Abstract
Objective
Complement C3 and other components of the alternative pathway are higher in individuals with obesity. Moreover, C3 has been identified as a risk factor for cardiovascular disease. This study investigated whether, and how, a weight‐loss intervention reduced plasma C3, activated C3 (C3a), and factor D and explored potential biological effects of such a reduction.
Methods
The study measured plasma C3, C3a, and factor D by ELISA and measured visceral adipose tissue, subcutaneous adipose tissue, and intrahepatic lipid by magnetic resonance imaging in lean men (n = 25) and men with abdominal obesity (n = 52). The men with obesity were randomized to habitual diet or an 8‐week dietary weight‐loss intervention.
Results
The intervention significantly reduced C3 (−0.15 g/L [95% CI: −0.23 to −0.07]), but not C3a or factor D. The C3 reduction was mainly explained by reduction in visceral adipose tissue but not subcutaneous adipose tissue or intrahepatic lipid. This reduction in C3 explained a part of the weight‐loss‐induced improvement of markers of endothelial dysfunction, particularly the reduction in soluble endothelial selectin and soluble intercellular adhesion molecule.
Conclusions
Diet‐induced weight loss in men with abdominal obesity could be a way to lower plasma C3 and thereby improve endothelial dysfunction. C3 reduction may be part of the mechanism via which diet‐induced weight loss could ameliorate the risk of cardiovascular disease in men with abdominal obesity.
Study Importance.
What is already known?
People with obesity have higher concentrations of plasma complement factor D (adipsin) and complement C3.
What does this study add?
We herein showed, in men with abdominal obesity, that a randomized‐controlled, diet‐induced weight‐loss intervention resulted in a reduction of C3, which was explained by the reduction of VAT and, in turn, explained part of the weight loss‐induced improvement in markers of endothelial dysfunction.
How might these results change the direction of research of the focus of clinical practice?
Reduction in complement C3, with subsequent improvement in endothelial dysfunction, may be part of the mechanism by which diet‐induced weight loss ameliorates cardiovascular disease risk in men with abdominal obesity.
INTRODUCTION
Obesity is a global epidemic, and the number of persons with excess body weight now approaches 2 billion worldwide, with one third being people with obesity (1, 2). In particular, excess visceral adipose tissue (VAT) is associated with low‐grade inflammation and it may contribute to development of cardiovascular disease (CVD) (3, 4) and type 2 diabetes (5, 6).
Obesity‐induced endothelial dysfunction is one of the mechanisms via which obesity contributes to cardiometabolic diseases (7, 8). Vascular endothelial cells are a major target of inflammatory damage (9). Vascular endothelial dysfunction is a hallmark of the early stages of most CVD characterized by a higher expression of biomarkers such as soluble vascular cell adhesion molecule‐1 (sVCAM‐1), soluble intercellular adhesion molecule‐1 (sICAM‐1), and soluble endothelial selectin (sE‐selectin) (10).
The complement system is an intricate protein network that is part of the innate immune system. The endothelial lining of all blood vessels is, by virtue of its location, in close contact with circulating complement components. Several complement factors can directly or indirectly target the endothelium (9, 11). Endothelial cells express anaphylatoxin receptors and complement regulators on their surface and they are a direct target of complement (12, 13).
A substantial number of complement factors are produced in adipose tissue (14), and this production may be higher in individuals with obesity. In humans, plasma concentrations of factor D and complement C3, two main circulating components of the alternative complement pathway, are strongly associated with adiposity (15), and both are produced in adipose tissue (16, 17). The anaphylatoxin C3a was also reported to be positively associated with BMI (18). C3a is released by C3 upon complement activation (19), and it may directly activate endothelial cells via the C3a receptor (20). There are indications that the plasma concentrations of some of these complement components may change upon changes in body weight. Circulating C3 was higher in obesity and it decreased after nonrandomized weight loss in patients with obesity (21, 22), whereas this was not the case for factor D (22). In addition, the concentrations of C3, C3a, and factor D, as well as other components of the alternative complement pathway, were lower in persons with anorexia (22). Whereas C3 and factor D increased upon weight gain in anorexia, the C3a level remained comparable to that at low weight (22). Moreover, C3 was identified as key marker for changes in body fat as found in a human proteomics study (23). In addition, in an observational human cohort, we have shown that factor D, C3, and C3a were strongly associated with adiposity, but only changes in C3 were associated with changes in BMI over time (15).
The aim of our study was to evaluate whether a diet‐induced weight‐loss intervention reduced circulating concentrations of complement C3, factor D, and/or C3a in a post hoc evaluation of a previously published trial in apparently healthy men with abdominal obesity (24, 25). We also evaluated whether weight‐loss‐induced changes in complement, if observed, were explained by a reduction of specific fat depots, i.e., subcutaneous adipose tissue (SAT), VAT, and/or intrahepatic lipid (IHL). We additionally explored whether changes in circulating complement components, if any, could explain the previously published observation that diet‐induced weight loss improved markers of endothelial dysfunction (25, 26).
METHODS
Study cohort
As described before (24, 25), White men were recruited via advertisements in local newspapers or among participants involved in earlier studies. They were included if they met the following inclusion criteria: age between 18 and 65 years; weight change < 3 kg within the previous 3 months; nonsmokers; without diabetes; without CVD; no drug or alcohol abuse; no use of medication known to affect lipid or glucose metabolism or hypertension; and no participation in another biomedical trial during the past 30 days. A total of 25 normal weight (waist circumference < 94 cm) men and 53 men with abdominal obesity (waist circumference = 102‐110 cm) completed the baseline measurements. The men with obesity were allocated into two age groups (18‐49 years or 50‐65 years). Men in the same age group were randomly divided into the weight‐stable control group or the weight‐loss group. Three men did not complete the weight‐loss study, and one violated the protocol (flowchart in Supporting Information Figure S1). All participants gave written informed consent before entering the study. The study was approved by the Medical Ethics Committee of the Maastricht University Medical Center, performed in accordance with the Declaration of Helsinki, and registered at ClinicalTrials.gov as NCT01675401.
Study design
At the start of the study, all men with normal weight and with abdominal obesity underwent baseline measurements at the research facilities. Details of the intervention have been published before (25). Briefly, participants in the weight‐loss group visited our research dietitian every week and had a very low‐calorie diet (Modifast; Nutrition and Sante Benelux) for at least 4 weeks, under strict guidance. After the very low‐calorie diet period, participants were provided with a calorie‐restricted diet in line with the Dutch dietary guideline for 1 to 2 weeks. In week 7 and week 8, the participants were kept in energy balance (weight‐maintenance period). Participants in the weight‐stable control group maintained their normal diet, physical activities, and alcohol consumption, and they were also monitored by the dietitian through the whole period.
Clinical measurements
As published previously (24), a 3.0‐T Philips Achieva magnetic resonance imaging (MRI) scanner with a dedicated 16‐element torso coil (XLTorso coil; Philips Healthcare) was used to assess SAT and VAT volumes. Two‐dimensional T1‐weighted turbo spin‐echo images were acquired centered at the top of the L4 vertebral body. Images were analyzed offline with dedicated software (Hippo Fat; IFC CNR).
The same MRI scanner and coil were used to assess IHL content through mDixon imaging. Images from two 6‐mm‐thick transverse sections through the liver were acquired using a two‐dimensional three‐point T1‐fast field echo mDixon pulse sequence to correct for T2* relaxation. The intrahepatic fat percentage was calculated in three regions of interest within the liver parenchyma, carefully avoiding blood vessels. The fat content was expressed as the weighted mean fat signal, divided by the sum of the weighted mean water and fat signal, as described before (24).
Blood analyses
After an overnight fast, blood was drawn through an intravenous catheter into NaF‐containing vacuum tubes (Becton, Dickinson, and Company) and ethylenediaminetetraacetic acid (EDTA)‐coated vacuum tubes (Becton, Dickinson, and Company) on ice. Within 30 minutes after blood sampling, the tubes were centrifuged at 1,300g for 15 minutes at 4°C to obtain plasma. Blood drawn in serum tubes (Becton, Dickinson, and Company) was allowed to clot for 30 minutes at 21°C and centrifuged at 1,300g for 15 minutes at 21°C. Plasma and serum aliquots were stored at −80°C until use.
Endothelial function markers (sVCAM‐1, sICAM‐1, sE‐selectin) were measured in EDTA plasma on a multiarray detection system based on electrochemiluminescence technology (SECTOR Imager 2400, Meso Scale Diagnostics), whereas von Willebrand factor (vWf) was assessed by enzyme‐linked immunosorbant assay (ELISA) in citrate plasma, all as previously described (25). The interassay coefficients of variation were 3.1%, 4.2%, 5.7%, and 7.2% for sICAM‐1, vWf, sE‐selectin, and sVCAM‐1, respectively.
To reduce the influence of the biological variability of each marker and achieve statistical efficiency, we standardized sum scores for endothelial dysfunction. To obtain standardized sum scores, we first standardized each individual biomarker, and then z scores were averaged into overall standardized endothelial dysfunction scores.
Complement factor D was measured in EDTA plasma using an Duo Set kit assay (R&D Systems), as described before (27). Complement C3 was measured in EDTA plasma using an MSD R‐plex Human Complement C3 Antibody Set (Meso Scale Diagnostics). The assay was performed according to the manufacturer's instruction, except for the use of a 1:20,000 instead of 1:300,000 dilution, which resulted in better stability of the measurements. Complement C3a was measured in EDTA plasma by ELISA (MicroVue C3a Plus EIA kit, Quidel Corp.) (19). The interassay coefficients of variation were 4.0%, 8.9%, and 4.2% for factor D, C3, and C3a, respectively.
Glucose concentrations were measured in NaF plasma (ABX, Horiba). Serum samples were analyzed for total cholesterol (CHOD‐PAP method; Roche Diagnostics), high‐density lipoprotein (HDL) cholesterol (precipitation method; Roche Diagnostics), triacylglycerol (GPO Trinder, Sigma‐Aldrich Corp.), and glycated hemoglobin (Bio‐Rad Laboratories, Inc.). Low‐density lipoprotein (LDL) cholesterol was calculated by using the Friedewald formula, and triacylglycerol was corrected for free glycerol (25).
Statistical analyses
Normally distributed variables are presented as mean (SD). Skewed variables are presented as median with interquartile range. Differences at baseline between men with normal weight and men with abdominal obesity were examined by an independent Student t test in case of normally distributed data or Mann–Whitney U test in case of a skewed distribution. One‐factor ANCOVA, using baseline measurements as covariates, was performed to evaluate the effect of the weight‐loss intervention. To take into account the age stratification in the randomization process, adjustment for age was performed, and an additional linear regression analysis was performed to investigate the association of changes in fat measures with changes in complement concentrations. Linear regression with adjustment for age was used to investigate the association of 1) the cross‐sectional association of BMI and measures of body composition with complement concentrations and 2) the associations of the intervention and changes in measures of body components with changes in complement concentrations. Multiple mediator analysis was used to study whether 1) a specific fat depot (i.e., SAT, VAT, and/or IHL) independently mediated the cross‐sectional association of BMI with plasma complement concentrations or 2) whether changes in a specific fat depot (i.e., SAT, VAT, and/or IHL) independently mediated the effect of the weight‐loss intervention on changes in complement concentrations. To further explore possible effects of weight‐loss‐induced changes in circulating complement on biomarkers of endothelial dysfunction, their associations were evaluated using linear regression, and single mediator analyses were done to investigate whether (changes in) complement concentrations significantly mediated the association of BMI or the intervention with (changes in) markers for endothelial dysfunction. Statistical analyses were performed using SPSS Statistics 25.0 (IBM Corp.). A two‐sided p value < 0.05 was considered statistically significant. Mediation analyses were conducted with the PROGRESS plug‐in for SPSS version 3.5.2 (Andrew F. Hayes, The Ohio State University). Bootstrapped confidence intervals (CI) (5,000 samplings) were generated, and effects were deemed significant when the CI did not include zero.
RESULTS
Study participants: comparison of participants with normal weight and with abdominal obesity
Men with normal weight (n = 25) and with abdominal obesity (n = 52) who completed the baseline measurements were analyzed, as reported before (24). Baseline characteristics of the participants are shown in Table 1. The median age was similar for the men with normal weight and the men with abdominal obesity. Anthropometric measures, which included weight, waist circumference, and BMI, were higher in the participants with abdominal obesity, as were VAT, SAT, and IHL (p < 0.001). The metabolic profile, that is, blood pressure, lipid metabolism (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), and glucose metabolism (fasting plasma glucose and glycated hemoglobin), was generally worse in men with abdominal obesity. Plasma sE‐selectin was significantly higher in men with abdominal obesity (p < 0.001), and sICAM‐1 also tended to be higher, albeit not statistically significantly (p = 0.086), whereas sVCAM and vWf were comparable between the two groups. Factor D (13%) and C3 (22%) were higher in men with abdominal obesity compared with lean men (p = 0.027 and p < 0.001, respectively), whereas plasma C3a did not statistically differ between lean men and men with abdominal obesity (p = 0.194).
TABLE 1.
Baseline characteristics of the study population
| Lean (n = 25) | Obesity (n = 52) | p value | |
|---|---|---|---|
| Age (y) | 53.7 (25.0‐61.6) | 51.8 (45.7‐60.7) | 0.965 |
| Body weight measures | |||
| Weight (kg) | 74.9 ± 8.3 | 96.9 ± 8.4 | <0.001 |
| Waist circumference (cm) | 84.9 ± 6.3 | 106.5 ± 3.6 | <0.001 |
| BMI (kg/m2) | 23.3 ± 1.8 | 30.1 ± 2.1 | <0.001 |
| Subcutaneous fat volume (L) a | 1.45 ± 0.51 | 3.09 ± 0.78 | <0.001 |
| Visceral fat volume (L) a | 0.89 ± 0.42 | 2.34 ± 0.72 | <0.001 |
| Intrahepatic lipid content (%) a | 3.43 (3.13‐3.78) | 4.96 (3.90‐7.86) | <0.001 |
| Blood pressure (mm Hg) | |||
| 24‐hour systolic blood pressure | 117.5 ± 8.8 | 123.4 ± 8.7 | 0.007 |
| 24‐hour diastolic blood pressure | 72.5 ± 9.4 | 80.4 ± 7.3 | <0.001 |
| Lipid metabolism status (mmol/L) | |||
| Total cholesterol | 4.55 ± 0.78 | 5.56 ± 0.97 | <0.001 |
| HDL cholesterol | 1.26 ± 0.26 | 1.11 ± 0.21 | 0.008 |
| LDL cholesterol b | 2.82 ± 0.70 | 3.68 ± 0.89 | <0.001 |
| Triglycerides | 0.95 (0.67‐1.11) | 1.66 (1.17‐2.19) | <0.001 |
| Glucose metabolism status | |||
| HbA1c (%) | 5.18 ± 0.37 | 5.30 ± 0.37 | 0.193 |
| Fasting plasma glucose (mmol/L) | 5.35 ± 0.29 | 5.64 ± 0.48 | 0.006 |
| Markers of endothelial dysfunction | |||
| sE‐selectin (ng/mL) | 70.4 ± 28.6 | 108.0 ± 44.6 | <0.001 |
| sICAM‐1(ng/mL) | 234.7 ± 37.7 | 255.0 ± 51.9 | 0.086 |
| vWf (%) | 125.9 ± 38.2 | 125.1 ± 44.2 | 0.937 |
| sVCAM‐1 (ng/mL) | 398.3 ± 82.8 | 413.5 ± 79.1 | 0.439 |
| Components of the alternative complement pathway | |||
| Factor D (mg/L) | 0.86 ± 0.17 | 0.97 ± 0.21 | 0.027 |
| C3 (g/L) | 1.29 ± 0.26 | 1.57 ± 0.24 | <0.001 |
| C3a (μg/L) | 32.5 (27.5‐38.5) | 35.4 (30.3‐47.7) | 0.194 |
Note: Data presented as mean ± SD (normal distribution) or median (IQR; skewed distribution), as partially published before reference (24). P values were obtained by independent Student t test or Mann–Whitney U test, when appropriate.
Abbreviations: HbA1c, glycated hemoglobin; sE‐selectin, soluble endothelial selectin; sICAM‐1, soluble intercellular adhesion molecule 1; sVCAM‐1, soluble vascular cell adhesion molecule 1; vWf, von Willebrand factor.
Analyzed in 24 lean men and 52 men with obesity.
In 25 lean men and 50 men with obesity.
Effect of the weight‐loss intervention on plasma concentrations of factor D, C3, and C3a
Table 2 and Supporting Information Figure S1 show the effect of the weight‐loss intervention on the complement components. The intervention significantly reduced plasma C3 (−0.15 g/L, 95% CI: −0.23 to −0.07 g/L; p < 0.001), which is approximately 10% of the baseline concentration. We also tested the effect of weight loss on plasma C3 using a repeated measures one‐way ANOVA. The p value for time × treatment was 0.011. No significant changes were observed in plasma factor D (−0.03 mg/L, 95% CI: −0.09 to 0.02) or C3a (3.74 μg/L, 95% CI: −2.50 to 9.98). Additional adjustment for age did not affect these results.
TABLE 2.
Plasma complement concentration before and after the 8‐week dietary intervention
| Weight‐stable control group a | Weight‐loss group b | Treatment effect | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | Mean change (95% CI) | p value | |
| Factor D (mg/L) | 0.96 ± 0.20 | 0.97 ± 0.19 | 0.99 ± 0.22 | 0.96 ± 0.20 | −0.03 (−0.09 to 0.02) | 0.237 |
| C3 (g/L) | 1.62 ± 0.22 | 1.58 ± 0.22 | 1.53 ± 0.25 | 1.37 ± 0.18 | −0.15 (−0.23 to −0.07) | <0.001 |
| C3a (μg/L) | 35.3 (32.3 to 46.7) | 35.0 (29.5 to 42.3) | 35.5 (26.9 to 49.4) | 40.0 (26.4 to 60.7) | 3.74 (−2.50 to 9.98) | 0.234 |
Note: Bold font represents statistically significant data. P value of treatment effect was obtained by one‐factor ANCOVA with baseline value as covariate. C3a concentrations at baseline and follow‐up were skewed distributed, whereas the change of C3a concentration showed a normal distribution. When C3a was ln‐transformed in a sensitivity analysis, the effect of the weight‐loss intervention was comparable.
n = 26.
n = 23.
Supporting Information Table S1 shows that, in the men with abdominal obesity, the changes in BMI, SAT, and VAT that were observed over the 8‐week follow‐up period were associated with the change in C3 (β = 0.042 to 0.156 g/L, p = 0.030 to p < 0.001). No significant associations were observed between changes in adiposity and changes in factor D or C3a.
Next, we evaluated whether the significant effects of the weight‐loss intervention on plasma C3 were attributable to changes in one of more of the individual fat depots. In the intervention study, only the change in VAT was associated with the change in C3, independent of age and independent of the other fat depots (Table 3). In multiple mediator models, we subsequently observed that the association between the weight‐loss intervention and change in C3 was substantially and independently mediated by change in VAT (C3: −0.147 g/L, 95% CI: −0.285 to −0.001), but not by changes in SAT or IHL (Figure 1A).
TABLE 3.
Multivariate linear associations of the (changes in) different fat depots (independent variables) with (changes in) C3 (g/L, dependent variable)
| Δ C3 (95% CI) | p value | C3 (95% CI) | p value | ||
|---|---|---|---|---|---|
| Intervention study a | |||||
| Δ Subcutaneous fat (L) | Model 1 | 0.103 (0.005 to 0.200) | 0.040 | ||
| Model 2 | −0.034 (−0.163 to 0.095) | 0.600 | |||
| Δ Visceral fat (L) | Model 1 | 0.157 (0.075 to 0.239) | <0.001 | ||
| Model 2 | 0.173 (0.054 to 0.292) | 0.005 | |||
| Δ Intrahepatic lipid (%) | Model 1 | 0.019 (−0.003 to 0.042) | 0.093 | ||
| Model 2 | 0.003 (−0.021 to 0.026) | 0.827 | |||
| Cross‐sectional study b | |||||
| Subcutaneous fat (L) | Model 1 | 0.121 (0.068 to 0.174) | <0.001 | ||
| Model 2 | 0.055 (0.003 to 0.108) | 0.040 | |||
| Visceral fat (L) | Model 1 | 0.199 (0.141 to 0.256) | <0.001 | ||
| Model 2 | 0.112 (0.036 to 0.189) | 0.005 | |||
| Ln Intrahepatic lipid (%) | Model 1 | 0.308 (0.193 to 0.422) | <0.001 | ||
| Model 2 | 0.161 (0.039 to 0.282) | 0.011 |
Note: Bold font represents statistically significant data. Model 1: adjusted for age. Model 2: additionally adjusted for the other two fat depots.
N = 49.
N = 78.
FIGURE 1.
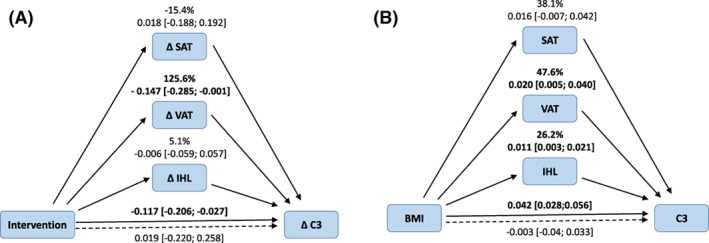
Multiple mediator models to determine the contribution of the different fat depots (SAT, VAT, and IHL) on the difference (or change) in C3. (A) Multiple mediator model in which ΔVAT, but not ΔSAT or ΔIHL, was an independent mediator of the association between the weight‐loss intervention and ΔC3 (n = 49). (B) Multiple mediator model adjusted for age in which VAT and ln‐transformed IHL, but not SAT, were significant mediators of the cross‐sectional association between BMI and plasma C3 (n = 76). IHL, intrahepatic lipid; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue [Color figure can be viewed at wileyonlinelibrary.com]
In the cross‐sectional linear regression analyses, SAT, VAT, and IHL were each associated with C3 concentration (Table 3). In multiple mediator models, the association between BMI, as a measure of generalized adiposity, and plasma C3 was independently mediated by VAT (0.020 g/L, 95% CI: 0.005‐0.040) and by IHL (0.011 g/L, 95% CI: 0.003‐0.021), but not by SAT (Figure 1B).
Effect of abdominal obesity and the weight‐loss intervention on markers of endothelial dysfunction via change in plasma C3
To explore the potential biological consequences of the effects of the weight‐loss intervention on alternative complement pathway components, we also investigated whether changes in complement were associated with changes in circulating markers of endothelial dysfunction. Table 4 shows that the association of the change in C3 was associated with the change in the overall endothelial dysfunction score (β = 2.500 SD, 95% CI: 0.866‐4.125). In line with this, the change in C3 also significantly associated with changes in sICAM (78 ng/mL, 95% CI: 37‐119) and sE‐selectin (83 ng/mL, 95% CI: 39‐127), but not vWF or sVCAM.
TABLE 4.
Linear association between change in plasma C3 concentration and changes in plasma concentrations of biomarkers for endothelial dysfunction
| Δ C3 (g/L) | ||
|---|---|---|
| 95% CI | p value | |
| Δ Endothelial dysfunction score | 2.500 (0.866 to 4.125) | 0.003 |
| Δ sE‐selectin (ng/mL) | 83.00 (39.17 to 126.83) | <0.001 |
| Δ sICAM‐1 (ng/mL) | 78.12 (36.94 to 119.29) | <0.001 |
| Δ vWF (%) | 17.21 (−30.77 to 65.18) | 0.474 |
| Δ sVCAM‐1 (ng/mL) | 29.00 (−67.11 to 125.11) | 0.547 |
Note: Bold font represents statistically significant data. Crude unstandardized associations between change in plasma C3 and changes in plasma endothelial biomarkers. Results are shown for all men with obesity who participated in the intervention study (n = 49).
Abbreviations: sE‐selectin, soluble endothelial selectin; sICAM‐1, soluble intercellular adhesion molecule 1; sVCAM‐1, soluble vascular cell adhesion molecule 1; vWf, von Willebrand factor.
We subsequently explored whether C3 could be a mediating variable in the associations of adiposity (BMI, cross‐sectional analyses) or the weight‐loss intervention with endothelial dysfunction. Table 5 shows the results of single mediation analyses in which we explored the mediating effect of C3 on the associations of either BMI or the weight‐loss intervention with the combined endothelial dysfunction score or the individual endothelial markers. The intervention‐induced change in C3 partially mediated the effect of the intervention on the overall score for endothelial dysfunction (−0.22 SD, 95% CI: −0.66 to 0.01), on sE‐selectin (−6.10 ng/mL, 95% CI: −14.2 to −0.92), and on sICAM (−6.65 ng/mL, 95% CI: −17.2 to −0.54). In subsequent sensitivity analyses, we evaluated the mediating effects of C3 on the associations of waist circumference and VAT with the endothelial dysfunction markers (Supporting Information Table S1). Additional adjustment for age did not affect any of the associations observed in the weight‐loss intervention.
TABLE 5.
The mediating effect of (changes in) C3 on the association of weight‐loss intervention or BMI with (changes in) markers of endothelial dysfunction (dependent variables) in simple mediator models
| Dependent | Independent: Intervention (Y/N) a | Independent: BMI (kg/m2) b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Path c | β | 95% CI | % d | Dependent | Path c | β | 95% CI | % d | |
| Δ EndDys score (SD) | c | −0.86 | (−1.39 to −0.33) | EndDys score (SD) | C | 0.06 | (0.00 to 0.12) | ||
| c′ | −0.64 | (−1.18 to −0.10) | c′ | 0.02 | (−0.05 to 0.08) | ||||
| a × b Δ C3 | −0.22 | (−0.66 to 0.01) | 26 | a × b C3 | 0.05 | (0.01 to 0.10) | 83 | ||
| Δ sE‐selectin (ng/mL) | C | −36.5 | (−48.9 to −24.1) | sE‐selectin (ng/mL) | C | 4.81 | (2.38 to 7.23) | ||
| c′ | −30.4 | (−42.8 to −17.9) | c′ | 3.88 | (1.14 to 6.63) | ||||
| a × b Δ C3 | −6.10 | (−14.2 to −0.92) | 17 | a × b C3 | 0.92 | (−0.60 to 2.60) | 19 | ||
| Δ sICAM (ng/mL) | c | −26.5 | (−39.9 to −13.2) | sICAM (ng/mL) | c | 3.13 | (0.27 to 5.98) | ||
| c′ | −19.9 | (−33.3 to −6.47) | c′ | 1.24 | (−1.91 to 4.39) | ||||
| a × b Δ C3 | −6.65 | (−17.2 to −0.54) | 25 | a × b C3 | 1.88 | (0.11 to 4.78) | 60 | ||
| Δ vWF (ng/mL) | C | −5.47 | (−20.5 to 9.60) | vWF (ng/mL) | c | −0.69 | (−3.08 to 1.71) | ||
| c′ | −3.02 | (−19.2 to 13.2) | c′ | −1.96 | (−4.64 to 0.72) | ||||
| a × b Δ C3 | −2.44 | (−16.6 to 5.61) | a × b C3 | 1.27 | (−0.44 to 3.19) | ||||
| Δ sVCAM (ng/mL) | C | 3.47 | (−28.3 to 35.2) | sVCAM (ng/mL) | Cc | 1.25 | (−3.64 to 6.14) | ||
| c′ | 7.64 | (−26.6 to 41.9) | c′ | −1.99 | (−7.38 to 3.41) | ||||
| a × b Δ C3 | −4.17 | (−15.2 to 7.63) | a × b C3 | 3.24 | (−0.89 to 6.29) | ||||
Note: Bold font represents statistically significant data.
Abbreviations: EndDys score, endothelial dysfunction score; sE‐selectin, soluble endothelial selectin; sICAM‐1, soluble intercellular adhesion molecule 1; sVCAM‐1, soluble vascular cell adhesion molecule 1; vWf: von Willebrand factor.
N = 49.
N = 77.
c is the total effect, that is, the regression coefficient of the association of BMI or the intervention as independent and the respective marker of endothelial dysfunction as outcome, c′ is the direct effect, and a × b is the indirect effect via (changes in) plasma C3.
The proportion mediated effect [a × b/c] was calculated only when the total effect (c path) was significant.
DISCUSSION
This randomized controlled dietary weight‐loss intervention study in men with abdominal obesity has several main observations. First, the weight‐loss intervention reduced complement C3, but not factor D or C3a. Second, the effect of the intervention on plasma C3 was partially explained by change in VAT. Third, the effect of the weight‐loss intervention on plasma markers of endothelial dysfunction was partly mediated by change in C3.
The weight‐loss intervention reduced the plasma concentration of C3. In addition, in the cross‐sectional comparison, the C3 concentration was significantly higher in men with obesity than in the normal weight group, as was reported previously (15, 22). The observed reduction in C3 after the weight‐loss intervention agrees with reports from nonrandomized, noncontrolled weight‐loss trials in men and women with various degrees of obesity (all with BMI > 40 kg/m2) (21, 22, 26). A reduction in C3 generally results from either hypoproduction, i.e., less production of C3 in tissue, including adipose tissue, or hyperconsumption, i.e., reduction of C3 upon activation of the C3 cascade, when C3 is converted into C3a and C3b. Because plasma C3a in our study did not change with the weight‐loss intervention, we consider it most likely that the decrease in C3 with weight‐loss intervention resulted from decreased C3 production rather than increased consumption. As the reduction in C3 with a weight‐loss intervention was mainly explained by VAT, we speculate that the weight‐loss intervention reduced the production of C3 in this adipose tissue depot. It could be argued that there may be an indirect effect as well, e.g., via reduction of C3 production in the liver, which is the main source of plasma C3 (17). However, the most likely mechanism via which a reduction in VAT would lead to a lower hepatic C3 production would be via a decrease in circulating inflammatory factors (26), which were unaffected in our study (27). Also, the change in IHL did not contribute to the association between the weight‐loss intervention and change in plasma C3, independent of change in VAT. A reduction in plasma C3 could result in a reduced potential for activation of the alternative complement pathway and of the common amplification loop. However, there may also be effects that are independent of canonical complement activation. As an example, C3 can directly interact with fibrinogen (28). Incorporation of C3 into fibrin clots can contribute to hypofibrinolysis and, therefore, to enhanced thrombosis risk (28).
In our study, the diet‐induced change in C3 partly explained the beneficial effect of the weight‐loss intervention on the endothelial markers sE‐selectin and sICAM. A cross‐sectional association of C3 with these markers was reported before (26). Our results suggest that a weight‐loss‐induced reduction in C3 reduces endothelial dysfunction in apparently healthy men with abdominal obesity. These results may have potential relevance in light of the worldwide SARS‐CoV‐2 epidemic. Severe COVID‐19 is characterized by, among others, inflammation, endothelial dysfunction, and thrombotic microangiopathy (29, 30), and men with obesity have a high risk to develop severe COVID‐19 upon infection (31). Inhibition of C3 activation can improve outcomes in severe COVID‐19 (32), and we speculate that reduction in plasma C3 via diet‐induced weight loss might be beneficial in the prevention of severe COVID‐19 in men with obesity.
In contrast to plasma C3, the concentration of the other complement factors that were studied, factor D and C3a, did not change with a weight‐loss intervention. At baseline, factor D was slightly higher in participants with obesity than in one with normal weight, which is in line with previous publications (15, 33) although not always significant (22). Given the profound expression of factor D in adipose tissue, one might intuitively expect the plasma concentration of factor D to be responsive to weight loss, but our current data do not support that premise. In fact, the change in factor D concentration with a weight‐loss intervention was small and not significant, which is in agreement with what was found before (22).
It was previously reported that the expression of factor D in adipose tissue may differ between men and women. In women, factor D was inversely correlated with BMI in SAT, but not in VAT, whereas, in men, a similar inverse correlation with SAT was present, but a positive correlation was observed in VAT (34). Based on these cross‐sectional observations, we speculate that, in men with obesity, a weight‐loss intervention would decrease the expression (and production) of factor D by adipocytes in VAT, but this may be counteracted by a concomitant increase in factor D expression (and production) by SAT, the total result of which would be the observed, nonsignificant effect of a weight‐loss intervention on circulating factor D in men with obesity. In addition, in vitro studies showed that factor D, as well as C3a, may be active contributors to lipid accumulation in adipocytes, rather than merely products of adipose tissue, which would provide an additional explanation for the fact that they are not responsive or are less responsive to diet‐induced weight loss (35).
Although baseline C3a was approximately 9% higher in men with abdominal obesity compared with lean men, this difference was not statistically significant. Previous publications have reported higher C3a in people with obesity than in lean individuals, but these reports have often concerned studies in persons with extreme levels of obesity, with obesity‐related comorbidities such as metabolic syndrome, and/or with larger numbers of participants (15, 36, 37, 38). This may explain why our current findings that C3a did not change upon a weight‐loss intervention in men with abdominal obesity contrasts with a previous report that, in women, plasma C3a was reduced upon extreme weight loss resulting from bariatric surgery (38). However, our current observations are in agreement with the observation that C3a was neither reduced in women with anorexia nor increased upon weight gain (22). In addition, we previously reported that, in our current weight‐loss intervention study, the concentrations of several plasma biomarkers of systemic inflammation were not altered by the weight‐loss intervention (25). C3a is an inflammatory factor (39), and our observation that C3a was not changed is therefore in line with these previous findings.
This study has several strengths. First, it is a randomized and controlled weight‐loss intervention. Second, the intervention is combined with a cross‐sectional comparison of lean participants and participants with abdominal obesity, allowing for a comprehensive evaluation. Third, participants were apparently healthy and had moderate obesity, thereby representing a large group of persons in our society. Fourth, the MRI data allowed us to look beyond abdominal obesity per se and to evaluate the independent effects of distinct fat depots. Our study also has limitations. First, no measurements were available between baseline and follow‐up; therefore, we cannot determine the order of events that resulted from the weight‐loss intervention. Also, our observation that the concentration of C3a was not altered in plasma does not necessarily exclude the possibility that the weight‐loss intervention did, to some extent, affect local generation of C3a. Local C3 activation can induce endothelial dysfunction (12, 40), but C3 may also be produced by endothelial cells, at least in vitro (41, 42). Therefore, reverse causation cannot be fully excluded. Last, our study involved White men, which prohibits extension of the findings to women and to other ethnicities.
CONCLUSION
In conclusion, we showed that a diet‐induced weight‐loss intervention reduced the plasma concentrations of complement C3 in men with abdominal obesity. This reduction in C3 was mainly explained by the reduction in VAT. In turn, the reduction in C3 partly explained the weight‐loss‐associated improvement of plasma biomarkers of endothelial dysfunction, in particular sE‐selectin and sICAM. Reduction in C3 can be one of the mechanisms via which a diet‐induced weight‐loss intervention could reduce the risk of obesity‐associated diseases such as CVD and type 2 diabetes.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
YHAMK, AJHMH, JP, PJJ, RPM, CGS, and CDAS designed the study. YHAMK performed the measurements included in this article. SJ performed the analyses. SJ wrote the manuscript under supervision of MMJvG. All authors critically read and commented on the manuscript.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We thank dietitians NAWM Wystyrk and DJ Luiten for the dietary counseling of the individuals in this trial, PMG Niessen (Department of Internal Medicine, Maastricht University Medical Center) for assistance during the execution of this study, CJAW van Gool (Department of Epidemiology, Maastricht University) for epidemiological counseling, and MEK for designing the MRI protocol. Data described in the manuscript will be made available upon request and approval.
Jin S, Kusters YHAM, Houben AJHM, et al. A randomized diet‐induced weight‐loss intervention reduces plasma complement C3: Possible implication for endothelial dysfunction. Obesity (Silver Spring). 2022;30(7):1401‐1410. doi: 10.1002/oby.23467
Funding informationThis work was supported by research grant CH001 from the Top Institute of Food and Nutrition, a public‐private partnership on precompetitive research in food and nutrition. The public partners are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. SJ was supported by the China Scholarship Council.
REFERENCES
- 1. Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10(suppl 1):S4‐S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66:7‐12. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Luo W, Wang J, et al. Obesity‐induced endothelial dysfunction is prevented by deficiency of P‐selectin glycoprotein ligand‐1. Diabetes 2012;61:3219‐3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bluher M. Adipokines—removing road blocks to obesity and diabetes therapy. Mol Metab. 2014;3:230‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyko EJ, Fujimoto WY, Leonetti DL, Newell‐Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465‐471. [DOI] [PubMed] [Google Scholar]
- 6. Nusrianto R, Ayundini G, Kristanti M, et al. Visceral adiposity index and lipid accumulation product as a predictor of type 2 diabetes mellitus: the Bogor cohort study of non‐communicable diseases risk factors. Diabetes Res Clin Pract. 2019;155:‐107798. doi: 10.1016/j.diabres.2019.107798 [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Wang Q, Venugopal J, et al. Obesity‐induced endothelial dysfunction is prevented by neutrophil extracellular trap inhibition. Sci Rep 2018;8:4881. doi:10.1038/s41598‐018‐23256‐y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial dysfunction in obesity‐induced inflammation: molecular mechanisms and clinical implications. Biomolecules. 2020;10:291. doi:10.3390/biom10020291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoie EB, Mcguire TR, Leuschen PM, Zach TL. Pentoxifylline inhibits tumor necrosis factor‐alpha induced synthesis of complement component C3 in human endothelial cells. Biol Pharm Bull. 2004;27:1670‐1673. [DOI] [PubMed] [Google Scholar]
- 10. Wojakowski W, Gminski J. Soluble ICAM‐1, VCAM‐1 and E‐selectin in children from families with high risk of atherosclerosis. Int J Mol Med. 2001;7:181‐185. [DOI] [PubMed] [Google Scholar]
- 11. Hertle E, Stehouwer CD, Van Greevenbroek MM. The complement system in human cardiometabolic disease. Mol Immunol. 2014;61:135‐148. [DOI] [PubMed] [Google Scholar]
- 12. Propson NE, Roy ER, Litvinchuk A, Kohl J, Zheng H. Endothelial C3a receptor mediates vascular inflammation and blood–brain barrier permeability during aging. J Clin Invest. 2021;131:e140966. doi: 10.1172/JCI140966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schraufstatter IU, Trieu K, Sikora L, Sriramarao P, Discipio R. Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J Immunol. 2002;169:2102‐2110. [DOI] [PubMed] [Google Scholar]
- 14. Gabrielsson BG, Johansson JM, Lonn M, et al. High expression of complement components in omental adipose tissue in obese men. Obes Res. 2003;11:699‐708. [DOI] [PubMed] [Google Scholar]
- 15. Xin Y, Hertle E, Van Der Kallen CJH, Schalkwijk CG, Stehouwer CDA, Van Greevenbroek MMJ. Longitudinal associations of the alternative and terminal pathways of complement activation with adiposity: the CODAM study. Obes Res Clin Pract. 2018;12:286‐292. [DOI] [PubMed] [Google Scholar]
- 16. White RT, Damm D, Hancock N, et al. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 1992;267:9210‐9213. [PubMed] [Google Scholar]
- 17. Morris KM, Aden DP, Knowles BB, Colten HR. Complement biosynthesis by the human hepatoma‐derived cell line HepG2. J Clin Invest. 1982;70:906‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age‐related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818‐5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hertle E, Van Greevenbroek MM, Arts IC, et al. Distinct associations of complement C3a and its precursor C3 with atherosclerosis and cardiovascular disease. The CODAM Study. Thromb Haemost. 2014;111:1102‐1111. [DOI] [PubMed] [Google Scholar]
- 20. Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003‐1014. [DOI] [PubMed] [Google Scholar]
- 21. Hernandez‐Mijares A, Banuls C, Bellod L, et al. Effect of weight loss on C3 and C4 components of complement in obese patients. Eur J Clin Invest. 2012;42:503‐509. [DOI] [PubMed] [Google Scholar]
- 22. Pomeroy C, Mitchell J, Eckert E, Raymond N, Crosby R, Dalmasso AP. Effect of body weight and caloric restriction on serum complement proteins, including factor D/adipsin: studies in anorexia nervosa and obesity. Clin Exp Immunol. 1997;108:507‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oberbach A, Bluher M, Wirth H, et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10:4769‐4788. [DOI] [PubMed] [Google Scholar]
- 24. Kusters YH, Schalkwijk CG, Houben AJ, et al. Independent tissue contributors to obesity‐associated insulin resistance. JCI Insight. 2017;2:e89695. doi: 10.1172/jci.insight.89695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joris PJ, Plat J, Kusters YH, et al. Diet‐induced weight loss improves not only cardiometabolic risk markers but also markers of vascular function: a randomized controlled trial in abdominally obese men. Am J Clin Nutr. 2017;105:23‐31. [DOI] [PubMed] [Google Scholar]
- 26. Nilsson B, Hamad OA, Ahlstrom H, et al. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur J Clin Invest. 2014;44:587‐596. [DOI] [PubMed] [Google Scholar]
- 27. Hertle E, Arts IC, Van Der Kallen CJ, et al. The alternative complement pathway is longitudinally associated with adverse cardiovascular outcomes. The CODAM Study. Thromb Haemost. 2016;115:446‐457. [DOI] [PubMed] [Google Scholar]
- 28. King RJ, Schuett K, Tiede C, et al. Fibrinogen interaction with complement C3: a potential therapeutic target to reduce thrombosis risk. Haematologica. 2021;106:1616‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID‐19: friend and foe? JCI Insight 2020;5:e140711. doi: 10.1172/jci.insight.140711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghebrehiwet B, Peerschke EI. Complement and coagulation: key triggers of COVID‐19‐induced multiorgan pathology. J Clin Invest. 2020;130:5674‐5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi‐center study. PLoS Negl Trop Dis 2020;14:e0008280. doi:10.1371/journal.pntd.0008280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mastellos DC, Pires Da Silva BGP, Fonseca BL, et al. Complement C3 vs C5 inhibition in severe COVID‐19: early clinical findings reveal differential biological efficacy. Clin Immunol. 2020;220:108598. doi: 10.1016/j.clim.2020.108598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Napolitano A, Lowell BB, Damm D, et al. Concentrations of adipsin in blood and rates of adipsin secretion by adipose tissue in humans with normal, elevated and diminished adipose tissue mass. Int J Obes Relat Metab Disord 1994;18:213‐218. [PubMed] [Google Scholar]
- 34. Xia Z, Cianflone K. Acylation‐stimulating protein precursor proteins in adipose tissue in human obesity. Metabolism. 2003;52:1360‐1366. [DOI] [PubMed] [Google Scholar]
- 35. Song NJ, Kim S, Jang BH, et al. Small molecule‐induced complement factor D (Adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One 2016;11:e0162228. doi:10.1371/journal.pone.0162228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mishra S, Gupta V, Mishra S, et al. Association of acylation stimulating protein and adiponectin with metabolic risk marker in north Indian obese women. Diabetes Metab Syndr 2019;13:2987‐2990. [DOI] [PubMed] [Google Scholar]
- 37. Yang Y, Lu HL, Zhang J, et al. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. Int J Obes 2006;30:439‐446. [DOI] [PubMed] [Google Scholar]
- 38. Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation‐stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594‐1602. [DOI] [PubMed] [Google Scholar]
- 39. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bottiger BW, Motsch J, Braun V, Martin E, Kirschfink M. Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Crit Care Med. 2002;30:2473‐2480. [DOI] [PubMed] [Google Scholar]
- 41. Kawakami Y, Watanabe Y, Yamaguchi M, Sakaguchi H, Kono I, Ueki A. TNF‐alpha stimulates the biosynthesis of complement C3 and factor B by human umbilical cord vein endothelial cells. Cancer Lett. 1997;116:21‐26. [DOI] [PubMed] [Google Scholar]
- 42. Klegeris A, Bissonnette CJ, Dorovini‐Zis K, Mcgeer PL. Expression of complement messenger RNAs by human endothelial cells. Brain Res. 2000;871:1‐6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


