Abstract
Aims
There has been a dramatic increase in hypoglycaemic agent expenditure. We assessed the variability in prescribing costs at the practice level and the relationship between expenditure and the proportion of patients achieving target glycaemic control.
Methods
We utilized national prescribing data from 406 general practices in Wales. This was compared against glycaemic control (percentage of patients achieving a HbA1c level < 59 mmol/mol in the preceding 12 months). Analyses were adjusted for the number of patients with diabetes in each general practice and the Welsh Index of Multiple Deprivation.
Results
There was considerable heterogeneity in hypoglycaemic agent spend per patient with diabetes, Median = £289 (IQR 247–343) range £31.1–£1713. Higher total expenditure was not associated with improved glycaemic control B(std) = −0.01 (95%CI –0.01, 0.002) p = 0.13. High‐spend practices spent more on SGLT2 inhibitors (16 vs. 9% p < 0.001) and GLP‐1 agonists (13 vs. 11% p < 0.001) and less on insulin (34 vs. 42% p < 0.001), biguanides (9 vs. 11% p = 0.001) and sulphonylureas (2 vs. 3% p < 0.001) than low spend practices. There were no differences in the pattern of drug prescribing between high spend practices with better glycaemic control (mean 68% of patients HbA1c <59 mmol/mol) and those with less good metabolic control (mean 58% of patients HbA1c <59 mmol/mol).
Conclusions
Spend on hypoglycaemic agents is highly variable between practices and increased expenditure per patient is not associated with better glycaemic control. Whilst newer, more expensive agents have additional benefits, in individuals where these advantages are more marginal widespread use of these agents has important cost implications.
Keywords: cost, HbA1c, Hypoglycaemic agents, primary care
Novelty Statement.
What is already known?
The cost and breadth of hypoglycaemic agents have increased dramatically in recent years.
What this study has found?
That expenditure per patient on diabetes is not associated with better outcomes with regard to glycaemic control.
What are the implications of the study?
More judicious selection of hypoglycaemic agents may enable optimal glycaemic control, without excessive costs.
1. INTRODUCTION
Globally there has been a dramatic increase in the number of individuals with type 2 diabetes (T2DM), which has quadrupled over the past 3 decades. 1 Approximately 1 in 11 adults worldwide now has diabetes, the vast majority of which have T2DM. 1 In tandem, there have been substantial increases in therapeutic options available for optimising glycaemic control in T2DM including glucagon‐like peptide 1 (GLP‐1) agonists, dipeptidyl peptidase‐4 (DPP‐4) inhibitors and sodium‐glucose co‐transporter 2 (SGLT‐2) inhibitors. 2 As a result, clinicians have the opportunity to be selective in therapeutic agents for hyperglycaemia and target therapies based on an individual's cardiovascular risk, or presence of heart failure or chronic kidney disease and ADA/EASD guidance has recommended that SGLT2 inhibitors and GLP‐1 agonists are used earlier in management. 3
The rising prevalence of diabetes and the advent of more expensive agents have substantially increased the cost of treating T2DM. In 2018, the annual prescription cost for glucose‐lowering agents exceeded £1 billion in the UK with almost one in 20 GP prescriptions related to diabetes treatment. 4 This figure is predicted to continue to increase substantially in the next 20 years and, therefore, poses an enormous challenge for healthcare resources. Extracting maximal benefit without excessive cost should therefore be a key focus of T2DM prescribing.
Like the rest of the UK, the prevalence of diabetes in Wales has been consistently increasing. Wales has the highest prevalence of diabetes in the UK at 209,015 individuals representing 8% of the population of which 90% have T2DM. In 2014, the All Wales Medicines Strategy Group (AWMSG) reported a sharp increase in expenditure on managing DM from £45 to £57 million between 2010 and 2014. The substantial rate of the annual expenditure increment on diabetes treatment has thus become a major public health concern.
UK NICE guidance for the pharmacological management of T2DM recommends the initiation of monotherapy with biguanides such as metformin or sulphonylurea as first‐ and second‐line treatments, respectively. 5 Over time, utilising these agents as monotherapy is often inadequate in aiding patients to achieve good glycaemic control. 6 , 7 Subsequently, if the HbA1c remains above 58 mmol/L, then the patient should commence dual therapy. 5 Most diabetologists and primary care physicians favour the addition of newer agents (GLP‐1 agonists, DPP‐IV inhibitors and SGLT2 inhibitors) rather than the addition of sulphonylureas to Metformin which significantly increases the early treatment cost per patient. Furthermore, there is now considerable debate as to whether these newer agents particularly the SGLT2 inhibitors could be potentially used instead of metformin as first‐line agents 8 and certainly their early use is encouraged in recent international guidance. 3 These changes to prescribing practices endorsed by the ADA/EASD thus have substantial long‐term cost implications. 3 Further costs can arise if ineffective drugs are not stopped when additional agents are added. 3
Annual prescription costs for an agent vary substantially. For instance, the relative cost of GLP‐1 agonists is 25‐fold higher than metformin and SGLT2 inhibitors are around 10‐fold higher than metformin. Furthermore, there is widespread variation in prescribing based on clinician's preferences and local policies. 6 In comparison to 2013–2014, the cost of prescribing these newer agents in 2014–2015 showed a 14% increase in costs within NHS Wales. 9 Theoretically, since more money has been spent on these medications, it might be expected that more patients should have achieved the appropriate HbA1c target post‐treatment although it should be noted these newer agents have additional advantages beyond glycaemic control.
The present study aims to assess the variability in expenditure at the practice level and also establish if there is a relationship between diabetes drug costs per patient and glycaemic outcomes. We will also provide information on the balance of spending across different drug categories that might be more cost‐effective with regard to glycaemic control. However, this will not recognise other aspects of management including cardiovascular, renal protection, weight loss and avoidance of hypoglycaemia. Whilst the needs of individual patients for diabetes medication vary. General practices in our region manage a median of 426 patients (IQR 288–561) and our analysis is at the general practice rather than individual patient level and adjusted comparisons according to a local deprivation index to overcome this variation as far as possible.
2. METHODS
2.1. Study population
In our study, all registered general practices (n = 473) across all seven Welsh health boards participating in the UK Quality and Outcomes Framework (QOF) Database were identified. We excluded 66 general practices (13.9%) due to either incomplete data entries on the QOF website (N = 48) or missing drug expenditure data (n = 18). Data on different parameters (DM001 and DM007) from the year 2018 were extracted from the QOF. DM001 refers to the number of adult patients with diabetes who are registered in each general practice. DM007 denotes the number of patients with the latest HbA1c level below 59 mmol/mol in the preceding 12 months, essentially highlighting the incidence of patients at the practice attaining target glycaemic control. The publicly accessible CASPA and QOF databases have been used extensively in several previous diabetes‐related studies. 9 , 10 No patient identifiable information was present in the extracted data.
2.2. Ethics statement
Data were fully anonymised and recorded as part of routine practice; as such, the study did not require ethical approval.
2.3. Comparison analysis system for prescribing audit (CASPA) data extraction
Comparison Analysis System for Prescribing Audit (CASPA) (NHS Wales Shared Services Partnership; version 1.0 15.0) is a database which compiles primary care prescription data from all GP surgeries across the seven Welsh health boards. 9 Using the CASPA database, we extracted annual values for the estimated total expenditure and the total number of items prescribed for the seven major categories of hypoglycaemic drug classes. The individual drug(s) analysed in our study were all listed in the British National Formulary (BNF) and dispensed in Welsh GP surgeries (n = 425) throughout each of the seven health boards in 2018. 7 classes of drugs were used (i) Biguanides (Metformin) (ii) Sulphonylureas (Gliclazide, Glibenclamide, Glipizide, Glimepiride, Tolbutamide) (iii) Thiazolidinediones (Pioglitazone, Rosiglitazone, Troglitazone) (iv) DPP4 inhibitors (Sitagliptin, Saxagliptin, Linagliptin, Vildagliptin, Alogliptin) (v) SGLT2 inhibitors (Dapagliflozin, Canagliflozin, Empagliflozin) (vi) GLP1‐ agonists (Liraglutide, Lixisenatide, Exenatide) (vii) Insulin (all short‐acting, intermediate‐acting, and long‐acting insulins).
All expenditure was measured in pounds sterling in the relevant year of data collection. For each general practice, we examined the percentage spent for each class of agents of the total expenditure for the 7 classes. We also assessed these against the proportion of patients with a target HbA1c level (<59 mmol/mol = 7.5%) in the preceding 12 months. Analyses were adjusted for a number of patients with diabetes in each general practice and the Welsh Index of Multiple Deprivation (IMD). IMD is the Welsh Government's official measure of relative deprivation for ‘small areas’ in Wales. 11 Each individual ‘small area’, known as a Lower‐layer Super Output Area (LSOA), is a Census geography with a population of approximately 1600 individuals. 11 IMD ranks each LSOA in Wales from 1 (most deprived) to 1909 (least deprived). The domains included in IMD are income, health, education, employment, housing, access to services, physical environment, and community safety. 11 The Welsh IMD data is typically updated every 3–5 years. We used the most recent IMD data, published in 2019, to determine IMD values for GP surgeries in the study. The IMD for each general practice was identified using the Welsh Government ‘Geography lookups’ spreadsheet, which was found on the Welsh Government website. Then, using each GP postcode from the QOF data, we identified a corresponding IMD value, which allows any postcode in Wales to be matched to a LSOA, and gives a corresponding IMD value.
2.4. Statistical analysis
To explore the potential association between expenditure and attaining metabolic target (proportion of patients with a HbA1c <59 mmol/mol) practices were divided into quadrants based on whether they were above or below the median cost per patient with diabetes (high prescribing cost/low prescribing cost) and whether they were above or below the median proportion of patients with a target HbA1c (high proportion meeting metabolic target/low proportion meeting metabolic target). Analysis was repeated with those individuals who did not have a HbA1c measure counted as a HbA1c > 59 mmol/mol.
We then undertook the analysis of the variation in percentage expenditure of each class of hypoglycaemic agent against the proportion of patients who attained a HbA1c <59 mmol/mol. We investigated the shape of the association between the percentage of total expenditure on each drug using ordinary least‐squares linear regression models with restricted cubic splines with five knots (quintiles). Cubic splines allow the models to capture the non‐linear relationship between expenditure and the proportion since we would expect increases at the lower end of the spending spectrum to yield great improvement in HbA1c, but these benefits might diminish at higher levels of spending. Five splines were used to accommodate this non‐linear relationship, but also to allow flexibility for floor and ceiling effects arising from using % which are capped at 0% and 100% by definition, but also by other thresholds arising from other drugs, for example, we would expect most general practices to have a baseline expenditure on insulin which limits the % which may be accounted for by other drugs. Analyses were adjusted for IMD and the number of patients in the practice with diabetes. IMD and amount spent per patient were standardised; these analyses are presented as per SD.
To compare the relative spending pattern between different oral hypoglycaemic agents by quadrants we used the Wilcoxon rank‐sum test. All statistical analyses were undertaken using STATA version 16 (STATACORP).
3. RESULTS
There was no significant difference between the 66 practices (13.9%) excluded and the final study dataset of 406 practices with regard to IMD (p = 0.13), number of patients with diabetes (p = 0.44) or total practice size (p = 0.87). Analysis of those practices missing expenditure data versus those with expenditure data also revealed no differences in HbA1c (p = 0.35) or IMD (p = 0.77) or a number of patients with diabetes mellitus (p = 0.82) or practice size (p = 0.54).
The vast majority of patients with diabetes at a general practice had a HbA1c measured in the previous year Median = 91.1% (IQR 84.9–95.2). Practices with a higher proportion of patients with a HbA1c measured had a lower ratio of HbA1c >59 mmoL/L B = −0.47 (95%CI –0.56, −0.39) p < 0.001. Areas of greater affluence as assessed by IMD had a tendency to have a higher ratio of patients with a HbA1c recorded B(std) = 0.10 (95%CI –0.002, 0.19) p = 0.06. There was no observed difference between the proportion of patients with a HbA1c measured and total hypoglycaemic agent expenditure (p = 0.15) or the number of patients with diabetes mellitus (p = 0.83) or practice size (p = 0.09).
In the final study dataset, the median number of patients with diabetes in a practice was 426 (IQR 288–561). The mean proportion of patients with a HbA1c measured having a target HbA1c (<59 mmol/mol) during the last year per practice was 0.65 (SD 0.08). There was considerable heterogeneity in the price spent per patient with diabetes, Median = £289 (IQR 247–343) range £31.1 ‐ £1713 (Figure 1, Figure S1). 8.1% of practices (n = 35) spent more than £500 per patient with diabetes however these practices represented 16.7% of total expenditure. 2.5% of practices spent more than £1000 and this resulted in 4.6% of total expenditure.
FIGURE 1.
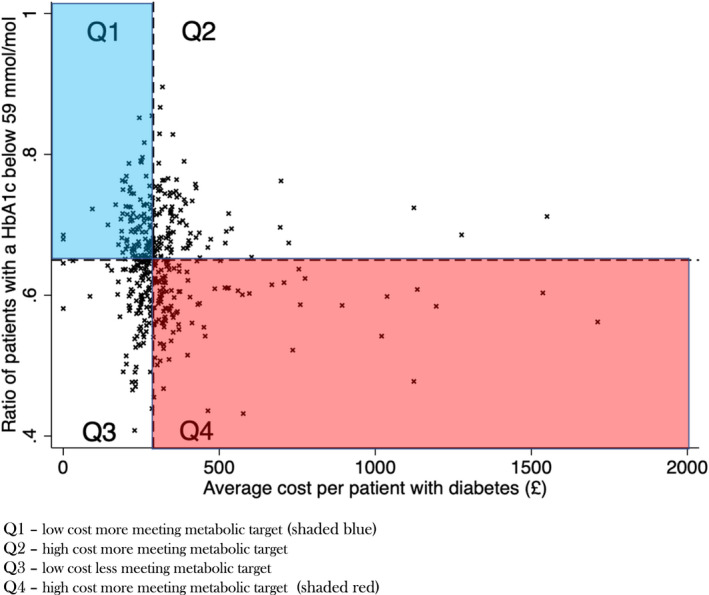
Scatter plot of proportion of patients with a HbA1c below 59 mmol/mol and average cost per patient with diabetes
Higher total expenditure per patient in general practice (across all seven drug types) was not associated with the proportion of patients attaining target HbA1c in the past 12 months B(std) = −0.08 (95%CI –0.17, 0.02) p = 0.13 which was similar after adjustment for IMD, number of patients with diabetes, practice size and proportion of patients with diabetes with a HbA1c measured B(std) = −0.07 (95%CI –0.15, 0.02) p = 0.13 (Figure 1). Practices with more patients with diabetes mellitus spent less per patient with diabetes; a one standard deviation increase in the number of patients with diabetes mellitus was associated with £31.6 lower expenditure per patient B(std) ‐31.6 (95%CI –49.5, −13.7) p < 0.001. No difference was seen in the proportion of patients having a target HbA1c in the past 12 months by the number of patients in the practice with diabetes B(std) 0.001 (95%CI –0.01, 0.01) p = 0.74. Areas with greater affluence as indicated by IMD did not spend substantially more per patient with diabetes B(std) = −8.65 (95%CI –26.9, 9.63) p = 0.35, but did have a higher proportion of patients meeting the target HbA1c. For one standard deviation increase in IMD, the ratio of patients achieving target Hba1c was 1% higher B(std) = 0.01 (95%CI 0.004, 0.02) p = 0.003. Areas with higher affluence had a lower proportion of patients in the practice with diabetes; those in the highest quintile of affluence had 5.4% of patients with diabetes versus 6.4% in the lowest quintile of affluence (p < 0.001). Repeat analysis of the percentage of total patients with diabetes with a HbA1c <59 mmol/mol (as opposed to those with a HbA1c measured) revealed no association between total cost and the proportion of patients attaining target HbA1c in the past 12 months B(std) = −0.06 (95%CI –0.21, 0.09) p = 0.45 after adjustment for IMD, number of patients with diabetes, practice size.
Biguanides (Metformin) represented the most numerous items prescribed (n = 1,366,399), followed by sulphonylureas (n = 495,417), insulin (n = 453,839), DPP‐IV inhibitors (n = 382,068), SGLT2 inhibitors (n = 314,522), GLP‐1 agonists (n = 83,199) and Thiazolidinediones (n = 49,100) (Table 1, Figure S2). Total costs, however, were very different. There was also widespread variation in percentage expenditure by general practice for the different drug classes (Table 1). Analysis of the total percentage expenditure for hypoglycaemic agents revealed that the majority of expenditure in individual GP practices is on insulin 38.4% (IQR 32.4–44.1) and also on DPPIV inhibitors 20.5% (IQR 15.8–24.6) (Table 1). Although the Biguanide class had the highest number of total prescriptions, as it is inexpensive it represented only 10.3% (IQR 8.8–12.0) of costs in GP surgeries, this was only slightly below SGLT2 inhibitors 11.9% (IQR 9.0–17.1) and GLP1—agonists 11.9% (IQR 9.5–15.5) (Table 1). Only a small percentage of expenditure was on Sulphonylureas 2.41% (IQR 1.7–3.1) despite their high number of prescriptions and Thiazolidinediones 0.3% (IQR 0.2–0.7) (Table 1).
TABLE 1.
Percentage of overall total cost for each agent, and comparison between Quadrant 1 (low cost, good outcome) and the other quadrants
| Agent | Number of items prescribed | Overall (% of total cost per practice) | Relationship to Quadrant 1 (low cost, good outcome) | p‐value | ||
|---|---|---|---|---|---|---|
| Median | IQR | |||||
| Overall | ||||||
| Insulin | 453,839 | 38.4 | 32.4–44.1 | |||
| DPP‐IV inhibitors | 382,068 | 20.5 | 15.8–24.6 | |||
| SGLT2 inhibitor | 314,522 | 11.9 | 9.0–17.1 | |||
| GLP‐1 agonist | 83,199 | 11.9 | 9.5–15.5 | |||
| Biguanide | 1,366,399 | 10.3 | 8.83–12.0 | |||
| Sulphonylurea | 495,417 | 2.41 | 1.7–3.13 | |||
| Thiazolidinediones | 49,100 | 0.3 | 0.16–0.65 | |||
| Quadrant 1 Low Cost, meeting metabolic target | ||||||
| Insulin | 41.2 | 37.2–45.6 | NA | |||
| DPP‐IV inhibitors | 20.6 | 16.9–23.9 | NA | |||
| SGLT2 inhibitor | 9.7 | 7.6–13.5 | NA | |||
| GLP‐1 agonist | 11.3 | 7.8–14.1 | NA | |||
| Biguanide | 11.4 | 9.8–13.0 | NA | |||
| Sulphonylurea | 3.0 | 2.3–3.7 | NA | |||
| Thiazolidinediones | 0.3 | 0.2–0.7 | NA | |||
| Quadrant 2 High Cost, meeting metabolic target | ||||||
| Insulin | 33.3 | 28.4–38.1 |
|
<0.001 | ||
| DPP‐IV inhibitors | 21.4 | 16.4–25.9 | 0.38 | |||
| SGLT2 inhibitor | 16 | 11.5–22.7 |
|
<0.001 | ||
| GLP‐1 agonist | 13.2 | 10.6–16.6 |
|
0.002 | ||
| Biguanide | 9.24 | 7.9–10.4 |
|
<0.001 | ||
| Sulphonylurea | 1.7 | 1.21–2.43 |
|
<0.001 | ||
| Thiazolidinediones | 0.4 | 0.2–0.7 | 0.42 | |||
|
Quadrant 3 Low Cost, not meeting metabolic target |
||||||
| Insulin | 43.2 | 39.0–47.6 | 0.13 | |||
| DPP‐IV inhibitors | 19.0 | 14.4–22.8 |
|
0.05 | ||
| SGLT2 inhibitor | 9.9 | 7.7–12.6 | 0.92 | |||
| GLP‐1 agonist | 11.3 | 8.7–15.5 | 0.40 | |||
| Biguanide | 11.7 | 10.3–12.7 | 0.62 | |||
| Sulphonylurea | 3.0 | 2.4–3.7 | 0.60 | |||
| Thiazolidinediones | 0.4 | 0.2–0.7 | 0.07 | |||
| Quadrant 4 High cost, not meeting metabolic target | ||||||
| Insulin | 35.8 | 28.7–40.4 |
|
<0.001 | ||
| DPP‐IV inhibitors | 21.0 | 15.8–25.4 | 0.94 | |||
| SGLT2 inhibitor | 14.1 | 10.9–23.1 |
|
<0.001 | ||
| GLP‐1 agonist | 11.5 | 8.1–15.3 | 0.49 | |||
| Biguanide | 9.26 | 6.98–10.6 |
|
<0.001 | ||
| Sulphonylurea | 2.1 | 1.4–2.6 |
|
<0.001 | ||
| Thiazolidinediones | 0.3 | 0.1–0.5 | 0.29 | |||
Practices were divided into quadrants based on whether they were above or below the median cost per patient with diabetes (high/low cost) and whether they were above or below the median proportion of patients with a target HbA1c (high proportion meeting metabolic target/low proportion meeting metabolic target): Quadrant 1 (low cost, high proportion meeting metabolic target), Quadrant 2 (high cost, high proportion meeting metabolic target), Quadrant 3 (low cost, low proportion meeting metabolic target) and Quadrant 4 (high cost, low proportion meeting metabolic target)—see Figure 1. The median spend and percentage of patients achieving the HbA1c target are summarised in Table 2. The distribution of spending per patient with diabetes between quadrants is shown in Figure 2a and the proportion of patients achieving a target HbA1c is shown in Figure 2b. Even within quadrants there was considerable variability; particularly at extremes with 6% of practices in Quadrant 4 spending more than £1000 per patient with diabetes more than 3 times the median (Figure 1, Figure S1).
TABLE 2.
Summary of median cost per patient with diabetes and the proportion of patients achieving target HbA1c (<59 mmol/mol)
| Quadrant | Median cost (£) | IQR | Proportion Hba1c less than 59 mmol/mol (SD) |
|---|---|---|---|
| 1 low cost more meeting metabolic target | 243 | 215–267 | 0.68 (0.05) |
| 2 high cost more meeting metabolic target | 344 | 314–395 | 0.69 (0.05) |
| 3 (low cost less meeting metabolic target) | 248 | 222–266 | 0.59 (0.05) |
| 4 (high cost less meeting metabolic target) | 340 | 312–450 | 0.58 (0.04) |
FIGURE 2.
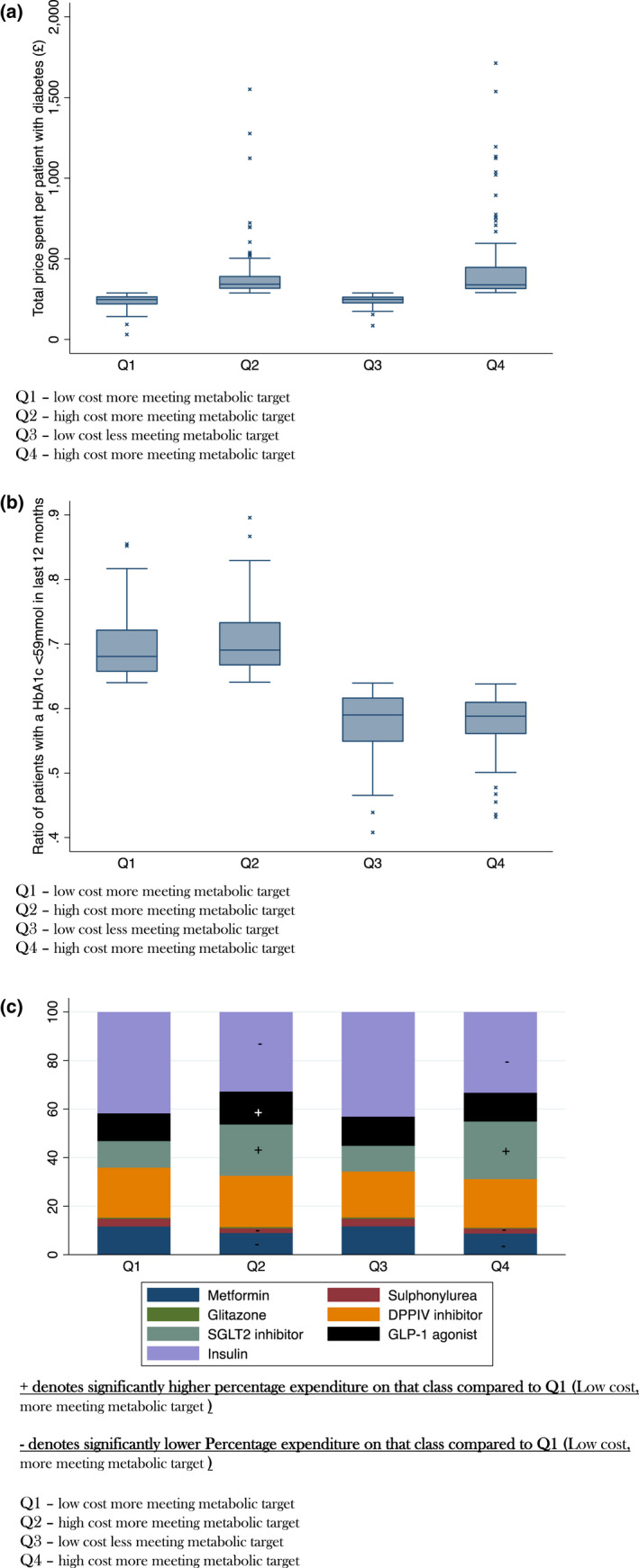
(a) Box Plot of Total cost per patient with a HbA1c by Quadrant. (b) Box Plot of proportion of patients with a HbA1c <59 mmol in the last 12 months by Quadrant. (c) Pie Chart of relative expenditure of hypoglycaemic agent class by Quadrant
Relative expenditure of each class of agent in each Quadrant was compared to Quadrant 1 (low cost, high proportion meeting metabolic target) (Table 1). Expenditure patterns in the two low‐cost quadrants (Q1, Q3) were remarkably similar and differed significantly from the two high‐cost quadrants (Q2 and Q4) (Table 1). Those in Q1 had higher relative expenditure on Biguanides 11.4% (IQR 9.8–23) versus 9.26% (IQR 6.98–10.6) p < 0.001 Sulphonylureas 3.0% (IQR 2.3–3.7) versus 2.1% (IQR 1.4–2.6) p < 0.001 and insulin 41.2% (IQR27.2–45.6) vs 35.8% (IQR28.7–40.4) in Q4 (high cost) (Table 1). In contrast, those in Q1 had lower relative expenditure on SGLT2‐inhibitors 9.7% (IQR 7.6–13.5) versus 14.1% (IQR 10.9–23.1) p < 0.001 (Table 1). No difference in relative expenditure was observed for the Thiazolidinediones (p = 0.29), DPP‐IV inhibitors (p = 0.94) or GLP‐1 agonists (p = 0.49). The relative percentage of expenditure for each drug class is shown in Figure 2c. We also observed differences in prescribing patterns between Quadrant 1 (low cost, high proportion meeting metabolic target), Quadrant 2 (high cost, high proportion meeting metabolic target), Those in Quadrant 1 had a high relative expenditure on Biguanides 11.4% (IQR 9.8–23) versus 9.24 (IQR 7.9–10.4) Insulin 41.2% (IQR27.2–45.6) versus 33.3 (IQR 28.4–38.1) and Sulphonylureas 3.0% (IQR 2.3–3.7) versus 1.7 (IQR 1.21–2.43) than Quadrant 2 and a lower expenditure on SGLT2 inhibitors 9.7% (IQR 7.6–13.5) versus 16% (IQR 11.5–22.7) and GLP‐1agonists 11.9% (IQR 9.5–15.5) versus 13.2% (10.6–16.6) (Table 1).
No clear differences were seen between Quadrant 1 (low cost, high‐proportion meeting metabolic target), and Quadrant 3 (low cost, low‐proportion meeting metabolic target) (Table 2). An analysis of practices with very high expenditure on diabetes medication (>£500 per patient – n = 35), showed a distinctive pattern dominated by particularly high usage of around 50% of SGLT2‐inhibitors, DPPIV inhibitors and GLP‐1 agonists (Figure 3. This is in stark contrast with other practices including those with higher than average prescribing costs (Figure 2c).
FIGURE 3.
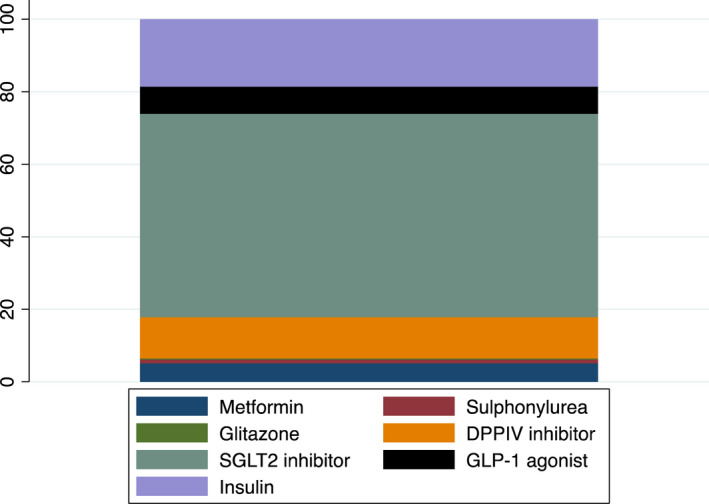
Practices spending more than £500 per patient – % expenditure of total
To illustrate the association between variation in percentage expenditure of each class of agent against the proportion of patients with a HbA1c <59 we used cubic spline plots (Figure 4). The curves identified that the best outcomes were generally observed between the second to fourth knots. Extremes of prescribing practice, both low and high were generally associated with lower HbA1c proportions with the exception of DPP‐IV inhibitors where a steady increase in expenditure on DPPIV inhibitors had a higher proportion of HbA1c < 59 mmol/mol (Figure 4) although further effects were modest and data relatively limited above 30% of expenditure. Importantly, a higher cost expenditure per patient with diabetes was not associated with a higher proportion of patients achieving a HbA1c of <59 mmol/mol in the preceding 12 months B(std) = −0.01 (95%CI –0.01, 0.002) p = 0.13 (Figure 5).
FIGURE 4.
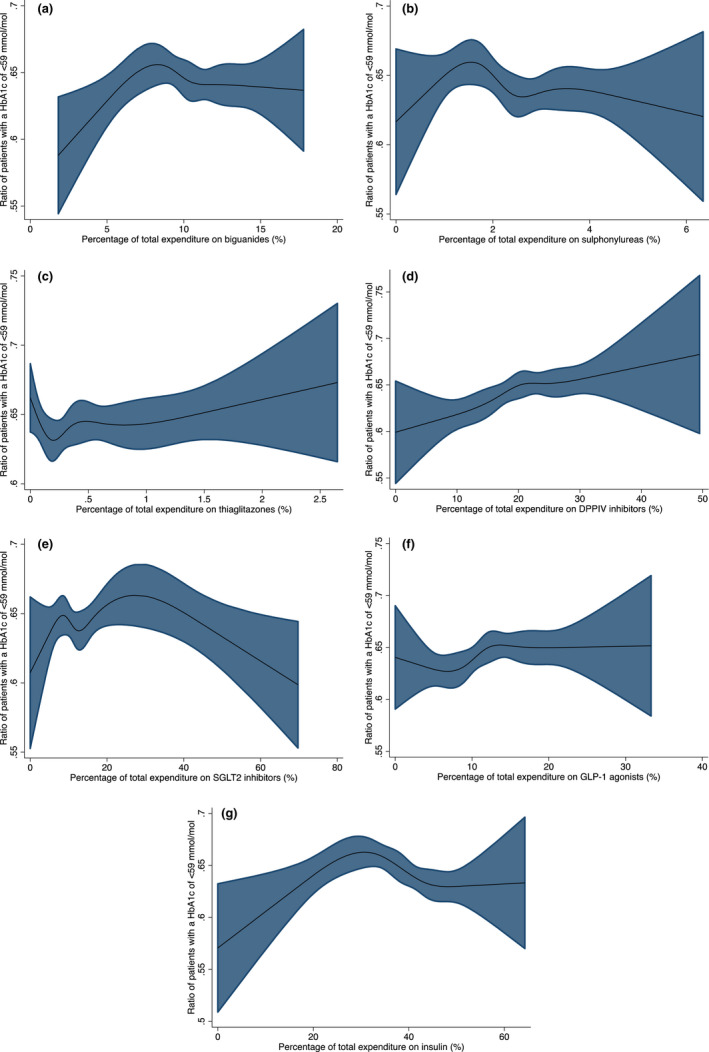
(a) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on biguanides. (b) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on sulphonylureas. (c) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on thiaglitazones. (d) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on DPPIV inhibitors. (e) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on SGLT2 inhibitors. (f) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on GLP‐1 agonists. (g) Proportion of Patients with a HbA1c <59 mmol/mol by the percentage of total expenditure on insulin
FIGURE 5.
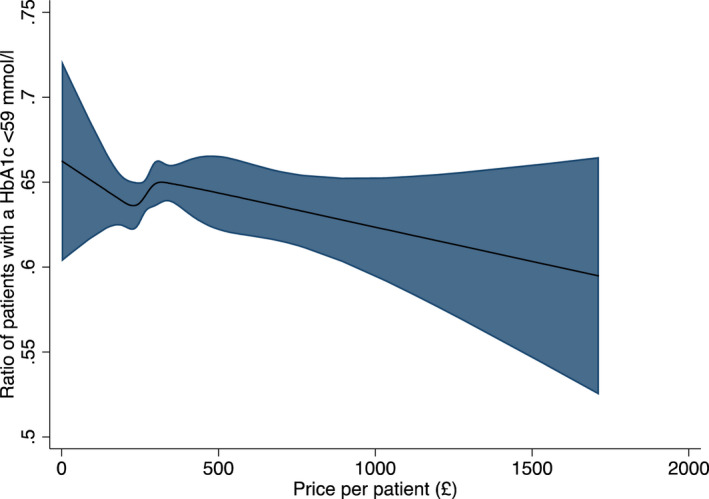
Cubic Splines analysis of the proportion of patients with a HbA1c <59 mmoL/L in the last 12 months against price per patient
4. DISCUSSION
In this nationwide study of primary care prescribing our results show there was no clear association at the practice level between spending per patient on hypoglycaemic agents and the proportion of patients attaining the last HbA1c less than 59 mmol/mol. There was also considerable variation in the prescribing costs per patient with diabetes between practices. Given that diabetes is such a common condition, and treatment is life‐long this variation in prescribing patterns within primary care has substantial cost implications at the population level.
Newer agents, particularly the SGLT2 inhibitors appear to have a substantial effect on prescribing costs (Figure 4). High‐cost practices had a higher relative expenditure on SGLT2 inhibitors (14.1–16%) compared to low‐cost practices (9.7–9.9%) p < 0.001 Given their attractive profile with regard to cardio‐renal protection, avoidance of hypoglycaemia and weight gain, prescriptions are likely to continue to increase substantially At present, there is still considerable use of the older medications; sulphonylureas currently represent the second most commonly prescribed agent (Table 1) so there is clear potential for costs to substantially rise if these current trends continue. Additional benefits were not assessed in this paper, and we have simply restricted our analysis to metabolic control.
Higher spending practices (Quadrants 2 and 4) spent almost £100 more a year per patient with diabetes. This difference equates to around £10–15 million per annum for the 210,000 patients with diabetes in Wales alone. 8.1% of practices spent more than £500 per patient with diabetes and these practices represented 16.7% of total expenditure. It is noteworthy that the drug‐spending profile of practices in low‐cost practices (Quadrants 1 and Quadrants 3) was similar regardless of whether patients were meeting metabolic target practices. Similarly, the drug‐spending profile of high‐cost practices (Quadrants 2 and Quadrants 4) was very similar regardless of whether patients were meeting metabolic target practices (Figure 2c). Thus, prescribing patterns for practices are similar for costs, but not for HbA1c outcomes. This raises the possibility that some individuals at least might have been poorly selected for these newer agents. This is consistent with the known considerable variation in the response of individual patients to different classes of hypoglycaemic agents. 12 For instance, patients may remain on ineffective drugs if not switched low cost, less meeting metabolic target, (Quadrant 3) or new drugs may be added without stopping ineffective ones or waiting to see the full effect of the initial therapies, or expensive drugs may have been continued despite a poor response (Quadrant 4). The patient‐level analysis would be required to resolve this which is beyond the scope of the current project.
Our modelling of the association between hypoglycaemic agent expenditure and metabolic benefit (Figures 4 and 5) showed that extremes of prescribing of any drug class, aside from the DPPIV inhibitors were associated with lower proportions of patients achieving a HbA1c < 59 mmol/mol in the preceding 12 months.
Taken together this suggests there is substantial cost‐saving potential in the management of type 2 diabetes. Deprescribing agents when they have not shown substantial benefit and reviewing/stopping some of the newer medications in patients where substantial benefits have not been observed could substantially reduce healthcare costs. Another potential cost‐saving strategy might be for higher spend practices to utilise more of the older and less expensive insulins if their insulin prescribing costs are particularly high at their practice.
Strengths of our analysis include the large population size (3 million people in Wales of which approximately 210,000 have diabetes), the large number of practices included (n = 426) and the limited bias introduced by using nationally collected data. However, our analysis is also subject to several caveats and limitations. Caution needs to be taken when interpreting our data, particularly with regard to the derived quadrants of hypoglycaemic agent costs and the proportion of patients with HbA1c less than or greater than 59 mmol/mol as these are observational in nature and the identified associations cannot be considered causal. It is therefore, possible that the observed variation in prescribing and HbA1c outcome is driven by variation in practice demographic profiles, including age, ethnic mix, obesity, socio‐economic status and the percentage of patients with type 1 diabetes rather than solely prescribing patterns.
For instance, general practices with more individuals with early diabetes (managed by diet and exercise or metformin alone) would bias our results by showing that low‐cost treatment is particularly effective at achieving a HbA1c of less than 59 mmol/mol as they would have many patients with good outcomes as measured by HbA1c at low cost and this would undermine the potential benefits observed with expenditure on other agents. Potentially greater screening for people with early diabetes in more affluent areas may also explain at least in part why areas of higher deprivation had fewer patients with a HbA1c of <59 mmol/mol. Another key limitation is that we cannot extrapolate from general practice data scores to the individual level without falling prey to the potential ecological fallacy. These influences are likely to be important as although the majority of patients in each practice had a HbA1c measured median of 91.1% (IQR 84.9–95.2), practices with a higher proportion of patients with HbA1c measured had a lower proportion of patients with a HbA1c >59 mmol/mol and local deprivation appeared to influence this. However, our sensitivity analysis where we assessed the effect of total expenditure in a practice against the proportion of patients of the total number with diabetes with a HbA1c ≤59 mmol/mol of the total population revealed similar results.
Additional limitations are that our analysis was undertaken at the practice level key data such as duration of diabetes for individuals was not available. Furthermore, we had insufficient data for analysis on 13.9% of practices. A small number of patients may have moved general practices and may appear more than once in our dataset. Our analysis is very dependent on individual drug costs, which will vary between regions and countries and there are additional indirect costs of blood glucose monitoring which are more likely to be used in patients on insulin and sulphonylureas which have not been taken into account here. However, it is uncommon to use regular glucose monitoring in individuals on sulphonylureas alone. Finally, our analysis did not distinguish between type‐1 and type‐2 diabetes, although the prescribing of oral hypoglycaemic drugs relates almost entirely to type‐2 diabetes.
In conclusion in this national analysis of primary care data in Wales, we have observed considerable heterogeneity in hypoglycaemic agent prescribing. In addition, the highest cost prescribing amongst practices was driven by substantially higher use of SGLT2 inhibitors (>50% vs. around 12.9% for the other practices). Substantial cost‐savings might be made with more considered prescribing, and consideration may need to be given to reducing the use of other costly agents that appear not to confer such additional benefits e.g. DPPIV inhibitors.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
Supporting information
Appendix S1
Taylor PN, Siah QZ, Marei O, et al. Prescribing costs of hypoglycaemic agents and associations with metabolic control in Wales; a national analysis of primary care data. Diabet Med. 2022;39:e14908. doi: 10.1111/dme.14908
Please send address for re‐prints: taylorpn@cardiff.ac.uk
REFERENCES
- 1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 2. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2018;41(12):2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stedman M, Lunt M, Livingston M, et al. The costs of drug prescriptions for diabetes in the NHS. The Lancet. 2019;393(10168):226‐227. [DOI] [PubMed] [Google Scholar]
- 5. Type 2 diabetes in adults: management, NICE guideline NG28. Published December 2015, last updated April 2017.
- 6. Curtis HJ, Dennis JM, Shields BM, et al. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes Metab. 2018;20(9):2159‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1):e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaccardi F, Khunti K, Marx N, Davies MJ. First‐line treatment for type 2 diabetes: is it too early to abandon metformin? Lancet. 2020;396(10264):1705‐1707. [DOI] [PubMed] [Google Scholar]
- 9. Keeping S, Deslandes PN, Haines KE, Routledge PA. Estimated versus observed expenditure associated with medicines recommended by the all Wales medicines strategy group. Pharmacoecon Open. 2019;3(3):343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forbes LJ, Marchand C, Doran T, Peckham S. The role of the quality and outcomes framework in the care of long‐term conditions: a systematic review. Br J Gen Pract. 2017;67(664):e775‐e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Welsh Index of Multiple Deprivation, 2020.
- 12. Dawed AY, Zhou K, Pearson ER. Pharmacogenetics in type 2 diabetes: influence on response to oral hypoglycemic agents. Pharmgenomics Pers Med. 2016;9:17‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


