Abstract
Autoimmune diseases and acute inflammation like sepsis cause significant morbidity and disability globally, and new targeted therapies are urgently needed. DNA repair and reactive oxygen species (ROS) pathways have long been investigated as targets for cancer treatment, but their role in immunological research has been limited. In this MiniReview, we discuss the DNA repair enzymes MTH1 and OGG1 as targets to treat both T cell‐driven diseases and acute inflammation. The MiniReview is based on a PhD thesis where both enzymes were investigated with cell and animal models. For MTH1, we found that its inhibition selectively kills activated T cells without being toxic to resting cells or other tissues. MTH1 inhibition also had an alleviating role in disease models of psoriasis and multiple sclerosis. We further identified a novel MTH1lowROSlow phenotype among activated T cells. Regarding OGG1, we demonstrated a mechanism of action of the OGG1 inhibitor TH5487, which prevents the assembly of pro‐inflammatory transcription factors and mitigates acute airway infection in mouse models of pneumonia. Hence, we propose both enzymes to be promising novel targets to treat inflammation and suggest that redox and DNA repair pathways could be useful targets for future immunomodulating therapies.
Keywords: DNA repair, drug discovery, immunology, reactive oxygen species, T cells
1. INTRODUCTION
Inflammatory diseases cause morbidity, disability, shortened life expectancy and economical strain on society globally, and the prevalence of many autoimmune and inflammatory conditions is increasing. 1 , 2 In addition to chronic inflammation, acute inflammation is another great medical challenge, where severe infections leading to organ dysfunction, sepsis, come with a high mortality and limited treatment options. 3 Several approaches to treat both acute and chronic inflammation have been tried over the past decades, and great strides have been made in the field of immunomodulating drugs. However, many of the existing therapies are broad and non‐disease specific, causing a wide range of side‐effects, such as life‐threatening infections and malignant diseases. 2
DNA damage response and DNA repair mechanisms have received an increasing amount of attention as potential targets for new therapeutics over the past years. As DNA lesions might lead to mutations and cell death, targeting DNA repair enzymes has been extensively utilized in the development of cancer treatments, like Poly (ADP‐ribose)‐polymerase 1 (PARP‐1) inhibitors. 4 Drugs such as alkylators, antimetabolites, topoisomerase inhibitors and replication inhibitors cause DNA damage and are already commonly used in clinical practice, side‐by‐side with DNA damage‐inducing radiotherapy. 4 However, just like reactive oxygen species (ROS) have taken a leap from traditionally being considered unconditionally harmful, to playing important roles in physiological cell signalling, 5 the role of oxidative DNA lesions and associated DNA repair enzymes is controversial, as they too might play an important role in cell signalling. 5 , 6 , 7
In this MiniReview, we discuss the findings of the PhD thesis “The DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation” 8 and associated literature. In the thesis, we investigated the connection of the nudix hydrolase MutT homologue 1 (MTH1 or NUDT1) and the DNA glycosylase 8‐oxoguanine glycosylase (OGG1) to inflammatory signalling. Both enzymes are involved in regulating 8‐oxo‐7,8‐dihydroguanine (8‐oxoG) in the DNA and might have important roles in immunological signalling and future therapies.
2. IMMUNE CELLS AND OXIDATIVE STRESS
Despite the risk of inducing DNA damage, ROS have been demonstrated to play an important role in cell signalling, not least in inflammatory processes 5 , 8 (Figure 1). Particularly T cells are dependent on ROS signalling, both regarding activation and differentiation into the different T helper subtypes. 9 , 10 , 11 Too low levels of ROS will inhibit T cell receptor (TCR) stimulation, whereas too high levels can be lethal. The different ROS stages in between have important signalling functions, affecting functions like T cell polarization and proliferation. 9 , 10 , 11
FIGURE 1.
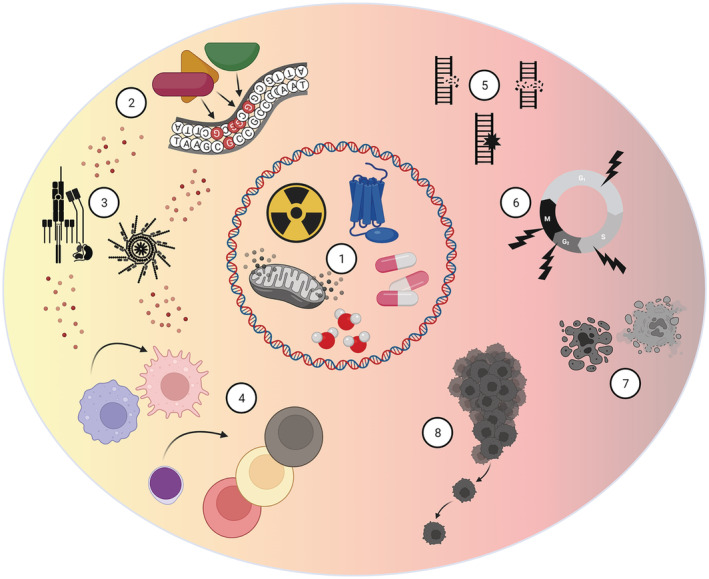
The controversial role of oxidative DNA modifications for immune signalling and cell survival. There are several unavoidable intrinsic and extrinsic sources of ROS and DNA damage. Examples of the intrinsic risk factors are normal cell metabolism, NOX enzymes and water molecules. Extrinsic factors include medications and ionizing radiation (1). Oxidized guanine can attract transcription factors in promoter regions of pro‐inflammatory genes, inducing gene transcription and inflammatory signalling (2). Cellular pro‐inflammatory signalling, like TCR stimulation and assembly of the inflammasome, can both promote and be induced by ROS (3). ROS affects proliferation and polarization of T cells into different subsets, as well as activation of other immune cells (4). However, excessive ROS might lead to DNA strand breaks and mutations (5). This can further lead to cell cycle arrest (6) and cell death (7) or cancer (8). Figure created with BioRender.com
Another important factor connected to ROS and T cell proliferation is the metabolic profile of the cells, which differs between the different T cell populations. 12 , 13 Naïve T cells rely mainly on oxidative phosphorylation (OXPHOS), whereas effector T cells undergo a glycolytic switch, the Warburg effect, in favour of glycolysis. 12 However, OXPHOS is still crucial for both memory T cells and regulatory T cells. 13 The glycolytic switch that T cells undergo affects their ROS status and also gives them some metabolic similarities to several types of cancer cells. 14 Considering these traits and the plasticity among T cells, 15 targeting ROS and DNA repair could have a significant impact on the phenotype of the T cells.
In addition to their impact on T cells, ROS and DNA damage response have important effects on the innate immune system. 6 , 7 Different macromolecules in the cell can be oxidized due to elevated ROS levels, and oxidation of the DNA can lead to epigenetic changes and ultimately DNA strand breaks. 16 Various damage sensors, mediators, transducers, and effectors can all be activated depending on the type of DNA lesions and the cell cycle phase. Clinically, patients with different types of DNA repair enzyme defects can exhibit symptoms like inflammation and accelerated ageing and often an increased cancer risk. An example of this is Ataxia telangiectasia, where the DNA repair and cell cycle kinase Ataxia telangiectasia mutated (ATM), activated by replication stress and DNA double strand breaks, is mutated. 17 Another important DNA repair enzyme is PARP‐1 that also repairs strand breaks and has been considered a promising target in cancer treatment. 4 However, PARP‐1 has several important effects in inflammatory signalling, through activating NF‐kB and downstream signalling, and the downstream effects of ATM are also strongly associated with inflammatory signalling. 7 , 18
Taken together, DNA repair pathways and ROS signalling play an important role in immune cells, and the redox dependent repair enzymes could be important novel targets for new therapeutics beyond cancer, against acute inflammation and T cell‐driven diseases.
3. THE DNA REPAIR ENZYMES MTH1 AND OGG1
Among the four DNA bases, guanine has the lowest redox potential, making it vulnerable to oxidation and leading to the formation of 8‐oxoG, which is important for inflammatory signalling. 19 8‐oxoG in the DNA can be detrimental for replicating cells, as it can mimic thymine. This can lead to the formation of an 8‐oxoG·A pair from 8‐oxoG·C during a first round of replication, and after a second round to a T·A pair instead of the original G·C. To avoid this, 8‐oxoG can be excised by the DNA glycosylase OGG1 before replication, or alternatively, the erroneous adenine can be removed by another glycosylase, the MutY homologue. 20 Thus, OGG1 is of great importance for the cells due to its role in excising 8‐oxoG from the DNA, therefore limiting mutations and strand breaks.
In addition to the glycosylase OGG1, the cells also have enzymes to prevent incorporation of new 8‐oxoG into the DNA from the nucleotide pool. MTH1 is a nudix hydrolase that sanitizes the nucleotide pool from oxidized deoxynucleoside triphosphates (dNTPs), by turning oxidized dNTPs into monophosphates (dNMPs). 21
Hence, both MTH1 and OGG1 are important for genomic stability and cell survival by regulating the amount of 8‐oxoG in the DNA (Figure 2). Both enzymes might also have additional roles in immune signalling.
FIGURE 2.
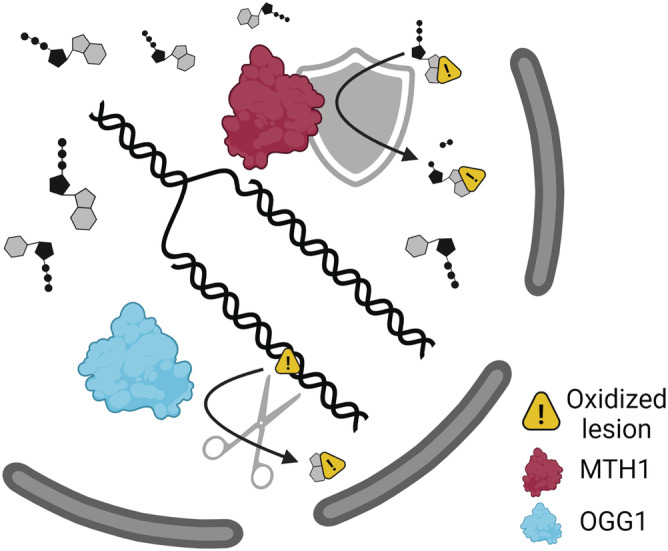
MTH1 and OGG1 eliminate 8‐oxoG from the DNA. MTH1 is a nudix hydrolase that turns oxidized dNTPs into dNMPs, therefore preventing their incorporation into the DNA. OGG1 is a DNA glycosylase that excises 8‐oxoG from the DNA. Figure created with BioRender.com and reprinted with permission and carefully modified from the thesis “the DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation” 8
4. MTH1 IS UP‐REGULATED IN BOTH CANCER CELLS AND RAPIDLY DIVIDING T CELLS
Limited numbers of studies have been performed regarding the role of MTH1 in inflammation, despite it being known since 1997 that MTH1 levels are increased in activated peripheral blood cells as compared to unstimulated cells. 22 The MTH1 levels were even higher in leukaemic cells, 22 as cancer cells have an altered redox balance and increased DNA lesions. Thus, MTH1 has been studied extensively in the context of cancer, as it has been suggested that many cancer cells have an increased need for detoxification by MTH1, making them vulnerable to MTH1 inhibition. However, the complete mechanism of action and role as a therapeutic target is still under investigation, as comprehensively reviewed by others. 23 , 24
In concordance with the findings that activated leukocytes have elevated MTH1 levels, 22 we showed that the MTH1 inhibitor TH1579 (also OXC‐101 or Karonudib) selectively kills activated CD3+ T cells, without having any detectable effect on resting T cells. 8 , 25 , 26 The correlation between ROS status and MTH1 levels was also determined, where the majority of in vitro CD3/CD28‐stimulated T cells had a higher ROS status and higher MTH1 levels than resting ones, whereas MTH1 inhibition drove the population towards an MTH1lowROSlow phenotype. This subset of T cells was not sensitive to MTH1 inhibition by TH1579 but was interestingly viable and proliferating, although not to the same extent as the MTH1highROShigh T cells. 25 We could thus conclude that MTH1 and ROS levels correlate with each other in T cells, but they are not indispensable for all activated T cells.
The mechanism of action of MTH1 inhibition in the sensitive T cells might not be fully known yet. In the context of cancer, emerging data suggest that the MTH1 protein could have a direct effect on mitosis. 27 Indeed, we showed that MTH1 inhibition induced DNA damage with increased 8‐oxoG levels and γH2AX foci in activated T cells, leading to cell cycle arrest in G2/M and apoptosis. Elevated levels of phosphorylated histone H3 suggested that also the mitotic spindle was disrupted as part of the toxicity. 25 However, the mitotic effect seen in cancer cells could alternatively be due to an MTH1‐independent effect by the inhibitors, as some studies have suggested regarding other MTH1 inhibitors. 28
In the context of inflammatory diseases, the elevated MTH1 levels seem to be highly specific for pro‐inflammatory cells, as the results suggest not only a prominent gap in sensitivity between resting and activated T cells but also an increased level in psoriatic skin as compared to normal skin tissue. 29 Furthermore, MTH1‐positive immune cells were overrepresented in liver samples of patients with autoimmune hepatitis and correlated with disease severity, although the hepatocytes barely expressed MTH1. 26 Altogether, this supports the hypothesis that MTH1 could be a promising target to treat inflammation and that its inhibition would be highly specific for the pro‐inflammatory cells.
The toxicity of the MTH1 inhibitor TH1579 has been assessed thoroughly, and the inhibitor is already in clinical phase I trials for solid tumours and leukaemia (NCT03036228 and NCT04077307, respectively). Data suggest that the drug candidate is well tolerated and has beneficial pharmacokinetic features. 30 In a rat model, it was demonstrated that any bone marrow suppressing effects are reversible, 25 and interestingly, T cells from TH1579‐treated mice were able to proliferate again upon treatment withdrawal. 25 The finding indicates that effector memory T cells are highly sensitive and suppressed upon treatment, which could further indicate that a significant part of the MTH1highROShigh T cells is effector memory T cells. However, this remains to be investigated.
MTH1 knockout mice are viable and healthy, with a slightly elevated risk of developing tumours, 31 and interestingly, MTH1 deficient T cells are able to proliferate, indicating that an important part of proliferating T cells can manage without MTH1. 26 This could support the claim that an MTH1 inhibitor would be more selective for pro‐inflammatory cells and associated with less side‐effects than many established T‐cell killing drugs.
5. MTH1 AS AN IMMUNOMODULATOR
Taken together, the role of MTH1 for inflammation is not fully known yet. The data described above suggest that inhibition of MTH1 selectively kills pro‐inflammatory T cells, indicating that MTH1 inhibitors like TH1579 could be used for similar indications as other T cell suppressing agents, like methotrexate and azathioprine. Just like these established drugs, TH1579 was initially designed for cancer treatment, where a T cell suppressive effect would be controversial, although not uncommon among many successful chemotherapies. However, it has been shown that MTH1 inhibition does not impair the ability of tumour infiltrating lymphocytes or in vitro‐stimulated T cells to kill cancer cells. 8 , 32 It could instead be speculated that the effect on T cell subsets and the increased ROS and DNA damage that the treatment induces in the target tissues could enhance the cytotoxic activity of T cells in cancer settings, but that remains to be further investigated. The role of an MTH1lowROSlow T cell phenotype in the context of cancer has not yet been explored.
Considering the redox dependency of T cell polarization, 9 , 10 , 11 and the association between MTH1 and oxidative stress in T cells, 25 it would be logical to assume that MTH1 inhibition could have a polarizing effect on T cell subsets. It was already demonstrated that IL‐17 and IL‐17‐producing γδT cells are suppressed upon activation, whereas Foxp3+ cells are rather unaffected. 26 , 29 In addition, the effector memory T cells were seemingly more sensitive than naïve T cells. This was demonstrated both by investigating CD44+CD62L+ murine T cells 25 , 26 and by investigating the proliferation of memory‐specific splenocytes after a treatment period with TH1579. 25 Whether this effect on different subtypes distinguishes MTH1 inhibitors from established T cell killing agents or not remains to be seen, but it illustrates that there might be important correlations between DNA repair and oxidative DNA modulations and certain immune phenotypes.
6. MTH1 INHIBITORS HAVE PROMISING EFFECTS IN DISEASE MODELS
When challenging MTH1 inhibitors in disease models of T cell‐driven diseases, a clinical effect was observed in murine models of psoriasis, 29 autoimmune hepatitis 26 and the multiple sclerosis model experimental autoimmune encephalomyelitis. 25 The MTH1 inhibitor alleviated disease progress and disease status. Most importantly, the effect was beneficial also when given after disease onset and not only prophylactically. 25
The fact that the inhibitor showed promising effects in several disease models could indicate usefulness in many T cell‐driven diseases, in addition to cancer treatment. The effect on T cells does not seem to be a disadvantage in cancer settings 8 , 32 but could instead possibly be an advantage by immunomodulation without preventing cytotoxic T cells from eliminating target cells and by direct cancer cell toxicity. What other potential indications there are for MTH1 inhibition remains to be investigated. Possibly, also, combinations of oncological and inflammatory conditions could be a subject for this, like Graft‐versus‐host‐disease after allogeneic stem cell transplantation.
In conclusion, MTH1 inhibitors are yet in an early stage of clinical trials but have shown promising pharmacokinetic and toxicologic properties, 25 , 30 with clinical efficacy yet to be investigated in further inflammatory settings. The effect of MTH1 inhibition seems to be highly specific for pro‐inflammatory T cells, driving the surviving T cells towards an MTH1lowROSlow phenotype and alleviating autoimmune disease (Figure 3).
FIGURE 3.
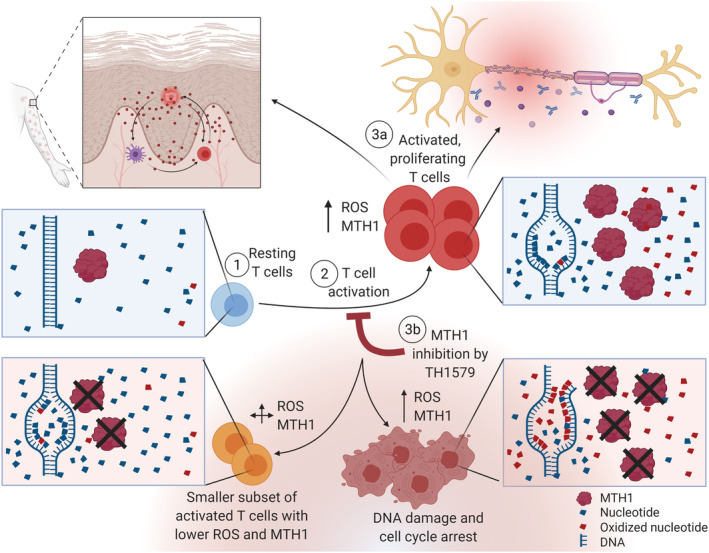
Inhibition of MTH1 alleviates T cell‐driven diseases by killing pro‐inflammatory cells and driving the T cell population towards an MTH1lowROSlow phenotype. When resting T cells (1) get activated (2), their ROS and MTH1 levels increase, ameliorating their survival, proliferation, cytokine secretion and cytotoxic activity in autoimmune diseases (3a). Upon MTH1 inhibition, the MTH1highROShigh cells die, driving the T cell population towards another phenotype, MTH1lowROSlow T cells (3b), with reduced disease activity in disease models of T cell‐driven diseases like psoriasis and experimental autoimmune encephalomyelitis. Figure created with BioRender.com and reprinted with permission from the thesis “The DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation” 8
7. OGG1 IS ASSOCIATED WITH INFLAMMATION
Just like MTH1, the other target reviewed in this MiniReview—OGG1—is up‐regulated in inflammatory diseases like multiple sclerosis and inflammatory bowel disease. 33 , 34 Several animal models have suggested that OGG1 knockout mice are inherently resistant to inflammation, like sepsis and airway inflammation. 35 , 36 The OGG1 knockout mice have an increased amount of 8‐oxoG in their genome and a slightly increased tumour rate but are otherwise viable and grow old. 37 This has made OGG1 an interesting drug target, and several OGG1 inhibitors have been developed with promising results. 38 , 39 , 40
Using murine in vivo models of aseptic pneumonia induced with lipopolysaccharide (LPS) and tumour necrosis factor‐α (TNF‐α), we showed that the OGG1 inhibitor TH5487 (other name OXC‐201) rapidly suppresses neutrophil infiltration in the lungs. 40 The effect was strongest when given prophylactically, but a clear, time‐dependent effect was also seen when given up to 9 h after TNF‐α stimulation, when the neutrophil infiltration was already increased.
These results could indicate that OGG1 inhibition alters early inflammatory signalling and that the effect on the neutrophils is secondary, due to decreased levels of chemotactic cytokines. The data also show that many pro‐inflammatory genes are suppressed both in vivo and in vitro, 40 which could support this claim. However, the direct effect on neutrophils has not been studied with TH5487. Therefore, a direct neutrophil‐dependent effect, in addition to an indirect chemotactic effect through cytokine suppression, cannot be excluded.
We demonstrated a broad cytokine suppressing effect on both murine cells and human cells, where OGG1 inhibition diminished a wide array of pro‐inflammatory gene expression. Importantly, we also knocked out OGG1 with CRISPR/Cas9 to prove that the cytokine suppressing effect in knockout cells was comparable to the inhibitor‐treated cells. 40
The exact mechanism of how OGG1 is involved in inflammation has been studied extensively before, and it has been suggested that the interaction of OGG1 to its substrate 8‐oxoG in DNA is needed for inflammatory signalling and ameliorates gene transcription. 41 Not only the catalytic activity is important but also the DNA‐binding site of OGG1 is thought to play an important role, by assembling the transcription machinery. 40 , 41 This was demonstrated using the OGG1 inhibitor TH5487, which prevented OGG1 from binding to the guanine‐rich promoter regions of pro‐inflammatory genes, inhibiting the expression of pro‐inflammatory genes. 40 It was also later confirmed that a catalytically dead mutant of OGG1, K249Q, able to bind to 8‐oxoG but not excise it from the DNA, resulted in even higher pro‐inflammatory gene expression. 42 However, this does not exclude additional downstream effects of OGG1 on inflammation.
8. SUPPRESSING INFLAMMATION WITHOUT SUPPRESSING LYMPHOCYTES
In addition to acute pneumonia induced by LPS or TNF‐α, emerging data suggest that OGG1 inhibition by TH5487 attenuates lung fibrosis in a bleomycin model of pulmonary fibrosis. 43 This could indicate that also chronic inflammation can be mitigated by OGG1 inhibition. One challenge with immunosuppression in the context of both acute and chronic inflammation is that the immunosuppression itself can have adverse effects. It is widely known that immunosuppression caused by medication, infections like HIV or even ageing can lead to life‐threatening infections and cancer in the long run. 2 , 44 It is thus important to not suppress immunity too strongly or non‐specifically when developing new clinically relevant drug candidates for inflammatory diseases.
In the bleomycin and LPS/TNF‐α models, no infectious agent was used, and so a comparable effect of anti‐inflammatory drugs like dexamethasone would have been expected, which was also demonstrated with preliminary data. 8 , 43 However, albeit being indispensable anti‐inflammatory drugs, corticosteroids impose severe risks of opportunistic or exacerbated infections and have long been considered controversial in the context of acute infectious inflammation, like sepsis. 45 , 46 One important consequence of corticosteroids is the disadvantageous effect on T cells, where dexamethasone suppresses T cell proliferation and differentiation. 47 Interestingly, OGG1 inhibition with TH5487 did not suppress T cells in concentrations relevant for immunosuppression. 8 , 48 This could indicate a clear advantage of OGG1 inhibitors over corticosteroids, where OGG1 inhibition could rapidly suppress acute inflammation, but without suppressing the lymphocytes (Figure 4).
FIGURE 4.
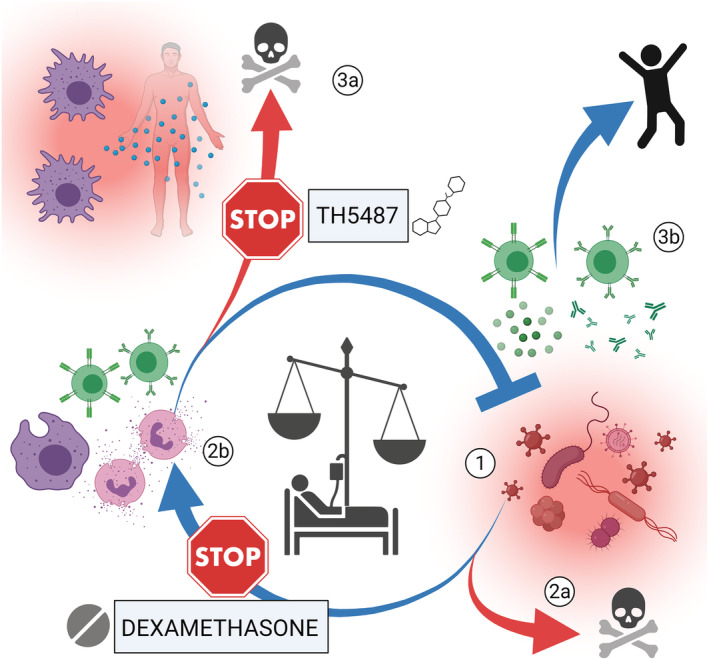
OGG1 inhibition could suppress inflammation like dexamethasone, but without suppressing lymphocytes. When pathogens enter the body (1), a proper immune response (2b) is needed to avoid death (2a). In sepsis, the immune response is dysregulated and disproportional to the microbes, leading to organ failure and potentially death (3a). With a properly regulated immune reaction, where B and T cells play important roles, the pathogen can instead be cleared out (3b). Dexamethasone is known to have a wide immunosuppressing effect, but it also suppresses the T cells, which makes it controversial in infectious inflammation. Our studies suggest that the OGG1 inhibitor is non‐toxic to T cells, which could be a great advantage in infectious diseases. Figure created with BioRender.com and reprinted with permission from the thesis “The DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation” 8
To further support this hypothesis, a study from 2020 shows that OGG1 inhibition has a beneficial effect in a bacterial murine model of pneumonia, where Pseudomonas aeruginosa was used as the infectious agent. 49 This is an important finding, as it could benchmark a difference to corticosteroids, by being fully suitable for infectious diseases. As TH5487 and other OGG1 inhibitors have additionally been suggested to inhibit viral replication, 50 it would be intriguing to challenge OGG1 inhibitors in viral pneumonia models, to investigate whether OGG1 inhibition could be a superior alternative to corticosteroids for viral and bacterial pneumonia in the future. This could potentially be an important additional tool in the arsenal of treatment options against current and future infectious diseases.
9. CONCLUSION
The DNA repair enzymes MTH1 and OGG1 emerge as interesting targets to treat inflammatory conditions like T cell‐driven diseases and acute inflammation. For both targets, cellular mechanisms of action and efficacy in vivo have been demonstrated using small‐molecule inhibitors, with promising results. The toxicity of the compounds was generally low, and both targets can be knocked out in mice with minimal consequences and no effect on viability, which is promising as new treatment options with few side‐effects are needed. If OGG1 inhibition would indeed suppress inflammation with the same efficacy as corticosteroids, but with less side‐effects, it would be a very useful therapy. There are a vast amount of T cell killing agents available today, but most of them are not specific for activated T cells, but instead all rapidly dividing cells, which could give MTH1 inhibition an important role among immunomodulating drugs.
In addition to investigating the drug candidates, the thesis “The DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation” also proposed a mechanism of how inhibition of OGG1 prevents the assembly of the transcription machinery, providing more insight to how one of the most common oxidative lesions of the DNA induces inflammation, and how it can be alleviated. The thesis and articles therein also demonstrated some novel T cell biology with the identification of proliferating MTH1lowROSlow T cells. The significance of such a subgroup is yet to be elucidated, as is its association to the different already known T cell subsets. Considering all currently used cancer drugs that induce DNA damage and affect DNA repair, 4 it would be intriguing to investigate the MTH1 levels of T cells of both treated and untreated patients.
DNA repair pathways and DNA damage‐inducing agents have classically been investigated mainly for cancer therapeutics, both with chemotherapeutics and radiotherapy, but many established cancer therapeutics could also have immunomodulating effects as part of their therapeutic actions. This could partly explain the occasional clinical mismatch between tumour characterization and patient response. It is therefore intriguing that DNA repair and immunity are gaining more and more attention over the years, and potentially, drugs affecting the DNA repair pathways will constitute an important group of immunomodulating therapeutics in the future.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGEMENTS
The supervisors of this PhD project are acknowledged for their work: Thanks to Prof. Thomas Helleday, Dr. Ulrika Warpman Berglund, Assoc. Prof. Karin Cederbrant, Dr. Christina Kalderén, Dr. Torkild Visnes, Dr. Lars Bräutigam and Dr. Roland Fiskesund. Thank you, Petra Marttila for writing support on this MiniReview. Thanks to all co‐workers of the Helleday laboratory and the collaborators in the groups of Prof. Istvan Boldogh and Prof. Charlotta Enerbäck. Thanks to all blood donors who donated blood in the Stockholm county area, Sweden, during 2015–2021, and allowed their buffy coats to be used for research.
Karsten S. Targeting the DNA repair enzymes MTH1 and OGG1 as a novel approach to treat inflammatory diseases. Basic Clin Pharmacol Toxicol. 2022;131(2):95‐103. doi: 10.1111/bcpt.13765
Funding informationThe work of this MiniReview was funded by the Department of Clinical Immunology and Transfusion Medicine, Karolinska University Hospital, Stockholm, Sweden. The previous PhD work was funded by the Karolinska Institute, Stockholm, Sweden, via its CSTP programme and research internship programme for medical doctors (forskar‐AT), while the articles of the PhD thesis were funded by sources specified in each article.
Funding information Department of Clinical Immunology and Transfusion Medicine, Karolinska University Hospital; Karolinska Institute
REFERENCES
- 1. Abate KH, Abebe Z, Abil OZ, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet (British Edition). 2018;392(10159):1789‐1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63‐80. doi: 10.1016/j.cell.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 3. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. The Lancet (British Edition). 2018;392(10141):75‐87. doi: 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 4. Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8(3):193‐204. doi: 10.1038/nrc2342 [DOI] [PubMed] [Google Scholar]
- 5. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363‐383. doi: 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- 6. Poliezhaieva T, Ermolaeva MA. DNA damage in protective and adverse inflammatory responses: Friend of foe? Mech Ageing Dev. 2017;165(Pt A):47‐53. doi: 10.1016/j.mad.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 7. Pateras IS, Havaki S, Nikitopoulou X, et al. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol Ther. 2015;154:36‐56. doi: 10.1016/j.pharmthera.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 8. Karsten S. The DNA repair enzymes MTH1 and OGG1 as targets to treat inflammation. Inst för onkologi‐patologi/Dept of Oncology‐Pathology; 2021. [Google Scholar]
- 9. Yarosz EL, Chang C‐H. The role of reactive oxygen species in regulating T cell‐mediated immunity and disease. Immune Network. 2018;18(1):e14. doi: 10.4110/in.2018.18.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. doi: 10.1186/s12929-015-0194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simeoni L, Bogeski I. Redox regulation of T‐cell receptor signaling. Biol Chem. 2015;396(5):555‐568. doi: 10.1515/hsz-2014-0312 [DOI] [PubMed] [Google Scholar]
- 12. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345‐1360. doi: 10.1084/jem.20151159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927‐8930. doi: 10.1158/0008-5472.CAN-06-1501 [DOI] [PubMed] [Google Scholar]
- 15. Loo TT, Gao Y, Lazarevic V. Transcriptional regulation of CD4(+) TH cells that mediate tissue inflammation. J Leukoc Biol. 2018;104(6):1069‐1085. doi: 10.1002/JLB.1RI0418-152RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB j. 2003;17(10):1195‐1214. doi: 10.1096/fj.02-0752rev [DOI] [PubMed] [Google Scholar]
- 17. Zaki‐Dizaji M, Akrami SM, Azizi G, Abolhassani H, Aghamohammadi A. Inflammation, a significant player of Ataxia‐Telangiectasia pathogenesis? Inflamm Res. 2018;67(7):559‐570. doi: 10.1007/s00011-018-1142-y [DOI] [PubMed] [Google Scholar]
- 18. Pazzaglia S, Pioli C. Multifaceted role of PARP‐1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non‐cancer diseases. Cells (Basel, Switzerland). 2019;9(1):41. doi: 10.3390/cells9010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ba X, Aguilera‐Aguirre L, Rashid QTAN, et al. The role of 8‐oxoguanine DNA glycosylase‐1 in inflammation. Int J Mol Sci. 2014;15(9):16975‐16997. doi: 10.3390/ijms150916975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Rosa M, Johnson SA, Opresko PL. Roles for the 8‐oxoguanine DNA repair system in protecting telomeres from oxidative stress. Front Cell Dev Biol. 2021;9:758402. doi: 10.3389/fcell.2021.758402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakumi K, Furuichi M, Tsuzuki T, et al. Cloning and expression of Cdna for a human enzyme that hydrolyzes 8‐Oxo‐Dgtp, a mutagenic substrate for DNA‐synthesis. J Biol Chem. 1993;268(31). PMID: 23524‐23530. [PubMed] [Google Scholar]
- 22. Oda H, Nakabeppu Y, Furuichi M, Sekiguchi M. Regulation of expression of the human MTH1 gene encoding 8‐oxo‐dGTPase. Alternative splicing of transcription products. J Biol Chem. 1997;272(28):17843–17850. doi: 10.1074/jbc.272.28.17843 [DOI] [PubMed] [Google Scholar]
- 23. Yin Y, Chen F. Targeting human MutT homolog 1 (MTH1) for cancer eradication: current progress and perspectives. Acta Pharmaceutica Sinica B. 2020;10(12):2259‐2271. doi: 10.1016/j.apsb.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samaranayake GJ, Huynh M, Rai P. MTH1 as a chemotherapeutic target: the elephant in the room. Cancer. 2017;9(5):47. doi: 10.3390/cancers9050047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karsten S, Fiskesund R, Zhang X‐M, et al. MTH1 as a target to alleviate T cell driven diseases by selective suppression of activated T cells. Cell Death Differ. 2021;29(1):246‐261. doi: 10.1038/s41418-021-00854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y, Hua X, Huang B, et al. MutT homolog 1 inhibitor karonudib attenuates autoimmune hepatitis by inhibiting DNA repair in activated T cells. Hepatol Commun. 2021. doi: 10.1002/hep4.1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gad H, Mortusewicz O, Rudd SG, et al. MTH1 promotes mitotic progression to avoid oxidative DNA damage in cancer cells. bioRxiv. 2019. doi: 10.1101/575290 [DOI] [Google Scholar]
- 28. Kawamura T, Kawatani M, Muroi M, et al. Proteomic profiling of small‐molecule inhibitors reveals dispensability of MTH1 for cancer cell survival. Sci Rep. 2016;6(1):26521. doi: 10.1038/srep26521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bivik Eding C, Köhler I, Verma D, et al. MTH1 inhibitors for the treatment of psoriasis. J Investig Dermatol. 2021;141(8):2037‐2048.e4. doi: 10.1016/j.jid.2021.01.026 [DOI] [PubMed] [Google Scholar]
- 30. Warpman Berglund U, Sanjiv K, Gad H, et al. Validation and development of MTH1 inhibitors for treatment of cancer. Ann Oncol. 2016;27(12):2275‐2283. doi: 10.1093/annonc/mdw429 [DOI] [PubMed] [Google Scholar]
- 31. Teruhisa T, Akinori E, Hisato I, et al. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8‐Oxo‐dGTPase. Proc Natl Acad Scie ‐ PNAS. 2001;98(20) 11456–11461: doi: 10.1073/pnas.191086798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Einarsdottir BO, Karlsson J, Soderberg EMV, et al. A patient‐derived xenograft pre‐clinical trial reveals treatment responses and a resistance mechanism to karonudib in metastatic melanoma. Cell Death Dis. 2018;9(8):810. doi: 10.1038/s41419-018-0865-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahraian MA, Soltani BM, Behmanesh M. Alteration of OGG1, MYH and MTH1 genes expression in relapsing‐remitting multiple sclerosis patients. Physiology and Pharmacology. 2017;21(2):129‐136. [Google Scholar]
- 34. Kumagae Y, Hirahashi M, Takizawa K, et al. Overexpression of MTH1 and OGG1 proteins in ulcerative colitis‐associated carcinogenesis. Oncol Lett. 2018;16(2):1765‐1776. doi: 10.3892/ol.2018.8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, Szabo C. Potential role for 8‐oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005;19(2):290‐292. doi: 10.1096/fj.04-2278fje [DOI] [PubMed] [Google Scholar]
- 36. Li GP, Yuan KF, Yan CG, et al. 8‐Oxoguanine‐DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL‐4 in cells and in mice. Free Radic Biol Med. 2012;52(2):392‐401. doi: 10.1016/j.freeradbiomed.2011.10.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klungland A, Rosewell I, Hollenbach S, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96(23):13300‐13305. doi: 10.1073/pnas.96.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donley N, Jaruga P, Coskun E, Dizdaroglu M, McCullough AK, Lloyd RS. Small molecule inhibitors of 8‐oxoguanine DNA glycosylase‐1 (OGG1). ACS Chem Biol. 2015;10(10):2334‐2343. doi: 10.1021/acschembio.5b00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Y‐k T, Auld D, Ji D, et al. Potent and selective inhibitors of 8‐oxoguanine DNA glycosylase. J Am Chem Soc. 2018;140(6):2105‐2114. doi: 10.1021/jacs.7b09316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Visnes T, Cazares‐Korner A, Hao WJ, et al. Small‐molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science. 2018;362(6416):834. doi: 10.1126/science.aar8048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ba X, Boldogh I. 8‐oxoguanine DNA glycosylase 1: beyond repair of the oxidatively modified base lesions. Redox Biol. 2018;14:669‐678. doi: 10.1016/j.redox.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hao W, Wang J, Zhang Y, et al. Enzymatically inactive OGG1 binds to DNA and steers base excision repair toward gene transcription. FASEB J. 2020;34(6):7427‐7441. doi: 10.1096/fj.201902243R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanner L, Single AB, Bonghir RKV, et al. Small‐molecule‐mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis. bioRxiv. 2021. doi: 10.1101/2021.02.27.433075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta‐analysis. Lancet. 2007;370(9581):59‐67. doi: 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 45. Ni YN, Liu YM, Wang YW, Liang BM, Liang ZA. Can corticosteroids reduce the mortality of patients with severe sepsis? A systematic review and meta‐analysis. Am J Emerg Med. 2019;37(9):1657‐1664. doi: 10.1016/j.ajem.2018.11.040 [DOI] [PubMed] [Google Scholar]
- 46. Cooper AS. Corticosteroids for treating sepsis in children and adults. Crit Care Nurse. 2020;40(4):83‐84. doi: 10.4037/ccn2020588 [DOI] [PubMed] [Google Scholar]
- 47. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone‐induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. doi: 10.1186/s40425-018-0371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Visnes T, Benítez‐Buelga C, Cázares‐Körner A, et al. Targeting OGG1 arrests cancer cell proliferation by inducing replication stress. Nucleic Acids Res. 2020;48(21):12234‐12251. doi: 10.1093/nar/gkaa1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin S, Lin P, Wu Q, et al. Small‐molecule inhibitor of 8‐oxoguanine DNA glycosylase 1 regulates inflammatory responses during pseudomonas aeruginosa infection. J Immunol (1950). 2020;205(8):2231‐2242. doi: 10.4049/jimmunol.1901533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pettke A, Tampere M, Pronk R, et al. Broadly active antiviral compounds disturb zika virus progeny release rescuing virus‐induced toxicity in brain organoids. Viruses. 2021;13(1):37. doi: 10.3390/v13010037 [DOI] [PMC free article] [PubMed] [Google Scholar]


