Abstract
Background
The gut microbiome and its metabolites can impact brain health and are altered in Parkinson's disease (PD) patients. It has been recently demonstrated that PD patients have reduced fecal levels of the potent epigenetic modulator butyrate and its bacterial producers.
Objectives
Here, we investigate whether the changes in the gut microbiome and associated metabolites are related to PD symptoms and epigenetic markers in leucocytes and neurons.
Methods
Stool, whole blood samples, and clinical data were collected from 55 PD patients and 55 controls. We performed DNA methylation analysis on whole blood samples and analyzed the results in relation to fecal short‐chain fatty acid concentrations and microbiota composition. In another cohort, prefrontal cortex neurons were isolated from control and PD brains. We identified genome‐wide DNA methylation by targeted bisulfite sequencing.
Results
We show that lower fecal butyrate and reduced counts of genera Roseburia, Romboutsia, and Prevotella are related to depressive symptoms in PD patients. Genes containing butyrate‐associated methylation sites include PD risk genes and significantly overlap with sites epigenetically altered in PD blood leucocytes, predominantly neutrophils, and in brain neurons, relative to controls. Moreover, butyrate‐associated methylated‐DNA regions in PD overlap with those altered in gastrointestinal (GI), autoimmune, and psychiatric diseases.
Conclusions
Decreased levels of bacterially produced butyrate are related to epigenetic changes in leucocytes and neurons from PD patients and to the severity of their depressive symptoms. PD shares common butyrate‐dependent epigenetic changes with certain GI and psychiatric disorders, which could be relevant for their epidemiological relation. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: Parkinson's disease, DNA methylation, microbiome, epigenetics, gut brain axis
Parkinson's disease (PD) is a neurodegenerative disorder that is typically characterized by motor impairments due to the death of dopaminergic neurons located within the substantia nigra pars compacta. 1 A hallmark of PD is the aggregation of misfolded α‐synuclein protein in both the central and peripheral nervous system, forming aggregates or Lewy bodies. 2 The discovery of α‐synuclein aggregates in the enteric nervous system, coupled with the early gastrointestinal (GI) symptoms of PD (eg, constipation), has led to the hypothesis that the pathogenesis of PD may originate in the GI tract or at least outside of the central nervous system (CNS). 3 In addition, it is hypothesized that α‐synuclein aggregates can be transported in a prion‐like manner from the enteric nervous system to the CNS through the vagus nerve. 4 This hypothesis is supported by the decreased risk for PD among patients who have undergone a vagotomy. 5 Further, intertwining the GI system with PD is the finding that the presence of the gut microbiome is required for mice that overexpress α‐synuclein to develop motor deficits. 6 These same mice displayed increased motor deficits after receiving a fecal transplant from a PD patient compared to receiving transplant from a healthy donor. Moreover, the composition and function of the gut microbiome in PD patients have been shown to be significantly different from healthy controls in multiple studies. 7 , 8 , 9 , 10
Recent meta‐analyses suggest that a reduced abundance of short‐chain fatty acid (SCFA) producing bacteria is one of the most consistent findings in the PD microbiome composition across studies. 11 , 12 SCFAs are saturated fatty acids produced via the fermentation of dietary fiber by certain colonic bacteria. 13 The deficiency of SCFAs has been implicated in multiple diseases such as autoimmune disorders, cancer, metabolic syndromes, and neurological disorders. 14 Fecal samples from PD patients have been shown to harbor significantly lower concentrations of the SCFAs acetate, propionate, and butyrate when compared to healthy controls. 15 Beyond its central role as the main energy source for colonocytes, butyrate, specifically, has been implicated as an important bacterial metabolite due to its role as a strong endogenous histone deacetylase (HDAC) inhibitor indirectly affecting DNA methylation, 16 allowing it to epigenetically alter the gene expression of multiple cell types. 17 Butyrate is also the ligand of some free fatty acid receptors that are critical for inflammation regulation and secretion of peptide hormones. 18 Several studies have demonstrated the ability of butyrate to reduce the inflammatory properties of both innate and adaptive immune cells through inhibiting reactive oxygen species release and inflammatory cytokine production and inducing activated immune cell apoptotic mechanisms. 19 , 20 , 21 In addition to its immune‐modulating properties, butyrate has been shown in multiple in vivo studies to influence the CNS through decreasing blood–brain barrier permeability, decreasing microglial activation, and relieving anxiety and depression, which are both common prodromal PD symptoms. 22 , 23 , 24 , 25 Butyrate has also shown its impact on astrocyte gene expression in vitro and neuroprotective effects in PD mouse models. 26 , 27 , 28 Some studies also suggested deleterious effects of SCFAs. 6 , 29 , 30 However, the possible impact of the altered butyrate level observed in PD patients on epigenome status as well as on clinical symptoms has yet to be elucidated.
To test whether butyrate impacts epigenetic markers in the blood and brain of PD patients, and whether this is associated with symptom severity, we performed DNA methylation profiling in whole blood samples and neuronal tissue from two cohorts of PD patients and controls (Fig. 1) and related the findings to fecal gut microbiome and metabolite data and clinical symptoms.
FIG 1.
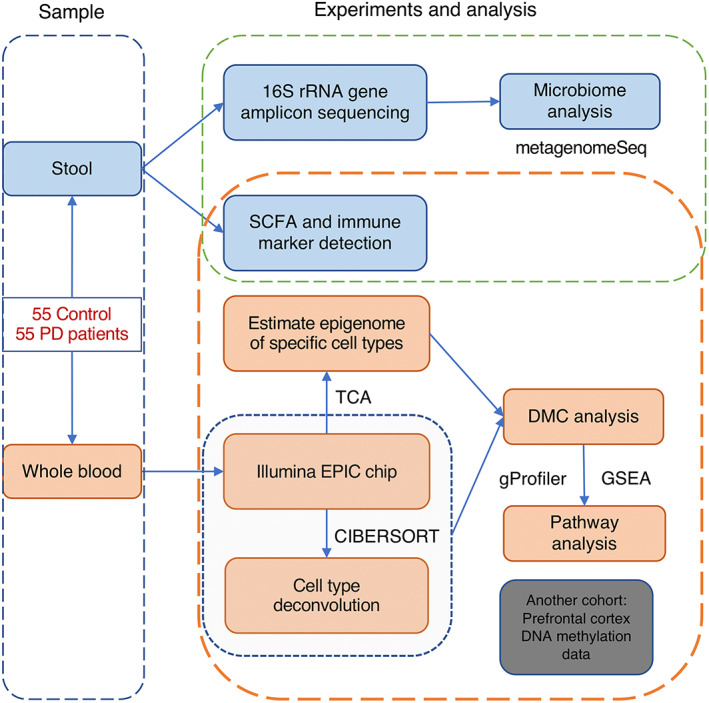
Outline of this study. [Color figure can be viewed at wileyonlinelibrary.com]
Patients and Methods
Human Samples and Metadata
Blood samples, clinical data, microbiome count data, inflammatory and permeability markers, and stool SCFA levels used in this study are from the Helsinki Parkinson microbiome cohort, and methodology of sampling, sample processing, and analysis have been described previously. 31 , 32 The study was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa. All participants provided informed consent. Human prefrontal cortex tissue for this study was obtained from the Parkinson's UK Brain Bank, NIH NeuroBioBank, and Michigan Brain Bank, with approval from the ethics committee of the Van Andel Research Institute (IRB 15025). Methodology of sampling, sample processing, and analysis have been described previously. 33 We used the differential methylated cytosine data (Supplementary File 5) in our analysis.
Statistical Analyses
We performed statistical analyses with R (v3.6.1),34 with packages such as metagenomeSeq (v 1.27.3) 35 for differential microbial data comparisons, ChAMP (v 2.20.1) 36 and Minfi (v 1.31.1) 37 for EPIC array analysis, tensor composition analysis (TCA) (v 1.1.0) 38 for epigenome estimation of immune cell types, limma (v 3.41.17) 39 for robust linear regression, and ggplot2 (v 3.3.1) 40 for data visualization. All the codes used for this work are publicly available: Github: https://github.com/AojiXie/PD_microbiome_DNA_methylation.
Genome‐Wide DNA Methylation Profiling
Whole‐genome DNA methylation profiling for each sample was performed on Illumina Methylation EPIC BeadChip microarrays at Van Andel Institute Genomic core. Bisulfite‐converted DNA samples (n = 136, including replicates) were randomized across arrays (eight samples per array). Data generated from the microarrays were preprocessed using Minfi (v 1.31.1). 37 Normalization was performed using Noob. 41 We confirmed that the sex of the individuals matched that inferred from the DNA methylome (minfi getSex() function).
The portions of immune cell types (CD8+T cell, CD4+T cell, B cell, NK, monocyte, and neutrophil) were estimated by CIBERSORT 42 (Fig. S2a) using whole blood‐specific markers as reference. 43 The filtering method in ChAMP was used to filter probes: probes that overlapped single nucleotide polymorphisms (SNPs) 44 (minor allele frequency >0.05) on the CpG or single‐base extension (95,485 probes), probes that aligned to multiple locations (42,558 probes), probes with a beadcount <3 in at least 5% of samples (3380 probes), MultiHit Start 45 (11 probes), probes located on X,Y chromosome (16,541 probes), NoCG Start (2953), and those that failed detectability (P > 0.01) (7951 probes) were excluded. After processing, 739,597 probes remained. Champ.svd() in ChAMP was used to test the batch effects. Those batch effects (array and slide positions) were corrected by ComBat. 46 After batch effects correction, the M value was ready for the subsequent statistical analysis. Cell‐type‐specific resolution epigenetics were performed using TCA. 38
Statistical Analysis for Differentially Methylated Sites
DNA methylation analysis involved robust linear regression models with empirical Bayes from the limma (v 3.41.17) statistical package. 39 P‐values were adjusted with a Benjamini–Hochberg correction for multiple testing, and those with false discovery rate (FDR) q < 0.05 were deemed significant.
Model
1: In Whole Blood Methylation Epigenome
Variable selection: cell‐type percentages were used as covariates because the whole blood contains several immune cell types in varying proportions. This variation might affect the interpretations of DNA methylation levels based on whole blood DNA. 47 Body mass index (BMI) and smoking history are used as covariates because their associations with whole blood DNA methylation have been observed. 48 , 49
M value ~ butyrate + age + sex + smoking history + BMI + CD4+T cell + CD8+T cell + B cell + monocyte + neutrophil.
2: In Cell‐Specific Epigenome
M value ~ butyrate + age + sex + smoking history + BMI.
Pathway Enrichment Analysis
To identify proximal interactions with gene targets, we used the GREAT (v4.0.4) software. 50 Gene annotation was performed for the gene targets of the significant cytosine sites in our analysis and for the background, consisting of gene targets for all cytosines included in our analysis. The background consisted of 18,455 genes. Pathway analysis of methylated cytosines altered in PD and correlated with butyrate level was performed using g:Profiler, 51 with networks determined by EnrichmentMap and clustered by AutoAnnotate in Cytoscape (v3.7.1). 52
Because enhancer elements dynamically regulate gene expression through three‐dimensional physical interactions, we analyzed chromatin interaction data to reveal the gene targets of enhancers relevant to the differential methylation sites linked to butyrate. For this analysis, we used promoter‐centric chromatin interactions identified in blood cell types. 53 Gene enrichment set test of different blood cell types was performed by GSEA 54 , 55 (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp) Reactome gene sets. Benjamini–Hochberg FDR q < 0.01 with minimal gene set less than 100 was used as the significant threshold.
Genetic–Epigenetic Correlation
Genetic–epigenetic analyses were performed using LD Score software 56 , 57 to estimate the correlations between butyrate‐associated methylated‐DNA (mDNA) regions and the genome‐wide association studies (GWAS) summary statistics of other diseases. To construct butyrate‐associated mDNA regions for linkage disequilibrium (LD) score regression analysis, SNPs within ±5000 bp of EPIC chip array sites were included, and the P‐values of methylation cytosines in butyrate linear model were assigned to those SNPs. If an SNP was within ±5000 bp of more than one methylated cytosine, the smallest P‐value was selected. The summary statistic of a 2019 PD GWAS study 58 was used in this analysis. For other diseases, we used the summary statistics that are provided in the LD Hub interface. 59 P < 0.05 was used as the significance threshold.
The common SNPs (with P < 0.05) between butyrate‐associated mDNA regions, PD GWAS summary statistics, and other diseases were extracted. GREAT 50 was used to obtain the gene annotation of those common SNPs with association rule: basal + extension: 5000 bp upstream, 1000 bp downstream, 600,000 bp maximum extension, curated regulatory domains included. Gene set enrichment analysis was performed by GSEA using Reactome gene sets 54 , 55 (https://www.gsea-msigdb.org/gsea/msigdb/index.jsp). Benjamini–Hochberg FDR q < 0.01 with minimal gene set less than 100 was used as the significant threshold.
Data Availability
Microbiota data are available at the European Nucleotide Archive (accession number: PRJEB27564) (https://www.ebi.ac.uk/ena/browser/view/PRJEB27564). Other data and files utilized in this study are available from the corresponding authors on reasonable request.
Results
Butyrate‐Producing Microbes Are Altered in PD and Correlate with Depressive Symptoms
First, we reanalyzed previously published 31 , 32 raw data on SCFA levels and 16S rRNA gene amplicon counts from stool samples of PD patients and healthy controls. The metagenomeSeq was used for differential microbial analysis. 35 Unlike RNAseq studies, most operational taxonomic units are rare (absent from a large number of samples) because of insufficient sequencing depth (undersampling) or some organisms being present only in a few samples. This sparsity can lead to strong biases when sequence read counts are tested for significant differences. Zero counts in samples with low coverage are misinterpreted as absent taxonomic features. The advantages of metagenomeSeq are the zero‐inflated Gaussian (ZIG) mixture model that removes testing biases resulting from undersampling and the cumulative‐sum scaling normalization method to avoid biases of uneven sequencing depth. 60 We can confirm significantly reduced butyrate levels (Benjamini–Hochberg FDR q < 0.05, robust linear regression) (Fig. APPENDIX S1a) and differential abundances of the genera Bifidobacterium, Butyricicoccus, Clostridium_XlVa, Lactobacillus, Prevotella, and Roseburia in PD patients (Benjamini–Hochberg FDR q < 0.05, metagenomeSeq ZIG model) (Fig. APPENDIX S1b). 31 Important new findings within the PD group are links between depressive symptoms as measured using the Geriatric Depression Scale (GDS‐15) and lower fecal butyrate levels (Benjamini–Hochberg FDR q < 0.05, robust linear regression) (Fig. 2A) as well as lower counts of the genera Prevotella, Romboutsia, and Roseburia and higher counts of the genera Deltaproteobacteria_unclassified (Benjamini–Hochberg FDR q < 0.05, metagenomeSeq ZIG model) (Fig. APPENDIX S1c; Fig. 2B). In line with these findings, we confirm a positive correlation of the genera Romboutsia and Roseburia with butyrate levels in PD patients (Fig. APPENDIX S1c). 32 In addition, no other symptoms, including GI (Rome‐III questionnaire; Wexner score), motor symptoms (the Unified Parkinson's Disease Rating Scale [UPDRS]), and nonmotor symptoms (Non‐Motor Symptoms Questionnaire [NMSQ] and Non‐Motor Symptoms Scale [NMSS]), were linked with butyrate in PD patients.
FIG 2.
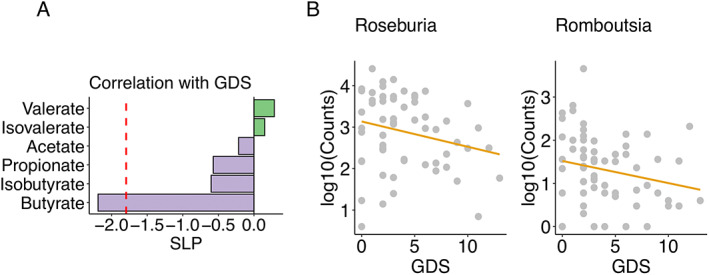
Butyrate and butyrate‐producing microbes are associated with PD depressive symptoms. (A) Short‐chain fatty acids change with Geriatric Depression Scale (GDS) total scores in PD patients (robust linear regression, adjusting for age, sex, smoking status, and BMI [body mass index]. Benjamini–Hochberg FDR [false discovery rate] q < 0.05 is used as the significant threshold). (B) The butyrate‐producing bacterial genera Roseburia and Romboutsia were negatively associated with GDS (Geriatric Depression Scale) total scores in PD patients. Benjamini–Hochberg FDR (false discovery rate) q < 0.05, signed logP: SLP. [Color figure can be viewed at wileyonlinelibrary.com]
Fecal Butyrate Levels Are Associated with Epigenetic Alterations in Leucocytes and Neurons of PD Patients
We identified 3195 CpG sites that correlated significantly with stool butyrate levels in PD patients (Benjamini–Hochberg FDR q < 0.05, robust linear regression) (Fig. 3A) and 2950 CpG sites that were significantly changed in PD relative to controls (Benjamini–Hochberg FDR q < 0.05, robust linear regression). In a previous study, we identified genes epigenetically altered in cortical neurons from PD patients. 33 Genes containing the butyrate‐associated methylated cytosines in blood cells significantly overlapped with those genes altered in PD patients' blood cells (relative to controls) and with those genes altered in PD patients' prefrontal cortex neurons (relative to controls) (genes containing modified cytosines at Benjamini–Hochberg FDR q < 0.05, Fisher's exact test P < 0.05) (Fig. 3B,C). Pathways involving those genes epigenetically altered in PD or with butyrate were identified and included neurodevelopment, cell development, synaptic transmission, metabolism, and signal transduction (Fig. 3D).
FIG 3.
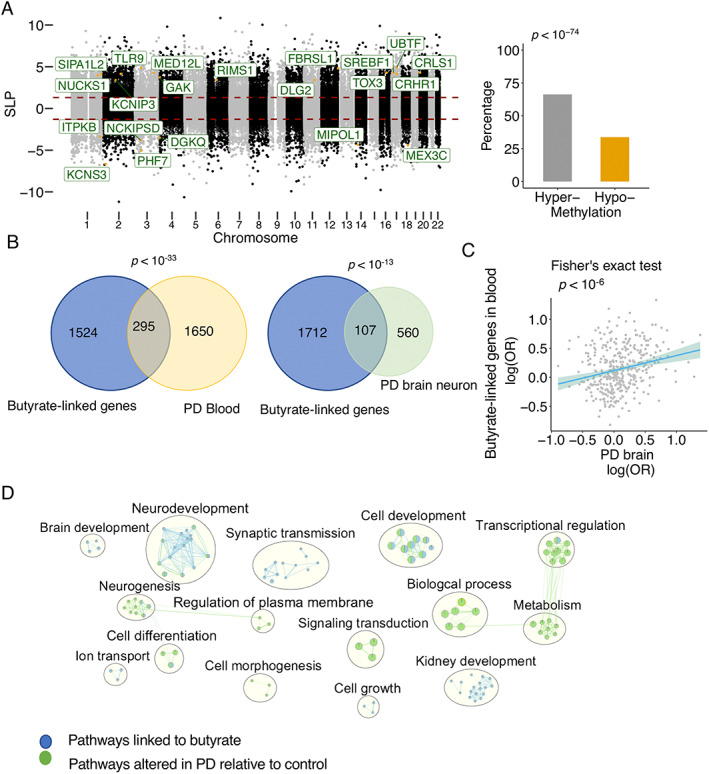
Links of butyrate to PD epigenome in brain and blood. DNA methylation analysis was performed by Illumina EPIC array in PD and control whole blood (n = 55 PD, 55 controls). (A) DNA methylation changes in the blood of PD patients associated with butyrate levels are identified (robust linear regression, 3195 cytosines at Benjamini–Hochberg FDR [false discovery rate] q < 0.05, after adjusting for age, sex, smoking status, BMI [body mass index], and blood cell types). PD risk genes (identified by GWAS [genome‐wide association studies]) with differential methylation are highlighted. The percentage of hypermethylated and hypomethylated cytosines is plotted (Fisher's test, P < 0.05), and butyrate level is positively associated with cytosine methylation in PD. (B) Genes in PD blood (295 genes) and PD prefrontal cortex (107 genes) are significantly converged on the genes linked to butyrate levels (Fisher's exact test, P < 0.05). (C) Epigenetic alterations in blood and brain converge on those methylation sites linked to butyrate. The log(odds ratio) of genes in PD blood is significantly correlated with the log(odds ratio) of those genes in PD prefrontal cortex (Fisher's exact test, P < 0.05). (D) Enrichment analysis of genes epigenetically linked to butyrate and altered in PD relative to control. Nodes are q < 0.05 pathways merged by EnrichmentMap in Cytoscape. [Color figure can be viewed at wileyonlinelibrary.com]
Epigenetic Links between Butyrate, Bacteria and Leucocytes Differ between Cell Types
To test if leucocyte epigenetic changes are associated with fecal butyrate, we used cell‐type‐specific resolution epigenetics, TCA, 38 to analyze the epigenome for each cell type (Fig. S2). To test the epigenetic links of butyrate on different immune cell types, we analyzed the significant methylation sites linked to butyrate in the epigenome of neutrophils, monocytes, CD8+T cells, CD4+T cells, and B cells. All cell types were linked differently to butyrate, with neutrophils and monocytes containing the largest number of genes that were epigenetically linked to butyrate (Fig. 4A). Levels of inflammatory stool cytokines were derived from a previously published study of the same samples. 32 We also analyzed the methylated cytosines that were correlated with blood inflammatory cytokine levels. There was a large overlap between the genes epigenetically linked to butyrate and those epigenetically associated with levels of inflammatory cytokines (TNF, IL6, CXCL8, IL4, IL1B, IL10, IFNg, IL13, IL12p70, IL2, and LBP) in both monocytes and neutrophils (Fisher's exact test, P < 0.05) (Fig. 4B). Pathway analysis shows that epigenetic alterations in three recently identified PD polygenic risk pathways 61 (neutrophil degranulation, metabolism of lipids, and innate immune system) in the monocyte and neutrophil epigenome were linked to butyrate levels (Fig. 4C). We also analyzed the links to other SCFAs, and our results showed that the genes epigenetically altered in neutrophils were most strongly associated with butyrate compared to other SCFAs (Fig. S3a) and that there were no significant PD polygenic risk pathways linked to other SCFAs in neutrophils (Fig. S3b). We further analyzed the significant methylation sites linked to bacterial genera that correlated with butyrate levels and/or depressive symptoms (Table APPENDIX S1). Like butyrate, Roseburia and Romboutsia were linked mostly to epigenetic alterations in innate immune cells. Prevotella and Deltaproteobacteria_unclassified showed stronger links to the epigenetic status of T cells. These results indicate that immune cell functions are specifically altered in PD patients and that they might be epigenetically altered by butyrate and gut microbiota.
FIG 4.
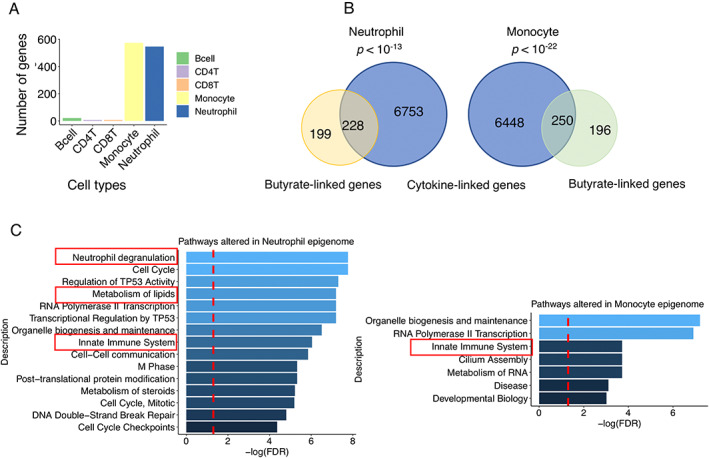
Blood cell types are differentially linked to butyrate. (A) Significant methylation sites (related gene numbers) linked to butyrate in the epigenome of neutrophils, monocytes, CD8+T cells, CD4+T cells, and B cells, respectively. (B) Epigenetically altered genes linked to cytokines overlap with those epigenetically linked to butyrate in monocytes and neutrophils. Fisher's exact test, P < 0.05. (C) Gene set enrichment analysis of the monocyte and neutrophil genes that are epigenetically altered and linked to butyrate. PD polygenic risk pathways, including innate immune systems, metabolism of lipids and neutrophil degranulation, are significantly altered. Significant threshold Benjamini–Hochberg FDR q < 0.05. [Color figure can be viewed at wileyonlinelibrary.com]
Epigenetic–Genetic Correlation between Diseases and Butyrate‐Associated mDNA Regions
To investigate the epigenetic–genetic correlation, we ran a linear regression model to compare the results of methylation analysis to the GWAS of the odds risk ratios for PD and other diseases, including GI (ulcerative colitis, Crohn's disease), autoimmune (rheumatoid arthritis), neurodegenerative (Alzheimer's disease), and psychiatric diseases (bipolar disorder, schizophrenia, and major depression), and our DNA methylation study, adjusting for LD scores. We found that butyrate‐associated mDNA regions are most strongly related to GWAS loci linked to PD and inflammatory bowel diseases. Further significant links were found with the GWAS loci of rheumatoid arthritis and bipolar disorder (linear regression, P < 0.05) (Fig. 5A). In contrast, Alzheimer's disease, schizophrenia, or major depression GWAS loci were not linked to butyrate‐associated mDNA regions. Pathway analysis was performed on the common genomic regions (butyrate‐associated mDNA regions, GWAS loci of PD, and each of the other diseases, respectively). All of the six PD polygenic risk pathways without known PD risk loci 61 were significantly altered in ulcerative colitis, Crohn's disease, rheumatoid arthritis, and bipolar disorder (FDR q < 0.05) (Fig. 5B). The top 15 significant pathways are shown in Figure S4. These results indicate that butyrate‐associated epigenetic changes may contribute to observed epidemiologic links between PD and GI, autoimmune, and certain psychiatric diseases.
FIG 5.
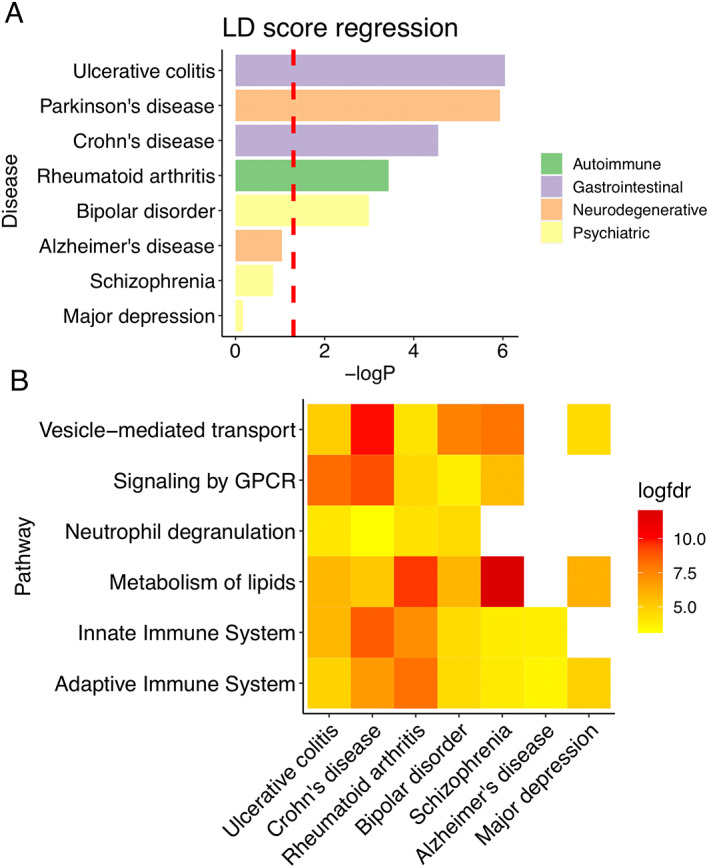
Epigenetic–genetic correlation between butyrate‐associated mDNA (methylated DNA) regions and the GWAS (genome‐wide association studies) of other diseases. (A) LD (linkage disequilibrium) score regression was performed between butyrate‐associated mDNA regions and the GWAS summary statistics of Parkinson's disease, ulcerative colitis, Crohn's disease, rheumatoid arthritis, bipolar disorder, Alzheimer's disease, schizophrenia, and major depression, respectively. P < 0.05 is used as the significant threshold. (B) Gene set enrichment analysis of the location of common genetic regions in Parkinson's disease, butyrate‐associated mDNA regions, and those diseases in panel A. The log(FDR) of six PD polygenetic risk pathways without known PD risk loci was plotted. 61 Benjamini–Hochberg FDR (false discovery rate) q < 0.05 is considered as significant threshold; white squares indicate nonsignificant associations. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
Several studies have found alterations in microbial composition and metabolites in PD, but the mechanisms linking these changes to PD and its symptoms are not well understood. 8 , 9 , 11 The bacterial metabolite butyrate is a strong HDAC inhibitor, with known epigenetic effects. 62 Several studies have shown the ability of butyrate to affect the innate and adaptive immune system as well as to alter the blood–brain barrier permeability. 23 , 27 , 63 This study is the first to examine the possible role of epigenetic changes as a link between gut microbiota, its metabolites, and the pathophysiology of neural and immune cells in PD.
In this study, genome‐wide DNA methylation profiling revealed significant epigenetic changes in the leucocytes and neurons of PD patients that overlap with genes, the methylation of which is linked to fecal butyrate levels, including loci in PD risk genes. 33 Furthermore, butyrate levels correlated most strongly with abundance of bacteria belonging to the genera Romboutsia and Roseburia, known SCFA producers.
A major finding of our study is that the epigenome of specific immune cell types in PD is differentially linked to fecal butyrate levels and fecal bacterial counts. In particular, neutrophil and monocyte epigenomes show the strongest links to butyrate. We have also shown that the neutrophil epigenome is linked less to other SCFAs. A recent study reported that neutrophil degranulation is potentially linked to PD risk. 61 Our gene set enrichment analysis suggests that butyrate‐linked epigenetic changes may impact this pathway. Several studies have reported an increase in neutrophils in PD patients, including studies using flow cytometric and epigenetic profiling approaches, which is consistent with our cell deconvolution results from methylation profiling. 64 However, our study for the first time decomposed the estimated epigenome of each cell type and investigated the epigenetic change from different immune cell types in PD and in connection to SCFAs. Our results suggest that butyrate may impact PD through epigenetic effects on innate immune cells and PD‐related genes.
A limitation of this study is that blood SCFA levels were not available. The exact dynamics that related fecal butyrate levels with blood leucocyte and brain epigenetics are not known. While a significant proportion of microbial‐released butyrate is rapidly taken up and consumed locally in the gut, butyrate can cross the epithelial barrier and enter the circulation via the portal vein. 65 Microbiota‐derived butyrate impacts histone acetylation in multiple tissues. 66 Whereas concentrations in the portal vein are still considerable, concentrations in peripheral blood appear to be relatively low. 65 Thus, leucocytes become exposed to butyrate mostly in the gut wall and portal vein, whereas the impact of butyrate in systemic venous blood can be expected to be less and influenced by liver function. Epigenetic changes in blood leucocytes may impact inflammation systemically and in the brain. 27 At physiological concentrations, butyrate's impact on brain metabolism and hippocampal neurogenesis has been shown in pigs. 67 Although our findings support the importance of epigenetic mechanisms, their relative impact on the physiological effects of butyrate in the brain as compared to other mechanisms remains to be established.
Interestingly, our results suggest that patterns of butyrate‐related epigenetic changes in PD are most similar to those found in inflammatory bowel disease and clearly less similar to those found in Alzheimer's disease. Although evidence is mixed for irritable bowel syndrome, 68 in particular inflammatory bowel diseases 69 have been related to an increased risk of PD and Alzheimer's disease, but associations are stronger for PD. 70 Our results suggest that microbiome‐related epigenetic modulation could be a mechanism relating GI disorders and PD. Also, bipolar disorder 71 has been related to an increased PD risk, and our results support a role for common epigenetic mechanisms in this context. In contrast, we could not find significant overlap with epigenetic patterns found in schizophrenia 72 and depression, 73 which points to a lesser impact of epigenetics relating these disorders to PD. Recent meta‐analyses suggest alterations in SCFA‐producing bacteria in several psychiatric disorders and Alzheimer's disease, warranting further research in this context. 74 , 75 Interestingly, some overlap was observed with epigenetic patterns of rheumatoid arthritis, which reportedly is associated with a decreased PD risk. 76 , 77 , 78 Rheumatoid arthritis has also been related to the gut microbiome, but changes have been somewhat contrary to those observed in PD, for example, increase in Prevotella abundance in arthritis but decrease in PD. 79
In this study, we partly reanalyzed microbiome and metabolite data with methods not used in the previous publications. 31 , 32 We observed that fecal bacterial butyrate is inversely correlated with depressive symptoms (GDS‐15) in PD patients. Although there is no correlation between bacterial butyrate and other nonmotor symptom–related scales that include depressive‐related items (UPDRS I, NMSS, and NMSQ), GDS‐15 is more specific for assessing depressive symptoms. Although we were able to reproduce PD‐related microbiota alterations and identify decreased butyrate levels using earlier methods, we gained important new insights. Fecal butyrate and counts of the genera Prevotella, Romboutsia, and Roseburia were negatively correlated with depressive symptoms in PD patients, potentially implicating bacterial metabolites in this important nonmotor PD symptom.
In sum, combining metabolite, microbiome, clinical data, and DNA methylation profiling, our study is the first to reveal a possible relation between gut microbiome metabolite production and epigenetic changes, implicating immune and neural pathways in PD patients with potential impact on depressive symptoms. Furthermore, our results point to microbiota‐dependent epigenetic modulation as a potential pathway linking inflammatory bowel diseases and PD. Further research on altered bacterial metabolism and its impact on host physiology may reveal new biomarkers and therapeutic targets for PD.
Author Roles
Conceptualization: A.X., E.E., P.L., J.G., L.L.M., and V.L.; data curation: E.E., V.T.E.A., M.C.H., K.R., L.P., P.A.B.P., M.G.T., P.A., and F.S.; formal analysis: AX; funding acquisition: J.A.P., P.B., L.B., F.S., and V.L.; investigation: P.B., L.B., F.S., and V.L.; methodology: A.X., L.B., and V.L.; visualization: A.X.; writing—original draft: A.X.; writing—review and editing: A.X., E.E., S.G., P.B., L.B., P.A.B.P., V.T.E.A., M.C.H., K.R., M.G.T., and F.S. The authors read and approved the final manuscript.
Full financial disclosures for the previous 12 months
P.B. has received commercial support as consultant from Axial Therapeutics, Calico, CuraSen, Fujifilm‐Cellular Dynamics Inc., IOS Press Partners, LifeSci Capital LLC, Lundbeck A/S, Idorsia, and Living Cell Technologies Ltd. He has received commercial support for grants/research from Lundbeck A/S and Roche. He has ownership interests in Acousort AB and Axial Therapeutics.
S.G. receives commercial support as consultant from Coleman Research and Biogen.
V.T.E.A., P.A.B.P., L.P., P.A., and F.S. have patents issued (FI127671B, EP3149205B1, and US10139408B2) and pending (US20190137493A1, US20210109098A1, and EP3789501A1) that are assigned to NeuroBiome Ltd.
F.S. is founder and CEO of NeuroInnovation Oy and NeuroBiome Ltd., is a member of the scientific advisory board, and has received consulting fees and stock options from Axial Biotherapeutics. F.S. has received grants from the Academy of Finland, the Hospital District of Helsinki and Uusimaa, OLVI‐Foundation, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Wilhelm and Else Stockmann Foundation, the Emil Aaltonen Foundation, the Yrjö Jahnsson Foundation, and Renishaw and honoraria from AbbVie, Orion, GE Healthcare, Merck, Teva, Bristol Myers Squibb, Sanofi, and Biogen.
M.G.T. is an ex‐employee of Xencor Inc. and coinventor of dominant‐negative TNF inhibitor (XPro1595) as well as consultant for INmune Bio and holds stock ownership in the company. She is a consultant for and/or collaborates with Longevity Biotech, Cerebral Therapeutics, Innoviva, iMetabolic Pharma, Amylyx, Biogen/IONIS, Nanobiotix, and Jaya. She serves on the Medical Scientific Advisory Board of the Alzheimer's Association and the World Parkinson Coalition and is advisor for the Weston Family Foundation, The Michael J. Fox Foundation for Parkinson's Research, and the Quebec Parkinson's Network. She is editor‐in‐chief of Nature's Parkinson's Disease and serves on the editorial boards of Science Advances, Experimental Neurology, Neurobiology of Disease, Journal of Neuroinflammation, Journal of Parkinson's Disease, and PLoS ONE. Her research is funded by The Michael J. Fox Foundation, the NIH, and the Parkinson's Foundation.
L.B. was supported by the Farmer Family Foundation and the Michigan State University Gibby and Friends versus Parky Parkinson's Disease Research for this project. Other support she received include NIH R01 MH118211 and MJFF grant 010296.
K.R., P.L., J.A.P., A.X., L.L.M., M.C.H., and E.E. have nothing to disclose.
Supporting information
APPENDIX S1. Supporting Information
Acknowledgments
We dedicate this paper in memory of our research adviser, mentor, and friend, Viviane Labrie. We thank the Van Andel Institute Genomics and Bioinformatics and Biostatistics Cores. Open access funding enabled and organized by Projekt DEAL.
Viviane Labrie and Filip Scheperjans should be considered joint senior authors.
Funding agencies: This work was supported by a Farmer Family Foundation grant award and a Gibby & Friends versus Parky Award to P.B., with L.B., J.A.P., and V.L. F.S. received funding from The Michael J. Fox Foundation for Parkinson's Research, the Academy of Finland (295724 and 310835), the Hospital District of Helsinki and Uusimaa (UAK1014004, UAK1014005, and TYH2018224), the Finnish Medical Foundation, and the Finnish Parkinson Foundation.
Data Availability Statement
Microbiota data are available at the European Nucleotide Archive (accession number PRJEB27564) (https://www.ebi.ac.uk/ena/browser/view/PRJEB27564). Other data and files utilized in this study are available from the corresponding authors upon reasonable request.
References
- 1. Alexander GE. Biology of Parkinson's disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci 2004;6:259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu L, Pu J. Alpha‐Synuclein in Parkinson's disease: from Pathogenetic dysfunction to potential clinical application. Parkinsons Dis 2016;2016:1720621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fitzgerald E, Murphy S, Martinson HA. Alpha‐Synuclein pathology and the role of the microbiota in Parkinson's disease. Front Neurosci 2019;13:369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma J, Gao J, Wang J, Xie A. Prion‐like mechanisms in Parkinson's disease. Front Neurosci 2019;13:552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svensson E, Horváth‐Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 2015;78:522–529. [DOI] [PubMed] [Google Scholar]
- 6. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and Neuroinflammation in a model of Parkinson's disease. Cell 2016;167:1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheperjans F, Aho V, Pereira PAB, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015;30:350–358. [DOI] [PubMed] [Google Scholar]
- 8. Pereira PAB, Trivedi DK, Silverman J, et al. Multiomics implicate gut microbiota in altered lipid and energy metabolism in Parkinson's disease. NPJ Parkinsons Dis 2022;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cirstea MS, Yu AC, Golz E, et al. Microbiota composition and metabolism are associated with gut function in Parkinson's disease. Mov Disord 2020;35:1208–1217. [DOI] [PubMed] [Google Scholar]
- 10. Boertien JM, Murtomäki K, Pereira PAB, et al. Gut microbiome alterations in fecal samples of treatment‐naïve de novo Parkinson's disease patients med Rxiv 2022. 10.1101/2022.02.18.22270887 [DOI] [Google Scholar]
- 11. Boertien JM, Pereira PAB, Aho VTE, Scheperjans F. Increasing comparability and utility of gut microbiome studies in Parkinson's disease: a systematic review. J Parkinsons Dis 2019;9:S297–S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta‐analysis of the Parkinson's disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis 2021;7:27–021–00156–z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D‐J, Bakker BM. The role of short‐chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013;54:2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silva YP, Bernardi A, Frozza RL. The role of short‐chain fatty acids from gut microbiota in gut‐brain communication. Front Endocrinol 2020;11:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unger MM, Spiegel J, Dillmann K‐U, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age‐matched controls. Parkinsonism Relat Disord 2016;32:66–72. [DOI] [PubMed] [Google Scholar]
- 16. Sarkar S, Abujamra AL, Loew JE, Forman LW, Perrine SP, Faller DV. Histone deacetylase inhibitors reverse CpG methylation by regulating DNMT1 through ERK signaling. Anticancer Res 2011;31:2723–2732. [PubMed] [Google Scholar]
- 17. Schilderink R, Verseijden C, de Jonge WJ. Dietary inhibitors of histone deacetylases in intestinal immunity and homeostasis. Front Immunol 2013;4:226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimura I, Ichimura A, Ohue‐Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev 2020;100:171–210. [DOI] [PubMed] [Google Scholar]
- 19. Liu Q, Shimoyama T, Suzuki K, Umeda T, Nakaji S, Sugawara K. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand J Gastroenterol 2001;36:744–750. [DOI] [PubMed] [Google Scholar]
- 20. Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corrêa‐Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short‐chain fatty acids. Clin Transl Immunol 2016;5:e73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Sun J, Wang F, et al. Sodium butyrate exerts neuroprotective effects by restoring the blood‐brain barrier in traumatic brain injury mice. Brain Res 2016;1642:70–78. [DOI] [PubMed] [Google Scholar]
- 24. Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant‐like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 2007;62:55–64. [DOI] [PubMed] [Google Scholar]
- 25. Duan C, Huang L, Zhang C, et al. Gut commensal‐derived butyrate reverses obesity‐induced social deficits and anxiety‐like behaviors via regulation of microglial homeostasis. Eur J Pharmacol 2021;908:174338 [DOI] [PubMed] [Google Scholar]
- 26. Spichak S, Donoso F, Moloney GM, et al. Microbially‐derived short‐chain fatty acids impact astrocyte gene expression in a sex‐specific manner. Brain Behav Immun Health 2021;16:100318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short‐chain fatty acids in microbiota‐gut‐brain communication. Nat Rev Gastroenterol Hepatol 2019;16:461–478. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Wang F, Liu S, et al. Sodium butyrate exerts protective effect against Parkinson's disease in mice via stimulation of glucagon like peptide‐1. J Neurol Sci 2017;381:176–181. [DOI] [PubMed] [Google Scholar]
- 29. Trapecar M, Communal C, Velazquez J, et al. Gut‐liver Physiomimetics reveal paradoxical modulation of IBD‐related inflammation by short‐chain fatty acids. Cell Syst 2020;10:223–239.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trapecar M, Wogram E, Svoboda D, et al. Human physiomimetic model integrating microphysiological systems of the gut, liver, and brain for studies of neurodegenerative diseases. Sci Adv 2021;7:eabd1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aho VTE, Pereira PAB, Voutilainen S, et al. Gut microbiota in Parkinson's disease: temporal stability and relations to disease progression. EBioMedicine 2019;44:691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aho VTE, Houser MC, Pereira PAB, et al. Relationships of gut microbiota, short‐chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener 2021;16:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li P, Ensink E, Lang S, et al. Hemispheric asymmetry in the human brain and in Parkinson's disease is linked to divergent epigenetic patterns in neurons. Genome Biol 2020;21:61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. R Core Team R: A language and environment for statistical computing. 2020; http://www.r-project.org/index.html (2020).
- 35. Paulson JN. metagenomeSeq: Statistical analysis for sparse high‐throughput sequencing. 2021; https://www.bioconductor.org/packages/devel/bioc/vignettes/metagenomeSeq/inst/doc/metagenomeSeq.pdf (2021).
- 36. Tian Y, Morris TJ, Webster AP, et al. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017;33:3982–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aryee MJ, Jaffe AE, Corrada‐Bravo H, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014;30:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rahmani E, Schweiger R, Rhead B, et al. Cell‐type‐specific resolution epigenetics without the need for cell sorting or single‐cell biology. Nat Commun 2019;10:3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA‐sequencing and microarray studies. Nucleic Acids Res 2015;43:e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer‐Verlag, Heidelberg; 2016. [Google Scholar]
- 41. Triche TJ Jr, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD. Low‐level processing of Illumina Infinium DNA methylation BeadArrays. Nucleic Acids Res 2013;41:e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salas LA, Koestler DC, Butler RA, et al. An optimized library for reference‐based deconvolution of whole‐blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol 2018;19:64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou W, Laird PW, Shen H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res 2017;45:e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nordlund J, Bäcklin CL, Wahlberg P, et al. Genome‐wide signatures of differential DNA methylation in pediatric acute lymphoblastic leukemia. Genome Biol 2013;14:r105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y, Parmigiani G, Johnson WE. ComBat‐seq: batch effect adjustment for RNA‐seq count data. NAR Genom Bioinform 2020;2:lqaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reinius LE, Acevedo N, Joerink M, et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 2012;7:e41361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He F, Berg A, Imamura Kawasawa Y, et al. Association between DNA methylation in obesity‐related genes and body mass index percentile in adolescents. Sci Rep 2019;9:2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sabogal C, Su S, Tingen M, Kapuku G, Wang X. Cigarette smoking related DNA methylation in peripheral leukocytes and cardiovascular risk in young adults. Int J Cardiol 2020;306:203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McLean CY, Bristor D, Hiller M, et al. GREAT improves functional interpretation of cis‐regulatory regions. Nat Biotechnol 2010;28:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raudvere U, Kolberg L, Kuzmin I, et al. G:profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 2019;47:W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reimand J, Isserlin R, Voisin V, et al. Pathway enrichment analysis and visualization of omics data using g:profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc 2019;14:482–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Javierre BM, Burren OS, Wilder SP, et al. Lineage‐specific genome architecture links enhancers and non‐coding disease variants to target gene promoters. Cell 2016;167:1369–1384.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mootha VK, Lindgren CM, Eriksson K‐F, et al. PGC‐1alpha‐responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273. [DOI] [PubMed] [Google Scholar]
- 56. Bulik‐Sullivan BK, Loh P‐R, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome‐wide association studies. Nat Genet 2015;47:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bulik‐Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nalls MA, Blauwendraat C, Vallerga CL, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta‐analysis of genome‐wide association studies. Lancet Neurol 2019;18:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng J, Erzurumluoglu AM, Elsworth BL, et al. LD hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 2017;33:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker‐gene surveys. Nat Methods 2013;10:1200–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bandres‐Ciga S, Saez‐Atienzar S, Kim JJ, et al. Large‐scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol 2020;140:341–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 1978;14:105–113. [DOI] [PubMed] [Google Scholar]
- 63. Bach Knudsen KE, Lærke HN, Hedemann MS, et al. Impact of diet‐modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018;10:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson's disease patients. Aging 2015;7:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota‐gut‐brain axis? Neurochem Int 2016;99:110–132. [DOI] [PubMed] [Google Scholar]
- 66. Krautkramer KA, Kreznar JH, Romano KA, et al. Diet‐microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell 2016;64:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Val‐Laillet D, Guérin S, Coquery N, et al. Oral sodium butyrate impacts brain metabolism and hippocampal neurogenesis, with limited effects on gut anatomy and function in pigs. FASEB J 2018;32:2160–2171. [DOI] [PubMed] [Google Scholar]
- 68. Mertsalmi TH, But A, Pekkonen E, Scheperjans F. Irritable bowel syndrome and risk of Parkinson's disease in Finland: a Nationwide registry‐based cohort study. J Parkinsons Dis 2021;11:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu F, Li C, Gong J, Zhu W, Gu L, Li N. The risk of Parkinson's disease in inflammatory bowel disease: a systematic review and meta‐analysis. Dig Liver Dis 2019;51:38–42. [DOI] [PubMed] [Google Scholar]
- 70. Fu P, Gao M, Yung KKL. Association of Intestinal Disorders with Parkinson's disease and Alzheimer's disease: a systematic review and meta‐analysis. ACS Chem Nerosci 2020;11:395–405. [DOI] [PubMed] [Google Scholar]
- 71. Faustino PR, Duarte GS, Chendo I, et al. Risk of developing Parkinson disease in bipolar disorder: a systematic review and meta‐analysis. JAMA Neurol 2020;77:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kuusimäki T, Al‐Abdulrasul H, Kurki S, et al. Increased risk of Parkinson's disease in patients with schizophrenia Spectrum disorders. Mov Disord 2021;36:1353–1361. [DOI] [PubMed] [Google Scholar]
- 73. Gustafsson H, Nordström A, Nordström P. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 2015;84:2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spichak S, Bastiaanssen TFS, Berding K, et al. Mining microbes for mental health: determining the role of microbial metabolic pathways in human brain health and disease. Neurosci Biobehav Rev 2021;125:698–761. [DOI] [PubMed] [Google Scholar]
- 75. Nikolova VL, Hall MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta‐analysis. JAMA Psychiat 2021;78:1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bacelis J, Compagno M, George S, et al. Decreased risk of Parkinson's disease after rheumatoid arthritis diagnosis: a nested case‐control study with matched cases and controls. J Parkinsons Dis 2021;11:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease: a population‐based case‐control study. Neurology 2009;73:1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li C, Ou R, Shang H. Rheumatoid arthritis decreases risk for Parkinson's disease: a Mendelian randomization study. NPJ Parkinsons Dis 2021;7:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013;2:e01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information
Data Availability Statement
Microbiota data are available at the European Nucleotide Archive (accession number: PRJEB27564) (https://www.ebi.ac.uk/ena/browser/view/PRJEB27564). Other data and files utilized in this study are available from the corresponding authors on reasonable request.
Microbiota data are available at the European Nucleotide Archive (accession number PRJEB27564) (https://www.ebi.ac.uk/ena/browser/view/PRJEB27564). Other data and files utilized in this study are available from the corresponding authors upon reasonable request.


