Abstract
Background
Studies concerning pediatric lichen sclerosus are limited, and, to date, there have been no studies comparing the course of lichen sclerosus in boys and girls. We sought to examine all publications on boys and girls with lichen sclerosus and assess and compare epidemiology, symptoms and signs, genetic background, risk factors, treatment, and prognosis.
Methods
A systematic search was performed in the Embase, Medline, Cochrane, and Web of Science databases. Inclusion criteria were information on children ages 0–18 years and a clinical or histologic diagnosis of lichen sclerosus. Literature from 1985 to 2021 was reviewed.
Results
A total of 1780 articles were retrieved from the search, of which 90 articles were eligible for inclusion. Boys and girls present similarly on many aspects; nonetheless, treatment and follow‐up are approached differently.
Conclusions
Though the clinical approach is often different, lichen sclerosus in boys and girls demonstrates many similarities. More research is needed, especially on follow‐up, to gain a better understanding of the course of lichen sclerosus and establish an advanced management plan for children.
Keywords: balanitis xerotica obliterans, children, lichen sclerosus, pediatric lichen sclerosus, phimosis
1. INTRODUCTION
Lichen sclerosus (LS), first described by Breisky (1885) and Hallopeau (1887), is a chronic inflammatory skin disease which predominantly affects the anogenital region. 1 , 2 LS is relatively common in postmenopausal women, with an estimated prevalence between 1:1000 and 1:60. 3 , 4 In men, LS seems less common 5 ; the reported ratio between men and women varies from 1:10 to 1:6. 6 , 7 The disease is well‐documented in adults; less is known regarding LS in children. In addition, many publications address LS in either boys or girls, but not both. Therefore, we aim to assess the literature on LS in both boys and girls. LS has been known by various synonyms such as white spot disease, kraurosis vulvae, lichen sclerosus et atrophicus vulvae or, in men, balanitis xerotica obliterans (BXO). For consistency, in this review, we uniformly use the term lichen sclerosus.
2. METHODS
In collaboration with a medical information specialist, a systematic literature search was performed in Embase, Medline, Cochrane, and Web of Science, using the terms shown in Appendix 1. We reviewed all articles from 1985 to July 2021. Articles were appraised according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines (PRISMA). 8 Screening was independently performed by two individuals (K.K. and E.M.). Publications were included based on title and abstract if they involved children up to age 18 with a clinical or histologic diagnosis LS. Discrepancies were discussed before inclusion. In the second round, inclusion was based on full‐length manuscripts. Exclusion criteria included commentaries, brief abstracts, case reports, expert opinions, full text unavailable, and unclear study methodology, not available in English or Dutch.
3. RESULTS
A total of 1780 articles were retrieved. After screening based on titles and abstracts, full texts of 300 publications were read. 89 articles met the inclusion criteria. The references in these articles were checked for relevant studies, yielding one additional publication, resulting in 90 included publications (Figure 1). Included articles were subsequently categorized into the following categories based on focus of content: epidemiology, clinical presentation, genetic background, risk factors, treatment, and prognosis (Table 1).
FIGURE 1.
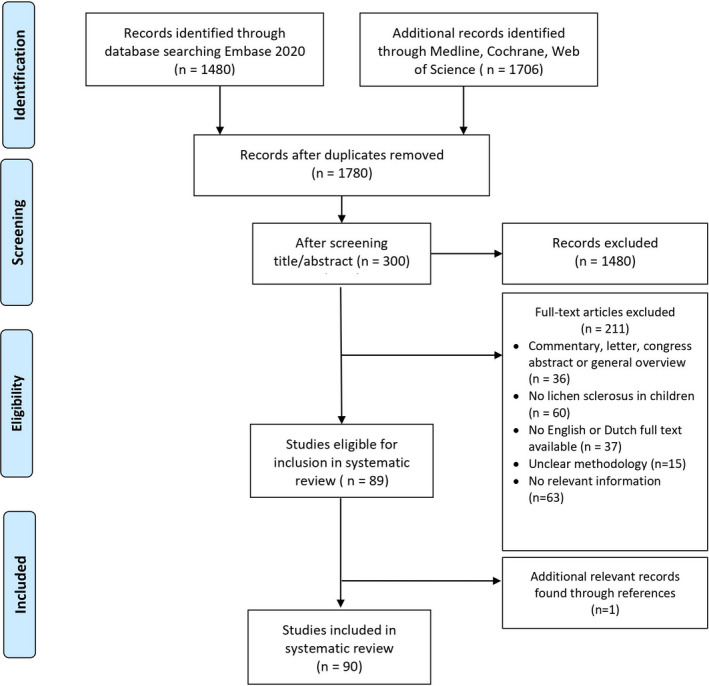
Flowchart of inclusions and exclusions in the systematic review of pediatric lichen sclerosus
TABLE 1.
Systematic review of the literature: comparison in outcome measures between boys and girls with lichen sclerosus
| Girls | Boys | |
|---|---|---|
| Average age at onset | 6.6 years 11 | 8.6 years 11 |
| Prevalence | 1:900 (2–16 years) 12 | 1:200 (0–14 years) 7 |
| Symptoms |
94.6% anogenital lesions 11 5.4% anogenital and/or extragenital 11 Pruritus, pain, dysuria, burning sensation 11 Constipation or gastrointestinal complaints relatively common (58–89%) 19 , 20 , 21 |
Mainly genital lesions, 0.4–6% extragenital 6 , 11 Ballooning of the foreskin, urine retention, dysuria, other urinary tract symptoms, and erectile pain. Pruritus is rare in boys 6 , 11 Secondary constipation uncommon 11 |
| Clinical features |
Hypopigmentation, hyperpigmentation, erythema, fissures, atrophy, keratotic papules 19 , 22 |
Hypopigmentation distal portion of the prepuce 35 Phimosis, scarring, secondary buried penis, balanitis |
| Histopathology | Hyperkeratosis (96%), basal vacuolization of keratinocytes (88%), lymphocytic exocytosis (91%), dermal sclerosis (99%) and epidermal atrophy (50%) 43 |
Hyperkeratosis (82.9%), hyalinization (100%), basal cell degeneration (56.1%), lymphocytic infiltration (100%), fibrin deposition (7.3%) 32 In 67% dermoepidermal clefts 44 |
| Diagnosis | Usually based on clinical features 39 | Usually based on histopathology 33 , 40 , 41 , 42 |
| Genetic background |
Turner syndrome with LS (prevalence 17.3%) 50 |
Unknown |
| Risk Factors |
Atopic constitution, vitiligo, thyroid disease, localized scleroderma, alopecia areata and rheumatic diseases 11 , 12 , 53 , 54 Celiac disease (case report) 55 History of urinary tract symptoms/urinary incontinence 21 , 27 |
Atopic constitution 57 Obesity 58 |
| Treatment |
Emollients 4 Ultrapotent topical corticosteroids: mainly clobetasol propionate 0.05% ointment (CP 0.05%) 59 Tacrolimus 0.03–0.1% (combined with CP 0.05% or as maintenance therapy). 11 , 65 , 66 , 67 , 68 Tacrolimus (and other topical calcineurin inhibitors), sometimes giving a brief burning sensation at initiation of treatment (16%) 11 Alternatively, for maintenance potent corticosteroid ointment intermittently or less potent corticosteroids 59 , 69 Surgery for scarring not advised. (Seldom in adolescents in cases of introital stenosis) 28 |
Circumcision with complete resection of foreskin 6 , 70 Urethral strictures: Single stage buccal mucosal inlay grafting 73 Preoperative/ postoperative therapy: Moderate or potent corticosteroids e.g., mometasone furoate 0.05% ointment or betamethasone ointment 74 , 84 or tacrolimus 0.1% 77 |
| Prognosis |
20–97% have recurrent signs and/or symptoms despite therapy (0–18 years) 78 Untreated LS may lead to scarring and possibly labial fusion 27 , 28 |
Recurrent phimosis, urethral strictures, and meatal stenosis Subsequent meatus surgery in 7–20% (urethral dilatation, meatoplasty) 33 , 35 , 82 , 83 , 84 When untreated, LS can lead to obstructive urinary tract complications or renal failure 85 , 87 |
3.1. Epidemiology
Data on the epidemiology of pediatric LS are limited. The true prevalence is difficult to estimate considering that many patients are asymptomatic, unaware, misdiagnosed, or hesitant to report their condition. 4 The incidence of pediatric LS (0–20 years old) in the general population is estimated at 0.04–0.06%, 9 , 10 compared with 0.1–0.6% in adults. 3 , 4 In adulthood, the incidence ratio for LS in women relative to men is higher (from 6:1 to 10:1); in children the female:male ratio is 1:1.7. 11
The estimated prevalence of LS in girls aged 2–16 years is 1:900, 12 with a mean age of onset of 6.6 years. 11 Girls aged 0–19 years comprise 4.1% of females with LS. 13 In boys age 0–14 years, the incidence is estimated at 1:200, with most cases found in 9–11 years old (mean age at onset 8.6 years). 7 , 11 , 14 , 15 , 16 , 17 , 18
3.2. Clinical features
In girls with LS, 94.6% present with anogenital lesions. The remaining 5.4% either have extragenital lesions only or have genital and extragenital involvement. 11 The most common symptoms in girls are pruritus, pain, dysuria, and a burning sensation in the anogenital area. 11 , 19 In 58–89% of the cases, constipation and gastrointestinal complaints are reported. 11 , 19 , 20 , 21 Clinical signs are hypopigmentation, erythema, fissures, hyperpigmentation, atrophy, ecchymosis, and/or keratotic papules. Often a “figure‐of‐8” pattern is reported affecting the labia minora, clitoris, and perianal region. 19 , 22 Erosions and can be present, often due to scratching. Vascular lesions such as angiokeratomas and telangiectasias are occasionally described and are usually asymptomatic. 23 , 24 , 25 , 26 In a later stage, anatomic changes can be seen, such as labial fusion. 27 , 28 Genital LS may be mistaken for sexual abuse. However, LS and sexual abuse are not mutually exclusive and, if suspected, abuse should be investigated. 20 , 29 , 30
The referral diagnoses for boys with LS are mostly clinical features such as phimosis (52%), balanitis (13%), or buried penis (10%), regardless of whether or not the LS has been recognized. 14 The incidence of LS in non‐circumcised boys with phimosis ranges from 2 to 95%, 31 the largest cohort studies reporting 10–50%. 15 , 18 , 32 , 33 Boys with an acquired phimosis may have a greater risk of having LS than boys with congenital phimosis. 17 , 31 , 34 Frequent symptoms subsequent to phimosis include urine retention, ballooning of the foreskin, dysuria, other urinary tract symptoms, and erectile pain. 6 , 11 Hypopigmentation of the distal portion of the prepuce, seen as a white (sclerotic) ring, mostly results in progressive phimosis. 35 Furthermore, erythema, pigmentary changes, telangiectasia, purpura, and scarring may be present. 11 , 36 We found no mention of scrotal skin being affected. The perianal region of boys is rarely affected. Therefore, secondary constipation is uncommon. 6 , 11 , 32 , 37 , 38 Extragenital LS is present in 0.4–6% of boys with LS. 6 , 11
In both girls and boys, the diagnosis is based on clinical signs and/or symptoms. In girls, clinical findings are paramount, and biopsies are generally not necessary. 39 In boys, histology is more often performed, but the correlation between the clinical and histopathological diagnosis varies, ranging from 53 to 88%, 33 , 40 , 41 , 42 often due to lack of recognition of LS in boys. Ghidini 42 found that an abnormal foreskin appearance together with a positive clinical history has a positive predictive value of 100% in boys with LS. A positive clinical history included the presence of autoimmune or allergic disease, having experienced at least one episode of balanitis or a lower urinary tract infection and the lack of improvement after steroids. Clinical examination alone led to an underestimation of LS in boys. 42
3.3. Histopathology
A large cohort study showed the histopathological characteristics of adult vulvar LS are also present in juvenile vulvar LS. 43 The main histopathological changes seen in girls are hyperkeratosis (96%), basal vacuolization of keratinocytes (88%), lymphocytic exocytosis (91%), dermal sclerosis (99%), and epidermal atrophy (50%). 43
Microscopic specimens of penile foreskin in boys with LS are comparable with vulvar LS. 32 Dermoepidermal clefts can be found in 87% of specimens due to lymphedema in the upper dermis. 44
3.4. Genetic background and risk factors
Several factors suggest that LS is an autoimmune disease with a genetic predisposition. 45 In 11–12% of LS cases a positive family history was reported, supporting a genetic component. 46 Most genetic studies have been conducted in adults, though several case‐control studies found HLA‐DQ7 (HLA‐DQ‐B1*301/04/09/10) in 50–66% of prepubertal girls with LS compared with 25–31% in controls. 45 , 47 , 48 , 49 Furthermore, LS is frequently seen in patients with Turner syndrome, with a prevalence of 17.3%. 50 In boys, the molecular evidence of specific gene expression patterns was found in congenital phimosis associated with LS, which is comparable with the existing literature on adult LS. 51 , 52
The association between LS and autoimmune disease is well‐established in women but less so in men. 45 In girls the most common concomitant autoimmune diseases that are reported are vitiligo, thyroid disease, localized scleroderma, alopecia areata, and rheumatic diseases. 11 , 12 , 53 , 54 One case series on 3 girls suggested a possible association with celiac disease. 55 Intriguingly, HLA‐DRB113 is more prevalent in LS patients without autoimmune disease compared with LS patients with autoimmune disease, suggesting a protective role of HLA‐DRB113 against autoimmune diseases in the presence of LS. 49 Baldo et al 56 detected circulating basement membrane zone protein antibodies in girls with LS. 56 Other factors associated with LS in children include a history of urinary tract symptoms and urinary incontinence in girls, 21 , 27 atopic constitution in both girls and boys, 12 , 57 and obesity in boys. 58
3.5. Treatment
In both girls and boys, moisturizing practices with emollients are crucial to reduce irritation and restore the skin barrier. 4 , 6
In girls, a systematic review of pediatric LS concluded that ultrapotent topical corticosteroid ointment, generally clobetasol propionate 0.05% (CP 0.05%), is the most effective treatment to induce remission, with improvement of symptoms and signs in 99% of patients within 4–12 weeks. 59 In most cases, treatment is applied for up to 3 months, after which intermittent maintenance therapy is often required or advised. Despite the initial therapy with CP 0.05%, overall recurrences in girls were reported in 67% within 1 year, and additional treatment was required in 37% after 3 months of therapy. 59 , 60 , 61 , 62 , 63 , 64 Tacrolimus 0.03%‐0.1% ointment is reported to produce improvement in signs and symptoms but may be less effective than CP 0.05%. 65 Tacrolimus is often used along with CP 0.05% or bridged during remission. 11 , 65 , 66 , 67 , 68 Anderson 65 found that tacrolimus 0.1% showed promise as an option for maintenance therapy. Initially tacrolimus can sometimes briefly cause a burning sensation upon application. 11 After treatment with potent topical corticosteroids (TCS), Ellis and Fischer 69 prescribed individualized maintenance regimes with moderate‐ or mild‐potency TCS. Scarring and progression was prevented in subjects adherent to maintenance. 69 Surgery is not advised for girls with LS. In adolescence, there might rarely be an indication for introitus‐plasty in non‐active LS. 28
In boys, circumcision with complete resection of the foreskin is still considered the ultimate treatment. 6 , 70 Two studies found preputioplasty combined with intralesional steroid injection to be an effective alternative, though with a higher relapse rate than circumcision. 71 , 72 In a study of 5 boys with urethral strictures secondary to LS, single stage buccal mucosal inlay grafting was effective, with a significant improvement of urinary flow rates. 73 Few studies have addressed corticosteroids as preoperative or postoperative therapy. In a double blind, placebo controlled trial (n = 40) of boys with histologically diagnosed early and intermediate stage LS, pre‐circumcision treatment with mometasone furoate 0.05% (MF 0.05%) resulted in clinical improvement in 41% after 5 weeks. 74 Folaranmi et al 75 conducted a literature search and included 89 boys with LS who were treated with topical corticosteroids with various regimes for an average of 2 years. In this study, the use of topical corticosteroids preoperatively (mainly MF 0.05% or betamethasone cream) prevented circumcision in up to 35% of the cases. 75 , 76 Furthermore, Ebert et al 77 showed treatment with tacrolimus 0.1% postoperatively twice daily for 3 weeks is a safe option for disease control (median follow‐up 13 months).
3.6. Prognosis
Little is known about the long‐term prognosis of pediatric LS. In a systematic review by Morrel et al, 78 the majority of girls experienced continued signs and symptoms after puberty, along with architectural changes and scarring. Overall, the results fluctuate between 20% and 97%. 69 , 78 , 79 , 80 , 81 Maintenance treatment with topical corticosteroids might improve long‐term sequelae. 69 , 78
In boys with LS, findings following circumcision include meatal stenosis, urethral strictures, and phimosis. Cohort studies show 7–20% of boys circumcised for LS subsequently need a meatal procedure in the form of a meatomy or meatoplasty within weeks to several months. 33 , 35 , 82 , 83 , 84 Arena et al 85 using uroflowmetry (UF) to evaluate the outcome of 75 circumcised patients with LS, found that 13.3% had a pathological UF after 1 year requiring progressive urethral dilatation or meatoplasty. 85 Neither an abnormal appearance of the meatus nor meatal intervention during the first surgery seems to have a significant effect on the need of a subsequent operation. 82 Snodgrass et al 86 found 40% of patients who had circumcision for meatal LS (complete excision, including total replacement of the involved urethra) still had a recurrence of the disease at a median of 2 years. The progression of LS in boys can lead to obstructive urinary tract complications, and in severe cases, even renal failure. 85 , 87
Regarding possible malignant transformation, squamous cell carcinoma (SCC) in pediatric LS has never been reported. However, adults LS patients are at risk for developing genital SCC later in life, as described by Spekreijse et al, the absolute risk in men with LS being between 0.00 and 0.91%, and in women between 0.21 and 3.88%. 88 This is particularly so for patients with a delayed diagnosis or partial treatment or response to topical corticosteroids. 88 , 89 , 90 It is unknown whether this risk is applicable when LS develops in childhood. 88 Howevere, six pediatric cases of vulvar melanoma have been described in conjunction with LS. 91 , 92 , 93 , 94 Clinicians should also be aware of the diagnostic pitfalls of pigmented vulvar lesions, especially in a background of LS where melanoma may be over‐diagnosed. 95
4. DISCUSSION
This review systematically assessed available literature on pediatric LS and, to our knowledge, is the first study focusing on and comparing girls and boys, as the literature tended to consider LS in girls and boys as separate entities. In published reports, boys are almost exclusively studied by urologists and girls by gynecologists and dermatologists. However, as this review shows, there are fewer differences between LS in girls and boys than generally assumed. Therefore, we propose to use the term lichen sclerosus in both sexes (discontinuing the term BXO in boys) and encourage multidisciplinary research.
To date, only one epidemiologic study has been performed to assess the prevalence of LS in girls, 12 whereas for boys many studies have been performed. 7 , 11 , 14 , 15 , 16 , 17 , 18 While in adulthood, the incidence ratio for LS in women is higher than in men, in children the female:male ratio is 1:1.7. 11 This reversal might be caused by detection bias, since boys are often referred due to clinical symptoms of phimosis. 7 Furthermore, circumcision at a young age might lead to less cases of LS in the adult male population. This is supported by evidence that the absence of childhood circumcision and presence of chronic inflammation (leading to phimosis) are risk factors for penile SCC. 96 Li et al 31 observed LS in asymptomatic boys undergoing circumcision for religious beliefs. It would be interesting to know the incidence of male LS in regions where circumcision is routinely performed versus regions where it is generally done for medical reasons.
Remarkably, one study found LS in 39.5% of failed hypospadias repairs. 97 We conjecture this might either be a consequence of the Koebner phenomenon from surgery or pre‐existing urethral epithelial inflammation. Other studies found incidences of LS after hypospadias repairs ranging between 0.4 and 2.7%. 98 , 99
Little is known about the prognosis of LS in both girls and boys. LS can be a chronic disease, as seen in the recurrence rate in both sexes after either initial topical therapy in girls or circumcision in boys. 33 , 35 , 69 , 72 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 97 , 98 , 99 As discussed earlier, literature often refers to meatal and urethral strictures post‐surgery as complications of the circumcision. In our practice, however, we note the meatus is involved prior to circumcision in nearly all cases. Therefore, meatal stenosis should not simply be regarded as a complication of circumcision in initial or recurrent LS. Furthermore, in our practice boys are treated with corticosteroids for a limited period of weeks to months following circumcision. Sometimes permanent maintenance therapy is required. In girls, maintenance treatment is generally prescribed.
More research is needed regarding several concepts: the scope of LS at time of diagnosis or surgery, disease course and the role of maintenance therapy in children. 82 , 83 , 84 The strengths of this study include: literature was found through a comprehensive systematic search of all publications addressing pediatric LS. Limitations are that most included studies were of level III‐IV evidence. However, a placebo controlled RCT would not be ethical. Therefore, systematic reviews are invaluable. Etiology and histopathology of LS are relatively well‐researched in adults, and some of these data may also be applicable to children, but that is a topic for further study.
5. CONCLUSION
Lichen sclerosus in childhood seems to develop in a similar fashion in both sexes, though the clinical approach is often different partly due to the division of care according to the patient's sex. Follow‐up is crucial to avoid future complications. More collaborative research is needed to improve understanding of the course of LS and to establish an advanced management plan for children.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. W.M. Bramer, information specialist, from the Erasmus MC Medical Library for developing and assisting with the search strategies.
1.
| Embase.com | 1480 | 1458 |
| Medline ALL ovid | 1071 | 193 |
| Web of science core collection | 574 | 108 |
| Cochrane CENTRAL register of trials | 61 | 21 |
| Total | 3186 | 1780 |
Embase.com
(‘lichen sclerosus et atrophicus’/de OR ‘vulva kraurosis’/de OR Phimosis/de OR ((lichen NEAR/3 (sclerosus OR sclerosis OR atrophicus OR scleroatrophic* OR sclera‐atrophic*)) OR (white‐spot* NEAR/3 disease*) OR kraurosis OR csillag OR (balanitis NEAR/3 (sclerotica OR xerotica) NEAR/3 obliteran*) OR (skin NEAR/3 papillary NEAR/3 degenerat*) OR Phimosis OR Parephimosis:ab,ti) AND (child/exp OR adolescent/exp OR adolescence/exp OR ‘child behavior’/de OR ‘child parent relation’/de OR pediatrics/exp OR childhood/exp OR ‘child nutrition’/de OR ‘infant nutrition’/exp OR ‘child welfare’/de OR ‘child abuse’/de OR ‘child advocacy’/de OR ‘child development’/de OR ‘child growth’/de OR ‘child health’/de OR ‘child health care’/exp OR ‘child care’/exp OR ‘childhood disease’/exp OR ‘child death’/de OR ‘child psychiatry’/de OR ‘child psychology’/de OR ‘pediatric ward’/de OR ‘pediatric hospital’/de OR ‘pediatric anesthesia’/de OR ‘pediatric intensive care unit’/de OR ‘neonatal intensive care unit’/de OR ‘prematurity’/de OR (adolescen* OR preadolescen* OR infan* OR newborn* OR (new NEXT/1 born*) OR baby OR babies OR neonat* OR prematur* OR pre‐matur* OR child* OR kid OR kids OR toddler* OR teen* OR boy* OR girl* OR minors OR underag* OR (under NEXT/1 (age* OR aging OR ageing)) OR juvenil* OR youth* OR kindergar* OR puber* OR pubescen* OR prepubescen* OR prepubert* OR pediatric* OR paediatric* OR school* OR preschool* OR highschool* OR suckling* OR PICU OR NICU OR PICUs OR NICUs OR premenarch*):ab,ti,kw) NOT [conference abstract]/lim AND [english]/lim NOT ([animals]/lim NOT [humans]/lim).
Medline ALL ovid
(exp Lichen Sclerosus et Atrophicus/OR Phimosis/OR (lichen ADJ3 (sclerosus OR sclerosis OR atrophicus OR scleroatrophic* OR sclera‐atrophic*)) OR (white‐spot* ADJ3 disease*) OR kraurosis OR csillag OR (balanitis ADJ3 (sclerotica OR xerotica) ADJ3 obliteran*) OR (skin ADJ3 papillary ADJ3 degenerat*) OR Phimosis OR Parephimosis).ab,ti.) AND (exp Child/OR exp Infant/OR exp Adolescent/OR exp “Child Behavior”/OR exp “Parent Child Relations”/OR exp “Pediatrics”/OR “Child Nutrition Sciences”/OR “Infant nutritional physiological phenomena”/OR exp “Child Welfare”/OR “Child Development”/OR exp “Child Health Services”/OR exp “Child Care”/OR “Child Rearing”/OR exp “Child development Disorders, Pervasive”/OR “Child Psychiatry”/OR “Child Psychology”/OR “Hospitals, Pediatric”/OR exp “Intensive Care Units, Pediatric”/OR (adolescen* OR preadolescen* OR infan* OR newborn* OR (new ADJ born*) OR baby OR babies OR neonat* OR prematur* OR pre‐matur* OR child* OR kid OR kids OR toddler* OR teen* OR boy* OR girl* OR minors OR underag* OR (under ADJ (age* OR aging OR ageing)) OR juvenil* OR youth* OR kindergar* OR puber* OR pubescen* OR prepubescen* OR prepubert* OR pediatric* OR paediatric* OR school* OR preschool* OR highschool* OR suckling* OR PICU OR NICU OR PICUs OR NICUs OR premenarch*).ab,ti,kw.) AND english.la. NOT (exp animals/NOT humans/).
Web of science core collection
TS=((((lichen NEAR/2 (sclerosus OR sclerosis OR atrophicus OR scleroatrophic* OR sclera‐atrophic*)) OR (white‐spot* NEAR/2 disease*) OR kraurosis OR csillag OR (balanitis NEAR/2 (sclerotica OR xerotica) NEAR/2 obliteran*) OR (skin NEAR/2 papillary NEAR/2 degenerat*) OR Phimosis OR Parephimosis)) AND ((adolescen* OR preadolescen* OR infan* OR newborn* OR (new NEAR/1 born*) OR baby OR babies OR neonat* OR prematur* OR pre‐matur* OR child* OR kid OR kids OR toddler* OR teen* OR boy* OR girl* OR minors OR underag* OR (under NEAR/1 (age* OR aging OR ageing)) OR juvenil* OR youth* OR kindergar* OR puber* OR pubescen* OR prepubescen* OR prepubert* OR pediatric* OR paediatric* OR school* OR preschool* OR highschool* OR suckling* OR PICU OR NICU OR PICUs OR NICUs OR premenarch*))) AND DT=(article) AND LA=(english).
Cochrane CENTRAL register of trials
(((lichen NEAR/3 (sclerosus OR sclerosis OR atrophicus OR scleroatrophic* OR sclera NEXT atrophic*)) OR (white NEXT spot* NEAR/3 disease*) OR kraurosis OR csillag OR (balanitis NEAR/3 (sclerotica OR xerotica) NEAR/3 obliteran*) OR (skin NEAR/3 papillary NEAR/3 degenerat*) OR Phimosis OR Parephimosis):ab,ti) AND ((adolescen* OR preadolescen* OR infan* OR newborn* OR (new NEXT/1 born*) OR baby OR babies OR neonat* OR prematur* OR pre NEXT matur* OR child* OR kid OR kids OR toddler* OR teen* OR boy* OR girl* OR minors OR underag* OR (under NEXT/1 (age* OR aging OR ageing)) OR juvenil* OR youth* OR kindergar* OR puber* OR pubescen* OR prepubescen* OR prepubert* OR pediatric* OR paediatric* OR school* OR preschool* OR highschool* OR suckling* OR PICU OR NICU OR PICUs OR NICUs OR premenarch*):ab,ti).
Kumar KS, Morrel B, van Hees CLM, van der Toorn F, van Dorp W, Mendels EJ. Comparison of lichen sclerosus in boys and girls: A systematic literature review of epidemiology, symptoms, genetic background, risk factors, treatment, and prognosis. Pediatr Dermatol. 2022;39:400–408. doi: 10.1111/pde.14967
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Breisky . Ein wenig beobachtet Form von Hautatrophie am Pudendum muliebre. Z Heilk. 1885;6:69. [Google Scholar]
- 2. Hallopeau H. Leçons clinique sur les maladies cutanées et syphilitiques. Union Méd. 1887;43:742. [Google Scholar]
- 3. Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14(1):27‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong LX, Sun GS, Teng JMC. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32(5):593‐599. [DOI] [PubMed] [Google Scholar]
- 5. Stühmer A. Balanitis xerotic obliterans (post‐operationem) und ihre Beziehungen zur "Kraurosis glandis et praeputii penis". Arch F Dermat U. 1928;165:343. [Google Scholar]
- 6. Nguyen ATM, Holland AJA. Balanitis xerotica obliterans: an update for clinicians. Eur J Pediatr. 2020;179(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 7. Chi CC, Kirtschig G, Baldo M, Brackenbury F, Lewis F, Wojnarowska F. Topical interventions for genital lichen sclerosus. Cochrane Database Syst Rev. 2011;2011(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 9. Kyriakis KP, Emmanuelides S, Terzoudi S, Palamaras I, Damoulaki E, Evangelou G. Gender and age prevalence distributions of morphea en plaque and anogenital lichen sclerosus. J Eur Acad Dermatol Venereol. 2007;21(6):825‐826. [DOI] [PubMed] [Google Scholar]
- 10. Kizer WS, Prarie T, Morey AF. Balanitis xerotica obliterans: epidemiologic distribution in an equal access health care system. South Med J. 2003;96(1):9‐11. [DOI] [PubMed] [Google Scholar]
- 11. Balakirski G, Grothaus J, Altengarten J, Ott H. Paediatric lichen sclerosus: a systematic review of 4516 cases. Br J Dermatol. 2020;182(1):231‐233. [DOI] [PubMed] [Google Scholar]
- 12. Powell J, Wojnarowska F. Childhood vulvar lichen sclerosus: an increasingly common problem. J Am Acad Dermatol. 2001;44(5):803‐806. [DOI] [PubMed] [Google Scholar]
- 13. Halonen P, Jakobsson M, Heikinheimo O, Gissler M, Pukkala E. Incidence of lichen sclerosus and subsequent causes of death: a nationwide Finnish register study. BJOG Int J Obstet Gynaecol. 2020;127(7):814‐819. [DOI] [PubMed] [Google Scholar]
- 14. Gargollo PC, Kozakewich HP, Bauer SB, et al. Balanitis xerotica obliterans in boys. J Urol. 2005;174(4 Part 1):1409‐1412. [DOI] [PubMed] [Google Scholar]
- 15. Pradhan A, Patel R, Said AJ, Upadhyaya M. 10 years’ experience in balanitis xerotica obliterans: a single‐institution study. Eur J Pediatr Surg. 2019;29(3):302‐306. [DOI] [PubMed] [Google Scholar]
- 16. Jayakumar S, Antao B, Bevington O, Furness P, Ninan GK. Balanitis xerotica obliterans in children and its incidence under the age of 5 years. J Pediatr Urol. 2012;8(3):272‐275. [DOI] [PubMed] [Google Scholar]
- 17. Jasaitiene D, Valiukeviciene S, Vaitkiene D, et al. Lichen sclerosus et atrophicus in pediatric and adult male patients with congenital and acquired phimosis. Medicina (Kaunas). 2008;44(6):460‐466. [PubMed] [Google Scholar]
- 18. Kiss A, Király L, Kutasy B, Merksz M. High incidence of balanitis xerotica obliterans in boys with phimosis: prospective 10‐year study. Pediatr Dermatol. 2005;22(4):305‐308. [DOI] [PubMed] [Google Scholar]
- 19. Dinh H, Purcell SM, Chung C, Zaenglein AL. Pediatric lichen sclerosus: a review of the literature and management recommendations. J Clin Aesthetic Dermatol. 2016;9(9):49‐54. [PMC free article] [PubMed] [Google Scholar]
- 20. Maronn ML, Esterly NB. Constipation as a feature of anogenital lichen sclerosus in children. Pediatrics. 2005;115(2):e230‐e232. [DOI] [PubMed] [Google Scholar]
- 21. Wang M, Arlen AM, Vash‐Margita A. characteristics among premenarchal girls with lichen sclerosus. J Low Genit Tract Dis. 2021;25(2):152‐157. [DOI] [PubMed] [Google Scholar]
- 22. Orszulak D, Dulska A, Niziński K, et al. Pediatric vulvar lichen sclerosus—a review of the literature. Int J Environ Res Public Health. 2021;18(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedland R, Ben‐Amitai D, Didkovsky E, Feinmesser M, Zvulunov A. Vascular lesions in genital lichen sclerosus in pediatric patients. Pediatr Dermatol. 2020;37(5):849‐852. [DOI] [PubMed] [Google Scholar]
- 24. Luzar B, Neil SM, Calonje E. Angiokeratoma‐like changes in extragenital and genital lichen sclerosus. J Cutaneous Pathol. 2009;36(5):540‐542. [DOI] [PubMed] [Google Scholar]
- 25. Vash‐Margita A, Smith YR, Rabah R, Quint EH. Adolescent vulvar angiokeratoma associated with lichen sclerosus. J Pediatr Adolesc Gynecol. 2019;32(4):440‐442. [DOI] [PubMed] [Google Scholar]
- 26. Di Altobrando A, Patrizi A, Bassi A, et al. Lichen sclerosus with enlarged vessels: a variant of lichen sclerosus in young girls. Pediatr Dermatol. 2021;38(1):318‐319. [DOI] [PubMed] [Google Scholar]
- 27. Ismail D, Owen CM. Paediatric vulval lichen sclerosus: a retrospective study. Clin Exp Dermatol. 2019;44(7):753‐758. [DOI] [PubMed] [Google Scholar]
- 28. Kalampalikis A, Ivanidou S, Michala L. Labial fusion in adolescence secondary to lichen sclerosus. J Obstet Gynaecol. 2021;41(4):647‐650. [DOI] [PubMed] [Google Scholar]
- 29. Handfield‐Jones SE, Hinde FRJ, Kennedy CTC. Lichen sclerosus et atrophicus in children misdiagnosed as sexual abuse. Br Med J. 1987;294(6584):1404‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ridley CM. Genital lichen sclerosus (lichen sclerosus et atrophicus) in childhood and adolescence. J R Soc Med. 1993;86(2):69‐75. [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Deng C, Peng Q. Underestimation of genital lichen sclerosus incidence in boys with phimosis: results from a systematic review. Pediatr Surg Int. 2018;34(11):1245‐1250. [DOI] [PubMed] [Google Scholar]
- 32. Hoare DT, Metcalfe P. An epidemiologic overview of a tertiary referral practice for male paediatric lichen sclerosus. Paediatr Child Health. 2020;25(4):241‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Celis S, Reed F, Murphy F, et al. Balanitis xerotica obliterans in children and adolescents: a literature review and clinical series. J Pediatr Urol. 2014;10(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 34. Mattioli G, Repetto P, Carlini C, Granata C, Gambini C, Jasonni V. Lichen sclerosus et atrophicus in children with phimosis and hypospadias. Pediatr Surg Int. 2002;18(4):273‐275. [DOI] [PubMed] [Google Scholar]
- 35. Becker K. Lichen sclerosus in boys. Dtsch Arztebl. 2011;108(4):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barton PG, Ford MJ, Beers BB. Penile purpura as a manifestation of lichen sclerosus et atrophicus. Pediatr Dermatol. 1993;10(2):129‐131. [DOI] [PubMed] [Google Scholar]
- 37. Hughes KE, Corbett HJ. Ultrasound evidence of bladder outlet obstruction secondary to lichen sclerosus et atrophicus in boys (balanitis xerotica obliterans). J Pediatr Surg. 2020;55(4):721‐725. [DOI] [PubMed] [Google Scholar]
- 38. Bale PM, Lochhead A, Martin HCO, Gollow I. Balanitis xerotica obliterans in children. Pediatr Pathol. 1987;7(5–6):617‐627. [DOI] [PubMed] [Google Scholar]
- 39. McCarthy S, MacEoin N, O'Driscoll M, O'Connor R, Heffron CCBB, Murphy M. Should we always biopsy in clinically evident lichen sclerosus? J Lower Genital Tract Dis. 2019;23(2):182‐183. [DOI] [PubMed] [Google Scholar]
- 40. Alyami FA, Bateni ZH, Odeh R, Farhat WA, Koyle M. Routine histopathological examination of the foreskin after circumcision for clinically suspected lichen sclerosus in children: is it a waste of resources? Can Urol Assoc J. 2018;12(5):E231‐E233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boksh K, Patwardhan N. Balanitis xerotica obliterans: has its diagnostic accuracy improved with time? JRSM Open. 2017;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghidini F, Virgone C, Pulvirenti R, Trovalusci E, Gamba P. Could a careful clinical examination distinguish physiologic phimosis from balanitis xerotica obliterans in children? Eur J Pediatr. 2020;180(2):591‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morrel B, Ewing‐Graham PC, van der Avoort IAM, Pasmans S, Damman J. Structured analysis of histopathological characteristics of vulvar lichen sclerosus in a juvenile population. Human Pathol. 2020;106:23‐31. [DOI] [PubMed] [Google Scholar]
- 44. Singh L, Sengar M, Goyal S, Mansi M, Khurana N, Mohta A. Childhood phimosis secondary to lichen sclerosus: is there a spatial pattern of histopathological changes? Am J Dermatopathol. 2018;40(11):824‐828. [DOI] [PubMed] [Google Scholar]
- 45. Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen Sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15(7):1429‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sherman V, McPherson T, Baldo M, Salim A, Gao XH, Wojnarowska F. The high rate of familial lichen sclerosus suggests a genetic contribution: an observational cohort study. J Eur Acad Dermatol Venereol. 2010;24(9):1031‐1034. [DOI] [PubMed] [Google Scholar]
- 47. Powell J, Wojnarowska F, Winsey S, Marren P, Welsh K. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol. 2000;142(3):481‐484. [DOI] [PubMed] [Google Scholar]
- 48. Marren P, Yell J, Charnock FM, Bunce M, Welsh K, Wojnarowska F. The association between lichen sclerosus and antigens of the HLA system. Br J Dermatol. 1995;132(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 49. Gao XH, Barnardo MC, Winsey S, et al. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB112 and its associated DRB112/DQB10301/04/09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB10301/04 and its associated DRB10301/04/DQB10201/02/03 haplotype protects from vulval lichen sclerosus. J Invest Dermatol. 2005;125(5):895‐899. [DOI] [PubMed] [Google Scholar]
- 50. Chakhtoura Z, Vigoureux S, Courtillot C, Tejedor I, Touraine P. Vulvar lichen sclerosus is very frequent in women with Turner syndrome. J Clin Endocrinol Metab. 2014;99(4):1103‐1104. [DOI] [PubMed] [Google Scholar]
- 51. Pilatz A, Altinkilic B, Schormann E, et al. Congenital phimosis in patients with and without lichen sclerosus: distinct expression patterns of tissue remodeling associated genes. J Urol. 2013;189(1):268‐274. [DOI] [PubMed] [Google Scholar]
- 52. Terlou A, Santegoets LA, van der Meijden WI, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA‐155. J Invest Dermatol. 2012;132(3 Pt 1):658‐666. [DOI] [PubMed] [Google Scholar]
- 53. Dennin MH, Stein SL, Rosenblatt AE. Vitiligoid variant of lichen sclerosus in young girls with darker skin types. Pediatr Dermatol. 2018;35(2):198‐201. [DOI] [PubMed] [Google Scholar]
- 54. Yalici‐Armagan B, Bostan E, Akdogan N, Ersoy‐Evans S. Paediatric lichen sclerosus et atrophicus: a retrospective analysis of 38 paediatric patients. Int J Clin Pract. 2021;75(10):e14661. [DOI] [PubMed] [Google Scholar]
- 55. Jacobs L, Gilliam A, Khavari N, Bass D. Association between lichen sclerosus and celiac disease: a report of three pediatric cases. Pediatr Dermatol. 2014;31(6):e128‐e131. [DOI] [PubMed] [Google Scholar]
- 56. Baldo M, Bhogal B, Groves RW, Powell J, Wojnarowska F. Childhood vulval lichen sclerosus: autoimmunity to the basement membrane zone protein BP180 and its relationship to autoimmunity: experimental dermatology. Clin Exp Dermatol. 2010;35(5):543‐545. [DOI] [PubMed] [Google Scholar]
- 57. Becker K, Meissner V, Farwick W, Bauer R, Gaiser MR. Lichen sclerosus and atopy in boys: coincidence or correlation? Br J Dermatol. 2013;168(2):362‐366. [DOI] [PubMed] [Google Scholar]
- 58. Fuchs ME, Beecroft N, Dajusta DG, McLeod DJ. The Association between BXO and obesity in boys undergoing circumcision. Glob Pediatr Health. 2017;4:2333794X1774274. 10.1177/2333794X17742749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mashayekhi S, Flohr C, Lewis FM. The treatment of vulval lichen sclerosus in prepubertal girls: a critically appraised topic. Br J Dermatol. 2017;176(2):307‐316. [DOI] [PubMed] [Google Scholar]
- 60. Casey GA, Cooper SM, Powell JJ. Treatment of vulvar lichen sclerosus with topical corticosteroids in children: a study of 72 children. Clin Exp Dermatol. 2015;40(3):289‐292. [DOI] [PubMed] [Google Scholar]
- 61. Cooper SM, Gao XH, Powell JJ, Wojnarowska F. Does treatment of vulvar lichen sclerosus influence its prognosis? Arch Dermatol. 2004;140(6):702‐706. [DOI] [PubMed] [Google Scholar]
- 62. Focseneanu MA, Gupta M, Squires KC, Bayliss SJ, Berk D, Merritt DF. The course of lichen sclerosus diagnosed prior to puberty. J Pediatr Adolesc Gynecol. 2013;26(3):153‐155. [DOI] [PubMed] [Google Scholar]
- 63. Patrizi A, Gurioli C, Medri M, Neri I. Childhood lichen sclerosus: a long‐term follow‐up. Pediatr Dermatol. 2010;27(1):101‐103. [DOI] [PubMed] [Google Scholar]
- 64. Smith YR, Quint EH. Clobetasol propionate in the treatment of premenarchal vulvar lichen sclerosus. Obstet Gynecol. 2001;98(4):588‐591. [DOI] [PubMed] [Google Scholar]
- 65. Anderson K, Ascanio NM, Kinney MA, Krowchuk DP, Jorizzo JL. A retrospective analysis of pediatric patients with lichen sclerosus treated with a standard protocol of class I topical corticosteroid and topical calcineurin inhibitor. J Dermatol Treat. 2016;27(1):64‐66. [DOI] [PubMed] [Google Scholar]
- 66. Li Y, Xiao Y, Wang H, Li H, Luo X. Low‐concentration topical tacrolimus for the treatment of anogenital lichen sclerosus in childhood: maintenance treatment to reduce recurrence. J Pediatr Adolesc Gynecol. 2013;26(4):239‐242. [DOI] [PubMed] [Google Scholar]
- 67. Mazzilli S, Diluvio L, Di Prete M, et al. Tacrolimus 0.03% ointment for treatment of paediatric lichen sclerosus: a case series and literature review. J Int Med Res. 2018;46(9):3724‐3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Athota K, Kolalapudi SA. Vulvar lichen sclerosus in children: a prospective study over a 3 year period. Ind J Paediatr Dermatol. 2021;22(2):131‐135. [Google Scholar]
- 69. Ellis E, Fischer G. Prepubertal‐onset vulvar lichen sclerosus: the importance of maintenance therapy in long‐term outcomes. Pediatr Dermatol. 2015;32(4):461‐467. [DOI] [PubMed] [Google Scholar]
- 70. Meuli M, Briner J, Hanimann B, Sacher P. Lichen sclerosus et atrophicus causing phimosis in boys: a prospective study with 5‐year followup after complete circumcision. J Urol. 1994;152(3):987‐989. [DOI] [PubMed] [Google Scholar]
- 71. Green PA, Bethell GS, Wilkinson DJ, Kenny SE, Corbett HJ. Surgical management of genitourinary lichen sclerosus et atrophicus in boys in England: a 10‐year review of practices and outcomes. J Pediatr Urol. 2019;15(1):45.e41‐45.e45. [DOI] [PubMed] [Google Scholar]
- 72. Wilkinson DJ, Lansdale N, Everitt LH, et al. Foreskin preputioplasty and intralesional triamcinolone: a valid alternative to circumcision for balanitis xerotica obliterans. J Pediatr Surg. 2012;47(4):756‐759. [DOI] [PubMed] [Google Scholar]
- 73. Ashraf J, Turner A, Subramaniam R. Single‐stage urethroplasty with buccal mucosal inlay graft for stricture caused by balanitis xerotica obliterans in boys: outcomes in the medium term. J Pediatr Urol. 2018;14(1):66.e61‐66.e65. [DOI] [PubMed] [Google Scholar]
- 74. Kiss A, Csontai A, Pirót L, Nyirády P, Merksz M, Király L. The response of balanitis xerotica obliterans to local steroid application compared with placebo in children. J Urol. 2001;165(1):219‐220. [DOI] [PubMed] [Google Scholar]
- 75. Folaranmi SE, Corbett HJ, Losty PD. Does application of topical steroids for lichen sclerosus (balanitis xerotica obliterans) affect the rate of circumcision? A systematic review. J Pediatr Surg. 2018;53(11):2225‐2227. [DOI] [PubMed] [Google Scholar]
- 76. Vincent MV, MacKinnon E. The response of clinical balanitis xerotica obliterans to the application of topical steroid‐based creams. J Pediatr Surg. 2005;40(4):709‐712. [DOI] [PubMed] [Google Scholar]
- 77. Ebert AK, Rösch WH, Vogt T. Safety and tolerability of adjuvant topical tacrolimus treatment in boys with lichen sclerosus: a prospective phase 2 study. Eur Urol. 2008;54(4):932‐937. [DOI] [PubMed] [Google Scholar]
- 78. Morrel B, van Eersel R, Burger CW, et al. The long‐term clinical consequences of juvenile vulvar lichen sclerosus: a systematic review. J Am Acad Dermatol. 2020;82(2):469‐477. [DOI] [PubMed] [Google Scholar]
- 79. Helm KF, Gibson LE, Muller SA. Lichen sclerosus et atrophicus in children and young adults. Pediatr Dermatol. 1991;8(2):97‐101. [DOI] [PubMed] [Google Scholar]
- 80. Loening‐Baucke V. Lichen sclerosus et atrophicus in children. Am J Dis Child. 1991;145(9):1058‐1061. [DOI] [PubMed] [Google Scholar]
- 81. Smith SD, Fischer G. Childhood onset vulvar lichen sclerosus does not resolve at puberty: a prospective case series. Pediatr Dermatol. 2009;26(6):725‐729. [DOI] [PubMed] [Google Scholar]
- 82. Homer L, Buchanan KJ, Nasr B, Losty PD, Corbett HJ. Meatal stenosis in boys following circumcision for lichen sclerosus (balanitis xerotica obliterans). J Urol. 2014;192(6):1784‐1788. [DOI] [PubMed] [Google Scholar]
- 83. Holbrook C, Tsang T. Management of boys with abnormal appearance of meatus at circumcision for balanitis xerotica obliterans. Ann R Coll Surg Engl. 2011;93(6):482‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leganés Villanueva C, Gander R, Royo Gomes G, Ezzeddine Ezzeddine M, López Paredes M, Asensio LM. Treatment of balanitis xerotica obliterans in pediatric patients. Cir Pediatr. 2020;33(2):79‐83. [PubMed] [Google Scholar]
- 85. Arena S, Russo T, Impellizzeri P, Parisi S, Perrone P, Romeo C. Utility of uroflowmetry during the follow‐up of children affected by balanitis xerotica obliterans (BXO). Arch Ital Urol Androl. 2018;9(2):123‐126. [DOI] [PubMed] [Google Scholar]
- 86. Snodgrass W, Blanquel JS, Bush NC. Recurrence after management of meatal balanitis xerotica obliterans. J Pediatr Urol. 2017;13(2):204.e201‐204.e206. [DOI] [PubMed] [Google Scholar]
- 87. Christman MS, Chen JT, Holmes NM. Obstructive complications of lichen sclerosus. J Pediatr Urol. 2009;5(3):165‐169. [DOI] [PubMed] [Google Scholar]
- 88. Spekreijse JJ, Streng BMM, Vermeulen RFM, Voss FO, Vermaat H, van Beurden M. The risk of developing squamous cell carcinoma in patients with anogenital lichen sclerosis: a systematic review. Gynecol Oncol. 2020;157(3):671‐677. [DOI] [PubMed] [Google Scholar]
- 89. Bleeker MC, Visser PJ, Overbeek LI, van Beurden M, Berkhof J. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25(8):1224‐1230. [DOI] [PubMed] [Google Scholar]
- 90. Halonen P, Jakobsson M, Heikinheimo O, Riska A, Gissler M, Pukkala E. Lichen sclerosus and risk of cancer. Int J Cancer. 2017;140(9):1998‐2002. [DOI] [PubMed] [Google Scholar]
- 91. La Spina M, Meli MC, De Pasquale R, et al. Vulvar melanoma associated with lichen sclerosus in a child: case report and literature review. Pediatr Dermatol. 2016;33(3):e190‐e194. [DOI] [PubMed] [Google Scholar]
- 92. Pinto A, McLaren SH, Poppas DP, Magro CM. Genital melanocytic nevus arising in a background of lichen sclerosus in a 7‐year‐old female: the diagnostic pitfall with malignant Melanoma. A literature review. Am J Dermatopathol. 2012;34(8):838‐843. [DOI] [PubMed] [Google Scholar]
- 93. El Shabrawi‐Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50(5):690‐694. [DOI] [PubMed] [Google Scholar]
- 94. Hassanein AM, Mrstik ME, Hardt NS, Morgan LA, Wilkinson EJ. Malignant melanoma associated with Lichen sclerosus in the vulva of a 10‐year‐old. Pediatr Dermatol. 2004;21(4):473‐476. [DOI] [PubMed] [Google Scholar]
- 95. Bussen SS. Melanocytic proliferations associated with lichen sclerosus in adolescence. Arch Gynecol Obstet. 2009;280(6):1039‐1040. [DOI] [PubMed] [Google Scholar]
- 96. Thomas A, Necchi A, Muneer A, et al. Penile cancer. Nat Rev Disease Prim. 2021;7:1. [DOI] [PubMed] [Google Scholar]
- 97. Sultan M, El‐Shazly M, Elsherif E, Younes S, Selim M. Role of urethral plate and fossa navicularis biopsies in the detection of balanitis xerotica obliterans in boys undergoing redo hypospadias repair. Arab J Urol. 2017;15(4):326‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kumar MVK, Harris DL. Balanitis xerotica obliterans complicating hypospadias repair. Br J Plast Surg. 1999;52(1):69‐71. [DOI] [PubMed] [Google Scholar]
- 99. Uemura S, Hutson JM, Woodward AA, Kelly JH, Chow CW. Balanitis xerotica obliterans with urethral stricture after hypospadias repair. Pediatr Surg Int. 2000;16(1–2):144‐145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


