Summary
Several recently published trials investigate novel therapies for relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL). To estimate the benefit of these therapies in the real‐world setting, comprehensive data on patients treated in clinical routine are needed. We report outcomes for 736 R/R DLBCL patients identified among all curatively treated DLBCL patients in Sweden in the period 2007–2014. Survival and associations with disease characteristics, second‐line treatment and fulfilment of chimaeric antigen receptor (CAR) T‐cell trial criteria were assessed. Median overall survival (OS) was 6.6 months (≤70 years 9.6 months, >70 years 4.9 months). Early relapse (≤12 months) was strongly associated with selection of less intensive treatment and poor survival. Among patients of at most 70 years of age, 63% started intensive second‐line treatment and 34% received autologous stem cell transplantation (ASCT). Two‐year OS among transplanted patients was 56% (early relapse ≤12 months 40%, late relapse >12 months 66%). A minority of patients 76 years (n = 178/506, 35%) fitted CAR T trial criteria. Median progression‐free survival (PFS) for patients with early relapse fitting trial criteria was 4.8 months. In conclusion, most R/R DLBCL manifest early and are often ineligible for or cannot complete intensive regimens resulting in dismal survival. Real‐world patients eligible for CAR T trials also did poorly, providing a benchmark for efficacy of novel therapies.
Keywords: clinical research, epidemiology, non‐Hodgkin lymphoma, stem cell transplantation, tumour immunotherapy
INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is the most common lymphoid neoplasm in the western world and is characterized by an aggressive clinical presentation. 1 , 2 , 3 Most patients are cured with anthracycline‐containing immunochemotherapy, 4 , 5 , 6 , 7 but approximately one in four experience primary refractory disease or relapse (R/R DLBCL) with subsequent poor prognosis. 8 Intensive multi‐agent induction chemotherapy followed by consolidation with high‐dose therapy and autologous stem cell transplantation (ASCT) is standard treatment for young, fit patients (<65–70 years) with chemosensitive R/R DLBCL. 9 , 10 , 11 , 12 , 13 Transplant‐ineligible patients are usually treated with less intensive regimens and face a dismal prognosis. 10
Current knowledge about outcomes of R/R DLBCL is mainly derived from patients treated in clinical trials or at academic centres with selected younger patient populations. 11 , 14 , 15 , 16 Although previous studies have shown heterogeneity in treatment selection and outcomes, 14 , 16 , 17 comprehensive evaluations of outcomes in non‐selected R/R DLBCL patients of all ages are missing. Several novel therapies are being investigated or have been approved already for R/R DLBCL including chimaeric antigen receptor (CAR) T‐cell therapy, 18 , 19 , 20 , 21 , 22 , 23 , 24 anti‐CD19 antibodies, 25 and bispecific antibodies, 26 , 27 all with encouraging response rates. However, these new therapies are expensive and, for CAR T in particular, logistically challenging. Therefore, identification of the patients who benefit most from these therapies versus those who are likely to do well with standard treatment is critical. 28 Recent publications have raised concern that patients enrolled in CAR T trials are substantially different from real‐world R/R DLBCL patients, threatening the generalizability of results to clinical routine. 28 , 29 With recently presented pivotal phase 3 trials of CAR T against standard‐care second line for large B‐cell lymphomas, there is a need for population‐based outcome data to provide appropriate benchmarking and understand the impact of trial selection criteria on real‐world outcomes. 22 , 23 , 24
In this study, detailed outcomes of a large population‐based cohort of 736 R/R DLBCL patients treated in Sweden during the period 2007–2018 are reported and the impact of CAR T trial eligibility is assessed.
METHODS
Study population
The study base consisted of all patients diagnosed with primary DLBCL in the period 2007–2014 registered in the national Swedish Lymphoma Register who received curative‐intent treatment (defined as anthracycline‐containing but also accepting replacement of anthracycline with etoposide). Medical charts for all 3549 patients were reviewed to identify refractoriness or relapse in 2007–2018, as previously described. 8 Primary refractory disease was defined as stable or progressive disease (SD/PD) as best response to primary treatment. We identified 853 patients with R/R DLBCL and those who were alive were asked for informed consent for study participation. Fifteen patients (1.8%) did not consent and were excluded. Another 102 patients (12%) were excluded due to limited access to medical charts or missing information for several main variables. To ascertain that the final study cohort was representative of all identified R/R DLBCL patients, we compared median age and overall survival (OS) in the two cohorts and found these to be similar (Figure S1). The regional ethics committee in Stockholm approved the study (Dnr 2015/2028–31/2).
Clinical characteristics and outcome
Detailed information regarding clinical characteristics, second and further treatment lines and treatment response was collected from medical charts and a secondary international prognostic index (IPI) score was calculated. During the study period, patients were evaluated with positron emission tomography–computed tomography (PET‐CT) or CT.
Second‐line treatments were categorized as intensive chemotherapy, remission‐inducing chemotherapy, palliative therapy or no active antitumoural therapy. Intensive chemotherapy included DHAP (dexamethasone, high‐dose cytarabine, cisplatin), ICE (iphosphamide, carboplatin, etoposide), GDP (gemcitabine, dexamethasone, cisplatin) and high‐dose methotrexate and/or high‐dose cytarabine). Remission‐inducing chemotherapy included GemOx (gemcitabine, oxaliplatin), IME (iphosphamide, methotrexate, etoposide) and bendamustine. Palliative therapy consisted of single‐agent intravenous (IV) chemotherapy, oral chemotherapy or radiotherapy.
The Swedish health system ensures access to specialized health care in oncology/haematology to all residents. During the study period, standard of care in Sweden for R/R DLBCL patients with first relapse was, for patients under 70 years of age, platinum‐based chemotherapy with the aim of consolidation with ASCT. For patients older than 70 years standard of care was remission‐inducing chemotherapy or palliative treatment depending on performance status and comorbidities.
Information regarding cell of origin based on the Hans algorithm was collected from pathology reports. 30 The cohort was linked with the National Patient and Cancer Registers to add data regarding comorbidity and cancer history. The Charlson comorbidity index (CCI) was calculated based on registered disease codes during 10 years before R/R DLBCL. 31
Eligibility to CAR T therapy in the clinical trial setting was retrospectively evaluated among patients at first R/R disease, based on inclusion and exclusion criteria in the ZUMA‐7, 22 TRANSFORM 23 and ZUMA‐1 trials. 19 , 21 To be considered eligible, patients should have started second‐line intensive or remission‐inducing therapy (the latter was allowed since this is standard for patients older than 70 years of age). Inclusion criteria used were Eastern Cooperation Oncology Group (ECOG) 0–1, neutrophils above 1 × 109, lymphocytes more than 100/μl, thrombocytes above 50 × 109, glomerular filtration rate (GFR) above 45 ml/min, no significant hepatic, renal, cardiac or pulmonary comorbidity or autoimmune condition requiring therapy. Organ function could not be addressed beyond existing comorbidity. Exclusion criteria were prior malignancy within 2 years, human immunodeficiency virus (HIV), active hepatitis B/C virus (HBV/HCV), central nervous system (CNS) disease including CNS involvement of lymphoma at relapse. To address eligibility to ASCT (as in ZUMA‐7 and TRANSFORM), we considered patients aged 18–70 years separately. Since criteria related to time to relapse differed by trial, we evaluated R/R disease within and beyond 12 months separately.
Statistics
The primary outcome was overall survival (OS) defined as time from date of R/R disease until date of death of any cause or end of follow‐up (March 31 2019). OS was estimated using the Kaplan–Meier method overall and separately by clinical characteristics and fulfilment of trial criteria. Cox regression models were used to assess the association between clinical characteristics and OS. Multivariable models were adjusted for the assumed causal structure relating the respective characteristics/exposures to outcome (Table S1). The proportional‐hazards assumption was assessed using the Schoenfeld residuals. Secondary outcomes were progression‐free survival (PFS, defined as time from date of R/R disease until progression, death or end of follow‐up), and selection to intensive second‐line chemotherapy and ASCT, evaluated using multivariable logistic regression and Cox regression models, respectively. The proportion of patients not older than 70 years of age who received ASCT was estimated non‐parametrically using the Aalen–Johansen method in the presence of the competing risk of death. The Kaplan–Meier method was further used to estimate OS and PFS from ASCT among patients not older than 70 years of age. The statistical analyses were performed using STATA version 16 (Stata Corp., College Station, TX, USA).
RESULTS
Patient characteristics and treatment
A total of 736 R/R DLBCL patients were included. Median age was 71 years (range 18–99), and 60% (n = 438) were men (Table 1). The majority of patients relapsed within 12 months of primary diagnosis (n = 457, 62%), and 208 (28%) were primary refractory. A majority (57%) had stage IV disease and 37% had secondary IPI 3–5 with similar distributions in patients aged not older than 70 vs. over 70 years of age. Germinal centre B (GCB) subtype (n = 278, 38%) was more common than non‐GCB (n = 158, 22%) (information was however missing for the remaining patients). Among patients no more than 70 years old, 63% (n = 220) started intensive second‐line regimens, mostly DHAP/DHAO (41%, n = 90) or ICE (37%, n = 81), whereas 18% (n = 64) received remission‐inducing regimens and 19% (n = 66) palliative or no active treatment (Figure S2). Overall, 41% (n = 296) also received anti‐CD20 antibody at relapse [63% (n = 161) among intensively treated patients]. Among patients not older than 70 years who received intensive second‐line treatment, the overall response rate (ORR) was 56% [30% complete remission (CR; n = 65), 26% partial remission (PR, n = 57)], whereas 5% (n = 10) had SD and 26% (n = 57) PD. Response was not evaluated in 31 patients (14%), most of whom died within 6 months, indicating lack of response.
TABLE 1.
Clinical characteristics of all patients with relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) and separately by age ≤70 years and >70 years at relapse
| All R/R patients, n = 736 | Patients aged ≤70 years at relapse, n = 350 | Patients aged >70 years at relapse, n = 386 | |
|---|---|---|---|
| Sex | |||
| Men | 438 (60) | 211 (60) | 227 (59) |
| Women | 298 (40) | 139 (40) | 159 (41) |
| Age at relapse | |||
| ≤50 | 52 (7) | 52 (15) | – |
| 51–60 | 92 (12) | 92 (26) | – |
| 61–70 | 206 (28) | 206 (59) | – |
| 71–80 | 247 (34) | – | 247 (64) |
| >80 | 139 (19) | – | 139 (36) |
| Time to relapse | |||
| ≤6 months | 239 (32) a | 127 (36) | 112 (29) |
| >6–12 months | 218 (30) | 98 (28) | 120 (31) |
| >12–18 months | 84 (11) | 40 (11) | 44 (11) |
| >18–24 months | 53 (7) | 22 (6) | 31 (8) |
| >24 months | 142 (19) | 63 (18) | 79 (20) |
| Charlson Comorbidity Index (CCI) | |||
| 0 | 341 (46) | 204 (58) | 137 (36) |
| 1 | 122 (17) | 46 (13) | 76 (20) |
| 2+ | 273 (37) | 100 (29) | 173 (45) |
| LDH at relapse | |||
| Elevated | 330 (45) | 182 (52) | 148 (38) |
| Normal | 248 (34) | 104 (30) | 144 (37) |
| Missing | 158 (22) | 64 (18) | 94 (24) |
| Stage at relapse | |||
| I | 66 (9) | 28 (8) | 38 (10) |
| II | 98 (13) | 56 (16) | 42 (11) |
| III | 72 (10) | 37 (11) | 35 (9) |
| IV | 421 (57) | 199 (57) | 222 (58) |
| Missing | 79 (11) | 30 (9) | 49 (13) |
| Extranodal sites at relapse b | |||
| 0 | 310 (42) | 151 (43) | 159 (41) |
| 1 | 297 (40) | 136 (39) | 161 (42) |
| 2 or more | 128 (18) | 62 (18) | 66 (17) |
| Missing | 1 (0.0) | 1 (0.3) | 0 (0) |
| Performance status at relapse | |||
| 0 | 243 (33) | 128 (37) | 115 (30) |
| 1 | 289 (39) | 139 (40) | 150 (39) |
| 2 | 65 (9) | 30 (9) | 35 (9) |
| 3 | 32 (4) | 7 (2) | 25 (6) |
| 4 | 15 (2) | 6 (2) | 9 (2) |
| Missing | 92 (12) | 40 (11) | 52 (14) |
| IPI at relapse | |||
| 0 | 19 (2.6) | 19 (5.4) | 0 (0) |
| 1 | 89 (12.1) | 47 (13.4) | 42 (10.9) |
| 2 | 192 (26.1) | 95 (27.1) | 97 (25.1) |
| 3 | 179 (24.3) | 88 (25.1) | 91 (23.6) |
| 4 | 86 (11.7) | 30 (5.6) | 56 (14.5) |
| 5 | 9 (1.2) | 3 (0.9) | 6 (1.6) |
| Missing | 162 (22.0) | 68 (19.4) | 94 (24.4) |
| Molecular subtype | |||
| GCB | 278 (38) | 145 (41) | 133 (34) |
| Non‐GCB | 158 (22) | 85 (24) | 73 (19) |
| Unclassifiable/missing | 300 (41) | 120 (34) | 180 (47) |
| Second‐line treatment type | |||
| Intensive regimens | 255 (35) | 220 (63) | 35 (9) |
| DHAP/DHAO | 98 (38) | 90 (41) | 8 (23) |
| ICE | 89 (35) | 81 (37) | 8 (23) |
| GDP | 11 (4) | 8 (4) | 3 (9) |
| HD‐Mtx and/or HD‐AraC | 50 (20) | 37 (17) | 13 (37) |
| Other intensive | 7 (3) | 4 (2) | 3 (9) |
| Remission‐inducing regimens | 204 (28) | 64 (18) | 140 (36) |
| GemOx | 7 (3) | 1 (12) | 6 (4) |
| IME/MIME/IMVP‐16 | 109 (53) | 46 (72) | 63 (45) |
| Bendamustine | 51 (25) | 8 (12) | 43 (31) |
| CHOP | 21 (10) | 6 (9) | 15 (11) |
| VAdriaC | 10 (5) | 0 (0) | 10 (7) |
| Other remission‐inducing | 6 (3) | 3 (5) | 3 (2) |
| Palliative treatment | 147 (20) | 29 (8) | 118 (31) |
| Only radiotherapy | 76 (52) | 17 (59) | 59 (50) |
| Palliative IV chemotherapy | 26 (18) | 6 (21) | 20 (17) |
| Palliative oral chemotherapy | 45 (31) | 6 (21) | 39 (33) |
| No active treatment | 130 (18) | 37 (11) | 93 (24) |
| Immunotherapy c | |||
| Yes | 296 (40) | 142 (41) | 221 (57) |
| No | 363 (49) | 183 (52) | 113 (29) |
| Missing | 77 (10) | 25 (7) | 52 (14) |
Palliative IV chemotherapy included single‐agent regimens such as cyclophosphamide, gemcitabine or vinblastine. Palliative oral chemotherapy included mainly trophosphamide and chlorambucil. Proportions may add up to 99% or 101% due to rounding.
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; DHAP/O, dexamethasone, high‐dose cytarabine, cisplatin/oxaliplatin; GCB, germinal centre B; GDP, gemcitabine, dexamethasone, cisplatin; GemOx, gemcitabine, oxaliplatin; HD‐Mtx and/or HD‐AraC, high‐dose methotrexate and/or high‐dose cytarabine; ICE, iphosphamide, carboplatin, etoposide; IME, iphosphamide, methotrexate, etoposide; IPI, International prognostic index; IV, intravenous; LDH, lactate dehydrogenase; VAdriaC, vincristine, doxorubicin, cyclophosphamide.
208 patients were primary refractory with stable or progressive disease as best response to primary therapy.
In the whole cohort 118 patients had involvement of the central nervous system at relapse.
A majority received rituximab, and three received ofatumumab.
Among patients over 70 years old, 9% (n = 35) were selected for intensive regimens whereas 36% received remission‐inducing therapy (n = 140) and 55% (n = 211) palliative or no active treatment (Table 1). Among those who received remission‐inducing therapy, ORR was 42% [24% CR (n = 34), 18% PR (n = 25)]. Five patients more than 70 years old could receive ASCT consolidation.
Survival
Overall, outcomes were poor with a median OS of 6.6 months [95% confidence interval (CI): 5.8–7.9] and 2‐year OS of 27% (95% CI: 24–30) (Figure 1A). Median PFS for all patients was 3.9 months (95% CI: 3.5–4.6) and 2‐year PFS was 19% (95% CI: 16–22) (Figure S3). Among patients not older than 70 years old, median OS was 9.6 months (95% CI: 8.0–11.8) and 2‐year OS was 35% (95% CI: 30–40) and among patients older than 70 years median OS was 4.9 months (95% CI: 4.1–5.7) and 2‐year OS was 19% (95% CI: 16–23) (Figure 1A). When stratified by time from primary diagnosis to R/R disease, the 2‐year OS ranged from 19% (95% CI: 14–24) for patients with R/R disease within 6 months, to 55% (95% CI: 47–63) for patients relapsing after more than 2 years (Figure 1B). For patients with primary refractory disease, median OS was 4.4 months (95% CI: 3.8–5.0) and the 2‐year OS was 14% (95% CI: 10–19) (Figure S4). When stratified by secondary IPI (Figure 1C), 2‐year OS was only 7% (95% CI: 3–14) for high‐risk (4, 5), vs 50% (95% CI: 40–59) for low‐risk IPI (0–1). In multivariable analyses, time to relapse, stage, performance status and secondary IPI were independent predictors of survival overall and among patients aged not older than 70 and more than 70 years of age (Table S2).
FIGURE 1.
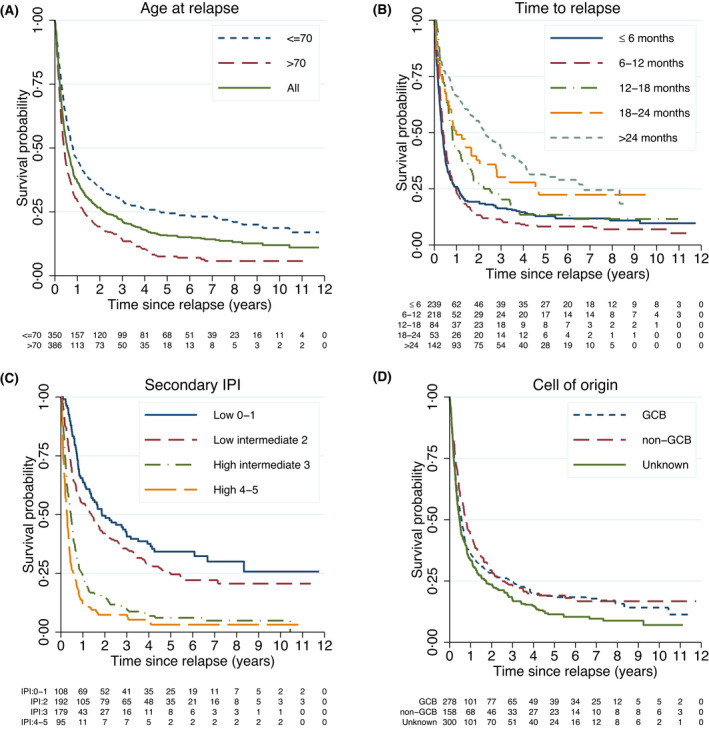
Overall survival among all relapsed/refractory diffuse large B‐cell lymphoma patients (R/R DLBCL) (n = 736). (A) Stratified by age of at most 70 and older than 70 years at relapse. (B) Stratified by time to first relapse. (C) Stratified by secondary IPI score. * (D) Stratified by cell of origin. ** GCB, germinal centre B; IPI, international prognostic index. *, Based on age, stage, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase level and number of extranodal sites at time of relapse/refractoriness. **, Information regarding cell of origin was based on immunohistochemistry mostly from primary diagnosis and categorized using the Hans algorithm
Intensive second‐line therapy and ASCT
Among patients of at most 70 years of age, R/R disease within 6 months ago, ECOG 2–4, high secondary IPI (4, 5), age 61–70 years and CCI score 1 were associated with a lower probability of receiving intensive second‐line therapy (Figure 2A). Among those starting intensive second‐line, 47% (n = 103/220) proceeded to ASCT, with relapse within 12 months, age 61–70 and secondary IPI 2–3 (but not higher) predicting a lower probability of ASCT (Figure 2B).
FIGURE 2.
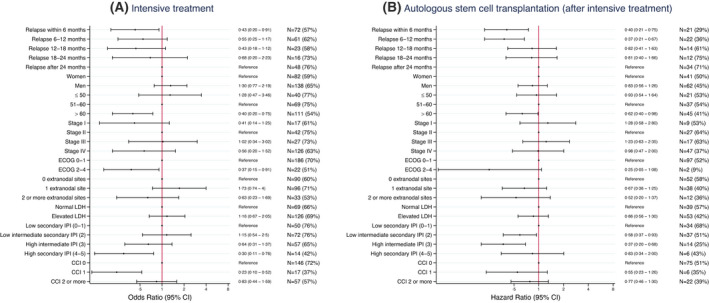
Disease characteristics and probability of receiving intensive second‐line treatment and going through autologous stem cell transplantation. (A) Associations between disease characteristics and probability of receiving intensive second‐line treatment among patients with relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) of at most 70 years of age, estimated using multivariable logistic regression [odds ratios and 95% confidence intervals (CI)]. (B) Associations between disease characteristics and probability of going through ASCT after intensive treatment, among patients with relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) of at most 70 years of age, estimated using Cox regression (hazard ratios, 95% CI). CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; IPI, international prognostic index; LDH, lactate dehydrogenase
Among all patients of at most 70 years of age, 34% completed ASCT (early relapse: 22%, late relapse: 57%) (Figure 3). Median age among ASCT‐treated patients was 59 years. Most patients had their transplant in second line (93%), whereas eight patients were transplanted in later lines. Most received carmustine, etoposide, cytarabine and melphalan (BEAM) as conditioning regimen (74%). Two‐year OS from transplantation was 56% (95% CI: 46–64) (Figure 4), median PFS was 16.4 months (10.8–35.8) and 2‐year PFS was 48% (38–57) (Figure S5). Survival following ASCT differed significantly by time to relapse and secondary IPI, but not age, stage or cell of‐origin (Figure 4, Table S3). For patients transplanted for early relapse, 2‐year OS was 40% (95% CI: 26–53) and median PFS 5.0 months (95% CI: 3.8–12.9), whereas patients transplanted for late relapse had a 2‐year OS of 66% (95% CI: 54–76) and a median PFS of 30.6 months (95% CI: 15.9–49.4) (Figure 4B, Figure S5).
FIGURE 3.
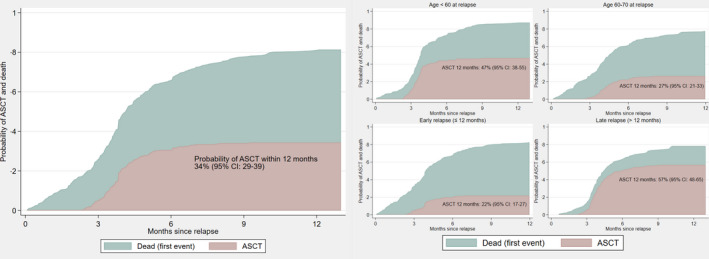
Proportion of patients with relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) of at most 70 years of age who received autologous stem cell transplantation (ASCT). (A) Among all patients. (B) Among patients with age under 60 years at relapse. (C) Among patients with age 60–70 years at relapse. (D) Among patients with relapse within 12 months. (E) Among patients with relapse later than 12 months. Proportions were estimated in the presence of the competing risk of death as first event. CI, confidence interval
FIGURE 4.
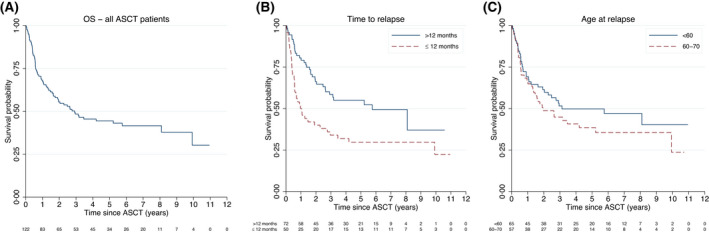
Overall survival (OS) for all relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) patients of at most 70 years at relapse who underwent autologous stem cell transplantation (ASCT). (A) Among all patients. (B) Among patients with early relapse (≤12 months from primary diagnosis) and late relapse (>12 months) separately. (C) Among patients aged less than 60 or 60–70 years separately. Patients were followed from date of ASCT until death of any cause
Fulfilment of CAR T trial criteria
In the retrospective assessment of CAR T therapy trial eligibility, age limits were set to 18–76 years (n = 506, 69%) (Figure S6). In this group, 379 patients (75% of all ≤76 years) started an IV second‐line therapy, of whom 178 patients (35% of all ≤76 years) also fulfilled the selected trial inclusion and exclusion criteria (Figure S6). Fewer patients with early relapse fitted trial criteria (n = 94/320, 29%) compared with patients with late relapse (n = 84/186, 45%). Younger patients (≤70 years) fitted trial criteria more often (n = 130/350, 37%). Among patients not older than 76 years old with early relapse, median PFS was 4.8 months (95% CI: 2.9–6.3) for those who fitted trial criteria, and 3.1 months (95% CI: 2.6–3.8) for all patients who started any IV second‐line therapy (Figure 5). Similarly, only small differences in PFS were seen in other patient subgroups by age and early/late relapse comparing those who fitted trial criteria with all who could start any IV second‐line therapy (Figure 5). The 2‐year OS for those who fitted trial criteria was 27% (95% CI: 18–36).
FIGURE 5.
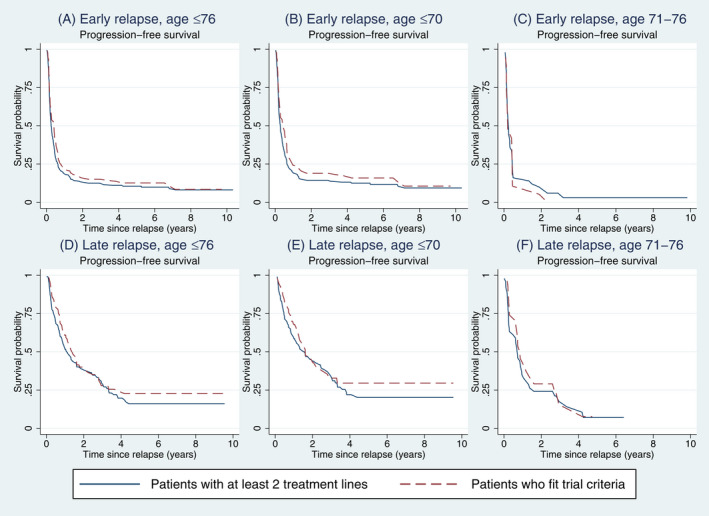
Progression‐free survival among all relapsed/refractory diffuse large B‐cell lymphoma (R/R DLBCL) patients of 76 years of age or younger who received any intravenous (IV) second‐line treatment (i.e. received at least 2 treatment lines) and among those who fulfilled chimaeric antigen receptor (CAR) T‐cell trial criteria. (A) Among all patients of at most 76 years who received at least two treatment lines (solid line) and among those who fulfilled CAR T‐cell trial criteria (dashed line). (B) Among patients of at most 70 years with early relapse. (C) Among patients of 71–76 years with early relapse. (D) Among all patients of at most 76 years who received at least two treatment lines (solid line) and among those who fulfilled CAR T‐cell trial criteria (dashed line). (E) Among patients of 71–76 years with late relapse. (F) Among patients of 71–76 years with late relapse.
DISCUSSION
This is the largest population‐based study of R/R DLBCL patients reported to date. Results confirm generally poor outcomes in the era prior to cellular therapies but also point to substantial outcome variation, highly relevant for clinicians facing difficult treatment decisions for R/R DLBCL patients. Importantly, patients with primary refractory disease had a 2‐year survival of 14% as compared with 66% in patients not older than 70 years old with late relapse treated with intensive second‐line therapy and ASCT. In unselected older R/R DLBCL patients (>70 years), seldom in focus in previous studies, the 2‐year OS was only 19%, underscoring the high need of new therapies for this group in particular. In a novel approach addressing eligibility of CAR T therapy in second line, we note that fulfilment of trial criteria among patients not older than 76 years old with early relapse was not associated with a clear PFS benefit. This is relevant information when estimating the potential value of CAR T therapies in the broader real‐world setting and assessing the external validity of recently published CAR T phase 3 trials. 22 , 24
There is a lack of population‐based studies of R/R DLBCL outcomes encompassing the entire spectrum of time to relapse and age group. Whereas previous studies have focused on patients younger than 65–70 years old, 11 , 14 the majority of the patients in our cohort were over 70 years of age at first relapse. This group was unlikely to receive curative‐intent salvage therapy and, as expected, their median OS was short, 4.9 months. Few clinical trials have targeted older patients but in a phase 2 study of transplant‐ineligible patients (median 72 years), Cazelles et al. reported a median OS of 10 months following treatment with rituximab (R)‐GemOx. 32 In a randomized study comparing polatuzumab vedotin and R‐bendamustine against R‐bendamustine alone in patients 30–86 years old, median OS was 12.4 months in the experimental arm vs 4.7 months in the standard arm. 15 In the recent ZUMA‐7 22 and TRANSFORM 23 randomized phase 3 trials comparing CAR T products with standard second‐line therapy for large B‐cell lymphoma, subgroup analyses showed that CAR T was more efficacious than standard therapy for both elderly (aged ≥65) and younger patients. In the single‐arm trials of CAR T in third or later lines including patients up to 76 years old 18 , 19 ORRs for patients above and below 65 years were also similar, again suggesting that CAR T therapy is safe and effective regardless of patient age unlike standard ASCT salvage therapy. In a study by Nastoupil et al. reporting on real‐world outcomes of patients treated with CAR T in third or later lines, patients aged 60 years or older even had better outcomes than younger patients. 33 Taken together, these findings highlight the enormous potential for CAR T and other new treatments among older patients with R/R DLBCL (representing the majority of R/R DLBCL patients).
The first pivotal CAR T trials for R/R DLBCL in third or later lines reporting CR rates of 40%–60% 18 , 19 , 20 were single‐arm trials without comparators, and therefore, the external generalizability of the results has been questioned. 29 In a real‐world study of 69 CAR T‐treated patients and 146 patients treated with standard of care in third‐line, Sermer et al. reported that some patients indeed achieve long‐term remissions with standard therapy. 28 Others have also identified the need of robust real‐world data for comparison of trials of novel agents in R/R DLBCL. 14 , 34 In this nationwide study of consecutive R/R DLBCL patients, we defined subpopulations of patients using typical CAR T trial criteria to enhance comparability with trial results. In ZUMA‐7, a superior median event‐free survival (EFS) of 8.3 months in the CAR T arm was reported versus 2 months in the standard arm (median PFS 14.7 vs 3.7 months). 22 In an interim analysis of TRANSFORM, median EFS was 10.1 months in the CAR T arm vs 2.3 months with standard of care (median PFS 14.8 vs 5.7 months). 23 In contrast, the BELINDA trial did not report any benefit of CAR T (median EFS 3 months in both arms); however the groups were not completely balanced. 24 Interestingly, median PFS among patients not older than 76 years of age with early relapse fulfilling trial criteria in our study (representing 35% of all patients ≤76 years) was in the same range (4.8 months) as those reported for the standard‐care arms in the phase 3 CAR T trials. Furthermore, fulfilment of trial criteria was not associated with any notable PFS advantage when compared with all patients 18–76 years old that could start any IV second‐line therapy (median PFS 3.1 months). This suggests that patient selection for the CAR T trials has not led to enrolment of patients that are widely different from those treated in a routine clinical setting and that the external generalizability of the studies is likely to be high. The short PFS in both these groups underscores the potential for improved outcomes with CAR T and other novel agents in second line for early relapse in routine care.
In our study, we further confirmed the strong association between short time to relapse and low probability of receiving standard second‐line therapy and ASCT (among patients ≤70 years), and short survival (all ages). Overall, 2‐year OS ranged from 19% among patients with R/R disease within at most 6 months versus 55% for patients with relapse after more than 2 years. The CORAL trial reported similar patterns with 3‐year OS varying from 39% to 64% if relapse manifested within or after 12 months. 11 Another study of 331 patients with early R/R DLBCL treated at 15 US academic centres (75% <65 years) reported that only 40% could proceed to ASCT consolidation after intensive salvage therapy, 35 compared with 34% among patients of 70 years old or youger in our study. In a single‐centre US study, Farooq et al. reported that less than 50% of 244 patients treated for R/R large B‐cell lymphomas could receive ASCT consolidation, but outcomes were encouraging among those who did, with a 4‐year post‐ASCT survival of 51% for late relapses, 16 compared with 66% in our study. Therefore, it seems fair to conclude that standard‐of‐care intensive second‐line therapy with ASCT consolidation still has a role for young fit patients with late relapses.
The most evident explanation for the prognostic importance of time to relapse is an underlying tumour biology associated with reduced chemosensitivity resulting in short remissions or even no response to first‐line immunochemotherapy. The bio‐CORAL study demonstrated that the ABC subtype had worse outcome, implying prognostic importance of cell of origin also in the relapse setting. 36 In our study, we detected no difference in outcome for non‐GCB compared with GCB, although missing data limited interpretation. Several additional studies have not shown a prognostic value of cell of origin in the relapse setting. 16 , 37 , 38 In the past few years, landmark studies have suggested new molecular DLBCL subgroups based on comprehensive genetic sequencing and functional aspects with prognostic implications at primary diagnosis. 39 , 40 , 41 , 42 If these also have prognostic value at relapse is however yet to be shown.
Strengths of our study include the large sample size and truly population‐based design combined with detailed data from a comprehensive medical chart review. The Swedish system with unique personal identification numbers and national health care registration further ensured complete follow‐up regarding survival. A weakness is that data were collected retrospectively and thus information was sometimes missing in the medical charts, e.g. on biological characteristics such as cell of origin. Also, we could not distinguish high‐grade B‐cell lymphoma with MYC, BCL2 and/or BCL6 rearrangements since this subgroup was not defined in routine care during the entire study period. This also means that patients were treated according to what was considered standard of care in 2007–2018 and that the results do not reflect recently approved agents in the R/R DLBCL setting.
In summary, in this large study of consecutive R/R DLBCL patients, we illustrate the vast heterogeneity in tolerance and outcomes with standard second‐line regimens and note good standard‐care outcomes among young patients with late relapse. The lack of outcome benefit for patients fulfilling the trial criteria underscores the enormous potential for novel therapies, primarily for early relapse and among older patients.
CONFLICT OF INTEREST
El‐Galaly: previous employment by Roche Ltd (Basel) and speakers fee from Abbvie. Jerkeman: research support from Abbvie, AstraZeneca, Janssen, Gilead, BMS and Roche. Honoraria from Abbvie, AstraZeneca, Janssen, Novartis, Incyte, EUSApharma, Gilead, BMS and Roche. Sander: speaker’s fee from Roche and Sanofi. Smedby: research support from Janssen Pharmaceutical NV and Takeda. Harrysson, Eloranta, Ekberg, Andersson: no relevant conflicts to declare.
AUTHOR CONTRIBUTIONS
Authors Sara Harrysson, Karin E. Smedby, Sandra Eloranta and Sara Ekberg designed the study, managed the data collection and analysed the data. Gunilla Enblad, Per‐Ola Andersson and Mats Jerkeman contributed to data collection and design of the study. Tarec C. El‐Galaly, Kristina Sonnevi and Birgitta Sander provided input on the study design. Sara Harrysson and Karin E. Smedby wrote the manuscript and all authors revised and approved the final version of the manuscript.
Supporting information
TABLE S1
TABLE S2
TABLE S3
FIGURE S1
FIGURE S2
FIGURE S3
FIGURE S4
FIGURE S5
FIGURE S6
ACKNOWLEDGEMENTS
This work was supported partly through the Swedish Cancer Society and partly through a public–private real‐world evidence collaboration between Karolinska Institutet and Janssen Pharmaceutical NV. The funding bodies supported the data collection but did not have a role in the study design, data analyses or manuscript writing/decision to publish.
Harrysson S, Eloranta S, Ekberg S, Enblad G, El‐Galaly TC, Sander B, et al. Outcomes of relapsed/refractory diffuse large B‐cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line—A population‐based study of 736 patients. Br J Haematol. 2022;198:267–277. 10.1111/bjh.18197
First preliminary results of this study were presented at ASH 2019 and 2020.
Contributor Information
Sara Harrysson, Email: sara.harrysson@ki.se.
Karin E. Smedby, Email: karin.ekstrom.smedby@ki.se.
REFERENCES
- 1. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. [DOI] [PubMed] [Google Scholar]
- 2. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346:1937–47. [DOI] [PubMed] [Google Scholar]
- 3. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B‐cell lymphoma outcome prediction by gene‐expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. [DOI] [PubMed] [Google Scholar]
- 4. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346:235–42. [DOI] [PubMed] [Google Scholar]
- 5. Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol. 2006;24:3121–7. [DOI] [PubMed] [Google Scholar]
- 6. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP‐like chemotherapy plus rituximab versus CHOP‐like chemotherapy alone in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. [DOI] [PubMed] [Google Scholar]
- 7. Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33. [DOI] [PubMed] [Google Scholar]
- 8. Harrysson S, Eloranta S, Ekberg S, Enblad G, Jerkeman M, Wahlin BE, et al. Incidence of relapsed/refractory diffuse large B‐cell lymphoma (DLBCL) including CNS relapse in a population‐based cohort of 4243 patients in Sweden. Blood Cancer J. 2021;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018;182:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol. 2018;31:209–16. [DOI] [PubMed] [Google Scholar]
- 11. Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol. 2014;32:3490–6. [DOI] [PubMed] [Google Scholar]
- 13. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–5. [DOI] [PubMed] [Google Scholar]
- 14. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B‐cell lymphoma: results from the international SCHOLAR‐1 study. Blood. 2017;130:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab Vedotin in relapsed or refractory diffuse large B‐cell lymphoma. J Clin Oncol. 2020;38:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farooq U, Maurer MJ, Thompson CA, Thanarajasingam G, Inwards DJ, Micallef I, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front‐line immunochemotherapy. Br J Haematol. 2017;179:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maurer MJ, Jakobsen LH, Mwangi R, Schmitz N, Farooq U, Flowers CR, et al. Relapsed/refractory international prognostic index (R/R‐IPI): an international prognostic calculator for relapsed/refractory diffuse large B‐cell lymphoma. Am J Hematol. 2021;96:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 19. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med. 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B‐cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. [DOI] [PubMed] [Google Scholar]
- 21. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1‐2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second‐line therapy for large B‐cell lymphoma. N Engl J Med. 2022;386(7):640–54. [DOI] [PubMed] [Google Scholar]
- 23. Kamdar MSS, Arnason JE. Lisocabtagene Maraleucel (liso‐cel), a CD19‐directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second‐line (2L) treatment in patients (pts) with relapsed or refractory (R/R) large B‐cell lymphoma (LBCL): results from the randomized phase 3 transform study. Blood. 2021;138(Supplement 1):91.33881503 [Google Scholar]
- 24. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second‐line tisagenlecleucel or standard care in aggressive B‐cell lymphoma. N Engl J Med. 2022;386(7):629–39. [DOI] [PubMed] [Google Scholar]
- 25. Salles G, Duell J, González Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B‐cell lymphoma (L‐MIND): a multicentre, prospective, single‐arm, phase 2 study. Lancet Oncol. 2020;21:978–88. [DOI] [PubMed] [Google Scholar]
- 26. Hutchings M, Morschhauser F, Iacoboni G, Carlo‐Stella C, Offner FC, Sureda A, et al. Glofitamab, a novel, bivalent CD20‐targeting T‐cell‐engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B‐cell lymphoma: a phase I trial. J Clin Oncol. 2021;39:1959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma: an open‐label, phase 1/2 study. Lancet. 2021;398:1157–69. [DOI] [PubMed] [Google Scholar]
- 28. Sermer D, Batlevi C, Palomba ML, Shah G, Lin RJ, Perales MA, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4:4669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apaydin EA, Richardson AS, Baxi S, Vockley J, Akinniranye O, Larkin J, et al. Differences in lymphoma patients between chimeric antigen receptor T‐cell therapy trials and the general population. Clin Exp Med. 2021;22:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. [DOI] [PubMed] [Google Scholar]
- 31. Ludvigsson JF, Appelros P, Askling J, Byberg L, Carrero JJ, Ekström AM, et al. Adaptation of the Charlson comorbidity index for register‐based research in Sweden. Clin Epidemiol. 2021;13:21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cazelles C, Belhadj K, Vellemans H, Camus V, Poullot E, Gaulard P, et al. Rituximab plus gemcitabine and oxaliplatin (R‐GemOx) in refractory/relapsed diffuse large B‐cell lymphoma: a real‐life study in patients ineligible for autologous stem‐cell transplantation. Leuk Lymphoma. 2021;62:2161–8. [DOI] [PubMed] [Google Scholar]
- 33. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard‐of‐care axicabtagene ciloleucel for relapsed or refractory large B‐cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38:3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rutherford SC, Leonard JP. Lymphoma "benchmark" or "bench‐smudge"? Blood. 2017;130:1778–9. [DOI] [PubMed] [Google Scholar]
- 35. Costa LJ, Maddocks K, Epperla N, Reddy NM, Karmali R, Umyarova E, et al. Diffuse large B‐cell lymphoma with primary treatment failure: ultra‐high risk features and benchmarking for experimental therapies. Am J Hematol. 2017;92:161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The germinal center/activated B‐cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B‐cell lymphoma: a bio‐CORAL study. J Clin Oncol. 2011;29:4079–87. [DOI] [PubMed] [Google Scholar]
- 37. Gu K, Weisenburger DD, Fu K, Chan WC, Greiner TC, Aoun P, et al. Cell of origin fails to predict survival in patients with diffuse large B‐cell lymphoma treated with autologous hematopoietic stem cell transplantation. Hematol Oncol. 2012;30:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyman H, Jantunen E, Juvonen E, Elonen E, Böhm J, Kosma VM, et al. Impact of germinal center and non‐germinal center phenotypes on overall and failure‐free survival after high‐dose chemotherapy and auto‐SCT in primary diffuse large B‐cell lymphoma. Bone Marrow Transplant. 2008;42:93–8. [DOI] [PubMed] [Google Scholar]
- 39. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phelan JD, Young RM, Webster DE, Roulland S, Wright GW, Kasbekar M, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lacy SE, Barrans SL, Beer PA, Painter D, Smith AG, Roman E, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a haematological malignancy research network report. Blood. 2020;135:1759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37:551–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
TABLE S2
TABLE S3
FIGURE S1
FIGURE S2
FIGURE S3
FIGURE S4
FIGURE S5
FIGURE S6


