Abstract
The global impacts of climate change are evident in every marine ecosystem. On coral reefs, mass coral bleaching and mortality have emerged as ubiquitous responses to ocean warming, yet one of the greatest challenges of this epiphenomenon is linking information across scientific disciplines and spatial and temporal scales. Here we review some of the seminal and recent coral‐bleaching discoveries from an ecological, physiological, and molecular perspective. We also evaluate which data and processes can improve predictive models and provide a conceptual framework that integrates measurements across biological scales. Taking an integrative approach across biological and spatial scales, using for example hierarchical models to estimate major coral‐reef processes, will not only rapidly advance coral‐reef science but will also provide necessary information to guide decision‐making and conservation efforts. To conserve reefs, we encourage implementing mesoscale sanctuaries (thousands of km2) that transcend national boundaries. Such networks of protected reefs will provide reef connectivity, through larval dispersal that transverse thermal environments, and genotypic repositories that may become essential units of selection for environmentally diverse locations. Together, multinational networks may be the best chance corals have to persist through climate change, while humanity struggles to reduce emissions of greenhouse gases to net zero.
Keywords: climate change, conservation, coral bleaching, coral reefs, corals, global warming, mesoscale sanctuaries, networks, protected reefs, refugia, thermal stress
With the growing severity of marine heatwaves, mass coral bleaching and mortality has become widespread. Yet, our understanding of coral bleaching and its cascading consequences is incomplete. One of the greatest challenges of this epiphenomenon is integrating findings from different disciplines and across biological and spatial scales. Here, we synthesize seminal and recent coral‐bleaching discoveries, evaluate which data and processes can improve predictive models, and provide a conceptual framework that integrates studies across scales. An integrative approach across biological and spatial scales will not only advance coral‐reef science but will also provide necessary information to guide conservation efforts.
1. INTRODUCTION
The relationship between scleractinian corals and their photosynthetic microalgal symbionts has allowed corals to build coral reefs for millions of years. In recent decades however thermal‐stress events have increased in frequency and intensity resulting in widespread coral bleaching (Glynn, 1996; Heron et al., 2016; Figure 1). Coral bleaching represents the breakdown of a long co‐evolutionary relationship between the coral host and its photosynthetic symbionts (Coles & Jokiel, 1977; Gates et al., 1992; Goreau, 1964; Hoegh‐Guldberg, 1999; LaJeunesse et al., 2018; Rädecker et al., 2021; Weis, 2008). This breakdown leads to the visual whitening of corals through the loss of intracellular microalgal symbionts (Box 1), which can result in coral mortality and changes in reef communities over large regions (Hughes et al., 2018; McClanahan et al., 2020; Stuart‐Smith et al., 2018). Such changes reduce the goods and services that reefs provide, including their capacity to keep up with sea‐level rise (Perry et al., 2013; van Woesik & Cacciapaglia, 2021), and thereby protect coastal communities from storm waves (Ferrario et al., 2014). Yet, our understanding of coral bleaching resulting from thermal stress and its cascading consequences on coral reefs is incomplete. We are just beginning to understand the role of molecular, genetic, and phenotypic traits in determining which individuals, species, and populations of corals are likely to survive.
FIGURE 1.
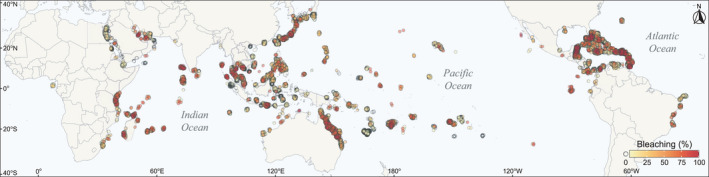
Global coral bleaching from 1980 to 2020. Coral bleaching was calculated as a percentage of the coral colonies that were bleached at the time of survey, from 11,068 sites in 89 countries (n = 23,298; data from van Woesik & Kratochwill, 2022)
BOX 1. The coral bleaching phenomenon.
The term ‘bleaching’ was coined to describe the visible paling of the coral surface, as the white skeleton becomes visible through the animal's translucent tissue that has lost pigmentation and symbionts (Glynn, 1983; Goreau, 1964). Coral bleaching is synonymous with the breakdown of the symbiotic relationship between the coral host and its microalgal symbionts (family Symbiodiniaceae). Yet, bleaching does not imply a physiological pathology per se because bleaching can result from multiple biotic and abiotic stressors, including freshwater, disease, pollutants, UV radiation, and suboptimal seawater temperatures (Glynn, 1996; Goreau, 1964). Most recent coral‐bleaching events are caused by anomalously high seawater temperatures (Glynn, 1996; Hoegh‐Guldberg, 1999; Hughes et al., 2018; Sully et al., 2019) resulting from climate change. Under extreme temperature stress, coral tissue deterioration or detachment from the skeleton can occur, often leading to mortality (Gates et al., 1992; Leggat et al., 2019). In many cases, however, the coral tissue remains intact during bleaching, albeit severely compromised and deprived of a nutrition source due to the loss of symbiotic microalgae that provide energy from photosynthesis to the coral host. Without sufficient heterotrophic compensation (Grottoli et al., 2006; Levas et al., 2016) or high enough energy reserves (Anthony et al., 2009), the bleached coral may eventually die from starvation.
Processes involved in the establishment and maintenance of the coral‐microalgal symbiosis provide insights into bleaching mechanisms. On establishment of symbiosis, the microalgal cells are taken into the coral gastrodermis, coral immune responses are repressed (Voolstra et al., 2009), and the cells are incorporated into the symbiosome (Davy et al., 2012). There the microalgal symbionts are maintained in a vegetative, immobile, nutrient‐limited state that stimulates the release of excess photosynthetic carbon for the coral host to harvest (Barott et al., 2015; Jokiel et al., 1994). Recognition and signaling between both partners are crucial for maintaining nutritional demands (Rädecker et al., 2021). Under environmental stress, translocation of microalgal photosynthates to the coral host slows (Hughes et al., 2010), reducing the coral's primary source of organic carbon, signaling starvation and amino acid digestion (Rädecker et al., 2021).
The onset of physiological stress and bleaching is strongly dependent on the rate of heating, the accumulated thermal stress, and the maximum temperature (Middlebrook et al., 2010; Savary et al., 2021; Voolstra et al., 2020). Thermal stress affects multiple processes in both partners, resulting in direct impairment of key cellular functions, homeostasis, and nutrition (Rädecker et al., 2021; Roach et al., 2021a). In the microalgal symbiont, several factors have been implicated as pressure points during thermal stress, especially when combined with high irradiance. These factors include photosystem II repair, thylakoid membrane stability, and both photosynthetic and heterotrophic carbon assimilation pathways (Hughes et al., 2010; Iglesias‐Prieto et al., 1992; Tchernov et al., 2004; Warner et al., 1999).
Disrupted photosynthetic and mitochondrial electron flow leads to elevated reactive‐oxygen and nitrogen species. These disruptions alter redox homeostasis, create oxidative stress (Brown et al., 2002; Krueger et al., 2014; Lesser, 1997), and trigger a coral‐innate‐immune response. Transcriptomic analyses show that oxidative stress disrupts calcium (Ca2+) homeostasis, which leads to altered cytoskeletal and cell‐adhesion, reduced calcification, and expression of stress‐response genes (DeSalvo et al., 2008; Rodriguez‐Lanetty et al., 2009)—however, gene‐network analyses indicate that additional, but less understood, mechanisms are also involved in coral bleaching (Dixon et al., 2020; Rose et al., 2016). Persistent disruption to cellular homeostasis under thermal stress can cause both the coral and the microalgal symbiont to undergo necrotic and apoptotic cell death (Dunn et al., 2012; Lesser & Farrell, 2004) and can lead to fatal coral bleaching.
Although numerous studies collect coral‐bleaching data (Box 2), these studies are rarely integrated across biological levels of organization. Still, understanding variation in thermal tolerance among individuals at the molecular and physiological levels is essential for elucidating a population's vulnerability at the ecological level. Similarly, variable responses to thermal stress may provide insight into physiological and molecular mechanisms and highlight adaptive potential at different geographic scales. Therefore, there is a need for a conceptual framework that connects environmental conditions to coral‐bleaching responses across biological, spatial, and temporal scales. Such a framework should integrate individual‐based molecular and physiological responses with coral populations and communities. Here, we explore potential links across biological disciplines that may increase our understanding of coral‐bleaching responses and their impact on coral‐reef ecosystems.
BOX 2. Coral‐bleaching data and metadata needs.
Field studies and monitoring on coral reefs usually focus on capturing the intensity and extent of coral bleaching under varying environmental conditions and determine where corals are most likely to bleach and recover given different local and regional conditions. In the field, bleaching is commonly recorded as the proportion of bleached coral colonies, or as a percentage of coral cover. Some field estimates use either the presence or absence of bleaching or qualitative categories, which are convenient but restrict analytical approaches harnessing the strength of continuous variables. As bleaching is a dynamic process that varies spatially and temporally (Brown et al., 2002; Castillo et al., 2012; Wall et al., 2021), the timing of field surveys is often dictated by logistical constraints and may not coincide with peak bleaching. This presents challenges to accurately observing the full extent of bleaching because recently dead corals may not necessarily be attributed to bleaching, potentially leading surveys to underestimate bleaching if the survey is conducted after some mortality has occurred.
In situ ecological bleaching data are collected using a variety of methods (Figure 2), including rapid surveys, quantitative transects, and photographic censuses. These data are sometimes supplemented by in situ measurements of the photosynthetic responses of microalgal symbionts and by sampling of corals for further laboratory diagnoses to determine the densities and types of symbionts, and various molecular, cellular, and physiological responses. Yet, species‐level identification is still problematic for many corals (e.g., Pocillopora, massive Porites species, and the highly speciose genus Acropora) and for their symbionts belonging to Symbiodiniaceae. These unknown species boundaries can jeopardize geographic comparisons of coral bleaching. We, therefore, encourage in situ surveys at high taxonomic resolution and the implementation of a collection of specimen vouchers as routine procedures, which would allow taxonomic comparisons.
Bleaching data can also be obtained by autonomous underwater vehicles and aerial imagery from drones, low altitude flights (i.e., helicopters and airplanes), and satellites (Dornelas et al., 2019; Drury et al., 2022; Hedley et al., 2016). These methods vary in their spatial extent and accuracy of bleaching detection. Although satellite imagery may revolutionize broad‐scale monitoring, especially for remote reefs (Xu et al., 2020), such data have several limitations, including being informative only for the shallowest parts of the reef and providing little, if any, taxonomic resolution (Hedley et al., 2012). Therefore, there is a need to integrate coral‐bleaching datasets at multiple biological, spatial, and temporal scales (Figure 2) and match those data with appropriate environmental predictors to make survey results scalable across large geographic regions.
Data integration and geographic comparisons also require detailed metadata, which are frequently missing from datasets (McLachlan et al., 2020), making spatial and temporal comparisons and meta‐analyses difficult. At a minimum, metadata should include the date of sampling, the coordinates of the study site, the depth of survey, and the sampling method (Grottoli et al., 2021). Additionally, when coral samples are collected, information about habitat characteristics, such as contact with turf or macroalgae, as well as variables such as water temperature, photosynthetically active radiation, salinity, and dissolved oxygen is helpful to provide context for the interpretation of physiological and molecular responses (Grottoli et al., 2021). To facilitate metadata collection, coral‐bleaching studies could adopt the Darwin Core standard, which describes biodiversity data and outlines the ‘Minimal Information about any(x) Sequence’ (MIxS) standard (Yilmaz et al., 2011), used widely in genomics and microbial ecology. An example of a tool that meets both standards is the Genomic Observatories Metadatabase (Deck et al., 2017) (GEOME, https://geome‐db.org/), which stores metadata archives that are permanently linked to ‐omics resources, stored at the National Center of Biotechnology Information's (NCBI) Sequence Read Archive. In addition, gene expression data can be deposited and maintained in NCBI’s Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), and physiological and ecological data can be deposited in archives such as BCO‐DMO (https://www.bco‐dmo.org). Open, transparent, and linked access to all raw data, sequence data, metadata, and analytical code are needed to integrate data across disciplines and accelerate coral‐bleaching discoveries.
Elucidating coral‐bleaching responses is critical in determining which habitats and oceanic regions might serve as climate‐change refugia and how we could manage them. Building on past reviews on coral bleaching (Glynn, 1996; Brown, 1997; Baker et al., 2008; Suggett & Smith, 2020), we update the relevant information and synthesize recent coral‐bleaching discoveries from an ecological, physiological, and molecular perspective. This synthesis aims towards providing a conceptual framework that integrates coral‐bleaching responses across biological scales in a way that sheds light on how best to identify climate‐change refugia and possible management strategies. We also recommend which data, metadata, and processes are critical for: (i) improving predictive models of coral resilience (Box 2) and (ii) refining the capacity to detect inter‐connected networks of reefs for the establishment of mesoscale sanctuaries (thousands of km2) in which high levels of coral genetic diversity, phenotypic adaptation, thermal tolerance, and resilience are most likely. Incorporating models of coral resilience and refugia into sanctuary planning will help to improve the ecosystem‐level resilience of local marine reserves and expand multinational mesoscale sanctuaries. Multinational mesoscale sanctuaries have the potential to simultaneously protect coral reefs from local and regional scale stressors, bridge territorial boundaries, and give corals and coral reefs a fighting chance of coping with climate change because they can preserve standing genetic diversity while maintaining connectivity between reefs through larval dispersal across contrasting environmental and thermal gradients.
2. ENVIRONMENTAL DRIVERS OF CORAL BLEACHING
Coral bleaching is a general response to stress (Box 1). The most widespread cause of recent broad‐scale coral bleaching is anomalously warm seawater resulting from climate change (Glynn, 1996; Hoegh‐Guldberg, 1999; Hughes et al., 2018). Coral bleaching is most common in localities with a high intensity and high frequency of thermal‐stress anomalies (McClanahan et al., 2020), and is less common in localities with highly variable seawater temperatures (Safaie et al., 2018; Sully et al., 2019; but see Klepac & Barshis, 2020). In the past 20 years, the most extensive coral bleaching has been recorded between latitudes 15° and 20°, north and south (Sully et al., 2019). Reefs in the equatorial western Pacific Ocean have experienced relatively less coral bleaching than elsewhere, despite prevalent high seawater temperatures (McClanahan et al., 2020; Sully et al., 2019). However, in recent decades this region has warmed less rapidly than other ocean regions (Kleypas et al., 2008), most likely because negative feedback loops limit maximum temperatures (Clement et al., 1996).
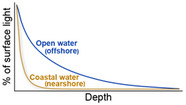
Although anomalously high seawater temperatures are the main driver of coral bleaching (Brown et al., 2000; Glynn, 1983), there are other environmental factors that can exacerbate or mitigate coral bleaching during thermal stress. Most notably, light intensity, nutrient concentrations, zooplankton availability, and water‐flow rates are key factors mediating bleaching responses. Experimental studies under elevated temperatures indicate that coral bleaching is reduced when light levels are low (Lesser & Farrell, 2004; Takahashi et al., 2004). Field studies corroborate these findings, showing that under similarly elevated seawater temperatures, coral bleaching is more likely on clear‐water reefs than on turbid reefs (Morgan et al., 2017; Teixeira et al., 2019; van Woesik et al., 2012). Light is also reduced by clouds, which may provide corals some relief when experiencing thermal stress (Gonzalez‐Espinosa & Donner, 2021; Mumby et al., 2001). Additionally, light intensity also declines with depth (Table 1), and although corals have been observed bleaching at depths >40 m (Frade et al., 2018; Williams & Bunkley‐Williams, 1990), depth may attenuate the severity of coral bleaching (Hoeksema, 1991; Smith et al., 2014; Muir et al., 2017; but see Smith et al., 2016; Venegas et al., 2019).
TABLE 1.
Conceptual summaries of general responses of coral holobionts to thermal stress and relevant environmental factors. Processes are described and several references are listed, where Temperature = °C, Irradiance = photosynthetically available radiation, Depth = m, Thermal stress = degree heating weeks (DHW; https://coralreefwatch.noaa.gov/product/5km/index_5km_dhw.php), and Exposure time = days. DHW is a measure of the accumulated bleaching heat stress during the most recent 12‐week period. Coral bleaching tends to occur when DHW values reach 4°C‐weeks, and at 8°C‐weeks bleaching is generally widespread and followed by substantial mortality
| Process description | Response | References |
|---|---|---|
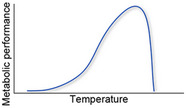
| Hypothetical thermal metabolic performance curve of corals, with the optimum close to the upper thermal limit. When corals are exposed to temperature above their optimum, it causes a decline in metabolic function |
|
Pörtner (2012), Sinclair et al. (2016) |
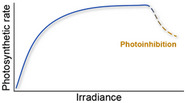
| Photosynthetic rate increases with irradiance (light) availability up to a threshold, beyond which photoinhibition (a process whereby photosynthesis is impeded) occurs. Thermal stress can accelerate photoinhibition |
|
Iglesias‐Prieto et al. (1992), Warner et al. (1999), Takahashi and Murata (2008) |
| Light attenuates exponentially with depth. In turbid, nearshore reefs light attenuates more rapidly than in clear, offshore reefs. Since high light can exacerbate thermal stress, corals in low light conditions may suffer less bleaching |
|
van Woesik et al. (2012), Morgan et al. (2017), Kahng et al. (2019) |
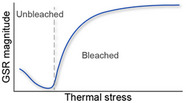
| Eigengene expression of the generalized stress response gene‐network module. This generalized gene‐expression response was similar under different stressors (in Acropora spp.) and illustrates the underlying response to thermal stress |
|
Rose et al. (2016), Wright et al. (2019), Dixon et al. (2020) |
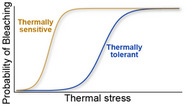
| Corals bleach as the intensity of thermal stress increases. Some genotypes, species, and localities are more sensitive than others to thermal stress. How corals respond depends on the innate and adapted thermal tolerance of individuals and populations |
|
Glynn (1996), Hoegh‐Guldberg (1999), Loya et al. (2001) |
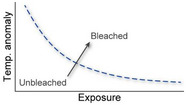
| Coral bleaching is a function of cumulative exposure to thermal stress, which depends on duration, rate, and intensity of exposure. Coral mortality tends to increase as exposure increases |
|
Berkelmans (2002), Berkelmans et al. (2004), Middlebrook et al. (2010) |
| Some coral genotypes and species adjust to thermal stress through acclimatization, adaptation, and epigenetics |
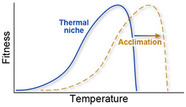
|
Pörtner (2012), Matz et al. (2018), Jury and Toonen (2019), Liew et al. (2020) |
Thermal‐stress relief also may be provided by hydrology, for example, strong currents, upwelling, internal waves, and temperature stratification (Glynn, 1996; Riegl & Piller, 2003; Wyatt et al., 2020). While coral bleaching is reduced by moderate to high water‐flow rates (Fifer et al., 2021; Nakamura et al., 2003), a recent study suggests that those effects may be temporary (Page et al., 2021). By contrast, unbalanced forms and ratios of nutrient concentrations can increase coral bleaching susceptibility (Morris et al., 2019; Wiedenmann et al., 2013). There is some evidence that elevated nutrient levels may indeed play a role in coral bleaching (Donovan et al., 2020), with short‐term anomalously high‐nutrient concentrations exacerbating bleaching during thermal stress (DeCarlo et al., 2020). Yet, the effects of nutrients on coral bleaching are complex (Lesser, 2021) and may vary with nutrient type, concentration, stoichiometry, and the duration of exposure (Dobson et al., 2021; Kitchen et al., 2020). For example, persistently high nutrient concentrations may lead to corals increasing their tissue thickness (Barkley et al., 2018); a characteristic that often leads to higher resilience to thermal stress (Loya et al., 2001). Nonetheless, disentangling the compounding influences of nutrients, turbidity, light, and flow from thermal stress is especially challenging for bleaching research, and therefore additional studies are needed to decipher their individual and synergistic effects (Lesser, 2021).
3. DIFFERENTIAL THERMAL‐STRESS SUSCEPTIBILITY OF CORALS
For this review, we regard thermal stress as the increase in water temperature that can, or eventually will, trigger coral bleaching. Coral species naturally differ in their susceptibility to thermal stress and subsequent bleaching (Berkelmans et al., 2004; Hoeksema, 1991; Loya et al., 2001; Marshall & Baird, 2000). Molecular markers are increasingly used to resolve differences among similar coral species and their associated microalgal symbionts to clarify why they differ in susceptibility to thermal stress (Burgess et al., 2021; Hume et al., 2019; LaJeunesse et al., 2018; Rowan & Powers, 1991; Sampayo et al., 2008). Although most corals associate with specific microalgal symbionts (Hume et al., 2020; LaJeunesse et al., 2009), thermal stress can increase the proportion of thermally tolerant symbionts (Baker et al., 2004; Berkelmans & van Oppen, 2006). For example, Durusdinium symbionts often have the highest thermal tolerance (LaJeunesse et al., 2018), with some local exceptions such as in the Persian‐Arabian Gulf (Hume et al., 2016). Yet over the long term, shuffling to more thermally tolerant symbiont species is typically unsustainable (Hume et al., 2020; LaJeunesse et al., 2009) as hosting thermally tolerant symbionts may come at a physiological cost—reducing both calcification (Grottoli et al., 2014; Little et al., 2004) and reproductive output (Jones & Berkelmans, 2011)—most likely because of suboptimal transfer of nutrients and photosynthates (Rädecker et al., 2018). However, during annually recurring coral‐bleaching episodes, shuffling to thermally tolerant symbionts may become essential (Lewis et al., 2019).
The coral's microbial community (e.g., bacteria, archaea, fungi, and viruses, etc.) also plays a role in thermal susceptibility (Peixoto et al., 2021; Reshef et al., 2006; Ziegler et al., 2017). For example, microalgae harbored by thermally–sensitive corals were more prone to viral infections under warmer temperatures (Levin et al., 2017). Furthermore, bleached corals showed evidence of increased viral loads compared with non‐bleached conspecific corals (Messyasz et al., 2020). Thus, the lytic cycle of some viruses that infect Symbiodiniaceae may be triggered by increased temperatures, which then compromises the fidelity of the symbiosis, initiating, accelerating, or worsening bleaching. By contrast, endolithic microbes may provide corals with an additional source of energy during bleaching (Fine & Loya, 2002; Pernice et al., 2020). Understanding the role of the symbiotic community in shaping the thermal susceptibility of the coral holobiont offers researchers the prospect of microbiome manipulation to boost the thermal tolerance of corals (Doering et al., 2021; Santoro et al., 2021; Voolstra et al., 2021a).
Hallmarks of bleaching tolerance can also entail heterotrophic plasticity (Grottoli et al., 2006; Levas et al., 2016) and high–energy reserves (Anthony et al., 2009). Corals that have high feeding rates, or those that are heterotrophically plastic, tend to have higher survivorship and recovery rates from bleaching than those that have low feeding rates and limited heterotrophic plasticity (Grottoli et al., 2006; Levas et al., 2013). Additionally, some corals resist thermal stress by inherently frontloading the expression of genes involved in heat shock proteins, antioxidant enzymes, and innate immunity (Barshis et al., 2013; Voolstra et al., 2021b), or by dynamically regulating response genes through transcriptome plasticity (Kenkel & Matz, 2017). Other thermally tolerant corals rapidly adjust their transcriptional response to thermal stress, followed by a rapid return to baseline gene expression (Savary et al., 2021; Seneca & Palumbi, 2015). For microalgal symbionts, thermal stress elicits down‐regulation of genes involved in photosynthesis and up‐regulation of genes involved in photoinhibition (Bellantuono et al., 2019; Savary et al., 2021). The response of gene expression in hospite symbionts to thermal stress is often surprisingly subtle compared with the host (Barshis et al., 2014; Leggat et al., 2011)—a phenomenon that still requires further explanation considering that many postulated bleaching mechanisms start with stress to the microalgal symbiont (but see Bellantuono et al., 2019; Voolstra et al., 2021b).
Seascape genomics approaches have begun to identify genes and gene variants of both corals and their microalgal symbionts putatively associated with thermal tolerance (Selkoe et al., 2016; Selmoni et al., 2020). Such approaches typically use genome‐wide marker sequencing (e.g., RAD‐Seq or whole genome sequencing) to evaluate allele frequency differences at thousands of loci among corals based on comparison of sites with differing thermal environments. Although such studies are still restricted to only a few coral species, these data are collected at localities with differing thermal environments to pinpoint genetic variants and localities that potentially contribute to increased thermal tolerance. While these studies can potentially provide geospatial context to the adaptive potential of corals to thermal stress (Liggins et al., 2019; Selmoni et al., 2020), care must be taken to avoid circular reasoning by projecting adaptive probabilities onto the same environmental layers that were used to create the probabilities. Recent investigations of genome‐wide associations suggest that the microalgal symbionts and environmental differences among reef localities play a prominent role in the variation of coral bleaching (Fuller et al., 2020).
For some corals that survive bleaching, repeated exposure to thermal stress can increase thermal tolerance (Brown et al., 2000, 2002; Grottoli et al., 2014; Maynard et al., 2008), but for others, it may reduce thermal tolerance (Grottoli et al., 2014; Wall et al., 2021). Although the mechanism for such “memory,” or legacy effects, is still unknown, recent studies have demonstrated that epigenetic mechanisms (i.e., notably DNA methylation as well as histone variants and their post‐translational modifications) regulate gene expression and stress‐repair mechanisms in response to thermal stress (Eirin‐Lopez & Putnam, 2019). Whether these modifications play a role in multi‐generational adaptation is still debated (Dixon et al., 2018; Torda et al., 2017). Nevertheless, epigenetic modifications occur during endosymbiosis (Li et al., 2018) and are associated with seasonal thermal changes (Rodríguez‐Casariego et al., 2020), suggesting that epigenetic mechanisms may influence the responses and acclimatization of corals to thermal stress (Liew et al., 2020).
FIGURE 2.
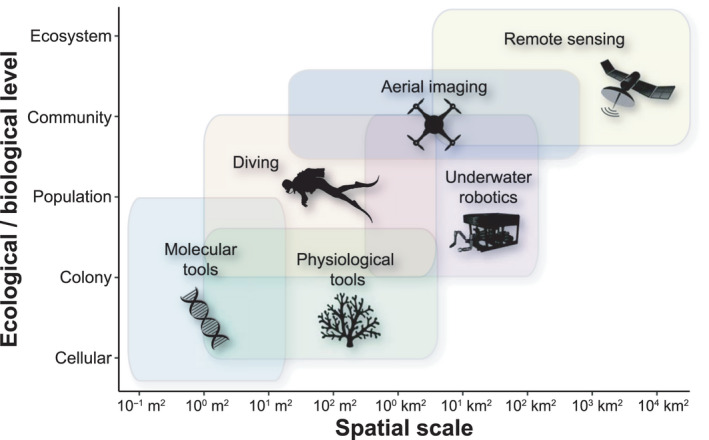
The diversity of approaches used to investigate coral bleaching across spatial and biological scales
4. LINKING CORAL‐BLEACHING RESPONSES ACROSS SCALES
Linking the probabilistic responses of corals across biological scales to environmental stress can be formulated using hierarchical approaches (Clark & Gelfand, 2006) that can capture responses and integrate data from different disciplines while accounting for multiple sources of uncertainty (Cressie et al., 2009). Effectively examining patterns and processes while preserving information and uncertainty across scales can be achieved by introducing latent variables to hierarchical Bayesian models (Clark & Gelfand, 2006). Latent variables represent unobserved quantities that are estimated using data observed at different scales. Such approaches can be used to either scale up or scale down measurements across ecosystems. For example, Wilson et al. (2011) integrated field data of vegetation biomass collected at cm to m scales with satellite imagery at the km scale. Using a similar framework, Keil et al. (2013) downscaled bird species occurrence data from 30 to 5 km. A similar approach can take advantage of spatially nested data on coral bleaching that are being collected at a variety of scales (Table 2).
TABLE 2.
Suggested processes and levels of organization to be considered within an integrated research framework to examine the effects of coral bleaching across biological scales
| Processes | Scales | Research questions | Hypotheses | Benefits |
|---|---|---|---|---|
| Carbon assimilation | Cells to colonies | How does carbon assimilation, through photosynthesis and heterotrophy, relate to bleaching susceptibility and post‐bleaching responses? | Constraints on rates of carbon assimilation will affect bleaching susceptibility, coral energy reserves, and life histories. |
Quantifying rates of carbon assimilation will increase the predictability of local, regional, and species‐specific susceptibility to bleaching and recovery. (Anthony et al., 2009; Grottoli et al., 2014; Hughes et al., 2010) |
| Calcification | Cells to ecosystems | How do thermal stress and bleaching affect coral calcification and reef carbonate production? | Changes in individual colony calcification scale to the carbonate production of reefs. |
Clarifying the effect of bleaching on coral calcification and reef‐scale carbonate production will benefit the accuracy of reef‐accretion models in the context of sea‐level rise. (Allemand et al., 2011; Cantin et al., 2010; Perry et al., 2013; van Woesik & Cacciapaglia, 2021). |
| Stress response gene networks | Genes to colonies | Is there a universal signature of stress tolerance (e.g., a generalized stress response)? | Tolerance of one stressor may predict tolerance of other stressors. |
Resolving whether thermal tolerance comes at a cost and whether there are trade‐offs will improve multi‐stress predictions. (Bay et al., 2017; Dixon et al., 2020; Rose et al., 2016; Wright et al., 2019) |
| Microalgal symbiosis | Colonies to populations | How fixed or flexible are coral host‐algal symbiont associations? What drives genetic diversity and divergence in symbiont populations and how rapidly can they adapt? | Symbionts adapt to specific host–environment combinations. |
Shedding new light on population biology of Symbiodiniaceae will improve predictions of symbiosis stability and holobiont responses. (Baker et al., 2004; LaJeunesse et al., 2018; Little et al., 2004; Rädecker et al., 2021) |
| Microbial interactions | Colonies to ecosystems | What are the roles of coral‐ and reef‐associated microbes, and can they serve as biomarkers for colony and ecosystem resilience? | The type and abundance of microbes associated with corals and reef ecosystems are indicative of reef state and resilience. |
Characterizing microbial communities is amenable to large‐scale assessment. Probiotic applications may aid colony resilience and recovery. (Peixoto et al., 2021; Voolstra et al., 2021; Ziegler et al., 2017) |
| Demographic processes | Colonies to populations | How do demographic processes, such as survival, growth, and reproduction, respond to and recover from thermal stress? | Constraints on energy reserves and metabolic rates of coral colonies have cascading repercussions on coral populations. |
Quantifying the effects of thermal stress on demography will enhance the predictability of population and community trajectories. (Cant et al., 2020; Cornwell et al., 2021; Edmunds et al., 2014) |
| Heritability | Genes to populations | How heritable is acclimatization and what is the role of epigenetics in shaping adaptation to thermal stress? | Acclimatization to thermal stress is an adaptive trait that is heritable. |
Identifying individuals harboring genetic markers predictive of thermal resilience would be useful for reef restoration and conservation efforts. (Bairos‐Novak et al., 2021; Kenkel et al., 2015; Palumbi et al., 2014; Voolstra et al., 2021a) |
| Adaptation based on genetic diversity | Genes to communities | What role does standing genetic diversity have on population and community persistence? | High genetic diversity will invariably increase adaptive potential. |
Understanding the extent and functionality of diverse genetic repositories will benefit conservation efforts and the establishment of protected reef networks. |
| Adaptation based on locality | Genes to ecosystems | Which localities are the most thermally sensitive or resilient and which are undergoing the most rapid adjustments to thermal stress? | History, geography, habitat, life‐history traits, and hydrodynamics influence connectivity and thermal tolerance. |
Knowing the extent and patterns of genetic connectivity, and where directional selection is occurring is paramount to management and conservation planning. (Balbar & Metaxas, 2019; Beger et al., 2015; Hock et al., 2017; Selkoe et al., 2016; Selmoni et al., 2020) |
Experimental work also requires transdisciplinary approaches. While studies typically measure nutritional status, gene activity, or population trajectories in isolation, the nutritional status of corals influences cellular function (Rädecker et al., 2021) and their survival through coral‐bleaching events (Levas et al., 2016). These interactions, and both their environmental and genetic basis, can be explored through reciprocal transplant experiments of coral fragments, common garden experiments, or through “identical twin” study designs (e.g., Dubé et al., 2021; Voolstra et al., 2020; Ziegler et al., 2017). Such experiments, using physiological measurements alongside transcriptomic and epigenetic markers across sites, can be highly informative in an ecological context by ascribing the relative role of genetics, acclimatization, and measures of fitness to temperature regimes, geography, and bleaching histories (Berkelmans & van Oppen, 2006; Dixon et al., 2015; Kenkel & Matz, 2017; Palumbi et al., 2014). These interdisciplinary studies can also estimate the heritability of physiological, microbial, and molecular characteristics of corals (Bairos‐Novak et al., 2021; Dubé et al., 2021; Jury et al., 2019; Kenkel et al., 2015), expediting discovery since heritability is the foundation of adaptive potential and is also an essential piece of information for predictive models (Logan et al., 2021; McManus et al., 2021) (Table 2). Such experimental work, however, should be expanded to include environmental variability, on multiple temporal scales, to better encompass natural conditions (Ziegler et al., 2021).
Since physiology and ecology interact at the individual coral‐colony level, the physiological responses of individual colonies to environmental conditions determine their relative fitness and reproductive success. Together, the vitality of individuals (i.e., growth, maintenance, and reproduction rates) determines the population growth rate after a coral‐bleaching event. Therefore, life history and demographic models may best connect coral physiology with ecology (Cant et al., 2020; Edmunds et al., 2014). There is also considerable value in collaborative efforts across regions and ocean basins. Data from experiments using a common framework can provide the material for meaningful meta‐studies (Grottoli et al., 2021), help to establish phenotypic reaction norms, and standardized effect sizes for comparative responses (Voolstra et al., 2021b; Ziegler et al., 2021). For example, using a standardized assessment of thermal limits of phenotypes (Voolstra et al., 2020) will provide reliable information and geographical context on the adaptive potential of coral populations. Repeatedly running the same experimental designs will establish a workable link between genotype, phenotype, and the environment. Such studies could investigate multifactorial effects, foster scientific exchange, and facilitate data integration to provide a holistic view of coral responses to the increasing risks of thermal stress from global climate change. Together, such research will accelerate discoveries and lead to improved models and predictions of coral bleaching and its impact on coral‐reef ecosystems (Table 2).
Another viable step towards successful interdisciplinary integration is to focus on a few key processes, such as calcification and carbon assimilation, and to track those processes from molecules to reef structures (Table 2). Using calcification as a universal currency is timely, particularly as thermal stress reduces both calcification and growth rates (Cantin et al., 2010; Cornwell et al., 2021; Davis et al., 2021), and carbonate production is an emergent property of all reef processes (Perry et al., 2013). At the cellular level, calcium‐ion transporters, carbonic anhydrases, and skeletal organic‐matrix proteins are all involved in coral calcification processes (Allemand et al., 2011). At the organismal level, calcification can be measured using the buoyant weight technique (Jokiel et al., 1978). At the reef scale, net ecosystem calcification can be measured using the total alkalinity anomaly method or estimated in the field using carbonate budgets (Perry et al., 2013; van Woesik & Cacciapaglia, 2021) (Table 2). Similarly, carbon assimilation can be studied across scales because carbon budgets link photosynthesis, respiration, heterotrophy, calcification, energy reserves, and fecundity to population responses and net primary production. Identifying the geographical extent of carbonate‐production rates, in the form of reef accretion rates and carbon assimilation is of utmost societal importance in the context of ocean warming and rising sea levels, especially for low‐lying island nations that are immediately affected by sea‐level rise.
At geographical scales, rapid advances in seascape genomics are predicting the adaptive potential of coral populations to thermal stress in a spatially explicit manner (Liggins et al., 2019; Selkoe et al., 2016; Selmoni et al., 2020). These methods associate genetic variance along environmental gradients in the context of gene flow (Selmoni et al., 2020) and require strong integration across disciplines using accurately defined environmental characteristics, biophysical modeling of larval dispersal, and genomics. Additionally, downscaled, high‐resolution temperature outputs from global climate models that predict future climate scenarios (Dixon et al., 2022; van Hooidonk et al., 2015) can improve the accuracy of predictions of latitudinal range shifts and highlight possible locations of climate‐change refugia.
5. LOOKING FORWARD
There is growing evidence that some reefs have predictably higher or lower risks of coral bleaching over successive events (Cheung et al., 2021; Thompson & van Woesik, 2009). This suggests that some of the heterogeneity seen within bleaching events is spatially conserved and, therefore, may be amenable to management. There is, however, a need to determine the inherent spatial and temporal variability of thermal tolerance of corals to provide insights into why and where certain genotypes, phenotypes, species, or localities exhibit differential responses to thermal stress. In the short term, we need time‐series studies across coral genotypes to examine changes in regulatory capacity, gene expression, and physiology before, during, and after coral bleaching. In the long term, we need to assess the likelihood of acclimatization and adaptation to increasing thermal stress for different species across habitats and oceanic regions (Coles et al., 2018; Logan et al., 2021; Matz et al., 2020; Walsworth et al., 2019). We also need more information on microbial communities and nutritional pathways involving coral symbionts, and how those communities and pathways influence symbiosis and physiological changes of the coral host that result in different bleaching responses (Claar et al., 2020; Santoro et al., 2021).
There is also a need for a better understanding of factors contributing to post‐bleaching responses and coral recovery (Claar et al., 2020; Donovan et al., 2021; Leinbach et al., 2021). The after‐effects of thermal stress on coral homeostasis are pervasive and corals can continue to lose energy reserves for weeks to months after temperatures have returned to “normal” (Leinbach et al., 2021; Rodrigues & Grottoli, 2007; Figure 3). Consequently, calcification rates do not return to “normal” for months after bleaching (Rodrigues & Grottoli, 2006). Other post‐bleaching ramifications may include the following: (i) increased disease susceptibility (Muller et al., 2008), (ii) destabilization of the coral's bacterial and viral communities (Messyasz et al., 2020; Ziegler et al., 2017), (iii) reductions in antibiotic properties of coral mucus (Ritchie, 2006), (iv) prolonged dependence on heterotrophically derived carbon (Hughes & Grottoli, 2013), (v) suppression of the coral immune system (Pinzón et al., 2015), and (vi) reductions in reproductive output (Fisch et al., 2019; Leinbach et al., 2021; Ward et al., 2000) that can be suppressed years after bleaching (Johnston et al., 2020; Levitan et al., 2014; Figure 3).
FIGURE 3.
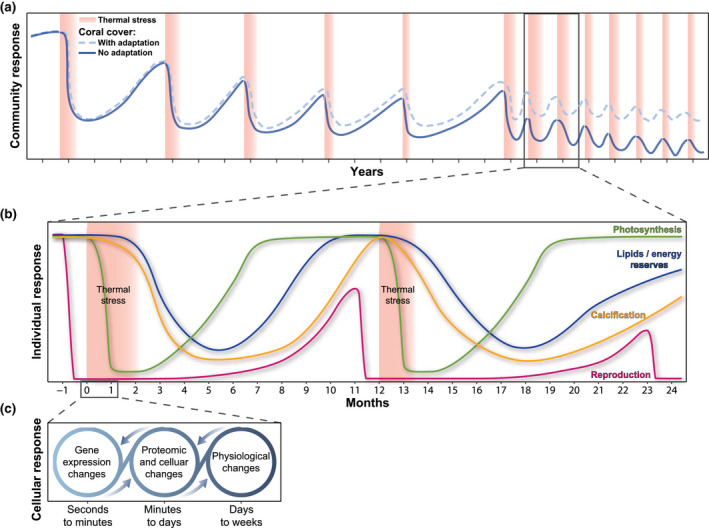
Coral responses to thermal stress at different temporal and biological scales. (a) Coral community response, reflected as overall living–coral coverage, to thermal stress and its hypothetical potential to adapt to future thermal stress; (b) Individual responses to thermal stress, showing characteristic responses of photosynthesis, calcification, reproduction, and lipid reserves; (c) Cellular responses of corals to thermal stress, where gene expression is typically considered to translate the encoded genomic potential into the resultant phenotype through proteins being expressed that in turn underlie the measured physiological change. In (a) the terms “with adaptation” and “no adaptation” refer to the potential of corals to adapt, considering both potential societal (i.e., reductions in emissions of greenhouse gases) and evolutionary (i.e., increases in thermal tolerance) adjustments
The ramifications of coral bleaching also influence community structure, architectural complexity, and ecosystem functioning of coral reefs, as bleaching may alter species composition (Loya et al., 2001; van Woesik et al., 2011), recruitment (Hughes et al., 2019), and structural complexity (Alvarez‐Filip et al., 2009). Some coral species that bleach have a high capacity for individual‐colony and population recovery, suggesting that resilience, and not only resistance, is a viable pathway to survival and persistence (Voolstra et al., 2021b). However, we are only beginning to understand the dependencies among coral physiology and genetics, nutritional pathways, and symbiont communities in the months and years following bleaching events, and the implications that post‐thermal stress responses have on the long‐term persistence of coral‐reef ecosystems.
There is an emergence of new tools to facilitate the assessment of reef communities during and following coral‐bleaching events. Such tools include surveys using drones and autonomous underwater vehicles, and recent advances in photogrammetry (e.g., structure‐from‐motion) to generate orthomosaics, which will be especially powerful when combined with developments in robotics, artificial intelligence, and machine learning (Dornelas et al., 2019; Roach et al., 2021b; Yuval et al., 2021). If collected repeatedly, these high‐resolution three‐dimensional reef‐scale archives can provide population‐level vital rates used in demographic models to make predictions of population trajectories under different environmental conditions (Cant et al., 2020; Edmunds et al., 2014). These population trajectories will provide further insight when placed into context with bio‐geophysical oceanographic models, some of which are readily accessible (Thompson et al., 2018). Advances in physical oceanographic modeling and remote sensing of air‐sea heat fluxes have provided information on reef‐heat budgets, which aim to predict temperature variability at spatial scales relevant to corals (Reid et al., 2020; Wyatt et al., 2020). Reef‐heat budgets were previously limited by a need for high‐quality bathymetry data, which are now resolved by pulsed laser Light Detection and Ranging (LiDAR), down to the scale of coral colonies. These broad‐scale applications of technologies will enhance the predictability of coral bleaching caused by anomalously high seawater temperatures.
Although the loss of corals through recent bleaching events should not be understated, there is a growing realization that some coral populations may evolve and adapt to increasing seawater temperatures (Bay et al., 2017; Coles et al., 2018; Matz et al., 2018; Wright et al., 2019). From an evolutionary perspective, thermal tolerance has genetic underpinnings (Barshish et al., 2013; Bellantuono et al., 2012; Dixon et al., 2015) and coral populations can evolve elevated stress tolerance, for example in the form of phenotypic plasticity in gene expression (Kenkel & Matz, 2017). In addition, based on the standing genetic diversity the extent of pre‐existing adaptive variants in the coral host, or the microalgal symbionts (Logan et al., 2021), may be large enough in some coral species and in some localities (Bay et al., 2017) to provide the prerequisite for positive selection (Hume et al., 2016). Indeed, recent models have shown that adaptive evolution to thermal stress can be rapid in corals (Bay et al., 2017; Matz et al., 2018). However, we currently have a limited understanding of the standing genetic diversity of different coral species and populations, and of a selection coefficient associated with thermal resilience.
Innovative solutions have also been proposed to actively facilitate coral adaptations to ocean warming, including assisted evolution or direct manipulations of the coral holobiont's adaptive response (National Academies of Sciences, Engineering, & Medicine, 2019; van Oppen et al., 2015; Voolstra et al., 2021a). Novel molecular methods will help resolve cellular responses to thermal stress, provide the ability to manipulate genomes (e.g., CRISPR, cas9), and test hypotheses and putative mechanisms involved in thermal stress (Cleves et al., 2020). These practices and their capacity to scale up both spatially and across functional groups will be dependent on our understanding of the relationships among the holobiont genome, the phenotypic responses to thermal stress, and the rapidly changing environment. The development of biomarkers for thermal tolerance will allow the identification of coral genotypes that are most likely to survive elevated temperatures, and, therefore, determine which genotypes are best suited for restoration efforts (Roach et al., 2021a). The spatially explicit consideration of genomic data, combined with detailed and standardized phenotypic responses and environmental characterizations, helps identify which heat‐tolerant individuals, species, and reefs will best tolerate and adapt to climate change (Drury et al., 2022; Voolstra et al., 2021a). Such efforts will provide the scientific foundation to help direct conservation priorities.
6. FROM SCIENCE TO MANAGEMENT ACTION
Traditional forms of marine reserves designed to protect local diversity may have limited ability to enhance thermal tolerance or protect corals from global stressors (Bruno et al., 2019). These earlier approaches are being replaced by calls for networks of sites protecting metacommunities, connected through larval dispersal (Gaines et al., 2010; Gajdzik et al., 2021; Krueck et al., 2017; Mumby et al., 2011; Walsworth et al., 2019), although most of that literature stems from fishes and non‐coral invertebrates. As thermal‐stress events continue to reduce population sizes and fragment metacommunities, it is becoming imperative to identify both barriers to dispersal, due to immigrant inviability or phenotype–environment mismatches (Marshall et al., 2010; Nosil et al., 2005; Shlesinger & Loya, 2021), and dispersal corridors and stepping‐stone sites that connect reef systems (Crandall et al., 2012; Hock et al., 2017). Such networks may facilitate coral recovery and enhance resilience and connectivity (Cheung et al., 2021). Strategically investing conservation efforts in networks of highly connected sites (Beyer et al., 2018; Krueck et al., 2017), while also prioritizing sites with high genetic diversity and reproductive potential (Beger et al., 2014; Hock et al., 2017), may be instrumental in sustaining coral populations through climate change (Morelli et al., 2020).
Reef connectivity is increasingly being considered in the design and establishment of marine protected areas (Beger et al., 2015; Magris et al., 2016; Crandall et al., 2019; but see Balbar & Metaxas, 2019). For example, when Singapore enacted its first coral‐reef marine park, authorities explicitly chose areas with both high reproductive potential and high genetic diversity (Afiq‐Rosli et al., 2021). Moreover, studies along the Australian Great Barrier Reef showed that a small fraction of reefs can serve both as potential thermal refugia and as ‘robust source reefs’ for a substantial portion of the reef network (Cheung et al., 2021; Hock et al., 2017). Similarly, in The Bahamas, estimates showed that larval supply could traverse some (but not all) scales of thermal environments (Mumby et al., 2011). Recent advances, however, go beyond the inclusion of possible thermal refugia and connectivity and incorporate potential evolutionary adaptations to climate change (Logan et al., 2021; Matz et al., 2020; McManus et al., 2021). For example, Beger et al. (2014) showed that incorporating genetic information related to diversity and connectivity may significantly change spatial conservation priorities. Likewise, Walsworth et al. (2019) provided theoretical evidence that simply protecting possible thermal refugia without accounting for the preservation of diversity can lead to the collapse of coral populations under future climatic conditions. By contrast, networks that protected habitat and genetic heterogeneity, the fuel for adaptation, were more likely to persist through climate change (Walsworth et al., 2019). For example, Hume et al. (2016) showed that the prevalent microalgal symbiont of corals in the Persian‐Arabian Gulf was selected from the pool of available genotypes present around the Arabian Sea, which is evolutionary much older (in the order of millions of years) than the Persian‐Arabian Gulf (thousands of years). Therefore, connectivity through larval dispersal, which may traverse distinct thermal environments and diverse genetic repositories, can lead to genotypes present at one location becoming essential units of selection at other locations under changing environments.
There is still, however, some disconnect between spatially–explicit models and coral responses in the field. For example, Matz et al. (2020) showed coral persistence in relatively high–latitude sites in the western Pacific Ocean under climate change, and McManus et al. (2021) showed unlikely persistence at low‐latitude sites in the Coral Triangle. Yet, in situ data are showing that some sites in the Coral Triangle are faring better than elsewhere (McClanahan et al., 2020; Sully et al., 2019). The discrepancy between some of the predictive models and field data might arise from the models not capturing the full extent of habitat heterogeneity or not reflecting the diversity in coral community response. There is, therefore, a need for concerted effort to refine spatially–explicit predictive models to include more habitat heterogeneity and more species to accurately capture field responses. Better‐informed models, more comprehensive real‐world data, and a framework for incorporating genetic information are needed to optimize efforts for marine conservation and reserve design (Beger et al., 2014; Gajdzik et al., 2021; Mumby et al., 2011; Walsworth et al., 2019). Our cross‐scale assessment here suggests that multiple features may improve coral persistence by protecting reef locations from local pollutants and regional land‐use changes that: (i) include multiple species of coral populations that are resistant to climate change; (ii) will become suitable for corals in the near future (i.e., range extensions); (iii) support high intra‐ and inter‐species diversity (i.e., evolutionary hotspots); (iv) support steep and diverse environmental gradients (i.e., temperature, depth, currents, turbidity, etc.), and thereby presumably harbor high levels of genetic diversity through the process of local adaptation; (v) host viable adult breeding stocks; and (vi) preserve highly connected sites to maintain meta‐population corridors. Yet, capturing these features in combination will require protection at scales larger than typically considered by management, leading us to recommend multinational mesoscale sanctuaries as networks of protected areas promoting coral‐reef resilience and survival in the face of climate change.
7. MESOSCALE SANCTUARIES
Some studies suggest that mitigating climate change at the global scale is the only path to conserve coral reefs (Bruno et al., 2019; Eakin et al., 2019), whereas other studies suggest that effective local management can also help sustain coral reefs (Claar et al., 2020; Donovan et al., 2021; Ortiz et al., 2018). Here we suggest “mesoscale sanctuaries” as a third option to conserve coral reefs. Some mesoscale sanctuaries (at a scale of thousands of km2) already exist, for example, the Micronesia Challenge, but they are rare across national boundaries. Multinational networks of protected areas would be designed and enforced to protect corals from local and regional disturbances through climate change. Such mesoscale sanctuaries should incorporate localities in which reef corals can persist in and potentially expand from in the future (Beyer et al., 2018). To “climate‐proof” reefs requires conserving both coral‐reef habitats and genetic diversity that can serve as the raw material for positive selection. Although traditional marine reserves are designed to protect local biodiversity and prevent over‐harvesting, additional mesoscale sanctuaries may be essential to preserve both the genetic diversity necessary to fuel evolutionary adaptation of coral holobionts, and large enough populations that can function as a source of migrants across climatic gradients (Hoffman et al., 2017). Therefore, mesoscale networks that span across national boundaries (i.e., multinational mesoscale sanctuaries) are recommended to protect diverse habitats and genetic heterogeneity that will provide coral populations with the best chance to persist through climate change.
Although there are currently several large marine protected areas in the oceans (Toonen et al., 2013; Wilhelm et al., 2014), and a multi‐national approach has been suggested for the Red Sea to boost conservation in the face of climate change (Gajdzik et al., 2021; Kleinhaus et al., 2020), there is only one transboundary agreement between the Republic of Kiribati and the United States in the central Pacific Ocean to facilitate collaboration across marine park boundaries (Friedlander et al., 2016). By contrast, multinational mesoscale sanctuaries have been successfully implemented on land. For example, in 2011 in Africa, at the convergence of the borders of Angola, Botswana, Namibia, Zambia, and Zimbabwe, the Kavango‐Zambezi Transfrontier Conservation Area (>500,000 km2) was created to protect and conserve Africa's endangered wildlife. A similar conservation area (>200,000 km2) is being planned for parts of Mozambique, South Africa, and Zimbabwe. Comparable multinational mesoscale sanctuaries for coral reefs would provide the impetus to expand, interconnect, and network local marine protected areas over broader spatial scales. We are therefore suggesting not only to increase in‐country conservation efforts but also to link those efforts across national boundaries. Since mesoscale sanctuaries would span across international waters, they would require multinational coordination (Beger et al., 2015; Gajdzik et al., 2021; Kleinhaus et al., 2020) and considerable investment into strengthening the scientific and management capacity of all parties involved in hosting the sanctuaries (Barber et al., 2014; Stefanoudis et al., 2021; Trisos et al., 2021).
Identifying where to implement mesoscale sanctuaries needs concerted scientific effort across all biological and social disciplines, although the groundwork towards this goal is already in place. Recent studies have identified several areas as potential climate‐change refugia in northwestern Indonesia, the central Philippines, Malaysia, French Polynesia (Beyer et al., 2018; Cacciapaglia & van Woesik, 2015; Hoegh‐Guldberg et al., 2018), the northern Red Sea (Fine et al., 2013; Osman et al., 2018), Hawaii (Jury & Toonen, 2019), Cuba and The Bahamas (Beyer et al., 2018). Similarly, Beger et al. (2015) identified many reefs in the Coral Triangle as essential for coral‐reef conservation through climate change. Mesoscale sanctuaries may also benefit from the inclusion of deep‐water, mesophotic reefs (Bridge et al., 2013; Soares et al., 2020), which could potentially help maintain coral populations and genetic diversity, although demographic connectivity (Bongaerts et al., 2017; Serrano et al., 2014; Shlesinger & Loya, 2021) and species overlap (Montgomery et al., 2021; Morais & Santos, 2018) across large depth gradients might be rather limited. The geographical patterns of possible climate‐change refugia align with geological studies, showing that past extinction events were less extensive for equatorial‐dwelling marine species than they were for marine species at higher latitudes (Penn et al., 2018). Yet, other paleo studies suggest that corals retracted their equatorial ranges during the last interglacial when the oceans were warmer than today (Kiessling et al., 2012), suggesting that contemporary mesoscale sanctuaries might be best located at mid‐latitudes. In combination, these studies indicate the need for equatorial and mid‐latitude mesoscale sanctuaries and an urgent need for coordinated scientific efforts and synthesis to identify the optimal location of mesoscale sanctuaries to maximize conservation resources and reduce the risk of widespread coral‐reef collapse through climate change.
There is still, however, a considerable societal barrier to management action. While most coral‐reef science is published by scientists from high‐income countries, most coral reefs are under the jurisdiction of low‐income countries (Stefanoudis et al., 2021). The persistence of “parachute science,” whereby scientists from high‐income countries collect data in coral‐rich low‐income nations without engaging local communities, tends to reduce the likelihood that the science will lead to an effective policy (Trisos et al., 2021). Without genuine collaboration and engagement, researchers are missing opportunities to build capacity and connections in the management agencies that are tasked with protecting coral reefs (Barber et al., 2014). The research associated with thermal stress on coral reefs and the optimal ways to design marine reserves needs to be an inclusive global effort to ensure that the science is rapidly translated into effective management at all geographic scales. Taking an integrative approach across biological scales, using for example hierarchical models, to estimate major coral‐reef processes will not only rapidly advance coral‐reef science but will also provide the necessary scientific information to optimize decision‐making and conservation efforts.
8. CONCLUDING REMARKS
Climate change is increasing the frequency and intensity of coral‐bleaching events and is changing the composition, architectural complexity, and functioning of coral reefs. Under this reality, the future of coral reefs may appear grim. Nonetheless, and despite global declines, it seems that many coral reefs still host enough genetic diversity for adaptation and for perhaps recovery in some form. The best way to support the resilience, adaptation, and recovery of coral reefs is to urgently reduce global emissions of greenhouse gases while working cooperatively to create both local and mesoscale coral‐reef sanctuaries. It is imperative to know which coral species and which reefs to prioritize for protection, based on their adaptive potential and innate resilience. Taking a broad transdisciplinary approach to investigate coral bleaching will improve predictive models, help mitigate the risks, and bolster management and conservation efforts to preserve coral reefs through climate change. Alongside the urgent global need to reduce emissions of greenhouse gases, all possible local and multinational actions should be made to conserve coral reefs—one of the most wondrous ecosystems on the planet—into the future.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS CONTRIBUTION
RvW and TS conceptualized the manuscript. All authors contributed to writing and editing parts of the manuscript. RvW and TS wrote the first and final drafts and all authors reviewed the final draft prior to submission.
ACKNOWLEDGMENTS
We thank the National Science Foundation OCE 1838667 and OCE 1829393, for the funding that brought this group together to discuss interdisciplinary research on coral bleaching during a weeklong virtual workshop of the Coral Bleaching Research Coordination Network (CBRCN) in May 2021. We thank Maria Beger and an anonymous reviewer for their helpful comments. We also thank Sandra van Woesik and Derya Akkaynak for their editorial comments. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the authors and do not necessarily reflect the views of NOAA or the Department of Commerce.
van Woesik, R. , Shlesinger, T. , Grottoli, A. G. , Toonen, R. J. , Vega Thurber, R. , Warner, M. E. , Marie Hulver, A. , Chapron, L. , McLachlan, R. H. , Albright, R. , Crandall, E. , DeCarlo, T. M. , Donovan, M. K. , Eirin‐Lopez, J. , Harrison, H. B. , Heron, S. F. , Huang, D. , Humanes, A. , Krueger, T. , … Zaneveld, J. (2022). Coral‐bleaching responses to climate change across biological scales. Global Change Biology, 28, 4229–4250. 10.1111/gcb.16192
REFERENCES
- Afiq‐Rosli, L. , Wainwright, B. J. , Gajanur, A. R. , Lee, A. C. , Ooi, S. K. , Chou, L. M. , & Huang, D. (2021). Barriers and corridors of gene flow in an urbanized tropical reef system. Evolutionary Applications, 14(10), 2502–2515. 10.1111/eva.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allemand, D. , Tambutté, É. , Zoccola, D. , & Tambutté, S. (2011). Coral calcification, cells to reefs. In Dubinsky Z., & Stambler N. (Eds.), Coral reefs: An ecosystem in transition (pp. 119–150). Springer. 10.1007/978-94-007-0114-4_9 [DOI] [Google Scholar]
- Alvarez‐Filip, L. , Dulvy, N. K. , Gill, J. A. , Côté, I. M. , & Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: Region‐wide declines in architectural complexity. Proceedings of the Royal Society B: Biological Sciences, 276(1669), 3019–3025. 10.1098/rspb.2009.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony, K. R. N. , Hoogenboom, M. O. , Maynard, J. A. , Grottoli, A. G. , & Middlebrook, R. (2009). Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Functional Ecology, 23(3), 539–550. 10.1111/j.1365-2435.2008.01531.x [DOI] [Google Scholar]
- Bairos‐Novak, K. R. , Hoogenboom, M. O. , van Oppen, M. J. H. , & Connolly, S. R. (2021). Coral adaptation to climate change: Meta‐analysis reveals high heritability across multiple traits. Global Change Biology, 27(22), 5694–5710. 10.1111/gcb.15829 [DOI] [PubMed] [Google Scholar]
- Baker, A. C. , Glynn, P. W. , & Riegl, B. (2008). Climate change and coral reef bleaching: An ecological assessment of long‐term impacts, recovery trends and future outlook. Estuarine, Coastal and Shelf Science, 80(4), 435–471. 10.1016/j.ecss.2008.09.003 . [DOI] [Google Scholar]
- Baker, A. C. , Starger, C. J. , McClanahan, T. R. , & Glynn, P. W. (2004). Corals’ adaptive response to climate change. Nature, 430(7001), 741. 10.1038/430741a [DOI] [PubMed] [Google Scholar]
- Balbar, A. C. , & Metaxas, A. (2019). The current application of ecological connectivity in the design of marine protected areas. Global Ecology and Conservation, 17, e00569. 10.1016/j.gecco.2019.e00569 [DOI] [Google Scholar]
- Barber, P. H. , Ablan‐Lagman, M. C. A. , Ambariyanto, A. , Berlinck, R. G. S. , Cahyani, D. , Crandall, E. D. , Ravago‐Gotanco, R. , Juinio‐Meñez, M. A. , Mahardika, I. G. N. , Shanker, K. , Starger, C. J. , Toha, A. H. A. , Anggoro, A. W. , & Willette, D. A. (2014). Advancing biodiversity research in developing countries: The need for changing paradigms. Bulletin of Marine Science, 90(1), 187–210. 10.5343/bms.2012.1108 [DOI] [Google Scholar]
- Barkley, H. C. , Cohen, A. L. , Mollica, N. R. , Brainard, R. E. , Rivera, H. E. , DeCarlo, T. M. , Lohmann, G. P. , Drenkard, E. J. , Alpert, A. E. , Young, C. W. , Vargas‐Ángel, B. , Lino, K. C. , Oliver, T. A. , Pietro, K. R. , & Luu, V. H. (2018). Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016). Communications Biology, 1, 177. 10.1038/s42003-018-0183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barott, K. L. , Venn, A. A. , Perez, S. O. , Tambutteeé, S. , Tresguerres, M. , & Somero, G. N. (2015). Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 112(2), 607–612. 10.1073/pnas.1413483112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barshis, D. J. , Ladner, J. T. , Oliver, T. A. , & Palumbi, S. R. (2014). Lineage‐specific transcriptional profiles of Symbiodinium spp. unaltered by heat stress in a coral host. Molecular Biology and Evolution, 31(6), 1343–1352. 10.1093/molbev/msu107 [DOI] [PubMed] [Google Scholar]
- Barshis, D. J. , Ladner, J. T. , Oliver, T. A. , Seneca, F. O. , Traylor‐Knowles, N. , & Palumbi, S. R. (2013). Genomic basis for coral resilience to climate change. Proceedings of the National Academy of Sciences of the United States of America, 110(4), 1387–1392. 10.1073/pnas.1210224110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay, R. A. , Rose, N. H. , Logan, C. A. , & Palumbi, S. R. (2017). Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Science Advances, 3(11), e1701413. 10.1126/sciadv.1701413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger, M. , McGowan, J. , Treml, E. A. , Green, A. L. , White, A. T. , Wolff, N. H. , Klein, C. J. , Mumby, P. J. , & Possingham, H. P. (2015). Integrating regional conservation priorities for multiple objectives into national policy. Nature Communications, 6, 8208. 10.1038/ncomms9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger, M. , Selkoe, K. A. , Treml, E. , Barber, P. H. , Von Der Heyden, S. , Crandall, E. D. , Toonen, R. J. , & Riginos, C. (2014). Evolving coral reef conservation with genetic information. Bulletin of Marine Science, 90(1), 159–185. 10.5343/BMS.2012.1106 [DOI] [Google Scholar]
- Bellantuono, A. J. , Dougan, K. E. , Granados‐Cifuentes, C. , & Rodriguez‐Lanetty, M. (2019). Free‐living and symbiotic lifestyles of a thermotolerant coral endosymbiont display profoundly distinct transcriptomes under both stable and heat stress conditions. Molecular Ecology, 28(24), 5265–5281. 10.1111/mec.15300 [DOI] [PubMed] [Google Scholar]
- Bellantuono, A. J. , Granados‐Cifuentes, C. , Miller, D. J. , Hoegh‐Guldberg, O. , & Rodriguez‐Lanetty, M. (2012). Coral thermal tolerance: Tuning gene expression to resist thermal stress. PLoS One, 7(11), e50685. 10.1371/journal.pone.0050685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelmans, R. (2002). Time‐integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Marine Ecology Progress Series, 229, 73–82. 10.3354/meps229073 [DOI] [Google Scholar]
- Berkelmans, R. , De'ath, G. , Kininmonth, S. , & Skirving, W. J. (2004). A comparison of the 1998 and 2002 coral bleaching events on the Great Barrier Reef: Spatial correlation, patterns, and predictions. Coral Reefs, 23(1), 74–83. 10.1007/s00338-003-0353-y [DOI] [Google Scholar]
- Berkelmans, R. , & van Oppen, M. J. H. (2006). The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B: Biological Sciences, 273(1599), 2305–2312. 10.1098/rspb.2006.3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, H. L. , Kennedy, E. V. , Beger, M. , Chen, C. A. , Cinner, J. E. , Darling, E. S. , Eakin, C. M. , Gates, R. D. , Heron, S. F. , Knowlton, N. , Obura, D. O. , Palumbi, S. R. , Possingham, H. P. , Puotinen, M. , Runting, R. K. , Skirving, W. J. , Spalding, M. , Wilson, K. A. , Wood, S. , … Hoegh‐Guldberg, O. (2018). Risk‐sensitive planning for conserving coral reefs under rapid climate change. Conservation Letters, 11(6), e12587. 10.1111/conl.12587 [DOI] [Google Scholar]
- Bongaerts, P. , Riginos, C. , Brunner, R. , Englebert, N. , Smith, S. R. , & Hoegh‐Guldberg, O. (2017). Deep reefs are not universal refuges: Reseeding potential varies among coral species. Science Advances, 3(2), e1602373. 10.1126/sciadv.1602373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge, T. C. L. , Hughes, T. P. , Guinotte, J. M. , & Bongaerts, P. (2013). Call to protect all coral reefs. Nature Climate Change, 3(6), 528–530. 10.1038/nclimate1879 [DOI] [Google Scholar]
- Brown, B. E. (1997). Coral bleaching: Causes and consequences. Coral Reefs, 16, 129–138. 10.1007/s003380050249 [DOI] [Google Scholar]
- Brown, B. E. , Downs, C. A. , Dunne, R. P. , & Gibb, S. W. (2002). Exploring the basis of thermotolerance in the reef coral Goniastrea aspera . Marine Ecology Progress Series, 242, 119–129. 10.3354/meps242119 [DOI] [Google Scholar]
- Brown, B. E. , Dunne, R. P. , Goodson, M. S. , & Douglas, A. E. (2000). Bleaching patterns in reef corals. Nature, 404(6774), 142–143. 10.1038/35004657 [DOI] [PubMed] [Google Scholar]
- Bruno, J. F. , Côté, I. M. , & Toth, L. T. (2019). Climate change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Annual Review of Marine Science, 11(1), 307–334. 10.1146/annurev-marine-010318-095300 [DOI] [PubMed] [Google Scholar]
- Burgess, S. C. , Johnston, E. C. , Wyatt, A. S. J. , Leichter, J. J. , & Edmunds, P. J. (2021). Response diversity in corals: hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology, 102(6), e03324. 10.1002/ecy.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia, C. W. , & van Woesik, R. (2015). Reef‐coral refugia in a rapidly changing ocean. Global Change Biology, 21(6), 2272–2282. 10.1111/gcb.12851 [DOI] [PubMed] [Google Scholar]
- Cant, J. , Salguero‐Gómez, R. , Kim, S. W. , Sims, C. A. , Sommer, B. , Brooks, M. , Malcolm, H. , Pandolfi, J. M. , & Beger, M. (2020). The projected degradation of subtropical coral assemblages by recurrent thermal stress. Journal of Animal Ecology, 90(1), 233–247. 10.1111/1365-2656.13340 [DOI] [PubMed] [Google Scholar]
- Cantin, N. E. , Cohen, A. L. , Karnauskas, K. B. , Tarrant, A. M. , & McCorkle, D. C. (2010). Ocean warming slows coral growth in the central Red Sea. Science, 329(5989), 322–325. 10.1126/science.1190182 [DOI] [PubMed] [Google Scholar]
- Castillo, K. D. , Ries, J. B. , Weiss, J. M. , & Lima, F. P. (2012). Decline of forereef corals in response to recent warming linked to history of thermal exposure. Nature Climate Change, 2(10), 756–760. 10.1038/nclimate1577 [DOI] [Google Scholar]
- Cheung, M. W. M. , Hock, K. , Skirving, W. , & Mumby, P. J. (2021). Cumulative bleaching undermines systemic resilience of the Great Barrier Reef. Current Biology, 31(23), 5385–5392.e4. 10.1016/j.cub.2021.09.078 [DOI] [PubMed] [Google Scholar]
- Claar, D. C. , Starko, S. , Tietjen, K. L. , Epstein, H. E. , Cunning, R. , Cobb, K. M. , Baker, A. C. , Gates, R. D. , & Baum, J. K. (2020). Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nature Communications, 11, 6097. 10.1038/s41467-020-19169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J. S. , & Gelfand, A. E. (2006). Hierarchical modelling for the environmental sciences: Statistical methods and applications. Oxford University Press. [Google Scholar]
- Clement, A. C. , Seager, R. , Cane, M. A. , & Zebiak, S. E. (1996). An ocean dynamical thermostat. Journal of Climate, 9(9), 2190–2196. [DOI] [Google Scholar]
- Cleves, P. A. , Tinoco, A. I. , Bradford, J. , Perrin, D. , Bay, L. K. , & Pringle, J. R. (2020). Reduced thermal tolerance in a coral carrying CRISPR‐induced mutations in the gene for a heat‐shock transcription factor. Proceedings of the National Academy of Sciences of the United States of America, 117(46), 28899–28905. 10.1073/pnas.1920779117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles, S. L. , Bahr, K. D. , Rodgers, K. S. , May, S. L. , McGowan, A. E. , Tsang, A. , Bumgarner, J. , & Han, J. H. (2018). Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ, 6, e5347. 10.7717/peerj.5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles, S. L. , & Jokiel, P. L. (1977). Effects of temperature on photosynthesis and respiration in hermatypic corals. Marine Biology, 43(3), 209–216. 10.1007/bf00402313 [DOI] [Google Scholar]
- Cornwell, B. , Armstrong, K. , Walker, N. S. , Lippert, M. , Nestor, V. , Golbuu, Y. , & Palumbi, S. R. (2021). Widespread variation in heat tolerance and symbiont load are associated with growth tradeoffs in the coral Acropora hyacinthus in Palau. eLife, 10, e64790. 10.7554/eLife.64790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall, E. D. , Toonen, R. J. , Laboratory, T. , & Selkoe, K. A. (2019). A coalescent sampler successfully detects biologically meaningful population structure overlooked by F‐statistics. Evolutionary Applications, 12(2), 255–265. 10.1111/eva.12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall, E. D. , Treml, E. A. , & Barber, P. H. (2012). Coalescent and biophysical models of stepping‐stone gene flow in neritid snails. Molecular Ecology, 21(22), 5579–5598. 10.1111/mec.12031 [DOI] [PubMed] [Google Scholar]
- Cressie, N. , Calder, C. A. , Clark, J. S. , Ver Hoef, J. M. , & Wikle, C. K. (2009). Accounting for uncertainty in ecological analysis: The strengths and limitations of hierarchical statistical modeling. Ecological Applications, 19(3), 553–570. 10.1890/07-0744.1 [DOI] [PubMed] [Google Scholar]
- Davis, K. L. , Colefax, A. P. , Tucker, J. P. , Kelaher, B. P. , & Santos, I. R. (2021). Global coral reef ecosystems exhibit declining calcification and increasing primary productivity. Communications Earth & Environment, 2, 105. 10.1038/s43247-021-00168-w [DOI] [Google Scholar]
- Davy, S. K. , Allemand, D. , & Weis, V. M. (2012). Cell biology of cnidarian‐dinoflagellate symbiosis. Microbiology and Molecular Biology Reviews, 76(2), 229–261. 10.1128/mmbr.05014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo, T. M. , Gajdzik, L. , Ellis, J. , Coker, D. J. , Roberts, M. B. , Hammerman, N. M. , Pandolfi, J. M. , Monroe, A. A. , & Berumen, M. L. (2020). Nutrient‐supplying ocean currents modulate coral bleaching susceptibility. Science Advances, 6(34), eabc5493. 10.1126/sciadv.abc5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deck, J. , Gaither, M. R. , Ewing, R. , Bird, C. E. , Davies, N. , Meyer, C. , Riginos, C. , Toonen, R. J. , & Crandall, E. D. (2017). The Genomic Observatories Metadatabase (GeOMe): A new repository for field and sampling event metadata associated with genetic samples. PLoS Biology, 15(8), e2002925. 10.1371/journal.pbio.2002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo, M. K. , Voolstra, C. R. , Sunagawa, S. , Schwarz, J. A. , Stillman, J. H. , Coffroth, M. A. , Szmant, A. M. , & Medina, M. (2008). Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata . Molecular Ecology, 17, 3952–3971. 10.1111/j.1365-294X.2008.03879.x [DOI] [PubMed] [Google Scholar]
- Dixon, A. M. , Forster, P. M. , Heron, S. F. , Stoner, A. M. K. , & Beger, M. (2022). Future loss of local‐scale thermal refugia in coral reef ecosystems. PLOS Climate, 1(2), e0000004. 10.1371/journal.pclm.0000004 [DOI] [Google Scholar]
- Dixon, G. B. , Abbott, E. , & Matz, M. V. (2020). Meta‐analysis of the coral environmental stress response: Acropora corals show opposing responses depending on stress intensity. Molecular Ecology, 29(15), 2855–2870. 10.1111/mec.15535 [DOI] [PubMed] [Google Scholar]
- Dixon, G. B. , Davies, S. W. , Aglyamova, G. V. , Meyer, E. , Bay, L. K. , & Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science, 348(6242), 1460–1462. 10.1126/science.1261224 [DOI] [PubMed] [Google Scholar]
- Dixon, G. B. , Liao, Y. , Bay, L. K. , & Matz, M. V. (2018). Role of gene body methylation in acclimatization and adaptation in a basal metazoan. Proceedings of the National Academy of Sciences of the United States of America, 115(52), 13342–13346. 10.1073/pnas.1813749115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, K. L. , Levas, S. , Schoepf, V. , Warner, M. E. , Cai, W. J. , Hoadley, K. D. , Yuan, X. , Matsui, Y. , Melman, T. F. , & Grottoli, A. G. (2021). Moderate nutrient concentrations are not detrimental to corals under future ocean conditions. Marine Biology, 168(7), 98. 10.1007/s00227-021-03901-3 [DOI] [Google Scholar]
- Doering, T. , Wall, M. , Putchim, L. , Rattanawongwan, T. , Schroeder, R. , Hentschel, U. , & Roik, A. (2021). Towards enhancing coral heat tolerance: a “microbiome transplantation” treatment using inoculations of homogenized coral tissues. Microbiome, 9, 102. 10.1186/s40168-021-01053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, M. K. , Adam, T. C. , Shantz, A. A. , Speare, K. E. , Munsterman, K. S. , Rice, M. M. , Schmitt, R. J. , Holbrook, S. J. , & Burkepile, D. E. (2020). Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Proceedings of the National Academy of Sciences of the United States of America, 117(10), 5351–5357. 10.1073/pnas.1915395117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, M. K. , Burkepile, D. E. , Kratochwill, C. , Shlesinger, T. , Sully, S. , Oliver, T. A. , Hodgson, G. , Freiwald, J. , & van Woesik, R. (2021). Local conditions magnify coral loss after marine heatwaves. Science, 372(6545), 977–980. 10.1126/science.abd9464 [DOI] [PubMed] [Google Scholar]
- Dornelas, M. , Madin, E. M. P. , Bunce, M. , DiBattista, J. D. , Johnson, M. , Madin, J. S. , Magurran, A. E. , McGill, B. J. , Pettorelli, N. , Pizarro, O. , Williams, S. B. , Winter, M. , & Bates, A. E. (2019). Towards a macroscope: Leveraging technology to transform the breadth, scale and resolution of macroecological data. Global Ecology and Biogeography, 28(12), 1937–1948. 10.1111/geb.13025 [DOI] [Google Scholar]
- Drury, C. , Martin, R. E. , Knapp, D. E. , Heckler, J. , Levy, J. , Gates, R. D. , & Asner, G. P. (2022). Ecosystem‐scale mapping of coral species and thermal tolerance. Frontiers in Ecology and the Environment, 10.1002/fee.2483 [DOI] [Google Scholar]
- Dubé, C. E. , Ziegler, M. , Mercière, A. , Boissin, E. , Planes, S. , Bourmaud, C. A. F. , & Voolstra, C. R. (2021). Naturally occurring fire coral clones demonstrate a genetic and environmental basis of microbiome composition. Nature Communications, 12, 6402. 10.1038/s41467-021-26543-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, S. R. , Pernice, M. , Green, K. , Hoegh‐Guldberg, O. , & Dove, S. G. (2012). Thermal stress promotes host mitochondrial degradation in symbiotic cnidarians: Are the batteries of the reef going to run out? PLoS One, 7(7), e39024. 10.1371/journal.pone.0039024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin, M. C. , Sweatman, H. P. A. , & Brainard, R. E. (2019). The 2014–2017 global‐scale coral bleaching event: Insights and impacts. Coral Reefs, 38(4), 539–545. 10.1007/s00338-019-01844-2 [DOI] [Google Scholar]
- Edmunds, P. J. , Burgess, S. C. , Putnam, H. M. , Baskett, M. L. , Bramanti, L. , Fabina, N. S. , Han, X. , Lesser, M. P. , Madin, J. S. , Wall, C. B. , Yost, D. M. , & Gates, R. D. (2014). Evaluating the causal basis of ecological success within the scleractinia: An integral projection model approach. Marine Biology, 161(12), 2719–2734. 10.1007/s00227-014-2547-y [DOI] [Google Scholar]
- Eirin‐Lopez, J. M. , & Putnam, H. M. (2019). Marine environmental epigenetics. Annual Review of Marine Science, 11, 335–368. 10.1146/annurev-marine-010318-095114 [DOI] [PubMed] [Google Scholar]
- Ferrario, F. , Beck, M. W. , Storlazzi, C. D. , Micheli, F. , Shepard, C. C. , & Airoldi, L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nature Communications, 5, 3794. 10.1038/ncomms4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifer, J. , Bentlage, B. , Lemer, S. , Fujimura, A. G. , Sweet, M. , & Raymundo, L. J. (2021). Going with the flow: How corals in high‐flow environments can beat the heat. Molecular Ecology, 30(9), 2009–2024. 10.1111/mec.15869 [DOI] [PubMed] [Google Scholar]
- Fine, M. , Gildor, H. , & Genin, A. (2013). A coral reef refuge in the Red Sea. Global Change Biology, 19(12), 3640–3647. 10.1111/gcb.12356 [DOI] [PubMed] [Google Scholar]
- Fine, M. , & Loya, Y. (2002). Endolithic algae: an alternative source of photoassimilates during coral bleaching. Proceedings of the Royal Society B: Biological Sciences, 269, 1205–1210. 10.1098/rspb.2002.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch, J. , Drury, C. , Towle, E. K. , Winter, R. N. , & Miller, M. W. (2019). Physiological and reproductive repercussions of consecutive summer bleaching events of the threatened Caribbean coral Orbicella faveolata . Coral Reefs, 38(4), 863–876. 10.1007/s00338-019-01817-5 [DOI] [Google Scholar]
- Frade, P. R. , Bongaerts, P. , Englebert, N. , Rogers, A. , González‐Rivero, M. , & Hoegh‐Guldberg, O. (2018). Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nature Communications, 9(1), 3447. 10.1038/s41467-018-05741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander, A. M. , Wagner, D. , Gaymer, C. F. , Wilhelm, T. A. , Lewis, N. A. , Brooke, S. , Kikiloi, K. , & Varmer, O. (2016). Co‐operation between large‐scale MPAs: Successful experiences from the Pacific Ocean. Aquatic Conservation: Marine and Freshwater Ecosystems, 26, 126–141. 10.1002/aqc.2645 [DOI] [Google Scholar]
- Fuller, Z. L. , Mocellin, V. J. L. , Morris, L. A. , Cantin, N. , Shepherd, J. , Sarre, L. , Peng, J. , Liao, Y. , Pickrell, J. , Andolfatto, P. , Matz, M. , Bay, L. K. , & Przeworski, M. (2020). Population genetics of the coral Acropora millepora: Toward genomic prediction of bleaching. Science, 369(6501), eaba4674. 10.1126/science.aba4674 [DOI] [PubMed] [Google Scholar]
- Gaines, S. D. , White, C. , Carr, M. H. , & Palumbi, S. R. (2010). Designing marine reserve networks for both conservation and fisheries management. Proceedings of the National Academy of Sciences, 107(43), 18286–18293. 10.1073/pnas.0906473107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdzik, L. , DeCarlo, T. M. , Aylagas, E. , Coker, D. J. , Green, A. L. , Majoris, J. E. , Saderne, V. F. , Carvalho, S. , & Berumen, M. L. (2021). A portfolio of climate‐tailored approaches to advance the design of marine protected areas in the Red Sea. Global Change Biology, 27(17), 3956–3968. 10.1111/gcb.15719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates, R. D. , Baghdasarian, G. , & Muscatine, L. (1992). Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. The Biological Bulletin, 182(3), 324–332. 10.2307/1542252 [DOI] [PubMed] [Google Scholar]
- Glynn, P. W. (1983). Extensive “bleaching” and death of reef corals on the pacific coast of Panamá. Environmental Conservation, 10(2), 149–154. www.jstor.org/stable/44517481 [Google Scholar]
- Glynn, P. W. (1996). Coral reef bleaching: Facts, hypotheses and implications. Global Change Biology, 2(6), 495–509. 10.1111/j.1365-2486.1996.tb00063.x [DOI] [Google Scholar]
- Gonzalez‐Espinosa, P. C. , & Donner, S. D. (2021). Cloudiness reduces the bleaching response of coral reefs exposed to heat stress. Global Change Biology, 27(15), 3474–3486. 10.1111/gcb.15676 [DOI] [PubMed] [Google Scholar]
- Goreau, T. F. (1964). Mass expulsion of zooxanthellae from Jamaican reef communities after Hurricane Flora. Science, 145(3630), 383–386. 10.1126/science.145.3630.383 [DOI] [PubMed] [Google Scholar]
- Grottoli, A. G. , Rodrigues, L. J. , & Palardy, J. E. (2006). Heterotrophic plasticity and resilience in bleached corals. Nature, 440(7088), 1186–1189. 10.1038/nature04565 [DOI] [PubMed] [Google Scholar]
- Grottoli, A. G. , Toonen, R. J. , van Woesik, R. , Vega Thurber, R. , Warner, M. E. , McLachlan, R. H. , Price, J. T. , Bahr, K. D. , Baums, I. B. , Castillo, K. , Coffroth, M. A. , Cunning, R. , Dobson, K. , Donahue, M. , Hench, J. L. , Iglesias‐Prieto, R. , Kemp, D. W. , Kenkel, C. D. , Kline, D. I. , … Wu, H. C. (2021). Increasing comparability among coral bleaching experiments. Ecological Applications, 31(4), e02262. 10.1002/eap.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottoli, A. G. , Warner, M. E. , Levas, S. J. , Aschaffenburg, M. D. , Schoepf, V. , Mcginley, M. , Baumann, J. , & Matsui, Y. (2014). The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology, 20(12), 3823–3833. 10.1111/gcb.12658 [DOI] [PubMed] [Google Scholar]
- Hedley, J. D. , Roelfsema, C. M. , Chollett, I. , Harborne, A. R. , Heron, S. F. , Weeks, S. J. , Skirving, W. J. , Strong, A. E. , Eakin, M. C. , Christensen, T. R. L. , Ticzon, V. , Bejarano, S. , & Mumby, P. J. (2016). Remote sensing of coral reefs for monitoring and management: A review. Remote Sensing, 8(2), 118. 10.3390/rs8020118 [DOI] [Google Scholar]
- Hedley, J. D. , Roelfsema, C. M. , Koetz, B. , & Phinn, S. (2012). Capability of the Sentinel 2 mission for tropical coral reef mapping and coral bleaching detection. Remote Sensing of Environment, 120, 145–155. 10.1016/J.RSE.2011.06.028 [DOI] [Google Scholar]
- Heron, S. F. , Maynard, J. A. , van Hooidonk, R. , & Eakin, C. M. (2016). Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Scientific Reports, 6, 38402. 10.1038/srep38402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock, K. , Wolff, N. H. , Ortiz, J. C. , Condie, S. A. , Anthony, K. R. N. , Blackwell, P. G. , & Mumby, P. J. (2017). Connectivity and systemic resilience of the Great Barrier Reef. PLOS Biology, 15(11), e2003355. 10.1371/journal.pbio.2003355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh‐Guldberg, O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research, 50(8), 839–866. 10.1071/Mf99078 [DOI] [Google Scholar]
- Hoegh‐Guldberg, O. , Kennedy, E. V. , Beyer, H. L. , McClennen, C. , & Possingham, H. P. (2018). Securing a long‐term future for coral reefs. Trends in Ecology and Evolution, 33(12), 936–944. 10.1016/j.tree.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Hoeksema, B. W. (1991). Control of bleaching in mushroom coral populations (Scleractinia: Fungiidae) in the Java Sea: Stress tolerance and interference by life history strategy. Marine Ecology Progress Series, 74, 225–237. 10.3354/meps074225 [DOI] [Google Scholar]
- Hoffmann, A. A. , Sgrò, C. M. , & Kristensen, T. N. (2017). Revisiting adaptive potential, population size, and conservation. Trends in Ecology & Evolution, 32(7), 506–517. 10.1016/j.tree.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Hughes, A. D. , & Grottoli, A. G. (2013). Heterotrophic compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS One, 8(11), e81172. 10.1371/journal.pone.0081172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, A. D. , Grottoli, A. G. , Pease, T. K. , & Matsui, Y. (2010). Acquisition and assimilation of carbon in non‐bleached and bleached corals. Marine Ecology Progress Series, 420, 91–101. 10.3354/meps08866 [DOI] [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Baird, A. H. , Connolly, S. R. , Chase, T. J. , Dietzel, A. , Hill, T. , Hoey, A. S. , Hoogenboom, M. O. , Jacobson, M. , Kerswell, A. , Madin, J. S. , Mieog, A. , Paley, A. S. , Pratchett, M. S. , Torda, G. , & Woods, R. M. (2019). Global warming impairs stock–recruitment dynamics of corals. Nature, 568(7752), 387–390. 10.1038/s41586-019-1081-y [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Baird, A. H. , Connolly, S. R. , Dietzel, A. , Eakin, C. M. , Heron, S. F. , Hoey, A. S. , Hoogenboom, M. O. , Liu, G. , McWilliam, M. J. , Pears, R. J. , Pratchett, M. S. , Skirving, W. J. , Stella, J. S. , & Torda, G. (2018). Global warming transforms coral reef assemblages. Nature, 556(7702), 492–496. 10.1038/s41586-018-0041-2 [DOI] [PubMed] [Google Scholar]
- Hume, B. C. C. , Mejia‐Restrepo, A. , Voolstra, C. R. , & Berumen, M. L. (2020). Fine‐scale delineation of Symbiodiniaceae genotypes on a previously bleached central Red Sea reef system demonstrates a prevalence of coral host‐specific associations. Coral Reefs, 39(3), 583–601. 10.1007/s00338-020-01917-7 [DOI] [Google Scholar]
- Hume, B. C. C. , Smith, E. G. , Ziegler, M. , Warrington, H. J. M. , Burt, J. A. , LaJeunesse, T. C. , Wiedenmann, J. , & Voolstra, C. R. (2019). SymPortal: A novel analytical framework and platform for coral algal symbiont next‐generation sequencing ITS2 profiling. Molecular Ecology Resources, 19(4), 1063–1080. 10.1111/1755-0998.13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, B. C. C. , Voolstra, C. R. , Arif, C. , D’Angelo, C. , Burt, J. A. , Eyal, G. , Loya, Y. , & Wiedenmann, J. (2016). Ancestral genetic diversity associated with the rapid spread of stress‐tolerant coral symbionts in response to Holocene climate change. Proceedings of the National Academy of Sciences of the United States of America, 113(16), 4416–4421. 10.1073/pnas.1601910113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias‐Prieto, R. , Matta, J. L. , Robins, W. A. , & Trench, R. K. (1992). Photosynthetic response to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proceedings of the National Academy of Sciences of the United States of America, 89(21), 10302–10305. 10.1073/pnas.89.21.10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, E. C. , Counsell, C. W. W. , Sale, T. L. , Burgess, S. C. , & Toonen, R. J. (2020). The legacy of stress: Coral bleaching impacts reproduction years later. Functional Ecology, 34, 2315–2325. 10.1111/1365-2435.13653 [DOI] [Google Scholar]
- Jokiel, P. L. , Dubinsky, Z. , & Stambler, N. (1994). Results of the 1991 United States‐Israel workshop, “Nutrient limitation in the symbiotic association between zooxanthellae and reef‐building corals”. Pacific Science, 48(3), 215–218. [Google Scholar]
- Jokiel, P. L. , Maragos, J. E. , & Franzisket, L. (1978). Coral growth: Buoyant weight technique. In Stoddart D. R., & Johannes R. E. (Eds.), Coral reefs: Research methods (pp. 529–541). UNESCO. [Google Scholar]
- Jones, A. M. , & Berkelmans, R. (2011). Tradeoffs to thermal acclimation: Energetics and reproduction of a reef coral with heat tolerant Symbiodinium type‐D. Journal of Marine Biology, 2011, 185890. 10.1155/2011/185890 [DOI] [Google Scholar]
- Jury, C. P. , Delano, M. N. , & Toonen, R. J. (2019). High heritability of coral calcification rates and evolutionary potential under ocean acidification. Scientific Reports, 9, 20419. 10.1038/s41598-019-56313-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury, C. P. , & Toonen, R. J. (2019). Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proceedings of the Royal Society B: Biological Sciences, 286(1902), 20190614. 10.1098/rspb.2019.0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahng, S. E. , Akkaynak, D. , Shlesinger, T. , Hochberg, E. J. , Wiedenmann, J. , Tamir, R. , & Tchernov, D. (2019). Light, temperature, photosynthesis, heterotrophy, and the lower depth limits of mesophotic coral ecosystems. In Loya Y., Puglise K. A., & Bridge T. C. L. (Eds.), Mesophotic coral ecosystems (pp. 801–828). Springer. 10.1007/978-3-319-92735-0_42 [DOI] [Google Scholar]
- Keil, P. , Belmaker, J. , Wilson, A. M. , Unitt, P. , & Jetz, W. (2013). Downscaling of species distribution models: A hierarchical approach. Methods in Ecology and Evolution, 4(1), 82–94. 10.1111/J.2041-210X.2012.00264.X [DOI] [Google Scholar]
- Kenkel, C. D. , & Matz, M. V. (2017). Gene expression plasticity as a mechanism of coral adaptation to a variable environment. Nature Ecology & Evolution, 1, 14. 10.1038/s41559-016-0014 [DOI] [PubMed] [Google Scholar]
- Kenkel, C. D. , Setta, S. P. , & Matz, M. V. (2015). Heritable differences in fitness‐related traits among populations of the mustard hill coral, Porites astreoides . Heredity, 115(6), 509–516. 10.1038/hdy.2015.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling, W. , Simpson, C. , Beck, B. , Mewis, H. , & Pandolfi, J. M. (2012). Equatorial decline of reef corals during the last Pleistocene interglacial. Proceedings of the National Academy of Sciences of the United States of America, 109(52), 21378–21383. 10.1073/pnas.1214037110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen, R. M. , Piscetta, M. , de Souza, M. R. , Lenz, E. A. , Schar, D. W. H. , Gates, R. D. , & Wall, C. B. (2020). Symbiont transmission and reproductive mode influence responses of three Hawaiian coral larvae to elevated temperature and nutrients. Coral Reefs, 39(2), 419–431. 10.1007/s00338-020-01905-x [DOI] [Google Scholar]
- Kleinhaus, K. , Al‐Sawalmih, A. , Barshis, D. J. , Genin, A. , Grace, L. N. , Hoegh‐Guldberg, O. , Loya, Y. , Meibom, A. , Osman, E. O. , Ruch, J.‐D. , Shaked, Y. , Voolstra, C. R. , Zvuloni, A. , & Fine, M. (2020). Science, diplomacy, and the Red Sea’s unique coral reef: It’s time for action. Frontiers in Marine Science, 7, 90. 10.3389/fmars.2020.00090 [DOI] [Google Scholar]
- Klepac, C. N. , & Barshis, D. J. (2020). Reduced thermal tolerance of massive coral species in a highly variable environment. Proceedings of the Royal Society B: Biological Sciences, 287(1933), 20201379. 10.1098/rspb.2020.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas, J. A. , Danabasoglu, G. , & Lough, J. M. (2008). Potential role of the ocean thermostat in determining regional differences in coral reef bleaching events. Geophysical Research Letters, 35(3), L03613. 10.1029/2007gl032257 [DOI] [Google Scholar]
- Krueck, N. C. , Ahmadia, G. N. , Green, A. , Jones, G. P. , Possingham, H. P. , Riginos, C. , Treml, E. A. , & Mumby, P. J. (2017). Incorporating larval dispersal into MPA design for both conservation and fisheries. Ecological Applications, 27(3), 925–941. 10.1002/eap.1495 [DOI] [PubMed] [Google Scholar]
- Krueger, T. , Becker, S. , Pontasch, S. , Dove, S. , Hoegh‐Guldberg, O. , Leggat, W. , Fisher, P. L. , & Davy, S. K. (2014). Antioxidant plasticity and thermal sensitivity in four types of Symbiodinium sp. Journal of Phycology, 50(6), 1035–1047. 10.1111/jpy.12232 [DOI] [PubMed] [Google Scholar]
- LaJeunesse, T. C. , Parkinson, J. E. , Gabrielson, P. W. , Jeong, H. J. , Reimer, J. D. , Voolstra, C. R. , & Santos, S. R. (2018). Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology, 28(16), 2570–2580. 10.1016/j.cub.2018.07.008 [DOI] [PubMed] [Google Scholar]
- LaJeunesse, T. C. , Smith, R. T. , Finney, J. , & Oxenford, H. (2009). Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proceedings of the Royal Society B: Biological Sciences, 276(1676), 4139–4148. 10.1098/rspb.2009.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggat, W. P. , Camp, E. F. , Suggett, D. J. , Heron, S. F. , Fordyce, A. J. , Gardner, S. , Deakin, L. , Turner, M. , Beeching, L. J. , Kuzhiumparambil, U. , Eakin, C. M. , & Ainsworth, T. D. (2019). Rapid coral decay is associated with marine heatwave mortality events on reefs. Current Biology, 29(16), 2723–2730. 10.1016/j.cub.2019.06.077 [DOI] [PubMed] [Google Scholar]
- Leggat, W. P. , Seneca, F. , Wasmund, K. , Ukani, L. , Yellowlees, D. , & Ainsworth, T. D. (2011). Differential responses of the coral host and their algal symbiont to thermal stress. PLoS One, 6(10), e26687. 10.1371/journal.pone.0026687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinbach, S. E. , Speare, K. E. , Rossin, A. M. , Holstein, D. M. , & Strader, M. E. (2021). Energetic and reproductive costs of coral recovery in divergent bleaching responses. Scientific Reports, 11, 23546. 10.1038/s41598-021-02807-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser, M. P. (1997). Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs, 16(3), 187–192. 10.1007/s003380050073 [DOI] [Google Scholar]
- Lesser, M. P. (2021). Eutrophication on coral reefs: What is the evidence for phase shifts, nutrient limitation and coral bleaching. BioScience, 71(12), 1216–1233. 10.1093/biosci/biab101 [DOI] [Google Scholar]
- Lesser, M. P. , & Farrell, J. H. (2004). Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs, 23(3), 367–377. 10.1007/s00338-004-0392-z [DOI] [Google Scholar]
- Levas, S. J. , Grottoli, A. G. , Hughes, A. , Osburn, C. L. , & Matsui, Y. (2013). Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: Implications for resilience in mounding corals. PLoS One, 8(5), e63267. 10.1371/journal.pone.0063267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levas, S. J. , Grottoli, A. G. , Schoepf, V. , Aschaffenburg, M. , Baumann, J. , Bauer, J. E. , & Warner, M. E. (2016). Can heterotrophic uptake of dissolved organic carbon and zooplankton mitigate carbon budget deficits in annually bleached corals? Coral Reefs, 35(2), 495–506. 10.1007/s00338-015-1390-z [DOI] [Google Scholar]
- Levin, R. A. , Voolstra, C. R. , Weynberg, K. D. , & van Oppen, M. J. H. (2017). Evidence for a role of viruses in the thermal sensitivity of coral photosymbionts. ISME Journal, 11(3), 808–812. 10.1038/ismej.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan, D. R. , Boudreau, W. , Jara, J. , & Knowlton, N. (2014). Long‐term reduced spawning in Orbicella coral species due to temperature stress. Marine Ecology Progress Series, 515, 1–10. 10.3354/meps11063 [DOI] [Google Scholar]
- Lewis, C. , Neely, K. , & Rodriguez‐Lanetty, M. (2019). Recurring episodes of thermal stress shift the balance from a dominant host‐specialist to a background host‐generalist zooxanthella in the threatened pillar coral, Dendrogyra cylindrus . Frontiers in Marine Science, 6, 5. 10.3389/fmars.2019.00005 [DOI] [Google Scholar]
- Li, Y. , Liew, Y. J. , Cui, G. , Cziesielski, M. J. , Zahran, N. , Michell, C. T. , Voolstra, C. R. , & Aranda, M. (2018). DNA methylation regulates transcriptional homeostasis of algal endosymbiosis in the coral model Aiptasia. Science Advances, 4(8), eaat2142. 10.1126/sciadv.aat2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, Y. J. , Howells, E. J. , Wang, X. , Michell, C. T. , Burt, J. A. , Idaghdour, Y. , & Aranda, M. (2020). Intergenerational epigenetic inheritance in reef‐building corals. Nature Climate Change, 10(3), 254–259. 10.1038/s41558-019-0687-2 [DOI] [Google Scholar]
- Liggins, L. , Treml, E. A. , & Riginos, C. (2019). Seascape genomics: Contextualizing adaptive and neutral genomic variation in the ocean environment. In Oleksiak M., & Rajora O. (Eds.), Population genomics: Marine organisms (pp. 171–218). Springer. 10.1007/13836_2019_68 [DOI] [Google Scholar]
- Little, A. F. , van Oppen, M. J. H. , & Willis, B. L. (2004). Flexibility in algal endosymbioses shapes growth in reef corals. Science, 304(5676), 1492–1494. 10.1126/science.1095733 [DOI] [PubMed] [Google Scholar]
- Logan, C. A. , Dunne, J. P. , Ryan, J. S. , Baskett, M. L. , & Donner, S. D. (2021). Quantifying global potential for coral evolutionary response to climate change. Nature Climate Change, 11, 537–542. 10.1038/s41558-021-01037-2 [DOI] [Google Scholar]
- Loya, Y. , Sakai, K. , Yamazato, K. , Nakano, Y. , Sambali, H. , & van Woesik, R. (2001). Coral bleaching: the winners and the losers. Ecology Letters, 4(2), 122–131. 10.1046/j.1461-0248.2001.00203.x [DOI] [Google Scholar]
- Magris, R. A. , Treml, E. A. , Pressey, R. L. , & Weeks, R. (2016). Integrating multiple species connectivity and habitat quality into conservation planning for coral reefs. Ecography, 39(7), 649–664. 10.1111/ecog.01507 [DOI] [Google Scholar]
- Marshall, D. J. , Monro, K. , Bode, M. , Keough, M. J. , & Swearer, S. (2010). Phenotype–environment mismatches reduce connectivity in the sea. Ecology Letters, 13(1), 128–140. 10.1111/j.1461-0248.2009.01408.x [DOI] [PubMed] [Google Scholar]
- Marshall, P. A. , & Baird, A. H. (2000). Bleaching of corals on the Great Barrier Reef: Differential susceptibilities among taxa. Coral Reefs, 19(2), 155–163. 10.1007/s003380000086 [DOI] [Google Scholar]
- Matz, M. V. , Treml, E. A. , Aglyamova, G. V. , & Bay, L. K. (2018). Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLoS Genetics, 14(4), e1007220. 10.1371/journal.pgen.1007220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz, M. V. , Treml, E. A. , & Haller, B. C. (2020). Estimating the potential for coral adaptation to global warming across the Indo‐West Pacific. Global Change Biology, 26(6), 3473–3481. 10.1111/gcb.15060 [DOI] [PubMed] [Google Scholar]
- Maynard, J. A. , Anthony, K. R. N. , Marshall, P. A. , & Masiri, I. (2008). Major bleaching events can lead to increased thermal tolerance in corals. Marine Biology, 155(2), 173–182. 10.1007/s00227-008-1015-y [DOI] [Google Scholar]
- McClanahan, T. R. , Maina, J. M. , Darling, E. S. , Guillaume, M. M. M. , Muthiga, N. A. , D’agata, S. , Leblond, J. , Arthur, R. , Jupiter, S. D. , Wilson, S. K. , Mangubhai, S. , Ussi, A. M. , Humphries, A. T. , Patankar, V. , Shedrawi, G. , Julius, P. , Ndagala, J. , & Grimsditch, G. (2020). Large geographic variability in the resistance of corals to thermal stress. Global Ecology and Biogeography, 29(12), 2229–2247. 10.1111/geb.13191 [DOI] [Google Scholar]
- McLachlan, R. H. , Price, J. T. , Solomon, S. L. , & Grottoli, A. G. (2020). Thirty years of coral heat‐stress experiments: A review of methods. Coral Reefs, 39(4), 885–902. 10.1007/s00338-020-01931-9 [DOI] [Google Scholar]
- McManus, L. C. , Forrest, D. L. , Tekwa, E. W. , Schindler, D. E. , Colton, M. A. , Webster, M. M. , Essington, T. E. , Palumbi, S. R. , Mumby, P. J. , & Pinsky, M. L. (2021). Evolution and connectivity influence the persistence and recovery of coral reefs under climate change in the Caribbean, Southwest Pacific, and Coral Triangle. Global Change Biology, 27, 4307–4321. 10.1111/gcb.15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messyasz, A. , Rosales, S. M. , Mueller, R. S. , Sawyer, T. , Correa, A. M. S. , Thurber, A. R. , & Vega Thurber, R. (2020). Coral bleaching phenotypes associated with differential abundances of nucleocytoplasmic large DNA viruses. Frontiers in Marine Science, 7, 555474. 10.3389/fmars.2020.555474 [DOI] [Google Scholar]
- Middlebrook, R. , Anthony, K. R. N. , Hoegh‐Guldberg, O. , & Dove, S. (2010). Heating rate and symbiont productivity are key factors determining thermal stress in the reef‐building coral Acropora formosa . Journal of Experimental Biology, 213(7), 1026–1034. 10.1242/jeb.031633 [DOI] [PubMed] [Google Scholar]
- Montgomery, A. D. , Fenner, D. , Donahue, M. J. , & Toonen, R. J. (2021). Community similarity and species overlap between habitats provide insight into the deep reef refuge hypothesis. Scientific Reports, 11, 23787. 10.1038/s41598-021-03128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, J. , & Santos, B. A. (2018). Limited potential of deep reefs to serve as refuges for tropical Southwestern Atlantic corals. Ecosphere, 9(7), e02281. 10.1002/ecs2.2281 [DOI] [Google Scholar]
- Morelli, T. L. , Barrows, C. W. , Ramirez, A. R. , Cartwright, J. M. , Ackerly, D. D. , Eaves, T. D. , Ebersole, J. L. , Krawchuk, M. A. , Letcher, B. H. , Mahalovich, M. F. , Meigs, G. W. , Michalak, J. L. , Millar, C. I. , Quiñones, R. M. , Stralberg, D. , & Thorne, J. H. (2020). Climate‐change refugia: Biodiversity in the slow lane. Frontiers in Ecology and the Environment, 18(5), 228–234. 10.1002/fee.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, K. M. , Perry, C. T. , Johnson, J. A. , & Smithers, S. G. (2017). Nearshore turbid‐zone corals exhibit high bleaching tolerance on the Great Barrier Reef following the 2016 ocean warming event. Frontiers in Marine Science, 4, 224. 10.3389/fmars.2017.00224 [DOI] [Google Scholar]
- Morris, L. A. , Voolstra, C. R. , Quigley, K. M. , Bourne, D. G. , & Bay, L. K. (2019). Nutrient availability and metabolism affect the stability of coral–Symbiodiniaceae symbioses. Trends in Microbiology, 27(8), 678–689. 10.1016/j.tim.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Muir, P. R. , Marshall, P. A. , Abdulla, A. , & Aguirre, J. D. (2017). Species identity and depth predict bleaching severity in reef‐building corals: Shall the deep inherit the reef? Proceedings of the Royal Society B: Biological Sciences, 284, 20171551. 10.1098/rspb.2017.1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, E. M. , Rogers, C. S. , Spitzack, A. S. , & van Woesik, R. (2008). Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs, 27(1), 191–195. 10.1007/s00338-007-0310-2 [DOI] [Google Scholar]
- Mumby, P. J. , Chisholm, J. R. M. , Edwards, A. J. , Andrefouet, S. , & Jaubert, J. (2001). Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Marine Ecology Progress Series, 222, 209–216. 10.3354/meps222209 [DOI] [Google Scholar]
- Mumby, P. J. , Elliott, I. A. , Eakin, C. M. , Skirving, W. , Paris, C. B. , Edwards, H. J. , Enríquez, S. , Iglesias‐Prieto, R. , Cherubin, L. M. , & Stevens, J. R. (2011). Reserve design for uncertain responses of coral reefs to climate change. Ecology Letters, 14(2), 132–140. 10.1111/J.1461-0248.2010.01562.X [DOI] [PubMed] [Google Scholar]
- Nakamura, T. , Yamasaki, H. , & van Woesik, R. (2003). Water flow facilitates recovery from bleaching in the coral Stylophora pistillata . Marine Ecology Progress Series, 256, 287–291. 10.3354/meps256287 [DOI] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . (2019). A decision framework for interventions to increase the persistence and resilience of coral reefs. The National Academies Press. 10.17226/25424 [DOI] [Google Scholar]
- Nosil, P. , Vines, T. H. , & Funk, D. J. (2005). Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution, 59(4), 705–719. 10.1111/j.0014-3820.2005.tb01747.x [DOI] [PubMed] [Google Scholar]
- Ortiz, J. C. , Wolff, N. H. , Anthony, K. R. N. , Devlin, M. , Lewis, S. , & Mumby, P. J. (2018). Impaired recovery of the great barrier reef under cumulative stress. Science Advances, 4(7), eaar6127. 10.1126/sciadv.aar6127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman, E. O. , Smith, D. J. , Ziegler, M. , Kürten, B. , Conrad, C. , El‐Haddad, K. M. , Voolstra, C. R. , & Suggett, D. J. (2018). Thermal refugia against coral bleaching throughout the northern Red Sea. Global Change Biology, 24(2), e474–e484. 10.1111/gcb.13895 [DOI] [PubMed] [Google Scholar]
- Page, C. E. , Leggat, W. , Heron, S. F. , Fordyce, A. J. , & Ainsworth, T. D. (2021). High flow conditions mediate damaging impacts of sub‐lethal thermal stress on corals’ endosymbiotic algae. Conservation Physiology, 9(1), coab046. 10.1093/conphys/coab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi, S. R. , Barshis, D. J. , Traylor‐Knowles, N. , & Bay, R. A. (2014). Mechanisms of reef coral resistance to future climate change. Science, 344(6186), 895–898. 10.1126/science.1251336 [DOI] [PubMed] [Google Scholar]
- Peixoto, R. S. , Sweet, M. , Villela, H. D. M. , Cardoso, P. , Thomas, T. , Voolstra, C. R. , Høj, L. , & Bourne, D. G. (2021). Coral probiotics: Premise, promise, prospects. Annual Review of Animal Biosciences, 9(1), 265–288. 10.1146/annurev-animal-090120-115444 [DOI] [PubMed] [Google Scholar]
- Penn, J. L. , Deutsch, C. , Payne, J. L. , & Sperling, E. A. (2018). Temperature‐dependent hypoxia explains biogeography and severity of end‐Permian marine mass extinction. Science, 362(6419), eaat1327. 10.1126/science.aat1327 [DOI] [PubMed] [Google Scholar]
- Pernice, M. , Raina, J. B. , Rädecker, N. , Cárdenas, A. , Pogoreutz, C. , & Voolstra, C. R. (2020). Down to the bone: The role of overlooked endolithic microbiomes in reef coral health. ISME Journal, 14(2), 325–334. 10.1038/s41396-019-0548-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C. T. , Murphy, G. N. , Kench, P. S. , Smithers, S. G. , Edinger, E. N. , Steneck, R. S. , & Mumby, P. J. (2013). Caribbean‐wide decline in carbonate production threatens coral reef growth. Nature Communications, 4, 1402. 10.1038/ncomms2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzón, J. H. , Kamel, B. , Burge, C. A. , Harvell, C. D. , Medina, M. , Weil, E. , & Mydlarz, L. D. (2015). Whole transcriptome analysis reveals changes in expression of immune‐related genes during and after bleaching in a reef‐building coral. Royal Society Open Science, 2, 140214. 10.1098/rsos.140214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner, H. O. (2012). Integrating climate‐related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem‐level changes. Marine Ecology Progress Series, 470, 273–290. 10.3354/meps10123 [DOI] [Google Scholar]
- Rädecker, N. , Pogoreutz, C. , Gegner, H. M. , Cárdenas, A. , Roth, F. , Bougoure, J. , Guagliardo, P. , Wild, C. , Pernice, M. , Raina, J. B. , Meibom, A. , & Voolstra, C. R. (2021). Heat stress destabilizes symbiotic nutrient cycling in corals. Proceedings of the National Academy of Sciences of the United States of America, 118(5), e2022653118. 10.1073/pnas.2022653118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rädecker, N. , Raina, J. B. , Pernice, M. , Perna, G. , Guagliardo, P. , Kilburn, M. R. , Aranda, M. , & Voolstra, C. R. (2018). Using Aiptasia as a model to study metabolic interactions in Cnidarian‐Symbiodinium symbioses. Frontiers in Physiology, 9, 214. 10.3389/fphys.2018.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, E. C. , Lentz, S. J. , DeCarlo, T. M. , Cohen, A. L. , & Davis, K. A. (2020). Physical processes determine spatial structure in water temperature and residence time on a wide reef flat. Journal of Geophysical Research: Oceans, 125(12), e2020JC016543. 10.1029/2020JC016543 [DOI] [Google Scholar]
- Reshef, L. , Koren, O. , Loya, Y. , Zilber‐Rosenberg, I. , & Rosenberg, E. (2006). The coral probiotic hypothesis. Environmental Microbiology, 8(12), 2068–2073. 10.1111/j.1462-2920.2006.01148.x [DOI] [PubMed] [Google Scholar]
- Riegl, B. , & Piller, W. E. (2003). Possible refugia for reefs in times of environmental stress. International Journal of Earth Sciences, 92(4), 520–531. 10.1007/s00531-003-0328-9 [DOI] [Google Scholar]
- Ritchie, K. B. (2006). Regulation of microbial populations by coral surface mucus and mucus‐associated bacteria. Marine Ecology Progress Series, 322, 1–14. 10.3354/meps322001 [DOI] [Google Scholar]
- Roach, T. N. F. , Dilworth, J. , Christian Martin, H. , Jones, A. D. , Quinn, R. A. , & Drury, C. (2021a). Metabolomic signatures of coral bleaching history. Nature Ecology & Evolution, 5(4), 495–503. 10.1038/s41559-020-01388-7 [DOI] [PubMed] [Google Scholar]
- Roach, T. N. F. , Yadav, S. , Caruso, C. , Dilworth, J. , Foley, C. M. , Hancock, J. R. , Huckeba, J. , Huffmyer, A. S. , Hughes, K. , Kahkejian, V. A. , Madin, E. M. P. , Matsuda, S. B. , McWilliam, M. , Miller, S. , Santoro, E. P. , Rocha de Souza, M. , Torres‐Pullizaa, D. , Drury, C. , & Madin, J. S. (2021b). A field primer for monitoring benthic ecosystems using structure‐from‐motion photogrammetry. Journal of Visualized Experiments, 170, e61815. 10.3791/61815 [DOI] [PubMed] [Google Scholar]
- Rodrigues, L. J. , & Grottoli, A. G. (2006). Calcification rate and the stable carbon, oxygen, and nitrogen isotopes in the skeleton, host tissue, and zooxanthellae of bleached and recovering Hawaiian corals. Geochimica Et Cosmochimica Acta, 70(11), 2781–2789. 10.1016/j.gca.2006.02.014 [DOI] [Google Scholar]
- Rodrigues, L. J. , & Grottoli, A. G. (2007). Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography, 52(5), 1874–1882. 10.4319/lo.2007.52.5.1874 [DOI] [Google Scholar]
- Rodríguez‐Casariego, J. A. , Mercado‐Molina, A. E. , Garcia‐Souto, D. , Ortiz‐Rivera, I. M. , Lopes, C. , Baums, I. B. , Sabat, A. M. , & Eirin‐Lopez, J. M. (2020). Genome‐wide DNA methylation analysis reveals a conserved epigenetic response to seasonal environmental variation in the staghorn coral Acropora cervicornis . Frontiers in Marine Science, 7, 560424. 10.3389/fmars.2020.560424 [DOI] [Google Scholar]
- Rodriguez‐Lanetty, M. , Harii, S. , & Hoegh‐Guldberg, O. (2009). Early molecular responses of coral larvae to hyperthermal stress. Molecular Ecology, 18(24), 5101–5114. 10.1111/j.1365-294X.2009.04419.x [DOI] [PubMed] [Google Scholar]
- Rose, N. H. , Seneca, F. O. , & Palumbi, S. R. (2016). Gene networks in the wild: Identifying transcriptional modules that mediate coral resistance to experimental heat stress. Genome Biology and Evolution, 8(1), 243–252. 10.1093/gbe/evv258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan, R. , & Powers, D. A. (1991). A molecular genetic classification of zooxanthellae and the evolution of animal‐algal symbioses. Science, 251(4999), 1348–1351. 10.1126/science.251.4999.1348 [DOI] [PubMed] [Google Scholar]
- Safaie, A. , Silbiger, N. J. , McClanahan, T. R. , Pawlak, G. , Barshis, D. J. , Hench, J. L. , Rogers, J. S. , Williams, G. J. , & Davis, K. A. (2018). High frequency temperature variability reduces the risk of coral bleaching. Nature Communications, 9, 1671. 10.1038/s41467-018-04074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo, E. M. , Ridgway, T. , Bongaerts, P. , & Hoegh‐Guldberg, O. (2008). Bleaching susceptibility and mortality of corals are determined by fine‐scale differences in symbiont type. Proceedings of the National Academy of Sciences of the United States of America, 105(30), 10444–10449. 10.1073/pnas.0708049105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, E. P. , Borges, R. M. , Espinoza, J. L. , Freire, M. , Messias, C. S. M. A. , Villela, H. D. M. , Pereira, L. M. , Vilela, C. L. S. , Rosado, J. G. , Cardoso, P. M. , Rosado, P. M. , Assis, J. M. , Duarte, G. A. S. , Perna, G. , Rosado, A. S. , Macrae, A. , Dupont, C. L. , Nelson, K. E. , Sweet, M. J. , … Peixoto, R. S. (2021). Coral microbiome manipulation elicits metabolic and genetic restructuring to mitigate heat stress and evade mortality. Science Advances, 7(33), 3088–3101. 10.1126/sciadv.abg3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary, R. , Barshis, D. J. , Voolstra, C. R. , Cárdenas, A. , Evensen, N. R. , Banc‐Prandi, G. , Fine, M. , & Meibom, A. (2021). Fast and pervasive transcriptomic resilience and acclimation of extremely heat‐tolerant coral holobionts from the northern Red Sea. Proceedings of the National Academy of Sciences of the United States of America, 118(19), e2023298118. 10.1073/pnas.2023298118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe, K. A. , D’Aloia, C. C. , Crandall, E. D. , Iacchei, M. , Liggins, L. , Puritz, J. B. , Von Der Heyden, S. , & Toonen, R. J. (2016). A decade of seascape genetics: Contributions to basic and applied marine connectivity. Marine Ecology Progress Series, 554, 1–19. 10.3354/meps11792 [DOI] [Google Scholar]
- Selmoni, O. , Rochat, E. , Lecellier, G. , Berteaux‐Lecellier, V. , & Joost, S. (2020). Seascape genomics as a new tool to empower coral reef conservation strategies: An example on north‐western Pacific Acropora digitifera . Evolutionary Applications, 13(8), 1923–1938. 10.1111/eva.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneca, F. O. , & Palumbi, S. R. (2015). The role of transcriptome resilience in resistance of corals to bleaching. Molecular Ecology, 24(7), 1467–1484. 10.1111/mec.13125 [DOI] [PubMed] [Google Scholar]
- Serrano, X. M. , Baums, I. B. , O’Reilly, K. , Smith, T. B. , Jones, R. J. , Shearer, T. L. , Nunes, F. L. D. , & Baker, A. C. (2014). Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Molecular Ecology, 23(17), 4226–4240. 10.1111/mec.12861 [DOI] [PubMed] [Google Scholar]
- Shlesinger, T. , & Loya, Y. (2021). Depth‐dependent parental effects create invisible barriers to coral dispersal. Communications Biology, 4, 202. 10.1038/s42003-021-01727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, B. J. , Marshall, K. E. , Sewell, M. A. , Levesque, D. L. , Willett, C. S. , Slotsbo, S. , Dong, Y. , Harley, C. D. G. , Marshall, D. J. , Helmuth, B. S. , & Huey, R. B. (2016). Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecology Letters, 19(11), 1372–1385. 10.1111/ele.12686 [DOI] [PubMed] [Google Scholar]
- Smith, T. B. , Glynn, P. W. , Maté, J. L. , Toth, L. T. , & Gyory, J. (2014). A depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology, 95(6), 1663–1673. 10.1890/13-0468.1 [DOI] [PubMed] [Google Scholar]
- Smith, T. B. , Gyory, J. , Brandt, M. E. , Miller, W. J. , Jossart, J. , & Nemeth, R. S. (2016). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Global Change Biology, 22, 2756–2765. 10.1111/gcb.13175 [DOI] [PubMed] [Google Scholar]
- Soares, M. O. , Araújo, J. T. , Ferreira, S. M. C. , Santos, B. A. , Boavida, J. R. H. , Costantini, F. , & Rossi, S. (2020). Why do mesophotic coral ecosystems have to be protected? Science of the Total Environment, 726, 138456. 10.1016/j.scitotenv.2020.138456 [DOI] [PubMed] [Google Scholar]
- Stefanoudis, P. V. , Licuanan, W. Y. , Morrison, T. H. , Talma, S. , Veitayaki, J. , & Woodall, L. C. (2021). Turning the tide of parachute science. Current Biology, 31(4), R184–R185. 10.1016/j.cub.2021.01.029 [DOI] [PubMed] [Google Scholar]
- Stuart‐Smith, R. D. , Brown, C. J. , Ceccarelli, D. M. , & Edgar, G. J. (2018). Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature, 560(7716), 92–96. 10.1038/s41586-018-0359-9 [DOI] [PubMed] [Google Scholar]
- Suggett, D. J. , & Smith, D. J. (2020). Coral bleaching patterns are the outcome of complex biological and environmental networking. Global Change Biology, 26(1), 68–79. 10.1111/gcb.14871 [DOI] [PubMed] [Google Scholar]
- Sully, S. , Burkepile, D. E. , Donovan, M. K. , Hodgson, G. , & van Woesik, R. (2019). A global analysis of coral bleaching over the past two decades. Nature Communications, 10, 1264. 10.1038/s41467-019-09238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S. , & Murata, N. (2008). How do environmental stresses accelerate photoinhibition? Trends in Plant Science, 13(4), 178–182. 10.1016/j.tplants.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Takahashi, S. , Nakamura, T. , Sakamizu, M. , van Woesik, R. , & Yamasaki, H. (2004). Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef‐building corals. Plant and Cell Physiology, 45(2), 251–255. 10.1093/pcp/pch028 [DOI] [PubMed] [Google Scholar]
- Tchernov, D. , Gorbunov, M. Y. , De Vargas, C. , Yadav, S. N. , Milligant, A. J. , Häggblom, M. , & Falkowski, P. G. (2004). Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proceedings of the National Academy of Sciences of the United States of America, 101(37), 13531–13535. 10.1073/pnas.0402907101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, C. D. , Leitão, R. L. L. , Ribeiro, F. V. , Moraes, F. C. , Neves, L. M. , Bastos, A. C. , Pereira‐Filho, G. H. , Kampel, M. , Salomon, P. S. , Sá, J. A. , Falsarella, L. N. , Amario, M. , Abieri, M. L. , Pereira, R. C. , Amado‐Filho, G. M. , & Moura, R. L. (2019). Sustained mass coral bleaching (2016–2017) in Brazilian turbid‐zone reefs: Taxonomic, cross‐shelf and habitat‐related trends. Coral Reefs, 38(4), 801–813. 10.1007/s00338-019-01789-6 [DOI] [Google Scholar]
- Thompson, D. M. , Kleypas, J. , Castruccio, F. , Curchitser, E. N. , Pinsky, M. L. , Jönsson, B. , & Watson, J. R. (2018). Variability in oceanographic barriers to coral larval dispersal: Do currents shape biodiversity? Progress in Oceanography, 165, 110–122. 10.1016/j.pocean.2018.05.007 [DOI] [Google Scholar]
- Thompson, D. M. , & van Woesik, R. (2009). Corals escape bleaching in regions that recently and historically experienced frequent thermal stress. Proceedings of the Royal Society B: Biological Sciences, 276(1669), 2893–2901. 10.1098/rspb.2009.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonen, R. J. , Wilhelm, T. A. , Maxwell, S. M. , Wagner, D. , Bowen, B. W. , Sheppard, C. R. C. , Taei, S. M. , Teroroko, T. , Moffitt, R. , Gaymer, C. F. , Morgan, L. , Lewis, N. , Sheppard, A. L. S. , Parks, J. , Friedlander, A. M. , & Tank, B. O. T. (2013). One size does not fit all: The emerging frontier in large‐scale marine conservation. Marine Pollution Bulletin, 77(1–2), 7–10. 10.1016/j.marpolbul.2013.10.039 [DOI] [PubMed] [Google Scholar]
- Torda, G. , Donelson, J. M. , Aranda, M. , Barshis, D. J. , Bay, L. , Berumen, M. L. , Bourne, D. G. , Cantin, N. , Foret, S. , Matz, M. , Miller, D. J. , Moya, A. , Putnam, H. M. , Ravasi, T. , van Oppen, M. J. H. , Thurber, R. V. , Vidal‐Dupiol, J. , Voolstra, C. R. , Watson, S.‐A. , … Munday, P. L. (2017). Rapid adaptive responses to climate change in corals. Nature Climate Change, 7(9), 627–636. 10.1038/nclimate3374 [DOI] [Google Scholar]
- Trisos, C. H. , Auerbach, J. , & Katti, M. (2021). Decoloniality and anti‐oppressive practices for a more ethical ecology. Nature Ecology & Evolution, 5(9), 1205–1212. 10.1038/s41559-021-01460-w [DOI] [PubMed] [Google Scholar]
- van Hooidonk, R. , Maynard, J. A. , Liu, Y. , & Lee, S. K. (2015). Downscaled projections of Caribbean coral bleaching that can inform conservation planning. Global Change Biology, 21(9), 3389–3401. 10.1111/gcb.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen, M. J. H. , Oliver, J. K. , Putnam, H. M. , & Gates, R. D. (2015). Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America, 112(8), 2307–2313. 10.1073/pnas.1422301112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , & Cacciapaglia, C. W. (2021). Thermal stress jeopardizes carbonate production of coral reefs across the western and central Pacific Ocean. PLoS One, 16(4), e0249008. 10.1371/journal.pone.0249008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , Houk, P. , Isechal, A. L. , Idechong, J. W. , Victor, S. , & Golbuu, Y. (2012). Climate‐change refugia in the sheltered bays of Palau: Analogs of future reefs. Ecology and Evolution, 2(10), 2474–2484. 10.1002/ece3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , & Kratochwill, C. (2022). A global coral‐bleaching database, 1980–2020. Scientific Data, 9(1), 20. 10.1038/s41597-022-01121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woesik, R. , Sakai, K. , Ganase, A. , & Loya, Y. (2011). Revisiting the winners and the losers a decade after coral bleaching. Marine Ecology Progress Series, 434, 67–76. 10.3354/meps09203 [DOI] [Google Scholar]
- Venegas, R. M. , Oliver, T. , Liu, G. , Heron, S. F. , Clark, S. J. , Pomeroy, N. , Young, C. , Eakin, C. M. , & Brainard, R. E. (2019). The rarity of depth refugia from coral bleaching heat stress in the western and central Pacific islands. Scientific Reports, 9(1), 19710. 10.1038/s41598-019-56232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra, C. R. , Buitrago‐López, C. , Perna, G. , Cárdenas, A. , Hume, B. C. C. , Rädecker, N. , & Barshis, D. J. (2020). Standardized short‐term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Global Change Biology, 26(8), 4328–4343. 10.1111/gcb.15148 [DOI] [PubMed] [Google Scholar]
- Voolstra, C. R. , Schwarz, J. A. , Schnetzer, J. , Sunagawa, S. , DeSalvo, M. K. , Szmant, A. M. , Coffroth, M. A. , & Medina, M. (2009). The host transcriptome remains unaltered during the establishment of coral–algal symbioses. Molecular Ecology, 18(9), 1823–1833. 10.1111/J.1365-294X.2009.04167.X [DOI] [PubMed] [Google Scholar]
- Voolstra, C. R. , Suggett, D. J. , Peixoto, R. S. , Parkinson, J. E. , Quigley, K. M. , Silveira, C. B. , Sweet, M. , Muller, E. M. , Barshis, D. J. , Bourne, D. G. , & Aranda, M. (2021a). Extending the natural adaptive capacity of coral holobionts. Nature Reviews Earth & Environment, 2(11), 747–762. 10.1038/s43017-021-00214-3 [DOI] [Google Scholar]
- Voolstra, C. R. , Valenzuela, J. J. , Turkarslan, S. , Cárdenas, A. , Hume, B. C. C. C. , Perna, G. , Buitrago‐López, C. , Rowe, K. , Orellana, M. V. , Baliga, N. S. , Paranjape, S. , Banc‐Prandi, G. , Bellworthy, J. , Fine, M. , Frias‐Torres, S. , & Barshis, D. J. (2021b). Contrasting heat stress response patterns of coral holobionts across the Red Sea suggest distinct mechanisms of thermal tolerance. Molecular Ecology, 30(18), 4466–4480. 10.1111/mec.16064 [DOI] [PubMed] [Google Scholar]
- Wall, C. B. , Ricci, C. A. , Wen, A. D. , Ledbetter, B. E. , Klinger, D. E. , Mydlarz, L. D. , Gates, R. D. , & Putnam, H. M. (2021). Shifting baselines: Physiological legacies contribute to the response of reef corals to frequent heatwaves. Functional Ecology, 35(6), 1366–1378. 10.1111/1365-2435.13795 [DOI] [Google Scholar]
- Walsworth, T. E. , Schindler, D. E. , Colton, M. A. , Webster, M. S. , Palumbi, S. R. , Mumby, P. J. , Essington, T. E. , & Pinsky, M. L. (2019). Management for network diversity speeds evolutionary adaptation to climate change. Nature Climate Change, 9(8), 632–636. 10.1038/s41558-019-0518-5 [DOI] [Google Scholar]
- Ward, S. , Harrison, P. , & Hoegh‐Guldberg, O. (2000). Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. Proceedings 9th International Coral Reef Symposium, 2, 1123–1128. Bali, Indonesia.
- Warner, M. E. , Fitt, W. K. , & Schmidt, G. W. (1999). Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proceedings of the National Academy of Sciences of the United States of America, 96(14), 8007–8012. 10.1073/pnas.96.14.8007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis, V. M. (2008). Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. Journal of Experimental Biology, 211(19), 3059–3066. 10.1242/jeb.009597 [DOI] [PubMed] [Google Scholar]
- Wiedenmann, J. , D’Angelo, C. , Smith, E. G. , Hunt, A. N. , Legiret, F. E. , Postle, A. D. , & Achterberg, E. P. (2013). Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Climate Change, 3(2), 160–164. 10.1038/nclimate1661 [DOI] [Google Scholar]
- Wilhelm, T. A. , Sheppard, C. R. C. , Sheppard, A. L. S. , Gaymer, C. F. , Parks, J. , Wagner, D. , & Lewis, N. (2014). Large marine protected areas – Advantages and challenges of going big. Aquatic Conservation: Marine and Freshwater Ecosystems, 24(S2), 24–30. 10.1002/AQC.2499 [DOI] [Google Scholar]
- Williams, E. H. J. , & Bunkley‐Williams, L. (1990). The world‐wide coral reef bleaching cycle and related sources of coral mortality. Atoll Research Bulletin, 335, 1–63. 10.5479/si.00775630.335.1 [DOI] [Google Scholar]
- Wilson, A. M. , Silander, J. A. , Gelfand, A. , & Glenn, J. H. (2011). Scaling up: Linking field data and remote sensing with a hierarchical model. International Journal of Geographical Information Science, 25(3), 509–521. 10.1080/13658816.2010.522779 [DOI] [Google Scholar]
- Wright, R. M. , Mera, H. , Kenkel, C. D. , Nayfa, M. , Bay, L. K. , & Matz, M. V. (2019). Positive genetic associations among fitness traits support evolvability of a reef‐building coral under multiple stressors. Global Change Biology, 25(10), 3294–3304. 10.1111/gcb.14764 [DOI] [PubMed] [Google Scholar]
- Wyatt, A. S. J. , Leichter, J. J. , Toth, L. T. , Miyajima, T. , Aronson, R. B. , & Nagata, T. (2020). Heat accumulation on coral reefs mitigated by internal waves. Nature Geoscience, 13(1), 28–34. 10.1038/s41561-019-0486-4 [DOI] [Google Scholar]
- Xu, Y. , Vaughn, N. R. , Knapp, D. E. , Martin, R. E. , Balzotti, C. , Li, J. , Foo, S. A. , & Asner, G. P. (2020). Coral bleaching detection in the Hawaiian Islands using spatio‐temporal standardized bottom reflectance and planet dove satellites. Remote Sensing, 12(19), 3219. 10.3390/rs12193219 [DOI] [Google Scholar]
- Yilmaz, P. , Kottmann, R. , Field, D. , Knight, R. , Cole, J. R. , Amaral‐Zettler, L. , Gilbert, J. A. , Karsch‐Mizrachi, I. , Johnston, A. , Cochrane, G. , Vaughan, R. , Hunter, C. , Park, J. , Morrison, N. , Rocca‐Serra, P. , Sterk, P. , Arumugam, M. , Bailey, M. , Baumgartner, L. , … Glöckner, F. O. (2011). Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nature Biotechnology, 29(5), 415–420. 10.1038/nbt.1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval, M. , Alonso, I. , Eyal, G. , Tchernov, D. , Loya, Y. , Murillo, A. C. , & Treibitz, T. (2021). Repeatable semantic reef‐mapping through photogrammetry and label‐augmentation. Remote Sensing, 13(4), 659. 10.3390/rs13040659 [DOI] [Google Scholar]
- Ziegler, M. , Anton, A. , Klein, S. G. , Rädecker, N. , Geraldi, N. R. , Schmidt‐Roach, S. , Saderne, V. , Mumby, P. J. , Cziesielski, M. J. , Martin, C. , Frölicher, T. L. , Pandolfi, J. M. , Suggett, D. J. , Aranda, M. , Duarte, C. M. , & Voolstra, C. R. (2021). Integrating environmental variability to broaden the research on coral responses to future ocean conditions. Global Change Biology, 27(21), 5532–5546. 10.1111/gcb.15840 [DOI] [PubMed] [Google Scholar]
- Ziegler, M. , Seneca, F. O. , Yum, L. K. , Palumbi, S. R. , & Voolstra, C. R. (2017). Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Communications, 8, 14213. 10.1038/ncomms14213 [DOI] [PMC free article] [PubMed] [Google Scholar]


