Abstract
Objective
To characterize the effects of blocking calcitonin gene‐related peptide (CGRP) activity in a mouse model of gastrointestinal transport.
Background
Migraine management using CGRP modulating therapies can cause constipation of varying frequency and severity. This variation might be due to the different mechanisms through which therapies block CGRP activity (e.g., blocking CGRP, or the CGRP receptor) with antibodies or receptor antagonists. The charcoal meal gastrointestinal transit assay was used to characterize constipation produced by these modes of therapy in transgenic mice expressing the human receptor activity–modifying protein 1 (hRAMP1) subunit of the CGRP receptor complex.
Methods
Male and female hRAMP1 mice were dosed with compound or vehicle and challenged with a charcoal meal suspension via oral gavage. The mice were then humanely euthanized and the proportion of the length of the large intestine that the charcoal meal had traveled indicated gastrointestinal transit.
Results
Antibody to the CGRP receptor produced % distance traveled (mean ± standard deviation) of 31.8 ± 8.2 (4 mg/kg; p = 0.001) and 33.2 ± 6.0 (30 mg/kg; p < 0.001) compared to 49.7 ± 8.3 (control) in female mice (n = 6–8), and 35.6 ± 13.5 (30 mg/kg, p = 0.019) compared to 50.2 ± 14.0 (control) in male mice (n = 10). Telcagepant (5 mg/kg, n = 8) resulted in % travel of 30.6 ± 14.7 versus 41.2 ± 8.3 (vehicle; p = 0.013) in male mice. Atogepant (3 mg/kg, n = 9) resulted in % travel of 30.6 ± 12.0, versus 41.2 ± 3.7 (control; p = 0.030) in female mice. The CGRP antibody galcanezumab (n = 7–10; p = 0.958 and p = 0.929) did not have a statistically significant effect.
Conclusions
These results are consistent with reported clinical data. Selectively blocking the CGRP receptor may have a greater impact on gastrointestinal transit than attenuating the activity of the ligand CGRP. This differential effect may be related to physiologically opposing mechanisms between the CGRP and AMY1 receptors, as the CGRP ligand antibody could inhibit the effects of CGRP at both the CGRP and AMY1 receptors.
Keywords: amylin, antibody, CGRP, constipation, galcanezumab, gastrointestinal transit
Abbreviations
- AMY1
amylin 1 receptor
- cAMP
cyclic adenosine monophosphate
- CGRP
calcitonin gene‐related peptide
- CHO
Chinese hamster ovary
- CLR
calcitonin receptor–like receptor
- CT
calcitonin
- FDA
Food and Drug Administration
- GI
gastrointestinal
- RAMP1
receptor activity–modifying protein 1
- RCP
receptor component protein
INTRODUCTION
Since the discovery that migraine attacks are associated with elevated jugular blood levels of calcitonin gene‐related peptide (CGRP), the role of this peptide in migraine has been extensively studied. 1 Both CGRP and the CGRP receptor are prominently expressed in the trigeminovascular system, and are key components in the pathophysiology of migraine. 1 , 2 , 3 In May 2018, erenumab, a humanized monoclonal antibody that binds to the human CGRP receptor, was approved by the US Food and Drug Administration (FDA) for the prevention of migraine. Its approval was soon followed by the approval of humanized monoclonal antibodies that bind to CGRP, galcanezumab and fremanezumab, in September of the same year, and most recently, by eptinezumab in February 2020 for the prevention of migraine. 3 Recently, the small molecule CGRP receptor antagonists ubrogepant and rimegepant were approved in the United States for the acute treatment of migraine attacks. 4 , 5 Randomized clinical trials have shown that the humanized antibodies to CGRP and the CGRP receptor present a favorable safety profile. It is therefore notable that labeling changes for erenumab were mandated with a warning for constipation with serious consequences. 6 There were cases that required hospitalization, including cases for which surgery was necessary. During the fairly short period that these treatments for migraine have been on the market, a differential response between those that bind to the CGRP neuropeptide and those that bind to the CGRP receptor with regard to the incidence of constipation was observed. The FDA Adverse Event Reporting System, containing data from Q3 of 2018 through Q4 of 2020, reports 103 cases of constipation with fremanezumab, 333 with galcanezumab, and 2561 with erenumab. Data from randomized clinical trials did not accurately predict this disparity. Constipation was reported in 1% (70 mg dose) and 3% (140 mg dose) of patients receiving erenumab, versus 1% on placebo. 6 Constipation rates with fremanezumab were 0.5% versus 1% for placebo, 7 and for galcanezumab 120 and 240 mg, the rates were 1% and 1.5%, versus 0.6% for placebo. 8 However, prospective real‐world analyses of erenumab have reported constipation rates of 20% and 43%. 9 , 10 The small molecule CGRP receptor antagonists atogepant 11 and telcagepant 12 had reported constipation rates of 6.6% and 3.8%, respectively, compared to 2.2% and 1.6% with placebo in the respective studies.
While its role in the trigeminovascular system is important in the pathology of migraine, CGRP is also widely distributed throughout the central and peripheral nervous system, including enteric innervation, and may mediate numerous physiologic effects. Activation of the CGRP receptor causes smooth muscle relaxation. The vasodilatory effect of CGRP is well known, 13 , 14 and has been used as a biomarker for evaluating the effects of blockers of CGRP. CGRP is present in nerve fibers throughout the myenteric plexus in the human ileum and stomach, and CGRP receptors are present in the human stomach, ileum, and colon. 15 The intraperitoneal and intracerebroventicalar injection of rat or human α‐CGRP produced diarrhea in mice, which was prevented by pretreatment with the small‐molecule CGRP receptor antagonist olcegepant or with a CGRP antibody. 16 A recent clinical study was conducted to examine the effect of 2 h infusions of CGRP (1.5 μg/min) performed on two different days with healthy human volunteers as a model for testing new anti‐migraine drugs. 17 An unexpected observation of that study was that on both study days, 97% (27/29 patients) of participants reported gastrointestinal (GI) symptoms such as rumbling, stomach pain, nausea, diarrhea, and an urge to defecate. 17
Emerging evidence indicates that CGRP can act through two different receptors, both members of the calcitonin receptor family. 18 The eponymous CGRP receptor consists of three components: calcitonin receptor–like receptor (CLR) with 7‐transmembrane domains; the receptor activity–modifying protein 1 (RAMP1) that confers specificity for CGRP binding; and the receptor component protein (RCP), which is responsible for effective coupling to G‐proteins. 18 , 19 Dimerization of calcitonin receptor (CT) protein with RAMP1 produces the amylin1 (AMY1) receptor, based on its affinity for amylin. However, this receptor has similar affinity for CGRP as does the canonical CGRP receptor itself, and may be considered a second CGRP receptor. 18 , 19 Amylin and CGRP show similar agonist potencies in cyclic adenosine monophosphate (cAMP) assays using rat and human CT/RAMP1. 18 Like CGRP, amylin and its receptor, AMY1, are widely expressed and are found in the stomach and intestines. 20 Systemic administration of amylin to rats produced dose‐dependent inhibition of gastric emptying and GI transit. 20 Conversely, the administration of an AMY1 antagonist, AC187, doubled the rate of gastric emptying in rats. 21 It is conceivable that CGRP can mediate two opposing effects, facilitation and inhibition of GI motility, through its actions at the CGRP and AMY1 receptors, respectively. We hypothesize that selective inhibition of CGRP receptors increases the incidence of constipation, whereas inhibition of both CGRP and AMY1 receptors, or of the effects of CGRP at these two receptors, retains the balance of pharmacological effects on gastric emptying and GI transit. We believe that maintaining this balance lessens constipation.
We explore these mechanisms to provide a mechanistic rationale for differential occurrence of constipation in patients on CGRP and CGRP receptor blockers.
METHODS
Generation of humanized hRAMP1 cell lines and transgenic animals
The generation of stable cell lines has been presented earlier. 22 A CHO‐K1 cell line was generated to stably express the human CGRP receptor (hCGRP receptor) components human CLR (hCLR; CALCRL; NM_005795.5) and hRAMP1 (NM_001308353). Likewise, a CHO‐K1 cell line was generated to stably express the mouse CGRP receptor (mCGRP receptor) components mouse CLR (mCLR; Calcrl; NM_018782) and mRAMP1 (NM_001168392). In a similar fashion, CHO‐K1 cell lines that stably express the human or mouse amylin receptor (hAMY1 or mAMY1 receptor) were generated by, respectively, using either human CT (CALCR, CTa; NM_001742.3) and hRAMP1 (NM_001308353) or mouse CT (CALCR, CT1a; NM_001042725.1) and mRAMP1 (NM_001168392). In addition, mCRL and hRAMP1 subunits were used to create a stable cell line expressing a mouse/human hybrid CGRP receptor (m/hCGRP receptor). Simultaneous transfection of cells with pcDNA3.1‐based expression constructs for both subunits was used to generate all cell lines. cAMP assay was used to identify stable clones expressing functional CGRP and AMY1 receptors.
GENERATION OF HUMANIZED RAMP1 MICE
A mouse model expressing human RAMP1 protein was generated by intercrossing a constitutive knock‐out strain of the mouse Ramp1 gene (C57BL/6NTac‐Ramp1 em1Tac ) with a transgenic strain expressing the human RAMP1 gene from a bacterial artificial chromosome (BAC) transgene (Tg[RAMP1,‐DsRed]Tac).
The Ramp1 knock‐out allele was generated by deleting exon 1 and around 1.5 kb of the proximal promoter using CRISPR/Cas9, which should result in loss of function of the Ramp1 gene by preventing transcription of the Ramp1 mRNA. The exact modification of the Ramp1 locus was confirmed by sequencing. Germline transmission of the Ramp1 allele containing the deletion of exon 1 was confirmed in G1 generation again by polymerase chain reaction assays and sequencing.
To generate a strain containing a human RAMP1 BAC transgene, a BAC construct containing the entire human RAMP1 gene and approximately 15 kb upstream and approximately 20 kb downstream sequence was generated by fusing parts of human BACs RP11‐232C21 (RPCI‐11 BAC library; Source BioScience) and CTD‐2554I14 (CalTechD BAC library; Thermo Fisher Scientific). The BAC construct was additionally modified by introducing an internal ribosomal entry site (IRES) and a DsRed sequence between the stop codon and the 3′ untranslated region of the human RAMP1 gene. The modified BAC construct should result in the expression of a chimeric transcript harboring the human RAMP1 sequence fused to the IRES and DsRed sequences resulting in co‐expression of the RAMP1 and DsRed proteins under the control of the human RAMP1 promoter.
We eventually intercrossed the two lines to homozygous for both mouse RAMP1 KO and BAC‐hRAMP1 transgene. All studies in this report were done by using double homozygous humanized RAMP1 mice.
Assays performed with cell lines—Functional cAMP assay
CHO‐K1 cells with hCGRP receptors, mCGRP receptors, m/hCGRP receptors, hAMY, or mAMY1 receptors were dissociated with enzyme‐free cell dissociation solution (Specialty Media, S‐014‐B=), then suspended in a (1: 2 v.v‐1) mixture of 100 ml Hanks' Balanced Salt Solution with 5 mM HEPES, 0.1% bovine serum albumin, 0.1 mM ascorbic acid, and 200 ml Dulbecco's Modified Eagle Medium with 300 μl of 500 mM 3‐isobutyl‐1‐methylxanthine. The assay was performed in 0.5 ml black polystyrene 96‐well plates (Costar). Each well contained approximately 5000 cells and 1.7 nM hCGRP for the hCGRP receptor cAMP assay or 1 nM hCGRP (or mCGRP) for mCGRP and m/hCGRP receptor cAMP assays. The AMY1 receptor assays were performed in white polystyrene 384‐well tissue‐culture treated plates (Corning). For the hAMY1 receptor assay, each well contained 2 K cells and 25 pM freshly solubilized human amylin; for the mAMY1 receptor assay, each well contained 10 K cells and 60 nM mouse amylin as challenge dose. The reaction was carried out in the presence of various concentrations of CGRP receptor antagonists. After incubation for 1 h at room temperature, cAMP levels were determined using an homogenous time resolved fluorescence cAMP assay kit (Cisbio). The raw data were converted to cAMP amount (pmole/well) using a cAMP standard curve generated for each experiment. Relative IC50 values were calculated from the top‐bottom range of the concentration response curve using a four‐parameter logistic curve fitting program (GENEDATA SCREENER® v12.0.4), and K b values were estimated as agonist‐corrected IC50 values using the Cheng‐Prusoff equation: K b = (IC50)/(1 + [(Agonist)/EC50]). An independent replicate for the in vitro experiments was defined as cells obtained from individual cell flasks with individual dilution series and potency assessment completed on separate days.
Intestinal transit test
Humanized RAMP1 male or female mice aged between 2.5 to 6 months were used in the experiments. Same gender mice were used in each individual study. The animals were housed in standard cages in a 12‐h light/dark cycle at constant temperature (22 ± 2°C) and relative humidity (65 ± 5%). Animals had free access to food and water. There were two sets of studies conducted, Small Intestinal Transit and Large Intestinal Transit. Charcoal meal, which was made of 10% carbon powder and 5% arabic gum in distilled water, was used as a marker. Experiments were conducted between the hours of 09:00 a.m. and 03:00 p.m. in the vivarium utilizing randomized treatment procedures. All treatment groups were spread across several days and individual doses were not tested sequentially. Sample sizes were targeted to run between 6 and 10 mice per dose, based on our own experience with the model, and limitations in the supply of the transgenic mice. Animal protocols were reviewed and approved by the Eli Lilly and Company Institutional Animal Care and Use Committee.
Small intestinal transit
Small intestinal transit was measured by a modification of previously described procedures. 23 , 24 , 25 The animals were deprived of food but were allowed free access to drinking water for approximately 18 h before the experiment. Each mouse was orally administered 0.3 ml of charcoal meal. The animal was then sacrificed by cervical dislocation 30 min after the marker administration. The abdomen was opened, and the intestine was removed from the pyloric junction to the ileocecal valve, and represented the experimental unit considered in this part of the study. The distance traveled by the head of the marker and the total length of the small intestine were measured. Small intestinal transit (%/30‐min) was expressed as a percentage of the distance traveled by the head of the marker relative to the total length of the small intestine.
Large intestinal transit
The animals were allowed free access to food and water. Each mouse was orally administered 0.4 ml of charcoal meal and after 90 min was sacrificed by cervical dislocation. The abdomen was opened, and the entire intestine was removed from the pyloric junction to the end of the rectum. The distance traveled by the charcoal meal from the cecocolic orifice of the cecum to the head of the marker represented the primary outcome measure. The experimental unit was defined as the length of intestine from the cecocolic orifice of the cecum through the anal canal. Colon intestinal transit (%/90‐min) was expressed as a percentage of the distance traveled by the head of the marker relative to the total length of the large intestine.
Statistical analyses
Animals were randomly allocated to treatment groups. The mean (± standard deviation [SD]) for each group of mice were determined and compared among groups. Pair‐wise comparisons were made utilizing paired t‐test (two‐tailed), and comparisons among groups were performed with one‐way analysis of variance followed by Dunnett's post hoc multiple comparisons test. GraphPad Prism software was used for statistical analysis and generation of graphs. Parametric statistical tests were used because the measures were continuous and Q‐Q plots confirmed that the data were normally distributed. Comparisons with p < 0.05 were considered statistically significant. No statistical power calculation was conducted prior to the study. Sample size was based on previous experience with this assay and availability of humanized RAMP1 mice. Female and male mice were not used in the same study to avoid possible gender‐related differences in intestinal transit rates. All data that were collected are presented in this article. Overall, two mice were removed due to dosing issues, and thus prior to data collection, one animal was removed as an outlier.
Drugs and dosing
The antibody treatments and their respective control antibodies were the CGRP antibody galcanezumab and its control immunoglobulin IgG4 PAA antibody (LSN2835015), the CGRP receptor antibody (IBA340), and its control mIgG1 antibody (IBA291). The CGRP receptor antibody (IBA340) was prepared by combining the variable region (antigen binding site) of erenumab with a mouse IgG1 backbone. The antibodies were administered by sc injection 72 h prior to the oral gavage with charcoal meal. The experimenters were not blinded to the treatments administered.
Telcagepant (5 mg/kg) was given by oral gavage 30 min prior to the charcoal meal. Atogepant (0.03, 0.3 and 3.0 mg/kg) was administered via oral gavage 30 min prior to charcoal meal. Telcagepant and atogepant both utilized a 10% acacia with 0.05% anti‐foaming agent as the vehicle solution.
RESULTS
Functional characterization of CGRP and amylin at human CGRP and AMY1 receptors, and of antibodies
We have previously shown that the hCGRP and hAMY1 receptors expressed in CHO‐K1 cells were fully functional in the cAMP assay. 20 CGRP was a full agonist at the hCGRP and the hAMY1 receptors expressed in CHO‐K1 cell lines, with EC50 values in the picomolar range. Amylin acted as a full agonist at the hAMY1 receptor as well.
Functional assays using cell lines expressing hRAMP1/hCLR, hRAMP1/hCT, mRAMP1/mCLR or hRAMP1/mCLR were used to assess the ability of the test antibodies to block the activity of CGRP on receptors expressing mouse and human subunits. Galcanezumab was approximately equipotent in blocking CGRP activity in cell lines expressing the human CGRP receptor, human AMY1 receptor, murine CGRP receptor, as well as the human/murine hybrid CGRP receptor, as indicated by K b values in the picomolar range (Table 1). In contrast, the CGRP receptor antibody IBA340 was effective only in preparations containing the hCGRP and hybrid hRAMP1/mCLR receptors, but not the murine receptor (Table 1). Both control antibodies had no functional activity at the highest concentrations evaluated (Table 1).
TABLE 1.
K b values in functional assay
| hRAMP1/hCLR (n = 5) | hRAMP1/hCT (n = 3) | mRAMP1/mCLR (n = 5) | hRAMP1/mCLR (n = 5) | |
|---|---|---|---|---|
| CGRP Ab (galcanezumab) | 16.56 ± 3.87 pM | 25.73 ± 2.44 pM | 20.75 ± 2.03 pM | 19.49 ± 0.80 pM |
| IgG4 PAA Control Ab | >100 nM | >50 nM | >100 nM | >100 nM |
| CGRP receptor Ab (IBA340) | 139.56 ± 28.34 pM | >50 nM | >100 nM | 882.74 ± 43.70 pM |
| mIgG1 control Ab (IBA291) | >100 nM | >50 nM | >100 nM | >100 nM |
Note: n indicates the number of independent replicates; inactive results are presented as > the highest concentration; h indicates human and m indicates mouse protein or receptor.
Abbreviations: Ab, antibody; CGRP, calcitonin gene‐related peptide; CLR, calcitonin receptor–like receptor; CT, calcitonin; IgG, immunoglobulin; RAMP1, receptor activity–modifying protein 1.
TABLE 2.
Summary of statistical analysis
| Fig. # | Analysis type and animal numbers |
|---|---|
| 1A |
Unpaired two‐tailed t‐test: t = 1.53, df = 10, p = 0.158 IBA291: 87.3 ± 10.6 (mean ± SD) IBA340: 75.7 ± 15.2 (mean ± SD) |
| 1B |
Unpaired two‐tailed t‐test: t = 0.16, df = 10, p = 0.875 IBA291: 80.2 ± 18.1 (mean ± SD) IBA340: 78.6 ± 15.3 (mean ± SD) |
| 2A |
One‐way ANOVA: F (2,19) = 11.6, p < 0.001. Dunnett's multiple comparisons test: **p = 0.01, ***p < 0.001 versus IBA291. One outlier was removed from IBA291 group IBA291: 49.7 ± 8.3 (mean ± SD) IBA340 (4 mg/kg): 31.8 ± 8.2 (mean ± SD) IBA340 (30 mg/kg): 33.2 ± 6.0 (mean ± SD) |
| 2B |
One‐way ANOVA: F (2,27) = 4.29. Dunnett's multiple comparisons test: *p = 0.019 IBA291: 50.21 ± 14.01 (mean ± SD) IBA340 (4 mg/kg): 47.3 ± 6.2 (mean ± SD) IBA340 (30 mg/kg): 35.6 ± 13.5 (mean ± SD) |
| 3A |
Unpaired two‐tailed t‐test: t = 0.054, df = 13, p = 0.958. One animal was removed from the Galcanezumab group due to error dosing charcoal meal IgG4PAA: 36.6 ± 6.2 (mean ± SD) Galcanezumab (4 mg/kg): 36.8 ± 7.70 (mean ± SD) |
| 3B |
Unpaired two‐tailed t‐test: t = 0.10, df = 18, p = 0.929 IgG4PAA: 38.0 ± 18.2 (mean ± SD) Galcanezumab (30 mg/kg): 38.7 ± 12.1 (mean ± SD) |
| 4 |
Unpaired two‐tailed t‐test: t = 2.84, df = 14, *p = 0.013 vehicle: 48.1 ± 9.3 (mean ± SD) Telcagepant (5 mg/kg): 30.64 ± 14.74 (mean ± SD) |
| 5A |
One‐way ANOVA: F (2,24) = 1.02. p = 0.229 vehicle: 41.2 ± 8.3 (mean ± SD) Atogepant (0.03 mg/kg): 36.1 ± 17.9 (mean ± SD) Atogepant (0.3 mg/kg): 30.0 ± 12.2 (mean ± SD) |
| 5B |
Unpaired two‐tailed t‐test: t = 2.39, df = 15, *p = 0.030. One animal was removed from the Atogepant group due to a dosing issue. vehicle: 41.2 ± 3.7 (mean ± SD) Atogepant (3 mg/kg): 30.6 ± 12.0 (mean ± SD) |
Abbreviations: ANOVA, analysis of variance; IgG, immunoglobulin; SD, standard deviation.
Small intestine transit rate
Groups of six male mice expressing hRAMP1 received injections of 4 mg/kg and 30 mg/kg sc of the CGRP receptor antibody IBA340 or the control antibody IBA291 72 h prior to gavage with charcoal meal. There were no statistically significant differences in the distance the charcoal meal traveled in the small intestine between the CGRP and control antibodies at either dose (Figure 1).
FIGURE 1.
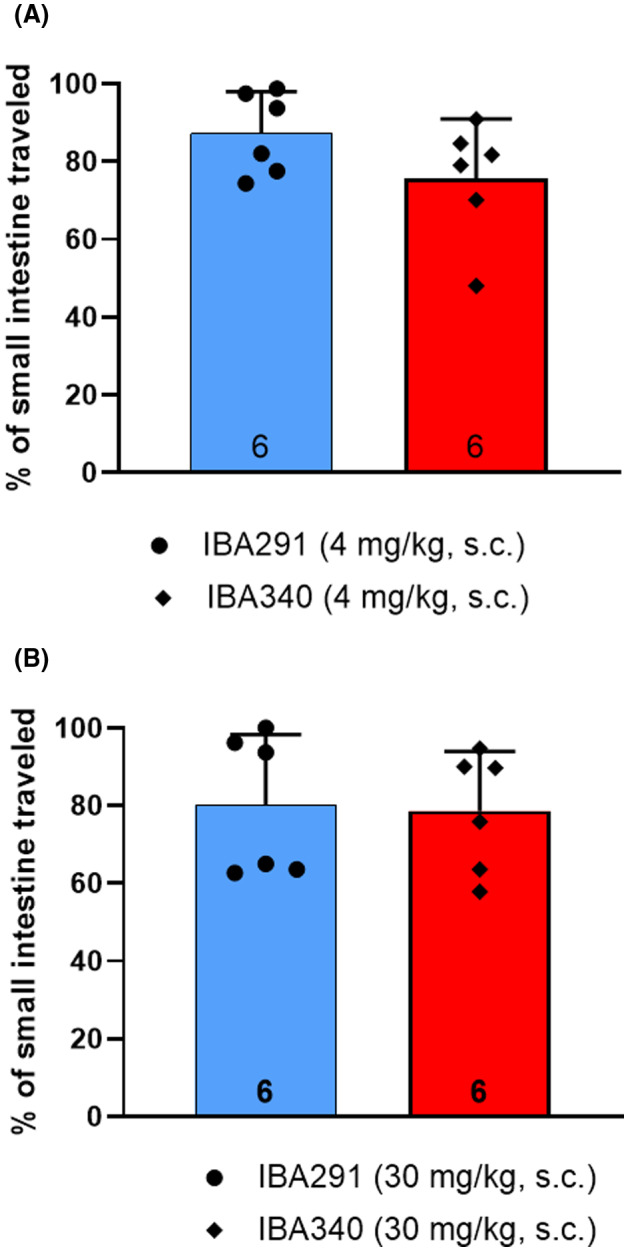
Distance charcoal meal traveled in small intestine. The calcitonin gene‐related peptide (CGRP) receptor antibody IBA340 or the control antibody IBA291 were given in sc doses of 4 mg/kg (A) and 30 mg/kg (B) to male humanized receptor activity–modifying protein 1 (RAMP1) mice (n = 6 per group) 72 h prior to the administration of the charcoal meal. The distance traveled in the small intestine was measured 30 min after charcoal meal gavage. Group data represented as mean ± standard deviation (n number at base of each bar). See Table 2 for detailed statistical analyses.
Transit rates in large intestine
Groups of six to eight female mice expressing hRAMP1 were pretreated (72 h) with 4 and 30 mg/kg of the CGRP receptor antibody IBA340 or 10 mg/kg of the control antibody IBA291. Both the 4 mg/kg (p < 0.01) and the 30 mg/kg (p < 0.001) doses caused a statistically significant reduction in the portion of large intestine containing charcoal meal, indicating reduced motility of the large intestine (Figure 2).
FIGURE 2.
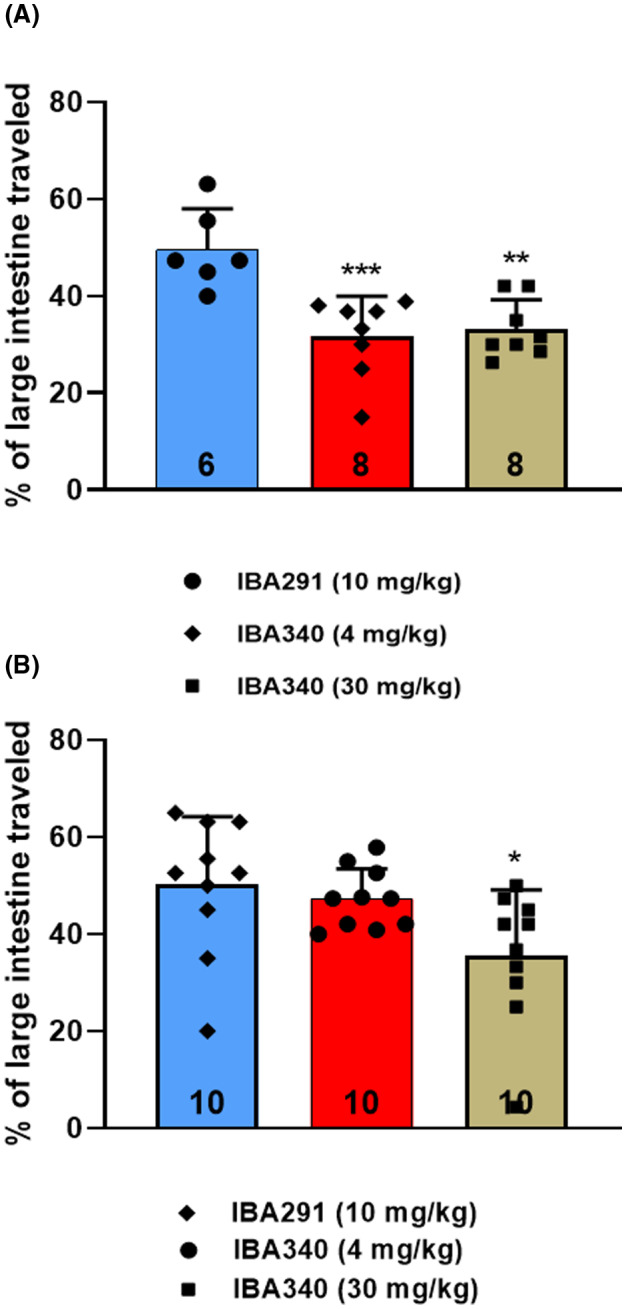
Distance charcoal meal traveled in large intestine. The calcitonin gene‐related peptide (CGRP) receptor antibody IBA340 were given in sc doses of 4 and 30 mg/kg or the control antibody IBA291 in a dose of 10 mg/kg to female (A; n = 6–8) and male (B; n = 10) humanized receptor activity–modifying protein 1 (RAMP1) mice 72 h prior to charcoal meal administration. The distance traveled in the large intestine was measured 90 min after charcoal meal gavage. Group data represented as mean ± standard deviation (n number at base of each bar). **p = 0.001, ***p < 0.001 versus IBA291. See Table 2 for detailed statistical analyses.
Groups of 10 male mice expressing hRAMP1 were pretreated (72 h) with 4 mg/kg and 30 mg/kg sc of the CGRP receptor antibody IBA340, or with 10 mg/kg of the control antibody IBA291. Large intestines were harvested and measured after 90 min. The higher dose of IBA340 produced a statistically significant (p = 0.019) reduction in the fraction of large intestine containing charcoal meal (Figure 2). The lower dose had a numerically reduced percentage of large intestine containing the meal, but this was not statistically significant.
Doses of 4 mg/kg and 30 mg/kg sc of galcanezumab or control antibody (IgG4 PAA) were given to groups of 7 to 10 female hRAMP1 mice 72 h prior to charcoal meal. The 4 mg/kg dose of galcanezumab had previously been shown to be efficacious in studies showing suppression of CGRP activity in vivo. 26 There were no differences in the portion of large intestine containing charcoal meal compared to the antibody control at either dose evaluated (Figure 3).
FIGURE 3.
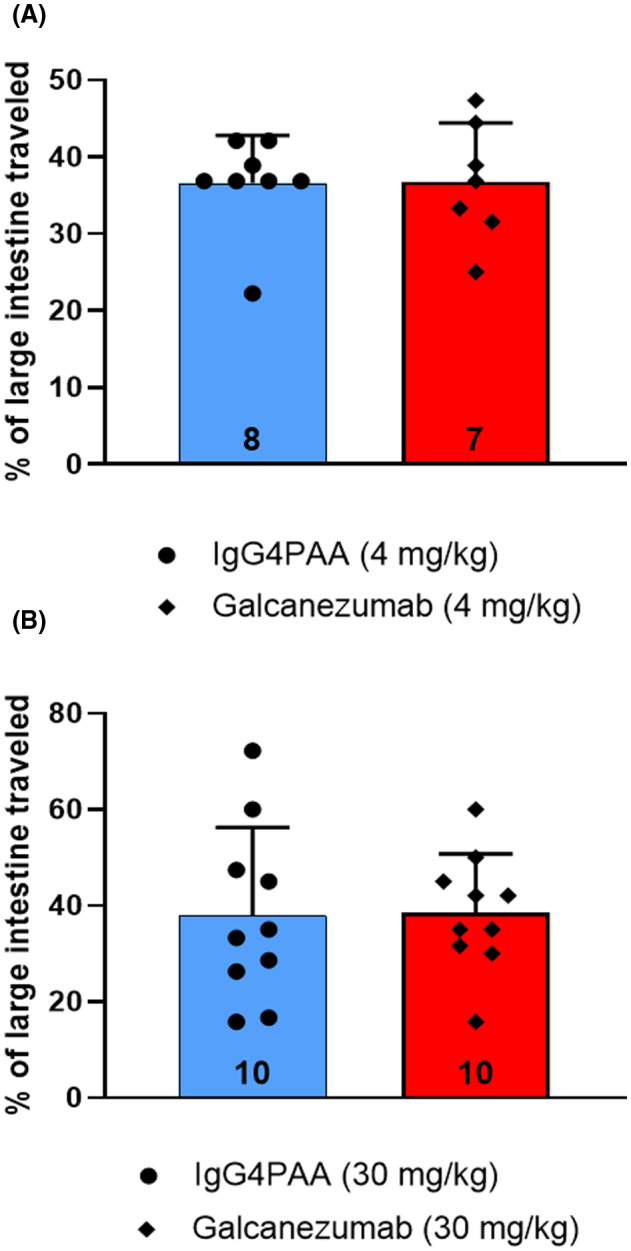
Distance charcoal meal traveled in large intestine following administration of galcanezumab or the control antibody immunoglobulin 4 PAA in doses of 4 mg/kg sc (A) and 30 mg/kg, sc (B) to (n = 7–10) humanized receptor activity–modifying protein 1 (RAMP1) female mice 72 h prior to charcoal meal administration. The distance traveled in the large intestine was measured 90 min after charcoal meal gavage. Group data represented as mean ± standard deviation (n number at base of each bar). See Table 2 for detailed statistical analyses.
Exposure and binding studies were conducted to inform the selection of telcagepant dose and its timing relative to charcoal meal gavage. The unbound plasma concentration of telcagepant achieved after a dose of 5 mg/kg, po at the time of charcoal meal gavage, 30 min after drug administration, was 7.7 nM. Telcagepant had a K b value of 1.1 nM when evaluated in a cell line expressing the hybrid human RAMP1/murine CLR receptor. 20 The ratio of unbound plasma concentration to the K i value in the “humanized” cell line was approximately 7 (7.7 nM/1.1 nM = 7), indicating in vivo exposures adequate to block the CGRP receptor. The CGRP receptor antagonist telcagepant, 5 mg/kg po, was given 30 min prior to charcoal meal gavage to groups of eight male hRAMP1 mice. Telcagepant‐treated mice showed a significantly (p = 0.013) smaller proportion of the large intestine with charcoal meal compared to the vehicle‐treated group (Figure 4), indicating a slowing of the gastrointestinal transit.
FIGURE 4.
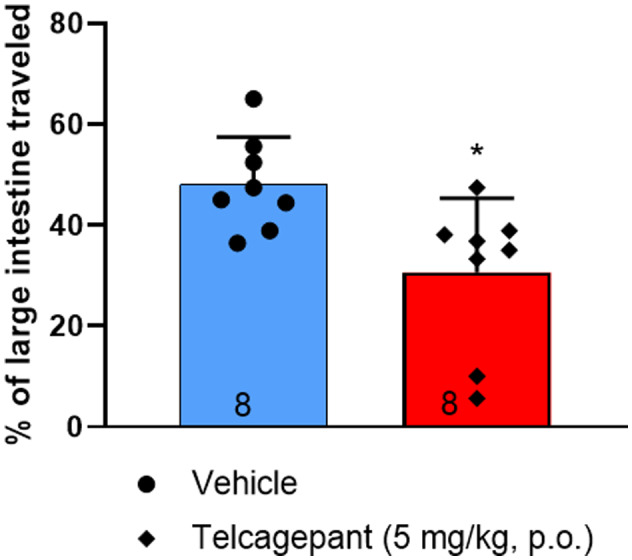
Distance charcoal meal traveled in large intestine following administration of telcagepant (5 mg/kg, po) or vehicle (n = 8) to humanized receptor activity–modifying protein 1 (RAMP1) male mice 30 min prior to charcoal meal administration. The distance traveled in the large intestine was measured 90 min after charcoal meal gavage. Group data represented as mean ± S standard deviation D (n number at base of each bar). *p < 0.05. See Table 2 for detailed statistical analyses.
Atogepant was given orally to female in two different sets of experiments. In the first run, mice received either vehicle (n = 9) or atogepant (0.03 or 0.3 mg/kg; n = 9) and in the second run, female mice received either vehicle (n = 8) or atogepant (3 mg/kg; n = 9) 30 min prior to charcoal meal gavage. Female mice receiving 0.03 and 0.3 mg/kg of atogepant showed a reduction in the proportion of large intestine containing charcoal meal relative to the vehicle‐treated mice that did not reach statistical significance (Figure 5A). In contrast, the atogepant‐treated female mice treated with a higher dose (3 mg/kg) showed a significantly (p = 0.030) smaller proportion of the large intestine with charcoal meal compared to the vehicle‐treated group (Figure 5B). Atogepant is reported to have a K i value of 0.015 nM at the human CGRP receptor. 27 The unbound plasma exposure to Ki ratios was calculated to be 7.3 and 72 for the 0.03 and 3.0 mg/kg doses, respectively, indicating these doses were adequate to inhibit the CGRP receptor.
FIGURE 5.
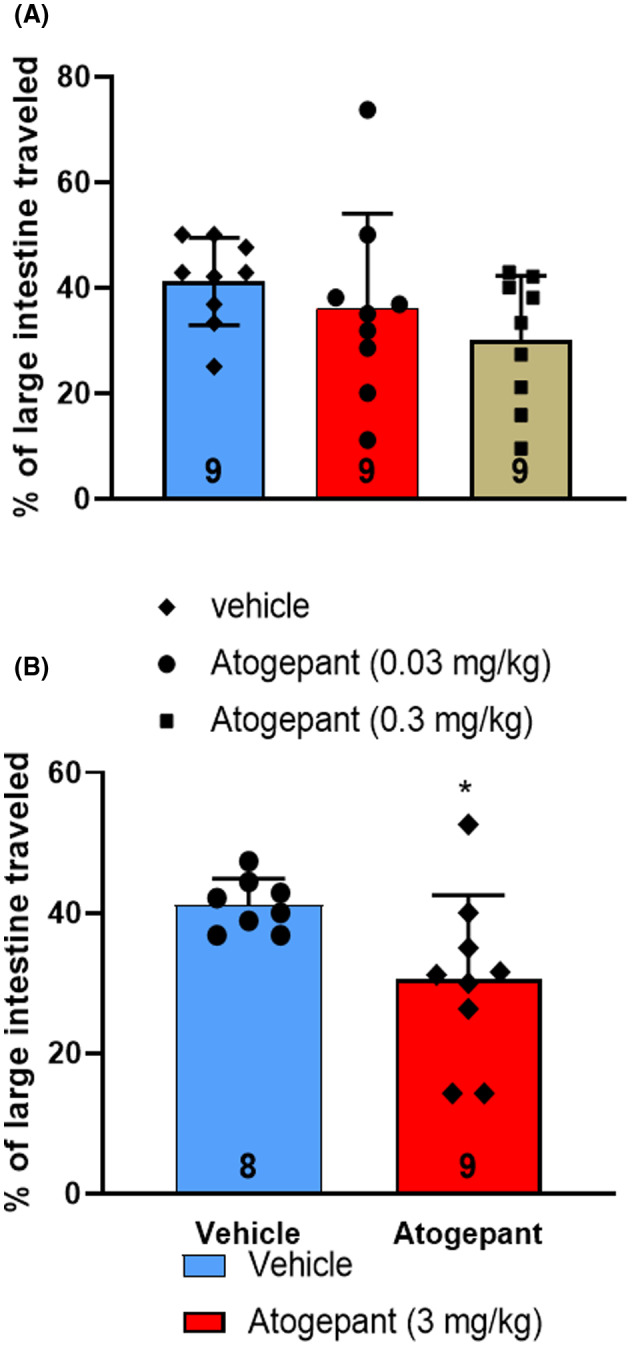
Distance charcoal meal traveled in large intestine of female humanized receptor activity–modifying protein 1 (RAMP1) mice following oral administration of atogepant or vehicle (A; 0.03 and 0.3 mg/kg) and (B; 3 mg/kg) 30 min prior to charcoal meal administration. The trial using 3 mg/kg of atogepant was run at a different time and with a different control group than the one run with 0.03 and 0.3 mg/kg of atogepant; consequently, these two trials are presented separately as (A) and (B). The distance traveled in the large intestine was measured 90 min after charcoal meal gavage. Group data represented as mean ± standard deviation (n number at base of each bar). *p < 0.05. See Table 2 for detailed statistical analyses.
DISCUSSION
The measurement of charcoal meal transit through the GI system is a commonly used animal model to assess the potential of drugs to produce diarrhea or constipation. 24 , 25 , 28 The results of the present investigation suggest that therapies that selectively block the activation of the CGRP receptor may result in constipation, as indicated by the significantly reduced travel of charcoal meal through the large intestine, whereas those that reduce activation of both the CGRP and AMY1 receptors together by reducing the overall pool of available CGRP ligand do not significantly alter GI transit, or produce constipation.
Species differences in affinity of compounds for the CGRP receptor can make preclinical studies challenging. For example, erenumab has picomolar affinity for the human CGRP receptor, but has more than 5000‐fold less affinity for the rat CGRP receptor. 29 , 30 Small‐molecule CGRP antagonists also show a >100‐fold difference in affinities between the human and rat CGRP receptors. 31 Studies employing site‐directed mutations and recombinant human/rat CRLR/RAMP1 receptors found that species selectivity for CGRP antagonists is directed exclusively by the RAMP1 component. 31 A recent study using Cos‐7 cells that were transfected with plasmids that encode mouse CLR/CTR and RAMPs found that the mouse receptors had reduced specificity for ligands compared to the human receptors. 32 The investigators suggested that there is a need for new ligands to differentiate these complexes in mice. 32 These concerns are mitigated in part by the fact that this study was performed with mice expressing human RAMP1. The functional in vitro assays demonstrated that the CGRP receptor antibody and antagonists could potently inhibit effects at the hRAMP1/mCLR hybrid receptor.
There are two approaches used for the treatment of migraine by blocking CGRP activity: reducing the overall availability of the neuropeptide itself—by sequestering CGRP with a monoclonal antibody, and blocking the activity of the CGRP receptor—either with an antibody or a pharmacologic receptor antagonist. CGRP binds with equal affinity to its eponymous receptor as well as to the AMY1 receptor, and it binds to the AMY1 receptor with equal affinity as amylin. 18 , 33 Thus, the AMY1 receptor may be thought of as an alternative CGRP receptor, 18 , 33 or a dual receptor for CGRP and amylin. 34
Therefore, one endogenous ligand can act at two different receptors that have opposing effects with regard to gastrointestinal motility. In human gastrointestinal tissue, mRNA for both CLR and RAMP1 were found in the stomach, ileum, and colon. 15 Nerve fibers of the myenteric plexus and nerve fibers throughout longitudinal and circular smooth muscle expressed immunoreactivity for CGRP, with immunoreactivity for CLR in close proximity. 15 Studies performed with mouse or rat intestinal tissue show a role for CGRP in GI motility. In isolated tissue preparations, CGRP inhibits electrically induced contractions of guinea pig ileum and relaxes unstimulated rat duodenum. 35 , 36 In isolated rat colonic preparations, CGRP dose‐dependently blocked isometric contractions and decreased resting muscle tone of isolated rat colon. 37 Knocked‐down expression of CLR in myenteric neurons and nerve fibers innervating circular smooth muscle significantly attenuated the effect of CGRP on colonic muscle tone, indicating that this effect is mediated through the CGRP receptor. 37 The CGRP receptor antagonist CGRP8–37 blocks ascending contraction and descending relaxation of intestinal responses to mucosal stimulation. 15 Systemic administration of CGRP to mice produced diarrhea that was blocked by the CGRP receptor antagonist olcegepant and by antibodies to the receptor. 16 Infusions of CGRP given over 2 h to healthy volunteers resulted in symptoms of increased GI activity in nearly all subjects. 17
Amylin was first identified in pancreatic islet cells of patients with type 2 diabetes, and was called islet amyloid polypeptide (IAPP). 21 , 38 It is secreted with insulin and acts in glucose regulation, in part by reducing gastric emptying. 21 , 38 Like CGRP, it is also expressed in many tissues in addition to the stomach and intestines, which include the lungs, peripheral nervous system, and the brain. 20 The iv injection of amylin radiolabeled with 125I resulted in high levels of radioactivity in the lung parenchyma and in the villi of the small intestinal mucosa, and elevated levels in kidney cortical tissue, whereas radioactivity was low and remained similar to blood levels in other tissue. 39 The uptake of amylin in lung and intestine was inhibited by administration of unlabeled amylin or CGRP. These results indicated that amylin was bound to receptors in these tissue, and likely exerted biological activity therein. 39 In rats, the intracerebroventricular (1–4 μg) or sc (25–100 μg/kg) administration dose‐dependently inhibited gastric emptying and intestinal transit of charcoal meal. 20 The amylin antagonist AC187 accelerates gastric emptying in animal models. 21 The amylin analog pramlintide, indicated for patients with diabetes, is contraindicated for patients with gastroparesis. 40
If CGRP can mediate opposing effects on GI motility through actions on the CGRP and the AMY1 receptors, then therapies that selectively block the CGRP receptor could disrupt a balance between these two actions. By blocking the promotility effects, without decreasing the activity of the AMY1 receptor, the balance is shifted to reduced GI motility, and therefore, constipation. Erenumab is more than 5000‐fold more selective for the CLR/RAMP1 receptor over the other human calcitonin family receptors, including the AMY1 receptor. 29 , 30 The affinity of erenumab for the CGRP receptor is similar to that of telcagepant. 29 , 30 Telcagepant is also considered to be a selective antagonist at the CGRP receptor, but its selectivity is less than that of erenumab. It is approximately 40‐fold more selective for the CGRP receptor over the AMY1 receptor. 18 In contrast, inactivation of CGRP with an antibody selective for the neuropeptide, such as galcanezumab, minimizes the activation of both the CGRP and AMY1 receptors by CGRP. The results presented here are consistent with this proposition. Selective blockade of the CGRP receptor shifted the balance, which was manifest as decreased travel in the large intestine, modeling constipation. Reducing overall CGRP availability (galcanezumab) did not produce significant changes in GI motility. A recent study using human embryonic kidney cells transfected with human CGRP and AMY1 receptors found that both erenumab and telcagepant blocked signaling induced by human amylin acting at the AMY1 receptor, in addition to their antagonistic activities at the CGRP receptor. 41 Moreover, it was found that erenumab, unlike fremanezumab, bound to and internalized both the CGRP and the AMY1 receptors. 41 It should be noted, however, that these experiments were run in a manner that does not allow the determination of relevant potency differences for erenumab at the CGRP and AMY1 receptors to be quantitatively determined as extremely high agonist concentrations were utilized, which limits the applicability of this report. In contrast, the potency differences from the functional assay reported in Table 1 for IBA340 strongly support selectivity for “erenumab” at the CGRP receptor versus the AMY1 receptor.
The results of the present investigation are consistent with the proposed mechanisms of action and results from the clinic. Treatments that can reduce activation of both the CGRP and AMY1 receptors did not inhibit GI transit, whereas those that selectively blocked CGRP receptors resulted in inhibition of GI transit. This observation is consistent with the constipation that has been reported to occur with erenumab, 6 atogepant, 11 and telcagepant. 12
A limitation of the study is the use of the charcoal meal GI transit assay lacks resolution, and this does not provide sufficient mechanistic information. Consequently, it is unclear if the effects observed here are due to constipation or reflect a delay in colonic transit. Another limitation is that this assay measures GI motility, without accounting for fecal output or stool water content. These factors could influence interpretation of the data regarding constipation, and should be considered in future studies. Finally, the experimenters were not blinded to the treatments, so the possibility of a bias exists.
AUTHOR CONTRIBUTIONS
Study concept and design: Kirk W. Johnson, Xia Li, Baolin Li, Beverly A. Heinz. Acquisition of data: Xiaofang Huang, Jianliang Yu, Xia Li, Baolin Li. Analysis and interpretation of data: Kirk W. Johnson, Xia Li, Baolin Li, Beverly A. Heinz. Drafting of the manuscript: Kirk W. Johnson, Xia Li. Revising it for intellectual content: Kirk W. Johnson, Xia Li, Baolin Li, Beverly A. Heinz, Jianliang Yu, Xiaofang Huang. Final approval of the completed manuscript: Kirk W. Johnson, Xia Li, Baolin Li, Beverly A. Heinz, Jianliang Yu, Xiaofang Huang.
CONFLICTS OF INTEREST
The authors are, or have been at the time of the study, employees of Eli Lilly and Company, and may own Lilly stock.
ACKNOWLEDGMENTS
Courtney Alexander of Eli Lilly and Company played a key role in the generation and separation of the CGRP receptor antibody. Michael H. Ossipov, PhD, of Evidera, provided medical writing services. Eli Lilly and Company contracted Evidera PPD for medical writing and editorial services.
Johnson KW, Li X, Huang X, Heinz BA, Yu J, Li B. Characterization of transit rates in the large intestine of mice following treatment with a CGRP antibody, CGRP receptor antibody, and small molecule CGRP receptor antagonists. Headache. 2022;62:848‐857. doi: 10.1111/head.14336
REFERENCES
- 1. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goadsby PJ. Primary headache disorders: five new things. Neurol Clin Pract. 2019;9:233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the trigeminal system in migraine. Headache. 2019;59:659‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott LJ. Ubrogepant: first approval. Drugs. 2020;80:323‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biohaven Pharmaceuticals I . Rimegepant: Highlights of Prescribing Information. Biohaven Pharmaceuticals; 2020. [Google Scholar]
- 6. Amgen Inc . AIMOVIG: Highlights of Prescribing Information. Amgen Inc; 2019. [Google Scholar]
- 7. Silberstein SD, McAllister P, Ning X, et al. Safety and tolerability of Fremanezumab for the prevention of migraine: A pooled analysis of phases 2b and 3 clinical trials. Headache. 2019;59:880‐890. [DOI] [PubMed] [Google Scholar]
- 8. Bangs ME, Kudrow D, Wang S, et al. Safety and tolerability of monthly galcanezumab injections in patients with migraine: Integrated results from migraine clinical studies. BMC Neurol. 2020;20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real‐world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanaan S, Hettie G, Loder E, Burch R. Real‐world effectiveness and tolerability of erenumab: A retrospective cohort study. Cephalalgia. 2020;40:1511‐1522. [DOI] [PubMed] [Google Scholar]
- 11. Clinicaltrials.gov . Identifier NCT02848326, Efficacy, Safety, and Tolerability of Multiple Dosing Regimens of Oral Atogepant (AGN‐241689) in Episodic Migraine Prevention. National Library of Medicine (US); 2018. [Google Scholar]
- 12. Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958‐966. [DOI] [PubMed] [Google Scholar]
- 13. Smillie SJ, Brain SD. Calcitonin gene‐related peptide (CGRP) and its role in hypertension. Neuropeptides. 2011;45:93‐104. [DOI] [PubMed] [Google Scholar]
- 14. MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD. Wiping out CGRP: Potential cardiovascular risks. Trends Pharmacol Sci. 2016;37:779‐788. [DOI] [PubMed] [Google Scholar]
- 15. Cottrell GS, Alemi F, Kirkland JG, Grady EF, Corvera CU, Bhargava A. Localization of calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) in human gastrointestinal tract. Peptides. 2012;35:202‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaiser EA, Rea BJ, Kuburas A, et al. Anti‐CGRP antibodies block CGRP‐induced diarrhea in mice. Neuropeptides. 2017;64:95‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falkenberg K, Bjerg HR, Olesen J. Two‐hour CGRP infusion causes gastrointestinal hyperactivity: Possible relevance for CGRP antibody treatment. Headache. 2020;60:929‐937. [DOI] [PubMed] [Google Scholar]
- 18. Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: Function and expression of the AMY1 receptor. Ann Clin Transl Neurol. 2015;2:595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene‐related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158:543‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clementi G, Caruso A, Cutuli VM, de Bernardis E, Prato A, Amico‐Roxas M. Amylin given by central or peripheral routes decreases gastric emptying and intestinal transit in the rat. Experientia. 1996;52:677‐679. [DOI] [PubMed] [Google Scholar]
- 21. Gedulin BR, Jodka CM, Herrmann K, Young AA. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul Pept. 2006;137:121‐127. [DOI] [PubMed] [Google Scholar]
- 22. Bohn KJ, Li B, Huang X, et al. CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br J Pharmacol. 2017;174:1826‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittelstadt SW, Hemenway CL, Spruell RD. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J Pharmacol Toxicol Methods. 2005;52:154‐158. [DOI] [PubMed] [Google Scholar]
- 24. Guillaume P, Provost D, Lacroix P. Gastrointestinal models: Intestinal transit, gastric emptying, and ulcerogenic activity in the rat. Curr Protoc Pharmacol 2008, 42, 5.3.1–5.3.12. Chapter 5:Unit5 3. [DOI] [PubMed] [Google Scholar]
- 25. Peracchia F, Bianchi G, Fiocchi R, Petrillo P, Tavani A, Manara L. Central and peripheral inhibition of gastrointestinal transit in rats: Narcotics differ substantially by acting at either or both levels. J Pharm Pharmacol. 1984;36:699‐701. [DOI] [PubMed] [Google Scholar]
- 26. Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene‐related peptide for the treatment of osteoarthritis‐related pain. Osteoarthr Cartil. 2014;22:578‐585. [DOI] [PubMed] [Google Scholar]
- 27. Dubowchik GM, Conway CM, Xin AW. Blocking the CGRP pathway for acute and preventive treatment of migraine: The evolution of success. J Med Chem. 2020;63:6600‐6623. [DOI] [PubMed] [Google Scholar]
- 28. Peddireddy M. In vivo methods for evaluation of drugs for the treatment of gastrointestinal motility disorders. Indian J Pharm Educ Res. 2010;44:42‐48. [Google Scholar]
- 29. Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther. 2016;356:223‐231. [DOI] [PubMed] [Google Scholar]
- 30. Garces F, Mohr C, Zhang L, et al. Molecular insight into recognition of the CGRPR complex by migraine prevention therapy Aimovig (Erenumab). Cell Rep. 2020;30:1714‐1723.e1716. [DOI] [PubMed] [Google Scholar]
- 31. Mallee JJ, Salvatore CA, LeBourdelles B, et al. Receptor activity‐modifying protein 1 determines the species selectivity of non‐peptide CGRP receptor antagonists. J Biol Chem. 2002;277:14294‐14298. [DOI] [PubMed] [Google Scholar]
- 32. Garelja ML, Bower RL, Brimble MA, et al. Pharmacological characterisation of mouse calcitonin and calcitonin receptor‐like receptors reveals differences compared with human receptors. Br J Pharmacol. 2022;179:416‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Edvinsson L, Grell A‐S, Warfvinge K. Expression of the CGRP family of neuropeptides and their receptors in the trigeminal ganglion. J Mol Neurosci. 2020;70:930‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hay DL. Amylin. Headache. 2017;57(Suppl 2):89‐96. [DOI] [PubMed] [Google Scholar]
- 35. Cottrell GS, Roosterman D, Marvizon JC, et al. Localization of calcitonin receptor‐like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239‐255. [DOI] [PubMed] [Google Scholar]
- 36. Maggi CA, Meli A. The sensory‐efferent function of capsaicin‐sensitive sensory neurons. Gen Pharmacol. 1988;19:1‐43. [DOI] [PubMed] [Google Scholar]
- 37. Clifton MS, Hoy JJ, Chang J, et al. Role of calcitonin receptor‐like receptor in colonic motility and inflammation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G36‐G44. [DOI] [PubMed] [Google Scholar]
- 38. Hay DL, Walker CS. CGRP and its receptors. Headache. 2017;57:625‐636. [DOI] [PubMed] [Google Scholar]
- 39. Stridsberg M, Tjalve H, Wilander E. Whole‐body autoradiography of 123I‐labelled islet amyloid polypeptide (IAPP). Accumulation in the lung parenchyma and in the villi of the intestinal mucosa in rats. Acta Oncol. 1993;32:155‐159. [DOI] [PubMed] [Google Scholar]
- 40. AstraZeneca Pharmaceuticals LP . SYMLIN: Highlights of Prescribing Information. AstraZeneca Pharmaceuticals; 2014. [Google Scholar]
- 41. Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia. 2021;41:499‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]


