Abstract
Stroke is the leading cause of disability and death. When blood flow is restored after prolonged ischemia and hypoxia, it leads to excessive production of reactive oxygen species (ROS), increased local inflammation, and apoptosis, which are the cause of most cerebral ischemia reperfusion injury (CIRI), leading to secondary brain tissue damage. Edaravone dexborneol is a novel neuroprotective agent consisting of edaravone and borneol. Studies have shown that it has synergistic antioxidant and anti‐inflammatory effects. However, whether Edaravone dexborneol stimulates the Nrf2/HO‐1 pathway to regulate NADPH oxidase 2 (NOX2) remains unclear. In this study, wild‐type (WT) mice and Nrf2 knockout (KO) mice were used to investigate the antioxidant, anti‐inflammatory, and anti‐apoptotic effects of Edaravone dexborneol on CIRI and its mechanism. The cognitive function of mice was evaluated with the Morris water maze (MWM), test and the cell structures of hippocampus were observed by hematoxylin and eosin (H&E) staining. Nrf2, HO‐1, and NOX2 proteins and apoptosis‐related proteins Bcl‐2, Bax, and Caspase 3 were detected by western blotting. Nrf2, HO‐1, NOX2, and inflammatory factors TNF‐α, IL‐1β, IL‐4, and IL‐10 were detected by real‐time polymerase chain reaction. The results showed that Edaravone dexborneol treatment improved learning and memory performance, neuronal damage, and enhanced antioxidant, inflammation, and apoptosis in CIRI mice. In addition, Edaravone dexborneol induced the activation Nrf2/HO‐1 signaling pathway activation while inhibiting NOX2 expression. Overall, these results indicate that Edaravone dexborneol ameliorates CIRI‐induced memory impairments by activating Nrf2/HO‐1 signaling pathway and inhibiting NOX2.
Keywords: cerebral ischemia reperfusion injury, Edaravone dexborneol, inflammation, NADPH oxidase 2, Nrf2/HO‐1 pathway
1. INTRODUCTION
Stroke is the second leading cause of death, including ischemic stroke, which accounts for approximately 79% of all strokes globally, and hemorrhagic stroke [1]. Most ischemic strokes occur when blood flow is stopped due to a blood clot (thrombosis or embolism) that causes vascular insufficiency. The current available treatment is through endovascular recanalization and intravenous thrombolysis, which both reduce disability, but with a time critical [2]. Cerebral ischemia reperfusion injury (CIRI) may occur after blood flow reestablished, leading to a series of pathological changes, such as oxidative stress, inflammation, ionic imbalance, and bioenergetics failure [3], and eventually ends with neuronal loss and progressive learning and memory impairment, which results in vascular cognitive impairment (VCI) [4]. Thus, improving the pathological mechanism after recanalization is an important means to reduce brain tissue damage. Although the specific mechanisms of VCI have not been completely cleared, oxidative stress plays a pivotal player in the signaling cascade of CIRI and has been reported as one of the important mechanisms of cerebral injury [5].
Nrf2, as a key transcription factor, involves cellular antioxidant damage. Increased expression of NAD(P)H‐quinone oxidoreductase‐1 (NQO‐1), heme oxygenase‐1 (HO‐1), and glutamate‐cysteine ligase catalytic subunit (Gclc) has been often used to measure Nrf2 transcription factor activation [6]. Nrf2/HO‐1 plays an important role in CIRI injury, and the activation of Nrf2/HO‐1 pathway can reduce oxidative stress and inflammation caused by ischemia reperfusion injury, restore neurological function defects, and reduce the volume of cerebral infarction [7]. Mitochondria and NADPH oxidase are the main sources of reactive oxygen species (ROS) [8]. A large number of studies have confirmed that NADPH oxidase 2 (NOX2) is the main source of ROS in the brain [9]. Wei et al. found that Nrf2 suppresses ROS overproduction by regulating NOX2 and may promote repair angiogenesis in ischemic retinopathy and other cardiovascular conditions [10]. In addition, in vivo and in vitro studies, CIRI models have confirmed that by mediating Nrf2, NOX4 could be regulated against oxidative stress and neuronal apoptosis [11]. Therefore, antioxidant therapy targeting Nrf2 and NOX2 may play an important role in the treatment of CIRI‐induced injury.
Edaravone, an effective free radical scavenger, scavenges hydroxyl‐free radical, peroxy‐free radical, and superoxide‐free radicals, relieves cerebral edema, inhibits oxidative damage and delayed neuronal death, sequentially improves ischemic stroke [12, 13, 14]. The drug has been recommended by Chinese and Japanese stroke care guidelines for the treatment of acute ischemic stroke (AIS) [15, 16]. (+)‐Bornel, a naturally lipid‐soluble bicyclic terpene chemical, has been found to inhibit the production of inflammatory factors and antioxidant stress and preserve brain function in preclinical investigations [17, 18]. Edaravone dexborneol (EDB), comprised of edaravone and (+)‐bornel in a 4:1 ratio, has been tested as a novel neuroprotective agent. Pharmacological research showed that compared with edaravone bornel alone, EDB had synergistic effects and had more protective effects on CIRI [19]. Phase II and Phase III studies on the safety and efficacy of EDB versus edaravone in patients with AIS showed a positive trend of improved functional outcomes when administered of EDB within 48 h after AIS and 90 days after AIS [20, 21]. However, so far, no study has examined the potential efficacy of EDB on cognitive function after CIRI treatment and whether the protective effects of EDB in CIRI are correlated to oxidative stress.
In the current study, we investigated whether EDB might protect CIRI‐induced cognitive impairment by improving CIRI‐induced oxidative stress, inflammation, and apoptosis. These results suggest that EDB may have an effective therapeutic agent on CIRI injury.
2. MATERIALS AND METHODS
2.1. Animal treatments
Nrf2 knockout (KO) mice were obtained from Dr Thomas W. Kensler laboratory (Johns Hopkins University, Baltimore, America). Male ICR mice (6–8 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (China). ICR WT (Nrf2+/+) and Nrf2 KO (Nrf2−/−) mice were obtained through breeding of heterozygotes (Nrf2+/−). All the animals were raised in an environment with temperature of 22 ± 1°C, relative humidity of 50 ± 1%, a light/dark cycle of 12/12 h, and were allowed free access to water and food. The experimental procedures were carried out in accordance with the requirements and principles of the Animal Care Management Committee of Hebei General Hospital (license number: SCXK2016‐0006).
2.2. Chemicals
EDB, a compound preparation comprising edaravone and (+)‐borneol in a 4:1 ratio. Edaravone (Lot No. Fd‐242a‐200 601) and dexborneol (Lot No. S‐S044–190102) were provided by Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd. (Nanjing, China). The extract was directly diluted with saline.
2.3. Establishment of a mice model of CIRI
Repeated CIRI was performed under aseptic conditions via bilateral common carotid artery occlusion (BCCAO) according to the previously described method [22] with some modifications. The mice were anesthetized with 10% chloral hydrate (300 mg/kg, ip). After being anesthetized, mice were placed in a supine position, the skin was disinfected with iodine and a vertical mid‐line incision was made in the neck. The bilateral common carotid arteries of mice were exposed with a mid‐line ventral incision, carefully isolated from the vagal nerves. The bilateral common carotid arteries were ligated with 4–0 silk sutures for 20 min. At the end of the occlusion period, the suture was untied to ensure blood flow. After the blood flow had been restored for 10 min, the bilateral common carotid arteries were ligated again for 20 min. This pattern was repeated three times. After the operation, all the mice were kept in cages with plenty of food and water after their vital signs were stable. For sham‐operated animals, only bilateral common carotid arteries were isolated without ischemia reperfusion.
2.4. Groups and animal treatments
The mice were randomly distributed into six groups: sham (sham‐operated + saline) group, CIRI (CIRI + saline) group, CIRI + EDB (CIRI + 10 mg/kg of EDB treatment model group) group, KO sham (KO sham‐operated + saline) group, KO CIRI (KO CIRI + saline) group, KO CIRI + EDB (KO CIRI + 10 mg/kg of EDB treatment model group) group. Each group was investigated 4 weeks after the operation. EDB (dissolved with 1,2 propanediol and saline) or saline was administered via intraperitoneal injection everyday (24 h) after CIRI.
2.5. Morris water maze test (MWM test)
All the mice were given the MWM test to assess their spatial memory and learning abilities 4 weeks after surgery. The water maze (Mobile Datum Information Technology Co., Ltd, Shanghai, China) consisted of a diameter of 120 cm and a height of 45 cm. The test water temperature was set at 23 ± 1°C. The escape platform was submerged 1 cm under the surface of the water. Simultaneously, the camera system automatically recorded the escape latency and transfer parameters to an electronic image analyzer (Mobile Datum Information Technology Co., Ltd, Shanghai, China). The time taken to find the platform (escape latency) was recorded for each mouse. If the mice failed to locate the platform within the allotted time (60 s), they were guided by an experimenter and placed on the platform for 15 s. In this case, the escape latency was recorded as 60 s. All experimental mice were trained four times for five consecutive days. On the sixth day of the MWM test, the mice entered the spatial probe test. The platform was removed, and the experimental mouse was released from the quadrant opposite to the target quadrant and allowed to search for the platform for 60 s. The time each mouse spent in each quadrant and the number of times across the platform were recorded.
2.6. Pathological examination by hematoxylin and eosin (H&E) staining
After the behavioral evaluation, mice were anesthetized in abdominal cavity (10% chloral hydrate [300–400 mg/kg, ip]). Rapid cardiac perfusion was first performed with isotonic sodium chloride solution, followed by rapid perfusion for about 2 min and slow perfusion for approximately 8 min with 4% paraformaldehyde (PFA). Then, the brain was decapitated, removed, and placed in a 4% PFA solution at 4°C for 24 h. After fixation, paraffin embedding was performed routinely. Serial coronal sections approximately 8 μm thick and stained with H&E were obtained from the specimens according to the manufacturer's instructions. Changes in neuronal morphology were assessed by examining H&E‐stained histological sections.
2.7. Examination of oxidative stress parameters (SOD and MDA)
The activities of SOD and the levels of MDA were measured by using commercial assay kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China).
2.8. Western blot analysis
Following behavioral testing, mice from each group were randomly chosen to be decapitated under deep anesthesia, and the bilateral hippocampal tissues were quickly dissected on the ice board. Protein was extracted with RIPA buffer (Solar bio, Beijing, China) following the manufacturer's instructions. The tissues were subjected to centrifugation at 4°C, 12 000 rpm for 10 min. Samples were separated using 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membranes (PVDF) (Millipore Corporation, United States). Then, 5% skim milk in 0.1% Tween 20 in TBS (TBST) was used to block nonspecific binding for 1 h at room temperature. The blots were incubated overnight at 4°C with antibodies against Nrf2 (1:1000 Proteintech), HO‐1 (1:1000 Proteintech), NOX2 (1:1000 Proteintech), Bax (1:1000 ZEN BIO), Bcl‐2(1:1000 ZEN BIO), Caspase‐3 (1:1000 ZEN BIO), β‐actin (1:3000 ZEN BIO) as the loading control. On the next day, the membranes were washed with TBST (10 min × 3) and incubated with the secondary antibody (goat anti‐rabbit IgG, 1:3000, ZEN BIO) for 1 h. After washing the membranes, the relative densities of the protein bands were analyzed using an Odyssey imaging system (Li‐Cor Biosciences, Lincoln, NE, USA) and quantified using ImageJ analysis software (National Institutes of Health, Bethesda, MD).
2.9. Real‐time polymerase chain reaction (RT‐PCR)
Total RNA was isolated from hippocampus tissues using TRIzol reagents (Shanghai Generay Biotech Co., Ltd) and reverse transcribed into complementary DNA (cDNA) according to manufacturer's instructions. The cDNA was amplified using the Reverse Transcription System (Promega Corporation, an affiliate of Promega [Beijing] Biotech Co., Ltd.) and the specific primers on a Roche Light Cycler 480II system (Roche Diagnostics GmbH, Mannheim, Germany). The relative abundance of mRNA transcripts was calculated using the 2−ΔΔCT method. The specific primers used for the current study are listed in Table 1.
TABLE 1.
Primers sequences for RT‐PCR
| Primers for RT‐PCR (5′–3′) | ||
|---|---|---|
| Nrf2 | Forward | AAAGCACAGCCAGCACATTC |
| Reverse | TGGGATTCACGCATAGGAGC | |
| HO‐1 | Forward | GAACCCAGTCTATGCCCCAC |
| Reverse | GGCGTGCAAGGGATGATTTC | |
| NOX2 | Forward | GTCACACCCTTCGCATCCATTCTCAAGTCAGT |
| Reverse | CTGAGACTCATCCCAGCCAGTGAGGATG | |
| TNF‐α | Forward | CTTCTGTCTACTGAACTTCGGG |
| Reverse | CAGGCTTGTCACTCGAATTTTG | |
| IL‐1β | Forward | GAAATGCCACCTTTTGACAGTG |
| Reverse | TGGATGCTCTCATCAGGACAG | |
| IL‐4 | Forward | CGAATGTACCAGGAGCCATATC |
| Reverse | TCTCTGTGGTGTTCTTCGTTG | |
| IL‐10 | Forward | AGCCGGGAAGACAATAACTG |
| Reverse | GGAGTCGGTTAGCAGTATGTTG | |
| GAPDH | Forward | ATGTTCCAGTATGACTCCACTCACG |
| Reverse | GAAGACACCAGTAGACTCCACGACA | |
2.10. Statistical analysis
Statistical analysis was performed by the SPSS software 22.0. All data were performed using the GraphPad Prism 8.0 software package and presented as the mean ± standard error of the mean (SEM). Intergroup differences in the escape latency in the MWM training task were evaluated using repeated measures two‐way analysis of variance (ANOVA), followed by Fisher's LSD test for multi‐group comparisons. The other results were compared among groups using one‐way ANOVA. Differences were considered significant at p < 0.05.
3. RESULTS
3.1. EDB improved learning and memory in CIRI mice
The MWM test was conducted to assess recognition memory and spatial learning and memory in mice. As shown in Figure 1b, all mice that were tested showed a progressive reduction in escape latency with training, which demonstrated that the mice learned the location where the platform had been placed. Compared with the sham group, the escape latency of mice in the CIRI group was significantly prolonged, suggesting that CIRI had a negative effect on learning. The administration of EDB mitigated the poor performances and the escape latency of mice in the CIRI+EDB group was significantly shortened, suggesting that this drug may improve CIRI‐induced cognitive impairment. Compared with the KO sham group, the escape latency of KO CIRI group was prolonged. Compared with the KO CIRI group, the escape latency of the KO CIRI + EDB group was shortened, but the difference was not statistically significant. Subsequent comparisons showed significant differences in the time target quadrant and the number of times crossing the platform between groups during the probe trials (Figure 1c–d). Mice in the CIRI group and KO CIRI group spent less time in the target quadrant and the number of times crossing the platform than mice in the sham group and KO sham group. Furthermore, significant improvements were observed in the EDB‐treated groups. However, differences were not observed between EDB treated with KO mice. These results indicated that EDB could alleviate CIRI‐induced memory impairment in mice.
FIGURE 1.
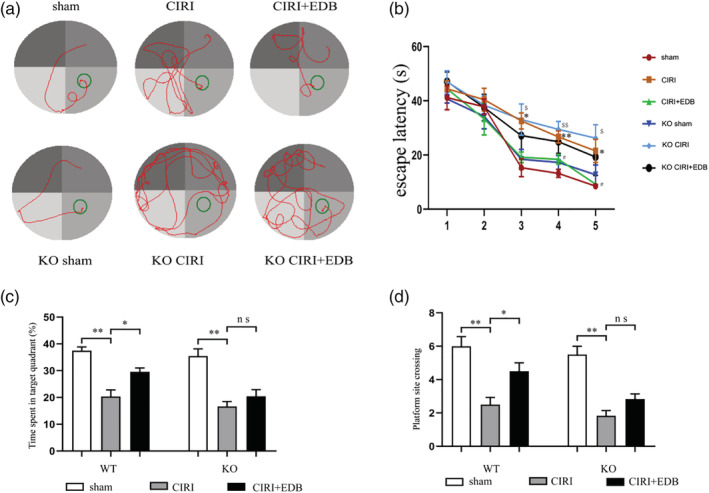
Effect of EDB on spatial reference memory in the MWMT in mice. (a) Representative swimming tracks of mice at Day 5 in different groups. (b) Mean daily escape latencies (time from the starting to the hidden platform). (c) The percentage of time spent in the target quadrant during the probe trial. (d) Numbers of crossing platform site spent in target quadrant during the probe trials. (*) significant difference (* P < 0.05 and ** P < 0.01) vs. sham group; (#) significant difference (# P < 0.05) vs. CIRI group; ($) significant difference ($ P < 0.05 and $$ P < 0.01) vs. KO sham group;* P < 0.05;** P < 0.01; ns no statistical significance. N = 12 mice per group
3.2. EDB significantly attenuated pathological change in the CA1 region of the hippocampus
Representative H&E staining was shown in Figure 2. The results revealed that the pyramidal neurons in the CA1 region of the hippocampus of mice were highly ranked in order, clearly stained, and moderate in size with normal micro structure in the sham group and KO sham group. Mice in the CIRI group and KO CIRI group showed obvious pathological changes, with loose arrangement of neurons and shallow staining, as well as obvious atrophy and loss of neurons. The administration of EDB reversed these morphological changes, but there were no notable changes in neuronal morphology in the KO CIRI + EDB group.
FIGURE 2.
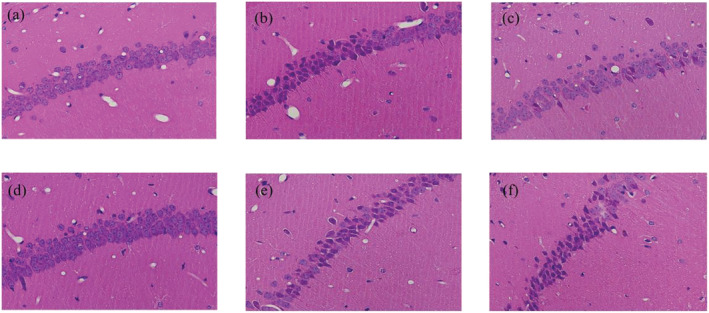
Hematoxylin and eosin (H&E) staining shows the effect of EDB on morphological changes in the mice hippocampus CA1 area. (a) Sham group. (b) CIRI group. (c) CIRI + EDB group. (d) KO sham group. (e) KO CIRI group. (f) KO CIRI + EDB group (magnification, ×400)
3.3. EDB alleviated oxidative damage in the hippocampus of mice with CIRI
To further evaluate the efficacy of reducing oxidative stress after EDB treatment, we measured the biochemical markers of oxidative stress parameters, including MDA level and SOD activity. As shown in Figure 3, the level of MDA was significantly increased, and the activity of SOD was significantly decreased in the hippocampus of the CIRI group compared to those in the hippocampus of the sham group, so oxidative stress injury was induced by CIRI treatment. Treatment of EDB showed a significant decrease in the level of MDA, meanwhile the activity of SOD was significantly increased in the EDB group compared with those in the CIRI group. In contrast, the improvement of SOD activity and MDA level was suppressed by Nrf2 KO. The level of MDA was significantly increased and the activity of SOD was significantly decreased in the hippocampus of the KO CIRI group compared to those in the hippocampus of the KO sham group. However, there is no significant difference between the KO CIRI group and the KO CIRI + EDB group. All the above results demonstrated that EDB had anti‐antioxidant activity and was able to reduce the oxidative damage induced by CIRI. Moreover, Nrf2 is involved in the regulation of oxidative stress.
FIGURE 3.
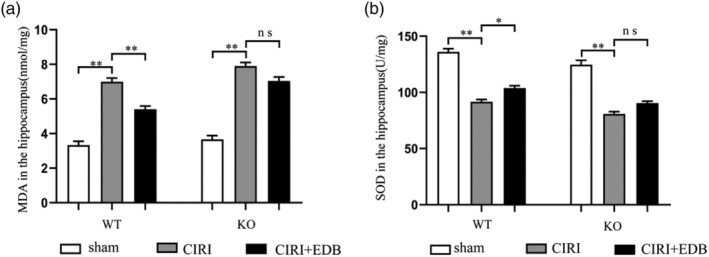
Effect of EDB on the expression of SOD and MDA in the hippocampus in mice with cerebral ischemia reperfusion injury evaluated. * P < 0.05; ** P < 0.01; ns no statistical significance. N = 12 mice per group
3.4. EDB prevents CIRI‐induced inflammation
We tested mRNA levels of pro‐inflammatory cytokines (TNF‐α and IL‐1β) and anti‐inflammatory cytokines (IL‐4 and IL‐10) by RT‐PCR in the hippocampus of WT and Nrf2 KO mice. Overall, the mRNA levels of TNF‐α and IL‐1β were increased, and the mRNA levels of IL‐4 and IL‐10 were decreased in the hippocampus during CIRI, which could be reversed after EDB treatment (Figure 4a–d). In the deletion of Nrf2, the mRNA levels of TNF‐α and IL‐1β in the KO CIRI group were increased. Conversely, the mRNA levels of IL‐4 and IL‐10 were significantly decreased, but there was no significant improvement after EDB treatment (Figure 4a–d). This suggested that EDB treatment might not improve CIRI‐induced inflammation in the Nrf2 deficiency.
FIGURE 4.
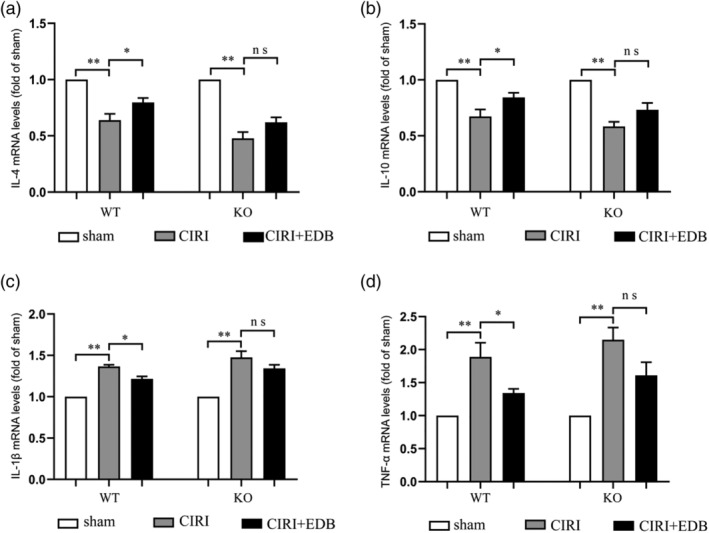
Effect of EDB on the expression of TNF‐α, IL‐1β, IL‐4, and IL‐10 in the hippocampus in mice with cerebral ischemia reperfusion injury evaluated by RT‐PCR (real‐time polymerase chain reaction). * P < 0.05; ** P < 0.01; ns no statistical significance. N = 6 mice per group
3.5. EDB attenuated CIRI‐induced cell apoptosis
To explore the mechanism of EDB on CIRI‐induced apoptosis, the anti‐apoptotic protein Bcl‐2 and pro‐apoptotic protein Bax, Caspase 3, which played crucial roles in regulating cell apoptosis were analyzed by western blot (Figure 5). In the CIRI group, the protein expression of Bcl‐2 was markedly lower compared to sham group, while the protein levels of Bax and Caspase 3 were significantly higher, and all of which were reversed by treatment with EDB. Furthermore, the effects of EDB on cell apoptosis may be related to Nrf2 KO. The KO CIRI group obviously decreased Bcl‐2 level and increased Bax and Caspase 3 levels, while there was a tendency for the KO CIRI + EDB group to show improvements compared with the KO CIRI group, but no statistical significance was achieved. Overall, these data demonstrated the hypothesis that the EDB played an essential role in apoptosis induced by CIRI mice, and the apoptotic effect may be related to Nrf2.
FIGURE 5.
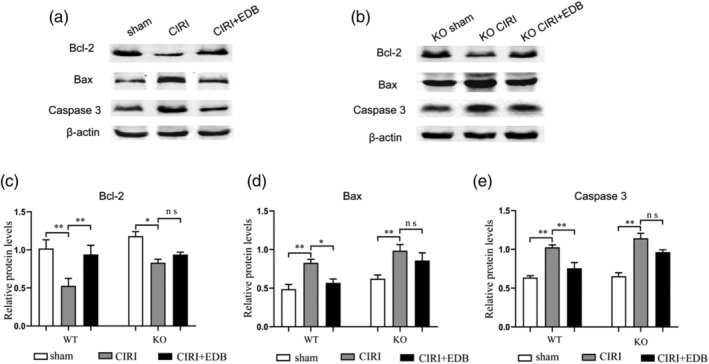
Effect of EDB on the expression of Bcl‐2, Bax, and Caspase 3 in the hippocampus in mice with cerebral ischemia reperfusion injury evaluated by western blot. ** P < 0.05; ** P < 0.01; ns no statistical significance. Bcl‐2, B cell lymphoma 2; Bax, Bcl‐2‐associated X; n = 6 mice per group
3.6. Regulation of NOX2 by EDB is achieved partially through activating the Nrf2/HO‐1 pathway
Western blot and RT‐PCR were used to detect the expressions of Nrf2, HO‐1, and NOX2 (Figure 6). Compared with the sham group, the expressions of Nrf2 and HO‐1 in the hippocampal of CIRI group were increased, and these two proteins were further increased after EDB treatment (Figure 6c, d, f, and g). On the contrary, CIRI induced a significant increase in NOX2 protein, which was alleviated after EDB treatment (Figure 6e,h). Moreover, we determined that compared with KO Sham group, Nrf2, HO‐1, and NOX2 proteins in hippocampal tissues of KO CIRI group increased, and the expression of NOX2 was further increased compared with the wild‐type CIRI group. After EDB treatment, Nrf2 and HO‐1 proteins further increased, while NOX2 protein was decreased, but there was no statistical significance.
FIGURE 6.
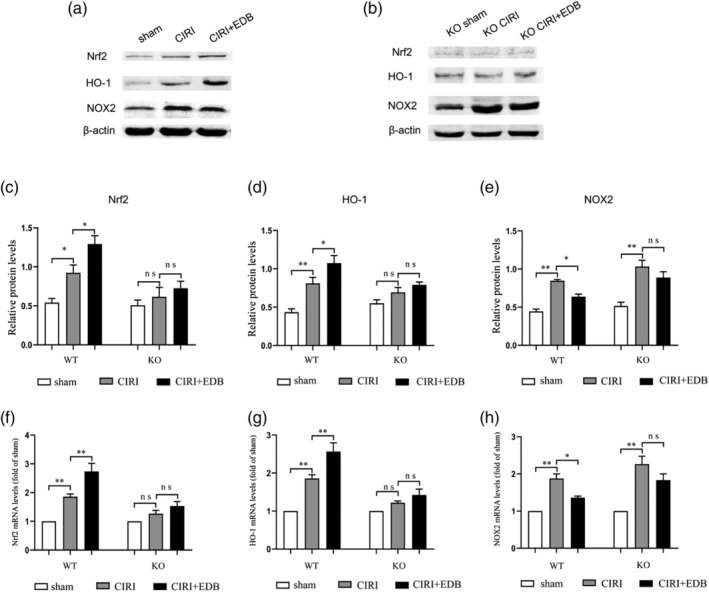
Effect of EDB on the expression of Nrf2, HO‐1, and NOX2 in the hippocampus in mice with cerebral ischemia reperfusion injury evaluated by western blot and RT‐PCR (real‐time polymerase chain reaction). * P < 0.05; ** P < 0.01; ns no statistical significance. N = 6 mice per group
4. DISCUSSION
After the stage of ischemia reperfusion, blood flow of the ischemic tissue restores, and ROS production increases, causing oxidative stress [23], promoting local inflammatory response, DNA damage, and further damaging cell structure and leading to cell death [24, 25]. Therefore, the ability to control oxidative stress and inflammation would be highly beneficial. Studies have confirmed that Nrf2 plays a significant role in regulating ROS and inflammation.
Edaravone—an antioxidant drug—has been shown to improve the outcomes of ischemic stroke through scavenging hydroxyl‐, peroxyl‐, and superoxide‐free radicals, reducing ROS production, oxidative stress and cognitive impairment after stroke [14, 26]. However, using edaravone, a neuroprotective drug, is far from satisfactory [27], and multitarget therapy strategies with antioxidant and anti‐inflammatory effects are urgently needed in clinical stroke management. More recently, (+) ‐bornel, a naturally occurring terpene and dicyclic organic compound, has been tested in preclinical models and shown to have powerful neuroprotective effects through multiple molecular pathways in ischemia reperfusion injury, such as reduction of ROS, improved of NO signaling pathway, inhibition of inflammatory process and apoptosis, alleviated the blood brain barrier [28]. Using an ischemia/reperfusion rat model, the researchers found that the expression of pro‐inflammatory mediator iNOS and TNF‐α and the level of free radical ONOO─ were significantly decreased with EDB treatment [19]. In our country for a multicenter, randomized, double‐blind, comparative, the phase II and phase III clinical trials, they found, when EDB or edaravone was administered within 48 h after AIS, compared with edaravone, 90‐day good functional outcomes favored in EDB group, especially in female patients [20, 21]. The pathophysiological mechanism of CIRI is complex, involving factors such as free radicals, inflammatory response, apoptosis, and blood–brain barrier damage [29]. It is theoretically believed that using two different agents to target different steps of ischemic injury may be superior to a single agent in preventing ischemia [30]. It is concluded that EDB may be more effective than any single drug in treating CIRI. The purpose of this study was to further explore the neuroprotective mechanism of synthetic drug EDB on CIRI.
In the current study, we confirmed the beneficial effects of EDB treatment against CIRI, and an interesting and striking point is that Nrf2 plays a key role in CIRI. Mice showed significant memory and learning disabilities after CIRI, but their memory and learning improved significantly after treatment with EDB. It is shown that compared with KO CIRI mice, symptoms were relieved after EDB treatment, but there was no significant statistical significance. In addition, H&E staining is the most widely used technique in pathology. The results showed that the brain tissues of CIRI group and KO CIRI group showed obvious pathological changes, with obvious atrophy and loss of neurons. After EDB treatment, the cortical and hippocampal structures of wild‐type mice were intact, and the number of neurons was increased, but the KO group was not significantly improved after EDB treatment. These results strongly suggest that EDB may have a neuroprotective role, and the critical role underlying Nrf2 in CIRI, helping to reduce neuronal damage and improve learning and memory disorders.
Oxidative stress plays a key role in secondary brain injury induced by reperfusion [23, 31]. In CIRI oxidative stress injury, MDA is one of the products of lipid per oxidation, which can reflect the degree of lipid per oxidation [32]. SOD is a component of the antioxidant defense mechanism and facilitates the removal or reduction of peroxides [33]. Yan et al. [5] confirmed that Astaxanthin could reduce oxidative stress in CIRI model by downregulating MDA level and upregulating SOD level, which was consistent with our research results. The excessive production of inflammatory mediators induced by CIRI oxidative stress can further accelerate brain damage. Inhibition of CIRI‐induced activation of pro‐inflammatory cytokines TNF‐α and IL‐1β, and enhancement of anti‐inflammatory cytokines IL‐4 and IL‐10 can improve neuronal excitability and neuronal survival, which may improve functional recovery after cerebral ischemia injury [34, 35]. In addition, CIRI oxidative stress induces excess levels of ROS production and induces apoptosis through the mitochondrial pathway [36, 37, 38]. Inhibition of apoptosis protein expression has been shown to protect neurons from CIRI [39, 40]. In this study, we found that EDB improves nerve injury by regulating oxidative stress levels, inflammatory cytokines, and apoptosis levels.
The Nrf2/HO‐1 pathway has been reported to play a vital role in regulating oxidative stress, inflammation, and the apoptosis of neurons [41, 42, 43]. Nrf2 regulates ROS production by mitochondria and NADPH oxidase [44]. In addition, activation of Nrf2 has been demonstrated to attenuate oxidative stress and neuro inflammation by inhibiting the NF‐κB pathway [45, 46, 47]. Nox2, a member of the NADPH oxidase family, is a major contributor to ROS production and ischemic stroke [48]. Previous studies have shown that inhibition of NOX2 can prevent CIRI‐induced oxidative stress and apoptosis by inhibiting ROS production [9, 49]. Studies have suggested that there is a relationship between Nrf2 and NOX2, Nrf2 KO exhibited a significant exacerbation of NOX2 induction in oxygen‐induced retinopathy [10]. Combined regulation of Nrf2 and NOX2 genes improves motor and cognitive function and decreased traumatic brain injury [50]. However, whether the neuroprotective effect of EDB on CIRI is related to the molecular mechanism of Nrf2‐mediated NOX2 remains to be further explored. In our study, we found that EDB regulates NOX2 protein in CIRI models by activating Nrf2/HO‐1 signaling pathway. Furthermore, Nrf2 KO could block the inhibitory effects of EDB on oxidative stress, inflammation, and apoptosis in CIRI mice. These data strongly suggest that EDB inhibited the NOX2 expression by stimulating Nrf2/HO‐1 signaling pathway.
In summary, the current study proves that EDB has a significant protective effect on CIRI‐induced nerve injury in mice, which may regulate NOX2 protein through the Nrf2/HO‐1 pathway, regulate oxidative stress, and inhibit inflammation and cell apoptosis. These findings provide novel prospects for EDB as a potential treatment for use in the treatment of ischemic stroke.
CONFLICT OF INTEREST
The authors have no relevant financial or non‐financial interests to disclose.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lili Xu and Yaran Gao. The first draft of the manuscript was written by Lili Xu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT
This study was performed in line with the principles of the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). The ethical approval was granted by the Ethics Committee of Hebei General Hospital (No: SCXK2016‐0006). All efforts were made to minimize the number of animals used and their suffering. All study participants provided informed consent, and the study design was approved by the appropriate ethics review board.
ACKNOWLEDGMENTS
We thank Dr Thomas W. Kensler laboratory (Johns Hopkins University, Baltimore, America) for providing Nrf2−/− mice for vivo in studies and Dr Chunyan Li (Second Hospital of Hebei Medical University, Shijiazhuang, China) for providing technical support for this study. This work was supported by the Hebei Provincial High‐end Talents Funding Project (6833452 and 83587216), 2019 Hebei Provincial Government funded clinical Talents Training Project (Ji CAI Society: 2019‐139‐5), and Project of Introducing Talents to Hebei Province in 2020 (Ji Ke special Letter: 2020‐19‐2).
Xu L, Gao Y, Hu M, et al. Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO‐1 signaling pathway in mice. Fundam Clin Pharmacol. 2022;36(5):790‐800. doi: 10.1111/fcp.12782
Funding information Project of Introducing Talents to Hebei Province, Grant/Award Number: 2020‐19‐2; 2019 Hebei Provincial Government funded clinical Talents Training Project, Grant/Award Number: 2019‐139‐5; Hebei Provincial High‐end Talents Funding Project, Grant/Award Numbers: 83587216, 6833452
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789‐1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8 [DOI] [PubMed] [Google Scholar]
- 3. Khatri R, Mckinney AM, Swenson B, McKinney AM, Janardhan V. Blood‐brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52‐S577. doi: 10.1212/WNL.0b013e3182697e70 [DOI] [PubMed] [Google Scholar]
- 4. Chung E, Iwasaki K, Mishima K, Egashira N, Fujiwara M. Repeated cerebral ischemia induced hippocampal cell death and impairments of spatial cognition in the rat. Life Sci. 2002;72(4–5):609‐61919. doi: 10.1016/S0024-3205(02)02269-5 [DOI] [PubMed] [Google Scholar]
- 5. Xue Y, Qu Z, Fu J, et al. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res Bull. 2017;131:221‐228. doi: 10.1016/j.brainresbull.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 6. Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50(2):77‐97. doi: 10.1152/physiolgenomics.00041.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Meng F. Effects of icariside II on brain tissue oxidative stress and Nrf2/HO‐1 expression in rats with cerebral ischemia‐reperfusion injury1. Acta Cir Bras. 2019;34(2):e201900208. doi: 10.1590/s0102-8650201900208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524‐551. doi: 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahles T, Brandes RP. Which NADPH oxidase isoform is relevant for ischemic stroke? The case for nox 2. Antioxid Redox Signal. 2013;18(12):1400‐141717. doi: 10.1089/ars.2012.4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei Y, Gong J, Xu Z, Duh EJ. Nrf2 promotes reparative angiogenesis through regulation of NADPH oxidase‐2 in oxygen‐induced retinopathy. Free Radic Biol Med. 2016;99:234‐243. doi: 10.1016/j.freeradbiomed.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dai Y, Zhang H, Zhang J, Yan M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2‐mediated inhibition of the NOX4/ROS/NF‐κB pathway. Chem Biol Interact. 2018;284:32‐40. doi: 10.1016/j.cbi.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 12. Nakamura T, Kuroda Y, Yamashita S, et al. Edaravone attenuates brain edema and neurologic deficits in a rat model of acute intracerebral hemorrhage. Stroke. 2008;39(2):463‐4699. doi: 10.1161/STROKEAHA.107.486654 [DOI] [PubMed] [Google Scholar]
- 13. Effect of a novel free radical scavenger, edaravone (MCI‐186), on acute brain infarction. Randomized, placebo‐controlled, double‐blind study at multicenters. Cerebrovasc Dis. 2003;15(3):222‐2299. doi: 10.1159/000069318 [DOI] [PubMed] [Google Scholar]
- 14. Zhang N, Komine‐Kobayashi M, Tanaka R, Liu M, Mizuno Y, Urabe T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke. 2005;36(10):2220‐22255. doi: 10.1161/01.STR.0000182241.07096.06 [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Liu M, Pu C. 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(3):302‐320. doi: 10.1177/1747493017694391 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI‐186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268(3):1597‐1604604. [PubMed] [Google Scholar]
- 17. Liu R, Zhang L, Lan X, et al. Protection by borneol on cortical neurons against oxygen‐glucose deprivation/reperfusion: involvement of anti‐oxidation and anti‐inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience. 2011;176:408‐41919. doi: 10.1016/j.neuroscience.2010.11.029 [DOI] [PubMed] [Google Scholar]
- 18. Wang HY, Xu LX, Xiao Y, Han J. Spectrophotometric determination of dapsone in pharmaceutical products using sodium 1,2‐naphthoquinone‐4‐sulfonic as the chromogenic reagent. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(12):2933‐29399. doi: 10.1016/j.saa.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 19. Wu HY, Tang Y, Gao LY, et al. The synergetic effect of edaravone and borneol in the rat model of ischemic stroke. Eur J Pharmacol. 2014;740:522‐53131. doi: 10.1016/j.ejphar.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Wang Y, Wang A, et al. Safety and efficacy of edaravone dexborneol versus edaravone for patients with acute ischaemic stroke: a phase II, multicentre, randomised, double‐blind, multiple‐dose, active‐controlled clinical trial. Stroke Vasc Neurol. 2019;4(3):109‐114. doi: 10.1136/svn-2018-000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu J, Wang A, Meng X, et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double‐blind, comparative trial. Stroke. 2021;52(3):772‐780. doi: 10.1161/STROKEAHA.120.031197 [DOI] [PubMed] [Google Scholar]
- 22. Wang H. Establishment of an animal model of vascular dementia. Exp Ther Med. 2014;8(5):599‐1603. doi: 10.3892/etm.2014.1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orellana‐Urzúa S, Rojas I, Líbano L, Rodrigo R. Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des. 2020;26(34):4246‐4260. doi: 10.2174/1381612826666200708133912 [DOI] [PubMed] [Google Scholar]
- 24. Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650‐1667. doi: 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 25. Amin EF, Rifaai RA, Abdel‐Latif RG. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative‐inflammatory‐apoptotic pathway. Fundam Clin Pharmacol. 2020;34(5):548‐558. doi: 10.1111/fcp.12548 [DOI] [PubMed] [Google Scholar]
- 26. Sun YY, Li Y, Wali B, et al. Prophylactic edaravone prevents transient hypoxic‐ischemic brain injury: implications for perioperative neuroprotection. Stroke. 2015;46(7):1947‐195555. doi: 10.1161/STROKEAHA.115.009162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Enomoto M, Endo A, Yatsushige H, Fushimi K, Otomo Y. Clinical effects of early edaravone use in acute ischemic stroke patients treated by endovascular reperfusion therapy. Stroke. 2019;50(3):652‐658. doi: 10.1161/STROKEAHA.118.023815 [DOI] [PubMed] [Google Scholar]
- 28. Chen ZX, Xu QQ, Shan CS, et al. Borneol for regulating the permeability of the blood‐brain barrier in experimental ischemic stroke: preclinical evidence and possible mechanism. Oxid Med Cell Longev. 2019;2019:2936737. doi: 10.1155/2019/2936737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang M, Chen Z, Yang L, Ding L. Sappanone A protects against inflammation, oxidative stress and apoptosis in cerebral ischemia‐reperfusion injury by alleviating endoplasmic reticulum stress. Inflammation. 2021;44(3):934‐945. doi: 10.1007/s10753-020-01388-6 [DOI] [PubMed] [Google Scholar]
- 30. Pérez De La Ossa N, Dávalos A. Neuroprotection in cerebral infarction: the opportunity of new studies. Cerebrovasc Dis. 2007;24(Suppl 1):153‐1566. doi: 10.1159/000107391 [DOI] [PubMed] [Google Scholar]
- 31. Ya BL, Liu Q, Li HF, et al. Uric acid protects against focal cerebral ischemia/reperfusion‐induced oxidative stress via activating Nrf2 and regulating neurotrophic factor expression. Oxid Med Cell Longev. 2018;2018:6069150. doi: 10.1155/2018/6069150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang RX, Li S, Sui X. Sodium butyrate relieves cerebral ischemia‐reperfusion injury in mice by inhibiting JNK/STAT pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1762‐1769. doi: 10.26355/eurrev_201902_17138 [DOI] [PubMed] [Google Scholar]
- 33. Wang FJ, Wang SX, Chai LJ, Zhang Y, Guo H, Hu LM. Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol Sin. 2018;39(6):998‐1011. doi: 10.1038/aps.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu H, Wang B, Cui N, Zhang Y. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROS‐dependent p38 MAPK and protects against cerebral ischemia‐reperfusion injury. Mol Med Rep. 2018;17(5):6639‐6646. doi: 10.3892/mmr.2018.8666 [DOI] [PubMed] [Google Scholar]
- 35. Jin Y, Wei F, Dai X, Qi M, Ma Y. Anti‐inflammatory effect of 4‐methylcyclopentadecanone in rats submitted to ischemic stroke. Fundam Clin Pharmacol. 2018;32(3):270‐278. doi: 10.1111/fcp.12348 [DOI] [PubMed] [Google Scholar]
- 36. Kanda T, Matsuoka S, Yamazaki M, et al. Apoptosis and non‐alcoholic fatty liver diseases. World J Gastroenterol. 2018;24(25):2661‐2672. doi: 10.3748/wjg.v24.i25.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu S, Wang C, Cheng Q, et al. An active component of Achyranthes bidentata polypeptides provides neuroprotection through inhibition of mitochondrial‐dependent apoptotic pathway in cultured neurons and in animal models of cerebral ischemia. PLoS ONE. 2014;9(10):e109923. doi: 10.1371/journal.pone.0109923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl‐2 proteins and apoptosis: an update. Arch Toxicol. 2015;89(3):289‐317. doi: 10.1007/s00204-014-1448-7 [DOI] [PubMed] [Google Scholar]
- 39. Liu MB, Wang W, Gao JM, Li F, Shi JS, Gong QH. Icariside II attenuates cerebral ischemia/reperfusion‐induced blood‐brain barrier dysfunction in rats via regulating the balance of MMP9/TIMP1. Acta Pharmacol Sin. 2020;41(12):1547‐1556. doi: 10.1038/s41401-020-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu L, Xu H, Zhang W, Chen Z, Li W, Ke W. Circular RNA circCCDC9 alleviates ischaemic stroke ischaemia/reperfusion injury via the Notch pathway. J Cell Mol Med. 2020;24(24):14152‐14159. doi: 10.1111/jcmm.16025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han M, Hu L, Chen Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO‐1 pathway in rats with cerebral ischemia‐reperfusion injury. Drug Des Devel Ther. 2019;13:2923‐2931. doi: 10.2147/DDDT.S216156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang C, Hu Y, Lyu H, et al. Neuroprotective effects of 1‐O‐hexyl‐2,3,5‐trimethylhydroquinone on ischaemia/reperfusion‐induced neuronal injury by activating the Nrf2/HO‐1 pathway. J Cell Mol Med. 2020;24(18):10468‐10477. doi: 10.1111/jcmm.15659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sivandzade F, Bhalerao A, Cucullo L. Cerebrovascular and neurological disorders: protective role of NRF2. Int J Mol Sci. 2019;20(14). doi: 10.3390/ijms20143433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kovac S, Angelova PR, Holmström KM, et al. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850(4):794‐801. doi: 10.1016/j.bbagen.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross‐talk between Nrf2 and NF‐κB response pathways. Biochem Soc Trans. 2015;43(4):621‐6266. doi: 10.1042/BST20150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamauchi K, Nakano Y, Imai T, et al. A novel nuclear factor erythroid 2‐related factor 2 (Nrf2) activator RS9 attenuates brain injury after ischemia reperfusion in mice. Neuroscience. 2016;333:302‐31010. doi: 10.1016/j.neuroscience.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 47. Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF‐қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69(14):2345‐236363. doi: 10.1007/s00018-012-1011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zuo ML, Wang AP, Song GL, Yang ZB. miR‐652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed Pharmacother. 2020;124:109860. doi: 10.1016/j.biopha.2020.109860 [DOI] [PubMed] [Google Scholar]
- 50. Chandran R, Kim T, Mehta SL, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab. 2018;38(10):1818‐1827. doi: 10.1177/0271678X17738701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


