Abstract
Patients with acute venous thromboembolism (VTE) require anticoagulant therapy to prevent recurrent VTE and death, which exposes them to an inherent increased risk of bleeding. Identification of patients at high risk of bleeding, and mitigating this risk, is an essential component of the immediate and long‐term therapeutic management of VTE. The bleeding risk can be estimated by either implicit judgment, weighing individual predictors (clinical variables or biomarkers), or by risk prediction tools developed for this purpose. Management of bleeding risk in clinical practice is, however, far from standardized. International guidelines are contradictory and lack clear and consistent guidance on the optimal management of bleeding risk. This report of the ISTH subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease summarizes the evidence on the prediction of bleeding in VTE patients. We systematically searched the literature and identified 34 original studies evaluating either predictors or risk prediction models for prediction of bleeding risk on anticoagulation in VTE patients. Based on this evidence, we provide recommendations for the standardized management of bleeding risk in VTE patients.
Keywords: anticoagulant treatment, major bleeding, prediction model, risk assessment, venous thromboembolism
1. INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a common cardiovascular disease, affecting 1–2 per 1000 individuals per year worldwide. 1 , 2 Timely diagnosis and initiation of anticoagulant treatment is critical as historical studies have reported mortality rates up to 30% when acute VTE is left untreated. 3 To prevent thrombus progression and early recurrences once a diagnosis of VTE is established, anticoagulant treatment begins with an initiation phase that lasts for 5–21 days, depending on the anticoagulant regimen selected, and is continued for at least 3 months (i.e., the treatment phase). 4 After completion of the treatment phase, the decision to continue anticoagulation is driven mainly by the estimated risk of recurrent VTE. Current guidelines advise to consider continuation of anticoagulant therapy among patients with major persistent risk factors (e.g., cancer or antiphospholipid syndrome) or when VTE occurs in the absence of transient risk factors (i.e., unprovoked VTE). 4 , 5 , 6
Although anticoagulant therapy is highly effective at reducing the risk of recurrent VTE, this comes at the expense of an increased risk of bleeding complications. The risk of major bleeding is greatest during the initial 3 months of treatment with an estimated incidence of 2%, and a case fatality rate of 11.3%, comparable to the case fatality rate of recurrent VTE. 7 , 8 , 9 , 10 For patients who receive anticoagulant drugs for more than 3 months, the reported risk of major bleeding is 1.3–2.2 per 100 patient‐years (reaching a 5‐year cumulative risk of 6.3%) with a case fatality rate of 5.1%–12% in patients treated with vitamin K antagonists (VKAs). 7 , 11 This risk is considerably lower when patients are treated with a direct oral anticoagulant (DOAC) approximating 0.72–1.6 events/100 patient years, while evidence on cumulative risks beyond 24 months are still sparse. 11 , 12 , 13 These numbers highlight the importance of harm‐to‐benefit assessment of long‐term anticoagulant treatment, for which the risk of VTE recurrence is weighed against the risk of bleeding. In addition, identification of potentially modifiable bleeding predictors may provide physicians tools to diminish an individual patient's bleeding risk during follow‐up. 9
Although current clinical guidelines on the management of VTE advocate the assessment of bleeding risk during treatment decisions, 4 , 5 specific guidance on how to assess bleeding risk is not standardized, and guidance to assess bleeding risk at least annually is based upon expert consensus. 4 In this communication by the Standardization Subcommittee (SSC) on Predictive and Diagnostic Variables of the International Society on Thrombosis and Haemostasis (ISTH), we aimed to summarize the published evidence on currently identified predictors and prediction tools for bleeding risk assessment in VTE patients using anticoagulant therapy, and propose recommendations for standardization of the management of risk of bleeding in VTE patients in clinical practice.
2. METHODS
2.1. Search strategy
A systematic PubMed search was performed including combinations of Medical Subject Headings terms and title/abstract terms which included Medline records, PubMed Central records, and the NCBI Bookshelf for studies in all languages until March 18, 2021. The complete search string is available in the Appendix S1 in supporting information.
2.2. Study selection
Studies were eligible for inclusion if they fulfilled the following criteria: (1) any retrospective or prospective cohort study or clinical trial; (2) inclusion of adult patients with objectively confirmed acute lower or upper extremity DVT or acute PE, 14 treated with any anticoagulant at therapeutic dose (unfractionated heparin, low molecular weight heparin, VKA, or DOAC) or reduced dose DOAC; (3) included unselected patients or targeting a specific population (e.g., cancer patients, elderly patients, unprovoked VTE); (4) evaluation of predictors or risk prediction models for major bleeding, either with short or long term follow‐up; and (5) written in English language. Studies were excluded from this analysis in case they did not involve a VTE population (e.g., atrial fibrillation [AF]); included pediatric patients; did not primarily assess predictors or scoring systems for prediction of major bleeding; were review articles or meta‐analyses; or involved case series (<50 patients) or case reports.
Studies were screened for eligibility using Covidence software. Titles were directly imported into Covidence from the search databases and duplicates were removed. Eight authors (PLE, SCW, CM, PEM, JBH, KW, FAK, and HRE) independently screened the titles and abstracts in duplicate of all citations identified by the search strategy. Disagreements were resolved by a third reviewer. Three authors (PLE, FAK, and HRE) subsequently independently assessed full texts of the selected articles following screening and determined the final list of included studies. All disagreements were resolved by discussion and consensus during several online meetings.
2.3. Data extraction
Included studies underwent systematic data extraction into a predefined standardized data capture tool by two authors (PLE and FAK). Data extraction included first author name, year of publication, and predictors studied or prediction model studied. If a bleeding prediction model was studied we captured: the number of variables included in the model, time period in which the model was evaluated (categorized as within the initial 3 months or beyond 3 months of anticoagulant treatment), type of anticoagulant used, and patient subgroups in which the models were evaluated. For individual predictors, the strength of the association with major bleeding was graded as: limited (odds ratio [OR] 1–2), moderate (OR 1–5), or strong (OR >5). Individual predictors were classified as modifiable, potential modifiable, or non‐modifiable. For the definition of major bleeding, we adopted and extracted the definition provided in the individual studies.
2.4. Recommendations
Based on the evidence identified by data extraction, recommendations were formed using best available evidence and discussed during several panel meetings. During the online SSC session at the 2021 ISTH Congress, the 392 participants were surveyed about these recommendations (Appendix S2 in supporting information). A final consensus meeting was organized in which all panel members voted on formulation and level of the recommendations. Recommendations vary in strength given they are based on best available evidence and consensus formation. The wording “we recommend” indicates a strong guidance statement, whereby we advise that the practice should be adopted in most cases. The wording “we suggest” reflects a weak guidance statement, whereby we advise that the practice may be adopted.
3. SUMMARY OF EVIDENCE
3.1. Study selection
The search strategy yielded 740 references after removal of duplicates; 705 were excluded after review of titles and abstracts, leaving 35 references that were assessed for eligibility by full text review. Of those, 10 studies were excluded for reasons shown in Figure 1. Using citation searching, another nine references were identified that fulfilled the inclusion criteria. Therefore, a total of 34 studies were used for data extraction.
FIGURE 1.
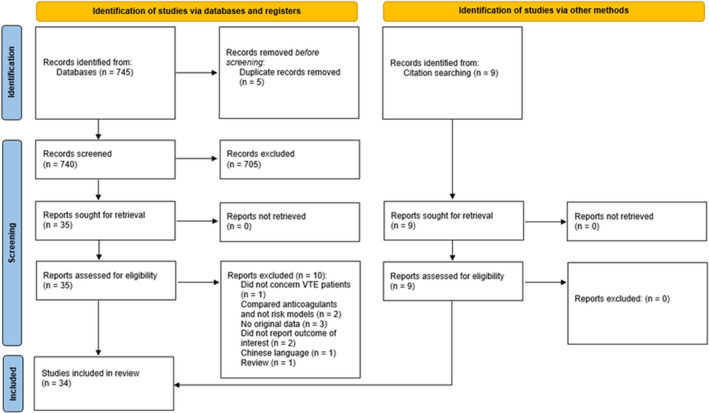
Flowchart of included studies. VTE, venous thromboembolism. http://www.prisma‐statement.org/
3.2. Studies assessing individual predictors
A total of 17 studies were eligible to evaluate the association between 14 individual predictors and major bleeding (Table 1). Of these predictors, two predictors were defined as modifiable (use of platelet inhibitors or non‐steroidal anti‐inflammatory drugs [NSAIDs] and hypertension), eight were defined as potentially modifiable (renal insufficiency, cancer, anemia, frequent falls, liver disease, thrombocytopenia, labile international normalized ratio [INR], and diabetes) and four were non‐modifiable (prior bleeding, advanced age, sex, and prior stroke). Of all predictors, prior bleeding, renal insufficiency, cancer, anemia, and advanced age were most consistently associated with limited to moderately increased risk of bleeding across studies.
TABLE 1.
Overview of individual predictors of major bleeding identified in VTE studies
| Variable | Relevant studies | Strength of association (univariate) | |||
|---|---|---|---|---|---|
| No association | Limited (OR 1–2) | Moderate (OR 2–5) | Strong (OR >5) | ||
| Prior bleeding | 15, 18, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 | IIIII | IIIIIIIIII | ||
| Renal insufficiency | 15, 18, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 43 | I | IIIIIII | IIIII | II |
| Cancer | 15, 18, 19, 31, 32, 33, 34, 35, 39, 40, 41, 43 | IIII | II | IIIIII | |
| Anemia | 15, 32, 33, 35, 36, 37, 38, 40, 41, 43 | II | III | IIIIII | |
| Elderly | 15, 18, 19, 30, 32, 33, 35, 36, 37, 39, 40, 41 | II | IIIIII | IIII | |
| Use of platelet inhibitors or non‐steroidal anti‐inflammatory drugs | 15, 18, 30, 31, 32, 33, 34, 37, 38, 40 | III | IIIII | II | |
| Hypertension | 15, 30, 31, 34, 37, 38, 40, 41 | III | IIII | I | |
| Frequent falls | 32, 34, 37 | III | |||
| Liver disease | 31, 33, 36, 37, 41 | II | III | ||
| Sex | 18, 19, 31, 33, 36, 37, 38, 39, 40, 41 | IIIII | IIII | ||
| Prior stroke | 31, 32, 33, 34, 36, 37, 38, 40, 41 | IIIII | III | ||
| Thrombocytopenia | 32, 37, 41 | I | I | I | |
| Labile INR | 30, 32, 34 | II | I | ||
| Diabetes | 32, 33, 34, 37, 38, 40, 41 | IIIII | II | ||
Note: Predictors are ranked on strength of association. Modifiable (green), potentially modifiable (gray), and non‐modifiable (white) predictors are color coded.
Abbreviations: INR, international normalized ratio; OR, odds ratio; VTE, venous thromboembolism.
3.3. Risk prediction models
In total, 17 bleeding risk prediction models were identified (Table 2). Of these models, ten were originally derived in VTE patients, and seven in patients with non‐valvular AF. The studies evaluating these models differed considerably in design (prospective data collection, retrospective data collection, registry, clinical trial), case mix, follow‐up duration, and definitions of predictors and major bleeding. There were no studies designed to prospectively assess and compare two or more prognostic models, let alone to use a prognostic model to guide decision making.
TABLE 2.
Overview of bleeding risk prediction models developed for or tested in VTE patients
| Model | Number of variables | Outcome | External evaluation | Evaluation in subgroups | Time period | Anticoagulants | Definition of major bleeding | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective | Prospective | Overall | Unprovoked | Cancer | Elderly | First 3 months | Beyond 3 months | VKA | Anti‐Xa | Anti‐IIa | ||||
| VTE‐BLEED | 6 | 2‐level | 43, 44, 45, 46, 47 | 35, 36, 48, 49, 50, 51 | X | X | X | X | X | X | X | X | ISTH major bleeding | |
| RIETE | 6 | 3‐level | 33, 34, 46, 47, 52, 53, 54, 55, 56 | 36, 51, 57, 58 | X | X | X | X | X | X | X | Investigator‐reported overt bleeding requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal or spinal location, or leading to death | ||
| Nieuwenhuis | 4 | 3‐level | 56, 59 | ‐‐ | X | X | X | Bleeding that leads to death, to interruption of treatment, to blood transfusion, or to a decrease in hemoglobin level of >2.42 g/dl | ||||||
| Seiler | 7 | 3‐level | ‐ | ‐ | X | X | X | X | ISTH major bleeding | |||||
| Einstein score | 3 different models proposed | No threshold provided | 41 | ‐ | X | X | Median 183 days | X | X | ISTH major bleeding | ||||
| Hokusai score | 5 | No threshold provided | ‐ | ‐ | X | X | Median 267 days | X | X | ISTH major bleeding | ||||
| Martinez | 15 | 2‐level | ‐ | ‐ | X | X | X | ISTH major bleeding | ||||||
| Kuijer | 3 | 3‐level | 33, 41, 47, 52, 53, 54, 55, 56 | 19, 57, 58, 60 | X | X | X | X | X | X | X | ≥2 g/dl drop in hemoglobin, requiring transfusion of ≥2 units of blood, intracranial or retroperitoneal location, or warranting permanent treatment discontinuation | ||
| ACCP risk table | 18 | 3‐level | 33, 34, 41, 55, 56 | 32, 48, 58 | X | X | X | X | X | No derivation study | ||||
| HAS‐BLED | 7 | 3‐level | 34, 41, 43, 46, 47, 52, 54, 61 | 30, 31, 48, 58 | X | X | X | X | X | X | X | Any bleeding requiring hospitalization and/or causing a decrease in hemoglobin level of >2 g/L and/or requiring blood transfusion that was not a hemorrhagic stroke. | ||
| HEMORR2‐HAGES | 11 | 2‐level | 34, 46, 52, 54 | 58 | X | X | X | X | X | X | X | Hospitalization for hemorrhage, as determined by Medicare claims. | ||
| ATRIA | 5 | 3‐level | 34, 47 | 58 | X | X | X | X | X | X | X | Fatal bleeding, bleeding requiring transfusion of ≥2 U packed blood cells, or bleeding into a critical anatomic site (e.g., intracranial, retroperitoneal). | ||
| Shireman | 8 | 3‐level | ‐ | 58 | X | X | X | X | Hospitalization for GI bleed or intracranial bleed | |||||
| ORBIT | 5 | 3‐level | 41, 47 | ‐ | X | X | X | X | ISTH major bleeding | |||||
| Landefeld and Goldman | 5 | 3‐level | 53, 59 | 57 | X | X | X | X | Bleeding that is (1) fatal, (2) life‐threatening, (3) potentially life‐threatening, (4) led to severe blood loss, (5) led to surgical treatment, or (6) led to moderate blood loss that was acute or subacute, not explained by trauma or surgery | |||||
| mOBRI (Beyth) | 4 | 3‐level | 33, 34, 41, 52, 53, 62 | 57, 58 | X | X | X | X | X | X | X | Overt bleeding that led to the loss of at least 2.0 units in 7 days or less, or was otherwise life‐threatening (e.g., intracranial bleeding) | ||
| PE‐SCARD | 3 | 3‐level | ‐ | ‐ | X | X | ISTH major bleeding | |||||||
Abbreviations: ACCP, American College of Chest Physicians; GI, gastrointestinal; ISTH, International Society on Thrombosis and Haemostasis; HAS‐BLED, Hypertension/Abnormal liver or renal function/Stroke/Bleeding/Labile INR/Elderly/Drugs or Alcohol; mOBRI, Modified Outpatient Bleeding Risk Index; ORBIT, Outcomes Registry for Better Informed Treatment; PE‐SCARD, Pulmonary Embolism Syncope, Anemia, Renal Dysfunction; RIETE, Registro Informatizado de Enfermedad Tromboembolico; VTE, venous thromboembolism; VTE‐BLEED, Venous Thromboembolic Disease & Bleeding.
Predictors that were most frequently included in the prediction models were age, previous bleeding, active cancer, renal insufficiency, anemia, and history of hypertension. Most scores were evaluated in studies with a follow‐up duration of 3–6 months. The VTE‐BLEED (Venous Thromboembolic Disease & Bleeding) score, 15 HAS‐BLED (Hypertension/Abnormal liver or renal function/Stroke/Bleeding/Labile INR/Elderly/Drugs or Alcohol) score, 16 ACCP (American College of Chest Physicians) risk table, 17 RIETE (Registro Informatizado de Enfermedad Tromboembolico) score, 18 and the Kuijer score 19 are the most extensively studied. Details on the validation of these scores are provided in Table 3. Until now, none of these models has been applied in a prospective outcome study.
TABLE 3.
Overview of status of most extensively studied bleeding prediction models in VTE patients
| VTE‐BLEED | HAS‐BLED | ACCP risk table | RIETE | Kuijer | |
|---|---|---|---|---|---|
| External evaluation in study with prospective data collection | Yes | Yes | Yes | Yes | Yes |
| Association with risk of fatal/intracranial bleeding established | Yes | No | No | Yes | No |
| Association with risk of recurrent VTE established | Yes | No | No | No | No |
| Prospective validation in outcome study | No | No | No | No | No |
Abbreviations: ACCP, American College of Chest Physicians; HAS‐BLED, Hypertension/Abnormal liver or renal function/Stroke/Bleeding/Labile INR/Elderly/Drugs or Alcohol; RIETE, Registro Informatizado de Enfermedad Tromboembolico; VTE, venous thromboembolism; VTE‐BLEED, Venous Thromboembolic Disease & Bleeding.
To date, no prospective outcome studies have been performed directly comparing the performance of bleeding risk models in the VTE population. Consequently, the SSC panel was unable to compare the discriminative performance between models.
4. DISCUSSION
Bleeding risk assessment is an essential part of clinical decision‐making in the management of anticoagulation for VTE. Despite accumulating evidence for bleeding risk prediction, current VTE management guidelines lack specific recommendations on the management of bleeding predictors. Therefore, this SSC summarized the evidence and developed guidance on this topic.
When considering predictors associated with anticoagulant‐associated bleeding, an important step is to differentiate modifiable from non‐modifiable predictors. Given that the risk of bleeding is highest during the initiation and treatment phases of anticoagulant therapy, identifying modifiable predictors to decrease the bleeding risk is most relevant when VTE is diagnosed. 10 , 20 We therefore recommend that a first bleeding risk assessment should take place upon diagnosis of VTE and before introduction of anticoagulant treatment for newly diagnosed VTE regardless of where the diagnosis occurs. Of the (potentially) modifiable predictors, hypertension, use of concomitant antiplatelet therapy or NSAIDs, anemia, and renal insufficiency were most consistently associated with higher bleeding risk. Although the association between antiplatelet agents and major bleeding in VTE patients was classified as limited in most studies, as patients using antiplatelet therapy were often excluded from the original studies, a recent meta‐analysis provides further support for this association, demonstrating that the combination of antiplatelet therapy with VKA or DOACs in VTE patients increases the risk of major bleeding (OR 1.79; 95% confidence interval [CI]: 1.22–1.63). 21 Furthermore, in the AF population, both dual and triple anticoagulant therapy have been found to be associated with a substantially increased bleeding risk. 22 As this predictor is easily identifiable and avoidable, we recommend reviewing the indication for dual or triple anticoagulant therapy in any VTE patients, both at initiation of anticoagulant therapy and during follow‐up. Although anemia is a symptom of bleeding rather than usually directly related to bleeding, and blood transfusion is unlikely to mitigate the bleeding risk, identifying and treating the cause of anemia may prevent bleeding complications. Identification of non‐modifiable predictors is helpful to inform patients and physicians about the absolute risk of events and determine net clinical benefit of treatment, which is particularly relevant for extended treatment. Furthermore, in AF patients, it has been shown that successful targeting of modifiable risk factors can be achieved by relatively simple interventions and can lead to reclassification of bleeding risk. 9 , 23 , 24 , 25 Particularly, changes in risk factors over time were found to be more predictive for major bleeding than risk factors measured at baseline. 23 , 24 Uncontrolled hypertension during follow‐up of anticoagulant therapy has been associated with an increased risk for intracranial hemorrhage. 26 Also, a decline in renal function has been associated with an increased bleeding risk, 27 whereas improvement in renal function has been demonstrated to reduce the risk of anticoagulant related bleeding. 28 Of note, VTE patients have a wider age range including younger patients with fewer co‐morbidities compared to the AF population, which may confound above assumptions.
With regard to risk prediction tools, we found an extensive list of models with largely overlapping components. As the impact of these models has not been tested in a VTE outcome study, we cannot recommend using bleeding risk prediction tools as stand‐alone entities to define the optimal treatment duration. Instead, we suggest that these should be applied to estimate bleeding risk for extended‐phase anticoagulation, and to plan the frequency of follow‐up. Patients with a higher risk for bleeding may benefit from more frequent re‐assessment. At follow‐up, a standardized inquiry reviewing new modifiable predictors for bleeding should be performed, the anticoagulant agent indication/dose reviewed, and the decision to continue extended‐phase anticoagulation reevaluated. This provides essential input for shared decision making, for which risks and benefits of treatment continuation should be weighed. For this purpose, we suggest using more extensively validated prediction scores in long‐term follow‐up of VTE patients, which have demonstrated consistent predictive capability across patient subgroups including patients treated with VKA or DOACs. Currently, VTE‐BLEED, the RIETE score, and the ACCP risk table among other models have been externally validated in separate populations from the derivation population (Tables 2 and 3). The panelists also agreed that future validation studies and prospective outcome studies are needed to confirm the clinical utility of these models over a wide range of VTE patients.
In recent years, several therapeutic strategies have been tested for extended‐phase VTE treatment, including reduced‐dose DOACs. A meta‐analysis found that reduced‐dose DOACs were as effective as full‐dose treatment in preventing recurrent VTE at 1 year, with a trend toward fewer bleeding complications (relative risk [RR] 0.74; 95% CI: 0.52–1.05). 29 It should be stated that this strategy has only been evaluated in two of the four DOACs (apixaban and rivaroxaban) and that outcome data are not available beyond 1 year of follow‐up. Definite evidence for a benefit of this strategy has thus not been provided. Nonetheless, for patients with a higher bleeding risk, adherence to a strategy with reduced‐dose DOAC is regarded an attractive option, and is endorsed by the CHEST guideline on antithrombotic therapy for VTE. 4 , 20
To date, no randomized trials or management studies have been performed evaluating the impact of treatment decisions based on standardized bleeding risk assessment in VTE patients. The strength of the guidance statements is therefore based on review of the available data and consensus among the committee. The decision to continue anticoagulation is ultimately informed by estimation of the net clinical benefit of treatment for the patient taking into account the estimated risk reduction for recurrent thrombosis afforded by anticoagulation counterbalanced with the burden of anticoagulant therapy.
RECOMMENDATIONS
We recommend estimation of a patient's bleeding risk at diagnosis of acute VTE before anticoagulation therapy is initiated.
We recommend bleeding risk assessment after completion of the treatment phase, and during the extended‐phase anticoagulant therapy for VTE, at least annually; in patients at high risk of bleeding, the evaluation should be more frequent.
We recommend that bleeding risk assessment includes identification of the indication of concomitant use of platelet inhibitors or non‐steroidal anti‐inflammatory drugs and avoiding concomitant use if possible. We suggest that other (potentially) modifiable predictors should be identified early and targeted, in particular hypertension.
We suggest routine standardized bleeding risk assessment during extended‐phase anticoagulation. Preferably, this includes combining measurement of individual predictors with the use of a validated prediction model to support anticoagulation management decisions. For this purpose, we identify that the VTE‐BLEED, the RIETE score, and the ACCP risk table are among those most externally validated. Future validation studies and prospective outcome studies are needed to confirm the clinical utility of these models.
5. RESEARCH PRIOTITIES
Many bleeding risk assessment tools have been developed to predict bleeding among patients treated for VTE. Future research should prioritize validation of existing models, with attentiveness to vulnerable patient groups diagnosed with VTE, such as frail elderly or patients with cancer. Most importantly, interventional or management studies applying bleeding risk assessment tools in the decision‐making process are urgently required. Clinical decision support and electronic alerts may improve routine standardized bleeding risk assessment. We encourage the use of structured bleeding risk assessment in any trial evaluating the long‐term management of VTE.
6. CONCLUSION
Based on current evidence, we provide recommendations for the standardized management of bleeding risk in VTE patients during both the short‐ and long‐term follow‐up, and propose research priorities to improve our understanding and allow for solid guideline recommendations on this topic.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. PLE and FAK drafted the manuscript. All other authors provided intellectual input for revisions of the draft. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
SCW, DC, KDW, CM, JBH, PEM, GJW, DMS, PLE, and HRE report nothing to disclose. FAK discloses received research support from: Bayer, BMS, Boehringer‐Ingelheim, MSD, Daiichi‐Sankyo, Actelion, The Netherlands Organisation for Health Research and Development, The Dutch Thrombosis Association, and The Dutch Heart Foundation. In addition, FAK was involved with the derivation of one of the bleeding models evaluated in this manuscript, namely the VTE‐BLEED model.
Supporting information
Appendix S1
Appendix S2
den Exter PL, Woller SC, Robert‐Ebadi H, et al.. Management of bleeding risk in patients who receive anticoagulant therapy for venous thromboembolism: Communication from the ISTH SSC Subcommittee on Predictive and Diagnostic Variables in Thrombotic Disease. J Thromb Haemost. 2022;20:1910‐1919. doi: 10.1111/jth.15776
Manuscript handled by: Joost Meijers
Final decision: Joost Meijers, 25 May 2022
Funding information
No funding for this study was provided.
REFERENCES
- 1. Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers. 2015;1:15006. doi: 10.1038/nrdp.2015.6 [DOI] [PubMed] [Google Scholar]
- 2. Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028. doi: 10.1038/nrdp.2018.28 [DOI] [PubMed] [Google Scholar]
- 3. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877‐905. doi: 10.1378/chest.121.3.877 [DOI] [PubMed] [Google Scholar]
- 4. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545‐e608. doi: 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. 2020;41:543‐603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 6. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14:1480‐1483. doi: 10.1111/jth.13336 [DOI] [PubMed] [Google Scholar]
- 7. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta‐analysis. Ann Intern Med. 2003;139:893‐900. doi: 10.7326/0003-4819-139-11-200312020-00007 [DOI] [PubMed] [Google Scholar]
- 8. Carrier M, Le GG, Wells PS, Rodger MA. Systematic review: case‐fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578‐589. doi: 10.1059/0003-4819-152-9-201005040-00008 [DOI] [PubMed] [Google Scholar]
- 9. Klok FA, Huisman MV. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood. 2020;135:724‐734. doi: 10.1182/blood.2019001605 [DOI] [PubMed] [Google Scholar]
- 10. Klok FA, Kooiman J, Huisman MV, Konstantinides S, Lankeit M. Predicting anticoagulant‐related bleeding in patients with venous thromboembolism: a clinically oriented review. Eur Respir J. 2015;45:201‐210. doi: 10.1183/09031936.00040714 [DOI] [PubMed] [Google Scholar]
- 11. Khan F, Tritschler T, Kimpton M, et al. Long‐term risk for major bleeding during extended oral anticoagulant therapy for first unprovoked venous thromboembolism: a systematic review and meta‐analysis. Ann Int Med. 2021;174:1420‐1429. doi: 10.7326/m21-1094 [DOI] [PubMed] [Google Scholar]
- 12. Castellucci LA, Cameron C, Le Gal G, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta‐analysis. Bmj. 2013;347:f5133. doi: 10.1136/bmj.f5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:320‐328. doi: 10.1111/jth.12485 [DOI] [PubMed] [Google Scholar]
- 14. Le Gal G, Carrier M, Castellucci LA, et al. Development and implementation of common data elements for venous thromboembolism research: on behalf of SSC subcommittee on official communication from the SSC of the ISTH. J Thromb Haemost. 2021;19:297‐303. doi: 10.1111/jth.15138 [DOI] [PubMed] [Google Scholar]
- 15. Klok FA, Hösel V, Clemens A, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48:1369‐1376. doi: 10.1183/13993003.00280-2016 [DOI] [PubMed] [Google Scholar]
- 16. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173‐180. doi: 10.1016/j.jacc.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 17. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for vte disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e419S‐e494S. doi: 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruíz‐Giménez N, Suárez C, González R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost. 2008;100:26‐31. doi: 10.1160/th08-03-0193 [DOI] [PubMed] [Google Scholar]
- 19. Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457‐460. doi: 10.1001/archinte.159.5.457 [DOI] [PubMed] [Google Scholar]
- 20. Klok FA, Ageno W, Ay C, et al. Optimal follow‐up after acute pulmonary embolism: a position paper of the European society of cardiology working group on pulmonary circulation and right ventricular function, in collaboration with the European society of cardiology working group on atherosclerosis and vascular biology, endorsed by the European respiratory society. Eur Heart J. 2021;43:183‐189. doi: 10.1093/eurheartj/ehab816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valeriani E, Porreca E, Weitz JI, Schulman S, Candeloro M, Di Nisio M. Impact of concomitant antiplatelet therapy on the efficacy and safety of direct oral anticoagulants for acute venous thromboembolism: systematic review and meta‐analysis. J Thromb Haemost. 2020;18:1661‐1671. doi: 10.1111/jth.14807 [DOI] [PubMed] [Google Scholar]
- 22. Nv R, Heide‐Jørgensen U, Lijfering WM, Dekkers OM, Sørensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139:775‐786. doi: 10.1161/CIRCULATIONAHA.118.036248 [DOI] [PubMed] [Google Scholar]
- 23. Peterson D, Geison E. Pharmacist interventions to reduce modifiable bleeding risk factors using HAS‐BLED in patients taking Warfarin. Fed Pract. 2017;34:S16‐S20. [PMC free article] [PubMed] [Google Scholar]
- 24. Chao TF, Lip GYH, Lin YJ, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow‐up and Delta HAS‐BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768‐777. doi: 10.1055/s-0038-1636534 [DOI] [PubMed] [Google Scholar]
- 25. Bohm M, Brueckmann M, Eikelboom JW, et al. Cardiovascular outcomes, bleeding risk, and achieved blood pressure in patients on long‐term anticoagulation with the thrombin antagonist dabigatran or warfarin: data from the RE‐LY trial. Eur Heart J. 2020;41:2848‐2859. doi: 10.1093/eurheartj/ehaa247 [DOI] [PubMed] [Google Scholar]
- 26. Toyoda K, Yasaka M, Uchiyama S, et al. Blood pressure levels and bleeding events during antithrombotic therapy: the bleeding with antithrombotic therapy (BAT) study. Stroke. 2010;41:1440‐1444. doi: 10.1161/strokeaha.110.580506 [DOI] [PubMed] [Google Scholar]
- 27. Bonde AN, Lip GY, Kamper AL, et al. Renal function and the risk of stroke and bleeding in patients with atrial fibrillation: an observational cohort study. Stroke. 2016;47:2707‐2713. doi: 10.1161/strokeaha.116.014422 [DOI] [PubMed] [Google Scholar]
- 28. Nijenhuis VJ, Peper J, Vorselaars VMM, et al. Prognostic value of improved kidney function after transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol. 2018;121:1239‐1245. doi: 10.1016/j.amjcard.2018.01.049 [DOI] [PubMed] [Google Scholar]
- 29. Vasanthamohan L, Boonyawat K, Chai‐Adisaksopha C, Crowther M. Reduced‐dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2018;16:1288‐1295. doi: 10.1111/jth.14156 [DOI] [PubMed] [Google Scholar]
- 30. Kooiman J, van Hagen N, Iglesias Del Sol A, et al. The HAS‐BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PloS One. 2015;10:e0122520. doi: 10.1371/journal.pone.0122520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown JD, Goodin AJ, Lip GYH, Adams VR. Risk stratification for bleeding complications in patients with venous thromboembolism: application of the HAS‐BLED bleeding score during the first 6 months of anticoagulant treatment. J Am Heart Assoc. 2018;7:e007901. doi: 10.1161/JAHA.117.007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palareti G, Antonucci E, Mastroiacovo D, et al. The American college of chest physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism. J Thromb Haemost. 2018;16:1994‐2002. doi: 10.1111/jth.14253 [DOI] [PubMed] [Google Scholar]
- 33. Scherz N, Mean M, Limacher A, et al. Prospective, multicenter validation of prediction scores for major bleeding in elderly patients with venous thromboembolism. J Thromb Haemost. 2013;11:435‐443. doi: 10.1111/jth.12111 [DOI] [PubMed] [Google Scholar]
- 34. Poli D, Antonucci E, Testa S, Cosmi B, Palareti G, Ageno W. The predictive ability of bleeding risk stratification models in very old patients on vitamin K antagonist treatment for venous thromboembolism: results of the prospective collaborative EPICA study. J Thromb Haemost. 2013;11:1053‐1058. doi: 10.1111/jth.12239 [DOI] [PubMed] [Google Scholar]
- 35. Nishimoto Y, Yamashita Y, Morimoto T, et al. Validation of the VTE‐BLEED score's long‐term performance for major bleeding in patients with venous thromboembolisms: from the COMMAND VTE registry. J Thromb Haemost. 2020;18:624‐632. doi: 10.1111/jth.14691 [DOI] [PubMed] [Google Scholar]
- 36. Martinez C, Katholing A, Wallenhorst C, Cohen AT. Prediction of significant bleeding during vitamin K antagonist treatment for venous thromboembolism in outpatients. Br J Haematol. 2020;189:524‐533. doi: 10.1111/bjh.16383 [DOI] [PubMed] [Google Scholar]
- 37. Gaboreau Y, Zenatti N, Vermorel C, Imbert P, Bosson JL, Pernod G. Anemia and bleeding in patients receiving anticoagulant therapy for venous thromboembolism: comment. J Thromb Thrombolysis. 2018;46:84‐87. doi: 10.1007/s11239-018-1668-4 [DOI] [PubMed] [Google Scholar]
- 38. Gómez‐Cuervo C, Rivas A, Visonà A, et al. Predicting the risk for major bleeding in elderly patients with venous thromboembolism using the Charlson index. findings from the RIETE. J Thromb Thrombolysis. 2021;51:1017‐1025. doi: 10.1007/s11239-020-02274-6 [DOI] [PubMed] [Google Scholar]
- 39. White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep‐venous thrombosis. Am J Med. 1999;107:414‐424. doi: 10.1016/s0002-9343(99)00267-3 [DOI] [PubMed] [Google Scholar]
- 40. Nieuwenhuis HK, Albada J, Banga JD, Sixma JJ. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood. 1991;78:2337‐2343. [PubMed] [Google Scholar]
- 41. Di Nisio M, Raskob G, Buller HR, et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost. 2017;117:784‐793. doi: 10.1160/TH16-11-0830 [DOI] [PubMed] [Google Scholar]
- 42. van Rein N, le Cessie S, van Vliet IP, et al. Increased risk of major bleeding after a minor bleed during treatment with vitamin K antagonists is determined by fixed common risk factors. J Thromb Haemost. 2016;14:948‐952. doi: 10.1111/jth.13306 [DOI] [PubMed] [Google Scholar]
- 43. Kresoja KP, Ebner M, Rogge NIJ, et al. Prediction and prognostic importance of in‐hospital major bleeding in a real‐world cohort of patients with pulmonary embolism. Int J Cardiol. 2019;290:144‐149. doi: 10.1016/j.ijcard.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 44. Klok FA, Barco S, Konstantinides SV. External validation of the VTE‐BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb Haemost. 2017;117:1164‐1170. doi: 10.1160/TH16-10-0810 [DOI] [PubMed] [Google Scholar]
- 45. Klok FA, Barco S, Konstantinides SV. Evaluation of VTE‐BLEED for predicting intracranial or fatal bleeding in stable anticoagulated patients with venous thromboembolism. Eur Respir J. 2018;51. doi: 10.1183/13993003.00077-2018 [DOI] [PubMed] [Google Scholar]
- 46. Skowronska M, Furdyna A, Ciurzynski M, et al. D‐dimer levels enhance the discriminatory capacity of bleeding risk scores for predicting in‐hospital bleeding events in acute pulmonary embolism. Eur J Int Med. 2019;69:8‐13. doi: 10.1016/j.ejim.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 47. Vedovati MC, Mancuso A, Pierpaoli L, et al. Prediction of major bleeding in patients receiving DOACs for venous thromboembolism: a prospective cohort study. Int J Cardiol. 2020;301:167‐172. doi: 10.1016/j.ijcard.2019.11.105 [DOI] [PubMed] [Google Scholar]
- 48. Palareti G, Antonucci E, Ageno W, Mastroiacovo D, Poli D, Tosetto A. The American college of chest physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism: reply. J Thromb Haemost. 2018;16:2539‐2540. doi: 10.1111/jth.14300 [DOI] [PubMed] [Google Scholar]
- 49. de Winter MA, Dorresteijn JAN, Ageno W, et al. Estimating bleeding risk in patients with cancer‐associated thrombosis: evaluation of existing risk scores and development of a new risk score. Thromb Haemost. 2021. doi: 10.1055/s-0041-1735251 [DOI] [PubMed] [Google Scholar]
- 50. Klok FA, Presles E, Tromeur C, et al. Evaluation of the predictive value of the bleeding prediction score VTE‐BLEED for recurrent venous thromboembolism. Res Pract Thromb Haemost. 2019;3:364‐371. doi: 10.1002/rth2.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chopard R, Piazza G, Falvo N, et al. An original risk score to predict early major bleeding in acute pulmonary embolism: the syncope, anemia, renal dysfunction (PE‐SARD) bleeding score. Chest. 2021;160:1832‐1843. doi: 10.1016/j.chest.2021.06.048 [DOI] [PubMed] [Google Scholar]
- 52. Di Nisio M, Ageno W, Rutjes AW, Pap AF, Buller HR. Risk of major bleeding in patients with venous thromboembolism treated with rivaroxaban or with heparin and vitamin K antagonists. Thromb Haemost. 2016;115:424‐432. doi: 10.1160/TH15-06-0474 [DOI] [PubMed] [Google Scholar]
- 53. Kline JA, Jimenez D, Courtney DM, et al. Comparison of four bleeding risk scores to identify rivaroxaban‐treated patients with venous thromboembolism at low risk for major bleeding. Acad Emerg Med. 2016;23:144‐150. doi: 10.1111/acem.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klok FA, Niemann C, Dellas C, Hasenfuß G, Konstantinides S, Lankeit M. Performance of five different bleeding‐prediction scores in patients with acute pulmonary embolism. J Thromb Thrombolysis. 2016;41:312‐320. doi: 10.1007/s11239-015-1239-x [DOI] [PubMed] [Google Scholar]
- 55. Seiler E, Limacher A, Mean M, et al. Derivation and validation of a novel bleeding risk score for elderly patients with venous thromboembolism on extended anticoagulation. Thromb Haemost. 2017;117. doi: 10.1160/TH-17-03-0162 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Z, Lei J, Zhai Z, et al. Comparison of prediction value of four bleeding risk scores for pulmonary embolism with anticoagulation: a real‐world study in Chinese patients. Clin Respir J. 2019;13:139‐147. doi: 10.1111/crj.12993 [DOI] [PubMed] [Google Scholar]
- 57. Piovella C, Dalla Valle F, Trujillo‐Santos J, et al. Comparison of four scores to predict major bleeding in patients receiving anticoagulation for venous thromboembolism: findings from the RIETE registry. Int Emerg Med. 2014;9:847‐852. doi: 10.1007/s11739-014-1073-8 [DOI] [PubMed] [Google Scholar]
- 58. Riva N, Bellesini M, Di Minno MN, et al. Poor predictive value of contemporary bleeding risk scores during long‐term treatment of venous thromboembolism. A multicentre retrospective cohort study. Thromb Haemost. 2014;112:511‐521. doi: 10.1160/TH14-01-0081 [DOI] [PubMed] [Google Scholar]
- 59. Wester JP, de Valk HW, Nieuwenhuis HK, et al. Risk factors for bleeding during treatment of acute venous thromboembolism. Thromb Haemost. 1996;76:682‐688. [PubMed] [Google Scholar]
- 60. Keller K, Münzel T, Hobohm L, Ostad MA. Predictive value of the Kuijer score for bleeding and other adverse in‐hospital events in patients with venous thromboembolism. Int J Cardiol. 2021;329:179‐184. doi: 10.1016/j.ijcard.2020.11.075 [DOI] [PubMed] [Google Scholar]
- 61. Rief P, Raggam RB, Hafner F, et al. Calculation of HAS‐BLED score is useful for early identification of venous thromboembolism patients at high risk for major bleeding events: a prospective outpatients cohort study. Semin Thromb Hemost. 2018;44:348‐352. doi: 10.1055/s-0037-1607433 [DOI] [PubMed] [Google Scholar]
- 62. Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arc Int Med. 2003;163:917‐920. doi: 10.1001/archinte.163.8.917 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


