Abstract
Background and Methods
Racial and socioeconomic disparities in receipt of adjuvant chemotherapy affect patients with pancreatic cancer. However, differences in receipt of neoadjuvant chemotherapy among patients undergoing resection are not well‐understood. A retrospective cross‐sectional cohort of patients with resected AJCC Stage I/II pancreatic ductal adenocarcinoma was identified from the National Cancer Database (2014–2017). Outcomes included receipt of neoadjuvant versus adjuvant chemotherapy, or receipt of either, defined as multimodality therapy and were assessed by univariate and multivariate analysis.
Results
Of 19 588 patients, 5098 (26%) received neoadjuvant chemotherapy, 9624 (49.1%) received adjuvant chemotherapy only, and 4757 (24.3%) received no chemotherapy. On multivariable analysis, Black patients had lower odds of neoadjuvant chemotherapy compared to White patients (OR: 0.80, 95% CI: 0.67–0.97) but no differences in receipt of multimodality therapy (OR: 0.89, 95% CI: 0.77–1.03). Patients with Medicaid or no insurance, low educational attainment, or low median income had significantly lower odds of receiving neoadjuvant chemotherapy or multimodality therapy.
Conclusions
Racial and socioeconomic disparities persist in receipt of neoadjuvant and multimodality therapy in patients with resected pancreatic adenocarcinoma.
Discussion
Policy and interventional implementations are needed to bridge the continued socioeconomic and racial disparity gap in pancreatic cancer care.
Keywords: healthcare disparity, multimodal treatment, neoadjuvant chemotherapy, pancreas cancer, socioeconomic factors
1. INTRODUCTION
Despite improvements in surgical and multidisciplinary treatment, the prognosis of pancreatic cancer remains poor. It is the fourth leading cause of cancer‐related mortality in the United States. 1 Additionally, significant racial disparities have been demonstrated in pancreatic cancer incidence, receipt of treatment, and mortality. Black patients suffer an approximately 1.4‐fold higher incidence of pancreatic cancer than patients of other races, and are diagnosed at a younger age and with more advanced disease. 2 , 3 Other patient factors including advanced age, lower socioeconomic status, lack of health insurance, and treatment at nonacademic, low‐volume institutions have been associated with decreased likelihood of receipt of stage‐specific treatment. 3 , 4 , 5 , 6 , 7 , 8
Currently, the standard of care for American Joint Commission on Cancer (AJCC) Stage I and II pancreatic cancer consists of the combination of surgical resection and systemic chemotherapy, otherwise referred to as multimodality therapy. 9 The use of preoperative, or neoadjuvant, chemotherapy for borderline resectable pancreatic cancer has been well demonstrated, and interest in the resectable setting has been growing. Theoretical advantages include immediate treatment of occult micrometastatic disease, improved patient selection, and better tolerance than chemotherapy administered in the postoperative setting. 10 , 11 , 12 , 13 Many patients have difficulty initiating or completing adjuvant chemotherapy due to postoperative complications or treatment toxicity; therefore, preoperative use may allow for improved rates of treatment completion. 14 , 15 Neoadjuvant chemotherapy use has steadily increased over the past two decades, from less than 5% in 2004 to more than 25% of patients undergoing pancreatectomy in 2016, and has recently been demonstrated to be efficacious in the treatment of resectable pancreatic cancer in a randomized, Phase II clinical trial. 16 , 17 However, studies have shown that its use is less prevalent in uninsured or underinsured patients, patients of lower socioeconomic status, and in patients treated at nonacademic facilities, thus raising concerns regarding disparities in care delivery. 8 , 16
The primary objective of this study was to evaluate differences in the receipt of (1) neoadjuvant chemotherapy and (2) multimodality therapy by race and health insurance status in patients with resected Stage I and II pancreatic cancer. The hypothesis was that racial/ethnic minority and uninsured/underinsured status are associated with failure to receive neoadjuvant chemotherapy and multimodality therapy.
2. MATERIALS AND METHODS
2.1. Study population and variables
Patient data were obtained from the American College of Surgeons and American Cancer Society‐sponsored National Cancer Database (NCDB), which collects hospital registry data from more than 1500 Commission on Cancer (CoC)‐accredited facilities in the United States, representing over 70% of new cancer diagnoses. 18 Data are coded and reported per established protocols by CoC trained and certified registrars.
Patients diagnosed with primary pancreatic cancer between 2014 and 2017 who underwent resection were included. Patients with a prior cancer diagnosis, histology other than adenocarcinoma, missing analytic stage, or analytic Stage III–IV as defined by the AJCC classification 7th (for diagnoses from 2014 to 2016) and 8th edition (for diagnoses in 2017) were excluded. 19 , 20 Histology codes were chosen for inclusion based on expert and literature review (Supplement). The study was deemed exempt by the Institutional Review Board. Results are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. 21
Clinical and demographic variables were chosen a priori from the available data points within the NCDB Participant User File. Demographic variables included age, race, ethnicity, sex, primary payor, median household income by ZIP code, percent not graduated from high school by ZIP code, distance traveled for care defined by the variable Great Circle Distance, geographic region of the treating facility, and rurality of county of residence. Clinical variables included Charlson/Deyo comorbidity score, facility type, primary tumor site (categorized as head, body, tail, or overlapping and not otherwise specified), grade, and analytic AJCC stage. Patients were subdivided by race/ethnicity cohorts as defined by the pre‐specified NCDB categories, namely Non‐Hispanic White (NHW), Non‐Hispanic Black (NHB), Hispanic, and Other. The primary outcome of interest was receipt of any neoadjuvant chemotherapy. Patients who received both preoperative and postoperative systemic therapy were considered to have received neoadjuvant chemotherapy. The secondary outcome of interest was receipt of multimodality therapy, defined as any systemic chemotherapy before or after resection. Receipt of radiation therapy was not included.
2.2. Statistical analysis
Descriptive statistics were used for categorical and continuous variables. For continuous variables, the number of nonmissing observations, median, and interquartile range is presented where applicable. The continuous variable distance traveled for care (Great Circle Distance) was recategorized by quartiles. Geographic region data which are provided in the Participant User File based on the US Census Bureau's nine divisions were recategorized into the US Census Bureau's four regions. 22 Missing data were treated as a separate category with no method for imputation used. The significance of cohort differences was assessed with χ 2 test for categorical variables and median test for continuous variables.
Logistic regression analyses were conducted to adjust for confounding factors for both outcomes. Covariates included race/ethnicity, primary payor, age, sex, median income by ZIP code, educational attainment defined by high school graduation by ZIP code, rurality of residence, distance traveled for care by quartile, geographic region, Charlson/Deyo comorbidity score, facility type, analytic stage, primary site, and grade. Odds ratios (OR) and 95% confidence intervals (CI) are presented as measures of strength of association and precision, respectively. All statistical tests were two‐sided. A pvalue <0.05 was considered statistically significant. The analyses excluded the missing/not available category if present for any of the covariates. A test for interaction was conducted for the covariates race and insurance. A post hoc sensitivity analysis utilizing the clinical stage variable in place of the analytic stage was performed. Analyses were performed with SPSS statistical software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0).
3. RESULTS
3.1. Demographics
Of the 423 482 patients in the NCDB pancreas data set, 19 588 met study inclusion criteria (Figure 1). Most patients were of NHW race (n = 15 397, 78.6%). Other demographic data are shown in Table 1.
Figure 1.
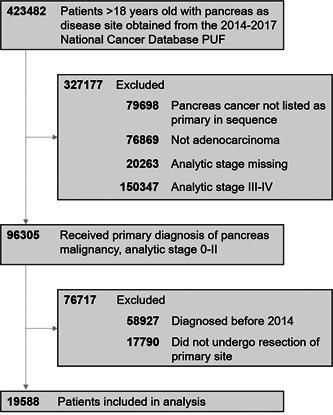
Cohort selection
Table 1.
Cohort demographics
| NHW | NHB | Hispanic | Other/unknown | Overall | |||
|---|---|---|---|---|---|---|---|
| Variable | Statistics or category | N = 15 397 (78.6%) | N = 1911 (9.8%) | N = 1096 (5.6%) | N = 1184 (6.0%) | N = 19 588 | p value |
| Age at diagnosis (years) | Median [IQR] | 67.00 [60.00–73.00] | 63.00 [57.00–70.00] | 65.00 [57.00–72.00] | 67.00 [58.00–74.00] | 66.00 [59.00–73.00] | <0.001 |
| Gender | Female | 7281 (47.3%) | 1112 (58.2%) | 562 (48.7%) | 407 (48.3%) | 9522 (48.6%) | <0.001 |
| Health insurance | Uninsured | 201 (1.3%) | 61 (3.2%) | 65 (5.9%) | 26 (3.2%) | 360 (1.8%) | <0.001 |
| Medicaid | 637 (4.1%) | 215 (11.3%) | 142 (13.0%) | 101 (12.0%) | 1106 (5.6%) | ||
| Medicare | 8515 (55.3%) | 841 (44.0%) | 456 (41.6%) | 364 (43.2%) | 10354 (52.9%) | ||
| Private | 5611 (36.4%) | 722 (37.8%) | 409 (37.3%) | 335 (39.7%) | 7202 (36.8%) | ||
| Other government | 243 (1.6%) | 44 (2.3%) | 7 (0.6%) | 8 (0.9%) | 310 (1.6%) | ||
| Not available | 190 (1.2%) | 28 (1.5%) | 17 (1.6%) | 9 (1.1%) | 256 (1.3%) | ||
| Median household income | Less than $40 227 | 1813 (11.8%) | 701 (36.7%) | 229 (20.9%) | 131 (11.1%) | 2874 (14.7%) | <0.001 |
| $40 227 to $50 353 | 2995 (19.5%) | 367 (19.2%) | 221 (20.2%) | 173 (14.6%) | 3756 (19.2%) | ||
| $50 354 to $63 332 | 3286 (21.3%) | 267 (14.0%) | 251 (22.9%) | 219 (18.5%) | 4023 (20.5%) | ||
| $63 333+ | 5315 (34.5%) | 339 (17.7%) | 268 (24.5%) | 513 (43.3%) | 6435 (32.9%) | ||
| Not available | 1988 (12.9%) | 237 (12.4%) | 127 (11.6%) | 148 (12.5%) | 2500 (12.8%) | ||
| Percent did not graduate from high school | 17.6% or more | 1931 (12.5%) | 656 (34.3%) | 466 (42.5%) | 203 (17.1%) | 3256 (16.6%) | <0.001 |
| 10.9%–17.5% | 3450 (22.4%) | 534 (27.9%) | 227 (20.7%) | 235 (19.8%) | 4446 (22.7%) | ||
| 6.3%–10.8% | 4130 (26.8%) | 347 (18.2%) | 179 (16.3%) | 298 (25.2%) | 4954 (25.3%) | ||
| Less than 6.3% | 3919 (25.5%) | 139 (7.3%) | 97 (8.9%) | 300 (25.3%) | 4455 (22.7%) | ||
| Not available | 1967 (12.8%) | 235 (12.3%) | 127 (11.6%) | 148 (12.5%) | 2477 (12.6%) | ||
| Geographic region | Northeast | 3326 (21.6%) | 311 (16.3%) | 188 (17.2%) | 309 (26.1%) | 4134 (21.1%) | <0.001 |
| Midwest | 4155 (27.0%) | 373 (19.5%) | 106 (9.7%) | 184 (15.5%) | 4818 (24.6%) | ||
| South | 5556 (36.1%) | 1089 (57.0%) | 442 (40.3%) | 331 (28.0%) | 7418 (37.9%) | ||
| West | 2253 (14.6%) | 111 (5.8%) | 335 (30.6%) | 339 (28.6%) | 3038 (15.5%) | ||
| Not available | 107 (0.7%) | 27 (1.4%) | 25 (2.3%) | 21 (1.8%) | 180 (0.9%) | ||
| Rurality | Metro | 12108 (78.6%) | 1692 (88.5%) | 1019 (93.0%) | 1034 (87.3%) | 15853 (80.9%) | <0.001 |
| Urban | 2322 (15.1%) | 160 (3.4%) | 55 (5.0%) | 98 (8.3%) | 2635 (13.5%) | ||
| Rural | 282 (1.8%) | 18 (0.9%) | 3 (0.3%) | 18 (1.5%) | 321 (1.6%) | ||
| Not available | 685 (4.4%) | 41 (2.1%) | 19 (1.7%) | 34 (2.9%) | 779 (4.0%) | ||
| Distance traveled for care by quartile (range in miles) | 1st (0–7) | 2924 (19.0%) | 670 (35.0%) | 377 (34.4%) | 352 (29.7%) | 4323 (22.1%) | <0.001 |
| 2nd (7–17.2) | 3311 (21.5%) | 452 (23.6%) | 286 (26.1%) | 286 (24.2%) | 4335 (22.1%) | ||
| 3rd (17.2–46.1) | 3637 (23.6%) | 295 (15.6%) | 155 (14.1%) | 213 (18.0%) | 4300 (22.0%) | ||
| 4th (46.1–4814.3) | 3697 (24.0%) | 272 (14.2%) | 164 (15.0%) | 199 (16.8%) | 4332 (22.1%) | ||
| Not available | 1828 (11.9%) | 222 (11.6%) | 114 (10.4%) | 134 (11.3%) | 2298 (11.7%) | ||
| Charlson/Deyo Score | 0 | 9930 (64.5%) | 1157 (60.5%) | 708 (64.6%) | 781 (66.0%) | 12576 (64.2%) | 0.01 |
| 1 | 3817 (24.8%) | 525 (27.5%) | 287 (26.2%) | 292 (24.7%) | 4921 (25.1%) | ||
| 2 | 1049 (6.8%) | 136 (7.1%) | 64 (5.8%) | 79 (6.7%) | 1328 (6.8%) | ||
| 3 or more | 601 (3.9%) | 93 (4.9%) | 37 (3.4%) | 32 (2.7%) | 763 (3.9%) | ||
| Facility type | Community | 612 (4.0%) | 59 (3.1%) | 42 (3.8%) | 59 (5.0%) | 772 (3.9%) | <0.001 |
| Comprehensive community | 4323 (28.1%) | 467 (24.4%) | 323 (29.5%) | 304 (25.7%) | 5417 (27.7%) | ||
| Academic | 8335 (54.1%) | 1065 (55.7%) | 585 (53.4%) | 654 (55.2%) | 10639 (54.3%) | ||
| Integrated | 2020 (13.1%) | 293 (15.3%) | 121 (11.0%) | 146 (12.3%) | 2580 (13.2%) | ||
| Not available | 107 (0.7%) | 27 (1.4%) | 25 (2.3%) | 21 (1.8%) | 180 (0.9%) | ||
| Primary site | Head | 11134 (72.3%) | 1369 (71.6%) | 825 (75.3%) | 850 (71.8%) | 14178 (72.4%) | 0.35 |
| Body | 1187 (7.7%) | 155 (8.1%) | 63 (5.7%) | 98 (8.3%) | 1503 (7.7%) | ||
| Tail | 1590 (10.3%) | 207 (10.8%) | 100 (9.1%) | 120 (10.1%) | 2017 (10.3%) | ||
| Overlapping/not otherwise specified | 1486 (9.7%) | 180 (9.5%) | 108 (9.9%) | 116 (9.8%) | 1890 (9.6%) | ||
| Grade | Well differentiated | 1249 (8.1%) | 179 (9.4%) | 85 (7.8%) | 78 (6.6%) | 1591 (8.1%) | <0.001 |
| Moderately differentiated | 6867 (44.6%) | 896 (46.9%) | 457 (41.7%) | 510 (43.1%) | 8730 (44.6%) | ||
| Poorly differentiated | 4201 (27.3%) | 496 (26.0%) | 362 (33.0%) | 359 (30.3%) | 5418 (27.7%) | ||
| Undifferentiated/anaplastic | 165 (1.1%) | 12 (0.6%) | 14 (1.3%) | 13 (1.1%) | 204 (1.0%) | ||
| Not available | 2915 (18.9%) | 328 (17.2%) | 178 (16.2%) | 224 (18.9%) | 3645 (18.6%) | ||
| Stage | 0 | 548 (3.6%) | 49 (2.6%) | 29 (2.6%) | 50 (4.2%) | 676 (3.5%) | 0.01 |
| 1 | 1948 (12.7%) | 251 (13.1%) | 136 (12.4%) | 180 (15.2%) | 2515 (12.8%) | ||
| 2 | 12901 (83.8%) | 1611 (84.3%) | 931 (84.9%) | 954 (80.6%) | 16397 (83.7%) |
Abbreviations: IQR, interquartile range; NHB, Non‐Hispanic Black; NHW, Non‐Hispanic White.
Racial cohorts differed in socioeconomic characteristics. On average, NHW patients traveled further for care than NHB and Hispanic patients (24.0% vs. 14.2% and 15.0%. respectively. in the fourth quartile of distance traveled). Compared to NHW patients, more NHB patients were uninsured (3.2% vs. 1.3%) or Medicaid‐insured (11.3% vs. 4.1%). More NHB patients resided in a region with lower median income (36.7% vs. 11.8% with median income <$40 227) and lower education level (34.3% vs. 12.5% residing in a ZIP code in which ≥17.6% did not graduate from high school). Similar trends were seen for Hispanic patients. Compared to NHW patients, more NHB and Hispanic patients resided in a metro area (78.6% vs. 88.5% and 93.0%, respectively). Additionally, compared to NHW patients, more NHB and Hispanic patients resided in the South (36.1% vs. 57.0% and 40.3%, respectively).
3.2. Receipt of therapy
Among the entire cohort, 5098 patients (26.0%) received neoadjuvant chemotherapy, 9624 patients (49.1%) received adjuvant chemotherapy only, with a total of 14 722 patients (75.1%) having received multimodality therapy of some type (Table 2). A total of 4757 patients (24.3%) received no systemic therapy. More NHW patients compared to NHB patients received neoadjuvant chemotherapy (26.9% vs. 23.3%). In addition, compared to NHW patients, more NHB and Hispanic patients received no systemic therapy (23.8% vs. 25.1% and 26.4%, respectively).
Table 2.
Percent receipt of therapy by racial/ethnic cohort
| NHW | NHB | Hispanic | Other/Unknown | Overall | |
|---|---|---|---|---|---|
| N = 15 397 (78.6%) | N = 1911 (9.8%) | N = 1096 (5.6%) | N = 1184 (6.0%) | N = 19 588 | |
| Neoadjuvant | 4143 (26.9%) | 445 (23.3%) | 224 (20.4%) | 187 (22.2%) | 5098 (26.0%) |
| Adjuvant | 7517 (48.8%) | 973 (50.9%) | 568 (51.8%) | 413 (49.0%) | 9624 (49.1%) |
| No systemic therapy | 3663 (23.8%) | 480 (25.1%) | 289 (26.4%) | 241 (28.6%) | 4757 (24.3%) |
| Unknown | 74 (0.5%) | 13 (0.7%) | 15 (1.4%) | 2 (0.2%) | 109 (0.6%) |
Abbreviations: NHW, non‐Hispanic White; NHB, non‐Hispanic Black.
3.3. Logistic regression: receipt of neoadjuvant chemotherapy
By multivariable logistic regression, compared to NHW patients, NHB patients had lower odds of receiving neoadjuvant chemotherapy (OR: 0.80, 95% CI: 0.67–0.97) (Table 3). Other factors independently associated with decreased odds of receipt of neoadjuvant chemotherapy included lack of insurance (OR: 0.55, 95% CI: 0.36–0.85), lower educational attainment (OR: 0.75, 95% CI: 0.64–0.89 for ZIP code where 10.9%–17.5% did not graduate from high school), and nonacademic facility type (OR: 0.73, 95% CI: 0.63–0.82 for comprehensive community; Figure 2). Stage I disease also had a higher odd of receipt of neoadjuvant chemotherapy compared to Stage II (OR: 2.87, 95% CI: 2.49–3.31). An interaction test between race and insurance in the receipt of neoadjuvant chemotherapy did not show any statistically significant associations.
Table 3.
Odds of receiving neoadjuvant chemotherapy versus strictly adjuvant chemotherapy by multivariable regression
| Factor | Odds ratio (OR) | 95% confidence interval (CI) | p value |
|---|---|---|---|
| Race | |||
| Non‐Hispanic White | Ref | — | — |
| Non‐Hispanic Black | 0.80 | 0.67–0.97 | 0.02 |
| Hispanic | 0.94 | 0.75–1.19 | 0.61 |
| Other | 1.00 | 0.78–1.29 | 0.99 |
| Insurance | |||
| Private | Ref | — | — |
| Uninsured | 0.55 | 0.36–0.85 | <0.01 |
| Medicaid | 0.88 | 0.70–1.10 | 0.26 |
| Medicare | 0.94 | 0.82–1.07 | 0.32 |
| Other government | 1.37 | 0.94–2.00 | 0.10 |
| Age | |||
| Per year increase | 0.98 | 0.97–0.99 | <0.001 |
| Sex | |||
| Female | Ref | — | — |
| Male | 1.00 | 0.91–1.11 | 0.97 |
| Income | |||
| ≥$63 333 | Ref | — | — |
| $50 354–$63 332 | 1.01 | 0.88–1.17 | 0.89 |
| $40 227–$50 353 | 1.00 | 0.84–1.18 | 0.99 |
| <$40 227 | 0.92 | 0.75–1.13 | 0.43 |
| Percent did not graduate from high school | |||
| <6.3% | Ref | — | — |
| 6.3%–10.8% | 0.89 | 0.77–1.02 | 0.09 |
| 10.9%–17.5% | 0.75 | 0.64–0.89 | <0.01 |
| ≥17.6% | 0.84 | 0.68–1.02 | 0.08 |
| Geographic region | |||
| Northeast | Ref | — | — |
| Midwest | 1.41 | 1.22–1.64 | <0.001 |
| South | 1.13 | 0.98–1.31 | 0.09 |
| West | 1.00 | 0.84–1.18 | 0.96 |
| Rurality | |||
| Metro | Ref | — | — |
| Urban | 1.00 | 0.85–1.18 | 0.99 |
| Rural | 0.81 | 0.54–1.21 | 0.30 |
| Distance traveled for care by quartile (range in miles) | |||
| 1st (0–7) | Ref | — | — |
| 2nd (7–17.2) | 1.15 | 1.00–1.33 | 0.06 |
| 3rd (17.2–46.1) | 1.31 | 1.13–1.52 | <0.001 |
| 4th (46.1–4814.3) | 1.98 | 1.68–2.33 | <0.001 |
| Charlson/Deyo Score | |||
| 0 | Ref | — | — |
| 1 | 1.03 | 0.92–1.15 | 0.65 |
| 2 | 1.08 | 0.88–1.32 | 0.47 |
| 3 or more | 0.98 | 0.74–1.28 | 0.85 |
| Facility type | |||
| Academic | Ref | — | — |
| Community | 0.91 | 0.70–1.17 | 0.44 |
| Comprehensive community | 0.73 | 0.64–0.82 | <0.001 |
| Integrated | 0.78 | 0.67–0.91 | <0.01 |
| Grade | |||
| Well differentiated | Ref | — | — |
| Moderately differentiated | 0.90 | 0.76–1.06 | 0.20 |
| Poorly differentiated | 0.86 | 0.72–1.02 | 0.09 |
| Undifferentiated/Anaplastic | 0.88 | 0.5–1.49 | 0.64 |
| Stage | |||
| 2 | Ref | — | — |
| 1 | 2.87 | 2.49–3.31 | <0.001 |
| Primary site | |||
| Head | Ref | — | — |
| Body | 0.44 | 0.34–0.56 | <0.001 |
| Tail | 0.93 | 0.74–1.18 | 0.57 |
| Overlapping/NOS | 0.99 | 0.83–1.17 | 0.89 |
Figure 2.
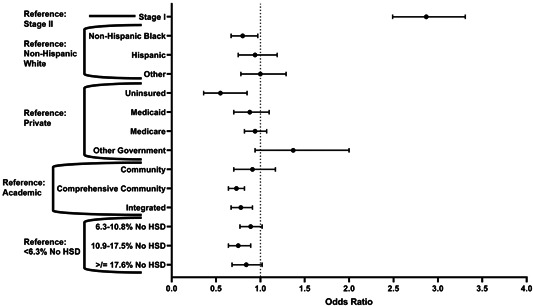
Odds of receiving neoadjuvant chemotherapy versus strictly adjuvant chemotherapy by multivariable regression, selected factors. HSD, high school degree
3.4. Logistic regression: receipt of multimodality therapy
Similar factors affected the receipt of multimodality therapy compared to resection alone when controlling for other confounding variables (Table 4). Lack of insurance (OR: 0.58, 95% CI: 0.42–0.79) and Medicaid insurance (OR: 0.54, 95% CI: 0.44–0.65) were both independently associated with decreased odds of receipt of multimodality therapy compared to those with private insurance (Figure 3). Additional factors independently associated with a decreased likelihood of multimodality therapy included lower educational attainment (OR: 0.74, 95% CI: 0.62–0.87), geographic region (OR: 0.74, 95% CI: 0.65–0.83), distance traveled (OR: 0.71, 95% CI: 0.62–0.81 for fourth quartile), and significant comorbidity (OR: 0.71, 95% CI: 0.62–0.81 for Charlson/Deyo comorbidity score of 3 or more). Race, however, was not statistically associated with a difference in likelihood of receiving multimodality therapy. An interaction test between race and insurance for the receipt of multimodality therapy also did not show any statistically significant associations.
Table 4.
Odds of Receiving multimodality therapy vs.versus surgery alone by multivariable regression
| Factor | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Race | |||
| Non‐Hispanic White | Ref | — | — |
| Non‐Hispanic Black | 0.89 | 0.77–1.03 | 0.11 |
| Hispanic | 0.94 | 0.78–1.12 | 0.49 |
| Other | 0.85 | 0.69–1.04 | 0.10 |
| Insurance | |||
| Private | Ref | — | — |
| Uninsured | 0.58 | 0.42–0.79 | <0.01 |
| Medicaid | 0.54 | 0.44–0.65 | <0.001 |
| Medicare | 1.01 | 0.90–1.13 | 0.89 |
| Other Government | 1.32 | 0.88–1.96 | 0.18 |
| Age | |||
| Per year increase | 0.94 | 0.93–0.95 | <0.001 |
| Sex | |||
| Female | Ref | — | — |
| Male | 1.04 | 0.95–1.13 | 0.41 |
| Income | |||
| ≥ $63 333 | Ref | — | — |
| $50 354 to $63 332 | 0.89 | 0.79–1.01 | 0.07 |
| $40 227 to $50 353 | 0.92 | 0.80–1.06 | 0.23 |
| <$40 227 | 0.87 | 0.74–1.03 | 0.10 |
| Percent did not graduate from high school | |||
| <6.3% | Ref | — | — |
| 6.3%–10.8% | 0.87 | 0.77–0.99 | 0.03 |
| 10.9%–17.5% | 0.71 | 0.62–0.82 | <0.001 |
| ≥17.6% | 0.74 | 0.62–0.87 | <0.001 |
| Geographic region | |||
| Northeast | Ref | — | — |
| Midwest | 1.08 | 0.94–1.24 | 0.27 |
| South | 0.74 | 0.65–0.83 | <0.001 |
| West | 0.85 | 0.74–0.99 | 0.03 |
| Rurality | |||
| Metro | Ref | — | — |
| Urban | 1.21 | 1.05–1.39 | <0.01 |
| Rural | 1.19 | 0.86–1.65 | 0.30 |
| Distance traveled for care by quartile (range in miles) | |||
| 1st (0–7) | Ref | — | — |
| 2nd (7–17.2) | 1.11 | 0.99–1.25 | 0.09 |
| 3rd (17.2–46.1) | 0.97 | 0.86–1.10 | 0.65 |
| 4th (46.1–4814.3) | 0.71 | 0.62–0.81 | <0.001 |
| Charlson/Deyo Score | |||
| 0 | Ref | — | — |
| 1 | 0.99 | 0.90–1.09 | 0.79 |
| 2 | 0.87 | 0.74–1.03 | 0.10 |
| 3 or more | 0.71 | 0.58–0.86 | <0.01 |
| Facility type | |||
| Academic | Ref | — | — |
| Community | 1.02 | 0.82–1.28 | 0.86 |
| Comprehensive community | 0.91 | 0.82–1.01 | 0.07 |
| Integrated | 0.98 | 0.86–1.12 | 0.81 |
| Grade | |||
| Well differentiated | Ref | — | — |
| Moderately differentiated | 0.93 | 0.85–1.02 | 0.11 |
| Poorly differentiated | 0.55 | 0.38–0.79 | <0.01 |
| Undifferentiated/Anaplastic | 0.96 | 0.83–1.11 | 0.54 |
| Stage | |||
| 2 | Ref | — | — |
| 1 | 35.55 | 18.67–67.70 | <0.001 |
| Primary site | |||
| Head | Ref | — | — |
| Body | 0.87 | 0.75–1.01 | 0.06 |
| Tail | 0.85 | 0.74–0.97 | 0.02 |
| Overlapping/NOS | 0.92 | 0.78–1.08 | 0.29 |
Abbreviation: NOS, not otherwise specified.
Figure 3.
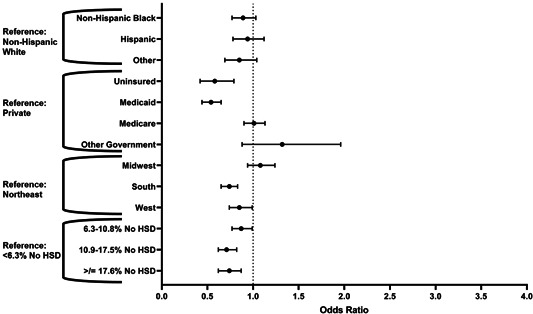
Odds of receiving multimodality therapy versus surgery alone by multivariable regression, selected factors. HSD, high school degree
3.5. Sensitivity analysis
Using clinical stage to define the cohort, a set of 14 916 patients underwent repeat analyses as described above. The demographic characteristics of this cohort are largely similar to the original cohort (Supplement) with a few exceptions. The clinical stage cohort had a higher proportion of NHB patients in the lowest income group compared to the original cohort. In addition, a much higher proportion of patients overall were designated Stage I (49.0% overall vs. 12.8%). Of the patients with a clinical Stage I, only 18.9% (n = 1384) had a concurrent analytic stage of I, whereas 88.8% (n = 6413) had an analytic stage of II.
Similar to the original analysis, the largest proportion of patients across all race/ethnicity groups received strictly adjuvant chemotherapy, although the proportions of patients who received some neoadjuvant therapy are higher than in the original analysis (29.3% compared to 26.2% overall).
On regression analysis, most factors did not change in degree or significance of their odds ratios. Some notable differences were found. NHB patients still have a lower likelihood of receiving neoadjuvant therapy compared to NHW patients but this finding loses significance (p = 0.16). Patients with worsening tumor grade still had lower likelihood of receiving neoadjuvant therapy and this finding became statistically significant. Finally, patients with clinical Stage I were much less likely to receive neoadjuvant therapy (OR: 0.44, 95% CI: 0.39–0.49). For the analysis of multimodality therapy, patients with clinical Stage I were less likely to receive multimodality therapy compared to resection alone (0.75, 95% CI: 0.68–0.83).
4. DISCUSSION
In this large, national cross‐sectional study of patients with resected AJCC Stage I and II pancreatic cancer, NHB patients, uninsured patients, and patients treated at nonacademic facility types had decreased odds of receiving neoadjuvant chemotherapy compared to NHW patients, privately insured patients, and treatment at academic facilities, respectively. Medicaid insurance and no insurance, geographic region, distance traveled, and lower educational attainment, but not race, were independently associated with a decreased likelihood of receiving multimodality therapy.
The growing addition of neoadjuvant chemotherapy to the treatment paradigm of pancreatic cancer has raised new concerns regarding sociodemographic disparities in treatment delivery. Previously demonstrated racial disparities for patients with resectable pancreatic cancer include disparities in receipt of surgery, systemic chemotherapy, and radiation, as well as multimodality therapy; 3 , 6 , 23 , 24 , 25 , 26 , 27 however to our knowledge, this is the first study to identify racial disparities in the receipt of neoadjuvant chemotherapy in early stage resected pancreas cancer. A recently published study of patients with Stage I/II pancreatic cancer did not identify significant racial differences in the use of neoadjuvant chemotherapy, although in that study, patients were compared by receipt of any neoadjuvant chemotherapy versus upfront resection rather than those receiving adjuvant chemotherapy. 16 The same study did find an insurance disparity, which is consistent with this study's findings. 16 In addition, their study found the use of neoadjuvant chemotherapy was much lower in the earlier years of their cohort, and steadily increased through 2016, 16 a finding that motivated our study to use a more contemporary cohort, that is, 2014–2017.
While the importance of systemic chemotherapy is undisputed in the treatment of pancreatic cancer, its timing as neoadjuvant versus adjuvant chemotherapy in patients undergoing curative surgical resection continues to be debated. Some studies have suggested that neoadjuvant chemotherapy can improve patient selection for surgery, and is better tolerated and equally effective compared to chemotherapy given in adjuvant setting. 10 , 11 , 12 The recently completed Phase II randomized SWOG S1505 study demonstrated the efficacy of neoadjuvant chemotherapy in the resectable pancreatic cancer population. 17 Systematic reviews and meta‐analyses have also shown a survival benefit of neoadjuvant chemotherapy compared with upfront surgery and adjuvant chemotherapy in patients with resectable or borderline resectable disease. 13 , 28 The most recent guidelines of the National Comprehensive Cancer Network do recommend neoadjuvant chemotherapy in all patients with borderline resectable disease, and either upfront surgical resection or neoadjuvant chemotherapy in those with resectable disease, with strong consideration for neoadjuvant chemotherapy in high‐risk patients. 9
Despite these recommendations, however, neoadjuvant chemotherapy has not been established as standard of care to date and was not standard of care during the time period of the study from 2014 to 2017. As a result, significant variation in clinical practice patterns exists, although its use has increased over time to nearly a quarter of patients treated at CoC‐accredited facilities. 16 The recently opened Alliance A021806 Phase III randomized trial of perioperative versus adjuvant chemotherapy for resectable pancreatic cancer should hopefully help settle this ongoing controversy. 29 Regardless of the outcome, it will be important to follow patterns of treatment including racial disparities, which are important to note and to address not only in standard of care treatment but also in emerging therapies, especially given the disproportionate burden of increased risk factors and socioeconomic disadvantage affecting patients of non‐White race as well as potential physician bias. 30 The findings of decreased likelihood for neoadjuvant therapy on sensitivity analysis for patients with clinical Stage I is consistent with ongoing preference for upfront resection for patients with presumed resectable disease.
In contrast, the use of systemic chemotherapy for resected PDAC is considered standard of care. 9 In this study, nearly 25% of patients did not receive any systemic therapy, which is concerning. After adjustment for covariates, racial disparities in receipt of multimodality therapy were no longer observed, which is in contrast to several other registry or population‐based studies of patients with resected PDAC. 23 , 24 , 25 A study of 223 465 patients from the NCDB (2004–2015) found that Black patients had marginally lower odds of receipt of any treatment (OR: 0.97, p = 0.04) although this included patients of all AJCC stages and receipt of resection as a possible treatment. 31 However, many of the existing studies are based on historical data from over 10 years ago and may not reflect contemporary practice. The present study was limited to the most recent era (2014–2017) to best observe more modern practice. The absence of race as an independent factor in this more contemporary timeframe may potentially represent a narrowing of the racial disparity gap in regard to multimodality therapy for pancreas cancer when controlling for other confounding factors. In general, racial disparities are narrowing across several cancer types, which may be attributable to many factors such as improvements in insurance coverage, institution‐specific interventions targeted towards addressing unconscious racial bias, 32 and the multifaceted efforts of various small and large organizations on improving research funding, advocacy, community awareness, and facilitating patient services.
While race is not modifiable, other modifiable risk factors were found to be independently associated with a decreased likelihood of receipt of multimodality therapy including health insurance, educational attainment, and geographic region. States in the South region of the United States have higher rates of poverty and lower rates of literacy among other socioeconomic disadvantages. 33 Importantly, the significance of socioeconomic disparities in the receipt of care in early pancreatic cancer is highlighted in the findings of this study and is consistent with prior studies. 6
One critical modifiable factor is insurance coverage. It is well established that uninsured patients have a decreased likelihood of receiving multimodality therapy. 4 , 5 , 27 Although increasing age and comorbidity burden have been associated with lower odds of receiving or completing adjuvant chemotherapy in the Medicare population, 14 , 27 Medicare insurance itself was not independently associated with decreased odds of receipt of multimodality therapy in this study, consistent with findings in prior NCDB studies. 34 For Medicaid‐insured and uninsured patients, healthcare reform aimed at improving coverage may alleviate this disparity. A study comparing over 6000 patients in Massachusetts to 41 000 patients in control states with pancreatic cancer found improved rates of resection for patients with government‐subsidized insurance or self‐pay status after healthcare reform, although concurrent systemic chemotherapy was not evaluated. 35 Overcoming the insurance barriers to healthcare access may also be achieved with safety‐net hospitals that see a high burden of Medicaid and uninsured patients. In a study of nearly 33 000 patients in the NCDB, no significant differences in receipt of multimodal therapy were seen for patients with pancreatic cancer between safety net and nonsafety net hospitals. 36
Other studies have shown that higher socioeconomic status and treatment at academic facilities are associated with improved survival. 31 , 37 Even within a single payer system such as in Canada, markers of socioeconomic disadvantage including rurality and median income are associated with treatment disparities. 38 While more granular data on specific socioeconomic factors—especially those not captured in the NCDB—are necessary to design targeted interventions, some efforts have shown success. One recent study from a large hospital system suggested that the implementation of a pancreatic cancer multidisciplinary clinic can standardize treatment decisions and thus eliminate socioeconomic‐based disparities in overall outcomes and survival. 39 A multifaceted approach involving nurse navigation, real‐time warnings on missed care, and regular race‐specific feedback eliminated treatment disparities at one institution for patients with lung cancer. 32 The efficacy of similar multipronged approaches for pancreas cancer treatment needs investigation. Patients of minority race are often doubly impacted by socioeconomic disadvantage; 30 however, according to several studies, when treated within a safety‐net, equal access integrated hospital system, disparities in treatment or survival were not observed. 31 , 40 , 41 Fortunately, postoperative outcomes also appear similar by race; a recently published NSQIP study found similar 30‐day outcomes in NHB and NHW patients following resection for pancreatic cancer. 42 While these results are promising, the ongoing disparities identified in this study suggest that more efforts are needed to ensure equivalent care delivery to all patients.
This study has several limitations. First, this is a retrospective cohort study and is limited by the quality of data abstraction by NCDB registrars as well as the availability of variables collected. For example, other socioeconomic factors beyond the variables included in the study are not provided. Second, it is comprised of data from Commission‐on‐Cancer‐accredited facilities only, and therefore may not be generalizable to other patient populations. Third, the association of the findings with dual eligibility insurance status could not be elicited through this model, thus potentially over‐ or underestimating the health insurance status as a covariate in assessing treatment disparities. Fourth, it is not possible to identify patients who were treated with neoadjuvant chemotherapy but did not ultimately undergo surgical resection due to disease progression or decline in performance status. Similarly, it is not possible to identify patients who are truly borderline resectable by anatomic definitions with the variables available in the NCDB. Therefore, this remains a confounder that cannot be accounted for to determine a more true association between receipt of resection by resectability and race. Additionally, a sizable proportion of patients was excluded from the study due to the missing stage. The direction and degree of bias resulting from missing data is not fully known; evidence suggests that survival outcomes are overestimated and that minority patients are underrepresented. 43 This limitation cannot be easily overcome with data imputation, which would also introduce error. Finally, although preference for one treatment modality for another should be informed by the ultimate outcome of survival benefit, survival analyses using cancer registry data such as the NCDB must be viewed cautiously due to the lack of detailed information on clinically relevant factors including types and doses of chemotherapy received. Studies comparing survival outcomes between randomized controlled trials and equivalent cohorts obtained from the NCDB found substantial discordance in hazard ratios as well as statistical significance of findings. 44 , 45 Although one might hope to use real‐world evidence from the NCDB to confirm effectiveness of an efficacious treatment from a controlled trial, some studies of nonefficacious treatments have actually demonstrated effectiveness using databases, 44 therefore calling into question the validity of using databases for survival analysis.
5. CONCLUSIONS
While neoadjuvant chemotherapy is emerging as a more frequently used treatment approach in pancreatic cancer, it has not been administered in equal frequency to all patients. NHB patients as well as uninsured patients have lower odds of receiving neoadjuvant chemotherapy. Perhaps more importantly, however, although race was not associated with differences in the receipt of multimodality therapy for resected pancreas cancer, socioeconomically disadvantaged patients, including those with lesser insurance, had a significantly lower likelihood of receiving what is considered standard of care. Fortunately, although race is not modifiable, these socioeconomic risk factors potentially are and emphasizes the need for policy and interventional implementations that address these factors to bridge the continued disparity gap in pancreatic cancer care—racial or otherwise.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
7.
6. ACKNOWLEDGMENTS
We would like to acknowledge Reba Bullard for her assistance with the preparation of this manuscript.
Hao S, Mitsakos A, Irish W, Tuttle‐Newhall JE, Parikh AA, Snyder RA. Differences in receipt of multimodality therapy by race, insurance status, and socioeconomic disadvantage in patients with resected pancreatic cancer. J Surg Oncol. 2022;126:302‐313. 10.1002/jso.26859
7.1. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the NCDB. Restrictions apply to the availability of these data, which were used under approval for this study. Data are available on request from the corresponding author with the permission of the NCDB.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Cervantes A, Waymouth EK, Petrov MS. African‐Americans and indigenous peoples have increased burden of diseases of the exocrine pancreas: a systematic review and meta‐analysis. Dig Dis Sci. 2019;64(1):249‐261. 10.1007/s10620-018-5291-1 [DOI] [PubMed] [Google Scholar]
- 3. Heller DR, Nicolson NG, Ahuja N, Khan S, Kunstman JW. Association of treatment inequity and ancestry with pancreatic ductal adenocarcinoma survival. JAMA Surg. 2020;155(2):e195047. 10.1001/jamasurg.2019.5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khawja SN, Mohammed S, Silberfein EJ, Musher BL, Fisher WE, Van Buren G 2nd. Pancreatic cancer disparities in African Americans. Pancreas. 2015;44(4):522‐527. 10.1097/MPA.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 5. Noel M, Fiscella K. Disparities in pancreatic cancer treatment and outcomes. Health Equity. 2019;3(1):532‐540. 10.1089/heq.2019.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lutfi W, Zenati MS, Zureikat AH, Zeh HJ, Hogg ME. Health disparities impact expected treatment of pancreatic ductal adenocarcinoma nationally. Ann Surg Oncol. 2018;25(7):1860‐1867. 10.1245/s10434-018-6487-5 [DOI] [PubMed] [Google Scholar]
- 7. Swords DS, Scaife CL. Decompositions of the contribution of treatment disparities to survival disparities in stage I‐II pancreatic adenocarcinoma. Ann Surg Oncol. 2020;28:3157‐3168. 10.1245/s10434-020-09267-y [DOI] [PubMed] [Google Scholar]
- 8. Dimou F, Sineshaw H, Parmar AD, Tamirisa NP, Jemal A, Riall TS. Trends in receipt and timing of multimodality therapy in early‐stage pancreatic cancer. J Gastrointest Surg. 2016;20:93‐103. Discussion 103. 10.1007/s11605-015-2952-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pancreatic adenocarcinoma. Updated 2021. Accessed March 12, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf;
- 10. Heinrich S, Lang H. Neoadjuvant therapy of pancreatic cancer: definitions and benefits. Int J Mol Sci. 2017;18(8):1622. 10.3390/ijms18081622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klaiber U, Leonhardt CS, Strobel O, Tjaden C, Hackert T, Neoptolemos JP. Neoadjuvant and adjuvant chemotherapy in pancreatic cancer. Langenbecks Arch Surg. 2018;403(8):917‐932. 10.1007/s00423-018-1724-8 [DOI] [PubMed] [Google Scholar]
- 12. Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant treatment for pancreatic cancer. Semin Oncol. 2019;46(1):19‐27. 10.1053/j.seminoncol.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 13. Versteijne E, Vogel JA, Besselink MG, et al. Meta‐analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946‐958. 10.1002/bjs.10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altman AM, Wirth K, Marmor S, et al. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26(12):4108‐4116. 10.1245/s10434-019-07602-6 [DOI] [PubMed] [Google Scholar]
- 15. Syed AR, Carleton NM, Horne Z, et al. Survival trends for resectable pancreatic cancer using a multidisciplinary conference: the impact of post‐operative chemotherapy. J Gastrointest Cancer. 2020;51(3):836‐843. 10.1007/s12029-019-00303-z [DOI] [PubMed] [Google Scholar]
- 16. Cloyd JM, Shen C, Santry H, et al. Disparities in the use of neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma. J Natl Compr Cancer Netw. 2020;18(5):556‐563. 10.6004/jnccn.2019.7380 [DOI] [PubMed] [Google Scholar]
- 17. Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab‐paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020;272(3):481‐486. 10.1097/SLA.0000000000004155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.About the National Cancer Database. Accessed March 21, 2020. https://www.facs.org/quality-programs/cancer/ncdb/about
- 19. Edge SB, Byrd SR, Compton CC, eds. AJCC Cancer Staging Manual. 7th ed. Springer‐Verlag; 2010. [Google Scholar]
- 20. Amin MB, Edge S, Greene F, et al., eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing, American Joint Commission on Cancer; 2017. [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 22.Geography Division. Census regions and divisions of the United States. Accessed May 5, 2021. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 23. Swords DS, Mulvihill SJ, Brooke BS, Skarda DE, Firpo MA, Scaife CL. Disparities in utilization of treatment for clinical stage I‐II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery. 2019;165(4):751‐759. 10.1016/j.surg.2018.10.035 [DOI] [PubMed] [Google Scholar]
- 24. Sanford NN, Aguilera TA, Folkert MR, et al. Sociodemographic disparities in the receipt of adjuvant chemotherapy among patients with resected stage I‐III pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2019;17(11):1292‐1300. 10.6004/jnccn.2019.7322 [DOI] [PubMed] [Google Scholar]
- 25. Wright MJ, Overton HN, Teinor JA, et al. Disparities in the use of chemotherapy in patients with resected pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2020;24(7):1590‐1596. 10.1007/s11605-019-04311-z [DOI] [PubMed] [Google Scholar]
- 26. Molina G, Clancy TE, Tsai TC, Lam M, Wang J. Racial disparity in pancreatoduodenectomy for borderline resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2021;28(2):1088‐1096. 10.1245/s10434-020-08717-x [DOI] [PubMed] [Google Scholar]
- 27. Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 2018;7(2):525‐535. 10.1002/cam4.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rangarajan K, Pucher PH, Armstrong T, Bateman A, Hamady Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta‐analysis. Ann R Coll Surg Engl. 2019;101(7):453‐462. 10.1308/rcsann.2019.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testing the use of the usual chemotherapy before and after surgery for removable pancreatic cancer. Accessed Mar 25, 2021. https://clinicaltrials.gov/ct2/show/NCT04340141
- 30.American Association for Cancer Research. AACR cancer disparities progress report 2020. 2020:1145.
- 31. Zhu F, Wang H, Ashamalla H. Racial and socioeconomic disparities in the treatments and outcomes of pancreatic cancer among different treatment facility types. Pancreas. 2020;49(10):1355‐1363. 10.1097/MPA.0000000000001688 [DOI] [PubMed] [Google Scholar]
- 32. Cykert S, Eng E, Walker P, et al. A system‐based intervention to reduce Black‐White disparities in the treatment of early stage lung cancer: a pragmatic trial at five cancer centers. Cancer Med. 2019;8(3):1095‐1102. 10.1002/cam4.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Explore census data. Accessed May 25, 2021. https://data.census.gov/cedsci/
- 34. Krishnan M, Ahmed A, Walters RW, Silberstein PT. Factors affecting adjuvant therapy in stage III pancreatic cancer‐analysis of the national cancer database. Clin Med Insights Oncol. 2017;11:1179554917728040. 10.1177/1179554917728040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loehrer AP, Chang DC, Hutter MM, et al. Health insurance expansion and treatment of pancreatic cancer: does increased access lead to improved care? J Am Coll Surg. 2015;221(6):1015‐1022. 10.1016/j.jamcollsurg.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhar VK, Hoehn RS, Kim Y, et al. Equivalent treatment and survival after resection of pancreatic cancer at safety‐net hospitals. J Gastrointest Surg. 2018;22(1):98‐106. 10.1007/s11605-017-3549-0 [DOI] [PubMed] [Google Scholar]
- 37. Riner AN, Underwood PW, Yang K, et al. Disparities in pancreatic ductal adenocarcinoma‐the significance of Hispanic ethnicity, subgroup analysis, and treatment facility on clinical outcomes. Cancer Med. 2020;9(12):4069‐4082. 10.1002/cam4.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kagedan DJ, Abraham L, Goyert N, et al. Beyond the dollar: influence of sociodemographic marginalization on surgical resection, adjuvant therapy, and survival in patients with pancreatic cancer. Cancer. 2016;122(20):3175‐3182. 10.1002/cncr.30148 [DOI] [PubMed] [Google Scholar]
- 39. Hoehn RS, Rieser CJ, Winters S, et al. A pancreatic cancer multidisciplinary clinic eliminates socioeconomic disparities in treatment and improves survival. Ann Surg Oncol. 2021;28:2438‐2446. 10.1245/s10434-021-09594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Markossian TW, O'Neal CM, Senkowski C. Geographic disparities in pancreatic cancer survival in a southeastern safety‐net academic medical center. Aust J Rural Health. 2016;24(2):73‐78. 10.1111/ajr.12200 [DOI] [PubMed] [Google Scholar]
- 41. Chang JI, Huang BZ, Wu BU. Impact of integrated health care delivery on racial and ethnic disparities in pancreatic cancer. Pancreas. 2018;47(2):221‐226. 10.1097/MPA.0000000000000981 [DOI] [PubMed] [Google Scholar]
- 42. Mitsakos AT, Dennis SO, Parikh AA, Snyder RA. Thirty‐day complication rates do not differ by race among patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2021;123(4):970‐977. 10.1002/jso.26383 [DOI] [PubMed] [Google Scholar]
- 43. Yang DX, Khera R, Miccio JA, et al. Prevalence of missing data in the national cancer database and association with overall survival. JAMA Netw Open. 2021;4(3):e211793. 10.1001/jamanetworkopen.2021.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karim S, Booth CM. Effectiveness in the absence of efficacy: Cautionary tales from real‐world evidence. J Clin Oncol. 2019;37(13):1047‐1050. 10.1200/JCO.18.02105 [DOI] [PubMed] [Google Scholar]
- 45. Kumar A, Guss ZD, Courtney PT, et al. Evaluation of the use of cancer registry data for comparative effectiveness research. JAMA Network Open. 2020;3(7):e2011985. 10.1001/jamanetworkopen.2020.11985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the NCDB. Restrictions apply to the availability of these data, which were used under approval for this study. Data are available on request from the corresponding author with the permission of the NCDB.


