Abstract
Cervical facet joint pain syndrome accounts for a great amount of cervical pain worldwide. This study aims to provide updated knowledge of cervical facet joint innervation with new anatomical findings. Twenty‐seven cervical facet joints and their innervating structures were dissected from five halves of three human neck specimens. Histologic staining was used to confirm that the samples were nervous tissues, and all samples were documented with photography. Histology: Thirty‐six assumed facet joint branch samples were obtained and stained. Twenty‐two of these were confirmed to be nervous tissue. Therefore, 61% of the samples were identified as facet joint branches. Of all samples, 28% were not nerves. Dissection: At least one medial branch was clearly identified at each dissected cervical level. At some cervical levels, more than one medial branch was found. Anatomical differences, such as a plexus‐like innervation in the high cervical region, were observed. Direct facet joint branches were also discovered. These branches originate directly from the dorsal root of the spinal nerve and were independent from medial branches during their direct pathway toward the facet joint. Direct cervical facet joint branches were identified and a more diverse innervation pattern than previously described of the cervical facet joints was found.
Keywords: anesthesiology, neck, nerve block, radiofrequency ablation, zygapophysial joints
1. INTRODUCTION
Pathology of the zygapophysial joints (Stallmeyer & Ortiz, 2002) (herein referred to as facet joints) causes cervical spine pain in a high number of adults, regardless of age. Therefore, cervical spine pain has a great impact on public health (Bovim et al., 1994; Bykowski & Wong, 2012; Croft et al., 2001; Kirpalani & Mitra, 2008; Manchikanti et al., 2008). Different methods regarding the diagnosis and treatment of chronic facet joint pain have been described previously (Bogduk, 2008; Bykowski & Wong, 2012; van Eerd et al., 2010). Local anesthetic injections were initially described as an option for verification of diagnostic (Sluijter & Koetsveld‐Baart, 1980) as well as therapeutic purposes for cervical facet joint pain (Barnsley & Bogduk, 1993; Barnsley, Lord, & Bogduk, 1993). A significant false‐ positive rate of facet joint pain diagnoses has been reported (Barnsley, Lord, Wallis, & Bogduk, 1993). Another study with placebo‐ controlled nerve blocks reported less false‐positive cases and suggested local infiltration of the zygapophysial joints before invasive interventions are conducted (Lord et al., 1996). In the last few decades, the promising technique of radiofrequency neurotomy was introduced (Lord et al., 1996; van Kleef & van Suijlekom, 2002). However, reports are inconsistent regarding the effectiveness of radiofrequency neurotomy as a treatment for facet joint pain. While some authors described promising results (Engel et al., 2016; van Suijlekom et al., 1998), even providing significantly better relief compared to placebo (Lord et al., 1996), others concluded that more research is needed, especially regarding the effectiveness of treatment in the cervical spine (Manchikanti et al., 2016).
To improve the outcome of radiofrequency neurotomy, as well as the success of local infiltration, it is important to have detailed anatomical knowledge of the treated region. To our best knowledge, there are two anatomical studies (Bogduk, 1982; Zhang et al., 2003) and one histologic study (Kallakuri et al., 2012) that previously investigated the innervation of the cervical facet joints. Bogduk (1982) was the first to describe the innervation pattern of the cervical facet joints, and his description is still used today in clinical and research settings. In addition to Bogduk, Zhang et al. (2003) also explored the innervation of the cervical facet joints. However, despite the benefits and knowledge gained, these two previous articles (Bogduk, 1982; Zhang et al., 2003) contain shortcomings: pictures are very limited and of insufficient quality and histological analyses were not performed to verify the tissues were correctly identified as nervous tissue and not connective tissues and/or small blood vessels. Thus, review of the cervical facet joint anatomy is required and may increase the success rate of radiofrequency neurotomy in the cervical spine, which appears to be less successful than procedures in the lumbar spine (Engel et al., 2016; Schofferman & Kine, 2004).
The aim of this study was to build upon previous knowledge by investigating the innervation pattern of the cervical facet joints of each level in detail. High‐quality pictures were taken of each dissection step to document the different innervation patterns. Facet joint branches were sampled and stained histologically to verify were nervous tissue.
2. MATERIALS AND METHODS
The necks of three human specimens from the Maastricht University Department of Anatomy and Embryology were dissected. All three dissected cadavers were male and were 67, 76, and 85 years of age at the time of conservation. No specimen had any known medical history of cervical injuries or surgical interventions. All human specimens in this study were persons who donated their body to Maastricht University to be used for educational and research purposes. Prior to death, each donor signed a handwritten and signed codicil. All codicils were kept at the Department of Anatomy and Embryology of the Faculty of Health, Medicine and Life Sciences at Maastricht University, The Netherlands as required by Dutch law for the use of dead bodies for scientific research and education (Hirsch Ballin, 1991).
All specimens were preserved in formalin within 24 hr of death following the normal preservation protocol of the Department. Three liters of formalin 37%, 10 l of ethanol 96%, 10 l of glycerin 40%, 25 l of tap water, and 2.4 g of thymol were mixed. Ten to fifteen liters of the fluid mixture were used for intra‐arterial injection of each body. After the first conservation process, the bodies were stored in the following fluid mixture for at least a month: 20 l of formalin 37%, 72 l of ethanol 96%, and 268 liters of tap water. After 1 month of initial conservation, the bodies were wrapped in formalin towels (100 ml formalin 37% in 4900 ml tap water), surrounded by plastic, and stored in a special cooling system (4°C).
2.1. Dissection
Five halves of three human neck specimens were dissected until the facet joints were visible and the innervating structures could be evaluated. A total of 27 facet joint levels were investigated, namely C1/C2 two times (venous plexus hampered a proper investigation of the other three) and C2/C3 to C6/C7 five times each. All necks were dissected from the skin down toward the facet joints with photo documentation in each step. All overlaying structures were dissected stepwise.
Once the area of primary interest has been reached, meaning the trapezius muscle has been removed and the splenius capitis muscle has been reflected laterally, further dissection was performed with the use of a binocular dissection microscope (ZEISS OPMI 1‐F) (magnification 0.4x 12.5 to 2.5x 12.5). Each new dissected level was documented by taking pictures with a camera attached to the microscope (0.4x 12.5; 0.6x 12.5, or 1.0x 12.5 depending on the level of dissection). The full dissection data set is publicly available (Büsken et al., 2021a, 2021b, 2021c, 2021d, 2021e, 2021f).
Once all visible assumed nervous structures surrounding the facet joints were identified and documented, the capsule of each facet was opened, and the joint was made visible to prove the correct location. In each specimen, the facet joints were opened one by one from cranially to caudally. Finally, all branches were dissected toward their origin. Interpretation of the findings was always evaluated by at least four different persons of which two professors from the department of anatomy and embryology of Maastricht University.
2.2. Histology
Histologic analyses were performed to verify that the structures identified as nerves were indeed nervous tissue. The branches identified were categorized, removed, and passed on for histologic staining. S100 staining was used as specific immunostaining for neuronal tissue, and Mayer Hematoxylin and Eosin (H&E) staining was used for standard tissue diagnosis (Mulisch & Welsch, 2015). Briefly, for S100 stains, a 10‐min peroxidases block was performed. S100 diluted 1:1000 in Teng T (10% goat serum) was incubated for 1 h at room temperature. A biotin labeled goat anti rabbit (1:1000 PBS/T) served as the secondary antibody (30 min, room temperature), to be visualized with streptavidin peroxidase and diaminobenzidine (DAB). Sciatic nerve samples served as positive control samples, not using the primary antibody as negative control samples. The characterization of the structures was conducted by two experienced histologists.
3. RESULTS
3.1. Histology
Thirty‐six potential facet branch samples were obtained and stained. S100 staining revealed 61% (22/36) of the samples to be of neural origin. Four samples (11%) could not be classified due to loss of the samples during histologic processing. Of the ten samples not of neural origin, eight samples (22%) mainly contained collagen, one sample (3%) contained an artery, and one sample (3%) contained an artery, a vein, and a small lymph vessel (Table 1). Nonetheless, very small nerve fibers were visible in most of the samples that were not classified as nerves. H&E staining, combined with the observable structure of the sample, suggests the small nerve fibers only innervated the other structures present (e.g., blood vessels) (Figure 1; Figure 2).
TABLE 1.
Results of the histologic analysis of suggested facet branches.
| Investigated samples | n | Arteries | Veins | Lymphatic vessels | Collagen fibers |
|---|---|---|---|---|---|
| Neural origin | 22 | ||||
| Non‐ neural origin | 10 | Found in 2 samples | Found in 1 sample | Found in 1 sample | Found in 8 samples |
| Lost during staining | 4 | ||||
| Total number of samples investigated | 36 |
FIGURE 1.
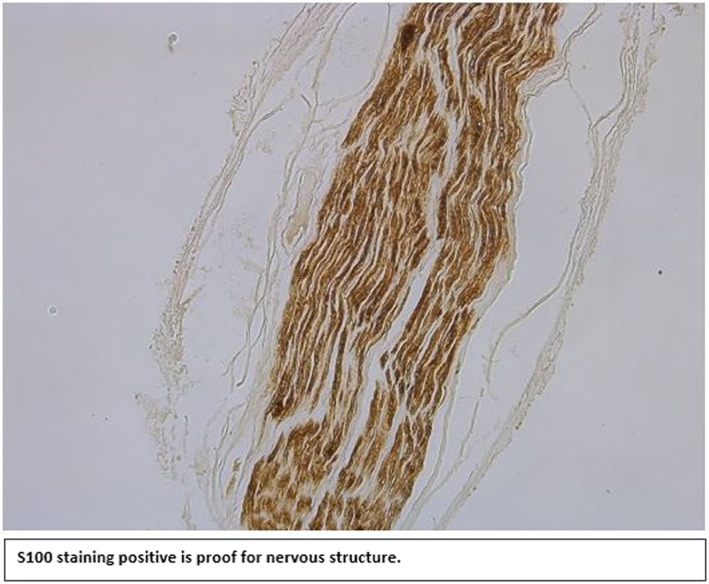
S100 staining positive is proof for nervous structure
FIGURE 2.
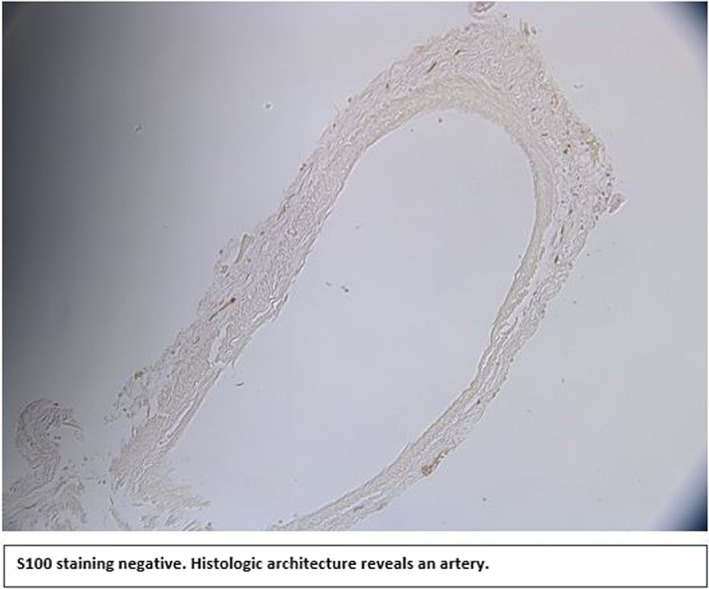
S100 staining negative. Histologic architecture reveals an artery
Therefore, 28% of the branches that were initially identified as potential facet joint branches were not actually nervous tissue. For example, in Figure 3, the right larger branch thought to be a facet joint branch (indicated by the black arrow) was actually an artery, whereas it was the small branch on the left that was of neural origin. In Figure 4, the upper structure was shown to be collagen, whereas the lower one was determined to be a facet joint nerve. All structures that were confirmed to be non‐nervous tissues were ruled out as possible facet joint branches. Only facet joint branches that were verified to be of nervous tissue origin by S100 staining are discussed below.
FIGURE 3.
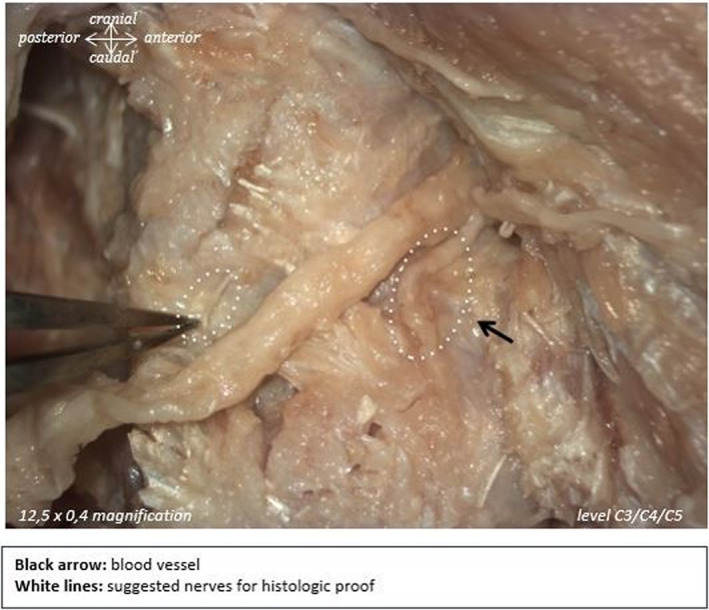
White dotted lines indicate suggested nerves for histologic proof. Black arrow indicates blood vessel
FIGURE 4.
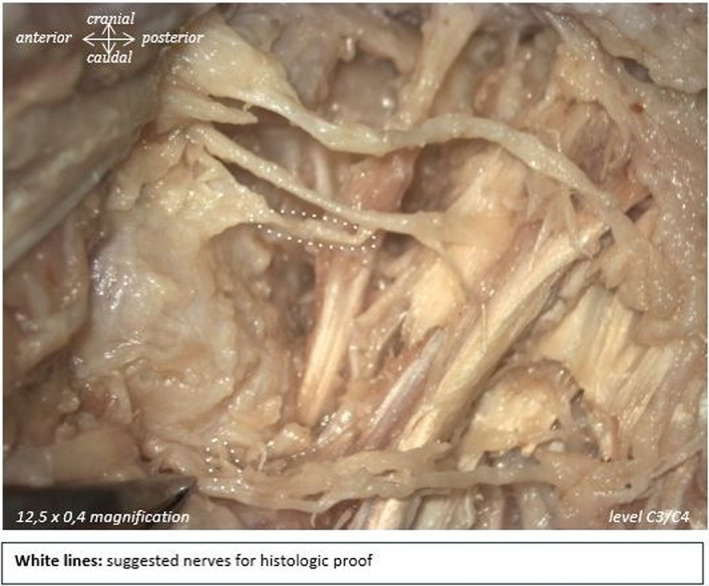
White dotted lines indicate suggested nerves for histologic proof
3.2. Dissection
At least one medial branch, defined as a nerve branch originating from the dorsal root of the spinal nerve that innervates the medial group of the cervical erector spinae muscle and the facet joints, was clearly identified on each cervical level. Medial branches were, on most occasions, accompanied by small blood vessels, and present at all dissected levels. Histological analyses revealed that sometimes two described medial branches share the same epineurium, thus raising the question of whether they must be described as one branch. Although not all discovered branches shared the same epineurium, in almost all cases, two branches could be identified as sharing collagenous connective tissue and could be traced back as two branches toward a shared origin from the spinal nerve (Büsken et al., 2021b, 2021f). Furthermore, connecting fibers between two medial branches could be made visible (Büsken et al., 2021b, 2021e). In some cases, even three medial branches were visible at the same level (Büsken et al., 2021a, 2021b).
All medial branches on all levels followed the same general pathway: originating from the dorsal root of the spinal nerve and following the pedicle toward the respective target muscles (multifidus muscle, semispinalis cervicis muscle). However, one medial branch was an exception to this pattern. This specific branch crossed straight over the facet joint itself instead of following the pedicle (Büsken et al., 2021d). Another branch could not be reached along the pedicle because of the presence of distinct osteophytes (Büsken et al., 2021f). Although only a few degenerative changes were evident during the dissection process, it was obvious that degeneration changed the course of nervous structures substantially.
In the higher cervical region (C1/C2, C2/C3, C3/C4), more than one medial branch was generally visible (Table 2 provides an overview of the variant innervations). This network of nerves was particularly visible around C1/C2 and C2/C3 levels, although the network spanned toward level C3/C4 in two specimens. Given this plexus‐like pattern, a good differentiation and classification of the different nerves was not always possible. In addition to the unpredictable network of nerves, a network of blood vessels and collagenous tissue was found in the high cervical region in all five dissected halves of the cervical spine, further complicating differentiation of structures (Figure 5A).
TABLE 2.
Overview over the discovered innervating nervous structures per facet level.
| N = 5 | Medial branches | Direct facet branch(es) (%) | Facet branches | Innvervation from different levels (%) | Plexus‐like structure (%) | Connection between branches (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 MB (%) | 2 MBs (%) | >2 MBs (%) | 1 fB (%) | ≥2 fBs (%) | |||||
| C1/C2 a | 0 | 0 | 40 | 0 | 0 | 40 | 0 | 60 | 0 |
| C2/C3 | 20 | 20 | 60 | 20 | 20 | 80 | 20 | 80 | 0 |
| C3/C4 | 0 | 80 | 20 | 40 | 40 | 40 | 20 | 40 | 20 |
| C4/C5 | 60 | 40 | 0 | 40 | 0 | 40 | 0 | 0 | 0 |
| C5/C6 | 40 | 60 | 0 | 80 | 40 | 40 | 0 | 0 | 0 |
| C6/C7 | 100 | 0 | 0 | 20 | 20 | 20 | 0 | 0 | 0 |
In three specimens, level C1/C2 has not been entirely dissected due to the overlying plexus‐like structure.
FIGURE 5.
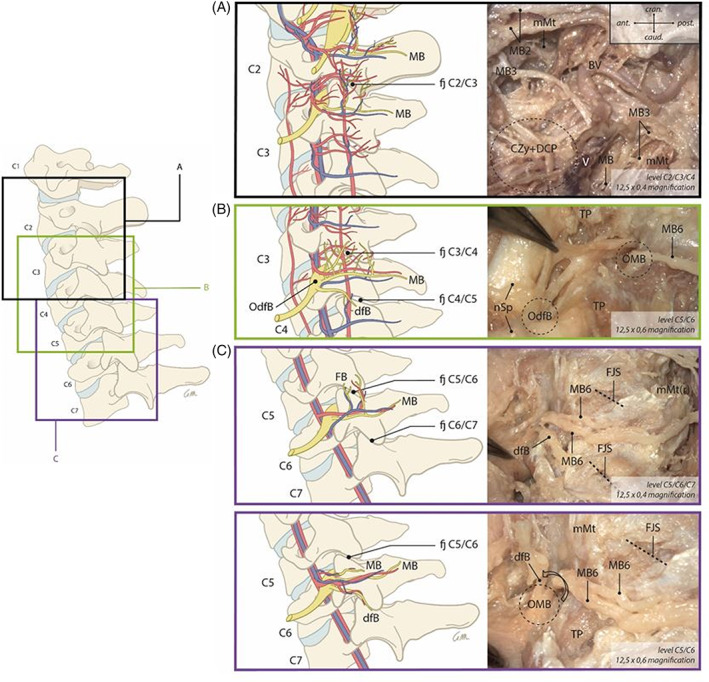
Gives an overview of several cervical facet joint innervation patterns. A, B, and C include example pictures taken during the dissection. They illustrate the clinical correlation of the described innervation patterns. *Please note that this figure provides a generic summary of all the discovered innervation patterns combined in one figure. Although all patterns have been found during dissections, not all have been found the exact way as shown in the figure and not all have been found in the dissected specimen
At lower cervical levels (C4/C5 and C5/C6), only one or two medial branches could be detected (Büsken, 2021f) (Büsken, 2021e). At level C6/C7, only one medial branch was found (Table 2). Although the medial branch pattern was similar for all lower cervical levels, the course of facet joint branches could not be predicted because it varied substantially between the different dissected cadavers. The appearance of facet joint branches, which originate from a medial branch (Büsken et al., 2021f), varied within the levels. In several cases, more than one facet joint branch was found to originate from the medial branches of the associated level (Büsken et al., 2021f). In other cases, no facet joint branches were found (refer to Table 2 for details). Some facet joints were innervated by branches that originate from two different cervical levels. In this study, those branches were only made visible in the high cervical region and mid cervical levels C3/C4 (Table 2).
Furthermore, direct facet joint branches coursing to the anterior joint capsule were discovered. These branches, like medial branches, originate directly from the dorsal root of the spinal nerve and are not entwined with any medial branches during their direct pathway toward the facet joint capsule (refer to Figure 5). Direct facet joint branches did not innervate muscles but only the facet joint. This shows the presence of an alternative innervation pattern of the cervical facet joints, independent from the medial branches. It must be noted that direct facet joint branches were not visualized at each facet level. They were mainly found in the mid and lower cervical regions (Table 2 and Figure 6).
FIGURE 6.
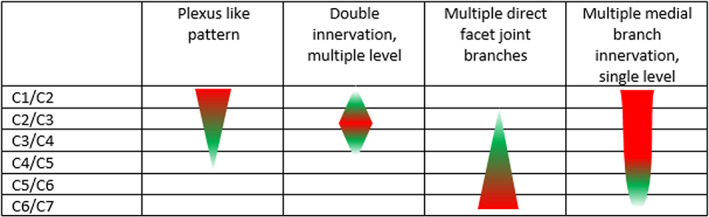
Simplified innervation overview (red: Most frequent, green less frequent); note the variations and individual patterns of innervation
In four out of twenty‐seven facet joints, no innervating branch toward the facet joint capsule (neither facet joint branch, nor direct facet joint branch) could be visualized macroscopically (Büsken et al., 2021e). Three out of the four cases were found in the same male specimen in whom only one side was dissected.
A great variation was noticed in the place of origin of the facet innervating branches from the dorsal root. In many cases, the origin from the dorsal root was clearly visible (Figure 5C; (Büsken et al., 2021e) and easy to reach during dissection, but in some cases the branching was situated deeper in the intervertebral foramen (Büsken et al., 2021b).
4. DISCUSSION
Historically, there has been some nomenclature variations used for the different nerves and branches in the spine. The present study uses the nomenclature introduced by Bogduk (1982) with some variations. The term articular branch, often used in reports in the current literature, has been replaced with facet joint branch. Additionally, the term direct facet joint branch is introduced to describe an innervation of the cervical facet joints originating directly from the dorsal root of the spinal nerve. To date, direct facet joint branches in the cervical spine have not been described.
Histological analyses revealed that 28% of the nerves initially thought to be nervous tissue only contained collagen fibers, highlighting the importance of verifying tissue composition histologically rather than relying solely on observations made during dissection. However, small nerve fibers that may contribute to the facet joint innervation might have been lost during the gross dissection, as they could have been mistaken for a different kind of tissue. Nonetheless, these histological staining results suggest caution should be taken when interpreting earlier studies on cervical facet joint nerve anatomy (Bogduk, 1982; Zhang et al., 2003). As previous studies did not include histological staining, it cannot be confidently ruled out that non‐nervous structure, such as vessels and connective tissue, were misinterpreted as nerves, or that some nerves may have been missed.
The overall medial nerve branch pattern reported here, generally matches with Bogduk's (1982) initial description. Bogduk (1982) defined the medial branches as branches that innervate the multifidus muscle (deep branch) as well as the semispinalis cervicis muscle (superficial branch). Comparable with the descriptions of Zhang et al. (2003), at least one medial branch, in some cases also up to three medial branches, per level were identified in the present study. Nonetheless, a differentiation between deep and superficial medial branches could not be made at all cervical spine levels. The course of the medial branch distally from the facet joint branches was not relevant for the innervation of the facet joints and was therefore not relevant for this study.
Comparable with previous reports (Bogduk, 1982; Zhang et al., 2003), facet joint branches were also identified (Büsken et al., 2021f). Bogduk (1982) described a common pattern of facet joint branches found at all levels (i.e., meaning one facet joint branch originating from the deep medial branch). Zhang et al. (2003) dissected the cervical spine of 14 adult cadavers but could only identify facet joint branches from C3 to C8 bilaterally in six of them. The presented study did not identify facet joint branches on all levels.
Bogduk (1982) described communicating branches that originate from a higher/lower segmental level and additionally innervate the facet joints besides the facet joint branches from the same segmental level as a common pattern. However, from the findings of the presented study, dual level innervation must be seen as an exception as innervation from two levels was present in only a few facet joints, specifically in the mid cervical region C3/C4. Although due to the plexus‐like pattern in the higher cervical levels, appearance of multilevel innervation from C1 to C3 is most likely, but most difficult to differentiate. In several cases, there were more than one medial branch present (Büsken et al., 2021a) and sometimes even interconnections between the medial branches were found (Büsken et al., 2021b, 2021e).
It is important to note that the present study revealed direct facet joint branches in addition to the already described innervation pattern, which differs from the findings of Zhang et al. (2003) and Bogduk (1982), who did not describe any direct branches (originating from the dorsal root of the spinal nerve directly) toward the facet joints, that coexist with the facet joint branches (articular branches) originating from the medial branches. Similar branches, referred to as descending branches, have already been described for the thoracic spine (Ishizuka et al., 2012). The study of Ishizuka et al. (2012) describes the descending branches as a direct innervation of the thoracic facet joint capsule in almost half of the dissected specimens. Those descending branches originate directly from the dorsal root of the spinal nerve. In the present study direct facet joint branches were consistently observed in the lower and middle cervical region in a regular manner (Büsken et al., 2021e) but were less frequently noticed in the higher cervical region in comparison to the middle and lower cervical regions (refer to Figure 5 and Table 2). This might be due to the more difficult topography (nerve plexus, venous plexus, and connective tissue) in this region. Consistent with the presented findings in the cervical spine, a standard pattern of descending branches was also not reported for the thoracic region (Ishizuka et al., 2012).
4.1. Clinical translation
Direct branches might be an explanation for recurrence of facet joint pain after successful medial branch radiofrequency intervention (Husted et al., 2008), as they are not intentionally targeted during radiofrequency ablation. Further, the finding of more than one medial branch, and/or more than one facet joint branch was found at multiple levels, might explain the described additional period of pain relief after repeated radiofrequency ablation (Engel et al., 2016), since a slightly different needle position during subsequent sessions might target additional nerves.
It is very important to point out the great difference in innervation of the facet joints between the higher cervical region and the lower cervical region. In the higher cervical region, a more plexus‐like, “chaotic” pattern was found, which makes targeted intervention very difficult. Due to the plexus‐like innervation pattern, even macroscopic tracing of facet joint nerves in this area was difficult, and surgical exploration would be even more problematic due to the large amount of blood vessels and collagen tissue. The lower cervical region shows a more structured innervation pattern, but still reveals great differences within its innervation pattern (refer to Figure 5 and Table 2), which may influence traditional treatment options negatively.
Great anatomic differences were also discovered in the facet joints themselves, such as the angle in which the two bony structures articulated or the presence of osteophytes. These are common phenomenon's in the elderly (Kettler et al., 2007) as well as in patients of all age groups (Masharawi et al., 2005; Masharawi et al., 2008). As seen in the presented study, the occurrence of degenerative changes can affect the position of the medial branches as well as their reachability during treatment. Even though this most likely affects only a limited number of (mostly elderly) patients, potential degenerative changes must be considered for invasive techniques such as medial branch neurotomy. Although identification of osteophytes or other degenerative changes can be detected with X‐ray or CT, it does not necessarily predict how the course of the nerve is affected. One nerve might lay under any degenerative changes (Büsken et al., 2021f), while another might change its course completely and run around any bony structures (Büsken et al., 2021e). Such degenerative changes might hamper the approach of target structures in contemporary methods of cervical radiofrequency ablation.
In some cases, the origin of the direct facet branches is situated deep, close to the intervertebral foramen and could barely be reached, which may prevent a neurotomy of such branches. The variations within the origin of the dorsal root are critical for the radiofrequency neurotomy intervention techniques (van Kleef & van Suijlekom, 2002). The goal of those techniques is to denervate the nerve as close to the origin of the dorsal root as possible without damaging the spinal nerve. If the medial branch is treated as close to its origin as possible, then the chance of denervating all following facet joint branches increases. Interventions not close enough to the origin of the medial branch might result in an increased likelihood of missing small facet joint branches as they branch throughout the course of the nerve.
Needle position is important, as McLain suggested in his study that damage to mechanoreceptors and/or nociceptors present in the facet joint capsule can have a significant impact on the stability of the spine, suggesting that those innervating structures must be spared during spinal interventions (McLain, 1993). Especially the multifidus muscle is suggested a key role in the spine stability due to its unique architecture (Ward et al., 2009). Mitsutake et al. found that cervical multifidus muscle denervation leads to postural instability (Mitsutake et al., 2016). Nevertheless, the number of reported complications is surprisingly low (Engel et al., 2016; van Eerd et al., 2010). Two studies that focused on possible post‐radiofrequency ablation instability did not find any signs of clinically relevant complications due to multifidus denervation muscle (Dreyfuss et al., 2009; Stegemöller et al., 2015). One study found significant more disc degeneration of levels affected by radiofrequency neurotomy compared to unaffected levels (Smuck et al., 2015).
4.2. Limitations
Although dissections were performed with the utmost diligence, errors resulting in structures being missed or origins changed are always possible. However, the potential effects of dissection errors on the results were limited by clearly documenting each step of the dissection process and by making structures as visible as possible using a dissection microscope. Furthermore, as very small facet joint branches that were surrounded by collagen and other structures were found during histological investigation, it cannot be ruled out that other small branches were damaged or overlooked during the dissection process. Additionally, the use of histological analyses revealed microscopically sized branches not otherwise detectable by eye. Because of the diverse innervation pattern, the likelihood of missed branches is reasonably high. The present study also did not investigate whether the identified direct facet branches consisted of sensory or rather exclusively autonomic fibers.
However, identifying the type of nerve fibers in the direct facet branches was not an aim of this study and may be investigated in future studies. Finally, the investigated subjects were relatively homogeneous (age (67–85 years), sex (all males)) and the sample size (five cervical halves from three specimens) was relatively small. This may complicate direct transfer of the presented findings into daily practice. More studies with greater sample sizes and a more heterogeneous samples concerning age and sex are needed to confirm the presented findings. Sex‐related difference cannot be concluded from our study. Future studies should specifically investigate the presence and role of direct facet joint branches, including detailed histologic analysis.
4.3. Conclusion
Dissection and histological analyses͓̀ were used to verify cervical facet joint innervation. A more diverse innervation pattern of the cervical facet joints than previously reported was found. Previously unknown direct cervical facet joint branches, a plexus like innervation pattern of the high cervical facet joints and less predictable appearance of facet joint branches were identified. The presence of direct cervical facet joint branches might play an important role in the treatment of chronic facet joint pain. The findings of this study may allow for a revision of current concepts regarding the innervation of the cervical facet joints and may be of great importance for the approaches and techniques used to treat chronic cervical facet joint pain. Practitioners should be aware of the great variations within the innervation of the cervical facet joints in each individual patient.
CONFLICT OF INTEREST
There is no conflict of interest known to the authors.
FUNDING INFORMATION
There has been no external financial or material support in the process of the presented study. Hence, there is no conflict of interest known to the authors toward any financial matter.
ACKNOWLEDGMENTS
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind's overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude (Iwanaga et al., 2021).
Greet Mommen: Special thanks to Greet Mommen, scientific illustrator at Maastricht University, Department of Anatomy and Embryology, for the realization of the amazing anatomical illustrations. Her great support was essential during the development of the visualization concept. www.greetmommen.be
Paul van Dijk: For his expertise concerning lab work and histological staining of the collected samples.
Adam Kositsky: For reviewing the English text for correct use of language.
Büsken, F. , Lataster, A. , & Herrler, A. (2022). The innervation of the cervical facet joints—an anatomical and histological approach. Clinical Anatomy, 35(6), 780–788. 10.1002/ca.23901
Funding information Maastricht University
REFERENCES
- Barnsley, L. , & Bogduk, N. (1993). Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Regional Anesthesia, 18(6), 343–350. [PubMed] [Google Scholar]
- Barnsley, L. , Lord, S. , & Bogduk, N. (1993). Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain, 55(1), 99–106. 10.1016/0304-3959(93)90189-v [DOI] [PubMed] [Google Scholar]
- Barnsley, L. , Lord, S. , Wallis, B. , & Bogduk, N. (1993). False‐positive rates of cervical zygapophysial joint blocks. The Clinical Journal of Pain, 9(2), 124–130. 10.1097/00002508-199306000-00007 [DOI] [PubMed] [Google Scholar]
- Bogduk, N. (1982). The clinical anatomy of the cervical dorsal rami. Spine (Phila Pa 1976), 7(4), 319–330. 10.1097/00007632-198207000-00001 [DOI] [PubMed] [Google Scholar]
- Bogduk, N. (2008). Evidence‐informed management of chronic low back pain with facet injections and radiofrequency neurotomy. The Spine Journal, 8(1), 56–64. 10.1016/j.spinee.2007.10.010 [DOI] [PubMed] [Google Scholar]
- Bovim, G. , Schrader, H. , & Sand, T. (1994). Neck pain in the general population. Spine (Phila Pa 1976), 19(12), 1307–1309. 10.1097/00007632-199406000-00001 [DOI] [PubMed] [Google Scholar]
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021a). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (right side, male, 76y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-right-side-male-76y
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021b). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (left side, male, 76y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-left-side-male-76y
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021c). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (right side, male, 67y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-right-side-male-67y
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021d). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (left side, male, 67y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-left-side-male-67y
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021e). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (right side, male, 86y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-right-side-male-86y
- Büsken, F. , Dirks, N. , Herrler, A. & Lataster, A. (2021f). A dorsolateral dissection of the cervical neck. Dissection neck from skin to facet joint (left side, male, 86y). Retrieved from https://anatomytool.org/content/dissection-neck-skin-facet-joint-left-side-male-86y
- Bykowski, J. L. , & Wong, W. H. (2012). Role of facet joints in spine pain and image‐guided treatment: A review. AJNR. American Journal of Neuroradiology, 33(8), 1419–1426. 10.3174/ajnr.A2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft, P. R. , Lewis, M. , Papageorgiou, A. C. , Thomas, E. , Jayson, M. I. V. , Macfarlane, G. J. , & Silman, A. J. (2001). Risk factors for neck pain: A longitudinal study in the general population. Pain, 93(3), 317–325. 10.1016/s0304-3959(01)00334-7 [DOI] [PubMed] [Google Scholar]
- Dreyfuss, P. , Stout, A. , Aprill, C. , Pollei, S. , Johnson, B. , & Bogduk, N. (2009). The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. PM & R: The Journal of Injury, Function, and Rehabilitation, 1(8), 719–722. 10.1016/j.pmrj.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Engel, A. , Rappard, G. , King, W. , & Kennedy, D. J. (2016). The effectiveness and risks of fluoroscopically‐guided cervical medial branch thermal radiofrequency Neurotomy: A systematic review with comprehensive analysis of the published data. Pain Medicine, 17(4), 658–669. 10.1111/pme.12928 [DOI] [PubMed] [Google Scholar]
- Hirsch Ballin, E. , de minister van justitie. (1991). Wet op de Lijkbezorging. Retrieved from https://wetten.overheid.nl/BWBR0005009/2015-07-01
- Husted, D. S. , Orton, D. , Schofferman, J. , & Kine, G. (2008). Effectiveness of repeated radiofrequency neurotomy for cervical facet joint pain. Journal of Spinal Disorders & Techniques, 21(6), 406–408. 10.1097/BSD.0b013e318158971f [DOI] [PubMed] [Google Scholar]
- Ishizuka, K. , Sakai, H. , Tsuzuki, N. , & Nagashima, M. (2012). Topographic anatomy of the posterior ramus of thoracic spinal nerve and surrounding structures. Spine (Phila Pa 1976), 37(14), E817–E822. 10.1097/BRS.0b013e31824b65ea [DOI] [PubMed] [Google Scholar]
- Iwanaga, J. , Singh, V. , Ohtsuka, A. , Hwang, Y. , Kim, H.‐J. , Moryś, J. , & Tubbs, R. S. (2021). Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clinical Anatomy, 34(1), 2–4. 10.1002/ca.23671 [DOI] [PubMed] [Google Scholar]
- Kallakuri, S. , Li, Y. , Chen, C. , & Cavanaugh, J. M. (2012). Innervation of cervical ventral facet joint capsule: Histological evidence. World Journal of Orthopedics, 3(2), 10–14. 10.5312/wjo.v3.i2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler, A. , Werner, K. , & Wilke, H. J. (2007). Morphological changes of cervical facet joints in elderly individuals. European Spine Journal, 16(7), 987–992. 10.1007/s00586-006-0275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpalani, D. , & Mitra, R. (2008). Cervical facet joint dysfunction: A review. Archives of Physical Medicine and Rehabilitation, 89(4), 770–774. 10.1016/j.apmr.2007.11.028 [DOI] [PubMed] [Google Scholar]
- Lord, S. M. , Barnsley, L. , Wallis, B. J. , McDonald, G. J. , & Bogduk, N. (1996). Percutaneous radio‐frequency neurotomy for chronic cervical zygapophyseal‐joint pain. The New England Journal of Medicine, 335(23), 1721–1726. 10.1056/nejm199612053352302 [DOI] [PubMed] [Google Scholar]
- Manchikanti, L. , Hirsch, J. A. , Kaye, A. D. , & Boswell, M. V. (2016). Cervical zygapophysial (facet) joint pain: Effectiveness of interventional management strategies. Postgraduate Medicine, 128(1), 54–68. 10.1080/00325481.2016.1105092 [DOI] [PubMed] [Google Scholar]
- Manchikanti, L. , Manchikanti, K. N. , Cash, K. A. , Singh, V. , & Giordano, J. (2008). Age‐related prevalence of facet‐joint involvement in chronic neck and low back pain. Pain Physician, 11(1), 67–75. [PubMed] [Google Scholar]
- Masharawi, Y. , Peleg, S. , Albert, H. B. , Dar, G. , Steingberg, N. , Medlej, B. , & Hershkovitz, I. (2008). Facet asymmetry in normal vertebral growth: Characterization and etiologic theory of scoliosis. Spine (Phila Pa 1976), 33(8), 898–902. 10.1097/BRS.0b013e31816b1f83 [DOI] [PubMed] [Google Scholar]
- Masharawi, Y. , Rothschild, B. , Salame, K. , Dar, G. , Peleg, S. , & Hershkovitz, I. (2005). Facet tropism and interfacet shape in the thoracolumbar vertebrae: Characterization and biomechanical interpretation. Spine (Phila Pa 1976), 30(11), E281–E292. 10.1097/01.brs.0000164098.00201.8d [DOI] [PubMed] [Google Scholar]
- McLain, R. F. (1993). Mechanoreceptor endings in human cervical facet joints. The Iowa Orthopaedic Journal, 13, 149–154. [PMC free article] [PubMed] [Google Scholar]
- Mitsutake, T. , Sakamoto, M. , Chyuda, Y. , Oka, S. , Hirata, H. , Matsuo, T. , Oishi, T. , & Horikawa, E. (2016). Greater cervical muscle fat infiltration evaluated by magnetic resonance imaging is associated with poor postural stability in patients with cervical Spondylotic radiculopathy. Spine (Phila Pa 1976), 41(1), E8–E14. 10.1097/brs.0000000000001196 [DOI] [PubMed] [Google Scholar]
- Mulisch, M. , & Welsch, U. (2015). Romeis ‐ Mikroskopische Technik. Berlin: Springer Spektrum. [Google Scholar]
- Schofferman, J. , & Kine, G. (2004). Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine (Phila Pa 1976), 29(21), 2471–2473. 10.1097/01.brs.0000143170.47345.44 [DOI] [PubMed] [Google Scholar]
- Sluijter, M. E. , & Koetsveld‐Baart, C. C. (1980). Interruption of pain pathways in the treatment of the cervical syndrome. Anaesthesia, 35(3), 302–307. 10.1111/j.1365-2044.1980.tb05102.x [DOI] [PubMed] [Google Scholar]
- Smuck, M. , Crisostomo, R. A. , Demirjian, R. , Fitch, D. S. , Kennedy, D. J. , & Geisser, M. E. (2015). Morphologic changes in the lumbar spine after lumbar medial branch radiofrequency neurotomy: A quantitative radiological study. The Spine Journal, 15(6), 1415–1421. 10.1016/j.spinee.2013.06.096 [DOI] [PubMed] [Google Scholar]
- Stallmeyer, M. J. , & Ortiz, A. O. (2002). Facet blocks and sacroiliac joint injections. Techniques in Vascular and Interventional Radiology, 5(4), 201–206. 10.1053/tvir.2002.36427 [DOI] [PubMed] [Google Scholar]
- Stegemöller, E. L. , Roper, J. , Hass, C. J. , & Kennedy, D. J. (2015). Changes in gait kinematics and lower back muscle activity post‐radiofrequency denervation of the zygapophysial joint: A case study. The Spine Journal, 15(6), e21–e27. 10.1016/j.spinee.2013.06.061 [DOI] [PubMed] [Google Scholar]
- van Eerd, M. , Patijn, J. , Lataster, A. , Rosenquist, R. W. , van Kleef, M. , Mekhail, N. , & Van Zundert, J. (2010). 5. Cervical facet pain. Pain Practice, 10(2), 113–123. 10.1111/j.1533-2500.2009.00346.x [DOI] [PubMed] [Google Scholar]
- van Kleef, M. , & van Suijlekom, J. A. (2002). Treatment of chronic cervical pain, brachialgia, and cervicogenic headache by means of radiofrequency procedures. Pain Practice, 2(3), 214–223. 10.1046/j.1533-2500.2002.02026.x [DOI] [PubMed] [Google Scholar]
- van Suijlekom, H. A. , van Kleef, M. , Barendse, G. A. , Sluijter, M. E. , Sjaastad, O. , & Weber, W. E. (1998). Radiofrequency cervical zygapophyseal joint neurotomy for cervicogenic headache: A prospective study of 15 patients. Functional Neurology, 13(4), 297–303. [PubMed] [Google Scholar]
- Ward, S. R. , Kim, C. W. , Eng, C. M. , Gottschalk, L. J. t. , Tomiya, A. , Garfin, S. R. , & Lieber, R. L. (2009). Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. The Journal of Bone and Joint Surgery. American Volume, 91(1), 176–185. 10.2106/jbjs.G.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Tsuzuki, N. , Hirabayashi, S. , Saiki, K. , & Fujita, K. (2003). Surgical anatomy of the nerves and muscles in the posterior cervical spine: A guide for avoiding inadvertent nerve injuries during the posterior approach. Spine (Phila Pa 1976), 28(13), 1379–1384. 10.1097/01.Brs.0000067095.75764.D3 [DOI] [PubMed] [Google Scholar]


