Abstract
Human colonic spirochetosis (CS) is usually due to Brachyspira pilosicoli or Brachyspira aalborgi infection. While traditionally considered to be commensal bacteria, there are scattered case reports and case series of gastrointestinal (GI) symptoms in CS and reports of colonic polyps with adherent spirochetes. We performed a systematic review and meta‐analysis investigating the association between CS and GI symptoms and conditions including the irritable bowel syndrome (IBS) and colonic polyps. Following PRISMA 2020 guidelines, a systematic search of Medline, CINAHL, EMBASE, and Web of Science was performed using specific keywords for CS and GI disease. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random‐effects model. Of 75 studies identified in the search, 8 case–control studies met the inclusion criteria for meta‐analysis and 67 case series studies met the inclusion criteria for pooled prevalence analysis. CS was significantly associated with diarrhea (n = 141/127, cases/controls, OR: 4.19, 95% CI: 1.72–10.21, P = 0.002) and abdominal pain (n = 64/65, OR: 3.66, 95% CI: 1.43–9.35, P = 0.007). CS cases were significantly more likely to have Rome III‐diagnosed IBS (n = 79/48, OR: 3.84, 95% CI: 1.44–10.20, P = 0.007), but not colonic polyps (n = 127/843, OR: 8.78, 95% CI: 0.75–103.36, P = 0.084). In conclusion, we found evidence of associations between CS and both diarrhea and IBS, but not colonic polyps. CS is likely underestimated due to suboptimal diagnostic methods and may be an overlooked risk factor for a subset of IBS patients with diarrhea.
Keywords: abdominal pain, diarrhea, gastrointestinal infection, IBS, sessile serrated polyps
Introduction
Although human intestinal spirochetosis was identified in 1967, 1 there is still ongoing debate regarding its pathogenic importance in humans. 2 Swine and poultry‐infecting intestinal spirochetes can induce severe colitis and diarrhea in those animals, 3 while species colonizing humans (the intestinal spirochetes Brachyspira pilosicoli and Brachyspira aalborgi ) are usually thought to induce few or no symptoms. 4 Our understanding of colonic spirochetosis (CS) has been hampered by the difficulty in working with these species. Brachyspira are fastidious, slow‐growing, anaerobic bacteria, hard to isolate and to grow in laboratory conditions. 5 As a result, the pathogenesis, transmission pattern, and risk factors of CS remain largely unknown. Studies utilizing transmission electron microscopy (TEM) show that spirochete may adhere to the epithelium, as the “head” of the spirochetes anchors between microvilli structures of the intestinal epithelium while the tail end is directed into the colonic lumen. 6 Although TEM studies using clinical tissues have found spirochetes present in macrophages 7 and close to mast cells, 8 the significance of this finding is unclear as there is little evidence of epithelial cell penetration by spirochetes. 9
The gold standard diagnosis of CS is based on its characteristic colonization of the epithelial surface, identified by routine histological examination of biopsies taken during colonoscopy. 10 Using hematoxylin and eosin (H&E) staining, colonized spirochetes are stained as light purple, while Warthin–Starry silver staining or specific immunohistochemistry (IHC) staining, using cross‐reactive anti‐ Treponema pallidum antibody, better highlight the presence of spirochetes from the background. 10 The limitation of histological diagnosis is that successful detection of spirochetes is largely dependent on the location of biopsy sampling and careful pathology examination, and the basophilic brush line from CS can be easily misinterpreted as a normal brush border structure on H&E staining. 11 While genomic screening of the gut microbiota is advancing rapidly and it has proven to be a powerful tool to investigate microbiota composition, 12 the “conventional” 16S rRNA sequencing approach is unable to detect human colonic spirochetes as the standard 16S rRNA primer sets are incompatible with spirochetes' 16S rRNA region. 13 Consequently, there are currently no non‐invasive routine diagnostic methods to diagnose human CS.
Recently, interest in CS has increased with reports of a possible association with diarrhea‐predominant irritable bowel syndrome (IBS‐D). 14 , 15 , 16 CS has also been observed with colonic polyps, but an association with adenoma formation is uncertain. 17 This systematic review and meta‐analysis aimed to determine whether human colonic spirochete infection is associated with specific gastrointestinal (GI) symptoms or GI diseases. We also aimed to identify risk factors associated with the infection and the results of treatment where data were available.
Methods
Search strategy
We followed the PRISMA guidelines for systematic reviews. 18 A protocol was developed before initiation of the systematic review (PROSPERO CRD42019124669). Electronic databases including Medline, CINAHL, EMBASE, and Web of Science were searched on June 31, 2021, with limitations set on human studies published between 1967 and the search date. Each database was searched with the same strategy: [spirochetosis OR spirochaetosis OR spirochete OR spirochaete OR spirochaetose OR Brachyspira aalborgi OR Brachyspira pilosicoli OR Serpulina pilosicoli ] AND [intestinal disease OR intestinal].
Study selection
After removal of duplicate studies, two independent reviewers (KF and GLB) screened titles and abstracts for relevance to the review topic. Following this, full texts of all remaining studies were assessed for suitability and relevance based on the review inclusion and exclusion criteria. The inclusion criteria were (i) studies in humans with intestinal (colonic) spirochete infection, (ii) case–control studies, case series, or original research studies of GI symptoms in intestinal (colonic) spirochete infection, and (iii) studies in the English language. Exclusion criteria were (i) reviews, (ii) single case reports, (iii) studies with no mention of patient symptoms, (iv) studies where full text was not available, and (v) studies not in the English language.
Data extraction
Data extraction was performed by two independent reviewers (KF and PMN). Disagreements were resolved by consensus. Data information were extracted where available using a standardized data extraction template and included (a) general: publication year, study type, number of cases, sex, age, travel/work/sex activity, and sexuality; (b) comorbidity: GI comorbidity, non‐GI comorbidity, and co‐infection; (c) GI symptoms: diarrhea, constipation, diarrhea/constipation mixed, abdominal pain, rectal bleeding, blood in stool, mucus in stool, vomiting, weight loss, fever, nausea, anemia or asymptomatic, and physical examination results when reported; (d) colonoscopy findings: normal colonoscopy, abnormal colonoscopy, and degree and location of the abnormalities; (e) histology findings: presence of spirochetes, location of spirochetes infection, mucosal inflammation, crypt changes, and presence of immune cells (plasma cell, lymphocyte, neutrophil, eosinophil, macrophage, and mast cells); (f) species specificity: Brachyspira genus, B. pilosicoli , and B. aalborgi ; (g) diagnostic method: histology, PCR, PCR target, PCR material, culture, culture material, and electron microscopy; and (h) treatment and outcomes: type of treatment, symptom relief, bacterial eradication, pathology recovery, and symptom reoccurrence.
Statistical analysis
For case–control studies, pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the risk correlation of CS and gender, GI symptoms, and GI diseases using a random‐effects model. 19 To test the heterogeneity of the studies included for analysis, Cochran's Q statistic was used, with P < 0.10 indicating significant effects of heterogeneity. Heterogeneity was assessed using the I 2 statistic with results of 0–25% (low), 25–75% (moderate), and > 75% (high) levels of heterogeneity. 20 Due to the small number of studies included in the meta‐analysis, publication bias was not assessed as most methods required at least 10 studies to perform a test. 21
For case series studies, the prevalence of sex, a range of GI symptoms, colonoscopy findings, diagnostic methods, mucosal inflammation, and treatment effects in reported CS cases were calculated using pooled prevalence rate (event rate [ER]) and 95% CIs using a random‐effects model. Heterogeneity of the studies were evaluated as described above. All analyses were performed with Comprehensive Meta‐analysis (version 3.0), Biostat, Englewood, NJ (2014).
Results
Study selection
Of the initial 2157 studies obtained from Medline, EMBASE, Web of Science, and CINAHL, 1079 texts were identified as duplicates. Of the remaining 1078 studies that were screened by title and abstract, 705 were excluded as animal studies, microbiological studies, or incorrect species of bacteria. A total of 373 papers then proceeded to full text screening, and of those, 207 studies were excluded as reviews, lacking patient symptom information, written in non‐English language, or had duplicated reports of the same patient cohorts. Ninety‐four single case reports were excluded. Three studies were added by hand search. In the end, 75 studies were included for data extraction. Eight case–control studies were included for meta‐analysis and 67 case series studies were included for pooled prevalence analysis (Fig. 1).
Figure 1.
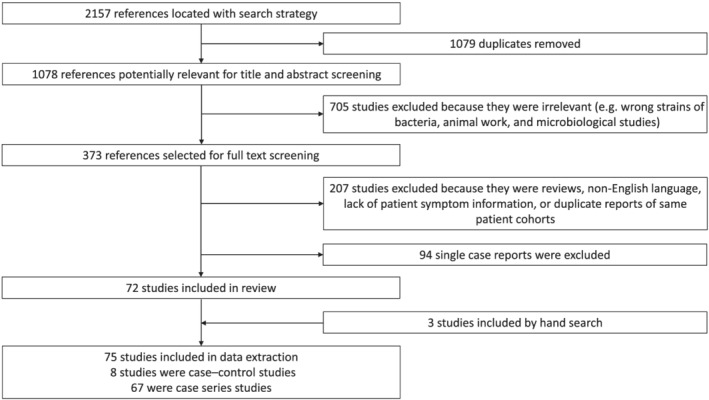
Study selection process. Flow diagram for the identification of studies included in the analysis.
Meta‐analysis of case–control studies
Study characterization
Of the eight case–control studies included (Table 1), in four studies (Walker et al., 14 Goodsall et al., 15 Alsaigh and Fogt, 22 and Higashiyama et al. 23 ), cases of CS and controls were identified in pathology databases based on histology findings; in Cooper et al., 24 cases were a cohort of homosexual males identified with CS while controls were male patients without CS; for Esteve et al., 25 cases were patients with chronic diarrhea collected prospectively who were later identified with CS, while controls were asymptomatic patients with colonic biopsies available in a pathology database; in Omori et al., 26 cases were patients with sessile serrated adenoma/polyps (SSA/P) while controls were non‐SSA/P patients; and in Jabbar et al., 16 cases were patients with IBS and controls were healthy volunteers. In all studies, CS was confirmed by histological examination.
Table 1.
Meta‐analysis of case–control studies characterization
| Paper | Year | Screening cohort selection criteria | CS patient/screening cohort | Selection criteria for control subjects | CS patient/control cohort | Data for meta‐analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Symptom | IBS | Polyps | ||||||
| Cooper et al. 24 | 1986 | Homosexual men with GI symptoms. | 5/8 | Age matched male patients with available colonic biopsy. | 0/5 | NA | NA | NA | NA |
| Alsaigh and Fogt 22 | 2002 | Pathology database. | 15/15 | Age and clinical indication matched patients with biopsy. | 0/30 | Yes | Diarrhea/rectal bleeding | NA | Yes |
| Esteve et al. 25 | 2006 | Prospective survey of patients with chronic watery diarrhea and CS patients identified in routine colonoscopy. | 11/11 | Patients with colonic biopsy taken due to rectal bleeding or polyps histology. | 0/100 | Yes | NA | NA | NA |
| Higashiyama et al. 23 , † | 2009 | Pathology database from 2005 to 2008. | 86/86 | Patient with colonic biopsy from 2005 to 2008. | 0/702 | NA | NA | NA | Yes |
| Omori et al. 26 | 2014 | Patients with sessile serrated adenoma/polyp identified by histology during 2008–2011. | 10/19 | Patients with biopsy excluding sessile serrated adenoma/polyp and cancer in 2011. | 14/172 | NA | NA | NA | Yes |
| Walker et al. 14 | 2015 | Pathology database. | 17/17 | Subjects with colonic biopsy from random population. | 0/17 | NA | Diarrhea/abdominal pain/rectal bleeding | Yes | Yes |
| Goodsall et al. 15 | 2017 | Pathology database. | 47/47 | Subjects with colonic biopsy from random population. | 0/48 | NA | Diarrhea/abdominal pain | NA | NA |
| Jabbar et al. 16 | 2020 | IBS patients diagnosed by Rome III criteria. | 19/62 | Healthy subjects with colonic biopsy. | 0/31 | Yes | Diarrhea | Yes | NA |
The Higashiyama et al. paper is an abstract.
CS, colonic spirochetosis; GI, gastrointestinal; IBS, irritable bowel syndrome; NA, not applicable.
In all studies, CS was confirmed by histological examination.
Demographic characterization
Sex
Three studies (Alsaigh and Fogt, 22 Esteve et al., 25 and Jabbar et al. 16 ) with no sex restrictions for recruitment were included providing n = 88 cases and n = 161 controls. CS cases were 1.84 times (95% CI = 0.11–29.91, P = 0.667) more likely to be male than female, although this was not significant. Heterogeneity of the studies was high (I 2 = 95.23, P < 0.001) (Fig. 2).
Figure 2.
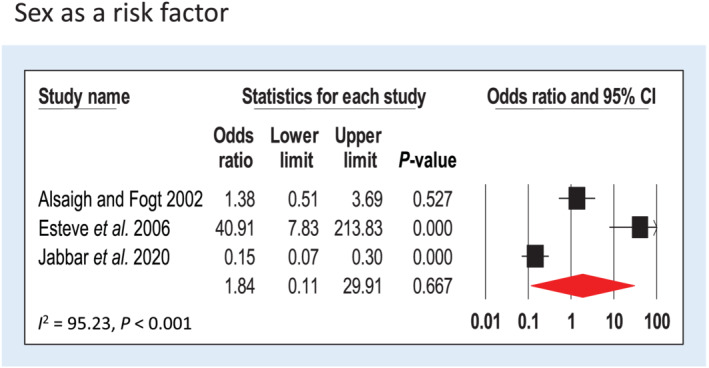
Forest plot of gender risk in colonic spirochetosis cases. Three case–control studies were included for analysis of male sex prevalence. Pooled odds ratios and 95% confidence intervals were calculated using a random‐effects model. Heterogeneity of the publications were tested with Cochran's Q statistic and I 2statistic. Publication bias was tested using the Egger's regression model with the effect of bias assessed using the fail‐safe number method. CI, confidence interval. [Color figure can be viewed at wileyonlinelibrary.com]
Age
Three studies (Alsaigh and Fogt, 22 Esteve et al., 25 and Jabbar et al. 16 ) with no age restrictions for recruitment were included providing n = 88 cases and n = 161 controls. The mean age of the cases and controls was 47.1 and 48.2 years (P = 0.94), respectively.
Gastrointestinal symptom in colonic spirochetosis cases
Diarrhea
Four studies assessed diarrhea in association with CS (Alsaigh and Fogt, 22 Walker et al., 14 Goodsall et al., 15 and Jabbar et al. 16 ). In total, n = 141 cases and n = 127 controls were included. CS was significantly associated with diarrhea; CS cases were more than three times more likely to have diarrhea compared with controls (OR: 4.19, 95% CI: 1.72–10.21, P = 0.002) (Fig. 3a). Heterogeneity of the studies was moderate (I 2 = 27.32, P = 0.25).
Figure 3.
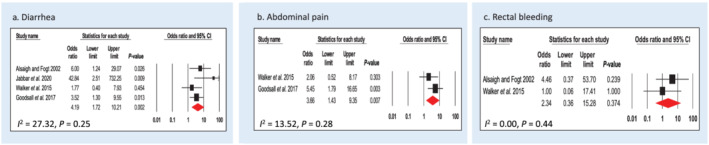
Forest plot of gastrointestinal symptoms risk in colonic spirochetosis cases. (a) Four case–control studies were included for analysis of diarrhea prevalence. (b) Three case–control studies were included for analysis of abdominal pain prevalence. (c) Two case–control studies were included for analysis of rectal bleeding prevalence. Pooled odds ratios and 95% confidence intervals were calculated using a random‐effects model. Heterogeneity of the publications were tested with Cochran's Q statistic and I 2statistic. Publication bias was tested using the Egger's regression model with the effect of bias assessed using the fail‐safe number method. For meta‐analysis of colonic spirochetosis and rectal bleeding, publication bias could not be tested as the minimal number of study for the Egger's test is 3. CI, confidence interval. [Color figure can be viewed at wileyonlinelibrary.com]
Abdominal pain
Two studies assessed abdominal pain in association with CS (Walker et al. 14 and Goodsall et al. 15 ). In total, n = 64 cases and n = 65 controls were included. CS cases were almost four times more likely to have abdominal pain (OR: 3.66, 95% CI: 1.43–9.35, P = 0.007) (Fig. 3b). Heterogeneity of the studies was low (I 2 = 13.52, P = 0.28).
Rectal bleeding
Two studies examined patients who self‐reported symptom of rectal bleeding (Alsaigh and Fogt 22 and Walker et al. 14 ). In total, n = 32 cases and n = 47 controls were included. CS cases were twice as likely to experience rectal bleeding (OR: 2.34, 95% CI: 0.36–15.28, P = 0.374) (Fig. 3c), although the reason for bleeding was not specified in these studies and the association was not significant. There was no heterogeneity in the studies (I 2 = 0.00, P = 0.44).
Gastrointestinal diseases in colonic spirochetosis cases
Irritable bowel syndrome
Two studies assessed diagnosis of IBS using Rome III criteria in association with CS (Walker et al. 14 and Jabbar et al. 16 ). In total, n = 79 cases and n = 48 controls were included. CS was significantly associated with IBS; CS cases are almost four times (OR: 3.84, 95% CI: 1.44–10.20, P = 0.007) more likely to have a diagnosis of IBS compared with controls (Fig. 4a). There was no heterogeneity in the studies (I 2 = 0.00, P = 0.71).
Figure 4.
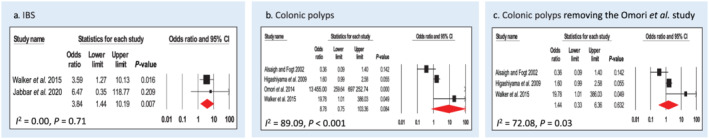
Forest plot of gastrointestinal disease/abnormality risk in colonic spirochetosis cases. (a) Two case–control studies were included for analysis of irritable bowel syndrome risk. (b) Four case–control studies were included for analysis of polyps risk. (c) Sensitivity analysis of polyps risk in colonic spirochetosis cases by removing the Omori et al. study from the meta‐analysis. Pooled odds ratios and 95% confidence intervals were calculated using a random‐effects model. Heterogeneity of the publications were tested with Cochran's Q statistic and I 2statistic. Publication bias was tested using the Egger's regression model with the effect of bias assessed using the fail‐safe number method. CI, confidence interval; IBS, irritable bowel syndrome. [Color figure can be viewed at wileyonlinelibrary.com]
Colonic polyps
Four studies assessed diagnosis of colonic polyps in association with CS (Alsaigh and Fogt, 22 Higashiyama et al., 23 Walker et al., 14 and Omori et al. 26 ). In Alsaigh and Fogt, Higashiyama et al., and Walker et al., the subtypes of polyps were not discriminated, while Omori et al. specifically investigated the correlation of CS and SSA/P in patient cohorts. In total, n = 127 cases and n = 843 controls were included. CS cases were almost nine times (OR: 8.78, 95% CI: 0.75–103.36, P = 0.084) more likely to have colonic polyps compared with controls, but this was not a significant finding (Fig. 4b). Heterogeneity of the studies was high (I 2 = 89.09, P < 0.001). After the removal of Omori et al., the OR for non‐specific polyps dropped to 1.44 (95% CI: 0.33–6.36, P = 0.632), with I 2 = 72.08, P = 0.03 (Fig. 4c).
Colonoscopy findings
Only one study (Alsaigh and Fogt 22 ) assessed colonoscopy findings in association with CS, although the definition of normal and abnormal colonoscopy was not specified in the paper. In total, n = 15 cases and n = 30 controls were analyzed. The OR of abnormal visible findings on colonoscopy was 0.87 (95% CI: 0.24–3.10, P = 0.828).
Case series
Pooled prevalence analysis
Sixty‐seven case series studies were included for pooled prevalence analysis. Results are shown in Table 2. In reported CS cases, the most common symptoms were diarrhea (39%) and abdominal pain (34%), followed by symptoms of bloating (29%), undefined rectal bleeding (21%), or a finding of blood in the stool (27%). We also observed that nearly half of the CS cases (48%) were reported to be asymptomatic with CS only identified because biopsies were taken during screening or polyp surveillance.
Table 2.
Pooled prevalence estimates of colonic spirochetosis‐positive patients in case series studies
| Event | Pooled study references | Cases | Number of event | Event rate/proportion | Heterogeneity |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Male gender | 1, 4, 6, 8, 9, 17, 28, 29, 48, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 | 2041 | 1409 | 0.68 (95% CI 0.63–0.73) | I 2 = 67.24%, P < 0.001 |
| Female gender | 1, 4, 6, 8, 9, 17, 28, 29, 48, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 | 2041 | 632 | 0.32 (95% CI 0.27–0.37) | I 2 = 67.24%, P < 0.001 |
| Travel before symptom onset | 29, 61, 66, 70, 81, 83, 84, 85, 86, 87, 88, 90, 94, 95 | 105 | 28 | 0.30 (95% CI 0.19–0.45) | I 2 = 29.10%, P = 0.14 |
| Childhood sexual abuse | 97 | 8 | 1 | 0.13 (95% CI 0.02–0.54) | I 2 = 0.00%, P = 1 |
| Frequent sexual activity | 85 | 4 | 1 | 0.25 (95% CI 0.03–0.76) | I 2 = 0.00%, P = 1 |
| Homosexual | 28, 48, 61, 70, 73, 77, 79, 80, 83, 85, 96, 98, 103 | 530 | 158 | 0.44 (95% CI 0.16–0.77) | I 2 = 86.83%, P < 0.001 |
| HIV+ | 4, 28, 48, 61, 62, 63, 64, 67, 70, 72, 75, 76, 79, 83, 93, 103, 104 | 1369 | 71 | 0.10 (95% CI 0.04–0.21) | I 2 = 88.64%, P < 0.001 |
| HIV− | 4, 28, 48, 63, 64, 67, 69, 70, 71, 72, 75, 76, 82, 83, 89 | 654 | 586 | 0.89 (95% CI 0.75–0.96) | I 2 = 85.58%, P < 0.001 |
| HIV status unknown | 1, 4, 6, 8, 17, 27, 29, 61, 62, 65, 66, 68, 74, 77, 81, 84, 85, 86, 87, 88, 89, 90, 91, 92, 94, 95, 96, 97, 98, 99, 100, 101, 102 | 429 | 411 | 0.90 (95% CI 0.84–0.93) | I 2 = 19.74%, P = 0.16 |
| GI abnormality | |||||
| Colonic polyps | 1, 9, 17, 27, 28, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 74, 75, 77, 79, 85, 94, 97, 105 | 1094 | 370 | 0.28 (95% CI 0.18–0.40) | I 2 = 89.77%, P < 0.001 |
| Diverticular disease | 8, 9, 17, 27, 62, 63, 64, 66, 67, 68, 69, 85, 105, 106 | 403 | 39 | 0.12 (95% CI 0.07–0.19) | I 2 = 47.67%, P = 0.02 |
| Inflammatory bowel disease | 4, 9, 28, 61, 62, 63, 65, 66, 72, 75, 76, 77, 79, 91, 92, 106 | 924 | 74 | 0.09 (95% CI 0.06–0.13) | I 2 = 52.59%, P = 0.007 |
| Cancer | 1, 9, 28, 64, 65, 66, 67, 68, 72, 75, 77, 105, 106 | 532 | 70 | 0.24 (95% CI 0.19–0.31) | I 2 = 87.85%, P < 0.001 |
| GI symptoms | |||||
| Diarrhea | 1, 4, 8, 9, 29, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 75, 76, 77, 78, 79, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 103, 104, 105, 107 | 1770 | 570 | 0.39 (95% CI 0.33–0.46) | I 2 = 74.44%, P < 0.001 |
| Abdominal pain | 1, 4, 6, 8, 9, 29, 60, 61, 62, 63, 64, 65, 66, 67, 68, 70, 71, 72, 75, 76, 78, 79, 81, 83, 84, 85, 86, 87, 88, 90, 92, 93, 94, 96, 97, 99, 101, 102, 104, 105 | 1645 | 436 | 0.34 (95% CI 0.26–0.43) | I 2 = 83.92%, P < 0.001 |
| Rectal bleeding | 1, 4, 8, 29, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 72, 75, 77, 82, 84, 85, 86, 87, 88, 90, 93, 94, 95, 97, 99, 105 | 705 | 114 | 0.21 (95% CI 0.15–0.27) | I 2 = 48.94%, P = 0.001 |
| Blood in stool | 4, 8, 27, 28, 66, 75, 76, 79, 82, 85, 87 | 576 | 174 | 0.27 (95% CI 0.20–0.35) | I 2 = 62.53%, P = 0.003 |
| Bloating | 29, 62, 88 | 7 | 2 | 0.29 (95% CI 0.07–0.68) | I 2 = 0.00%, P = 0.81 |
| Vomiting | 60, 79, 81, 84, 96, 99, 101 | 315 | 28 | 0.17 (95% CI 0.08–0.32) | I 2 = 55.47%, P = 0.04 |
| Weight loss | 8, 29, 61, 67, 70, 83, 85, 87, 93, 97, 99, 104 | 527 | 31 | 0.17 (95% CI 0.08–0.32) | I 2 = 58.75%, P = 0.005 |
| Anemia | 4, 61, 63, 66, 92, 94 | 112 | 8 | 0.10 (95% CI 0.03–0.26) | I 2 = 49.32%, P = 0.08 |
| Mucus in stool | 4, 8, 79, 87, 93, 95, 97 | 232 | 15 | 0.12 (95% CI 0.05–0.27) | I 2 = 35.50%, P = 0.17 |
| Asymptomatic | 4, 17, 28, 60, 62, 71, 72, 75, 76, 78, 89, 98, 104 | 1267 | 648 | 0.48 (95% CI 0.34–0.63) | I 2 = 90.67%, P < 0.001 |
| Colonoscopy findings | |||||
| Normal colonoscopy | 4, 8, 17, 29, 61, 63, 65, 69, 75, 82, 84, 85, 86, 87, 90, 91, 92, 94, 95, 96, 97, 101, 102 | 189 | 83 | 0.47 (95% CI 0.34–0.61) | I 2 = 49.19%, P = 0.007 |
| Abnormal colonoscopy | 4, 8, 17, 27, 29, 61, 63, 65, 69, 75, 82, 85, 86, 87, 88, 90, 91, 92, 93, 94, 95, 97, 99, 101, 102 | 273 | 109 | 0.45 (95% CI 0.29–0.61) | I 2 = 69.34%, P < 0.001 |
| Erythema | 4, 29, 69, 85, 88, 91 | 28 | 17 | 0.33 (95% CI 0.18–0.54) | I 2 = 34.34%, P = 0.18 |
| Hyperemia | 61, 97 | 21 | 18 | 0.67 (95% CI 0.01–1.00) | I 2 = 89.43%, P < 0.001 |
| Loss of vascular pattern | 69, 82 | 14 | 2 | 0.17 (95% CI 0.01–0.76) | I 2 = 61.89%, P = 0.11 |
| Pale mucosa | 85 | 3 | 1 | 0.25 (95% CI 0.03–0.76) | I 2 = 0.00%, P = 1.00 |
| Edema | 91, 97 | 7 | 4 | 0.46 (95% CI 0.02–0.97) | I 2 = 77.36%, P = 0.04 |
| Erosion | 61, 69, 92 | 21 | 32 | 0.70 (95% CI 0.06–0.99) | I 2 = 86.00%, P = 0.001 |
| Ulcer | 63, 87, 92, 93 | 26 | 7 | 0.23 (95% CI 0.06–0.56) | I 2 = 52.19%, P = 0.10 |
| Mucosal inflammation | 27, 69, 75, 86, 87, 97 | 48 | 35 | 0.28 (95% CI 0.09–0.60) | I 2 = 77.80%, P < 0.001 |
| Blood oozing | 85 | 3 | 1 | 0.25 (95% CI 0.03–0.76) | I 2 = 0.00%, P = 1.00 |
| Mucosal inflammation | |||||
| Inflammation presence | 4, 6, 8, 9, 28, 29, 48, 61, 62, 63, 64, 66, 67, 68, 69, 73, 75, 84, 86, 87, 88, 90, 91, 92, 93, 94, 95, 96, 97, 99, 100, 102, 105, 106 | 645 | 142 | 0.30 (95% CI 0.21–0.40) | I 2 = 62.76%, P < 0.001 |
| Lymphocyte presence | 4, 8, 94, 97 | 40 | 11 | 0.30 (95% CI 0.14–0.53) | I 2 = 25.99%, P = 0.25 |
| Eosinophil presence | 62, 84, 86, 87, 97 | 137 | 10 | 0.18 (95% CI 0.03–0.64) | I 2 = 80.79%, P < 0.001 |
| Neutrophil presence | 29, 62, 73, 86, 96 | 160 | 14 | 0.18 (95% CI 0.05–0.49) | I 2 = 67.55%, P < 0.001 |
| Mast cell presence | 8 | 2 | 2 | 0.83 (95% CI 0.19–0.99) | I 2 = 0.00%, P = 1.00 |
| Macrophage presence | 8, 29, 63, 67, 99 | 51 | 10 | 0.45 (95% CI 0.09–0.87) | I 2 = 74.63%, P = 0.003 |
| Crypt involvement | 8, 61, 62, 64, 69, 73, 86, 90, 93, 94 | 217 | 27 | 0.20 (95% CI 0.11–0.33) | I 2 = 54.40%, P = 0.02 |
| Diagnosis method | |||||
| By histology | 1, 4, 6, 8, 9, 17, 27, 28, 29, 48, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107 | 2183 | 1854 | 0.92 (95% CI 0.85–0.96) | I 2 = 70.44%, P < 0.001 |
| By PCR | 1, 4, 6, 8, 9, 17, 27, 28, 29, 48, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107 | 2104 | 289 | 0.15 (95% CI 0.08–0.25) | I 2 = 83.04%, P < 0.001 |
| By culture | 1, 4, 6, 8, 9, 17, 27, 28, 29, 48, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107 | 2104 | 321 | 0.08 (95% CI 0.04–0.14) | I 2 = 69.35%, P < 0.001 |
| Species prevalence | |||||
| Brachyspira pilosicolipresence | 28, 29, 61, 63, 64, 67, 70, 75, 76, 77, 78, 81, 83, 89, 90, 91, 92, 105 | 504 | 175 | 0.20 (95% CI 0.12–0.32) | I 2 = 69.32%, P < 0.001 |
| Brachyspira aalborgipresence | 28, 29, 61, 63, 64, 67, 70, 75, 76, 77, 78, 81, 83, 89, 90, 91, 92, 105 | 504 | 207 | 0.58 (95% CI 0.40–0.74) | I 2 = 82.78%, P < 0.001 |
| Metronidazole treatment | |||||
| One course of metronidazole/CS patient | 4, 8, 61, 63, 69, 79, 82, 83, 84, 85, 90, 92, 93, 94, 95, 96, 97, 99, 102, 103, 107 | 358 | 134 | 0.49 (95% CI 0.34–0.64) | I 2 = 60.08%, P < 0.001 |
| Symptom relief/metronidazole‐treated patient | 4, 61, 63, 69, 83, 84, 85, 90, 92, 93, 94, 97, 102, 103, 107 | 65 | 55 | 0.81 (95% CI 0.68–0.90) | I 2 = 0.00%, P = 0.95 |
| Bacteria eradication/metronidazole‐treated patient | 61, 63, 79, 85, 92, 94, 97, 103, 107 | 61 | 51 | 0.76 (95% CI 0.62–0.86) | I 2 = 0.00%, P = 0.55 |
| Pathology recovery/metronidazole‐treated patient | 61, 92, 93 | 20 | 19 | 0.84 (95% CI 0.52–0.96) | I 2 = 60.08%, P < 0.001 |
| Symptom relapse/metronidazole‐treated patient | 4, 63, 84, 85, 90, 93, 94 | 20 | 8 | 0.39 (95% CI 0.20–0.62) | I 2 = 60.08%, P < 0.001 |
CI, confidence interval; CS, colonic spirochetosis; GI, gastrointestinal.
In CS cases, a range of colonic diseases were assessed. Nearly one third had colonic polyps (29%), one quarter had colon cancer (24%), and one in ten had inflammatory bowel disease (9%) or diverticular disease (12%). In CS cases, the proportion of patients with a normal colonoscopy (47%) versus an abnormal colonoscopy (45%) were similar, which was in line with the case–control findings (the remaining 8% were missing data). Among CS cases with abnormal colonoscopy findings, 70% had erosions, 67% had hyperemia, 46% had edema, 33% had erythema, 28% had inflamed mucosa, 25% had pale mucosa, 25% had blood oozing, 23% had ulcers, and 17% had loss of the vascular pattern.
The majority of CS cases were diagnosed by histology (92%), while only 15% had specific PCR tests to confirm the species of infection, and only 8% were diagnosed by successful culture. Within those cases where species differentiation was examined, 20% were infected with B. pilosicoli , 58% were infected with B. aalborgi , and 22% reported undetermined Brachyspira genus.
Metronidazole was the most commonly prescribed treatment for CS. Nearly half of the CS patients (49%) received one course of metronidazole treatment (dose and frequency varied between studies). Among these patients, 81% had reported GI symptom relief (symptom assessment varied between studies), while 79% reported successful bacterial eradication in a follow‐up colonoscopy examination. Although most patients (84%) with CS‐related pathology experienced recovery after treatment, nearly 40% of these patients also reported symptom relapse between a few days to 15 months after treatment.
Anatomical locations of spirochetes were extracted from confirmed CS cases who underwent full colonoscopy and had biopsies taken from each section of the colon, or with colonoscopy that specified the biopsy locations. 11 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Biopsies taken from the ascending colon had the highest success rate (56%) for detecting spirochetes from these CS cases, followed by biopsies taken from the transverse colon (54%), descending colon (48%), sigmoid colon (47%), cecum (40%), and rectum (38%). In total, 964 biopsies were taken by colonoscopy in these confirmed CS cases, with 454 biopsies showing presence of spirochetes, providing a 47% successful detection rate (Fig. 5).
Figure 5.
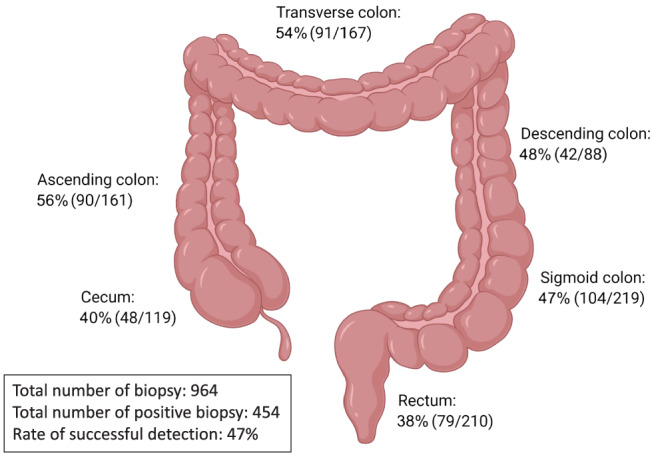
Anatomical location of positive‐spirochete biopsy in colonoscopy examination. Anatomical location of spirochetes in patients with colonic spirochetosis was recorded from studies that performed whole colon colonoscopy and had taken biopsies from each section of the colon, or studies that have specified the location which the biopsy had been taken. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
To our knowledge, this is the first meta‐analysis to investigate human colonic spirochetes infection and GI disease. Although spirochetes are generally considered to be commensals and largely ignored, the results of this review have identified a clear association between CS and diarrhea, abdominal pain, and IBS. However, no association between CS and the presence of polyps was identified. Importantly, we found that CS was strongly associated with IBS, a functional GI disorder that is characterized by abdominal pain and a change of bowel habits. 42 Although the etiology of IBS is still unclear, there is evidence that suggests GI infections may play a role in the initiation and development of IBS. 43 In both studies that directly reported an association of IBS with CS, subtle pathological changes were identified in CS patients, namely, increased eosinophils, mast cells, and lymphoid aggregates in the lamina propria. 14 , 16 These findings are consistent with low‐grade mucosal inflammation that has been observed in other IBS cohorts, although in these studies CS was not evaluated. 44 , 45 , 46 IBS patients usually have normal colonoscopy findings and therefore colonic biopsy is not indicated, which may be the reason that a direct involvement of CS in IBS has not previously been widely reported. Given histology is currently the gold standard for diagnosing CS, standardizing biopsy collection from patients with IBS for careful histological evaluation may reveal the true prevalence of CS in IBS cohorts.
In the meta‐analysis of case–control studies, we aimed to investigate the association of CS and GI symptoms. Due to the heterogeneity of GI symptoms reported in the available studies, we could only assess diarrhea, abdominal pain, and rectal bleeding by meta‐analysis with sufficient sample size. However, the pooled prevalence analysis of case series studies mirrored these findings with diarrhea, followed by abdominal pain, blood in stool, and rectal bleeding, the most commonly reported symptoms with CS. Limitation of the symptom analysis include possible selection bias and reporting bias. CS patients with symptoms may be more likely to seek healthcare and colonoscopy, which could increase the detection of CS compared with asymptomatic patients, and they may also be more likely to be reported and published. Therefore, it is interesting and important that we also assessed and found that 48% of the reported CS cases were asymptomatic and had undergone colonoscopy for polyp surveillance or population screening. It is plausible to assume this number may be higher in general population. Unfortunately, none of the included studies had characterized these cases in detail, and as a result, we were unable to delineate risk factors that may differentiate asymptomatic versus symptomatic disease in CS cases. Future studies are needed to comprehensively assess GI symptoms associated with CS with a thorough and standardized symptom evaluation including abdominal pain, diarrhea, rectal bleeding, blood in stool, weight loss, vomiting, bloating, mucus in stool, and anemia. Population studies are urgently needed to determine an accurate infection rate, symptom, and pathology profile of CS.
We also investigated the relationship between sex and CS, as sex differences have been reported in other infectious GI diseases. 47 CS was initially believed to be a sexually transmitted disease, with early work focusing predominantly on homosexual male cohorts. 48 Therefore, CS has largely been regarded as a male dominant disease. In the pooled prevalence analysis of case series, we found that the prevalence of male gender in current reported CS cases was 68%. However, the meta‐analysis showed that the OR of a male CS patient is only 1.84 compared with female; the difference was not statistically significant. Randomized population study like Walker et al. 14 reported that there was only a slight increase in the likelihood of being male (OR: 1.13) in CS patients. This likely reflects selection bias as past studies have focused on certain patient groups (i.e. male homosexuals). Sex differences in IBS subtypes have not been extensively studied, but previous meta‐analyses suggest that IBS‐D is more common in men over women, 49 although whether this is associated with a potentially higher rate of CS is unknown. Alternatively, spirochete infection in males may induce more severe symptoms than in females, as sex differences in infection are known to exist 49 and therefore lead to more male patients seeking care for infection.
There was no significant association between CS and colonic polyps. A sensitivity analysis showed that Omori et al. 26 was the source of the increased OR and the major contributor to the high heterogeneity of the analysis. Upon the removal of this study, the association of CS and colonic polyps was reduced from an OR of 8.78 to an OR of 1.44, although neither OR was significant. Interestingly, Omori et al. looked specifically at patients with SSA/P, while the remaining studies did not distinguish the polyp pathology subtypes. SSA/P is significantly associated with increased cancer incidence (OR: 1.77 vs OR: 1) and mortality (OR: 1.74 vs OR: 1) compared with a matched cohort at 10‐year follow‐up. 50 The case series study by Young et al., 51 which was similar to Omori et al., found that 28% (26/93) of patients with SSA/P had CS presence in their GI tract in an Australian population, while 2/4 patients with tubulovillous adenomas and 5/8 patients with colonic resection (reason unspecified) had CS. At the same time, we found that in the pooled prevalence analysis, 28% of patients with CS had colonic polyps, and notably 24% had colorectal cancer. This evidence suggests an association between CS and colonic polyps is possible, notably SSA/P and colorectal cancer. It was not possible to infer any causal relationship of CS and polyps with the available data. Firstly, patients with colon cancer and polyps are much more likely to receive surveillance colonoscopy, so there is a risk of detection bias driving the effect estimates for CS. Furthermore, it is known that colonic polyps and cancers exhibit altered mucosal microbiota, 52 , 53 , 54 and this may include an increase in spirochetes as a consequence of changes to the ecological niche. Given the clinical importance of colonic polyps and cancer, further research to clarify if there is an association between CS with polyps and cancer is warranted.
Only one case–control study assessed colonoscopy findings, indicating that this is a neglected topic and as such no association between CS and abnormal colonoscopy could be made. Given that the current gold standard for diagnosing CS is by histological examination of biopsies taken during colonoscopy, it remains important to characterize macroscopic features of CS infection in order to better inform endoscopists when to take targeted biopsies for CS. A published Digestive Diseases Week abstract 23 reported that red spots or hyperemia and a rough surface with loss of vascular pattern were features of CS at colonoscopy examination. Given that histology is still the gold standard for diagnosing CS, studies for the associations of CS and colonoscopy abnormalities are warranted.
Anatomical locations of spirochetes were assessed in the current case series study analysis. The result showed that the ascending colon and transverse colon had slightly higher positive rates of detecting spirochetes. However, within the confirmed CS cases, the ratio of positive biopsies to the total number of biopsies taken was only 0.47. This suggests that CS infection is patchy and that the current gold standard of diagnosis may be missing more than half of CS patients. 16S rRNA sequencing using stool samples would be an ideal screening method of intestinal microbiota components including spirochetes; however, 16S rRNA sequencing using common primer sets is unable to detect Brachyspira genus. 13 Specific PCR primers for Brachyspira genus have been developed by some groups, as well as species‐specific primers to B. pilosicoli and B. aalborgi, 55 , 56 and these approaches can be utilized for screening CS in stool samples. However, this requires a rather complicated protocol of stool sample collection to avoid environmental contamination and DNA extraction, so most clinical facilities would not be able to perform the test. Thus, more sensitive, specific, and non‐invasive routine diagnostic methods of CS are urgently needed, for example, a serological test.
One important question that we could not address in our meta‐analysis was whether there is a difference in pathogenicity between the two currently isolated species of human intestinal spirochetes, as none of the case–control studies and few case studies distinguished between the two CS species. As many studies used formalin‐fixed paraffin‐embedded tissue, the quality of DNA isolated from these tissues may be insufficient for subsequent PCR analysis in differentiating between these two species. Furthermore, the presence of yet to be isolated spirochetes species (e.g. Brachyspira hominis) may also contribute to this unclassified group of CS infection. 55 Interestingly, we noticed in early studies using culture methods, B. pilosicoli was believed to be the main species in human CS as it was easier to isolate, required a shorter incubation period, and was not as nutrient‐demanding as B. aalborgi . However, subsequent studies refuted this observation, and by PCR, B. aalborgi is more commonly reported in literature. Some studies 16 , 57 have shown that B. pilosicoli and B. aalborgi live in different niches in the human intestinal tract, with B. pilosicoli more “mucus‐associated” while B. aalborgi was more “membrane‐associated”; however, its correlation with symptoms, risk factors, or treatment response are still unclear. Thus, the pathological differences between B. pilosicoli and B. aalborgi are not fully investigated, and potential bias in their prevalence due to methodological limitations in identifying Brachyspira spp. need to be taken into account. More studies are needed to investigate specific factors such as the variation in colonic spirochete species colonization and population diversity, the host immune response, as well as lifestyle and dietary factors that may influence spirochetosis pathology and disease outcome. 58
Finally, we characterized the efficacy of metronidazole treatment in reducing CS‐associated GI symptoms to provide indirect evidence in support of the bacteria playing a pathogenic role and improve clinical guidance for treating CS. Despite being at the early stages of understanding CS, many antibiotics have been explored as a treatment for CS and the current consensus is to use metronidazole as standard. 59 Yet we found a proportion of metronidazole‐treated patients would report symptom relapses at follow‐up. Whether this is due to re‐infection from environmental sources of spirochetes or unsuccessful eradication of the primary infection is still largely unknown. Recently, Jabbar et al. 16 found that while the majority of spirochetes were eliminated with metronidazole treatment, some translocated from the colon surface to the colonic crypts and continued to reside within goblet cell granules. This may enable their continued survival despite antibiotic treatment and explain why CS recurs in many patients. A better understanding of spirochete antibiotic sensitivity profiles is therefore required to provide safer and more effective treatment approaches for CS. Eradication of the bacteria and therapeutic effects of antibiotic treatment should be evaluated through careful pathological assessment over an expanded period of time.
Overall, the limitations of our analysis include a relatively small sample size, and the variability between studies and therefore interpretation of the data, especially related to GI symptoms, must be validated directly. The strengths of this meta‐analysis include the comprehensive literature search strategy used to identify studies and the detailed review of each manuscript to obtain complete symptoms, pathology, and treatment data for analysis. In conclusion, patients with CS have a higher risk of a diagnosis of IBS, consistent with the increased risk of experiencing diarrhea and abdominal pain. Importantly, this may occur in the absence of abnormal endoscopy findings. CS may therefore represent a treatable infectious etiology for a proportion of IBS patients, and further study of their role in this condition is warranted.
Acknowledgment
Open access publishing facilitated by The University of Newcastle, as part of the Wiley ‐ The University of Newcastle agreement via the Council of Australian University Librarians.
Fan, K. , Eslick, G. D. , Nair, P. M. , Burns, G. L. , Walker, M. M. , Hoedt, E. C. , Keely, S. , and Talley, N. J. (2022) Human intestinal spirochetosis, irritable bowel syndrome, and colonic polyps: A systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology, 37: 1222–1234. 10.1111/jgh.15851.
Simon Keely and Nicholas J. Talley share equal contribution.
Declaration of conflict of interest: KF: None. GDE: None. PMN: None. GLB: None. MMW: Grant/research support: Prometheus Laboratories Inc (irritable bowel syndrome [IBS Diagnostic]), Commonwealth Diagnostics International (Biomarkers for FGIDs). SK: Grant/research support: National Health and Medical Research Council (Ideas Grant and Centre for Research Excellence), Viscera Labs (research contract), Microba Life Science (research contract). Consultant/advisory boards: Gossamer Bio (Scientific Advisory Board), Anatara Lifescience (Scientific Advisory Board), Microba Life Science (Consultancy). ECH: None. NJT: HVN National Science Challenge NZ, personal fees from Aviro Health (Digestive health) (2019), Anatara Life Sciences, Brisbane (2019), Allakos (gastric eosinophilic disease) (2021), Bayer [IBS] (2020), Danone (Probiotic) (2018), Planet Innovation (gas capsule IBS) (2020), Takeda, Japan (gastroparesis) (2019), twoXAR (2019) (IBS drugs), Viscera Labs, (USA 2021) (IBS‐diarrhea), Dr Falk Pharma (2020) (EoE), Censa, Wellesley MA USA (2019) (diabetic gastroparesis), Cadila PharmIncaceuticals (CME) (2019), Progenity Inc., San Diego (USA 2019) (intestinal capsule), Sanofi‐aventis, Sydney (2019) (probiotic), Glutagen (2020) (celiac disease), ARENA Pharmaceuticals (2019) (abdominal pain), IsoThrive (2021) (esophageal microbiome), BluMaiden (2021), Rose Pharma (2021) outside the submitted work; in addition, Dr. Talley has a patent Nepean Dyspepsia Index (NDI) 1998, Biomarkers of IBS licensed, a patent Licensing Questionnaires Talley Bowel Disease Questionnaire licensed to Mayo/Talley, a patent Nestec European Patent licensed, and a patent Singapore Provisional Patent “Microbiota Modulation Of BDNF Tissue Repair Pathway” issued. Committees: Australian Medical Council (AMC) [Council Member]; Australian Telehealth Integration Programme; MBS Review Taskforce; NHMRC Principal Committee (Research Committee) Asia Pacific Association of Medical Journal Editors. Boards: GESA Board Member, Sax Institute, Committees of the Presidents of Medical Colleges. Community group: Advisory Board, IFFGD (International Foundation for Functional GI Disorders). Miscellaneous: Avant Foundation (judging of research grants). Editorial: Medical Journal of Australia (Editor in Chief), Up to Date (Section Editor), Precision and Future Medicine, Sungkyunkwan University School of Medicine, South Korea, Med (Journal of Cell Press). Dr. Talley is supported by funding from the National Health and Medical Research Council (NHMRC) to the Centre for Research Excellence in Digestive Health and he holds an NHMRC Investigator grant.
Author contributions: Study concept and design: Kening Fan and Simon Keely. Acquisition of data: Kening Fan, Prema M. Nair, and Grace L. Burns. Analysis and interpretation of data: Kening Fan and Guy D. Eslick. Drafting of the manuscript: Kening Fan. Critical revision of the manuscript for important intellectual content: Guy D. Eslick, Simon Keely, Marjorie Walker, and Nicholas Talley. Statistical analysis: Guy D. Eslick. Study supervision: Simon Keely and Nicholas Talley.
Financial support: This study was supported by grants from the National Health and Medical Research Council (NHMRC; APP1170893).
Guarantor of the article: Nicholas Talley.
References
- 1. Harland WA, Lee FD. Intestinal spirochaetosis. Br Med J 1967; 3: 718–719. 10.1136/bmj.3.5567.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norris SJ. Hiding in plain sight: colonic spirochetosis in humans. J Bacteriol 2019; 201: e00465‐19. 10.1128/JB.00465-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hampson DJ. The spirochete Brachyspira pilosicoli, enteric pathogen of animals and humans. Clin Microbiol Rev 2018; 31: e00087‐17. 10.1128/CMR.00087-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anthony N, Blackwell J, Ahrens W, Lovell RD, Scobey M. Intestinal spirochetosis: an enigmatic disease. Gastroenterology 2012; 1: S599–S600. 10.1016/S0016-5085(12)62297-5 [DOI] [PubMed] [Google Scholar]
- 5. Brooke CJ, Riley TV, Hampson DJ. Evaluation of selective media for the isolation of Brachyspira aalborgi from human faeces. J Med Microbiol 2003; 52: 509–513. 10.1099/jmm.0.05105-0 [DOI] [PubMed] [Google Scholar]
- 6. Haleem A, Al‐Hindi H, Al Husseini H, Juboury M. Appendiceal spirochetosis: a light and electron microscope study of two cases. Ann Saudi Med 2003; 23: 216–219. 10.5144/0256-4947.2003.216 [DOI] [PubMed] [Google Scholar]
- 7. Antonakopoulos G, Newman J, Wilkinson M. Intestinal spirochaetosis: electron microscopic study of an unusual case. Histopathology 1982; 6: 477–488. 10.1111/j.1365-2559.1982.tb02744.x [DOI] [PubMed] [Google Scholar]
- 8. Gebbers JO, Ferguson DJ, Mason C, Kelly P, Jewell DP. Spirochaetosis of the human rectum associated with an intraepithelial mast cell and IgE plasma cell response. Gut 1987; 28: 588–593. 10.1136/gut.28.5.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delladetsima K, Markaki S, Papadimitriou K, Antonakopoulos GN. Intestinal spirochaetosis. Light and electron microscopic study. Pathol Res Pract 1987; 182: 780–782. 10.1016/S0344-0338(87)80042-0 [DOI] [PubMed] [Google Scholar]
- 10. Tesson JR, Fontainea R, Fumery M, Fontaine R, Gaudet LV, Attencourt C, Chatelain D. Immunohistochemical diagnosis of colonic spirochetosis with anti‐treponema antibody in patients consulting for chronic diarrhea. Results of a prospective study conducted in 137 patients. Ann Pathol 2019; 39: 280–285. 10.1016/j.annpat.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 11. Al Zoubi M, Rodriguez G, Kent P, Atallah C, Creticos C, Weisenberg E. Acute intestinal spirochetosis presenting as an IBD mimicker. Am J Gastroenterol 2017; 112: S823–S824. 10.14309/00000434-201710001-01506 [DOI] [Google Scholar]
- 12. Clarridge JE 3rd. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 2004; 17: 840–862. 10.1128/CMR.17.4.840-862.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thorell K, Inganas L, Backhans A et al. Isolates from colonic spirochetosis in humans show high genomic divergence and potential pathogenic features but are not detected using standard primers for the human microbiota. J Bacteriol 2019; 201: e00272‐19. 10.1128/JB.00272-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker MM, Talley NJ, Inganas L et al. Colonic spirochetosis is associated with colonic eosinophilia and irritable bowel syndrome in a general population in Sweden. Hum Pathol 2015; 46: 277–283. 10.1016/j.humpath.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 15. Goodsall TM, Talley NJ, Rassam L, Wood NK, Zala A, Jones M, Walker MM. Unique pathology of colonic spirochaetosis characterised by mucosal eosinophilia is linked to diarrhoea and IBS. Gut 2017; 66: 978–979. 10.1136/gutjnl-2016-312405 [DOI] [PubMed] [Google Scholar]
- 16. Jabbar KS, Dolan B, Eklund L et al. Association between Brachyspira and irritable bowel syndrome with diarrhoea. Gut 2020; 70: 1117–1129. 10.1136/gutjnl-2020-321466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coyne JD, Curry A, Purnell P, Coyne JD, Curry A, Purnell P, Haboubi NY. Colonic tubular adenomas and intestinal spirochaetosis: an incompatible association. Histopathology 1995; 27: 377–379. 10.1111/j.1365-2559.1995.tb01530.x [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
- 22. Alsaigh N, Fogt F. Intestinal spirochetosis: clinicopathological features with review of the literature. Colorectal Dis 2002; 4: 97–100. 10.1046/j.1463-1318.2002.00284.x [DOI] [PubMed] [Google Scholar]
- 23. Higashiyama M, Ogata S, Adachi Y et al. High prevalence of abnormal colonoscopical findings in human intestinal spirochetosis. Gastrointest Endosc 2009; 69: AB299. 10.1016/j.gie.2009.03.830 [DOI] [Google Scholar]
- 24. Cooper C, Cotton DW, Hudson MJ, Kirkham N, Wilmott FE. Rectal spirochaetosis in homosexual men: characterisation of the organism and pathophysiology. Genitourin Med 1986; 62: 47–52. 10.1136/sti.62.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esteve M, Salas A, Fernandez‐Banares F et al. Intestinal spirochetosis and chronic watery diarrhea: clinical and histological response to treatment and long‐term follow up. J Gastroenterol Hepatol 2006; 21: 1326–1333. 10.1111/j.1440-1746.2006.04150.x [DOI] [PubMed] [Google Scholar]
- 26. Omori S, Mabe K, Hatanaka K et al. Human intestinal spirochetosis is significantly associated with sessile serrated adenomas/polyps. Pathol Res Pract 2014; 210: 440–443. 10.1016/j.prp.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 27. Ogata S, Shimizu K, Nakanishi K. Human intestinal spirochetosis: right‐side preference in the large intestine. Ann Diagn Pathol 2015; 19: 414–417. 10.1016/j.anndiagpath.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 28. Sato H, Nakamura S, Habano W, Wakabayashi G, Adachi Y. Human intestinal spirochaetosis in northern Japan. J Med Microbiol 2010; 59: 791–796. 10.1099/jmm.0.017376-0 [DOI] [PubMed] [Google Scholar]
- 29. Padmanabhan V, Dahlstrom J, Maxwell L, Kaye G, Clarke A, Barratt PJ. Invasive intestinal spirochetosis: a report of three cases. Pathology 1996; 28: 283–286. 10.1080/00313029600169174 [DOI] [PubMed] [Google Scholar]
- 30. Ichimata S, Yoshizawa A, Kusakari M et al. Human intestinal spirochetosis in Japanese patients aged less than 20 years: histological analysis of colorectal biopsy and surgical specimens obtained from 479 patients. Pathol Int 2017; 67: 302–305. 10.1111/pin.12544 [DOI] [PubMed] [Google Scholar]
- 31. Green K, Harris C, Shuja A, Malespin M, de Melo S Jr. Intestinal spirochetosis: an obscure cause of lower gastrointestinal bleeding. Am J Gastroenterol 2017; 112: S1075–S1076. 10.14309/00000434-201710001-01945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guarda LA. Epigastric pain, nausea, vomiting, and diarrhea in a HIV‐positive man. Lab Med 2004; 35: 415–419. 10.1309/17NDFJWVENYEU31Q [DOI] [Google Scholar]
- 33. Guzman Rojas P, Catania J, Parikh J, Phung TC, Speth G. Intestinal spirochetosis in an immunocompetent patient. Cureus 2018; 10: e2328. 10.7759/cureus.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higashiyama M, Ogata S, Adachi Y et al. Human intestinal spirochetosis accompanied by human immunodeficiency virus infection: a case report. Acta Med Okayama 2009; 63: 217–221. [DOI] [PubMed] [Google Scholar]
- 35. Lin RK, Miyai K, Carethers JM. Symptomatic colonic spirochaetosis in an immunocompetent patient. J Clin Pathol 2006; 59: 1100–1101. 10.1136/jcp.2005.034900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Majid Z, Khalid MA, Khan SA, Achakzai I, Soomro G, Luck N. Intestinal spirocheteosis in a patient with celiac disease. J Coll Physicians Surg Pak 2019; 29: 173–174. 10.29271/jcpsp.2019.02.173 [DOI] [PubMed] [Google Scholar]
- 37. Matsuda H, Chinen K. Intestinal spirochetosis: an unusual cause of postantibiotic diarrhea. J Gen Fam Med 2018; 19: 215–216. 10.1002/jgf2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nishii S, Higashiyama M, Ogata S et al. Human intestinal spirochetosis mimicking ulcerative colitis. Clin J Gastroenterol 2018; 11: 145–149. 10.1007/s12328-017-0807-3 [DOI] [PubMed] [Google Scholar]
- 39. Takezawa T, Hayashi S, Adachi Y et al. Human intestinal spirochetosis in an immunocompromised host: evaluation of eradication therapy by endoscopy, histopathology and bacteriology. Clin J Gastroenterol 2012; 5: 69–73. 10.1007/s12328-011-0265-2 [DOI] [PubMed] [Google Scholar]
- 40. Tunuguntla A, Youngberg G, Sibley D et al. Intestinal spirochetosis: a poorly understood infection causing chronic diarrhea. Tenn Med 2004; 97: 75–76. [PubMed] [Google Scholar]
- 41. Yuki M, Emoto Y, Yoshizawa K, Yuri T, Tsubura A. Intestinal bacterial infection diagnosed by histological examination of endoscopic biopsy specimens. Case Rep Dermatol 2016; 10: 629–632. 10.1159/000452212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ford AC, Talley NJ. IBS in 2010: advances in pathophysiology, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2011; 8: 76–78. 10.1038/nrgastro.2010.216 [DOI] [PubMed] [Google Scholar]
- 43. Barbara G, Grover M, Bercik P, Corsetti M, Ghoshal UC, Ohman L, Rajilić‐Stojanović M. Rome Foundation Working Team report on post‐infection irritable bowel syndrome. Gastroenterology 2019; 156: 46–58.e7. 10.1053/j.gastro.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Talley NJ. What causes functional gastrointestinal disorders? A proposed disease model. Am J Gastroenterol 2020; 115: 41–48. 10.14309/ajg.0000000000000485 [DOI] [PubMed] [Google Scholar]
- 45. Philpott H, Gibson P, Thien F. Irritable bowel syndrome—an inflammatory disease involving mast cells. Asia Pac Allergy 2011; 1: 36–42. 10.5415/apallergy.2011.1.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goral V, Kucukoner M, Buyukbayram H. Mast cells count and serum cytokine levels in patients with irritable bowel syndrome. Hepatogastroenterology 2010; 57: 751–754. [PubMed] [Google Scholar]
- 47. Luo L, Gu Y, Wang X et al. Epidemiological and clinical differences between sexes and pathogens in a three‐year surveillance of acute infectious gastroenteritis in Shanghai. Sci Rep 2019; 9: 9993. 10.1038/s41598-019-46480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Law CL, Grierson JM, Stevens SM. Rectal spirochetosis in homosexual men—the association with sexual practices, HIV‐infection and enteric flora. Genitourin Med 1994; 70: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adeyemo MA, Spiegel BM, Chang L. Meta‐analysis: do irritable bowel syndrome symptoms vary between men and women? Aliment Pharmacol Ther 2010; 32: 738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song M, Emilsson L, Bozorg SR et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record‐linkage study. Lancet Gastroenterol Hepatol 2020; 5: 537–547. 10.1016/S2468-1253(20)30009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Young JP, Price TJ, Moore J, Ruszkiewicz AR. Human intestinal spirochetosis and its relationship to sessile serrated adenomas in an Australian population. Pathol Res Pract 2016; 212: 751–753. 10.1016/j.prp.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 52. Flemer B, Lynch DB, Brown JM et al. Tumour‐associated and non‐tumour‐associated microbiota in colorectal cancer. Gut 2017; 66: 633–643. 10.1136/gutjnl-2015-309595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dejea CM, Fathi P, Craig JM et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018; 359: 592–597. 10.1126/science.aah3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rezasoltani S, Asadzadeh Aghdaei H, Dabiri H, Akhavan Sepahi A, Modarressi MH, Nazemalhosseini Mojarad E. The association between fecal microbiota and different types of colorectal polyp as precursors of colorectal cancer. Microb Pathog 2018; 124: 244–249. 10.1016/j.micpath.2018.08.035 [DOI] [PubMed] [Google Scholar]
- 55. Westerman LJ, Stel HV, Schipper ME et al. Development of a real‐time PCR for identification of Brachyspira species in human colonic biopsies. PLoS ONE 2012; 7: e52281. 10.1371/journal.pone.0052281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mikosza ASJ, La T, Margawani KR, Brooke CJ, Hampson DJ. PCR detection of Brachyspira aalborgi and Brachyspira pilosicoli in human faeces. FEMS Microbiol Lett 2001; 197: 167–170. 10.1111/j.1574-6968.2001.tb10599.x [DOI] [PubMed] [Google Scholar]
- 57. Ogata S, Higashiyama M, Adachi Y et al. Imprint cytology detects floating Brachyspira in human intestinal spirochetosis. Hum Pathol 2010; 41: 249–254. 10.1016/j.humpath.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 58. Forgie AJ, Fouhse JM, Willing BP. Diet‐microbe‐host interactions that affect gut mucosal integrity and infection resistance. Front Immunol 2019; 10: 1802. 10.3389/fimmu.2019.01802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hampson DJ, Lugsomya K, La T, Phillips ND, Trott DJ, Abraham S. Antimicrobial resistance in Brachyspira—an increasing problem for disease control. Vet Microbiol 2019; 229: 59–71. 10.1016/j.vetmic.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 60. Barrett SP. Intestinal spirochaetes in a Gulf Arab population. Epidemiol Infect 1990; 104: 261–266. 10.1017/S0950268800059434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Calderaro A, Bommezzadri S, Gorrini C et al. Infective colitis associated with human intestinal spirochetosis. J Gastroenterol Hepatol 2007; 22: 1772–1779. 10.1111/j.1440-1746.2006.04606.x [DOI] [PubMed] [Google Scholar]
- 62. Carr NJ, Mahajan H, Tan KL, Sharma R. The histological features of intestinal spirochetosis in a series of 113 patients. Int J Surg Pathol 2010; 18: 144–148. 10.1177/1066896908330203 [DOI] [PubMed] [Google Scholar]
- 63. Graham RP, Naini BV, Shah SS, Arnold CA, Kannangai R, Torbenson MS, Lam‐Himlin DM. Treponema pallidum immunohistochemistry is positive in human intestinal spirochetosis. Diagn Pathol 2018; 13: 7. 10.1186/s13000-017-0676-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koteish A, Kannangai R, Abraham SC, Torbenson M. Colonic spirochetosis in children and adults. Am J Clin Pathol 2003; 120: 828–832. 10.1309/G7U6BD85W4G3WJ0J [DOI] [PubMed] [Google Scholar]
- 65. Lee FD, Kraszewsski A, Ciordon J. Intestinal spirohaetosis. Gut 1971; 12: 451–456. 10.1136/gut.12.2.126 [DOI] [Google Scholar]
- 66. Lindboe CF, Tostrup NE, Nersund R, Lindboe CF, Tostrup NE, Nersund R, Rekkavik G. Human intestinal spirochaetosis in mid‐Norway. A retrospective histopathological study with clinical correlations. Apmis 1993; 101: 858–864. 10.1111/j.1699-0463.1993.tb00192.x [DOI] [PubMed] [Google Scholar]
- 67. Mikosza ASJ, La T, Brooke CJ et al. PCR amplification from fixed tissue indicates frequent involvement of Brachyspira aalborgi in human intestinal spirochetosis. J Clin Microbiol 1999; 37: 2093–2098. 10.1128/JCM.37.6.2093-2098.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mooney EE, Casey M, Dervan PA. Intestinal spirochaetosis: pathological entity of no clinical significance? Ir J Med Sci 1988; 157: 324–325. [DOI] [PubMed] [Google Scholar]
- 69. O'Donnell S, Swan N, Crotty P, ODonnell S, Sangster D, OMorain C. Assessment of the clinical significance of intestinal spirochaetosis. J Clin Pathol 2008; 61: 1029–1033. 10.1136/jcp.2008.059204 [DOI] [PubMed] [Google Scholar]
- 70. Peruzzi S, Gorrini C, Piccolo G et al. Human intestinal spirochaetosis in Parma: a focus on a selected population during 2002–2005. Acta Biomed Ateneo Parmense 2007; 78: 128–132. [PubMed] [Google Scholar]
- 71. Petras R, Grindeland I, Katzin W. Colorectal intestinal spirochetosis in community practice: a retrospective review of 60 patients. Am J Gastroenterol 2009; 104: S170. 10.14309/00000434-200910003-00455 [DOI] [Google Scholar]
- 72. Saboorian M, Kinsey RS. Intestinal spirochetosis in the United States: a clinicopathologic study of colonic biopsy specimens. Am J Gastroenterol 2011; 2: S149. 10.14309/00000434-201110002-00380 [DOI] [Google Scholar]
- 73. Surawicz CM, Roberts PL, Rompalo A, Quinn TC, Holmes KK, Stamm WE. Intestinal spirochetosis in homosexual men. Am J Med 1987; 82: 587–592. 10.1016/0002-9343(87)90104-5 [DOI] [PubMed] [Google Scholar]
- 74. Takeuchi A, Jervis HR, Nakazawa H, Robinson DM. Spiral shaped organisms on the surface colonic epithelium of the monkey and man. Am J Clin Nutr 1974; 27: 1287–1296. 10.1093/ajcn/27.11.1287 [DOI] [PubMed] [Google Scholar]
- 75. Tanahashi J, Daa T, Gamachi A, Kashima K, Kondoh Y, Yada N, Yokoyama S. Human intestinal spirochetosis in Japan; its incidence, clinicopathologic features, and genotypic identification. Mod Pathol 2008; 21: 76–84. 10.1038/modpathol.3800987 [DOI] [PubMed] [Google Scholar]
- 76. Tateishi Y, Takahashi M, Horiguchi S‐I et al. Clinicopathologic study of intestinal spirochetosis in Japan with special reference to human immunodeficiency virus infection status and species types: analysis of 5265 consecutive colorectal biopsies. BMC Infect Dis 2015; 15: 13. 10.1186/s12879-014-0736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Threlkeld K, Trainer T, Adamson C et al. Molecular, histologic and clinical features of persistent intestinal spirochetosis. Lab Invest 2013; 1: 183A. [Google Scholar]
- 78. Trott DJ, Combs BG, Mikosza ASJ et al. The prevalence of Serpulina pilosicoli in humans and domestic animals in the Eastern Highlands of Papua New Guinea. Epidemiol Infect 1997; 119: 369–379. 10.1017/S0950268897008194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weisheit B, Bethke B, Stolte M. Human intestinal spirochetosis: analysis of the symptoms of 209 patients. Scand J Gastroenterol 2007; 42: 1422–1427. 10.1080/00365520701245629 [DOI] [PubMed] [Google Scholar]
- 80. McMillan A, Lee FD. Sigmoidoscopic and microscopic appearance of the rectal mucosa in homosexual men. Gut 1981; 22: 1035–1041. 10.1136/gut.22.12.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mikosza ASJ, Hampson DJ, Koopmans MPG, van Duynhoven YTHP. Presence of Brachyspira aalborgi and B. pilosicoli in feces of patients with diarrhea. J Clin Microbiol 2003; 41: 4492–4492. 10.1128/JCM.41.9.4492.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bhardwaj A, Yang Z, Lee TP. Human immunodeficiency virus: associated intestinal spirochetosis. Am J Gastroenterol 2012; 1: S489. 10.14309/00000434-201210001-01228 [DOI] [Google Scholar]
- 83. Calderaro A, Gorrini C, Peruzzi S, Piccolo G, Dettori G, Chezzi C. Occurrence of human intestinal spirochetosis in comparison with infections by other enteropathogenic agents in an area of the Northern Italy. Diagn Microbiol Infect Dis 2007; 59: 157–163. 10.1016/j.diagmicrobio.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 84. Carpentieri DF, Souza‐Morones S, Gardetto JS, Ross HM, Downey K, Ingebo K, Siaw E. Intestinal spirochetosis in children: five new cases and a 20‐year review of the literature. Pediatr Dev Pathol 2010; 13: 471–475. 10.2350/09-10-0725-CR.1 [DOI] [PubMed] [Google Scholar]
- 85. Cotton DWK, Kirkham N, Hicks DA. Rectal spirochaetosis. Br J Vener Dis 1984; 60: 106–109. 10.1136/sti.60.2.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. da Cunha Ferreira RM, Phillips AD, Stevens CR, da Cunha Ferreira RMC, Hudson MJ, Rees HC, Walker‐Smith JA. Intestinal spirochaetosis in children. J Pediatr Gastroenterol Nutr 1993; 17: 333–336. 10.1097/00005176-199310000-00020 [DOI] [PubMed] [Google Scholar]
- 87. Fowler G, Sharma S, Campbell D et al. Intestinal spirochaetosis: an infectious colitis with normal calprotectin. J Pediatr Gastroenterol Nutr 2019; 68: 466–467.30540713 [Google Scholar]
- 88. Gad A, Willen R, Furugard K, Willén R, Furugård K, Fors B, Hradsky M. Intestinal spirochaetosis as a cause of longstanding diarrhoea. Ups J Med Sci 1977; 82: 49–54. 10.3109/03009737709179059 [DOI] [PubMed] [Google Scholar]
- 89. Gil‐Setas A, Martinez‐Penuela JM, Escudero R et al. Six cases of human intestinal spirochetosis. Clin Microbiol Infect 2012; 3: 804–805. [Google Scholar]
- 90. Heine RG, Ward PB, Mikosza ASJ, Bennett‐Wood V, Robins‐Browne RM, Hampson DJ. Brachyspira aalborgi infection in four Australian children. J Gastroenterol Hepatol 2001; 16: 872–875. 10.1046/j.1440-1746.2001.t01-1-02543.x [DOI] [PubMed] [Google Scholar]
- 91. Iwamoto J, Adachi Y, Honda A, Monma T, Matsuzaki Y. The comparison of the intensity of human intestinal spirochetes between Brachyspira pilosicoli and Brachyspira aalborgi infections. J Clin Biochem Nutr 2019; 64: 86–90. 10.3164/jcbn.18-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Iwamoto J, Ogata S, Honda A et al. Human intestinal spirochaetosis in two ulcerative colitis patients. Intern Med 2014; 53: 2067–2071. 10.2169/internalmedicine.53.2386 [DOI] [PubMed] [Google Scholar]
- 93. Kostman JR, Patel M, Catalano E, Camacho J, Hoffpauir J, DiNubile MJ. Invasive colitis and hepatitis due to previously uncharacterized spirochetes in patients with advanced human‐immunodeficiency‐virus infection. Clin Infect Dis 1995; 21: 1159–1165. 10.1093/clinids/21.5.1159 [DOI] [PubMed] [Google Scholar]
- 94. Lemmens R, Devreker T, Hauser B, Degreef E, Goossens A, Vandenplas Y. Intestinal spirochetosis: a case series and review of the literature. Pediatr Gastroenterol Hepatol Nutr 2019; 22: 193–200. 10.5223/pghn.2019.22.2.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lo TCN, Heading RC, Gilmour HM. Intestinal spirochaetosis. Postgrad Med J 1994; 70: 134–137. 10.1136/pgmj.70.820.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Manjunath S, Thompson A. Intestinal spirochetosis: a “fuzzy” entity. Ann Dent 2012; 25: 372. [PMC free article] [PubMed] [Google Scholar]
- 97. Marthinsen L, Willen R, Carlen B, Lindberg E, Varendh G. Intestinal spirochetosis in eight pediatric patients from Southern Sweden—a clinical, histopathological and ultrastructural study. Apmis 2002; 110: 571–579. 10.1034/j.1600-0463.2002.11007809.x [DOI] [PubMed] [Google Scholar]
- 98. Tompkins DS, Waugh MA, Cooke EM. Isolation of intestinal spirochaetes from homosexuals. J Clin Pathol 1981; 34: 1385–1387. 10.1136/jcp.34.12.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. White J , Roche D, Chan YF, Mitchell EA. Intestinal spirochetosis in children: report of two cases. Pediatr Pathol 1994; 14: 191–199. 10.3109/15513819409024252 [DOI] [PubMed] [Google Scholar]
- 100. Yang M, Lapham R. Appendiceal spirochetosis. South Med J 1997; 90: 30–32. 10.1097/00007611-199701000-00006 [DOI] [PubMed] [Google Scholar]
- 101. Al‐Bozom IA, Al‐Rikabi AC. Colorectal spirochetosis. Saudi Med J 2000; 21: 1189–1191. [PubMed] [Google Scholar]
- 102. Anwar MA, Khan RU, Beg MA et al. Intestinal spirochetosis: symptomatic patients in the presence of no risk factors and improvement with metronidazole therapy. J Pak Med Stud 2013; 3: 115–117. [Google Scholar]
- 103. Lafeuillade A, Quilichini R, Benderitter T, Delbeke E, Dhiver C, Gastaut JA. Intestinal spirochaetosis in HIV infected homosexual men. Postgrad Med J 1990; 66: 253–254. 10.1136/pgmj.66.773.253-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McIntire M, Genta R. Intestinal spirochetosis is associated with diarrhea, weight loss, and abdominal pain in less than half of the infected subjects: a study of 447 patients and 1.2 million controls. Am J Gastroenterol 2013; 1: S168. [Google Scholar]
- 105. Jensen TK, Boye M, Ahrens P, Korsager B, Teglbjærg PS, Lindboe CF, Møller K. Diagnostic examination of human intestinal spirochetosis by fluorescent in situ hybridization for Brachyspira aalborgi, Brachyspira pilosicoli, and other species of the genus Brachyspira (Serpulina). J Clin Microbiol 2001; 39: 4111–4118. 10.1128/JCM.39.11.4111-4118.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nielsen RH, Orholm M, Pedersen JO, Hovind‐Hougen K, Stubbe Teglbjærg P, Hess Thaysen E. Colorectal spirochetosis: clinical significance of the infestation. Gastroenterology 1983; 85: 62–67. 10.1016/S0016-5085(83)80230-3 [DOI] [PubMed] [Google Scholar]
- 107. Dominguez C, Fetais AR, Jiang K. Unsuspected spirochetosis in receipts of bone marrow transplant: an institutional review of case series. Lab Invest 2017; 97: 389A. [Google Scholar]


