataract were evenly distributed through our sample population and no concentrations of corticosteroids were measured in the vitreous humour in all ponies, the influence of the presence of cataract on the results is deemed negligible.
In human medicine, an anatomic classification of uveitis is used (anterior, intermediate, posterior and panuveitis) and treatment is based on this classification. 39 , 40 In horses, current classification of ERU does not specifically differentiate between different anatomic locations and treatment often depends on the veterinarian's personal preference. 1 , 2 , 3 Currently, most veterinarians treat horses with ERU mainly with topical corticosteroids (dexamethasone or prednisolone), whether or not in combination with systemic corticosteroids, 1 , 2 , 3 knowing the latter may give rise to development of laminitis. 6 The results of this study suggest that a specific treatment protocol for every individual ERU patient might be warranted, based (partly) on the anatomic location of the disease.
Twenty‐one ponies were available for this study, which were already being used for an existing (terminal) orthopaedic research. If more ponies had been available additional time points could have been added to extend the period in which the pharmacokinetic curve was described. We expect the results to be similar in a larger sample population as the treatment and sampling were performed accurately and the LC/MS/MS is a very sensitive detection method for glucocorticoids.
As mentioned above, only a fixed number of ponies were available and this was one of the reasons we chose to treat both eyes in the same pony. The eyes were randomised, so we had both right and left eyes treated by the same formulation. Topical ophthalmic medication reaches the systemic circulation via the conjunctival sac or nasolacrimal duct and could possibly have affected the contralateral eye. 27 However, each sample was tested for both corticosteroids and no quantifiable amount of drugs were measured in the samples of the contralateral eye (in the eye treated with dexamethasone, no PRED concentrations were measured in both aqueous humour and vitreous humour and vice versa). Via the systemic circulation the vitreous chamber would reach higher concentrations than the aqueous humour, 16 , 18 and in our study, the concentrations of DEX and PRED in vitreous humour were below LOD in all ponies at all time points. Moreover, serum sample concentrations of both corticosteroids were below the LOD at all time points, so therefore, we believe no cross contamination between the eyes had occurred that interfered with the analysis.
Placing a subpalpebral lavage system in either the upper or lower conjunctival sac might have facilitated the topical treatment of all ponies. However, all ponies were easy to treat and as mentioned before the influence of differences in administered dose due to for example tearing was considered to be minimal. 27 , 35
Extrapolation of the results to patients with (inflammatory) ophthalmic disease should be done with caution as the blood‐aqueous barrier is compromised in these patients. All ponies in our study did not have overt inflammatory ophthalmic disease and were deemed to have intact blood‐ocular barriers. Studying the relation between concentrations of steroid anti‐inflammatory agents in ocular fluids after topical administration in diseased and inflamed equine eyes is warranted.
No tissue concentrations were measured in our study. However, fluid concentrations of the different ocular compartments showed to be representative for topical corticosteroids in a previous study. 12
In conclusion, it was demonstrated in this study that there was no difference in penetration of DEX and PRED into the aqueous humour because there was no difference in the concentration once adjusted for dose, and the PRED/DEX ratio was the same as the difference in dose (10 times higher for PRED).
The lack of penetration of DEX and PRED into the vitreous humour and undetectable serum concentrations suggest that other forms of administration (eg oral or subconjunctival) are required to reach the vitreous so could be indicated in cases of equine posterior uveitis. Further studies are indicated in order to determine the concentrations of corticosteroids in equine ocular fluids after systemic and/or subconjunctival treatment and the concentrations of corticosteroids in ocular fluids in horses with ophthalmic disease (ERU) after treatment with corticosteroids.
CONFLICT OF INTERESTS
No competing interests have been declared.
AUTHOR CONTRIBUTIONS
H Hermans and EMH van den Berg contributed to study design and study execution by preparing treatment of the ponies and sample collection, and data analysis and interpretation. IJM Slenter and MH Boevé contributed to study design and study execution by ophthalmic examination of the ponies. JC Vendrig and R Gehring contributed to study design and data analysis and interpretation. JL de Nijs‐Tjon performed the LC/MS/MS analysis. JCM Vernooij performed the statistical analysis. All authors contributed to the preparation of the manuscript and approved the final manuscript.
INFORMED CONSENT
Not applicable.
ETHICAL ANIMAL RESEARCH
This investigation was carried out as an adjunct to a large orthopaedic study which was approved by the Central Authority for Scientific Procedures on Animals (CCD) (project permit AVD108002015307). The Head of Animal Welfare Body Utrecht confirmed the additional interventions required for the current study were permitted.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/evj.13526.
Supporting information
Table S1
ACKNOWLEDGEMENTS
We thank Nikae te Moller, Inge van Geijlswijk, Janny de Grauw, Filipe Serra Bragança and Ralph Edwards for help in conducting and reporting this study.
Hermans H, van den Berg EMH, Slenter IJM, Vendrig DJC, de Nijs‐Tjon LJL, Vernooij JCM, et al. Penetration of topically administered dexamethasone disodium phosphate and prednisolone acetate into the normal equine ocular fluids. Equine Vet J. 2022;54:965–972. doi: 10.1111/evj.13526
The abstract is available in Portuguese in the Supporting Information section of the online version of this article
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in DataverseNL at www.dataverse.nl, https://doi.org/10.34894/XAFT9K.
REFERENCES
- 1. Gilger BC, Hollingsworth SR. Diseases of the uvea, uveitis and recurrent uveitis. In: Gilger BC, ed. Equine Ophthalmology. 3rd ed. John Wiley & Sons Incorporated; 2016:369–415. [Google Scholar]
- 2. Malalana F, Stylianides A, McGowan C. Equine recurrent uveitis: human and equine perspectives. Vet J. 2015;206:22–9. [DOI] [PubMed] [Google Scholar]
- 3. McMullen RJ, Fischer BM. Medical and surgical management of equine recurrent uveitis. Vet Clin North Am Equine Pract. 2017;33:465–81. [DOI] [PubMed] [Google Scholar]
- 4. Fischer BM, McMullen RJ, Reese S, Brehm W. Intravitreal injection of low‐dose gentamicin for the treatment of recurrent or persistent uveitis in horses: preliminary results. BMC Vet Res. 2019;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilger BC, Michau TM. Equine recurrent uveitis: new methods of management. Vet Clin North Am Equine Pract. 2004;20:417–27. [DOI] [PubMed] [Google Scholar]
- 6. Jordan VJ, Ireland JL, Rendle DI. Does oral prednisolone treatment increase the incidence of acute laminitis? Equine Vet J. 2017;49:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Awan MA, Agarwal PK, Watson DG, McGhee CNJ, Dutton GN. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br J Ophthalmol. 2009;93:708–13. [DOI] [PubMed] [Google Scholar]
- 8. Loftsson T, Sigurdsson HH, Hreinsdóttir D, Konrádsdóttir F, Stefánsson E. Dexamethasone delivery to posterior segment of the eye. J Incl Phenom Macrocycl Chem. 2007;57:585–9. [Google Scholar]
- 9. Weijtens O, Schoemaker RC, Romijn FP, Cohen AF, Lentjes EG, Van Meurs JC. Intraocular penetration and systemic absorption after topical application of dexamethasone disodium phosphate. Ophthalmology. 2002;109:1887–91. [DOI] [PubMed] [Google Scholar]
- 10. Kaiser T, Werner A, Bäumer W, Kietzmann M. Tissue distribution of dexamethasone in canine ocular compartments following topical application of dexamethasone‐21‐isonicotinate and oxytetracycline HCl. Vet Ophthalmol. 2008;11:335–9. [DOI] [PubMed] [Google Scholar]
- 11. Bessonova J, Meyer‐Lindenberg A, Bäumer W, Kietzmann M. Tissue distribution of dexamethasone in feline ocular structures following single topical application of dexamethasone as an ointment or suspension. Vet Ophthalmol. 2011;14:109–13. [DOI] [PubMed] [Google Scholar]
- 12. Chockalingam A, Xu L, Stewart S, LeMerdy M, Tsakalozou E, Fan J, et al. Protocol for evaluation of topical ophthalmic drug products in different compartments of fresh eye tissues in a rabbit model. J Pharmacol Toxicol Methods. 2019;96:9–14. [DOI] [PubMed] [Google Scholar]
- 13. McGhee CNJ, Watson DG, Midgley JM, Noble MJ, Dutton GN, Fern AI. Penetration of synthetic corticosteroids into human aqueous humour. Eye. 1990;4:526–30. [DOI] [PubMed] [Google Scholar]
- 14. Bäumer W, Reichenbecker F, Kietzmann M. Topische Arzneimittelanwendung am Pferdeauge unter besonderer Berücksichtung von Dexamethason. Praktischer Tierartz. 2008;89:738–43. [Google Scholar]
- 15. Weijtens O, Schoemaker RC, Lentjes EG, Romijn FP, Cohen AF, Van Meurs JC. Dexamethasone concentration in the subretinal fluid after a subconjunctival injection, a peribulbar injection, or an oral dose. Ophthalmology. 2000;107:1932–8. [DOI] [PubMed] [Google Scholar]
- 16. Kim L, Matsushima D, Widera G, Xu Y, Nyam K, Hosseini K, et al. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J. Ocul Pharmacol Ther. 2008;24:301–8. [DOI] [PubMed] [Google Scholar]
- 17. Weijtens O, Feron EJ, Schoemaker RC, Cohen AF, Lentjes EG, Romijn FP, et al. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am J Ophthalmol. 1999;128:192–7. [DOI] [PubMed] [Google Scholar]
- 18. Weijtens O, Schoemaker RC, Cohen AF, Romijn FP, Lentjes EG, van Rooij J, et al. Dexamethasone concentration in vitreous and serum after oral administration. Am J Ophthalmol. 1998;125:673–9. [DOI] [PubMed] [Google Scholar]
- 19. Watson D, Noble MJ, Dutton GN, Midgley JM, Healey TM. Penetration of topically applied dexamethasone alcohol into human aqueous humor. Arch Ophthalmol. 1988;106:686–7. [DOI] [PubMed] [Google Scholar]
- 20. Leibowitz HM, Berrospi AR, Kupferman A, Restropo GV, Galvis V, Alvarez JA. Penetration of topically administered prednisolone acetate into the human aqueous humor. Am J Ophthalmol. 1977;83:402–6. [DOI] [PubMed] [Google Scholar]
- 21. Stoppini R, Gilger BC. Equine ocular examination basic techniques. In: Gilger BC, ed. Equine Ophthalmology, 3rd ed. John Wiley & Sons Incorporated; 2016, 1–39. [Google Scholar]
- 22. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R‐project.org/ [Google Scholar]
- 23. Pinheiro J, Bates DM, DebRoy S, Sarkar D; R Core Team . Linear and Nonlinear Mixed Effects Models. R package version 3.1‐149. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://CRAN.R-project.org/package=nlme [Google Scholar]
- 24. Gaudio PA. A review of evidence guiding the use of corticosteroids in the treatment of intraocular inflammation. Ocul Immunol Inflamm. 2004;12:169–92. [DOI] [PubMed] [Google Scholar]
- 25. McGhee CN, Noble MJ, Watson DG, Dutton GN, Fern AI, Healey TM, et al. Penetration of topically applied prednisolone sodium phosphate into human aqueous humour. Eye. 1989;3(Pt 4):463–7. [DOI] [PubMed] [Google Scholar]
- 26. Cagini C, Cometa F, Torroni G, Pellegrino A, Pellegrino R, Cavallini GM. Dexamethasone disodium phosphate penetration into the human aqueous humor after topical application. Curr Eye Res. 2016;41:897–9. [DOI] [PubMed] [Google Scholar]
- 27. Regnier A. Clinical pharmacology and therapeutics. part 1: drug delivery and pharmacokinetics. In: Gelatt KN, Gilger BC, Kern TJ, eds. Veterinary Ophthalmology, 5th ed. John Wiley & Sons, Incorporated; 2013, 351–80. [Google Scholar]
- 28. Ferguson DC, Hoenig M. Glucocorticoids, mineralocorticoids, and adrenolytic drugs. In: Riviere JE, Papich MG, eds. Veterinary Pharmacology and Therapeutics, 10th ed. John Wiley & Sons, Incorporated; 2018, 729–62. [Google Scholar]
- 29. Asare K. Diagnosis and treatment of adrenal insufficiency in the critically ill patient. Pharmacotherapy. 2007;27:1512–28. [DOI] [PubMed] [Google Scholar]
- 30. Nourry H, Viard C, Cambourieu C, Warnet JM. A relevant choice for corticoid eye drops: solution or suspension? J Fr Ophtalmol. 2011;34:691–6. [DOI] [PubMed] [Google Scholar]
- 31. Diestelhorst M, Kwon KA, Süverkrup R. Dose uniformity of ophthalmic suspensions. J Cataract Refract Surg. 1998;24:672–7. [DOI] [PubMed] [Google Scholar]
- 32. Stringer W, Bryant R. Dose uniformity of topical corticosteroid preparations: difluprednate ophthalmic emulsion 0.05% versus branded and generic prednisolone acetate ophthalmic suspension 1%. Clin Ophthalmol. 2010;4:1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bar‐Ilan A, Neumann R. Basic considerations of ocular drug‐delivery systems. In: Zimmerman TJ, Kooner KS, Sharir M, Fechtner R, eds. Textbook of Ocular Pharmacology, 3rd ed. Lippincott‐Raven Publishers; 1997, 139–50. [Google Scholar]
- 34. Kurihara A, Ojima F, Tsurufuji S. Chemotactic factor production by rat polymorphonuclear leukocytes: stimulation with opsonized zymosan particles and inhibition by dexamethasone. Biochem Biophys Res Commun. 1984;119:720–5. [DOI] [PubMed] [Google Scholar]
- 35. Bachu RD, Chowdhury P, Al‐Saedi ZHF, Karla PK, Boddu SHS. Ocular drug delivery barriers—role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mcghee CNJ, Dean S, Danesh‐Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. [DOI] [PubMed] [Google Scholar]
- 37. Spiess BM, Nyikos S, Stummer E, Sahin A, Naegeli H. Systemic dexamethasone concentration in horses after continued topical treatment with an ophthalmic preparation of dexamethasone. Am J Vet Res. 1999;60:571–6. [PubMed] [Google Scholar]
- 38. Matthews AG. The lens and cataracts. Vet Clin North Am Equine Pract. 2004;20:393–415. [DOI] [PubMed] [Google Scholar]
- 39. Jabs DA, Nussenblatt RB, Rosenbaum JT, Atmaca LS, Becker MD, Brezin AP, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor SRJ, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica. 2010;224:46–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are openly available in DataverseNL at www.dataverse.nl, https://doi.org/10.34894/XAFT9K.


