Abstract
Abnormalities in type I procollagen genes (COL1A1 and COL1A2) are responsible for hereditary connective tissue disorders including osteogenesis imperfecta (OI), specific types of Ehlers‐Danlos syndrome (EDS), and COL1‐related overlapping disorder (C1ROD). C1ROD is a recently proposed disorder characterized by predominant EDS symptoms of joint and skin laxity and mild OI symptoms of bone fragility and blue sclera. Patients with C1ROD do not carry specific variants for COL1‐related EDS, including classical, vascular, cardiac‐valvular, and arthrochalasia types. We describe clinical and molecular findings of 23 Japanese patients with pathogenic or likely pathogenic variants of COL1A1 or COL1A2, who had either OI‐like or EDS‐like phenotypes. The final diagnoses were OI in 17 patients, classical EDS in one, and C1ROD in five. The OI group predominantly experienced recurrent bone fractures, and the EDS group primarily showed joint hypermobility and skin hyperextensibility, though various clinical and molecular overlaps between OI, COL1‐related EDS, and C1ROD as well as intrafamilial phenotypic variabilities were present. Notably, life‐threatening vascular complications (vascular dissections, arterial aneurysms, subarachnoidal hemorrhages) occurred in seven patients (41% of those aged >20 years) with OI or C1ROD. Careful lifelong surveillance and intervention regarding bone and vascular fragility could be required.
Keywords: COL1A1, COL1A2, COL1‐related overlap disorder, Ehlers‐Danlos syndrome, osteogenesis imperfecta
1. INTRODUCTION
Pathogenic variants in genes encoding two alpha chains of type I collagen (COL1A1 or COL1A2) are associated with several hereditary connective tissue disorders (HCTD), including autosomal dominant osteogenesis imperfecta (OI) type I − IV (MIM #166200, 166210, 259420, 166220) (Lim et al., 2017; Marini et al., 2017), postmenopausal osteoporosis (MIM #166710) (Grant et al., 1996), Caffey disease (MIM #114000) (Gensure et al., 2005), and four types of Ehlers‐Danlos syndrome (EDS): classical type (cEDS, MIM #130000), cardiac valvular type (cvEDS, MIM #225320), a subset of vascular type (vEDS, MIM #130050), and arthrochalasia type (aEDS, MIM #130060, 617821) (Brady et al., 2017; Malfait et al., 2017).
OI is characterized by bone fragility with multiple bone fractures, skeletal deformity, growth impairment, blue sclera, dentinogenesis imperfecta, late‐onset hearing loss, and normal intellectual development (Forlino & Marini, 2016). The clinical phenotypes of OI are highly variable, ranging from prenatally lethal with extremely severe bone deformities and multiple bone fractures to relatively asymptomatic with low bone mass and susceptibility to fractures. Approximately 85%–90% of OI cases show autosomal dominant inheritance caused by heterozygous pathogenic variants in COL1A1 or COL1A2 (Lim et al., 2017).
EDS is characterized by generalized joint hypermobility (GJH), skin hyperextensibility, and tissue fragility. cEDS is characterized by three major symptoms: joint hypermobility, skin hyperextensibility, and delayed wound healing. The majority of cEDS cases are caused by heterozygous pathogenic variants in COL5A1 or COL5A2, while a specific variant in COL1A1 (p.Arg312Cys) gives rise to cEDS with vascular fragility including rupture and dissection of medium‐sized arteries (Adham et al., 2020). vEDS is characterized by thin and translucent skin and tissue fragility of arteries and intestine; serious complications including vascular rupture and dissection as well as intestinal and uterine rupture occur in about 70% of patients. The main cause of vEDS is the presence of heterozygous pathogenic variants in a gene encoding type III procollagen (COL3A1); however, heterozygous pathogenic variants in COL1A1 (p.Arg312Cys; p.Arg574Cys; p.Arg1093Cys) are also associated with vEDS. aEDS is caused by heterozygous pathogenic variants that give rise to partial or complete loss of exon 6 in COL1A1 or COL1A2, leading to the inhibition of procollagen N‐terminal site cleavage and disruption of collagen fibril assembly and cross‐linking. Symptoms of aEDS include severe congenital GJH and bilateral hip dislocation, joint subluxation and dislocation, and hyperelastic or redundant skin. In addition to three major symptoms of cEDS, cvEDS is characterized by severe cardiac‐valvular defects, including aortic valve regurgitation, mitral valve prolapses, and subsequent left ventricular hypertrophy. Homozygous or compound heterozygous pathogenic variants in COL1A2 were found to be causal for cvEDS, which leads to the complete absence of pro‐alpha‐2 collagen chains and the generation of pro‐alpha‐1 homotrimers.
Recently, patients with mixed phenotypes of both OI and EDS have been found to harbor heterozygous pathogenic variants in COL1A1 or COL1A2 (Cabral et al., 2005; Malfait et al., 2013). In the series by Malfait et al. (2013), EDS‐related symptoms were more prominent than OI‐related symptoms in these patients. All variants were located near the N‐terminal helical region of the type I collagen alpha‐1 or alpha‐2 chain and were postulated to interfere with the cleavage of the procollagen N‐propeptide, which did not correspond to the variants of the other known COL1‐related types of EDS (cEDS, vEDS, aEDS, and cvEDS). Therefore, Malfait et al. (2013) proposed to name the condition “OI/EDS overlap syndrome.” Morlino et al. (2020) described another cohort of patients with similar features and suggested the term “COL1‐related overlap disorder (C1ROD),” considering a wide spectrum of bridging phenotypes between OI and EDS. Major criteria for molecular testing were proposed as follows: (1) blue sclera, (2) flat feet with valgus deformity of the hindfoot, (3) generalized joint hypermobility according to the age, and (4) significantly soft and doughy, and/or hyperextensible skin. Although many of the variants found in C1ROD were located in the N‐terminal helical region, several variants were located outside the region. Additional patients with C1ROD have been described (Budsamongkol et al., 2019; Foi et al., 2021; Gnoli et al., 2021).
The delineation of a wide clinical spectrum of C1ROD is insufficient because previously reported cohorts have recruited either patients with OI‐like phenotypes or those with EDS‐like phenotypes. Here, we report detailed and comprehensive clinical and molecular features of patients who met the criteria of C1ROD from a unique cohort, including both patients with OI‐like phenotypes and those with EDS‐like phenotypes. In addition, it is noteworthy that life‐threatening vascular events seemed to be associated with the condition.
2. MATERIALS AND METHODS
2.1. Ethical considerations
Blood samples and clinical information were collected after obtaining written informed consent from patients and/or their parents. This study was approved by the Ethics Committee at Shinshu University School of Medicine (Matsumoto, Japan) (#628, #4171).
2.2. Patient enrollment
Japanese patients who were clinically suspected to be patients with OI or EDS and were found to have pathogenic or likely pathogenic variants in COL1A1 or COL1A2 through our genetic investigation were enrolled. Clinical suspicion of OI was made based on the occurrence of recurrent bone fractures and/or family history of OI, and that of EDS was made based on the presence of joint hypermobility (indicated as Beighton score > 4 points) and skin hyperextensibility with or without bone fragility.
2.3. Genetic investigation
Genomic DNA was extracted from peripheral blood leukocytes of the patients using Gentra Puregene Blood Kit or QIAamp DNA Blood Mini Kit on QIAcube (Qiagen, Hilden, Germany). Next‐generation sequencing was performed on an Ion PGM™, Ion PGM™ Dx, or Ion GeneStudio™ S5 (Thermo Fisher Scientific, Waltham, MA, USA), using Ion AmpliSeq™ custom panels designed with Ion AmpliSeq™ Designer (https://www.ampliseq.com/) for 17 genes (version 1), 54 genes (version 2), 52 genes (version 3), 71 genes (version 4), and 52 genes (version 5) associated with HCTD, including COL1A1 and COL1A2 (Table S1). The sequencing data were mapped to human genome hg19 using Torrent Suite™ software (Thermo Fisher Scientific), and single‐nucleotide variants and small insertions/deletions were detected from the mapped data using the Torrent Suite™ plug‐in. The variants were annotated using SnpEff (http://pcingola.github.io/SnpEff/) (Cingolani et al., 2012). The candidate variant was confirmed by Sanger sequencing on an ABI 3130xl Genetic Analyzer using a BigDye™ Direct Cycle Sequencing Kit using M13 tailed primers and BigDye™ XTerminator Purification Kit (Thermo Fisher Scientific).
3. RESULTS
3.1. Clinical findings
Detailed and comprehensive clinical and molecular findings of the patients are shown in Table 1. Twenty‐three patients from 15 families aged 3–67 years were described: Eight were males (35%), and 15 were females (65%). Initial clinical suspicion was OI in 18 patients (#1 − #18; 78%) predominantly showing bone fragility and EDS in the remaining five patients (22%), who presented joint hypermobility and/or skin hyperextensibility: aEDS (patients #19, #20), cEDS (patient #21), vEDS (patient #22), and vEDS or OI (patient #23).
TABLE 1.
Clinical and molecular findings of 23 patients in this study
| Family | 1 | 2 | 3 | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 |
| Mother | Son | Mother | Daughter | Mother | Daughter | ||||
| Clinical suspicion | OI | OI | OI | OI | OI | OI | OI | OI | OI |
| Final diagnosis | OI type I | OI type I | OI type I | OI type I | OI unclassified | OI type I | OI type I | OI type I | OI unclassified |
| General | |||||||||
| Sex | F | F | F | M | F | F | F | F | M |
| Age (years) | 67 | 41 | 55 | 26 | 47 | 6 | 45 | 18 | 27 |
| Height (cm/SD) | 143/−0.6 | 160.8/+0.4 | 154.5/−0.5 | 173.5/+0.4 | 138.6/−3.7 | 111.4/−0.6 | 156.6/−0.3 | 157.5/0.0 | 152.8/−3.2 |
| Outcome | Deceased | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
| Skeletal | |||||||||
| Multiple bone fractures | + | + | + | + | + | + | + | + | + |
| Frequency of fractures | 8 | 10 | 7 | 6 | >50 | 2 | 2 | 6 | 5 |
| Position of fractures | LB, PT, FM, PB, FB | PH, EL, PF | FM, TB, SC, EL, PF | AK, EL, WR | FM, PH, PF, EL, PT | FM | SC, PF | SK, HM, EL, PH, KN | CX, HM, PT |
| Dental abnormality | − | − | + | − | − | − | NA | − | − |
| Joint hypermobility | NA | + | − | − | − | − | − | + | − |
| Beighton score | 6 | 2 | 4 | 2 | 8 | ||||
| Recurrent joint dislocation | + | − | − | + | − | − | − | + | + |
| Congenital hip dislocation | − | − | − | − | − | − | − | − | NA |
| Joint contracture | − | − | − | − | + | − | − | − | NA |
| Long bone deformity | − | − | − | − | + | − | − | − | + |
| Spinal deformity | + | + | − | + | − | − | − | + | + |
| Congenital clubfoot | − | − | − | − | − | − | − | − | NA |
| Flat feet | NA | + | + | − | + | + | + | + | + |
| Ruptures of tendon, ligament, or muscle | − | − | − | − | − | − | + | + | + |
| Intramuscular bleeding | NA | − | − | − | − | − | − | − | − |
| Joint pain | NA | NA | − | − | NA | NA | NA | + | NA |
| DXA(vertebral:g/cm2) | 0.548 | 1.132 | 0.743 | 0.961 | 0.547 | 0.432 | 0.645 | 1.145 | 0.936 |
| T‐score | −4.8 | −0.2 | −2.9 | −1.0 | −4.8 | − | 82 (%YAM) | − | −2.1 |
| Z‐score | −3.4 | −0.2 | −2.2 | −1.0 | −4.7 | − | 0 | −2.1 | |
| Skin | |||||||||
| Hyperextensibility | NA | NA | − | − | − | − | + | − | − |
| Fragility | NA | NA | − | − | + | + | + | + | − |
| Atrophic scars | NA | NA | − | − | − | − | − | − | − |
| Translucency | NA | + | − | − | NA | NA | + | − | − |
| Soft doughy skin | NA | NA | + | − | + | + | NA | NA | − |
| Piezogenic papules | NA | NA | NA | NA | − | − | + | − | NA |
| Easy bruising | NA | NA | − | − | − | − | − | − | + |
| Eye and ear | |||||||||
| Blue sclerae | + | + | + | + | + | + | + | + | + |
| Retinal detachment | NA | − | − | − | − | − | − | − | − |
| Refractive errors | NA | Myopia | Myopia | Myopia | − | − | Myopia | Myopia | + |
| Hearing impairment | + | + | + | − | + | − | + | + | − |
| Cardiovascular | |||||||||
| Vascular dissection | Ascending aorta | − | − | − | − | NA | − | NA | − |
| Arterial aneurysm | Lt. MCA | Bil. ICA | Rt. ICA | − | − | NA | Rt. MCA | NA | − |
| Cardiac valve disorder | AR | − | − | NA | − | NA | − | NA | MR, TR |
| Renal disease | PKD | URA | NA | NA | NA | NA | NA | NA | − |
| Hypertension | + | − | − | NA | − | − | + | − | NA |
| Others | SAH due to MCA rupture | SAH due to ICA rupture | SAH due to ICA rupture | Coronary‐pulmonary artery fistula | |||||
| Variant | |||||||||
| COL1A1(PV: OI) | COL1A1(PV: OI) | COL1A1(PV: OI, C1ROD) | COL1A1(PV: OI) | COL1A1(UV) | COL1A1(UV) | ||||
| c.779G > A | c.2829 + 1G > A | c.1243C > T | c.769G > A | c.1679del | c.572G > T | ||||
| p.Gly260Asp | p.Arg415* | p.Gly257Arg | p.Gly560Valfs*20 | p.Gly191Val | |||||
| Exon 11 | Intron 39 | Exon 19 | Exon 11 | Exon 25 | Exon 7 | ||||
| Family | 7 | 8 | 9 | 10 | 11 | |||
|---|---|---|---|---|---|---|---|---|
| Patient | #10 | #11 | #12 | #13 | #14 | #15 | #16 | #17 |
| Mother | Daughter | Elder brother | Younger brother | Father | Daughter | |||
| Clinical suspicion | OI | OI | OI | OI | OI | OI | OI | OI |
| Final diagnosis | OI type I | OI unclassified | OI unclassified | OI type I | OI type I | OI type I | OI type I | OI type I |
| General | ||||||||
| Sex | F | F | F | F | M | F | M | F |
| Age (years) | 37 | 3 | 26 | 14 | 16 | 9 | 41 | 8 |
| Height (cm/SD) | 151/−1.2 | 86.3/−1.7 | 133.9/−4.3 | 148.4/−1.5 | 150.3/−3.3 | 122/−1.8 | 168.5/−0.5 | 124.8/−1.8 |
| Outcome | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
| Skeletal | ||||||||
| Multiple bone fractures | + | − | + | + | + | + | + | − |
| Frequency of fractures | 5 | − | 12 | 5 | 5 | 2 | 5 | − |
| Position of fractures | RD, UL, FB | − | FM | TB, FB, EL, RD | TB, RD, UL, CB, PF | TB, HM | FM, PF | − |
| Dental abnormality | + | − | + | − | − | − | + | + |
| Joint hypermobility | − | − | + | + | − | − | − | − |
| Beighton score | 7 | 7 | 4 | 4 | ||||
| Recurrent joint dislocation | − | − | − | − | − | − | − | − |
| Congenital hip dislocation | − | − | − | − | − | − | − | − |
| Joint contracture | − | − | + | − | − | − | − | − |
| Long bone deformity | − | + | + | − | − | − | − | − |
| Spinal deformity | − | − | + | − | − | − | − | + |
| Congenital clubfoot | − | − | − | − | − | − | − | − |
| Flat feet | − | − | + | + | − | − | − | − |
| Ruptures of tendon, ligament, or muscle | − | − | − | − | − | − | − | − |
| Intramuscular bleeding | − | − | − | − | − | − | − | − |
| Joint pain | − | − | NA | − | − | − | − | − |
| DXA(vertebral:g/cm2) | NA | NA | 0.886 | 0.864 | 0.837 | 0.716 | 0.981 | 0.55 |
| T‐score | −1.9 | 80 (%YAM) | − | − | −0.9 | − | ||
| Z‐score | −1.5 | −1.7 | 0 | 0 | −0.9 | −1.5 | ||
| Skin | ||||||||
| Hyperextensibility | − | − | − | + | − | − | − | − |
| Fragility | − | − | − | − | − | − | − | − |
| Atrophic scars | − | − | − | − | − | − | − | − |
| Translucency | − | − | − | − | − | − | − | − |
| Soft doughy skin | − | − | − | + | − | − | − | − |
| Piezogenic papules | − | − | NA | NA | − | − | + | − |
| Easy bruising | + | − | − | − | − | − | − | − |
| Eye and ear | ||||||||
| Blue sclerae | + | + | + | + | + | + | + | + |
| Retinal detachment | − | − | NA | − | − | − | − | − |
| Refractive errors | − | − | Myopia | − | − | − | Myopia | NA |
| Hearing impairment | − | − | − | − | − | − | NA | − |
| Cardiovascular | ||||||||
| Vascular dissection | − | − | − | NA | − | − | − | NA |
| Arterial aneurysm | − | − | − | NA | − | − | − | NA |
| Cardiac valve disorder | − | − | − | NA | − | − | − | NA |
| Renal disease | − | − | NA | NA | − | − | Rt. renal cyst | NA |
| Hypertension | − | − | − | − | − | − | − | NA |
| Others | ||||||||
| Variant | ||||||||
| COL1A1(UV) | COL1A2(UV) | COL1A2(PV: OI) | COL1A2(PV: OI) | COL1A1(PV: OI) | ||||
| c.559del | c.1963G > C | c.693 + 1G > A | c.2314G > A | c.2362G > A | ||||
| p.Arg187Valfs*78 | p.Gly655Arg | p.Gly772Ser | p.Gly788Ser | |||||
| Exon 7 | Exon 32 | Intron 14 | Exon 38 | Exon 34 | ||||
| Family | 12 | 13 | 14 | 15 | ||
|---|---|---|---|---|---|---|
| Patient | #18 | #19 | #20 | #21 | #22 | #23 |
| Father | Daughter | Son | ||||
| Clinical suspicion | OI | aEDS | aEDS | cEDS | vEDS | vEDS or OI |
| Final diagnosis | C1ROD | C1ROD | C1ROD | cEDS | C1ROD | C1ROD |
| General | ||||||
| Sex | M | F | M | M | M | F |
| Age (years) | 34 | 4 | 2 | 18 | 47 | 45 |
| Height (cm/SD) | 163.2/−1.5 | 99.7/−0.9 | 85.2/0.0 | 143.8/−1.5 | 173/−0.1 | 135.5/−4.0 |
| Outcome | Alive | Alive | Alive | Alive | Alive | Alive |
| Skeletal | ||||||
| Multiple bone fractures | + | − | − | − | + | − |
| Frequency of fractures | 6 | − | − | − | 2 | 1 |
| Position of fractures | NA | − | − | − | EL | TB |
| Dental abnormality | − | − | − | − | − | − |
| Joint hypermobility | + | + | + | + | + | + |
| Beighton score | 9 | 9 | 8 | 6 | NA | NA |
| Recurrent joint dislocation | − | − | − | − | − | + |
| Congenital hip dislocation | − | + | − | − | − | − |
| Joint contracture | − | − | − | − | − | − |
| Long bone deformity | − | − | − | − | + | NA |
| Spinal deformity | − | + | NA | − | − | − |
| Congenital clubfoot | − | − | − | − | − | + |
| Flat feet | + | + | + | − | − | − |
| Ruptures of tendon, ligament, or muscle | − | − | − | − | − | − |
| Intramuscular bleeding | − | − | − | − | − | − |
| Joint pain | − | − | − | − | − | − |
| DXA(vertebral:g/cm2) | 1.048 | NA | NA | NA | 0.787 | 0.623 |
| T‐score | −0.5 | −0.8 | −3.5 | |||
| Z‐score | −0.5 | −0.8 | ||||
| Skin | ||||||
| Hyperextensibility | + | + | + | − | + | − |
| Fragility | − | − | − | − | + | + |
| Atrophic scars | − | − | − | + | − | − |
| Translucency | − | − | − | − | + | + |
| Soft doughy skin | − | + | + | + | + | + |
| Piezogenic papules | NA | + | + | − | NA | NA |
| Easy bruising | + | + | + | + | − | + |
| Eye and ear | ||||||
| Blue sclerae | + | + | + | − | + | + |
| Retinal detachment | − | − | NA | − | − | − |
| Refractive errors | Myopia | Myopia, astigmatism | NA | − | − | − |
| Hearing impairment | NA | − | − | − | − | NA |
| Cardiovascular | ||||||
| Vascular dissection | − | − | − | − | Bil. ICA | Bil. VA |
| Arterial aneurysm | − | − | − | − | − | AAA |
| Cardiac valve disorder | NA | − | − | − | − | − |
| Renal disease | NA | − | − | − | − | Lt. renal AVM |
| Hypertension | NA | NA | NA | − | − | NA |
| Others | Cerebral infarction | Lateral medullary infarction | ||||
| Variant | ||||||
| COL1A2(PV: OI, C1ROD) | COL1A1(PV: cEDS) | COL1A1(PV: OI) | COL1A1(UV) | |||
| c.432 + 4_432 + 7del | c.934C > T | c.658C > T | c.571G > T | |||
| p.Arg312Cys | p.Arg220* | p.Gly191Cys | ||||
| Intron 9 | Intron 14 | Exon 9 | Exon 7 | |||
Abbreviations: +, present; −, absent; AAA, abdominal aortic aneurysm; AK, ankle; AR, aortic valve regurgitation; AVM, arteriovenous malformation; Bil., bilateral; C1ROD, COL1‐related overlap disorder; CB, clavicle bone; CX, coxal bone; EL, elbow; F, female; FB, fibula; FM, femur; HM, humerus; ICA, inner carotid artery; KN, knee; LB, lumbar spine; Lt., left; M, male; MCA, middle cerebral artery; MR, mitral valve regurgitation; NA, not available; PB, pubis; PF, phalanx of foot; PH, phalanx of hand; PKD, polycystic kidney disease; PT, patella; PV, previously published variant; RD, radius; Rt., right; SAH, subarachnoidal hemorrhage; SC, scapula; SK, skull; TB, tibia; TR, tricuspid valve regurgitation; UL, ulna; URA, unilateral renal aplasia; UV, unpublished variant; VA, vertebral artery; WR, wrist; YAM, young adult mean.
In family #12, patient #19 was first clinically suspected to have aEDS based on two major criteria (congenital bilateral hip dislocation and skin hyperextensibility) plus GJH (without multiple dislocation/subluxation) and three minor criteria (kyphoscoliosis, atrophic scars, and easy bleeding). Later, her younger brother, patient #20, was also suspected of having aEDS based on symptoms similar to patient #19 including skin hyperextensibility, GJH (without multiple dislocation/subluxation), atrophic scars, and easy bleeding though he did not have congenital hip dislocation. However, her father, patient #18, was suspected of having OI type I because his main medical condition included multiple bone fractures. He had blue sclerae while also showing GJH and skin hyperextensibility.
Patient #21 was suspected of having cEDS based on two major criteria (skin hyperextensibility and atrophic scars, GJH) plus two minor criteria (easy bleeding and soft and doughy texture). Patient #22 was suspected of having vEDS based on an episode of internal carotid artery dissection and skin translucency. Though patient #23 was suspected of having vEDS based on an episode of vertebral artery dissection and congenital clubfeet, she also presented OI‐related symptoms (significant reduction of bone mineral density with an episode of bone fracture and blue sclera) and joint and skin features (joint hypermobility, easy bleeding, and soft and doughy texture) not typical for vEDS.
The final diagnosis was classical OI in 17 patients (#1 − #17; 74%), classified into “the OI group.” The final diagnoses were C1ROD in five patients (#18, #19, #20, #22, and #23; 22%) and COL1‐related cEDS in one patient (#21; 4%), both classified into “the EDS group.” All five patients with C1ROD met the criteria for submitting molecular testing to the diagnosis, as proposed by Morlino et al. (2020). Thirteen patients in the OI group were subclassified as having OI type I without long bone deformity and with blue sclerae. The remaining four patients (#5, #9, #11, and #12) were considered unclassified because they had both long bone deformities (also with short stature <2.0 SD in adult cases) and blue sclerae. The median height SD score was −1.2 in both groups, with the heights of five patients including one in the EDS group, below −2.0 SD. Only one patient in the OI group (#1) deceased due to vascular complications as mentioned below.
3.2. Molecular findings
Fifteen pathogenic or likely pathogenic variants were identified: 11 variants were in COL1A1 (NM_000088.3) (Figure 1a), and the remaining four were in COL1A2 (NM_000089.3) (Figure 1b). All variants were located within the triple helical domain of COL1A1 or COL1A2.
FIGURE 1.
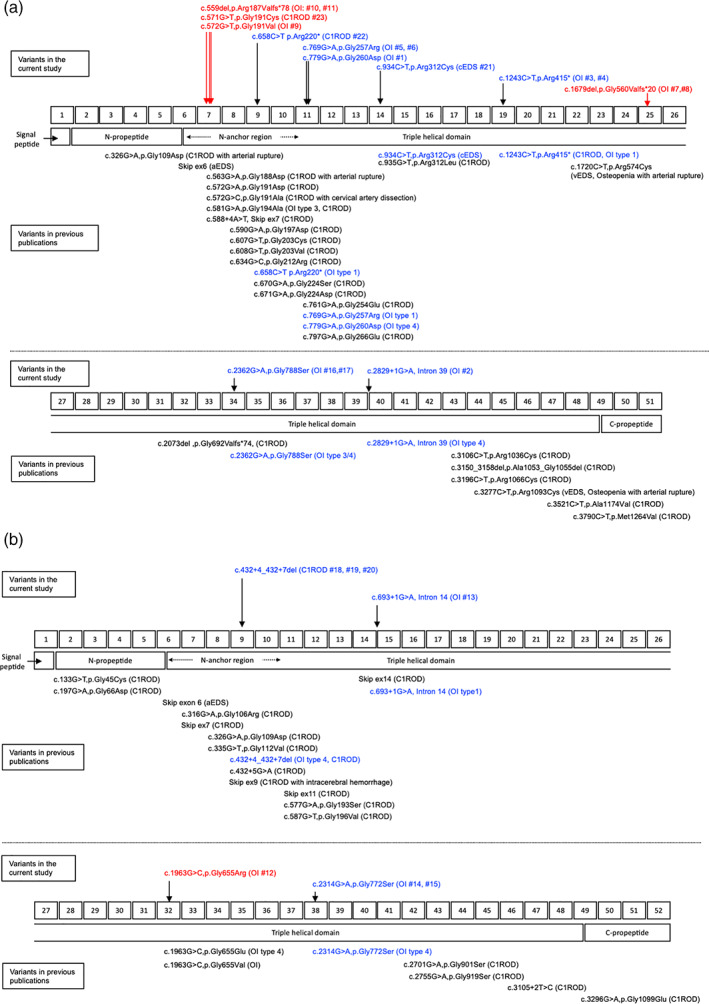
Schematic structure, location of domain organizations, and distribution of the mutations in COL1A1 (a) and COL1A2 (b). The numbers in the upper center rectangles indicate the exon numbers, and the rectangles in the lower center indicate the domain organizations of the proteins. Variants found in the current study are shown above the exon rectangles; unpublished variants are displayed in red, and previously published variants are displayed in blue. Variants in previous publications are shown below the domain rectangles. Variants both identified in the current study and previous publications are displayed in blue. aEDS, Ehlers‐Danlos syndrome arthrochalasia type; C1ROD, COL1‐related overlap disorder; OI, osteogenesis imperfecta; cEDS Ehlers‐Danlos syndrome classical type; vEDS, Ehlers‐Danlos syndrome vascular type
Among the COL1A1 variants, six were missense variants, four were nonsense or frameshift variants that resulted in premature stop codons, and one was a splice‐site variant. The final diagnoses of relevant patients were as follows: C1ROD in patient #22 with a nonsense variant (p.Arg220*) and patient #23 with a missense variant (p.Gly191Cys); cEDS in patient #21 with a missense variant (p.Arg312Cys) and OI in the remaining 13 patients (eight families) with four missense variants (p.Gly191Val, p.Gly257Arg, p.Gly260Asp, and p.Gly788Ser), three nonsense or frameshift variants (p.Arg187Valfs*78, p.Arg415*, and p.Gly560Valfs*20), or one splice‐site variant (c.2829 + 1G > A). Two missense variants (p.Gly191Cys and p.Gly191Val) and two frameshift variants (p.Arg187Valfs*78 and p.Gly560Valfs*20) were novel. According to the 2015 American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) guidelines (Richards et al., 2015), “p.Gly560Valfs*20” was classified as pathogenic and “p.Gly191Cys,” “p.Gly191Val,” and “p.Arg187Valfs*78” were classified as likely pathogenic. “p.Arg220*” associated with C1ROD (patient #22) was previously reported in a patient with OI type I (Körkkö et al., 1998), and “p.Arg415*” associated with OI (patient #3, #4) was previously reported in patients with OI type I or C1ROD (Morlino et al., 2020; Willing et al., 1996).
Among the COL1A2 variants, two were missense variants (p.Gly655Arg and p.Gly772Ser), one was a splice‐site variant (c.693 + 1G > A), and the remaining was a small deletion (c.432 + 4_432 + 7del). “p.Gly655Arg” was a novel variant, classified as pathogenic, according to the ACMG/AMP guidelines (Richards et al., 2015), and the others were reported previously. The final diagnoses of relevant patients were OI in patients with “p.Gly655Arg,” “p.Gly772Ser,” or “c.693 + 1G > A.” A small deletion variant (c.432 + 4_432 + 7del) was found in a family with a final diagnosis as C1ROD, including a father suspected of having OI (patient #18) and two children suspected of having aEDS (patients #19, #20). The variant was previously reported in patients with OI or C1ROD (Malfait et al., 2013; Marini et al., 2007).
3.3. Vascular complications
A total of ten vascular complications, including arterial dissections, vascular aneurysms, and/or arterial–arterial/arterial–venous fistulas, were identified in seven patients, 41% of those aged >20 years: five patients with the final diagnosis as OI type I (patients #1, #2, #3, #7, and #9) and two patients with C1ROD(patients #22, #23). All the seven patients had pathogenic or likely pathogenic variants in COL1A1: Three were missense variants (patient #1, p.Gly260Asp; patient #9, p.Gly191Val; and patient #23, p.Gly191Cys), three were nonsense/frameshift variants (patient #3, p.Arg415*; patient #7, p.Gly560Valfs*20; and patient #22, p.Arg220*), and one was a splice‐site variant (patient #2, c.2829 + 1G > A).
Vascular complications included a left middle cerebral artery aneurysm (Figure 2a), resulting in a left temporal and occipital lobe subarachnoid hemorrhage (SAH) (Figure 2b), and an acute aortic dissection (Figure 2c−e) in patient #1; SAH due to a ruptured internal carotid artery aneurysm in patients #2 and #3; an unruptured aneurysm of the right middle cerebral artery (Figure 2f) in patient #7; a coronary to pulmonary artery fistula in patient #9; bilateral internal carotid artery dissections (Figure 2g−j) in patient #22; and bilateral vertebral artery dissections, two saccular abdominal aortic aneurysms, and an aneurysmal‐type arteriovenous malformation in the left kidney in patient #23. Detailed clinical courses of patients #1, #2, #3, #7, #22, and #23 are provided in the Supplemental Information.
FIGURE 2.
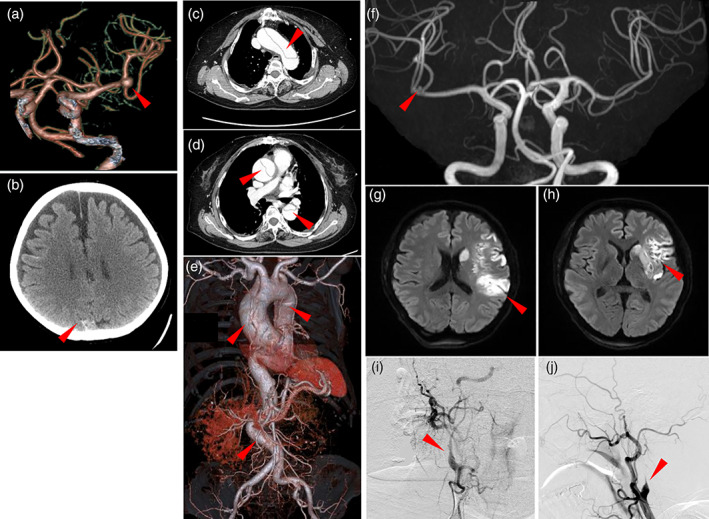
Radiographic findings of three patients with vascular complications. (a−e, patient #1) A brain enhanced computed tomography (CT)‐based angiography (a) shows a left inner carotid artery aneurysm (arrowhead). A brain CT reveals an occipital subarachnoid hemorrhage (b). Chest CT images (c, d) and chest to abdominal CT‐based angiography (e) show an extensive aortic dissection from the ascending aorta, aortic arch, and descending thoracic to abdominal aorta (arrowheads). (f, patient #7) A magnetic resonance imaging (MR)‐based angiography shows a right inner carotid artery aneurysm of 2 mm in diameter (f). (g−j, patient #23) A diffusion‐weighed brain MR imaging (g, h) shows a high intensity area in the left middle cerebral artery region. A digital subtraction angiography shows stenotic lesions (arrowheads) of the right (i) and left (j) inner carotid arteries
3.4. Histopathological investigation
Transmission electron microscopy (TEM)‐based histopathological evaluation of the skin specimens was performed on patients #22 (Figure 3a) and #23 (Figure 3b). Abnormal cauliflower‐like collagen fibrils with irregular margins were occasionally observed in both patients, similar to previous reports regarding C1ROD (Cabral et al., 2007; Malfait et al., 2013; Morlino et al., 2020).
FIGURE 3.
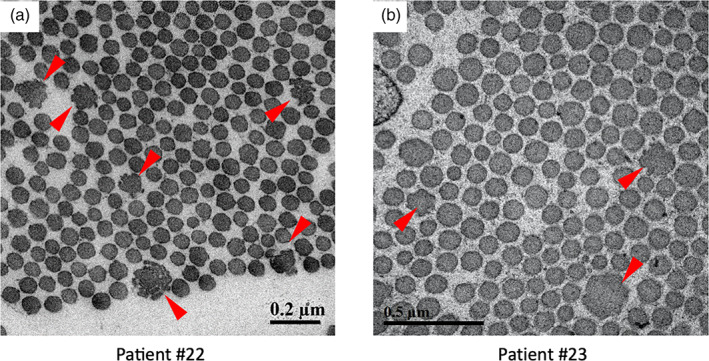
Transmission electron microscopic findings of skin specimens from patient #22 (a) and #23 (b). Occasional abnormal cauliflower‐like collagen fibrils with irregular margins (arrowheads) are noted in both images
4. DISCUSSION
We have presented clinical and molecular features of 23 patients from 15 families with pathogenic or likely pathogenic variants in COL1A1 or COL1A2, recruited based either on OI‐like or EDS‐like phenotypes. Whereas the initial clinical suspicion was OI, aEDS, cEDS, or vEDS, the final diagnoses were OI (type I or unclassified), COL1‐related cEDS, and C1ROD according to clinical and molecular findings. Phenotypic and molecular overlaps among patients in the OI group (patients #1 − #17) and those in the EDS group (patients #18 − #23), as well as intra‐familiar phenotypic variabilities, were noted. Life‐threatening vascular complications including arterial dissections and aneurysms occurred in both groups.
Patients in the OI group tended to show similar skeletal features to those in the EDS group besides recurrent bone fractures. Patients in the EDS group tended to show markedly higher frequencies in skin hyperextensibility, soft and doughy skin, and piezogenic papules than those in the OI group. Intrafamilial phenotypic variabilities included a presumably age‐dependent severity in a family in the OI group (family #4) and an age‐independent one in a family in the EDS group (family #12). In addition, patient #19 had a bilateral hip dislocation, a characteristic finding of aEDS, which suggests a phenotypical overlap between C1ROD and aEDS, as described by Morlino et al. (2020).
Regarding the variants identified both in the current study and in previous publications, six were found only in patients with OI (COL1A1: p.Gly257Arg, p.Gly260Asp, p.Gly788Ser, c.2829 + 1G > A, COL1A2: c.693 + 1G > A, and p.Gly772Ser), three in both OI and C1ROD (COL1A1: p.Arg220*, p.Arg415*, and COL1A2: c.432 + 4_432 + 7del), and one in cEDS (COL1A1: p.Arg312Cys). Pathogenic or likely pathogenic variants of patients with C1ROD included a missense (p.Gly191Cys) (patient #23) and a nonsense (p.Arg220*) (patient #22) variants in COL1A1 and a small deletion variant (c.432 + 4_432 + 7del) in COL1A2 (patients #18, #19, #20), all of which were located within the first 85 N‐terminal residues of the type I collagen helical domain. This region functions as the N‐terminal anchor for the stabilization and adequate folding of the collagen triple helix. Pathogenic variants in this lesion give rise to the conformational change of the procollagen N‐proteinase cleavage site and inhibition of normal N‐propeptide processing, leading to the decreased collagen fibril diameter and strength, ultimately resulting in the development of both OI and EDS overlap phenotypes (Cabral et al., 2005; Makareeva et al., 2006; Malfait et al., 2013). According to the current study and previous reports, most of the variants located in the N‐terminal propeptide or near the N‐terminal helical region (upstream of exon 14) were glycine substitution missense or exon skipping variants except for a nonsense (p.Arg220*) and arginine substitution missense variants. On the other hand, variants downstream of exon 14 included glycine and nonglycine substitution missense, nonsense, frameshift, and in‐frame deletion variants (Budsamongkol et al., 2019; Foi et al., 2021; Gnoli et al., 2021; Morlino et al., 2020). In view of these findings, a genotype–phenotype correlation underlying C1ROD seemed weaker than that in COL1‐related EDS. Multiple intralocus, extralocus, or epigenetic factors as well as nongenetic modifiers might contribute to the prominent EDS phenotype as the key features of C1ROD.
In the current study, four patients in the OI group and two in the EDS group suffered significant vascular complications (vascular dissection, arterial aneurysm, and subarachnoidal hemorrhage). Especially three patients with OI (patients #1, #2, and #3) developed SAH, and one (patient #1) developed aortic dissection leading to death. Vascular complications have been described as a rare but serious life‐threatening event in COL1‐related OI (Balasubramanian et al., 2019; Gaberel et al., 2016) and C1ROD (Feshchenko et al., 1998; Malfait et al., 2013; Mayer et al., 1996). Type I collagen, highly expressed in the myocardium, heart valves, chorda tendinea, and arterial walls, plays key role in maintaining the structural integrity and tensile strength of arterial walls (Folkestad et al., 2016; Vouyouka et al., 2001). Mimata et al. (1997) reported that type I and type III collagens are diffusely and homogeneously distributed in the luminal and abluminal layers in the cerebral aneurysmal wall. Etminan et al. (2014) revealed that structural remodeling of type I collagen is accelerated in a cerebral aneurysm, which contributes to the formation and progression of cerebral aneurysm. Moreover, McNeeley et al. (2012) revealed the histological change of cystic medial degeneration in the dissected aortic wall tissue of a patient with OI. Therefore, both COL1‐related OI and C1ROD, caused by defects in type I collagen biosynthesis, are likely to develop serious vascular complications. It remains challenging to explain the underlying pathology of the differences between the phenotypic severity and the prevalence of life‐threatening vascular complications; however, two independent contributing factors have been reported to affect the formation of cerebral aneurysms. Yoneyama et al. (2004) described that COL1A2 rs42524 single‐nucleotide polymorphism (SNP) in the triple‐helical domain was strongly related to the occurrence of cerebral aneurysms in a Japanese cohort; however, the SNP was not found in the current cohort. Moreover, Perrone et al. (2015) suggested autosomal dominant polycystic kidney disease (ADPKD) to be associated with an increased risk of cerebral aneurysm and aneurysmal SAH. The formation of cerebral aneurysms in patient #1 may have been derived and accelerated not only by a vascular fragility associated with the defect of type I collagen but also by ADPKD. An unruptured middle cerebral artery aneurysm detected in patient #7 could have been coincidental. In the general adult population cohort, the prevalence of unruptured intracranial aneurysms was 0.5%–3%, with a male‐to‐female ratio of 1:3 (Brown & Broderick, 2014). In contrast, in the current cohort, four of 13 adult patients (31%) were complicated by intracranial aneurysms. It is difficult to prove whether aneurysms developed due to the presence of the COL1A1 frameshift variant; however, given the high prevalence of the intracranial aneurysm in the current small cohort, we consider the occurrence of vascular events to be related to the pathogenic variants of COL1A1 or COL1A2.
There is no consensus regarding the use of beta‐blockers for the treatment or prevention of vascular complications in patients with COL1‐related OI or C1ROD. However, the efficacy of beta‐blockers has been recognized in the management of vascular lesions in patients with other HCTDs including Marfan syndrome, Loeys‐Diez syndrome, and vEDS (Baderkhan et al., 2021; MacCarrick et al., 2014; Shores et al., 1994). Beta‐blockers prevent hypertension and pulsatile aortic wall stress (Goldfinger et al., 2014), which could contribute to reducing the progression of arterial aneurysm and dissection in patients with HCTDs. Therefore, beta‐blockers might be a reasonable therapeutic or prophylactic option for vascular complications in patients with COL1‐related OI or C1ROD.
Several limitations exist for this study. First, the number of patients, especially in the EDS group, was small. Statistical comparison between the OI and EDS groups was considered difficult. However, the tendency of clinical symptoms could be recognized, such as high frequencies of joint hypermobility and skin hyperextensibility and a considerable frequency of vascular complications. Second, the collection of clinical symptoms and events could be incomplete, especially regarding possible age‐dependent events in younger patients. Children (patients #6, #11, #17, #19, and #20) of affected parents with recurrent bone fractures could experience fractures during adulthood. Still, other factors (e.g., optimization of life‐styles, pharmacological intervention) could have some effects on the occurrence of such events. In addition, patient #21, aged 18 years, with a recurrent variant “p.Arg312Cys” for cEDS susceptible to severe vascular involvement, could develop vascular complications in his adulthood. Further clinical and molecular investigations, including larger patient numbers from variable recruitment like the current study as well as longitudinal data collection, would be required to clarify the comprehensive picture of COL1‐related disorders.
In conclusion, the current cohort included additional patients with C1ROD, and various clinical and molecular overlaps between OI and C1ROD as well as intra‐familial phenotypic variabilities were present. Notably, life‐threatening vascular complications (vascular dissections, arterial aneurysms, and subarachnoidal hemorrhages) occurred in seven patients (41% of those aged >20 years), independently from the background HCTD‐related phenotypes. Careful lifelong surveillance and intervention could be required.
INSTITUTIONAL REVIEW BOARD STATEMENT
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee at Shinshu University School of Medicine (Matsumoto, Japan) (#628, #4171).
INFORMED CONSENT STATEMENT
Written informed consent was obtained from all patients or their guardians.
AUTHOR CONTRIBUTIONS
Ryojun Takeda and Tomoki Kosho designed the study. Tomomi Yamaguchi performed molecular investigation and interpreted the data with Tomoki Kosho. Shujiro Hayashi, Shinichirou Sano, Hiroshi Kawame, Sachiko Kanki, Hidekane Yoshimura and Yukio Nakamura collected clinical data. Ryojun Takeda combined clinical and molecular data and wrote the draft of the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
Research Program on Policy of Measures for Intractable/Rare Diseases (20FC1046) (2020–2) (TK), Ministry of Health, Labour and Welfare, Japan; Program for an Integrated Database of Clinical and Genomic Information (16kk0205001h0501, 16kk0205012h1001) (2016–2020) (TK) and the Initiative on Rare and Undiagnosed Diseases (IRUD) (19ek0109301h0002) (2018–2020) (TK), Japan Agency for Medical Research and Development (AMED); Division of Clinical Sequencing, Shinshu University School of Medicine, is an endowment division, supported with an unrestricted grant from BML Inc. and Life Technologies Japan Ltd.
CONFLICT OF INTEREST
Tomomi Yamaguchi and Tomoki Kosho are members of an endowed chair named “Division of Clinical Sequencing, Shinshu University School of Medicine,” sponsored by BML, Inc. and Life Technologies Japan Ltd. of Thermo Fisher Scientific Inc.
Supporting information
SUPPLEMENTARY FIGURE S1 Computed tomography findings of patient #1: transverse views (A, B), a coronal view (C), and a sagittal view (D). Irregularly dilated and tortuous descending thoracic to abdominal aorta (red arrowheads) and polycystic lesions in the liver and kidneys are noted.
SUPPLEMENTARY FIGURE S2 Clinical photographs and X‐ray images of patient #22. His skin shows mild hyperextensibility (A), translucency (B), and bruisability (C). Hypermobility of the metacarpophalangeal joint and the wrist was noted (D). Bilateral radii and ulnae show mild bowing (E, F).
Supplementary Table S1 Gene lists of Ion AmpliSeq Custom panels.
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We are grateful to the patients and their families for their cooperation during this study. We also thank Dr. K. Wakui, Ph.D., Dr. K. Takano, MD, Ph.D., Ms. Y. Takiguchi, and Dr. T. Fujikawa, MD, for their technical support and helpful discussion. We thank Editage (www.editage.com) for English language editing.
Takeda, R. , Yamaguchi, T. , Hayashi, S. , Sano, S. , Kawame, H. , Kanki, S. , Taketani, T. , Yoshimura, H. , Nakamura, Y. , & Kosho, T. (2022). Clinical and molecular features of patients with COL1 ‐related disorders: Implications for the wider spectrum and the risk of vascular complications. American Journal of Medical Genetics Part A, 188A:2560–2575. 10.1002/ajmg.a.62887
Funding information BML Inc.; Initiative on Rare and Undiagnosed Diseases, Grant/Award Number: 19ek0109301h0002; Japan Agency for Medical Research and Development; Life Technologies Japan Ltd.; Ministry of Health, Labour and Welfare; Program for an Integrated Database of Clinical and Genomic Information, Grant/Award Number: 16kk0205001h0501; 16kk0205012h1001
Contributor Information
Ryojun Takeda, Email: ryojun_takeda@icloud.com.
Tomoki Kosho, Email: ktomoki@shinshu-u.ac.jp.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Adham, S. , Dupuis‐Girod, S. , Charpentier, E. , Mazzella, J. M. , Jeunemaitre, X. , & Legrand, A. (2020). Classical Ehlers‐Danlos syndrome with a propensity to arterial events: A report on a French family with a COL1A1 p.(Arg312Cys) variant. Clinical Genetics, 97, 357–361. 10.1111/cge.13643 [DOI] [PubMed] [Google Scholar]
- Baderkhan, H. , Wanhainen, A. , Stenborg, A. , Stattin, E. L. , & Björck, M. (2021). Celiprolol treatment in patient with vascular Ehlers‐Danlos syndrome. European Journal of Vascular and Endovascular Surgery, 61, 326–331. 10.1016/j.ejvs.2020.10.020 [DOI] [PubMed] [Google Scholar]
- Balasubramanian, M. , Verschueren, A. , Kleevens, S. , Luyckx, I. , Perik, M. , Schirwani, S. , Mortier, G. , Morisaki, H. , Rodrigus, I. , Laer, L. V. , Verstraeten, A. , & Loeys, B. (2019). Aortic aneurysm/dissection and osteogenesis imperfecta: Four new families and review of the literature. Bone, 121, 191–195. doi: 10.1016/j.bone.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Brady, A. F. , Demirdas, S. , Fournel‐Gigleux, S. , Ghali, N. , Giunta, C. , Kapferer‐Seebacher, I. , Kosho, T. , Mendoza‐Londono, R. , Pope, M. F. , Rohrbach, M. , Van Damme, T. , Vandersteen, A. , van Mourik, C. , Voermans, N. , Zschocke, J. , & Malfait, F. (2017). The Ehlers‐Danlos syndromes, rare types. American Journal of Medical Genetics part C Seminars in Medical Genetics, 175, 70–115. 10.1002/ajmg.c.31550 [DOI] [PubMed] [Google Scholar]
- Brown, R. D. , & Broderick, J. P. (2014). Unruptured intracranial aneurysms: Epidemiology, natural history, management option, and familial screening. Lancet Neurology, 13, 393–404. 10.1016/S1474-4422(14)70015-8 [DOI] [PubMed] [Google Scholar]
- Budsamongkol, T. , Intarak, N. , Theerapanon, T. , Yodsanga, S. , Porntaveetus, T. , & Shotelersuk, V. (2019). A novel mutation in COL1A2 leads to osteogenesis imperfecta/Ehlers‐Danlos overlap syndrome with brachydactyly. Genes & Diseases, 6, 138–146. 10.1016/j.gendis.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral, W. A. , Makareeva, E. , Colige, A. , Letocha, A. D. , Ty, J. M. , Yeowell, H. N. , Pals, G. , Leikin, S. , & Marini, J. C. (2005). Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers‐Danlos syndrome by interference with N‐propeptide processing. Journal of Biological Chemistry, 280, 19259–19269. 10.1074/jbc.M414698200 [DOI] [PubMed] [Google Scholar]
- Cabral, W. A. , Makareeva, E. , Letocha, A. D. , Scribanu, N. , Fertala, A. , Steplewski, A. , Keene, D. R. , Persikow, A. V. , Leikin, S. , & Marini, J. C. (2007). Y‐position cysteine substitution in type I collagen (alpha1(I) R888C/p.R1066C) is associated with osteogenesis imperfecta/Ehlers‐Danlos syndrome phenotype. Human Mutation, 28, 396–405. 10.1012/humu.20456 [DOI] [PubMed] [Google Scholar]
- Cingolani, P. , Platts, A. , Coon, M. , Nguyen, T. , Wang, L. , Land, S. J. , Lu, X. , & Ruden, D. M. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso‐2; iso‐3. Fly, 6, 80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan, N. , Dreier, R. , Bucchholz, B. A. , Beseoglu, K. , Bruckner, P. , Matzenauer, C. , Torner, J. C. , Brown, R. D. , Steiger, H. J. , Hänggi, D. , & Macdonald, R. L. (2014). Age of collagen in intracranial saccular aneurysms. Stroke, 45, 1757–1763. 10.1161/STROKEAHA.114.005461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feshchenko, S. , Brinckmann, J. , Lehmann, H. W. , Koch, H. G. , Muller, P. K. , & Kugler, S. (1998). Identification of a new heterozygous point mutation in the COL1A2 gene leading to skipping of exon 9 in a patient with joint laxity, hyperextensibility of skin and blue sclera. Mutations in brief no.166. Online. Human Mutation, 12, 138. [DOI] [PubMed] [Google Scholar]
- Foi, M. , De Mazancourt, P. , Metay, C. , Carlier, R. , Allamand, V. , Gartioux, C. , Gillas, F. , Miri, N. , Jobic, V. , Mekki, A. , Richard, P. , Michot, C. , & Benistan, K. (2021). A novel COL1A1 variant in a family with clinical features of hypermobile Ehlers‐Danlos syndrome that proved to be a COL1‐related overlap disorder. Clinical Case Reports, 9, e04128. doi: 10.1002/ccr3.4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkestad, L. , Hald, J. D. , Gram, J. , Langdahl, B. L. , Hermann, A. P. , Diederichsen, A. C. , Abrahamsen, B. , & Brixen, K. (2016). Cardiovascular disease in patients with osteogenesis imperfecta – A nationwide, register‐based cohort study. International Journal of Cardiology, 225, 250–257. 10.1016/j.ijcard.2016.09.107 [DOI] [PubMed] [Google Scholar]
- Forlino, A. , & Marini, J. C. (2016). Osteogenesis imperfecta. The Lancet, 387, 1657–1671. 10.1016/S0140-6736(15)00728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaberel, T. , Rochey, A. , di Palma, C. , Lucas, F. , Touze, E. , & Emery, E. (2016). Ruptured intracranial aneurysm in patients with osteogenesis imperfecta: 2 familial cases and a systematic review of the literature. Neurochirurgine, 62, 317–320. 10.1016/j.neuchi.2016.07.004 [DOI] [PubMed] [Google Scholar]
- Gensure, R. C. , Mäkitie, O. , Barclay, C. , Chan, C. , Depalma, S. R. , Bastepe, M. , Abuzahra, H. , Couper, R. , Mundlos, S. , Sillence, D. , Ala kokko, L., Seidman, J. G., Cole, W. G., & Jüppner, H. (2005). A novel COL1A1 mutation in infantile cortical hyperostosis (Caffey disease) expands the spectrum of collagen‐related disorders. Journal of Clinical Investigation, 115, 1250–1257. 10.1172/JCI22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoli, M. , Brizola, E. , Tremosini, M. , Pedrini, E. , Maioli, M. , Mosca, M. , Bassotti, A. , Castronovo, P. , Giunta, C. , & Sangiorgi, L. (2021). COL1‐related disorders: Case report and review of overlapping syndromes. Frontiers in Genetics, 12(640), 558. 10.3389/fgene.2021.640558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger, J. Z. , Halperin, J. L. , Marin, M. L. , Stewart, A. S. , Eagle, K. A. , & Fuster, V. (2014). Thoracic aortic aneurysm and dissection. Journal of the American College of Cardiology, 64, 1725–1739. 10.1016/j.jacc.2014.08.025 [DOI] [PubMed] [Google Scholar]
- Grant, S. F. , Reid, D. M. , Blake, G. , Herd, R. , Fogelman, I. , & Ralston, S. H. (1996). Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nature Genetics., 14, 203–205. 10.1038/ng1096-203 [DOI] [PubMed] [Google Scholar]
- Körkkö, J. , Ala‐Kokko, L. , De Paepe, A. , Nuytinck, L. , Earley, J. , & Prockop, D. J. (1998). Analysis of the COL1A1 and COL1A2 genes by PCR amplification and scanning by conformation‐sensitive gel electrophoresis identifies only COL1A1 mutations in 15 patients with osteogenesis imperfecta type I: Identification of common sequences of null‐allele mutations. American Journal of Human Genetics, 62, 98–110. 10.1086/301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. , Grafe, I. , Alexander, S. , & Lee, B. (2017). Genetic causes and mechanisms of Osteogenesis Imperfecta. Bone, 102, 40–49. 10.1016/j.bone.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCarrick, G. , Blank, J. H., 3rd. , Bowdin, S. , El‐Hamamsy, I. , Frischmeyer‐Guerrerio, P. A. , Guerrerio, A. L. , Sponseller, P. D. , Loeys, B. , & Dietz, H. C., 3rd. (2014). Loeys‐Dietz syndrome: A primer for diagnosis and management. Genetics in Medicine, 16, 576–587. 10.1038/gim.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makareeva, E. , Cabral, W. A. , Marini, J. C. , & Leikin, S. (2006). Molecular mechanism of alpha 1(I)‐osteogenesis imperfecta/Ehlers‐Danlos syndrome: Unfolding of an N‐anchor domain at the N‐terminal end of the type I collagen triple helix. Journal of Biological Chemistry, 281, 6463–6470. 10.1074/jbc.M511830200 [DOI] [PubMed] [Google Scholar]
- Malfait, F. , Francomano, C. , Byers, P. , Belmont, J. , Berglund, B. , Black, J. , Bloom, L. , Bowen, J. M. , Brady, A. F. , Burrows, N. P. , Castori, M. , Cohen, H. , Colombi, M. , Demirdas, S. , De Backer, J. , De Paepe, A. , Fournel‐Gigleux, S. , Frank, M. , Ghali, N. , … Tinkle, B. (2017). The 2017 international classification of the Ehlers‐Danlos syndromes. American Journal of Medical Genetics part C Seminars in Medical Genetics, 175, 8–26. 10.1002/ajmg.c.31552 [DOI] [PubMed] [Google Scholar]
- Malfait, F. , Symoens, S. , Goemans, N. , Gyftodimou, Y. , Holmberg, E. , Lopez‐Gonzalez, V. , Mortier, G. , Nampoothiri, S. , & De Paepe, A. (2013). Helical mutations in type I collagen that affect the processing of the amno‐propeptide result in an Osteogenesis Imperfecta/Ehlers‐Danlos syndrome overlap syndrome. Orphanet Journal of Rare Disease, 8, 78. 10.1186/1750-1172-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini, J. C. , Forlino, A. , Bächinger, H. P. , Bishop, N. J. , Byers, P. H. , Paepe, A. , Fassier, F. , Fratzl‐Zelman, N. , Kozloff, K. M. , Krakow, D. , Montpetit, K. , & Semler, O. (2017). Osteogenesis Imperfecta. Nature Reviews Disease Primers, 3(17), 052. 10.1038/nrdp.2017.52 [DOI] [PubMed] [Google Scholar]
- Marini, J. C. , Forlino, A. , Cabral, W. A. , Barnes, A. M. , San Antonio, J. D. , Milgrom, S. , Hyland, J. C. , Körkkö, J. , Prockop, D. J. , d e Paepe, A. , Coucke, P. , Symoens, S. , Glorieux, F. H. , Roughley, P. J. , Lund, A. M. , Kuurila‐Svahn, K. , Hartikka, H. , Cohn, D. H. , Krakow, D. , Mottes, M. , Schwarze, U. , Chen, D. , Yang, K. , Kuslich, C. , Troendle, J. , Dalgleish, R. , & Byers, P. H. (2007). Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: Regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Human Mutation, 28, 209–221. 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, S. A. , Rubin, B. S. , Starman, B. J. , & Byers, P. H. (1996). Spontaneous multivessel cervical artery dissection in a patient with a substitution of alanine for glycine (G13A) in the alpha 1(I) chain of type I collagen. Neurology, 47, 552–556. 10.1212/wnl.47.2.552 [DOI] [PubMed] [Google Scholar]
- Mcneeley, M. F. , Dontchos, B. N. , Laflamme, M. A. , Hubka, M. , & Sadro, C. T. (2012). Aortic dissection in osteogenesis imperfecta: Case report and review of the literature. Emergency Radiology, 19, 553–556. doi: 10.1007/s10140-012-1044-1 [DOI] [PubMed] [Google Scholar]
- Mimata, C. , Kitaoka, M. , Nagahiro, S. , Iyama, K. , Hori, H. , Yoshioka, H. , & Ushio, Y. (1997). Differential distribution and expressions of collagens in the cerebral aneurysmal wall. Acta Neuropathologica, 94, 197–206. doi: 10.1007/s004010050694 [DOI] [PubMed] [Google Scholar]
- Morlino, S. , Micale, L. , Ritelli, M. , Rohrbach, M. , Zoppi, N. , Vandersteen, A. , Mackay, S. , Agolini, E. , Cocciadiferro, D. , Sasaki, E. , Madeo, A. , Ferraris, A. , Reardon, W. , Di Rocco, M. , Novelli, A. , Grammatico, P. , Marfait, F. , Mazza, T. , Hakim, A. , … Castori, M. (2020). COL1‐related overlap disorder: A novel connective tissue disorder incorporating the osteogenesis imperfecta/Ehlers‐Danlos syndrome overlap. Clinical Genetics, 97, 396–406. 10.1111/cge.13683 [DOI] [PubMed] [Google Scholar]
- Perrone, R. D. , Malek, A. M. , & Watnick, T. (2015). Vascular complications in autosomal dominant polycystic kidney disease. Nature Reviews Nephrology, 11, 589–598. 10.1038/nrneph.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, S. , Aziz, N. , Bale, S. , Bick, D. , Das, S. , Gastier‐Foster, J. , Grody, W. W. , Hegde, M. , Lyon, E. , Spector, E. , Voelkerding, K. , Rehm, H. L. , & ACMG Laboratory Quality Assurance Committee . (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine: official journal of the American College of Medical Genetics, 17, 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores, J. , Berger, K. R. , Murphy, E. A. , & Pyeritz, R. E. (1994). Progression of aortic dilatation and the benefit of long‐term beta‐adrenergic blockade in Marfan's syndrome. New England Journal of Medicine, 330, 1335–1341. 10.1056/NEJM199405123301902 [DOI] [PubMed] [Google Scholar]
- Vouyouka, A. G. , Pfeiffer, B. J. , Liem, T. K. , Taylor, T. K. , Mudaliar, J. , & Phillips, C. L. (2001). The role of type I collagen in aortic wall strength with a homotrimeric [α1(I)]3 collagen mouse model. Journal of Vascular Surgery, 33, 1263–1270. 10.1067/mva.2001.113579 [DOI] [PubMed] [Google Scholar]
- Willing, M. C. , Deschenes, S. P. , Slayton, R. L. , & Roberts, E. J. (1996). Premature chain termination is a unifying mechanism for COL1A1 null alleles in osteogenesis imperfecta type I cell strains. American Journal of Human Genetics, 59, 799–809. [PMC free article] [PubMed] [Google Scholar]
- Yoneyama, T. , Kasuya, H. , Onda, H. , Akagawa, H. , Hashiguchi, K. , Nakajima, T. , & Inoue, I. (2004). Collagen type 1 alpha2 (COL1A2) is the susceptible gene for intracranial aneurysms. Stroke, 35, 443–448. 10.1161/01.STR.0000110788.45858.DC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE S1 Computed tomography findings of patient #1: transverse views (A, B), a coronal view (C), and a sagittal view (D). Irregularly dilated and tortuous descending thoracic to abdominal aorta (red arrowheads) and polycystic lesions in the liver and kidneys are noted.
SUPPLEMENTARY FIGURE S2 Clinical photographs and X‐ray images of patient #22. His skin shows mild hyperextensibility (A), translucency (B), and bruisability (C). Hypermobility of the metacarpophalangeal joint and the wrist was noted (D). Bilateral radii and ulnae show mild bowing (E, F).
Supplementary Table S1 Gene lists of Ion AmpliSeq Custom panels.
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


