Abstract
Background
Apraglutide is a novel long‐acting glucagon‐like peptide‐2 (GLP‐2) analog designed for once‐weekly subcutaneous dosing, with the potential to increase fluid and nutrient absorption by the remnant intestine of patients who have short bowel syndrome (SBS) with intestinal insufficiency (SBS‐II) or intestinal failure (SBS‐IF). This trial investigated the safety and effects on intestinal absorption of apraglutide in patients with SBS‐II and SBS‐IF.
Methods
In this open‐label, phase 1 and 2 trial, adult patients with SBS‐II (n = 4) or SBS‐IF (n = 4) and a fecal output of ≥1500 g/day received once‐weekly subcutaneous 5 mg apraglutide for 4 weeks. Safety was the primary end point. Secondary end points included change from baseline in intestinal absorption of wet weight (indicative of fluid absorption), electrolytes, and energy (by bomb calorimetry) measured by inpatient metabolic balance studies.
Results
Common treatment‐related adverse events were decreased gastrointestinal (GI) stoma output (n = 6), stoma complications (n = 6), GI stoma complications (n = 5), nausea (n = 5), flatulence (n = 4), abnormal GI stoma output (n = 4), polyuria (n = 3), and abdominal pain (n = 3). The only treatment‐related serious adverse event (experienced in one patient) was abdominal pain. Apraglutide significantly increased wet weight and energy absorption by an adjusted mean of 741 g/day (95% CI, 194 to 1287; P = 0.015) and 1095 kJ/day (95% CI, 196 to 1994; P = 0.024), respectively. Sodium and potassium absorption significantly increased by an adjusted mean of 38 mmol/day (95% CI, 3 to 74; P = 0.039) and 18 mmol/day (95% CI, 4 to 32; P = 0.020), respectively.
Conclusion
Once‐weekly 5 mg apraglutide was well tolerated in patients with SBS‐II and SBS‐IF and significantly improved the absorption of fluids, electrolytes, and energy.
Keywords: apraglutide, glucagon‐like peptide‐2 analog, intestinal absorption, intestinal failure, intestinal insufficiency, research and diseases, short bowel syndrome
CLINICAL RELEVANCY STATEMENT
Patients with short bowel syndrome (SBS) have inadequate intestinal absorptive capacity. This leads to impaired quality of life from the burden of malabsorption and parenteral support (PS) administration. Apraglutide, a novel glucagon‐like peptide‐2 (GLP‐2) analog currently in development for SBS, targets the underlying issue of inadequate absorptive capacity. We present the results from an open‐label, phase 1 and 2 trial with once‐weekly subcutaneous apraglutide dosing for 4 weeks. The safety profile of apraglutide was similar to that observed for other GLP‐2 analogs. Most adverse events were of gastrointestinal origin and mild to moderate in severity. Apraglutide increased the absorption of fluid, energy, and electrolytes. The improvements in intestinal absorption could relieve symptoms of malabsorption and reduce PS dependence. With once‐weekly administration, apraglutide is also expected to improve treatment compliance and potentially quality of life.
INTRODUCTION
Short bowel syndrome (SBS) is a heterogeneous, complex, malabsorptive disorder caused by a clinically significant reduction in intestinal absorptive capacity. The knowledge and use of the proadaptive hormones in SBS has contributed substantially to the understanding and the treatment of intestinal failure associated with SBS (SBS‐IF), a neglected organ failure. 1 Glucagon‐like peptide‐2 (GLP‐2) analogs have been demonstrated to ameliorate gastrointestinal (GI) dysfunction by increasing fluid and nutrient absorption, as demonstrated by decreased parenteral support (PS) dependence ranging from days off to enteral autonomy. 2 , 3 , 4 These better clinical outcomes have the potential to contribute to significant improvements in patients' quality of life. 5 In 2012, teduglutide, a GLP‐2 analog, was approved for once‐daily treatment of patients with SBS. 6 , 7 To date, teduglutide is the only proadaptive agent available for SBS and has challenges that need to be overcome to maximize the full potential of GLP‐2 therapy. Tedulgutide is limited by a short half‐life of ~3–5 h, 8 , 9 which could lead to inconsistent fluid and nutrient absorption over 24 h. Daily subcutaneous (SC) administrations also represent a challenge for adherence and quality of life. Hence, there continues to be an unmet need for better, patient‐convenient, and effective treatments for patients with SBS.
Patients with extensive intestinal resections may experience disturbances in GI neuroendocrine feedback, which coordinates fluid and nutrient absorption. 10 Disturbances may include an impaired postprandial secretion of GLP‐2, normally produced by L‐cells in the terminal ileum and colon. 11 Lack of GLP‐2 may result in an accelerated GI emptying, 12 , 13 GI hypersecretion, 14 diminished intestinal blood flow, 15 disturbed immunological and barrier function, 16 , 17 and impaired mucosal growth. 2 The lack of intestinal adaptation in the remnant bowel contributes to the pathophysiological features of SBS, including frequent diarrheas or stoma emptyings, dehydration, electrolyte imbalances, malnutrition, and weight loss. 18 In patients with SBS‐IF, absorptive capacity is impaired to a degree in which PS is required to maintain health or growth. 19 Long‐term PS dependence may result in serious complications, such as catheter‐related bloodstream infections (CRBSIs) 20 , 21 and intestinal failure–associated liver disease. 22 In contrast to patients with SBS‐IF, patients who have SBS with intestinal insufficiency (SBS‐II) may be managed without PS because of their ability to compensate for their malabsorption with hyperphagia, metabolic adjustments, or symptomatic pharmacological treatments, including antidiarrheal and antisecretory medications. 19 However, patients with SBS‐II may be at risk of fluid and electrolyte imbalances, which necessitate repeated hospital admissions for PS administration. 23 Collectively, because of the clinically significant decrease in intestinal absorptive capacity, patients with SBS have an impaired quality of life, 24 , 25 , 26 significant morbidity and mortality, 27 , 28 and carry high healthcare costs. 29 The ultimate treatment goal for patients with SBS‐IF is to achieve enteral autonomy by enabling patients to absorb sufficient nutrients and fluids without the need for PS.
Apraglutide is a novel long‐acting, synthetic GLP‐2 analog with beneficial pharmacokinetic and pharmacodynamic properties. Preclinical studies show that apraglutide has low clearance, slow absorption after SC administration, and high protein binding compared with native GLP‐2 and other GLP‐2 analogs, resulting in a longer half‐life of ~72 h, making it suitable for once‐weekly dosing. 30 , 31 Apraglutide differs from human GLP‐2 by four amino acid substitutions. 32 Although native GLP‐2 is rapidly degraded by the enzyme dipeptidyl peptidase‐4 (DPP‐4), apraglutide has increased resistance to DPP‐4 breakdown, therefore also contributing to its prolonged half‐life. In cell‐based assays of receptor activation, apraglutide retained potency and selectivity at the GLP‐2 receptor, comparable to native GLP‐2 and teduglutide. 31 Apraglutide has also demonstrated significant trophic effects on the small intestine and enhanced nutrient absorption in animal models for SBS‐IF. 33 , 34 Apraglutide treatment may help patients regain enteral autonomy or reduce PS requirements, improve symptoms of malabsorption, and prevent patients with SBS‐II deteriorating to intermittent or chronic SBS‐IF.
We investigated the safety and efficacy of 5 mg apraglutide administered as once‐weekly SC injections in patients with SBS‐II and SBS‐IF by using metabolic balance studies in this trial. Metabolic balance studies quantify weight and volume, as well as the content of energy, macronutrients, and electrolytes of oral intake (ie, food and fluid intake and PS) and of the output (ie, ostomy output and diarrhea and urine production). Metabolic balance studies are considered as the gold standard for measuring intestinal absorption 35 and have been used as a routine clinical diagnostic tool at the clinical trial site. Herein, we report our learnings from these metabolic studies.
PARTICIPANTS AND METHODS
Trial design and participants
The trial was a single‐center, open‐label, phase 1 and 2 study performed at the Department of Intestinal Failure and Liver Diseases, Rigshospitalet, Copenhagen, Denmark. The Danish Medicines Agency and the Regional Committees on Health Research Ethics (project ID H‐17039730) approved the trial, and the study procedures were in accordance with the ethical standards of the Helsinki Declaration. A total of nine adult patients (aged ≥18 to ≤80 years) with SBS were screened, eight of whom enrolled in the trial: four patients had SBS‐II and four patients had SBS‐IF. Both subgroups of patients were included to investigate the safety and efficacy of apraglutide across the SBS spectrum. The main inclusion criteria were SBS secondary to surgical resection of the small intestine with or without a colon, at least 6 months since the last surgical bowel resection, and a severe degree of malabsorption, defined as a fecal wet weight output of ≥1500 g/day and a urine volume production of <2000 ml/day. Fecal output and urine volume production were confirmed during baseline examinations. Patients were excluded if they had clinical signs of active inflammatory bowel disease, a history of cancer within 5 years, or an inadequate hepatic, kidney, or heart function. Patients were also excluded if they had been hospitalized within 1 month before the screening visit or had received native GLP‐2, GLP‐2 analog, or any growth hormone within the last 3 months. The complete set of inclusion and exclusion criteria are described in the Supporting Information Materials and Methods section.
Patients were treated with once‐weekly 5‐mg apraglutide for 4 weeks. The dose was based on results from previous studies suggesting that a 5‐mg dose is sufficient for maintaining the pharmacological effect until the next dosing on day 7 and that the maximum pharmacological effect is already reached at a dose of 10‐mg, with no difference between 10‐, 25‐, and 50‐mg doses (data on file). 36 Apraglutide was provided as a freeze‐dried powder for reconstitution in sterile water before injection, and treatments were administered as SC injections in the abdominal area. First, second, and fourth apraglutide injections were performed at the hospital. The third dose could be administered at the hospital or in the patient's home, depending on the patient's preference.
Procedures
Safety assessments included observation for injection site reactions, vital signs, blood samples including antiapraglutide antibodies, electrocardiogram, urinalysis, and body weight (Table S1). Efficacy was evaluated by 72‐h metabolic balance studies performed at baseline and at the end of the treatment period (starting 1 day after the fourth/last apraglutide injection). Each 72‐h metabolic balance study was conducted during a 5‐day hospital stay. On the day of admission, patients were required to create a 24‐h drinking menu based on their habitual dietary fluid intake, which was to be followed during each metabolic balance study. On the second day of admission, the metabolic balance study was initiated after the patients had urinated and emptied their stoma bags or defecated. Patients were instructed to collect their fecal output, urine, and a precise duplicate of their dietary intake (fluids and solids separated) in respective containers covering 24‐h periods. Patients had free access to food. Daily PS (volume and content) and dietary fluid intake were kept constant during balance study periods to ensure that the baseline and posttreatment examinations were comparable in regard to measuring the treatment effect. Daily PS and compliance with the predefined drinking menu were documented during admissions.
Concomitant medications, including proton pump inhibitors, loperamide, and opiates, remained unchanged throughout the trial. The content of the containers was weighed, processed into dry matter, and analyzed as previously described 35 : energy by bomb calorimetry, nitrogen by Kjeldahl method, lipid by a modified Van de Kamer titration technique, carbohydrate by Englyst method, sodium and potassium by flame photometry, and calcium and magnesium by atomic absorption spectrometry. 37 Averages of the 72‐h collections were used in the analysis. Body weight was measured using a leveled platform scale. Body composition was measured at baseline and posttreatment using dual‐energy x‐ray absorptiometry (DXA) (Norland XR‐36 DXA densitometer; Norland). Blood samples for analysis of the fasting plasma concentration of citrulline, a suggested biomarker of enterocyte mass, 38 were collected at baseline and at the end of treatment. The analysis method is described in the Supporting Information Materials and Methods section. The trial was overseen by Larix, Copenhagen, Denmark (a contract research organization responsible for clinical trial management, monitoring, data management, and statistical analyses). This trial was registered at ClinicalTrials.gov (number: NCT03408132).
Outcomes
The primary end point of this trial was safety. An adverse event (AE) was defined as any untoward medical occurrence (sign, symptom, or disease), not necessarily causally related to treatment. A serious adverse event (SAE) was defined as an AE that resulted in death, was life‐threatening, required hospitalization, prolonged existing hospitalization, resulted in persistent or significant disability or incapacity, caused a congenital anomaly or birth defect, or was a medically important event. Secondary end points were absolute and relative changes from baseline in dietary intake, fecal excretion, and absorption of wet weight (indicative of fluid absorption), energy, macronutrients and electrolytes, urine production, urinary electrolyte excretion, body weight, lean body mass, fat mass, bone mineral content, and plasma citrulline. Only absolute changes are presented in the scope of this article except for changes from baseline in plasma citrulline.
Statistics
No formal sample size calculation was performed for this trial. Based on the observed safety and magnitude of effects in GLP‐2 analog phase 2 trials, 2 , 39 a total of eight patients completing the study was considered sufficient to provide adequate information about the general safety, tolerability, efficacy, and Pharmacodynamics (PD) at this stage. A statistical test of adjusted mean change from baseline to end of treatment was performed using a paired Student t test and no corrections were made for multiple comparisons. All statistical tests were done using a two‐sided test at a 5% significance level. Estimates were presented with approximate 95% CI and P values. SAS version 9.4 was used for the analysis. In addition, nonparametric testing was conducted for both the overall population and for subgroups (SBS‐II and IF). Results from parametric and nonparametric testing were comparable; therefore, nonparametric data are not reported here. Data from the subgroup analyses were presented descriptively.
RESULTS
A total of nine patients were screened between May 2, 2018, and August 27, 2019. One patient did not fulfill the inclusion criteria of a fecal output of ≥1500 g/day and a urine volume of <2000 ml/day (assessed during the baseline assessment). Hence, eight patients were dosed in the trial. All eight patients completed the trial and constituted the safety and full analysis set (Figure S1). The last patient's last visit was performed on October 27, 2019. The patient demographics and baseline characteristics are shown in Table 1. None of the patients had a reconstructable GI tract, and patients with SBS‐IF had been stable on PS. Two out of four patients with SBS‐II had a jejunostomy and remnant small bowel lengths of 140 and 150 cm. The remaining two patients had an ileostomy and remnant small bowl lengths of 200 and 230 cm. Two out of four patients with SBS‐IF had a jejunostomy and remnant small bowel lengths of 25 and 50 cm. The remaining two patients had an ileostomy with remnant small bowel lengths of 250 and 275 cm. No patients had a colon‐in‐continuity. Three patients had previously been treated with a GLP‐2 analog in clinical trials (≥6 months ago).
Table 1.
Demographics and baseline characteristics
| SBS‐II (n = 4) | SBS‐IF (n = 4) | Total (n = 8) | |
|---|---|---|---|
| Age, years, mean (SD) | 64.5 (3.4) | 57.5 (20.0) | 61.0 (13.8) |
| Sex, n (%) | |||
| Female | 2 (50) | 3 (75) | 5 (62.5) |
| Male | 2 (50) | 1 (25) | 3 (37.5) |
| Weight, kg, mean (SD) | 83.2 (18.5) | 62.5 (11.2) | 72.8 (18.0) |
| Body mass index, mean (SD) | 28.6 (5.1) | 22.7 (4.2) | 25.6 (5.4) |
| White race, n (%) | 4 (100) | 4 (100) | 8 (100) |
| PS volume, ml/day, mean (SD) | NA | 2230 (889) | NA |
| PS energy, kJ/day, mean (SD) | NA | 2823 (3579) | NA |
| Urine production, g/day, mean (SD) | 1423 (212) | 1370 (284) | 1397 (234) |
| Dietary intake, g/day, mean (SD) | 5710 (1519) | 3255 (1006) | 4482 (1773) |
| Fecal output, g/day, mean (SD) | 3419 (2015) | 3243 (1339) | 3331 (1587) |
| Plasma citrulline levels, µmol/L, mean (SD) | 43.7 (15.0) | 22.7 (15.5) | 33.2 (18.0) |
| Cause of resection, n (%) | |||
| Crohn's disease | 3 (75) | 0 | 3 (37.5) |
| Mesenteric vascular disease | 1 (25) | 1 (25) | 2 (25) |
| Surgical complications to ulcerative colitis | 0 | 3 (75) | 3 (37.5) |
| Disease characteristics | |||
| Small bowel length, cm, mean (SD) | 180 (42) | 155 (125) | 168 (87) |
| End‐jejunostomy, n (%) | 2 (50) | 2 (50) | 4 (50) |
| Ileostomy, n (%) | 2 (50) | 2 (50) | 4 (50) |
| Colon‐in‐continuity, n (%) | 0 | 0 | 0 |
| Concomitant medication, n (%) | |||
| Proton pump inhibitor | 4 (100) | 3 (75) | 7 (87.5) |
| Opioids or opioid agonists | 3 (75) | 3 (75) | 6 (75) |
| Loperamide | 2 (50) | 1 (25) | 3 (37.5) |
Note: Body mass index is calculated as weight in kilograms divided by height in meters squared. PS is scheduled PS at trial entry based on weekly average.
Abbreviations: NA, not applicable; PS, parenteral support; SBS‐IF, short bowel syndrome with intestinal failure; SBS‐II, short bowel syndrome with intestinal insufficiency.
Safety results
All patients experienced at least one treatment‐related AE (TRAE) (Table 2): most were mild to moderate. One patient experienced transient injection site reactions (local erythema and pruritus) after one injection, which were unrelated to the presence of antiapraglutide antibodies. A total of three SAEs occurred in two patients. One SAE, an event of abdominal pain requiring hospital admission, was assessed as related to the trial drug. The abdominal pain was conservatively treated, and the patient was discharged within 24 h. Temporary discontinuation of 7 days and rechallenge at a reduced dose allowed the patient to complete the trial. The remaining two SAEs were not considered related to apraglutide. They included one event of dehydration in a patient with SBS‐II and one occurrence of a CRBSI in a patient with SBS‐IF. Two additional patients required a dose reduction to complete the trial. One patient with SBS‐II had signs of fluid retention after the first drug administration, and therefore, the second and third administrations were given at reduced doses (2.5 mg). The fourth/last administration was given at the full dose (5 mg) without further complications. One patient experienced constipation after the first drug administration; the second administration was given at a reduced dose (2.5 mg). The full dose was reintroduced for the third and fourth/last administration without further complications. None of the patients discontinued the trial because of AEs, and no deaths occurred.
Table 2.
Common treatment‐related AEs
| SBS‐II (n = 4) | SBS‐IF (n = 4) | Total (n = 8) | |
|---|---|---|---|
| Any treatment‐related AEs | 4 | 4 | 8 (100) |
| GI stoma output decreased | 3 | 3 | 6 (75) |
| Stoma complication | 2 | 3 | 6 (75) |
| GI stoma complication | 3 | 2 | 5 (62.5) |
| Nausea | 1 | 4 | 5 (62.5) |
| GI stoma output abnormal | 3 | 1 | 4 (50) |
| Flatulence | 3 | 1 | 4 (50) |
| Polyuria | 2 | 1 | 3 (37.5) |
| Abdominal pain | 1 | 2 | 3 (37.5) |
| Hot flush | 2 | 0 | 2 (25) |
Note: Treatment‐related AEs occurring in ≥2 patients in either cohort. Data are number of patients (n) in the safety analysis set or n (%). The Medical Dictionary for Regulatory Activities preferred term “stoma complications” included the reported terms “increased stoma diameter” and “slower passage through stoma.” The preferred term “GI stoma complication” included the reported term “increased stoma protrusion”. The preferred term “GI stoma output abnormal” included the reported term “more solid stoma output.”
Abbreviations: AE, adverse event; GI, gastrointestinal; SBS‐IF, short bowel syndrome with intestinal failure; SBS‐II, short bowel syndrome with intestinal insufficiency.
All patients had negative results for antiapraglutide antibodies at screening. One patient with SBS‐IF developed a low titer of antiapraglutide antibodies during the trial. The patient had previously been treated with a GLP‐2 analog in a clinical trial setting in 2016. No relationship was observed between the antiapraglutide antibodies and plasma concentrations of apraglutide, the pharmacodynamic response, or the number or duration of AEs.
Efficacy end points
Wet weight
The individual changes from baseline in the wet weight of the dietary intake, fecal output, urine, and absorption are shown in Figure 1. Apraglutide did not change the wet weight of the dietary intake (Table 3). Apraglutide significantly increased intestinal absorption of wet weight by 741 g/day (95% CI, 194 to 1287; P = 0.015). Consistent with increased intestinal absorption, apraglutide significantly decreased fecal output by 680 g/day (95% CI, −1200 to −159; P = 0.018) and increased urine production by 560 g/day (95% CI, 72 to 1048; P = 0.030).
Figure 1.

Individual and mean changes from baseline to end of treatment in wet weight dietary intake, fecal output, intestinal absorption, and urine production. Dashed lines show patients with SBS‐II. Difference in grayscale shows individual patients. B, baseline; T, treatment; ∆, mean change from baseline (SD)
Table 3.
Absolute change from baseline in dietary intake, fecal and urine output, and absorption of wet weight, energy, macronutrients, and electrolytes
| Dietary intake | Fecal output | Urine output | Absorption | |||||
|---|---|---|---|---|---|---|---|---|
| Secondary end points | n = 8 | P value | n = 8 | P value | n = 8 | P value | n = 8 | P value |
| Wet weight, g/day | 61 (−84.0 to 207) | 0.352 | −680 (−1200 to −159) | 0.018 | 560 (72 to 1048) | 0.030 | 741 (194 to 1287) | 0.015 |
| Energy, kJ/day | 154 (−1006 to 1314) | 0.763 | −941 (−2438 to 556) | 0.181 | 1095 (196 to 1994) | 0.024 | ||
| Carbohydrate, kJ/day | 154 (−268 to 575) | 0.418 | −365 (−772 to 43) | 0.072 | 518 (112 to 924) | 0.019 | ||
| Lipid, kJ/day | 67 (−638 to 771) | 0.830 | −309 (−969 to 351) | 0.304 | 376 (61 to 691) | 0.026 | ||
| Protein, kJ/day | −41 (−274 to 191) | 0.688 | −145 (−497 to 207) | 0.362 | 104 (−205 to 412) | 0.453 | ||
| Sodium, mmol/day | −5 (−30 to 21) | 0.680 | −43 (−92 to 6) | 0.077 | 27 (5 to 49) | 0.024 | 38 (3 to 74) | 0.039 |
| Potassium, mmol/day | 4 (−5 to 12) | 0.337 | −15 (−32 to 3) | 0.086 | 13 (6 to 20) | 0.003 | 18 (4 to 32) | 0.020 |
| Magnesium, mmol/day | 1 (−2 to 3) | 0.561 | 0 (−9 to 9) | 0.961 | 1 (−1 to 2) | 0.411 | 0 (−9 to 9) | 0.930 |
| Calcium, mmol/day | −2 (−5 to 2) | 0.367 | −13 (−36 to 11) | 0.255 | 0 (−1 to 1) | 0.419 | 11 (−13 to 35) | 0.313 |
Note: Data are adjusted mean (95% CI). Calculations are based on changes from baseline to end of treatment, analyzed using a paired Student t test.
Abbreviation: n, number of patients in the full analysis set.
Electrolytes
Individual changes from baseline in electrolyte absorption are shown in Figure 2. The electrolyte content of dietary intake and fecal output did not change (Table 3). Apraglutide significantly increased absorption of sodium and potassium by 38 mmol/day (95% CI, 3 to 74; P = 0.039) and 18 mmol/day (95% CI, 4 to 32; P = 0.020), respectively (Table 3). Urinary sodium and potassium excretion increased by 27 mmol/day (95% CI, 5 to 49; P = 0.024) and 13 mmol/day (95% CI, 6 to 20; P = 0.003), respectively. There was no significant change in magnesium and calcium absorption or urinary excretion (Table 3).
Figure 2.

Individual and mean changes from baseline to end of treatment in intestinal absorption of potassium, sodium, magnesium, and calcium. Dashed lines show patients with SBS‐II. Difference in grayscale shows individual patients. B, baseline; T, treatment; ∆, mean change from baseline (SD)
Energy and macronutrients
Figure 3 shows the individual changes from baseline in the energy content of dietary intake, fecal output, and absorption. Apraglutide did not change total dietary energy intake or any individual macronutrient intake (Table 3). Compared with baseline, apraglutide significantly increased intestinal absorption of energy by 1095 kJ/day (95% CI, 196 to 1994; P = 0.024). The individual changes in macronutrient absorption are plotted in Figure 4. Carbohydrate and lipid absorption significantly increased by 518 kJ/day (95% CI, 112 to 924; P = 0.019) and 376 kJ/day (95% CI, 61 to 691; P = 0.026), respectively. There was no significant change from baseline in protein absorption (Table 3).
Figure 3.

Individual and mean changes from baseline to end of treatment in the energy dietary intake, fecal output, and intestinal absorption. Dashed lines show patients with SBS‐II. Difference in grayscale shows individual patients. B, baseline; T, treatment; ∆, mean change from baseline (SD)
Figure 4.
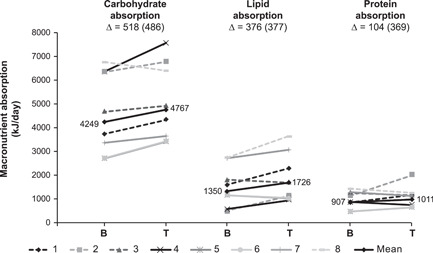
Individual and mean changes from baseline to end of treatment in intestinal absorption of macronutrients. Dashed lines show patients with SBS‐II. Difference in grayscale shows individual patients. B, baseline; T, treatment; ∆, mean change from baseline (SD)
Body weight and body composition
Body weight increased by 1.8 kg (95% CI, 0.4 to 3.1; P = 0.016) after 4 weeks of apraglutide treatment. Lean body mass significantly increased by 1.7 kg (95% CI, 0.8 to 2.6; P = 0.003) and fat mass decreased by 1.1 kg (95% CI, −2.1 to −0.0; P = 0.044). There was no significant change from baseline in bone mineral content.
Plasma citrulline
Compared with baseline, apraglutide significantly increased absolute and relative plasma concentration of citrulline by 15.2 µmol/L (95% CI, 3.3 to 27.1; P = 0.019) and 66% (95% CI, 3 to 128; P = 0.043), respectively.
DISCUSSION
In this phase 1 and 2 inpatient metabolic balance trial, once‐weekly 5 mg apraglutide, a next‐generation GLP‐2 analog, was safe and well tolerated in patients with SBS‐II and SBS‐IF after 4 weeks of treatment and showed positive effects on intestinal absorption. Most AEs were consistent with the known physiological effects of GLP‐2 agonism. Frequently reported related AEs were of GI origin and mainly mild to moderate. All stoma‐related AEs (including increased protrusion and diameter of the stoma) were mild in severity and were unlikely to have an impact on patient comfort or quality of life. The safety profile of apraglutide was comparable to native GLP‐2 and other GLP‐2 analogs. 2 , 39 , 40 Apraglutide caused few injection site reactions, demonstrating the benefit of once‐weekly dosing. Abdominal pain and nausea were transient and resolved with either continued treatment or a dose reduction. Since PS and dietary fluid intake were kept constant during balance periods, edema and polyuria were clinical signs of excess body fluid and would likely have resolved with PS reductions. Because of the short treatment period of 4 weeks, PS volume was only reduced if there were signs of fluid retention. In longer‐term studies of apraglutide, edema and polyuria is expected to resolve with adequate PS weaning. Some AEs were perceived as beneficial, including a more solid stoma output and a decreased stoma output. A more precise definition of an AE could have prevented beneficial effects from being reported as AEs. Overall, there was an equal distribution of common TRAEs in patients with SBS‐II and SBS‐IF. Nausea was more frequent in patients with SBS‐IF. The occurrence of nausea could not be explained by any underlying conditions, such as the presence of an abdominal stricture. Moreover, there was no relationship between nausea and dose adjusted for weight (VectivBio, 2021). Overall, results from this study suggest a favorable safety profile for apraglutide. However, longer‐term studies are required in larger numbers of patients before definite conclusions can be made.
Apraglutide significantly increased intestinal absorption of fluid, energy, and electrolytes (sodium and potassium). The improvements in fluid absorption were accompanied by a significant decrease in fecal wet weight and an increase in urine production and urinary electrolyte excretion (sodium and potassium). The results of our secondary end points should be interpreted with care because of the small sample size and the heterogeneous population. Based on algorithms used in phase 3 trials within the research field, the increases in urine production were clinically relevant, as they would enable PS reductions or help regain enteral autonomy in patients with SBS‐IF. 3 , 4 The improvements in absorption could eventually reduce the risk of developing intermittent or chronic IF in patients with SBS‐II and alleviate the symptom burden of malabsorption. For the first time, our study shows that beneficial effects can be achieved through once‐weekly treatment; previous phase 2 trials of other GLP‐2 agonists required daily injections. 2 , 39 Apraglutide's beneficial impact on fluid absorption has also been confirmed in another recently published phase 2 trial in which fluid absorption was assessed indirectly by increases in urine production. 41
The magnitude of effects of once‐weekly apraglutide on fluid, energy, and electrolyte absorption were comparable to those previously reported for daily native GLP‐2, teduglutide, and glepaglutide treatment. 2 , 38 , 40 Apraglutide is the first GLP‐2 analog to significantly improve absorption of energy across the whole patient spectrum in the SBS population when measured by bomb calorimetry, the gold standard laboratory method for quantifying intestinal energy absorption. Teduglutide failed to increase energy absorption except in patients with high dietary compliance or colon‐in‐continuity, 2 and glepaglutide only increased energy absorption when measured by the calculated sum of macronutrients. 39 In later stages of clinical development, decreasing PS energy requirements while maintaining body weight is considered indirect evidence of improved energy absorption. 4 The permanent effects on body composition and energy expenditure during PS energy reductions have not been studied. It is reassuring that key electrolytes such as sodium were seen to improve, which is especially important in patients with high stoma/fecal losses of sodium and may contribute to better hydration. Similarly, patients with colon‐in‐continuity are receiving PS not only for fluid and electrolyte losses but also for energy needs. It is encouraging that a once‐weekly administration over 4 weeks was able to demonstrate significant energy absorption. Teduglutide treatment is limited by a short half‐life of ~3–5 h, 8 , 9 which potentially leads to inconsistent fluid and nutrient absorption over 24 h. The pharmacokinetic profile of apraglutide allows for consistent exposure, which might explain the achievement of significance on improving effects on energy absorption.
Apraglutide significantly increased body weight and lean body mass while reducing fat mass, possibly representing improvements in hydration status. Previous trials show that increases in body weight and lean body mass often reflect transient fluid retention at the start of GLP‐2 analog treatment before the body has adjusted to an improved hydration status. 2 , 4 , 39 , 40 , 42 A concomitant decrease in fat mass was also found in the study of native GLP‐2. 40 Fat mass estimation errors by DXA may occur because of variations in soft tissue hydration. 43 Contrary to this trial, the phase 2 trial of apraglutide showed more diverse effects on body composition, with no uniform pattern of change. 41 This difference emphasizes that short‐term changes in body composition should be interpreted with care and may vary because of individual patient characteristics and evaluation methods.
Consistent with previous studies, 44 patients with SBS‐II presented with more pronounced hyperphagia than those with SBS‐IF. Although improvements in intestinal absorption were seen in both subgroups of patients, SBS‐II patients with jejunostomy (small bowel length of 140–150 cm with severe malabsorption and hyperphagia) had a greater response to treatment than those with SBS‐II and ileostomy (small bowel length of 200–230 cm with adequate absorption and less severe hyperphagia). Differences might be because of the small number of patients in this study. However, results are consistent with those from teduglutide studies, in which patients with the poorest remnant bowel function experienced the greatest absolute reduction in PS volume. 45 This might be explained by the decrease in endogenous postprandial GLP‐2 secretion in these patients due to resection of the terminal ileum and the colon. 11 Hence, differences found in this trial might also be due to between‐patient differences in baseline levels of endogenous postprandial GLP‐2 (although not measured in this trial) and/or due to differences between patients in drug exposure when adjusted for body weight (data not shown). Consequently, larger studies are required to assess treatment response in patients with similar baseline characteristics.
Apraglutide did not change the wet weight or energy content of dietary intake. Our findings support existing evidence that GLP‐2 does not significantly affect appetite or postprandial feeling of satiety in healthy participants. 46 , 47 A reduced dietary intake could be an undesired side effect in patients with SBS who depend on hyperphagia to compensate for the intestinal losses. In a study of long‐term GLP‐2 treatment, during which PS remained unchanged, decreases in fecal wet weight were accompanied by a decline in total wet weight intake. 48 This suggests that long‐term GLP‐2 treatment improved absorptive efficiency of the remnant intestine and allowed patients to reduce their dietary intake. Hence, apraglutide may reduce the need for compensatory hyperphagia in patients with severe SBS‐II.
Several actions of GLP‐2 may lead to increased fluid and energy absorption. GLP‐2 increases villus height and depth, leading to an increased mucosal surface area. 2 , 39 In this trial, apraglutide significantly increased plasma citrulline, a potential marker of enterocyte mass, 38 providing support for its expected proadaptive effects. Intestinal biopsies could have provided direct evidence for the intestinotrophic effect but were not performed in this trial. The extent to which morphological changes in the intestinal wall contribute to improvements in absorption is not known because GLP‐2 also inhibits GI motility, 12 , 13 , 14 reduces GI hypersecretion, 14 and stimulates mesenteric blood flow. 15
The average baseline plasma citrulline levels were 22.7 µmol/L (SD, 15.5) and 43.7 µmol/L (SD, 15.0) for patients with SBS‐IF and SBS‐II, respectively. The baseline citrulline level for patients with SBS‐IF was close to the threshold suggested by Crenn et al 38 of 20 µmol/L for permanent vs transient SBS‐IF (defined as being able to wean off PS 2 years within last intestinal surgery). However, a recent systematic review and meta‐analysis found that citrulline levels correlated well to small bowel length, but was a less reliable marker of functional absorptive capacity. 46
Limitations of this trial include the short duration, small numbers of patients, and the heterogeneous population. Moreover, we only enrolled patients with a jejunostomy/ileostomy. Patients with a colon‐in‐continuity have a distinct pathophysiological phenotype compared with patients with a jejunostomy/ileostomy, 45 and future metabolic balance studies could evaluate the effects of apraglutide in patients with a colon‐in‐continuity. Furthermore, we included only stable patients who did not have a reconstructable GI tract. Apraglutide may have therapeutic potential in the earlier stages of intestinal adaptation after surgery or as medical rehabilitation before final reconstructive surgery.
In conclusion, the next‐generation GLP‐2 analog apraglutide, administered at 5 mg once‐weekly for 4 weeks, increased intestinal absorption of fluid, electrolytes, and energy and was well tolerated. The rational design of apraglutide allows for its low clearance, slow absorption, and high protein binding compared with SC native GLP‐2 and other GLP‐2 analogs, resulting in a longer half‐life. Apraglutide acts as a full agonist of the GLP‐2 receptor, with potency and selectivity comparable to native GLP‐2.
Results of this metabolic balance study and emerging data suggest that the full spectrum of SBS patients, including patients with SBS‐II and SBS‐IF, may be appropriate candidates for treatment with apraglutide, a promising potential new treatment option.
A multicenter, multinational phase 3 trial to confirm the safety and efficacy of apraglutide has been initiated (trial to evaluate efficacy and safety of apraglutide in SBS‐IF [STARS], ClinicalTrials.gov Identifier: NCT04627025).
CONFLICTS OF INTEREST
Palle B. Jeppesen has served as a consultant and speaker for VectivBio AG and was the principal investigator in this study. Johanna Eliasson served as a consultant for VectivBio AG and was a study investigator. Mark K. Hvistendahl and Nanna Freund were study investigators. Federico Bolognani was, at the time of this study, an employee of VectivBio AG. Christian Meyer is an employee of VectivBio AG.
AUTHOR CONTRIBUTIONS
Johanna Eliasson, Mark K. Hvistendahl, Federico Bolognani, Christian Meyer, and Palle B. Jeppesen designed the research; Johanna Eliasson, Nanna Freund, and Palle B. Jeppesen conducted the research; Johanna Eliasson and Palle B. Jeppesen analyzed the data; Johanna Eliasson wrote the manuscript; Mark K. Hvistendahl, Nanna Freund, Federico Bolognani, Christian Meyer, and Palle B. Jeppesen provided a constructive review of the manuscript; Johanna Eliasson had primary responsibility for the final report. All authors read and approved the final manuscript.
Supporting information
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Eliasson J, Hvistendahl MK, Freund N, Bolognani F, Meyer C, Jeppesen PB. Apraglutide, a novel once‐weekly glucagon‐like peptide‐2 analog, improves intestinal fluid and energy absorption in patients with short bowel syndrome: An open‐label phase 1 and 2 metabolic balance trial. J Parenter Enteral Nutr. 2022;46:1639‐1649. 10.1002/jpen.2362
REFERENCES
- 1. Jeppesen PB. The long road to the development of effective therapies for the short gut syndrome: a personal perspective. Dig Dis Sci. 2019;64(10):2717‐2735. [DOI] [PubMed] [Google Scholar]
- 2. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX‐0600), a dipeptidyl peptidase IV resistant glucagon‐like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473‐1481. [DOI] [PubMed] [Google Scholar]
- 4. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo‐controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen K, Mu F, Xie J, et al. Impact of teduglutide on quality of life among patients with short bowel syndrome and intestinal failure. JPEN J Parenter Enteral Nutr. 2020;44(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Prescribing information. Gattex. Revised December 2012. Accessed February 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203441Orig1s000lbl.pdf
- 7.European Medicines Agency. Summary of product characteristics. Revestive. Updated May 2017. Accessed February 2021. https://www.ema.europa.eu/en/documents/overview/revestive-epar-summary-public_en.pdf
- 8. Marier JF, Beliveau M, Mouksassi MS, et al. Pharmacokinetics, safety, and tolerability of teduglutide, a glucagon‐like peptide‐2 (GLP‐2) analog, following multiple ascending subcutaneous administrations in healthy subjects. J Clin Pharmacol. 2008;48(11):1289‐1299. [DOI] [PubMed] [Google Scholar]
- 9. Marier JF, Mouksassi MS, Gosselin NH, Beliveau M, Cyran J, Wallens J. Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn's disease. J Clin Pharmacol. 2010;50(1):36‐49. [DOI] [PubMed] [Google Scholar]
- 10. Nightingale JM, Kamm MA, van der Sijp JR, et al. Disturbed gastric emptying in the short bowel syndrome. Evidence for a “colonic brake”. Gut. 1993;34(9):1171‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeppesen PB, Hartmann B, Hansen BS, Thulesen J, Holst JJ, Mortensen PB. Impaired meal stimulated glucagon‐like peptide 2 response in ileal resected short bowel patients with intestinal failure. Gut. 1999;45(4):559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wojdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon‐like peptide‐2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33(8):828‐832. [DOI] [PubMed] [Google Scholar]
- 13. Hvistendahl MK, Naimi RM, Enevoldsen LH, Madsen JL, Fuglsang S, Jeppesen PB. Effect of glepaglutide, a long‐acting glucagon‐like peptide‐2 analog, on gastrointestinal transit time and motility in patients with short bowel syndrome: findings from a randomized trial. JPEN J Parenter Enteral Nutr. 2020;44(8):1535‐1544. [DOI] [PubMed] [Google Scholar]
- 14. Wojdemann M, Wettergren A, Hartmann B, Hilsted L, Holst JJ. Inhibition of sham feeding‐stimulated human gastric acid secretion by glucagon‐like peptide‐2. J Clin Endocrinol Metab. 1999;84(7):2513‐2517. [DOI] [PubMed] [Google Scholar]
- 15. Bremholm L, Hornum M, Andersen UB, Hartmann B, Holst JJ, Jeppesen PB. The effect of glucagon‐like peptide‐2 on mesenteric blood flow and cardiac parameters in end‐jejunostomy short bowel patients. Regul Pept. 2011;168(1‐3):32‐38. [DOI] [PubMed] [Google Scholar]
- 16. Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP‐2‐driven improvement of gut permeability. Gut. 2009;58(8):1091‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon‐like peptide‐2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47(1):112‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38(1 suppl):8S‐13S. [DOI] [PubMed] [Google Scholar]
- 19. Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 20. Dibb M, Lal S. Home parenteral nutrition: vascular access and related complications. Nutr Clin Pract. 2017;32(6):769‐776. [DOI] [PubMed] [Google Scholar]
- 21. Fuglsang KA, Brandt CF, Scheike T, Jeppesen PB. Hospitalizations in patients with nonmalignant short‐bowel syndrome receiving home parenteral support. Nutr Clin Pract. 2020;35(5):894‐902. [DOI] [PubMed] [Google Scholar]
- 22. Bond A, Huijbers A, Pironi L, Schneider SM, Wanten G, Lal S. Review article: diagnosis and management of intestinal failure‐associated liver disease in adults. Aliment Pharmacol Ther. 2019;50(6):640‐653. [DOI] [PubMed] [Google Scholar]
- 23. Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55(2):175‐180. [DOI] [PubMed] [Google Scholar]
- 24. Carlsson E, Bosaeus I, Nordgren S. Quality of life and concerns in patients with short bowel syndrome. Clin Nutr. 2003;22(5):445‐452. [DOI] [PubMed] [Google Scholar]
- 25. Kalaitzakis E, Carlsson E, Josefsson A, Bosaeus I. Quality of life in short‐bowel syndrome: impact of fatigue and gastrointestinal symptoms. Scand J Gastroenterol. 2008;43(9):1057‐1065. [DOI] [PubMed] [Google Scholar]
- 26. Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut. 1999;44(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dibb M, Soop M, Teubner A, et al. Survival and nutritional dependence on home parenteral nutrition: three decades of experience from a single referral centre. Clin Nutr. 2017;36(2):570‐576. [DOI] [PubMed] [Google Scholar]
- 28. Messing B, Crenn P, Beau P, Boutron‐Ruault MC, Rambaud JC, Matuchansky C. Long‐term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117(5):1043‐1050. [DOI] [PubMed] [Google Scholar]
- 29. Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. 2003;17(6):931‐942. [DOI] [PubMed] [Google Scholar]
- 30. Bolognani F, Gal P, Moerland M, et al. The pharmakokinetic and pharmacodynamic relationship between apraglutide and citrulline: a randomized, placebo‐controlled, double‐blind study in healthy volunteers. Clin Nutr ESPEN. 2020;40:413‐414. [Google Scholar]
- 31. Hargrove DM, Alagarsamy S, Croston G, et al. Pharmacological characterization of apraglutide, a novel long‐acting peptidic glucagon‐like peptide‐2 agonist, for the treatment of short bowel syndrome. J Pharmacol Exp Ther. 2020;373(2):193‐203. [DOI] [PubMed] [Google Scholar]
- 32. Wiśniewski K, Sueiras‐Diaz J, Jiang G, et al. Synthesis and pharmacological characterization of novel glucagon‐like peptide ‐2 (GLP‐2) analogues with low systemic clearance. J Med Chem. 2016;59(7):3129‐3139. [DOI] [PubMed] [Google Scholar]
- 33. Pauline ML. Comparing the intestinotrophic effects of 2 glucagon‐like peptide‐2 analogues in the treatment of short‐bowel syndrome in neonatal piglets. JPEN J Parenter Enteral Nutr. 2021;45(3):538‐545. [DOI] [PubMed] [Google Scholar]
- 34. Martchenko SE, Sweeney ME, Dimitriadou V, Murray JA, Brubaker PL. Site‐ specific and temporal effects of apraglutide, a novel long‐acting glucagon‐like peptide‐2 receptor agonist, on intestinal growth in mice. J Pharmacol Exp Ther. 2020;373(3):347‐352. [DOI] [PubMed] [Google Scholar]
- 35. Jeppesen PB. Intestinal insufficiency and failure. Dan Med Bull. 2003;50(3):238‐261. [PubMed] [Google Scholar]
- 36. Bolognani F, Machacek M, Kruithof A, et al. Population model confirms predictable pharmacokinetic (PK) and pharmacodynamic (PD) profile for apraglutide: data from two randomized phase 1 studies. Gasterenterology. 2021;160(6):S738. [Google Scholar]
- 37. Jeppesen PB, Staun M, Tjellesen L, Mortensen PB. Effect of intravenous ranitidine and omeprazole on intestinal absorption of water, sodium, and macronutrients in patients with intestinal resection. Gut. 1998;43(6):763‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crenn P, Coudray‐Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119(6):1496‐1505. [DOI] [PubMed] [Google Scholar]
- 39. Naimi RM, Hvistendahl M, Enevoldsen LH, et al. Glepaglutide, a novel long‐acting glucagon‐like peptide‐2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4(5):354‐363. [DOI] [PubMed] [Google Scholar]
- 40. Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon‐like peptide 2 improves nutrient absorption and nutritional status in short‐bowel patients with no colon. Gastroenterology. 2001;120(4):806‐815. [DOI] [PubMed] [Google Scholar]
- 41. Eliasson J, Hvistendahl MK, Freund N, Bolognani F, Meyer C, Jeppesen PB. Apraglutide, a novel glucagon‐like peptide‐2 analog, improves fluid absorption in patients with short bowel syndrome intestinal failure: findings from a placebo‐controlled, randomized phase 2 trial. JPEN J Parenter Enteral Nutr . 2021. [DOI] [PMC free article] [PubMed]
- 42. Schwartz LK, O'Keefe SJD, Fujioka K, et al. Long‐term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7(2):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual‐energy X‐ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274(5):E808‐E816. [DOI] [PubMed] [Google Scholar]
- 44. Prahm AP, Brandt CF, Askov‐Hansen C, Mortensen PB, Jeppesen PB. The use of metabolic balance studies in the objective discrimination between intestinal insufficiency and intestinal failure. Am J Clin Nutr. 2017;106(3):831‐838. [DOI] [PubMed] [Google Scholar]
- 45. Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors associated with response to teduglutide in patients with short‐bowel syndrome and intestinal failure. Gastroenterology. 2018;154(4):874‐885. [DOI] [PubMed] [Google Scholar]
- 46. Fragkos KC, Forbes A. Citrulline as a marker of intestinal function and absorption in clinical settings: a systematic review and meta‐analysis. United European Gastroenterol J. 2018;6(2):181‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda. Medication Guide. GATTEX® (teduglutide). January 2021. Accessed March 2022. https://www.shirecontent.com/MEDGUIDE/PDFs/MG_Gattex_USA_ENG.pdf
- 48. Jeppesen PB, Lund P, Gottschalck IB, et al. Short bowel patients treated for two years with glucagon‐like Peptide 2: effects on intestinal morphology and absorption, renal function, bone and body composition, and muscle function. Gastroenterol Res Pract. 2009;2009:616054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.


