Abstract
The objective of this study was to review publications assessing cognitive functioning in patients with prostate cancer treated with androgen deprivation therapy. We conducted a systematic review of the literature published in PubMed, Embase, Web of Science, Cochrane Library, and PsycINFO up to February 2020. A total of 31 studies were included. Half of the studies (n = 16) demonstrated that androgen deprivation therapy in patients with prostate carcinoma did not result in a negative effect on cognitive functioning, however, still a substantial proportion of the studies (n = 11) reported a negative effect on cognitive functioning. In four studies the results were inconclusive. In the three studies using additional functional magnetic resonance imaging, no significant effect on neuropsychological tests was found, but grey matter volume, brain activity, and brain connectivity were affected. Given the substantial number of studies showing a significant negative effect of androgen deprivation therapy on cognitive functioning, clinicians should be aware of this side effect. Furthermore, future research should focus on the further examination of brain characteristics using functional magnetic resonance imaging, since these techniques might be more sensitive in detecting brain abnormalities as a result of androgen deprivation therapy.
Keywords: androgen deprivation therapy, cognitive functioning
Abbreviations & Acronyms
- ADT
androgen deprivation therapy
- AVLT
Auditory Verbal Learning Test
- BVMT‐R
Brief Visual Memory Test–Revised
- CCT
computerized cognitive training
- CNS
central nervous system
- COWAT
Controlled Oral Word Association Test
- CVLT
California Verbal Learning Test
- D‐KEFS
Delis–Kaplan Executive Function System
- DSST
Digit Symbol Substitution Test
- EBPM
Event‐Based Prospective Memory
- fMRI
functional magnetic resonance imaging
- GMV
grey matter volume
- HVLT‐R
Hopkins Verbal Learning Test–Revised
- HVOT
Hooper Visual Orientation Test
- JLOT
Judgement of Line Orientation Test
- LHRH
luteinizing hormone‐releasing hormone
- MMSE
Mini‐Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NA
not applicable
- NART
National Adult Reading Test
- NPA
neuropsychological assessment
- PI
proactive interference
- RAVLT
Rey Auditory Verbal Learning Test
- RCFT
Rey Complex Figure Test
- RCT
randomized controlled trial
- SCWT
Stroop Color Word Task
- SDMT
Symbol Digit Modalities Test
- SOPT
Subject Ordered Pointing Task
- TAVEC
Auditive Verbal Spanish Complutense Test
- TBPM
Time‐Based Prospective Memory
- TMT
Trail‐Making Test
- WAIS‐R
Wechsler Adult Intelligence Scale–Revised
- WML
white matter lesion
- WMS‐R
Wechsler Memory Scale–Revised
- WMV
white matter volume
Introduction
Prostate cancer is the second most common cancer worldwide, with 1.3 million cases in 2018 and a yearly death rate of approximately 300 000. 1 Prostate adenocarcinoma cells are, in general, initially testosterone‐sensitive. Consequently, approximately 50% of all patients are treated with ADT, predominantly by the use of LHRH. The majority of men undergo chemical castration with LHRH agonists or antagonists with or without antiandrogens. 2 The testicular production of testosterone is suppressed, as is the hypophyseal production of gonadotropins, especially luteinizing hormone, which stimulates testosterone production. ADT is usually given for many years or even lifelong. Several different side effects may occur during this therapy, for example, cardiovascular disease, osteoporosis, anemia, hot flushes, metabolic diseases such as insulin resistance, hyperlipoproteinemia, (central) adiposity, symptoms of a disturbed sexual life, loss of muscular tissue, and gynecomastia. 3 , 4
In the last two decades, attention has also been paid to potential disturbances in cognitive functioning in patients treated with ADT. 5 , 6 “Free” testosterone, and especially its more potent metabolite dihydrotestosterone (fourfold), as well as estradiol (which is formed by conversion from testosterone by the enzyme aromatase) and their receptors are found throughout the male brain. The receptors for these hormones are especially present in areas that are involved in cognition such as the thalamus, the hippocampus and the cerebral cortex. Their action follows several different pathways, such as activation of calcium channels, modulating neurotransmitters and decreased production of beta‐amyloid. 7 In animal studies, a positive effect of substitution with androgens or estrogens on cognitive functioning was found after castration. 8 In older men, testosterone levels decrease and a positive relationship was found between free testosterone levels and several different cognitive functions, such as working memory, verbal memory, and visuospatial abilities. 9 , 10 , 11 Of interest is the recent hypothesis that it is not the decrease of testosterone and estradiol in elderly men and women that is an etiological factor for the cognitive decline (especially in Alzheimer’s disease), but the elevated gonadotropins and their releasing hormone, because of the loss of negative feedback of the sex steroids. In that case, hormonal therapy with LHRH agonists or antagonists in prostate cancer could be a factor that slows down cognitive decline. 3 , 12 , 13
To the best of our knowledge, six review articles have appeared on the subject of cognitive function in patients with prostate carcinoma treated with ADT. The first review was by Nelson et al. 7 This review included nine relevant (small) studies and concluded that between 47% and 69% of men treated with ADT declined in at least one cognitive area, most often in visuospatial abilities and executive functioning. 7 In 2012, Jamadar et al. 14 selected 11 studies and reported that most of these had important limitations (e.g. small sample sizes, suboptimal control groups and baseline group differences in confounding factors). Nevertheless, it was concluded that the studies with the best controls suggested a potential negative impact of ADT on spatial memory and perhaps verbal memory. 14 McGinty et al. 15 conducted a systematic review and meta‐analysis in 2014, including 14 studies, and analyzing seven cognitive domains. Patients on ADT performed worse on visuomotor ability tasks compared to controls as well as their own baseline measurements. No significant effects were detected in the other domains (i.e. attention/working memory, executive functioning, language, verbal memory, visual memory, visuospatial ability). Mundell et al. 16 included 13 prospective studies in their review, and in five of these studies no effect on cognitive function was observed. In the other seven studies the evidence indicates that ADT adversely affects several different cognitive domains. 16 Treanor et al. 17 conducted a “review of reviews” including 28 reviews describing 20 primary studies published between 2003 and 2013. They found a prevalence rate of cognitive dysfunction varying from 10% to 69%. The domains impaired by ADT in prostate cancer patients included verbal memory, visuospatial abilities and executive functioning. 17 The most recent systematic review and meta‐analysis was conducted by Sun et al., 18 who reviewed 26 articles. Because of quality characteristics, only two prospective cohort studies and four retrospective cohort studies could be included in the meta‐analysis. The overall results on cognitive tests following ADT in prostate cancer patients were inconclusive in the two prospective cohort studies, and nonsignificant in the other four retrospective studies. 18
Considering the observation that the outcomes of the increasing number of studies on cognitive functioning in patients with prostate cancer treated with ADT are rather inconclusive, the aim of the present systematic review was to provide an overview of studies examining cognitive functioning in prostate cancer patients treated with ADT, including articles published up to 2020. Based on previous literature, we hypothesized that treatment with ADT using LHRH agonists or antagonists, and therefore exposure to low gonadotropins, might have a protective effect on cognitive functioning. Furthermore, we were interested in studies combining neuropsychological tests with modern techniques to detect abnormalities in the brain (i.e. structural, functional, metabolic) in patients with prostate cancer on ADT. 19 , 20 , 21 , 22 , 23 , 24
Methods
Search strategy and data extraction
The following electronic databases were searched: PubMed, Embase, Web of Science, Cochrane Library, and PsycINFO. The search was performed on 6 February 2020. A search strategy was composed focusing on patients with prostate cancer and the effect of LHRH treatment on their cognitive functioning. All relevant keyword variations were used, including free text. All duplicates were excluded. See Appendix S1 for the complete search strategy. Only original studies were included. Studies were eligible if: (i) patients with prostate cancer were addressed; (ii) treatment with LHRH agonists or antagonists was involved; (iii) cognitive functioning was assessed and reported; (iv) sample size was >10 patients; and (v) they were written in English. Case reports, letters, and reviews were excluded. Data extraction and eligibility were assessed by three independent investigators (IM Jazet, AE Meinders, and CD Andela). Inconsistencies were resolved by reaching consensus. All references were checked for additional papers. The following data were extracted: (i) sample size; (ii) age; (iii) design; (iv) treatment; (v) potential inclusion of controls; (vi) procedure; (vii) cognitive measures used; (viii) cognitive domains assessed; and (ix) outcome of the study.
Quality assessment
A quality assessment was performed on all included studies. Eleven items were identified: clear research objective, inclusion/exclusion criteria, population demographics, duration of treatment, sample size, design, control group included, cognitive domains assessed, validation of measures, test instruction described and discussion of limitations (Appendix S2). The total individual quality score ranged from 0 to 23 points (Table 1). The quality of the studies was assessed by two reviewers independently (AE Meinders and CD Andela), discrepancies were discussed and resolved by reaching consensus. The total scores were calculated as percentages (individual score/23 × 100%). The median of the scores was 70 and was used as a cut‐off point. Studies with a quality score ≥70 were considered high‐quality papers (n = 17 studies). Given the low number of studies, studies were not excluded based on their quality assessment score.
Table 1.
Quality assessment of the included studies
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 12 | Score | Quality score, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stone et al. 38 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 11 | 48 |
| Green et al. 51 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 14 | 61 |
| Cherrier et al. 44 | 1 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 1 | 1 | 1 | 14 | 61 |
| Salminen et al. 50 | 1 | 2 | 1 | 3 | 0 | 1 | 3 | 3 | 2 | 0 | 1 | 17 | 74 |
| Almeida et al. 41 | 1 | 2 | 2 | 3 | 0 | 1 | 0 | 3 | 2 | 1 | 1 | 16 | 70 |
| Green et al. 52 | 1 | 2 | 1 | 3 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 16 | 70 |
| Bussiere et al. 32 | 1 | 2 | 1 | 4 | 2 | 1 | 3 | 1 | 2 | 1 | 0 | 18 | 78 |
| Jenkins et al. 45 | 1 | 1 | 1 | 2 | 0 | 1 | 3 | 2 | 2 | 1 | 1 | 15 | 65 |
| Salminen et al. 43 | 1 | 2 | 2 | 3 | 0 | 1 | 0 | 3 | 2 | 1 | 1 | 16 | 70 |
| Joly et al. 28 | 1 | 2 | 2 | 4 | 1 | 1 | 2 | 3 | 2 | 0 | 1 | 19 | 83 |
| Clay et al. 34 | 1 | 1 | 0 | 4 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 15 | 65 |
| Cherrier et al. 42 | 1 | 2 | 1 | 2 | 0 | 1 | 3 | 2 | 2 | 1 | 1 | 16 | 70 |
| Alibhai et al. 49 | 1 | 2 | 2 | 3 | 1 | 1 | 3 | 3 | 2 | 0 | 1 | 19 | 83 |
| Jim et al. 27 | 1 | 2 | 2 | 2 | 0 | 1 | 3 | 2 | 2 | 1 | 1 | 17 | 74 |
| Matousek et al. 53 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 16 | 70 |
| Mohile et al. 40 | 1 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 1 | 13 | 57 |
| Chao et al. 19 | 1 | 2 | 1 | 1 | 0 | 1 | 3 | 2 | 2 | 1 | 1 | 15 | 65 |
| Chao et al. 21 | 1 | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 1 | 1 | 13 | 57 |
| Tan et al. 37 | 1 | 1 | 0 | 3 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 10 | 43 |
| Wiechno et al. 31 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 1 | 2 | 0 | 1 | 12 | 52 |
| Gonzalez et al. 47 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 2 | 2 | 0 | 1 | 19 | 83 |
| Okamoto et al. 36 | 1 | 1 | 0 | 3 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 10 | 43 |
| Yang et al. 29 | 1 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 2 | 0 | 1 | 17 | 74 |
| Yang et al. 30 | 1 | 2 | 2 | 1 | 0 | 1 | 3 | 3 | 1 | 1 | 1 | 16 | 70 |
| Alibhai et al. 39 | 1 | 2 | 2 | 4 | 2 | 1 | 3 | 3 | 2 | 0 | 1 | 21 | 91 |
| Gunlusoy et al. 48 | 1 | 2 | 2 | 3 | 2 | 1 | 2 | 3 | 2 | 0 | 1 | 19 | 83 |
| Morote et al. 35 | 1 | 2 | 0 | 1 | 2 | 1 | 0 | 2 | 1 | 0 | 0 | 10 | 43 |
| Ali Shah et al. 33 | 1 | 2 | 0 | 3 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 12 | 52 |
| Wu et al. 54 | 1 | 2 | 1 | 4 | 1 | 2 | 2 | 3 | 2 | 0 | 1 | 19 | 83 |
| Ceylan et al. 46 | 1 | 2 | 2 | 3 | 2 | 1 | 2 | 3 | 2 | 0 | 1 | 19 | 83 |
| Plata‐Bello et al. 24 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 0 | 1 | 15 | 65 |
Bold text indicates a high‐quality paper: score ≥70.
Results
Literature overview
The literature search identified 1111 single publications, of which 33 were eligible for inclusion (Fig. 1). One study was found to be a duplicate 25 and was excluded. Another study referred to data described in a previous study 26 and was therefore also excluded. A final number of 31 studies was included, covering a total number of 1526 unique patients treated with ADT. Of these studies, 18 studies were prospective studies, nine studies were case–control/cross‐sectional studies and four studies were RCTs (Table 2).
Fig. 1.
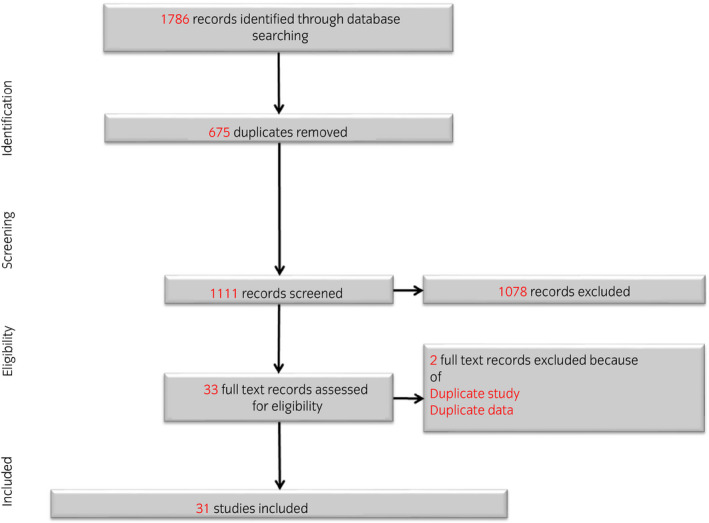
Flowchart literature selection process.
Table 2.
Literature table (N = 31)
| Reference | N † | Age, years‡ | Design | Treatment | Controls | Procedure | Cognitive measures | Cognitive domains | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Case‐control/cross‐sectional (n = 9) | |||||||||
| Jim et al. 27 | 48 | Mean 69 (51–87) | Case–control | Continuously treated with LHRH agonist alone or combined antiandrogen/LHRH agonist therapy for at least 6 months | 48 age‐ and education‐matched controls | Single visit for NPA |
HVLT‐R COWAT BVMT‐R Card Rotations test SDMT |
Memory Verbal fluency Visuospatial abilities Executive functioning |
Significantly more overall impairment (i.e. impairment in two or more tests) in patients compared to controls. Prior prostectomy was associated with impairment in immediate and delayed verbal memory in patients |
| Joly et al. 28 | 57 | Median 73, range 52–87 | Case–control | ≥3 months of ADT | 51 healthy age‐matched controls | Single visit for NPA | High‐Sensitivity Cognitive Screen |
Memory Attention/concentration Spatial ability Visual motor skills Language skills Self‐regulation/planning |
No difference in cognitive functioning between patients and controls |
| Yang et al. 29 | 33 | 68.85 ± 4.61 | Case–control |
6 months of ADT consisting of 50 mg bicalutamide once daily with an additional subcutaneous injection of 3.6 mg goserelin acetate once every 28 days following 2 weeks of bicalutamide therapy |
32 non‐ADT patients and 25 age‐ and education‐matched healthy controls | Single visit for NPA |
AVLT Digit span (WAIS‐III) SCWT TMT Verbal fluency MoCA |
Attention Concentration Executive functioning Visuospatial functions Memory Language Abstract thinking Naming Orientation Information processing Verbal fluency |
Patients on ADT performed worse on recognition, digit span forward, TMT B and the Stroop test compared to control groups |
| Yang et al. 30 | 43 | 69.28 ± 4.38 | Case–control | 6 months of ADT consisting of 50 mg bicalutamide once daily with an additional subcutaneous injection of 3.6 mg goserelin acetate once every 28 days following 2 weeks of bicalutamide therapy | 35 non‐ADT patients and 40 age‐ and education‐matched healthy controls | Single visit for NPA |
AVLT Digit span (WAIS‐III) SCWT TMT Verbal fluency MoCA EBPM TBPM |
Attention Concentration Executive functioning Visuospatial functions Memory Language Abstract thinking Naming Orientation Information processing Verbal fluency |
Patients on ADT performed worse on EBPM compared to control groups, with no significant differences in TBPM. Patients on ADT performed worse on attention, memory, information processing compared to control groups |
| Wiechno et al. 31 | 88 | Median 67, range 50–80 | Case–control | LHRH analogue, gosereline. 12 injections every 3 months. Was initiated within 3 months of radiotherapy completion | 61 with prostate cancer without hormonotherapy | Single visit for NPA | MMSE | General cognitive screening | No significant differences in cognitive functioning between patients on hormonotherapy and patients without hormonotherapy |
| Clay et al. 34 | 55 |
ST‐ADT: 74.4 ± 6.1, range 70.3–78.0 LT‐ADT: 73.1 ± 6.8, range 71.0–75.2 |
Cross‐sectional/case–control | ADT by orchiectomy, gonadotrophin‐releasing hormone agonists, antiandrogens, or a combination |
20 control subjects 25 non‐ADT patients |
Single visit for NPA in patients on short‐term ADT (<6 months) and patients on long‐term ADT (>6 months), non‐ADT patients, and controls | DSST (WAIS‐R) | Visuomotor skills | ADT did not have a significant effect on visuomotor function |
| Bussiere et al. 32 | 14 | Mean 66.9, range 50–80 | Case–control | Continuous ADT (with leuprolide acetate in 12 men and with orchiectomy in two men) without other concurrent adjuvant therapies, including chemotherapy | 16 healthy control men, matched for age, years of education and intelligence test (WAIS‐R vocabulary subtest) | NPA in patients on ADT for on average 1991 days | Word list‐learning test | Memory | Patients on ADT showed impairment in retention but normal encoding and retrieval processes |
| Ali Shah et al. 33 | 20 | Mean 72.1, range 57–85 | Cross‐sectional | ADT with LHRH agonist | NA | NPA in patients on early ADT (<4 months) and patients on late ADT (>4 months) | MMSE | General cognitive screening | No difference in cognitive functioning between early vs late ADT |
| Plata‐Bello et al. 24 | 50 | 78.3 ± 7.5 | Case–control | ≥6 months of ADT | 15 non‐ADT patients | NPA and MRI to examine GMV and WMV |
Word List Generation COWAT JLOT HVOT TMT BVMT TAVEC |
Verbal fluency Visuospatial ability Processing speed Memory |
No significant differences in cognitive functioning, GMV and WMV between patients and controls, but there was a negative relationship between ADT period and GMV |
| Prospective (n = 18) | |||||||||
| Morote et al. 35 | 308 | 71.2 ± 8.1, range 46–100 | Prospective, observational, multicenter, open‐label | LHRH analogue treatment, with bicalutamide 50 mg/day 2 weeks before and 2 weeks after the first LHRH administration | NA | NPA at baseline and after 6 months of treatment |
2‐part ad hoc test Digit Span (WAIS‐III) Benton Judgment of Line Orientation test Mental Rotation test Matrix Reasoning test (WAIS‐III) |
Memory Visuospatial ability Nonverbal analytical reasoning |
No significant change in cognitive functioning after LHRH analogues |
| Okamoto et al. 36 | 45 | 67.5 ± 3.5 | Prospective | 6‐month neoadjuvant ADT (i.e. leuprotide) with radiation therapy, followed by adjuvant ADT | NA | NPA before treatment, after 6 months and 12 months | MMSE | General cognitive screening | Treatment had no effect of MMSE scores. Lower MMSE scores were associated with low estradiol and cortisol and high androstenedione levels at 6 months |
| Tan et al. 37 | 50 | 71 (59–89) | Prospective | Leuprolide injection 30 mg every 4 months | NA | NPA before the first leuprolide injection and at 2, 4, 12 months |
MMSE CVLT |
General cognitive screening Memory |
No change from baseline to follow‐up on the MMSE. Verbal memory improved slightly, which was indicative of a practice effect |
| Almeida et al. 41 | 40 | 72.4 ± 7.5, range 44–83 | Prospective | Androgen blockade therapy (flutamide and leuprolide) for 36 weeks and followed‐up for another 18 weeks | NA | NPA at baseline and at week 4, 12, 24, 36, 42, 48 and 54 |
Cambridge Examination for Mental Disorders of the Elderly (CAMCOG) Word lists (WMS‐III) Verbal paired associations (WMS‐III) Visual reproduction (WMS‐III) Block design (WAIS‐III) |
Orientation Language Memory Attention Praxis Abstract thinking Perception Calculation Executive functioning Visuospatial ability |
Discontinuation of treatment is associated with better cognitive functioning, especially in verbal memory |
| Stone et al. 38 | 62 | Median 69 (55–80) | Prospective | First‐line hormone therapy cyproterone acetate 100 mg three times daily for 3 weeks followed by monthly injections with zoladex | NA | NPA prior to and following 3 months of treatment | Digit span (reverse) | Attention | No significant effects on the reverse digit span |
| Alibhai et al.§, 49 | 77 | 69.3 ± 6.9 |
Prospective Case–control |
ADT not further specified |
82 patients not receiving ADT 82 healthy controls |
NPA at baseline, 6 months and at 12 months |
Digit span (WAIS) Spatial span (WMS) TMT COWAT Card rotations test JLOT CVLT BVMT Conditional associative learning test Spatial working memory task D‐KEFS |
Attention Processing speed Verbal fluency Visuospatial ability Memory Executive functioning |
One test in immediate memory, working memory, and visuospatial ability were worse at 12 months in ADT users compared to control groups, while other test in these domain were not significantly different |
| Alibhai et al.§, 39 | 77 | Mean 68.9, median 16 (all included participants) |
Prospective Case–control |
73 patients used LHRH agonists alone, whereas four were receiving combined LHRH agonists and nonsteroidal antiandrogens | 82 non‐ADT patients and 82 healthy controls | NPA at baseline and on five occasions over 36 months |
Digit span (WAIS) Spatial span (WMS) TMT COWAT Card rotations test JLOT CVLT BVMT Conditional associative learning test Spatial working memory task D‐KEFS |
Attention Processing speed Verbal fluency Visuospatial ability Memory Executive functioning |
In patients ADT use was not associated with significant changes over time in any cognitive test compared with healthy controls |
| Cherrier et al. 42 | 20 | 62.05 ± 7.19 |
Prospective Case–control |
Intermitted ADT consisting of 9 months treatment with combined leuprolide and flutamide, followed by an off‐treatment period | 20 healthy controls matched for age and education | NPA at baseline and at 3 months and 9 months of ADT, and after 3 months of no treatment |
Puget sound route learning test Block design (WAIS‐R) Mental rotation test PI Story recall (WMS‐R) Verbal fluency test SCWT SOPT |
Memory Spatial ability Verbal fluency Executive functioning |
In ADT patients there was a significant decline at 3 months in spatial abilities (block design, mental rotation) and visual working memory (SOPT) |
| Mohile et al. 40 | 32 | Median 71.0, range 51–87 | Prospective | ADT with or without the addition of antiandrogen | NA | NPA at baseline and after 6 months of ADT |
TMT Digit span (WAIS‐III) COWAT RCFT HVLT‐R BVMT‐R Grooved pegboard and finger tapping test |
Attention Cognitive flexibility Verbal fluency Visuosptial abilities Memory Motor speed |
High prevalence of lower than expected cognitive performance at baseline. There was no significant difference in cognitive performance at baseline and at 6‐month follow‐up |
| Salminen et al. 50 | 25 | 64.4 ± 6.5, range 49–75 |
Prospective Case–control |
ADT was started with flutamide for 4 weeks, and LHRH analogue (s.c. 3 months four times) was added after 2 weeks | 52 healthy control subjects | NPA at baseline, at 6 months and at 12 months |
Similarities (WAIS) Digit span (WAIS) DSST (WAIS) Block design (WAIS) Object naming/recall Verbal fluency test Word list recall Benton visual recognition task Visual span (WMS) MMSE CogniSpeed software |
Language skills Verbal fluency Visuomotor skills Memory Attention Visuospatial abilities |
During follow‐up there was improvement in object recall and semantic memory. During longitudinal testing no impairment in cognitive functioning was found |
| Salminen et al. 43 | 23 | 65.0 ± 6.7, range 49–75 | Prospective | ADT started with flutamide 250 mg three times a day for 4 weeks, and LHRH analog (leuprolid 11, 25 mg subcutaneously, four times a year every 3 months) was added after 2 weeks | NA | NPA at baseline and at 6 and 12 months on ADT |
Similarities (WAIS) Digit span (WAIS) DSST (WAIS) Block design (WAIS) Object naming/recall Verbal fluency test Word list recall Benton visual recognition task Visual span (WMS) MMSE CogniSpeed software |
Language skills Verbal fluency Visuomotor skills Memory Attention Visuospatial abilities |
Visual memory and recognition speed were declined at 6 months. This decline was associated with a decline in estradiol during ADT Verbal fluency improved at 12 months |
| Chao et al. 19 | 15 | 69.0 ± 5.3 |
Prospective Case–control |
Receiving ADT for 6 months | 15 patients not receiving ADT, matched for age and education | NPA at baseline and after 6 months of treatment |
N‐back task Stop signal task MMSE |
Memory Cognitive control General screening |
No effect of treatment on cognitive functioning, but brain activation during cognitive control and functional brain connectivity (fMRI) were diminished after 6 months of treatment |
| Cherrier et al. 44 | 19 | Mean 65, range 51–81 (all included participants) |
Prospective Case–control |
9 months of leuprolide and flutamide followed by an off‐treatment period | 15 healthy community dwelling controls. | NPA at baseline, after 9 months of androgen suppression and after 3 months off‐treatment. |
Route test Block design Mental rotation test PI Story recall Verbal fluency test SCWT Grid arrays |
Memory Visuospatial abilities Verbal fluency Executive functioning Attention |
Patients declined on a measure of spatial rotation after 9 months of treatment. During off‐treatment period patients improved on a measure of verbal memory |
| Jenkins et al. 45 (pilot study) | 32 | 67.5 ± 4.7 |
Prospective Case–control |
LHRH agonist | 18 controls without prostate cancer | Before treatment, after 3 months or after completing drug treatment but before RT, and 9 months later |
NART Verbal fluency test RAVLT RCFT Mental rotation test Digit span (WMS‐III) Spatial span (WMS‐III) Kendrick assessment of cognitive ageing battery |
Intelligence Verbal fluency Memory Visuospatial ability Attention Processing speed |
After 3 months, LHRH therapy resulted in cognitive decline on at least one test (most frequently spatial memory and ability) in more patients compared to controls. There was no significant difference at 9‐month follow‐up |
| Ceylan et al. 46 | 72 | 67.27 ± 5.06 |
Prospective Case–control |
Complete ADT continuously for 12 months | 72 control patients who underwent radical prostatectomy | NPA at baseline and after 6 and 12 months | MoCA |
Attention Concentration Executive functioning Visuospatial functions Memory Language Abstract thinking Naming Orientation |
Patients and controls had worse post treatment scores. There were no differences between patients and controls |
| Chao et al. 21 | 12 | 69.1 ± 5.6 |
Prospective Case–control |
ADT consisted of LHRH agonist (Goserelin 10.8 mg subcutaneously every 90 days) after a lead‐in period for 2 weeks with bicalutamide 50 mg daily | 12 demographically matched controls | NPA and MRI of the brain at baseline and after 6 months of ADT |
N‐back task MMSE |
Memory General screening |
A decrease in GMV of the primary motor cortex was correlated with longer reaction time to target detection in the working memory task. There was no difference in working memory between patients and controls |
| Gonzalez et al. 47 | 58 | 67.31 ± 8.87 |
Prospective Case–control |
ADT for 12 months | 84 patient controls treated with prostectomy and 88 healthy controls. Both age and education matched | NPA at baseline and 6 and 12 months later |
HVLT‐R Logical memory (WMS‐III) Digit span (WMS‐III) Spatial span (WMS‐III) BVMT‐R Color trials SDMT COWA TIADL NART |
Intelligence Memory Attention Executive function |
ADT patients were more likely to demonstrate impaired cognitive performance within 6 and 12 months after starting ADT compared to controls |
| Gunlusoy et al. 48 | 78 | 67.12 ± 5.12 |
Prospective Case–control |
ADT treatment was an oral dose of 50 mg bicalutamide once daily with an additional subcutaneous injection of 10.8 mg goserelin acetate or 22.5 mg leuprolide acetate once every 3 months | 78 patients controls treated with radical prostectomy | NPA at baseline and after 6 and 12 months |
MoCA Frontal assessment battery |
Attention Concentration Executive functioning Visuospatial functions Memory Language Abstract thinking Naming Orientation Conceptualization Mental flexibility Programming Sensitivity of interference Inhibitory control Environmental autonomy |
Patients on ADT performed worse on post treatment test compared to patient controls, especially on language ability and short‐term memory capacity |
| RCT (n = 4) | |||||||||
| Green et al.§, 51 | 65 | 73.3 ± 6.4, range 56–86 | RCT | Leuprorelin or goserelin or cyproterone acetate | 15 patients in close clinical monitoring | Patients were randomly assigned to continuous leuprorelin or goserelin or cyproterone acetate or close clinical monitoring. NPA at baseline and before starting treatment, and 6 months later |
WMS AVLT RCFT Digit symbol (WAIS‐R) TMT COWAT SCWT WAIS‐R |
Memory Attention Executive functioning Intelligence |
24 of the 50 men randomized to active treatment demonstrated a significant decline in one or more tests. In the close clinical monitoring group no one demonstrated a decline |
| Green et al.§, 52 | 62 | 73.5 ± 6.4 | RCT |
Leuprorelin Goserelin Cyproterone acetate |
Close clinical monitoring group Community comparison group |
Before treatment and after 6 months and after 12 months |
WMS AVLT RCFT Digit symbol (WAIS‐R) TMT COWAT SCWT |
Memory Attention Executive functions |
Patients with pharmacologic treatment showed worse performance on verbal memory, coding and inhibitory tasks compared to the comparison groups |
| Matousek et al. 53 | 25 | 71.0 ± 8.8 | Prospective/RCT |
Phase 1: gonadotropin‐releasing hormone analogue + bicalutamide Phase 2: added micronized E2 1 mg/day |
Phase 2: added placebo | Prior to and after 3 months and after 6 months |
MMSE Mental rotations test Paper folding test Block design (WAIS‐III) Logical memory (WMS‐R) Verbal paired associations (WMS‐R) Verbal fluency test Digit symbol (WAIS‐III) Letter number sequencing task |
General cognitive screening Visuospatial abilities Memory Visuomotor skills Attention Verbal fluency |
No difference in cognitive functioning after 3 months of combined ADT. Added estradiol did not result into improvement of cognitive functioning |
| Wu et al. 54 | 60 | 66.6 ± 8.5 | RCT | ≥3 months of ADT | Usual care | Prior (T1) and directly after the home‐based CCT (T2), and after 8 weeks' follow‐up (T3) | CNS vital signs |
Attention Processing speed Motor speed Memory Cognitive flexibility |
CCT resulted in better reaction time, but worse verbal and visual memory. Memory was temporarily suppressed in the CCT group at T2, but normalized by T3 |
Number of patients with prostate carcinoma on ADT.
Mean ± standard deviation, or otherwise mentioned.
Duplicate sample.
Cognitive measures
In the 31 included studies, 17 different cognitive domains were examined using 61 different neuropsychological tests (Tables 2 and 3). The most frequently examined cognitive domain was memory (n = 26), followed by attention (n = 19), and visuospatial abilities (n = 18). The most frequently used test was Digit span (n = 10), followed by the MMSE (n = 9) which is a general cognitive screening, the COWAT (n = 7; assesses verbal fluency), as well as other verbal fluency tests (using letters or animal names; n = 6).
Table 3.
Neuropsychological tests used
| Memory | |
| PI | Verbal memory |
| Word lists (WMS‐III) | Verbal memory |
| Logical memory (WMS‐III) | Verbal memory |
| Verbal paired associations (WMS‐III) | Verbal memory |
| Story recall (WMS‐R) | Verbal memory |
| HVLT‐R | Verbal memory |
| Object naming/recall | Verbal memory |
| Word list recall | Verbal memory |
| CVLT | Verbal memory |
| Word list‐learning test | Verbal memory |
| PI | Verbal memory |
| RAVLT | Verbal memory |
| AVLT | Verbal memory |
| TAVEC | Verbal memory |
| BVMT‐R | Visual memory |
| Benton visual recognition task | Visual memory |
| Visual span (WMS) | Visual memory |
| Visual reproduction (WMS‐III) | Visual memory |
| Two‐part ad hoc test | Visual memory |
| Puget sound route learning test, route test | Spatial memory |
| Spatial working memory task | Spatial working memory |
| Conditional associative learning test | Working memory |
| N‐back task | Working memory |
| Letter–Number sequencing task | Working memory |
| EBPM | Event‐based memory |
| TBPM | Time‐based memory |
| Visuospatial ability | |
| Block design (WAIS‐R) | Visuospatial ability |
| Mental rotations test | Visuospatial ability |
| Card rotations test | Visuospatial ability |
| (Benton) JLOT | Visuospatial ability |
| HVOT | Visuospatial ability |
| Paper folding test | Visuospatial ability |
| Verbal fluency | |
| Verbal fluency test | Verbal fluency, executive functioning, attention |
| COWAT | Verbal fluency, executive functioning, attention |
| Word List Generation | Verbal fluency |
| Executive functions | |
| SCWT | Executive functioning, information processing |
| SOPT | Executive functioning, working memory |
| TMT | Attention, cognitive flexibility, processing speed |
| RCFT | Executive visuospatial planning abilities, visual memory |
| D‐KEFS color‐word interference test | Cognitive flexibility |
| Stop signal task | Cognitive control |
| Timed instrumental activities of daily living test | Executive functioning |
| Color trials | Executive functioning |
| Attention | |
| Digit span (WAIS‐III) | Attention, working memory |
| Spatial span (WMS‐R) | Attention, working memory |
| Grid arrays | Attention |
| Visuomotor skills | |
| Grooved pegboard and finger tapping test | Motor speed |
| DSST (WAIS) | Visuomotor skills, attention, executive functioning |
| SDMT | Visuomotor skills, attention, executive functioning |
| Language skills | |
| Similarities (WAIS) | Language skills |
| Intelligence | |
| Matrix reasoning test (WAIS‐III) | Nonverbal analytical reasoning |
| NART | General intelligence |
| WAIS‐R | General intelligence |
| Neuropsychological batteries | |
| Cambridge Examination for Mental Disorders of the Elderly (CAMCOG) | Cognitive screening |
| MMSE | Cognitive screening |
| Kendrick assessment of cognitive ageing battery | Processing speed |
| MoCA | Cognitive screening |
| Frontal assessment battery | Screening for frontal lobe function |
| High‐Sensitivity Cognitive Screen | Cognitive screening |
| WMS‐R | Memory assessment |
| CNS vital signs | Computer‐administered NPA tool |
| CogniSpeed software | Cognitive processing |
Single measurement of cognitive functioning of patients treated with ADT compared to control groups (case–control/cross‐sectional studies)
Nine studies examined cognitive functioning during a single measurement compared to other/control groups. These studies included a total of 408 unique patients. Their average age ranged from 66.9 to 78.3 years. They received treatment for at least 3–6 months. Of the included studies, seven were case–control studies, 24 , 27 , 28 , 29 , 30 , 31 , 32 one performed a cross‐sectional analysis (i.e. short‐term ADT vs long‐term ADT), 33 and one study used both. 34 Of the studies using a case–control design, three studies used a healthy matched control group, two studies included a patient control group, and three studies included a healthy matched control group, as well as a patient control group.
In five of the nine studies no significant differences were found in cognitive functioning between the patients on ADT and the specific control groups. 24 , 28 , 31 , 33 , 34 Clay et al. 34 did not find a difference in visuomotor performance between treated patients (ADT or orchiectomy) and healthy controls, as well as no difference between shorter treatment (<6 months) and longer treatment (>6 months) in patients. An unknown number of patients treated with orchiectomy was included, but no difference was found in visuomotor performance between patients with or without orchiectomy. 34 Interestingly, Plata‐Bello et al. 24 did not observe differences in cognitive functioning between prostate carcinoma patients on ADT and healthy controls, but they did observe a negative relationship between ADT period and GMV using MRI in 50 prostate cancer patients treated with ADT for 6 months. Also a positive association was found between age and WML burden in the ADT patients, but not in the control subjects. There were no significant differences in GMV and WML between patients and controls. 24
Four studies did observe a negative effect of ADT on cognitive functioning. 27 , 29 , 30 , 32 The study by Bussiere et al. 32 demonstrated that patients receiving ADT have impairment in retention, but normal encoding and retrieving processes in the memory domain. Two of the 14 included patients underwent orchiectomy as ADT. However, the results of the data remained the same after excluding the data of the patients who underwent orchiectomy. 32 In the study by Jim et al. 27 the patients on LHRH therapy displayed lower scores and higher rates of impairment on five of seven individual tests and a greater number of impaired tests, but the difference compared to the control subjects was not significant. However, the patient group displayed significantly greater overall impairment (defined as the percentage of individuals with impaired performance on two or more tests) then the control group. Prior prostatectomy was associated with impairment in immediate and delayed verbal memory in the ADT patients. 27 In the study by Yang et al., patients in the ADT group obtained significantly worse scores on several tests compared to the non‐ADT group and the healthy control group. No difference between the two control groups was found for the test results. 29 In the other study by Yang et al., patients on LHRH therapy performed worse on “EBPM,” on attention and memory and information processing, but not on “TBPM” compared to patient controls, as well as healthy controls. 30
Cognitive functioning during treatment with ADT (prospective studies)
Eighteen studies used a prospective design to evaluate the effect of ADT on cognitive functioning. These studies included a total of 968 unique patients. Their average age ranged from 64.4 to 72.4 years. Eleven of the 18 prospective studies included a control group (three studies included healthy controls as well as patients with prostate carcinoma not receiving ADT, five studies only included patient controls, and three studies only included healthy controls). The time to the last follow‐up measurement moment in these studies ranged from 3 to 36 months.
Nine of the 18 prospective studies did not find a significant effect of ADT on cognitive functioning. 19 , 21 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Although Mohile et al. 40 did not observe a decline in cognitive performance after 6 months of ADT, they did observe a high prevalence of lower than expected cognitive performance at baseline in patients with prostate cancer. Interestingly, Chao et al. 19 evaluated the effect of ADT on cognitive functioning, as well as on brain characteristics by using fMRI. They prospectively followed 15 patients with nonmetastatic prostate cancer treated with ADT and 15 patients with nonmetastatic prostate cancer without ADT; the patient groups were comparable in age and educational level. Patients were tested before and 6 months after starting ADT, and controls were tested twice with an interval of 6 months. The N‐back task (assessing working memory) and the stop‐signal task (assessing cognitive control) were used. The results for the N‐back task and the stop‐signal task were similar after 6 months compared with baseline in each group. However, significant associations were found between ADT use (vs nonuse) and decreased medial prefrontal cortical activation during cognitive control. This was also found for decreased connectivity between the medial prefrontal cortex and other regions involved in cognitive control. Thus, ADT for 6 months did not affect the selected tests for cognition, however, fMRI showed abnormalities in brain activations and brain connectivity during testing. 19 In 2013 the same research group reported the results of a structural MRI study of cerebral morphology in 12 prostate cancer patients before and after 6 months of ADT compared with 12 comparable patients not treated with ADT. The ADT group showed a decreased GMV in the frontopolar cortex, the dorsolateral prefrontal cortex and the primary motor cortex. These changes were not found in the control subjects. The decrease in GMV of the primary motor cortex related significantly to a longer reaction time to target detection in a working memory task, suggesting processing insufficiency. 21 Almeida et al. 41 assessed the effect of ADT and evaluated cognitive functioning for 36 weeks during treatment (on‐treatment period). Then ADT was stopped and cognitive functioning was evaluated during the following 18 weeks (off‐treatment period). During the on‐treatment period there were no clinically meaningful changes in cognitive functioning. However, after discontinuing ADT, performances on the verbal memory test improved while there were no differences on visuospatial tasks. The authors reported that the improvement in cognitive functioning after discontinuing ADT might be explained by the fact that, during the on‐treatment period, the potential learning effect of multiple testing was counterbalanced by the potential negative cognitive effect of a lack of sex hormones. After stopping ADT this learning effect dissipated. 41
Of the 18 prospective studies, a total of eight studies reported a decline in cognitive functioning after starting ADT. 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 These studies observed a decline in cognitive functioning during a follow‐up period of 3 months 42 , 44 , 45 or 12 months compared to controls. 43 , 46 , 47 , 48 , 49 Alibhai et al. 49 observed a decline in cognitive tests assessing immediate memory, working memory, and visuospatial ability after 12 months in patients treated with ADT compared to healthy controls; however, when comparing the percentage of participants that declined (>1 SD), there were no differences between patients and controls. Furthermore, Salminen et al. 43 observed a decline in cognitive functioning after 12 months of ADT (compared to baseline) which was also associated with a decline in estradiol. Interestingly, they observed an improvement in verbal fluency after 12 months of ADT. 43 Ceylan et al. 46 did observe a decline in cognitive functioning after a treatment period of 12 months compared to baseline, but this was not different from the control group. Cherrier et al. also evaluated the effect during off‐treatment. They observed that cognitive functioning declined after 3 months of ADT, but that there were no significant differences compared to baseline after 9 months of ADT or at 3 months after discontinuing ADT (at 12 months after the start of ADT). 42 , 44
Apparently one prospective study found improvement in cognitive functioning (i.e. object recall, semantic memory) after 12 months of ADT. 50
Effect of different treatment modalities on cognitive functioning (RCTs)
Four of the included studies were RCTs, of which three evaluated the effect of different ADT modalities, 51 , 52 , 53 and one evaluated the effect of CCT on cognitive functioning in prostate cancer patients on ADT. 54 The number of included patients with prostate cancer in the three studies evaluating ADT modalities ranged between 25 and 65 patients. The average participant age in these studies ranged between 71.0 and 73.5 years. In these studies, the control groups were either patients with prostate carcinoma treated with cyproterone acetate, close monitoring, or healthy men without prostate carcinoma.
In the study by Matousek et al., 53 after 3 months of combined ADT (ADT and bicalutamide), estradiol or placebo was added for 3 months. The authors did not observe any differences in cognitive test scores during the first 3 months of combined ADT. Furthermore, adding estradiol to the combined ADT in the following 3 months did not result in improvement of cognition. 53 In both studies by Green et al., 51 , 52 active hormonal therapy was accompanied by a decline of cognitive function (at 6 and 12 months) compared to the control groups.
The study by Wu et al. 54 did not show a consistent improvement of cognitive functioning after computerized neuropsychological training.
Discussion
The present systematic review demonstrates that half of the available studies in patients with prostate carcinoma treated with ADT using LHRH agonists or antagonists report a decline in cognitive functioning, while the other half did not show a negative effect on cognitive functioning, and only one study reported an improvement in cognitive functioning. Therefore, it can be postulated that the evidence for the hypothesis of a potential protective effect of ADT is not convincing.
Based on our quality assessment, it can be concluded that more than half of the included studies were of high quality. However, there was a large variety in the tests used (i.e. 61 different neuropsychological tests), making it difficult to compare the influence of ADT on specific cognitive function. Of the studies that observed impairment in cognitive functioning, memory was the most frequently impaired domain, followed by spatial abilities, executive functioning, language ability, attention, and information processing. The cognitive decline was observed after 3 months of ADT when such early measurements took place. Two studies also examined cognitive functioning during off‐treatment periods (periods ranging from 3 to 4.5 months) and observed improvement/normalization of cognitive functioning after an initial decline after starting ADT. One might argue that these are rather short periods of observation, knowing that the normalization of hormone levels after stopping treatment with LHRH agonists or antagonists can take considerably longer periods. Furthermore, for the examination of cognitive functioning during off‐treatment, it is important to establish normalization of the hypothalamic–hypophysial–testicular axis.
Of the included studies, only three combined cognitive tests with fMRI. 19 , 21 , 24 These studies showed that ADT for 6 months did not affect cognitive functioning (i.e. working memory, cognitive control); however, fMRI showed smaller GMVs in the frontopolar cortex, the dorsolateral prefrontal cortex, and the primary motor cortex, as well as impaired brain activation and brain connectivity during testing. 19 , 21 Furthermore, a longer duration of ADT was associated with smaller GMVs (whole brain), and older age was associated with more WMLs in prostate carcinoma patients on ADT. 24 Although not included in the present review, Cherrier et al. reported a preliminary study of fMRI of the brain in combination with cognitive testing in five ADT‐treated prostate cancer patients compared to seven healthy control subjects of the same age and education level. The tests were performed before and 9 months after ADT and twice with a 9‐month time interval in the controls. Patients treated with ADT showed a reduced blood oxygenation level‐dependent activation using fMRI, which was not found in the control subjects. Reduction in activation in the right parietal‐occipital regions was observed during the recall of the spatial location of objects and mental rotation. 22 In 2018, the same research group reported on the cerebral metabolic activity (using fluorine‐18 fluorodeoxyglucose positron emission tomography) before and 9 months after ADT in eight patients with prostate cancer. They found a decreased regional cerebral glucose metabolism in the cerebellum, posterior cingulate and medial hypothalamus bilaterally. Cortical glucose metabolism was associated positively and negatively with select cognitive tests. While on ADT, positive correlations were found between the posterior cingulate, left inferior parietal lobule and left mid‐temporal gyrus and spatial reasoning, and a negative correlation between the left inferior parietal lobule and verbal memory. 23 Considering these five publications using fMRI and positron emission tomography, it can be postulated that ADT can negatively influence characteristics of the brain (i.e. structure, function, metabolism).
In conclusion, there is no convincing evidence of the protective effect of treatment with LHRH agonists or antagonists on cognitive functioning in men with prostate cancer. Considering the substantial part of the studies reporting a decline in cognitive functioning after starting ADT, and the increasing number of studies showing that there might be a potential underlying substrate in the brain, it is important that clinicians are aware of this side effect. In the shared decision making when starting ADT, clinicians should discuss this potential side effect. This is in accordance with the recent updated guideline of the International Society of Geriatric Oncology on prostate cancer management in older patients. 55 When starting ADT this should be discussed with patients. Future (randomized) studies should focus on the effect of ADT on cognitive functioning in patients with prostate cancer using a valid neuropsychological test battery, together with innovative techniques to examine brain function, structure and metabolism, and potentially take into account difference between LHRH antagonists and LHRH agonists. 56
Conflict of interest
None declared.
Supporting information
Appendix S1. Search strategy.
Appendix S2. Quality assessment prostate cancer.
References
- 1. World Cancer Research Fund. Prostate cancer statistics. [Cited 3 May 2021.] Available from URL: https://www.wcrf.org/dietandcancer/prostate‐cancer‐statistics/
- 2. Crawford ED, Heidenreich A, Lawrentschuk N et al. Androgen‐targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019; 22: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crawford ED, Schally AV, Pinthus JH et al. The potential role of follicle‐stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol. Oncol. 2017; 35: 183–91. [DOI] [PubMed] [Google Scholar]
- 4. Nguyen C, Lairson DR, Swartz MD, Du XL. Risks of major long‐term side effects associated with androgen‐deprivation therapy in men with prostate cancer. Pharmacotherapy 2018; 38: 999–1009. [DOI] [PubMed] [Google Scholar]
- 5. Salminen EK, Portin RI, Koskinen A, Helenius H, Nurmi M. Associations between serum testosterone fall and cognitive function in prostate cancer patients. Clin. Cancer Res. 2004; 10: 7575–82. [DOI] [PubMed] [Google Scholar]
- 6. Sherwin BB. Steroid hormones and cognitive functioning in aging men: a mini‐review. J. Mol. Neurosci. 2003; 20: 385–93. [DOI] [PubMed] [Google Scholar]
- 7. Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer 2008; 113: 1097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 2006; 50: 18–26. [DOI] [PubMed] [Google Scholar]
- 9. Atwi S, McMahon D, Scharfman H, MacLusky NJ. Androgen modulation of hippocampal structure and function. Neuroscientist 2016; 22: 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hua JT, Hildreth KL, Pelak VS. Effects of testosterone therapy on cognitive function in aging: a systematic review. Cogn. Behav. Neurol. 2016; 29: 122–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saad F, Röhrig G, von Haehling S, Traish A. Testosterone deficiency and testosterone treatment in older men. Gerontology 2017; 63: 144–56. [DOI] [PubMed] [Google Scholar]
- 12. Blair JA, McGee H, Bhatta S, Palm R, Casadesus G. Hypothalamic‐pituitary‐gonadal axis involvement in learning and memory and Alzheimer’s disease: more than “just” estrogen. Front. Endocrinol. 2015; 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith MA, Bowen RL, Nguyen RQ, Perry G, Atwood CS, Rimm AA. Putative gonadotropin‐releasing hormone agonist therapy and dementia: an application of medicare hospitalization claims data. J. Alzheimers Dis. 2018; 63: 1269–77. [DOI] [PubMed] [Google Scholar]
- 14. Jamadar RJ, Winters MJ, Maki PM. Cognitive changes associated with ADT: a review of the literature. Asian J. Androl. 2012; 14: 232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGinty HL, Phillips KM, Jim HS et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta‐analysis. Support. Care Cancer 2014; 22: 2271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mundell NL, Daly RM, Macpherson H, Fraser SF. Cognitive decline in prostate cancer patients undergoing ADT: a potential role for exercise training. Endocr. Relat. Cancer 2017; 24: R145–R155. [DOI] [PubMed] [Google Scholar]
- 17. Treanor CJ, Li J, Donnelly M. Cognitive impairment among prostate cancer patients: an overview of reviews. Eur. J. Cancer Care 2017; 26: e12642. [DOI] [PubMed] [Google Scholar]
- 18. Sun M, Cole AP, Hanna N et al. Cognitive impairment in men with prostate cancer treated with androgen deprivation therapy: a systematic review and meta‐analysis. J. Urol. 2018; 199: 1417–25. [DOI] [PubMed] [Google Scholar]
- 19. Chao HH, Uchio E, Zhang S et al. Effects of androgen deprivation on brain function in prostate cancer patients – a prospective observational cohort analysis. BMC Cancer 2012; 12: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Ruiter MB, Schagen SB. Functional MRI studies in non‐CNS cancers. Brain Imaging Behav. 2013; 7: 388–408. [DOI] [PubMed] [Google Scholar]
- 21. Chao HH, Hu S, Ide JS et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients – an exploratory study. PLoS One 2013; 8: e72032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cherrier MM, Borghesani PR, Shelton AL, Higano CS. Changes in neuronal activation patterns in response to androgen deprivation therapy: a pilot study. BMC Cancer 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cherrier MM, Cross DJ, Higano CS, Minoshima S. Changes in cerebral metabolic activity in men undergoing androgen deprivation therapy for non‐metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2018; 21: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plata‐Bello J, Plata‐Bello A, Pérez‐Martín Y, Fajardo V, Concepción‐Massip T. Androgen deprivation therapy increases brain ageing. Aging 2019; 11: 5613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morote J, Tabernero ÁJ, Álvarez‐Ossorio JL et al. Cognitive function in patients on androgen suppression: a prospective, multicentric study. Actas Urol. Esp. 2018; 42: 114–20. [DOI] [PubMed] [Google Scholar]
- 26. Marzouk S, Naglie G, Tomlinson G et al. Impact of androgen deprivation therapy on self‐reported cognitive function in men with prostate cancer. J. Urol. 2018; 200: 327–34. [DOI] [PubMed] [Google Scholar]
- 27. Jim HS, Small BJ, Patterson S, Salup R, Jacobsen PB. Cognitive impairment in men treated with luteinizing hormone‐releasing hormone agonists for prostate cancer: a controlled comparison. Support Care Cancer 2010; 18: 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joly F, Alibhai SM, Galica J et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J. Urol. 2006; 176: 2443–7. [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Zhong F, Qiu J, Wang K. Cognitive function in Chinese prostate cancer patients on androgen‐deprivation therapy: a cross‐sectional study. Asia Pac. J. Clin. Oncol. 2015; 11: 277–81. [DOI] [PubMed] [Google Scholar]
- 30. Yang J, Zhong F, Qiu J, Cheng H, Wang K. Dissociation of event‐based prospective memory and time‐based prospective memory in patients with prostate cancer receiving androgen‐deprivation therapy: a neuropsychological study. Eur. J. Cancer Care 2015; 24: 198–204. [DOI] [PubMed] [Google Scholar]
- 31. Wiechno PJ, Sadowska M, Kalinowski T, Michalski W, Demkow T. Does pharmacological castration as adjuvant therapy for prostate cancer after radiotherapy affect anxiety and depression levels, cognitive functions and quality of life? Psychooncology 2013; 22: 346–51. [DOI] [PubMed] [Google Scholar]
- 32. Bussiere JR, Beer TM, Neiss MB, Janowsky JS. Androgen deprivation impairs memory in older men. Behav. Neurosci. 2005; 119: 1429–37. [DOI] [PubMed] [Google Scholar]
- 33. Ali Shah SI, Minhas UM, Nasir N. Impact of the duration of androgen deprivation therapy for prostate cancer on cognition: a study using mini‐mental state examination. Pak. J. Med. Health Sci. 2018; 12: 796–8. [Google Scholar]
- 34. Clay CA, Perera S, Wagner JM, Miller ME, Nelson JB, Greenspan SL. Physical function in men with prostate cancer on androgen deprivation therapy. Phys. Ther. 2007; 87: 1325–33. [DOI] [PubMed] [Google Scholar]
- 35. Morote J, Tabernero ÁJ, Álvarez Ossorio JL et al. Cognitive function in patients with prostate cancer receiving luteinizing hormone‐releasing hormone analogues: a prospective, observational, multicenter study. Int. J. Radiat. Oncol. Biol. Phys. 2017; 98: 590–4. [DOI] [PubMed] [Google Scholar]
- 36. Okamoto K, Sekine Y, Nomura M et al. Effects of a luteinizing hormone‐releasing hormone agonist on cognitive, sexual, and hormonal functions in patients with prostate cancer: relationship with testicular and adrenal androgen levels. Basic Clin. Androl. 2015; 25: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan WW, Heckman MG, Vishnu P et al. Effect of leuprolide on serum amyloid‐β peptide levels and memory in patients with prostate cancer with biochemical recurrence. Urology 2013; 81: 150–4. [DOI] [PubMed] [Google Scholar]
- 38. Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur. J. Cancer 2000; 36: 1134–41. [DOI] [PubMed] [Google Scholar]
- 39. Alibhai SM, Timilshina N, Duff‐Canning S et al. Effects of long‐term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer 2017; 123: 237–44. [DOI] [PubMed] [Google Scholar]
- 40. Mohile SG, Lacy M, Rodin M et al. Cognitive effects of androgen deprivation therapy in an older cohort of men with prostate cancer. Crit. Rev. Oncol. Hematol. 2010; 75: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow‐up study of the association between chemical castration, sex hormones, beta‐amyloid, memory and depression in men. Psychoneuroendocrinology 2004; 29: 1071–81. [DOI] [PubMed] [Google Scholar]
- 42. Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non‐metastatic prostate cancer. Psychooncology 2009; 18: 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salminen EK, Portin RI, Koskinen AI, Helenius HY, Nurmi MJ. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer 2005; 103: 1381–7. [DOI] [PubMed] [Google Scholar]
- 44. Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J. Urol. 2003; 170: 1808–11. [DOI] [PubMed] [Google Scholar]
- 45. Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005; 96: 48–53. [DOI] [PubMed] [Google Scholar]
- 46. Ceylan Y, Gunlusoy B, Koskderelioglu A, Gedizlioglu M, Degirmenci T. The depressive effects of androgen deprivation therapy in locally advanced or metastatic prostate cancer: a comparative study. Aging Male 2020; 23: 733–9. [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez BD, Jim HS, Booth‐Jones M et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen‐deprivation therapy: a controlled comparison. J. Clin. Oncol. 2015; 33: 2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gunlusoy B, Ceylan Y, Koskderelioglu A et al. Cognitive effects of androgen deprivation therapy in men with advanced prostate cancer. Urology 2017; 103: 167–72. [DOI] [PubMed] [Google Scholar]
- 49. Alibhai SM, Breunis H, Timilshina N et al. Impact of androgen‐deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J. Clin. Oncol. 2010; 28: 5030–7. [DOI] [PubMed] [Google Scholar]
- 50. Salminen E, Portin R, Korpela J et al. Androgen deprivation and cognition in prostate cancer. Br. J. Cancer 2003; 89: 971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Green HJ, Pakenham KI, Headley BC et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone‐releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002; 90: 427–32. [DOI] [PubMed] [Google Scholar]
- 52. Green HJ, Pakenham KI, Headley BC et al. Quality of life compared during pharmacological treatments and clinical monitoring for non‐localized prostate cancer: a randomized controlled trial. BJU Int. 2004; 93: 975–9. [DOI] [PubMed] [Google Scholar]
- 53. Matousek RH, Sherwin BB. A randomized controlled trial of add‐back estrogen or placebo on cognition in men with prostate cancer receiving an antiandrogen and a gonadotropin‐releasing hormone analog. Psychoneuroendocrinology 2010; 35: 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu LM, Amidi A, Tanenbaum ML et al. Computerized cognitive training in prostate cancer patients on androgen deprivation therapy: a pilot study. Support Care Cancer 2018; 26: 1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boyle HJ, Alibhai S, Decoster L et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur. J. Cancer 2019; 116: 116–36. [DOI] [PubMed] [Google Scholar]
- 56. Abufaraj M, Iwata T, Kimura S et al. Differential impact of gonadotropin‐releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta‐analysis of randomized controlled trials. Eur. Urol. 2021; 79: 44–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.
Appendix S2. Quality assessment prostate cancer.


