Abstract
Background
Children born to larger households have less allergic disease. T regulatory cell (Treg) development may be a relevant mechanism, but this has not been studied longitudinally.
Objective
We aim to (i) describe how prenatal and postnatal environmental factors are associated with Treg development and (ii) investigate whether serial Treg measures predict allergic outcomes at 1 year of age.
Methods
A birth cohort (n = 1074) with information on prenatal and postnatal early life factors. Both naïve Treg (nTreg) and activated Treg (aTreg) cell populations (as a proportion of CD4+ T cells) were available in 463 infants at birth (cord blood), 600 at 6 months, and 675 at 12 months. 191 infants had serial measures. Measures of allergic status at 12 months were polysensitization (sensitization to 2 or more allergens), clinically proven food allergy, atopic eczema, and atopic wheeze.
Results
Infants born to larger households (3 or more residents) had higher longitudinal nTreg proportions over the first postnatal year with a mean difference (MD) of 0.67 (95% CI 0.30–1.04)%. Higher nTreg proportions at birth were associated with a reduced risk of infant allergic outcomes. Childcare attendance and breastfeeding were associated with higher longitudinal nTreg proportions (MD 0.48 (95% CI 0.08–0.80)%.
Conclusion
Multiple prenatal and postnatal microbial factors are associated with nTreg and aTreg development. Larger household size was associated with higher nTreg at birth which in turn was associated with reduced allergic sensitization and disease at 12 months of age.
Keywords: food allergy, household size, hygiene hypothesis, infant atopic sensitization, T regulatory cells (Treg)
Abbreviations
- AOR
adjusted odds ratio
- aTreg
activated T regulatory cell
- AUC
area under curve
- BIS
Barwon Infant Study
- CI
confidence interval
- IgE
immunoglobulin E
- nTreg
naïve T regulatory cell
- SPT
skin prick testing
- Treg
T regulatory cell
Key Messages.
This study provides population‐based evidence for the links between household size, immune changes, and allergic disease in children, supportive of the “Hygiene Hypothesis.” Multiple early life environmental factors influence Treg development. Higher cord blood naïve Treg proportions predicted a reduced risk in infant polysensitization and food allergy, atopic eczema, and atopic wheeze. Prenatal boosting of naïve Treg cells may play a role in reducing early life allergic disease.
1. INTRODUCTION
Childhood allergic disease has increased over time. 1 In 1989, Strachan reported children born to larger households had less allergic disease. 2 This association has been well replicated. 1 The “Hygiene Hypothesis” proposes that a beneficial effect of larger household size is driven by sibling‐related and other environmental microbial exposures. 1 , 3 As well as larger household size, other candidate environmental microbial factors that may act as a proxy for environmental microbial load external to the child include dogs and farm animals. 4 , 5 , 6 Mounting evidence also implicates the potential role of the maternal 7 and infant gut microbiome. 7 , 8 Infant polysensitization, with atopic sensitization to multiple allergens, strongly predicts severe subsequent allergic disease. 9 , 10 , 11
FoxP3‐positive T regulatory (Treg) cells play a role in maintaining immune tolerance to foods and other environmental antigens. In early life, we reported that the CD4+FoxP3+ cells have a predominantly naïve (CD45RA+) phenotype. This CD4+CD45RA+FoxP3low population of Treg were shown by Miyara et al to be fully demethylated for the 5’ flanking region of the FoxP3 gene and potently suppressive. 12 Naïve Treg (nTreg) in circulation can transform into activated Treg (aTreg, CD4+CD45RA−FoxP3high) in response to antigenic immunostimulation. 12 , 13 We reported that a deficit of nTreg cells at birth predicts food allergy at 1 year, 14 , 15 , 16 with gene demethylation at the FOXP3 and TIGIT loci (functional Treg markers) also lower in sorted cord blood CD4+ T cells of food allergic infants. 16 Other studies have shown that low Treg (CD4+FOXP3+CD25+) proportions at birth predict egg allergy, 17 food sensitization, 18 and atopic dermatitis. 18 We recently reported that maternal and cord vitamin D levels were associated with increased proportions of nTreg, 19 and a number of external factors have been shown to upregulate overall number of Treg, including dog ownership 5 and farm milk, 4 but the influence of a full range of prenatal and postnatal environmental microbial factors on infant Treg development over the first postnatal year, and subsequent infant allergic outcomes, is yet to be evaluated.
Here, we aim to (i) describe how early life environmental factors are associated with infant thymic‐derived Treg and (ii) investigate whether serial measures of these Tregs predict allergic outcomes at 1 year of age. The emphasis of this paper is on the former, given that we and others have published on the prospective associations between early life Treg proportions and subsequent allergic disease. 14 , 15 , 16 , 17 , 18
2. MATERIALS AND METHODS
The Barwon Infant Study (BIS) is an Australian birth cohort study of 1074 infants recruited using an unselected antenatal sampling frame. 20 Women were recruited between June 2010 and June 2013. 20 Exclusion criteria included birth before 32 weeks of gestation, major congenital malformation and/or genetically determined disease, and/or serious illness. 20
Prenatal factors were recorded by questionnaire for trimesters 1 and 2 at enrolment (28–32 weeks gestation) and trimester 3 postnatally at 4 weeks. 20 These factors included some likely to be a proxy for environmental microbial exposure, such as pet ownership, household resident number by adult and child number, siblings by date of birth, and antibiotic and disinfectant use. Parent occupational social contact was assessed by the number of well or sick children, adults, or animals contacted daily through work. 21 Perinatal factors were collected from hospital records. Postnatal factors additionally included infant hygiene, breastfeeding, and childcare attendance. Comprehensive questionnaire, clinical, and biological measures were collected postnatally at birth, 4 weeks, and 3, 6, 9, and 12 months 20 with a relevant follow‐up at 4 years. 23
2.1. Blood sampling and monocyte isolation
Umbilical cord blood (up to 30 ml) was collected by syringe, and venous peripheral blood was collected at 6 and 12 months of age. Mononuclear cells were isolated by density gradient centrifugation (Lymphoprep, Axis‐Shield).
2.2. Measurement of treg subsets by flow cytometry
All mononuclear cell preparations were stained for flow cytometric analysis within 12 h of blood collection. Flow cytometry was used to characterize subpopulations of Treg as previously described. 14 , 15 All samples were analyzed by 3‐channel flow cytometry (FACSCalibur, Becton Dickinson). Characterization of the Treg subsets was performed in accordance with the method used by Miyara et al. with the CD4+CD45RA+FoxP3low subset defined as naïve Treg (nTreg) and CD4+CD45RA−FoxP3high defined as activated Treg (aTreg) 12 (Figure 1). Treg proportions are presented as a percentage of CD4+ T cells unless otherwise stated. We examined a serial Treg cohort (n = 191) and those with all available data (birth (n = 463), 6 months (n = 600), and 12 months (n = 675)). The former enabled comparison of serial Treg measures, and the latter allowed cross‐sectional analyses.
FIGURE 1.
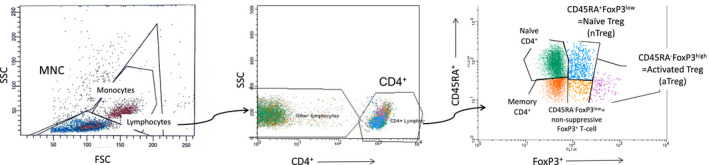
Gating strategies for regulatory T cells (Treg) in freshly collected cord blood samples. Umbilical cord blood (up to 30ml) was collected by syringe and immediately added to 10ml of RPMI 1640 (Gibco, Life Technologies) containing preservative‐free sodium heparin (Pfizer, final concentration of 10 IU/ml). Venous peripheral blood was collected at 6 and 12 months of age and added to a 15‐ml tube containing 100Ul preservative‐free sodium heparin (Pfizer, final concentration 10 IU/ml). Mononuclear cells (MNC) were isolated by density gradient centrifugation (Lymphoprep, Axis‐Shield). The MNC were stained with fluorochrome‐labelled monoclonal antibodies to CD4, CD45RA, and FoxP3. Events were gated to the lymphocytes (FSC and SSC plot) and then to the CD4+ T cells (FL‐2 and SSC plot). FoxP3+ subsets were then selected on the basis of CD45RA and FoxP3 expression (FL‐1 and FL‐3 plot). CD45RA+ FoxP3low populations were classified as naïve Treg (nTreg); the CD45RA− FoxP3high as activated Treg (aTreg); and CD45RA−FoxP3low as non‐suppressive FoxP3+ T‐cell; respectively
2.3. Infant allergic outcomes at age 1 year: polysensitization, food allergy, atopic eczema, and atopic wheeze
At 12‐month postnatal age, skin prick testing (SPT) was performed using Quintip® (Hollister‐Stier Laboratories) to the following allergens: cow's milk, egg white, peanut, sesame, cashew, dust mite (Dermatophagoides pteronyssinus 1), cat, dog, ryegrass, and Alternaria tenius (Stallergenes®). Sensitization to an allergen was defined as an SPT wheal size 2 mm or greater than the negative control (saline), in the presence of a positive histamine control as previously published. 7 Polysensitization was defined as sensitization to two or more allergens at 12 months of age. Immunoglobulin E (IgE)‐mediated food allergy was defined as a positive SPT at 1 year plus clinical history of a recent acute allergic reaction and/or formal in‐hospital open food challenge using predetermined stopping criteria. 7 At each postnatal review, parents were asked about wheeze and eczema symptoms since the last review. Atopic eczema at age one was defined as eczema plus sensitization. 7 Atopic wheeze at age one was defined as the presence of parent‐reported wheeze during the first year of life plus sensitization. 7 Similar outcomes were obtained when the children were aged 4 years. 23 The study was approved by Barwon Health Human Research and Ethics Committee (HREC 10/24), and written consent was provided by the parents or guardians for all participants.
2.4. Statistical analysis
Household sibling age at birth of index child was calculated using birth dates of participants and siblings. Most children had one or no siblings (Table 1), so homes with 3 or more residents during pregnancy were termed “larger household size.” The main forms of analysis were multiple linear or logistic regression. 24 , 25 Hierarchical multiple regression models 26 were used to examine how early life exposures related to Treg proportions longitudinally. An indicator for postnatal stage of the Treg measure, as well as a product term between it and the putative exposure, was added to assess trajectory in the first year. We examined how birth nTreg proportions predicted subsequent allergic outcomes at 1 year (polysensitization, food allergy, atopic eczema, and atopic wheeze) using receiver operating curve (ROC) analysis with Youden Index indicating optimal outcomes. 27 Mediation analysis was undertaken to determine whether cord blood nTreg proportions were a likely intermediate factor in a causal pathway between the selected exposures and allergic outcomes. 28 , 29 We calculated E values for key findings to evaluate the potential impact of unmeasured confounding. 30 , 31 We conducted propensity weighting analyses 24 to account for differences between those analyzed and non‐responders. Analyses were conducted in Stata 16.0 (StataCorp) and R version 3.6.1 (https://www.r‐project.org).
TABLE 1.
Characteristics of the Treg cohort sample with longitudinal nTreg measurements, compared with the full cohort
| Characteristic | Total Cohort N = 1074 | All with birth Treg measures N = 463 | nTreg at all 3 time points N = 191 |
|---|---|---|---|
| Prenatal factors | |||
| Parental allergy history (% (n/N)) | 83.9% (870/1037) | 86.20% (387/449) | 91.0% (171/188) |
| Number of residents (% (n/N)) | |||
| <3 | 39.3% (422/1074) | 36.3% (168/463) | 29.8% (57/191) |
| 3–4 | 55.2% (558/1074) | 57.0% (264/463) | 65.5% (125/191) |
| >4 | 8.75% (94/1074) | 6.7% (31/463) | 4.71% (9/191) |
| Number of older siblings (% (n/N)) | |||
| 0 | 45.0% (483/1073) | 40.0% (185/462) | 33.5% (64/191) |
| 1 | 34.9% (374/1073) | 39.8% (184/462) | 43.5% (83/191) |
| >1 | 20.1% (216/1073) | 20.1% (93/462) | 23.0% (44/191) |
| Pet dog during pregnancy (% (n/N)) | 55.4% (595/1074) | 51.6% (239/463) | 47.12% (90/191) |
| Maternal history of smoking (% (n/N)) | 16.0% (167/1043) | 16.6% (75/452) | 14.4% (27/188) |
| All 4 grandparents European (% (n/N)) | 94.7% (1010/1067) | 95.2% (439/461) | 96.7% (184/190) |
| SEIFA disadvantage* | |||
| Low (% (n/N)) | 33.3% (347/1041) | 33.1% (150/453) | 27.7% (52/188) |
| Medium (% (n/N)) | 33.3% (347/1041) | 29.8% (135/453) | 33.5% (63/188) |
| High (% (n/N)) | 33.3% (347/1041) | 37.1% (168/453) | 38.8% (73/188) |
| Perinatal factor | |||
| Sex | |||
| Male (% (n/N)) | 51.7% (519/1074) | 50.5% (234/463) | 50.8% (97/191) |
| Female (% (n/N)) | 48.3% (555/1074) | 49.5% (229/463) | 49.2% (94/191) |
| Caesarean section (% (n/N)) | 31.0% (333/1074) | 29.2% (135/463) | 30.9% (59/191) |
| Gestational age at birth (weeks) | 39.44 (1.52) | 39.50 (1.34) | 39.58 (1.2) |
| Labor time in hours | 6.07 (5.65) | 5.83 (5.85) | 5.72 (6.62) |
| Birthweight (kg) | 3.54 (0.51) | 3.58 (0.48) | 3.55 (0.44) |
| Birthweight Z score (unit) | 0.51 (0.9) | 0.57 (0.89) | 0.50 (0.83) |
| Postnatal factors | |||
| Pet dog during infancy (% (n/N)) | 54.4% (469/863) | 49.2% (189/384) | 48.7% (91/187) |
| Breastfeeding | |||
| Any breastmilk feeding at 6 months | 60.7% (603/993) | 60.6% (263/434) | 62.3% (119/191) |
| Any breastmilk feeding at 12 months | 37.8% (364/964) | 34.7% (145/418) | 32.5% (62/191) |
| Any breastfeeding | |||
| None | 2.3% (22/964) | 2.4% (10/418) | 2.1% (4/191) |
| <1 week | 2.9% (28/964) | 3.1% (13/418) | 3.1% (6/191) |
| 1 week to <6 months | 35.3% (340/964) | 35.4% (148/418) | 32.5% (62/191) |
| 6 to <12 months | 21.8% (210/964) | 24.4% (102/418) | 29.8% (57/191) |
| 12 months | 37.8% (364/964) | 34.7% (145/418) | 32.5% (62/191) |
| Exclusive breastfeeding up to 12 months | |||
| None | 70.6% (758/1074) | 68.9% (319/463) | 69.6% (133/191) |
| 1–4 | 20.6% (221/1074) | 22.3% (103/463) | 20.4% (39/191) |
| 5–8 | 4.9% (52/1074) | 4.9% (23/463) | 4.2% (8/191) |
| 9–12 | 4.0% (43/1074) | 3.9% (18/463) | 5.8% (11/191) |
|
Exclusive up to 12 months (completed months) (%(n/N)) |
18.8% (181/961) | 16.6% (69/417) | 18.9% (35/185) |
| Attended a formal childcare center in the first 12 months (% (n/N)) | 71.3% (417/585) | 71.7% (187/261) | 75.2% (94/125) |
| Avoiding egg by 6 months (% (n/N)) | 81.2 (675/831) | 80.8 (286/354) | 83.2 (134/161) |
| Avoiding peanut by 12 months (% (n/N)) | 34.5% (333/965) | 36.9% (156/422) | 37.6% (70/186) |
| Age at skin prick test (months) | 13.09 (0.89) | 13.02 (0.88) Socio‐Economic Indexes for Areas | 13.01 (0.93) |
| Cellular measures | |||
| Naïve T regulatory cells (nTreg) | |||
| Birth | ‐ | 4.45 (1.27) | 4.17 (1.27) |
| 6 months | ‐ | 5.50 (1.42) | 5.46 (1.41) |
| 12 months | ‐ | 5.53 (1.59) | 5.51 (1.82) |
| Activated T regulatory cells (aTreg) | ‐ | ||
| Birth | ‐ | 0.80 (0.48) | 0.72 (0.44) |
| 6 months | ‐ | 1.63 (0.81) | 1.56 (0.76) |
| 12 months | ‐ | 1.87 (1.05) | 1.97 (1.08) |
| 3% or more nTreg | ‐ | ||
| Birth (% (n/N)) | ‐ | 88.6% (410/463) | 84.3% (161/191) |
| 6 months (% (n/N)) | ‐ | 98.7% (592/600) | 100.0% (191/191) |
| 12 months (% (n/N)) | ‐ | 96.3% (650/675) | 99.5% (190/191) |
| Outcome measures | |||
| Polysensitization (% (n/N)) | 6.4% (52/814) | 7.8% (29/371) | 7.4% (16/186) |
| Food allergy (% (n/N)) | 7.6% (61/803) | 7.7% (28/365) | 8.6% (16/186) |
| Atopic eczema (% (n/N)) | 5.10% (54/1065) | 5.9% (27/458) | 6.4% (12/188) |
| Atopic wheeze (% (n/N)) | 5.10% (51/1006) | 5.9% (26/441) | 6.3% (12/190) |
Values are mean (SD) unless otherwise stated. *Socio‐Economic Indexes for Areas, values are low, medium, and high. Individuals were classified as Caucasian, values are low, medium, and high. Individuals were classified as Caucasian; values are low, medium, and high. Individuals were classified as Caucasian if both parents were born in Australia, Europe, UK, Northern America, or New Zealand (n = 503). Individuals were classified as Asian if both parents were born in South East Asia (n = 74). Those with one parent in each category were classified as mixed Asian‐Caucasian (n = 145).
3. RESULTS
3.1. Characteristics
Household size was generally small with only 10% of families having more than four residents (Table 1). We describe these 191 infants with Treg measures at birth, 6, and 12 months as “the serial Treg sub cohort.” Overall, there was a high correlation between household resident and sibling number (r = 0.76, p < .0001) and prenatal and postnatal dog ownership (r = 0.86, p < .0001).
3.2. Early life factors and T regulatory cell profile
We examined early life factors associated with Treg proportions at birth, 6, and 12 months for all available Treg measures at each single time point (Figure 2A–C) and also for the serial Treg subcohort (Figure 3 and S1).
FIGURE 2.
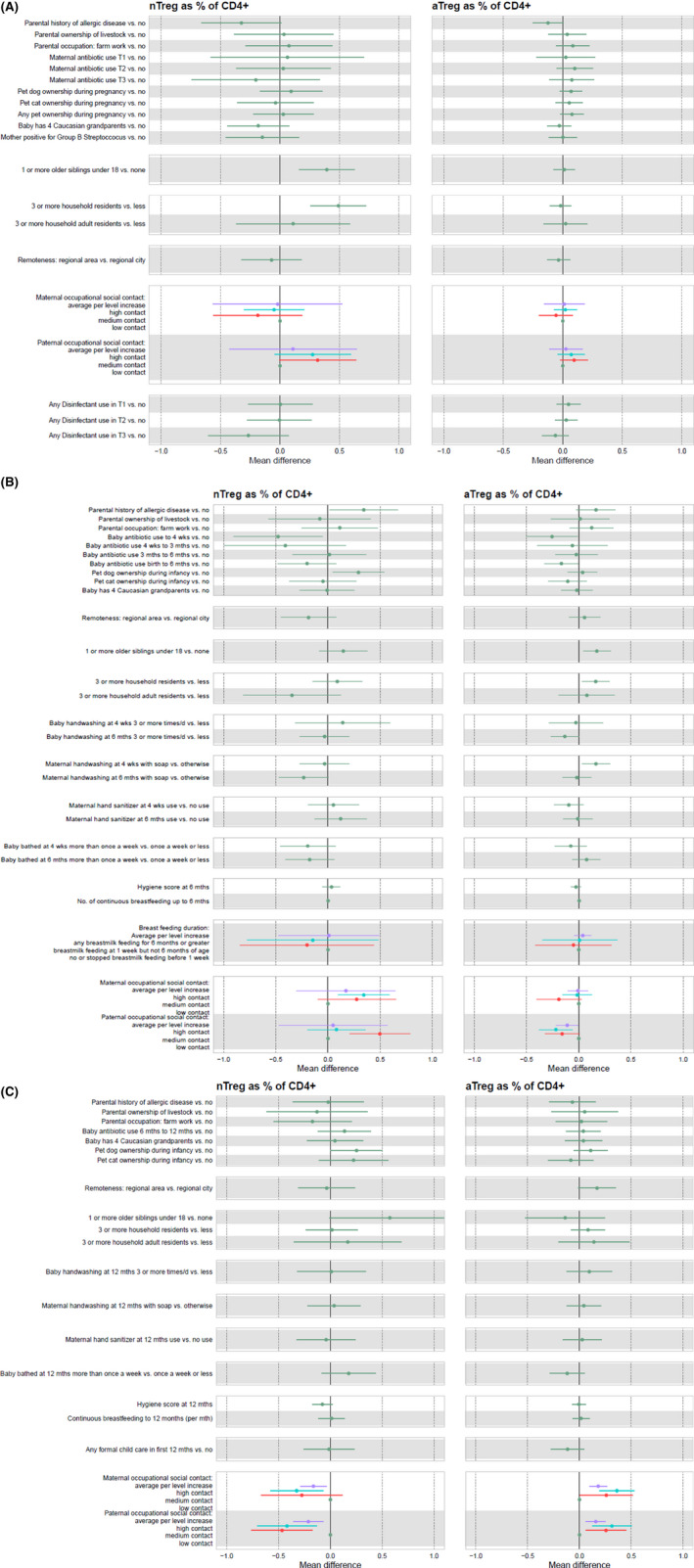
(A) Timepoint 1: birth. The associations between all measured microbial‐related early life factors and naïve and activated cord blood T regulatory cells as a proportion of CD4+ cells at birth (n = 463). Parental history of allergic disease: either parent has a history of allergic disease (asthma, eczema, hay fever, and food allergy), no otherwise. Parental ownership of livestock: family ownership of livestock in the prenatal period, no otherwise. Parental occupation: farm work—either parent has worked on a farm in the prenatal period, no otherwise. Maternal Antibiotic use in Trimester (T) 1/2/3: the mother took antibiotics during trimester 1, 2, or 3 (separate variables for each trimester), no otherwise. Pet dog ownership during pregnancy: presence of a household pet dog during the prenatal period, no otherwise. Pet cat ownership during pregnancy: presence of a household pet cat during the prenatal period, no otherwise. Any pet ownership during pregnancy: presence of any household pets, including dogs and cats during the prenatal period, no otherwise. Baby has four Caucasian grandparents: the child has all 4 Caucasian (British/Irish and European) grandparents, no otherwise. Mother positive for Group B Streptoccocus status: mother's GBS status at labour/birthwas positive, no otherwise. Number of siblings in T1&2: 1 or more siblings aged under 18 living in the home during T1T2, none otherwise. Household residents in T1&2: number of people of any age living at home in T1T2 is 3 or more, less otherwise. Household adult residents in T1&2: number of people aged over 18 years living in the home in T1T2 is 3 or more, less otherwise. Remoteness of location: lives in regional area or regional city, as the reference group. Maternal occupational social contact: levels Low, Medium, and High as outlined in 21 . Paternal occupational social contact: levels Low, Medium, and High as outlined in 21 . Disinfectant use in T1/2/3: values = none, <1 week, about 1 week, a few times per week, every day. (B). Timepoint 2: 6 months. The associations between microbial‐related early life factors and T regulatory cells as a proportion of CD4+ cells at 6 months of age (n = 600). Parental history of allergic disease: either parent has a history of allergic disease (asthma, eczema, hay fever, and food allergy), no otherwise. Parental ownership of livestock: ownership of livestock in the prenatal period, no otherwise. Parental occupation: farm work—either parent has worked on a farm in the prenatal period, no otherwise. Baby antibiotic use to 4 weeks: the baby took antibiotics in the first 4 weeks, no otherwise. Baby antibiotic use 4 weeks–3 months: the baby took antibiotics in the first 4 weeks–3 months, no otherwise. Baby antibiotic use 3–6 months: the baby took antibiotics in the first 3–6 months, no otherwise. Baby antibiotic use birth to 6 months: the baby took antibiotics between birth and 6 months, no otherwise. Pet dog ownership during infancy: presence of a household dog by 12‐month review, no otherwise. Pet cat ownership during infancy: presence of a household cat by 12‐month review, no otherwise. Baby has four Caucasian grandparents: the child has all four Caucasian (British/Irish and European) grandparents, no otherwise. Remoteness of location: lives in regional area or regional city, as the reference group. Number of siblings in T1&2: number of siblings aged under 18 living in the home during T1T2 is one or more, none otherwise. Household residents in T1&2: number of people of any age living at home in T1T2 is 3 or more, less otherwise. Household adult residents in T1&2: number of people aged over 18 years living in the home in T1T2 is 3 or more, less otherwise. Baby handwashing frequency at 4 weeks/6months: how often were baby's hands washed or wiped at 4 weeks and 6 months; three or more times/day compared to 2 times/day‐none at all. Maternal handwashing method at 4 weeks/6 months: Method of hand washing by the mother after changing baby's nappy at 4 weeks, and 6 months; wash hands with soap compared to don't clean hands, rinse with water only, wipe hands only, use a hand sanitizer. Maternal hand sanitizer use at 4 weeks/6 months: mother uses hand sanitizer after changing baby's nappy at 4 weeks, and 6 months, no otherwise. Baby bathing frequency at 4 weeks/6 months: how often baby is bathed; several times a week or once or more a day compared to once a week or less. Hygiene score at 6 months: baby's ALSPAC hygiene score at 6 months22. No. of continuous breastfeeding up to 6 months: the number of mothers who continuously breastfed their child up to 6 months. Breastfeeding duration: how long the mother breastfed for; any for 6 months or longer, breastmilk feeding at 1 week but not 6 months, no or stopped breastmilk feeding before 1 week. Maternal/Paternal occupational social contact: low or not elsewhere classified, medium, or high as per Ponsonby et al. 21 . (C). Timepoint 3: 12 months. The associations between microbial‐related early life factors and T regulatory cells as a proportion of CD4+ cells at 12 months of age (n = 675). Parental history of allergic disease: either parent has a history of allergic disease (asthma, eczema, hay fever, food allergy), no otherwise. Parental ownership of livestock: family ownership of livestock in the prenatal period, no otherwise. Parental occupation: farm work—either parent has worked on a farm in the prenatal period, no otherwise. Baby antibiotic use 6–12 months: the baby took antibiotics between 6 and 12 months, no otherwise. Baby has four Caucasian grandparents: the child has all 4 Caucasian (British/Irish and European) grandparents, no otherwise. Pet dog ownership during infancy: presence of a household dog by 12‐month review, no otherwise. Pet cat ownership during infancy: presence of a household cat by 12‐month review, no otherwise. Remoteness of location: lives in regional area or regional city, as the reference group. Number of siblings in T1&2: number of siblings aged under 18 living in the home during T1T2 is one or more, none otherwise. Household residents in T1&2: number of people of any age living at home in T1T2 is 3 or more, less otherwise Household adult residents in T1&2: number of people aged over 18 years living in the home in T1T2 is 3 or more, less otherwise. Baby handwashing frequency at 12 months: how often were baby's hands washed or wiped at 12 months; 3 or more times/day compared to 2 times/day‐none at all. Maternal handwashing method at 12 months: Method of hand washing by the mother after changing baby's nappy at 12 months; wash hands with soap compared to don't clean hands, rinse with water only, wipe hands only, use a hand sanitizer. Maternal hand sanitizer use at 12 months: mother uses hand sanitizer after changing baby's nappy at 12 months, no otherwise. Baby bathing frequency at 12 months: how often baby is bathed; several times a week or once or more a day compared to once a week or less. Hygiene score at 12 months: baby's ALSPAC hygiene score at 12 months 22 . Continuous breastfeeding to 12 months: baby was breastfed up to 12 months. Any formal childcare in first 12 months: baby received any regularly scheduled formal childcare in a childcare center in the first 12 months, no otherwise. Maternal/Paternal occupational social contact: low or not elsewhere classified, medium 21
FIGURE 3.
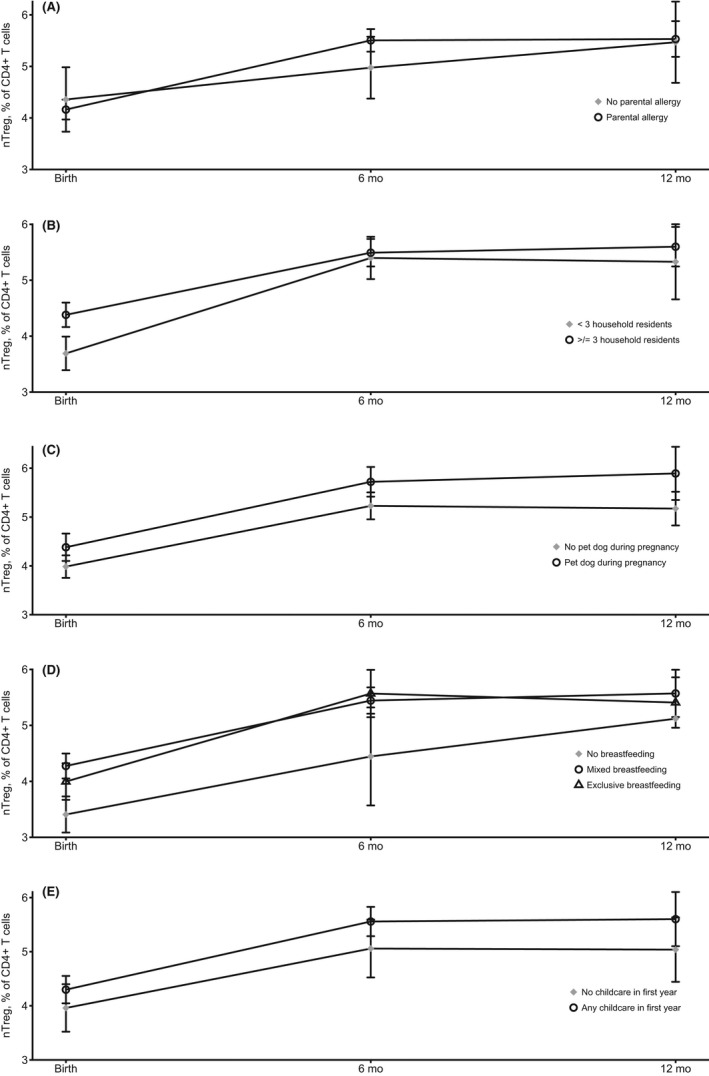
Trajectory of naïve T regulatory cells as a % of CD4+ cells in the first postnatal year by key prenatal factors (n = 191). (A) Infants with a positive parental history of allergy had a significant increase from birth to 6 months (p = .003) but not from birth to 12 months (p = .12). (B) Infants in larger households had a lower increase to 6 months (p = .01) and a lower increase from birth to 12 months (p = .006). (C) Infants with family dog ownership during pregnancy had no change in trajectory from birth to 6 months (p = .94) and birth to 12 months overall (p = .20). (D) Infants who received exclusive or mixed breastfeeding both had significant increases in trajectory from birth to 6 months (p = <.001) and birth to 12 months overall (p = <.001). (E) Infants who attended childcare at all in the first 12 months had significant increases in trajectory from birth to 6 months (p = <.001) and birth to 12 months overall (p = <.001). Adjusted for age at blood draw and sex
3.2.1. Naïve T regulatory cells (nTreg)
Overall, among infants with Treg measures at birth (n = 463), of the 19 prenatal environmental microbial factors examined, the strongest patterns were evident for larger family size (three or more residents) and siblings (one or more) being associated (p < .0001) with higher cord blood nTreg proportions (Figure 2A, Figure S1). A range of environmental microbial factors were associated with higher nTreg levels at the 6‐month (Figure 2B) and 12‐month (Figure 2C) time points. Compared with no breastfeeding, exclusive or mixed breastfeeding was associated with higher naïve Treg at 6 months (Table 2).
TABLE 2.
Association between breastfeeding and childcare and naïve T regulatory cells over the first year
| Longitudinal naïve Treg a | 6 months naïve Treg b | 12 months naïve Treg b | ||||
|---|---|---|---|---|---|---|
| Mean diff. (95% CI) | p value | Mean diff. (95% CI) | p value | Mean diff. (95% CI) | p value | |
| Breastfeeding | ||||||
| None, Mixed or Exclusive Breastfeeding | ||||||
| None | Ref. | Ref. | Ref. | |||
| Mixed | 0.78 (0.19, 1.37) | .01 | 1.14 (−0.06, 2.35) | 0.06 | 0.45 (−1.11, 2.01) | .57 |
| Exclusive | 0.69 (0.19, 1.37) | .03 | 1.20 (−0.01, 2.42) | 0.05 | 0.58 (−0.99, 2.16) | .47 |
| Any breastmilk feeding at 6 months | 0.09 (−0.22, 0.41) | .57 | 0.11 (−0.23, 0.45) | 0.53 | −0.16 (−0.61, 0.28) | .47 |
| Any breastmilk feeding at 12 months | −0.06 (−0.39, 0.26) | .70 | −0.04 (−0.41, 0.33) | 0.85 | −0.09 (−0.54, 0.36) | .68 |
| Duration exclusive breastmilk feeding (weeks) | −0.001 (−0.01, 0.01) | .81 | 0.0002 (0.01, 0.01) | 0.97 | −0.01 (−0.02, 0.008) | .44 |
| Childcare | ||||||
| Duration of childcare attendance in a center (weeks) in first 12 months | 0.004 (−0.01, 0.02) | .53 | −0.005 (−0.02, 0.01) | 0.56 | 0.004 (−0.02, 0.02) | .71 |
| Duration of childcare attendance any (weeks) in first 12 months | 0.003 (−0.01, 0.01) | .65 | 0.004 (−0.01, 0.02) | 0.54 | 0.003 (−0.01, 0.02) | .74 |
| Attended a formal childcare center in the first 12 months | 0.44 (0.08, 0.80) | .02 | 0.22 (−0.26, 0.70) | 0.37 | 0.31 (−0.29, 0.91) | .31 |
T Regulatory cells as a % of CD4+ cells.
Adjusted for gestational age and sex, serial treg cohort n = 191.
Adjusted for age at time point, sex, birth nTregs, birth cohort n = 463. Bold value indicate p<0.05 threshold.
3.2.2. Activated T regulatory cells (aTreg)
There was little association between environmental microbial factors and aTreg proportions at birth (n = 463) (Figure 2A and Table S1). Larger household size was associated with higher aTreg levels at age 6 months (Figure 2B). Dog ownership, as well as higher maternal and paternal occupational social contact, was associated with higher aTreg at 12 months with evidence of dose–response (Figure 2C).
3.3. Assessment of nTreg measures over multiple timepoints
We examined the longitudinal patterns of both nTreg and aTreg by trajectory analysis (Figure 3 and Figure S1) and demonstrate some differences by whether an early life factor was present or not.
We then examined how the key prenatal factors influenced overall longitudinal Treg proportions across all three timepoints in the first postnatal year. Infants from larger households had higher nTreg (p < .001) and aTreg (p = .007) proportions across the first postnatal year. Infants with a pet dog also had higher nTreg proportions throughout the first year (p = .01; Table 3). Infants who were breastfed had higher longitudinal nTreg and aTreg proportions (Table 2; Table S2). Infants who attended formal childcare in the first postnatal year had higher longitudinal nTreg proportions (p = .02; Table 2) but not aTreg proportions (p = .41; Table S2).
TABLE 3.
Association between microbial‐related factors and longitudinal naïve and activated T regulatory cells levels in the first year of life in the Treg cohort (n = 191)
| Naïve T regulatory cells* | Activated T regulatory cells* | |||
|---|---|---|---|---|
| Mean difference (95% CI) | p value | Mean difference (95% CI) | p value | |
| Prenatal Factors | ||||
| Parental allergy history | −0.22 (−0.85, 0.41) | .49 | −0.09 (−0.38, 0.21) | .57 |
| Residents (3 or more) | 0.67 (0.30, 1.04) | <.001 | 0.17 (0.05, 0.29) | .007 |
| Pet dog | 0.46 (0.10, 0.82) | .01 | 0.07 (−0.06, 0.20) | .30 |
| Occupational social contact (mother) | 0.16 (−0.21, 0.52) | .40 | 0.08 (−0.04, 0.21) | .20 |
| Occupational social contact (father) | 0.13 (−0.24, 0.50) | .49 | 0.03 (−0.11, 0.16) | .67 |
* T regulatory cells as a % of CD4+ cells. Bold value indicate p<0.05 threshold.
3.4. T regulatory cell profile and atopic disease at 1 year of age
Having previously reported higher birth nTreg proportions were associated with reduced food allergy at 1 year of age, 14 we conducted an ROC analysis to evaluate the predictive performance of higher birth nTreg proportions and resultant average Youden Index was a cutoff of 3% (Figure S2). Overall, infants with 3% or more nTreg at birth were markedly less likely to have positive allergic outcomes (Table 4).
TABLE 4.
Association between levels of naïve T regulatory cell levels, as a % of CD4+ cells and allergic outcomes at one postnatal year
| Naïve Treg cells (as a % of CD4+ cells) | Number | Polysensitization | Food allergy | Atopic eczema | Atopic wheeze | ||||
|---|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | p value | AOR (95% CI) | p value | AOR (95% CI) | p value | AOR (95% CI) | p value | ||
| All available measures | |||||||||
| Birth a ++ | 463/1074 | 0.85 (0.62, 1.17) | .32 | 0.65 (0.45, 0.93) | 0.02 | 0.68 (0.48, 0.96) | .03 | 0.80 (0.57, 1.12) | .19 |
| 6 months b | 600/1074 | 0.95 (0.72, 1.26) | .74 | 0.88 (0.69, 1.11) | 0.28 | 0.76 (0.58, 0.99) | .04 | 0.95 (0.72, 1.24) | .70 |
| 12 months c | 675/1074 | 1.01 (0.81, 1.25) | .95 | 0.89 (0.73, 1.09) | 0.27 | 0.89 (0.71, 1.10) | .27 | 0.97 (0.78, 1.20) | .77 |
| 3% or more at birth a + | 463/1074 | 0.42 (0.16, 1.07) | .07 | 0.21 (0.09, 0.50) | <0.001 | 0.26 (0.10, 0.64) | .004 | 0.46 (0.16, 1.34) | .15 |
| Treg cohort | |||||||||
| Birth a ++ | 191 | 0.68 (0.40, 1.13) | .14 | 0.55 (0.32, 0.95) | 0.03 | 0.47 (0.24, 0.89) | .02 | 0.49 (0.26, 0.92) | .03 |
| 6 months b | 191 | 1.23 (0.86, 1.76) | .25 | 0.93 (0.63, 1.37) | 0.73 | 0.81 (0.50, 1.30) | .38 | 0.96 (0.63, 1.46) | .85 |
| 12 months c | 191 | 1.19 (0.91, 1.56) | .20 | 0.92 (0.67, 1.26) | 0.60 | 0.96 (0.68, 1.35) | .81 | 0.90 (0.62, 1.29) | .56 |
| 3% or more at birth a + | 191 | 0.22 (0.07, 0.71) | .01 | 0.17 (0.06, 0.50) | 0.001 | 0.23 (0.07, 0.82) | .02 | 0.25 (0.07, 0.88) | .03 |
Model + was assessed to fit the data better than model ++:‐ model+ had an pseudo r‐squared = 0.06 and a log likelihood test p = .01 and model ++ had a pseudo r‐squared = 0.03 and a log likelihood test p = .11. Bold value indicate p<0.05 threshold.
Adjusted for gestational age and sex, in all with birth measure n = 463.
Adjusted for age at 6 month blood draw and sex, in all with 6 month measure n = 600.
Adjusted for age at 12 month skin prick test and sex, in all with 12 month measure n = 675.
3.5. Additional analyses
With multiple environmental microbial exposures per time period and four allergic outcomes, it was not feasible to undertake detailed confounder detection and evaluation analyses. 25 Therefore, we applied the E‐value approach. 30 , 31 For unmeasured confounding to have explained the association between 3% or more nTreg and reduced food allergy (Table 5), the unmeasured confounder would need to have a ninefold positive risk ratio for this nTreg profile and be associated with a ninefold lower risk of food allergy. To account for the association between larger household size and a twofold increase in the likelihood of this nTreg profile, a potential confounder would need to positively increase the likelihood of the nTreg profile 2 or more‐fold and also be associated with a 2‐ or more‐fold increase of a larger household. Table 5 shows that none of the potential confounders listed have these profiles, and thus, it is unlikely that these findings are due to unmeasured confounding.
TABLE 5.
Association between early life factors, a birth naïve T regulatory cell profile of 3% or more (as a proportion of CD4+ cells), household size, infant polysensitization, and food allergy at 12 months in all participants with nTreg at birth measured (n = 463)
| Characteristic | Naïve Treg 3% or more at birth OR* (95% CI) |
Residents (3 or more) OR* (95% CI) |
Polysensitization OR* (95% CI) |
Food allergy OR* (95% CI) |
Atopic Eczema OR* (95% CI) |
Atopic wheeze OR* (95% CI) |
|---|---|---|---|---|---|---|
| Prenatal factors | ||||||
| Parental allergy history (yes vs. no) | 0.97 (0.39, 2.44) | 0.88 (0.46, 1.70) | 1.19 (0.34, 4.14) | 1.88 (0.43, 8.25) | 1.11 (0.32, 3.88) | 1.68 (0.38, 7.46) |
| Number of residents (>=3 vs. <3) | 2.19 (1.20, 4.00) c | ‐ | 0.55 (0.25, 1.21) | 0.52 (0.23, 1.16) | 0.79 (0.34, 1.81) | 1.07 (0.44, 2.58) |
| Number of Siblings (>=1 vs. none) | 1.85 (1.02, 3.38) c | ** | 0.74 (0.34, 1.60) | 0.70 (0.32, 1.51) | 1.04 (0.46, 2.36) | 1.41 (0.59, 3.36) |
| Pet dog (yes vs. no) | 1.55 (0.84, 2.84) | 1.25 (0.81, 1.93) | 0.35 (0.15, 0.81) | 0.18 (0.06, 0.48) | 0.44 (0.19, 1.01) | 0.42 (0.17, 0.99) |
| Maternal social contact | 1.01 (0.72, 1.41) | 0.66 (0.51, 0.86) | 0.77 (0.50, 1.17) | 0.84 (0.55, 1.30) | 0.99 (0.64, 1.56) | 0.70 (0.44, 1.09) |
| Paternal social contact | 1.10 (0.74, 1.63) | 1.14 (0.86, 1.53) | 0.67 (0.40, 1.12) | 0.70 (0.42, 1.17) | 0.85 (0.50, 1.45) | 0.66 (0.38, 1.15) |
| Number of adults (more than 2 vs. rest) | 1.10 (0.71, 1.70) | ‐ | 0.89 (0.47, 1.69) | Omitted in Stata | 0.90 (0.47, 1.71) | 0.95 (0.50, 1.81) |
| SEIFA disadvantage (per % increase) | 0.92 (0.64, 1.32) | 1.41 (1.08, 1.82) | 1.30 (0.80, 2.13) | 1.11 (0.68, 1.81) | 0.82 (0.50, 1.34) | 1.74 (1.00, 3.02) |
| Maternal history of smoking (yes vs. no) | 1.40 (0.53, 3.75) | 0.37 (0.20, 0.68) | 1.13 (0.37, 3.45) | 0.82 (0.24, 2.85) | 1.66 (0.59, 4.66) | 0.90 (0.25, 3.16) |
| Perinatal factors | ||||||
| Gestational age (weeks) | 0.58 (0.44, 0.77) | 1.01 (0.86, 1.18) | 1.02 (0.77, 1.35) | 1.25 (0.92, 1.71) | 1.13 (0.83, 1.53) | 0.96 (0.72, 1.28) |
| Sex (Male vs. Female) | 1.33 (0.73, 2.42) | 1.17 (0.76, 1.79) | 1.82 (0.82, 4.02) | 1.05 (0.48, 2.27) | 1.36 (0.61, 3.02) | 2.70 (1.11, 6.60) |
| Caesarean section (Any vs. none) | 1.57 (0.77, 3.20) | 1.08 (0.67, 1.75) | 0.67 (0.28, 1.63) | 1.10 (0.48, 2.53) | 0.85 (0.40, 2.26) | 0.76 (0.31, 1.88) |
| Labor time (hour) a | 0.92 (0.88, 0.96) | 0.78 (0.73, 0.83) | 1.00 (0.94, 1.07) | 1.03 (0.98, 1.09) | 1.02 (0.96, 1.08) | 0.98 (0.92, 1.06) |
| Z scores birth | 1.12 (0.80, 1.57) | 1.64 (1.27, 2.13) | 0.77 (0.49, 1.22) | 0.77 (0.49, 1.21) | 0.80 (0.51, 1.27) | 0.76 (0.47, 1.23) |
| Birth nTreg 3% or more | ‐ | 2.19 (0.20, 3.99) | 0.43 (0.17, 1.07) | 0.19 (0.08, 0.44) | 0.30 (0.12, 0.74) | 0.58 (0.20, 1.63) |
| Postnatal factors | ||||||
| Breast feeding duration b | 1.07 (0.78, 1.48) | 1.26 (0.99, 1.59) | 1.27 (0.83, 1.94) | 1.09 (0.72, 1.65) | 0.76 (0.50, 1.16) | 1.11 (0.72, 1.72) |
| Avoid egg at 6m (yes vs. no) | 1.85 (0.86, 3.96) | 1.22 (0.66, 2.23) | 0.97 (0.31, 3.03) | 2.15 (0.48, 9.56) | 4.46 (0.58, 34.17) | 2.43 (0.55, 10.76) |
| Avoid peanut at 12m (yes vs. no) | 1.21 (0.64, 2.28) | 1.62 (1.01, 2.60) | 2.48 (1.15, 5.38) | 3.21 (1.45, 7.12) | 1.87 (0.85, 4.13) | 1.41 (0.62, 3.19) |
| Childcare in the first year | 1.23 (0.60, 2.51) | 1.08 (0.66, 1.76) | 0.97 (0.40, 2.35) | 1.43 (0.62, 3.29) | 0.86 (0.33, 2.20) | 0.92 (0.36, 2.38) |
*ORs adjusted for age at skin prick test and sex. **r = 0.85, p < .00001. Bold value indicate p<0.05 threshold.
Elective cesarean section =0 min.
Per category increase: <1 week, >1week but <6 months, >=6 months but <12 months, any at 12 months or older.
In those with vaginal births only, ORs 1.32 (0.68, 2.75) for number of residents and 3.92 (0.88, 17.48) for number of siblings 0–10 year.
Inverse probability weighting to rebalance the samples to better reflect women invited to enter the cohort at baseline did not alter the key findings above. Further, exclusion of cord blood samples with potential maternal contamination did not materially alter key findings. Lastly, we investigated how infant allergic outcomes predicted child allergic disease. Polysensitization at age 1 year was strongly associated with any allergic disease (food allergy, atopic eczema, and/or atopic asthma) at ages 4 years with an AOR of 9.26 (95% CI 2.46, 34.85), respectively. We have previously reported that food allergy at 1 year predicted subsequent hay fever and atopic wheeze. 23
4. DISCUSSION
Here, multiple environmental microbial factors were associated with Treg development at birth and during the first year of life. For the first time, to our knowledge, larger household size was associated with higher nTreg proportions at birth and in longitudinal analyses, it was associated with higher proportions of nTreg and aTreg over the first postnatal year. Higher nTreg proportions at birth but not 6 or 12 months predicted allergic outcomes by 12 months of age. Taken together, these findings indicate that inadequate prenatal immune priming is important in allergic disease development.
The major strength of this study is the availability of comprehensive environmental microbial and serial Treg measures over the first year of life, as well as objective infant allergic outcomes for a large population‐based cohort. While the longitudinal Treg measurements at all timepoints were limited to 191 infants, this sample is larger than most previous studies, particularly a recent study with serial Treg measures. 32 The availability of comprehensive data allowed an assessment of potential confounding and selection bias 33 that did not reveal these to be important explanations. In particular, the key associations were of high magnitude, reducing the risk of a contribution due to unmeasured confounding. 31 Limitations include the identification of additional subpopulations of Treg or alternate staining methods and the lack of functional suppressive assays. These aspects will be part of future studies; however, the nTreg and aTreg populations measured here have both previously been shown to be suppressive 12 which provides some functional relevance to the link between lower suppressive nTreg and allergic outcomes described here. In our past work on lower cord blood Treg proportions and subsequent food allergy, we also found in parallel that gene demethylation at the FOXP3 and TIGIT loci (markers of suppressive Treg function) was lower in the sorted CD4+ cells of food allergic infants. 16 Aditionally, we confirmed good correlations between our birth nTreg proportions and FoxP3 demethylation site (TSDR) (r = 0.50, p = .004, n = 31) and TIGIT demethylation (r = 0.43, p = .017, n = 31), indicating that the FoxP3 gene in the nTreg population that we measured is functionally stable.
The nTreg index of 3% or more requires replication in independent studies. We did not examine long‐term allergic outcomes, but infant polysensitization at 12 months is important in its own right and a key determinant of child allergic disease here and in other studies. 9 , 10
Our findings are consistent with past work, indicating that a lower Treg profile in the prenatal period 34 and at birth is associated with higher allergic disease risk. 18 Children born in a farming environment have higher Treg at birth. 35 Bacterial strain inoculation into pregnant rodents leads to FoxP3 Treg induction, but this must occur before the neonatal period. 35 Prenatal factors such as dog ownership and farm milk exposure have previously been related to upregulated nTreg proportions in cord blood. 18
The finding of a relative nTreg deficit at birth being associated with higher allergy risk is consistent with the concept of delayed maturation of T cell function in early life underlying the allergic phenotype. 36 , 37 Genetic risk of atopy is associated with delayed maturation of T cell competence. 36 Consistent with past work, 18 , 38 , 39 a positive parental allergy history was associated with lower cord blood nTreg and we describe, for the first time, a steeper trajectory of Treg maturation over the first six months. Compared with no breastfeeding, exclusive or any breastfeeding was associated with higher nTreg proportions at 6 months as well as higher longitudinal nTreg and aTreg proportions throughout the first year. A recent study (n = 38) reported that compared with non‐breastfed infants, exclusively breastfed infants had increased naïve Treg proportions 3 weeks after birth and a Treg‐dependent reduction in proliferative T cell antigenic responses. 40 We did not have Treg measures available during the first six months, when breastfeeding is most common, and lacked in vitro suppressive data, but our findings indicate more intensive studies are required for this issue. Childcare attendance was associated with higher longitudinal nTreg proportions in the first year.
Regarding aTreg postnatal development, parental occupational social contact was associated with higher aTreg at 12 months of age. This is a previously identified possible determinant of reduced type 1 diabetes 21 and higher child leukemia risk, 41 possibly through increased early infection, 21 , 41 reflecting that activated Treg cells develop in response to postnatal antigenic stimuli. 42
Several features indicating possible causality were present. 25 For example, high‐magnitude prospective associations were reported for larger family size and birth nTreg proportions and also the birth nTreg profile and reduced infant allergic outcomes with dose–response trends. These findings were consistent with past laboratory work. 43 , 44 The mechanism underlying the association between larger family size and increased nTreg in cord blood requires further elucidation. Relevant factors of a larger household include the presence of additional children and increased exposure to shared microbes, 45 a greater number of viral/bacterial infections, 46 reduced cleanliness, and the presence of pet/s. 47 Several protective immune factors have previously been related to birth order and protection from atopy. These include reduced maternal and cord IgE with subsequent pregnancies, 48 as well as dampened cord serum Th1 responses. 49 Additional research should, therefore, include evaluating the immune priming role of previous pregnancies in the mother 50 and the impacts of labor duration. 14 To elucidate the influence of factors on the development of Treg, further work on prenatal programming of Treg cells should include epigenetic studies. Aspects of the gut microbiota have also been shown to influence Treg development. 51 , 52 We have reported in this birth cohort that larger household size is associated with a higher abundance of maternal prenatal Prevotella copri, which is, in turn, associated with a markedly reduced risk of allergic disease at 12 months. 7 The influence of the maternal, as well as infant, gut microbiome on the infant immune profile requires further evaluation. 53
5. CONCLUSION
A range of prenatal and postnatal environmental microbial factors may work in concert to influence early Treg cell development. In this study, the most consistent of these was larger household size, which was associated with an increased proportion of nTreg in cord blood at birth, as well as with higher proportions of nTreg and aTreg throughout the first postnatal year. Higher cord blood nTreg proportions were associated with a reduced risk of allergic polysensitization, food allergy, atopic eczema, and atopic wheeze by one year of age. In utero programming of nTreg development may partly underlie the well‐replicated association between larger household size and reduced allergic disease. Our findings are consistent with recent calls for studies and trials targeting fetal immune development for the prevention of allergic disease. 54
AUTHOR CONTRIBUTIONS
Anne‐Louise Ponsonby: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (lead); Supervision (lead); Visualization (equal); Writing – original draft (lead); Writing – review & editing (lead). Fiona Collier: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (lead); Methodology (lead); Project administration (equal); Resources (equal); Supervision (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Martin O'Hely: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Investigation (equal); Methodology (lead); Supervision (equal); Visualization (lead); Writing – original draft (equal); Writing – review & editing (equal). Mimi Tang: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Writing – original draft (equal); Writing – review & editing (equal). Sarath Ranganathan: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Investigation (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Lawrence EK Gray: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (supporting). Ellen Morwitch: Data curation (equal); Formal analysis (lead); Investigation (equal); Methodology (equal); Visualization (lead); Writing – original draft (equal); Writing – review & editing (equal). Richard Saffery: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). David Burgner: Data curation (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Terence Dwyer: Conceptualization (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Peter D Sly: Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Leonard Harrison: Conceptualization (equal); Investigation (equal); Methodology (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal). Peter Vuillermin: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (lead); Methodology (lead); Project administration (equal); Resources (equal); Supervision (equal); Writing – original draft (equal); Writing – review & editing (equal).
CONFLICT OF INTEREST
All authors declare no support from any organization for the submitted work; Prof. Vuillermin, Prof. Ponsonby, Dr Tang, and Dr O’Hely report financial interest in Prevatex Pty Ltd, an organization that has an interest in the submitted work in the previous three years; Prof. Vuillermin, Prof. Ponsonby, and Dr Tang have a patent PCT/AU2019/050878 licensed to Prevatex and a patent PCT/AU2017/051453 licensed to Prevatex; no other relationships or activities that could appear to have influenced the submitted work, except Dr Tang who reports grants and speaker fees from Abbott Nutrition, speaker fees from Nestle Health Sciences, and consultancy fees from Bayer Pharmaceuticals, outside the submitted work.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13810.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Barwon Infant Study (BIS) participants for the generous contribution they have made to this project. We also thank the current and past staff for their efforts in recruiting and maintaining the cohort and in obtaining and processing the data and biospecimens. The BIS Investigator Group includes John Carlin, Toby Mansell, and Amy Loughman. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Ponsonby A‐L, Collier F, O’Hely M, et al. Household size, T regulatory cell development, and early allergic disease: a birth cohort study. Pediatr Allergy Immunol. 2022;33:e13810. doi: 10.1111/pai.13810
Editor: Jon Genuneit
Funding information
The establishment work and infrastructure for the BIS was provided by the Murdoch Children's Research Institute, Deakin University, and Barwon Health. Subsequent funding was secured from the National Health and Medical Research Council of Australia, The Shepherd Foundation, The Jack Brockhoff Foundation, the Scobie Trust, the Shane O’Brien Memorial Asthma Foundation, the Our Women's Our Children's Fund Raising Committee Barwon Health, the Rotary Club of Geelong, the Ilhan Food Allergy Foundation, Geelong Medical and Hospital Benefits Association (GMHBA) Ltd, The Gandel Foundation, The Percy Baxter Charitable Trust, Perpetual Trustees, the Gwenyth Raymond Trust, and Vanguard Investments Australia Pty Ltd. In‐kind support was provided by the Cotton on Foundation and CreativeForce. This work was partially supported by funds from the NHMRC‐funded Centre for Food and Allergy Research (CFAR). Study sponsors had no role regarding data access or this report. Research at Murdoch Children's Research Institute is supported by the Victorian Government's Operational Infrastructure Support Program. A‐L. Ponsonby, P. Vuillermin, D. Burgner, and P. Sly receive NHMRC fellowship support.
REFERENCES
- 1. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076‐1083. [DOI] [PubMed] [Google Scholar]
- 2. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rook GA. The hygiene hypothesis and the increasing prevalence of chronic inflammatory disorders. Trans R Soc Trop Med Hyg. 2007;101(11):1072‐1074. [DOI] [PubMed] [Google Scholar]
- 4. Ege MJ, Herzum I, Büchele G, et al. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol. 2008;122(2):407–412.e4. [DOI] [PubMed] [Google Scholar]
- 5. Gern JE, Reardon CL, Hoffjan S, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004;113(2):307‐314. [DOI] [PubMed] [Google Scholar]
- 6. Matricardi PM, Rosmini F, Panetta V, Ferrigno L, Bonini S. Hay fever and asthma in relation to markers of infection in the United States. J Allergy Clin Immunol. 2002;110(3):381‐387. [DOI] [PubMed] [Google Scholar]
- 7. Vuillermin PJ, O’Hely M, Collier F, et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat Commun. 2020;11(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Depner M, Taft DH, Kirjavainen PV, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med. 2020;26(11):1766‐1775. [DOI] [PubMed] [Google Scholar]
- 9. Baatenburg de Jong A, Dikkeschei LD, Brand PL. Sensitization patterns to food and inhalant allergens in childhood: a comparison of non‐sensitized, monosensitized, and polysensitized children. Pediatr Allergy Immunol. 2011;22(2):166‐171. [DOI] [PubMed] [Google Scholar]
- 10. Gabet S, Just J, Couderc R, Bousquet J, Seta N, Momas I. Early polysensitization is associated with allergic multimorbidity in PARIS birth cohort infants. Pediatr Allergy Immunol. 2016;27(8):831‐837. [DOI] [PubMed] [Google Scholar]
- 11. Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181(11):1200‐1206. [DOI] [PubMed] [Google Scholar]
- 12. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899‐911. [DOI] [PubMed] [Google Scholar]
- 13. Yuan X, Malek TR. Cellular and molecular determinants for the development of natural and induced regulatory T cells. Hum Immunol. 2012;73(8):773‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier F, Ponsonby AL, O'Hely M, et al. Naive regulatory T cells in infancy: associations with perinatal factors and development of food allergy. Allergy. 2019;74(9):1760‐1768. [DOI] [PubMed] [Google Scholar]
- 15. Collier FM, Tang ML, Martino D, et al. The ontogeny of naive and regulatory CD4(+) T‐cell subsets during the first postnatal year: a cohort study. Clin Transl Immunology. 2015;4(3):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang YX, Collier F, Naselli G, et al. Cord blood monocyte‐derived inflammatory cytokines suppress IL‐2 and induce nonclassic "T(H)2‐type" immunity associated with development of food allergy. Sci Transl Med. 2016;8(321). [DOI] [PubMed] [Google Scholar]
- 17. Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/‐) regulatory T cell function. J Allergy Clin Immunol. 2008;121(6):1460‐1466, 1466 e1461–1467. [DOI] [PubMed] [Google Scholar]
- 18. Hinz D, Bauer M, Röder S, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67(3):380‐389. [DOI] [PubMed] [Google Scholar]
- 19. Thorsen S, Collier F, Pezic A, et al. Maternal and cord blood 25‐Hydroxyvitamin D3 are associated with increased cord blood and naive and activated regulatory T cells: the Barwon Infant Study. J Immunol. 2021;206(4):874‐882. [DOI] [PubMed] [Google Scholar]
- 20. Vuillermin P, Saffery R, Allen KJ, et al. Cohort profile: the barwon infant study. Int J Epidemiol. 2015;44(4):1148‐1160. [DOI] [PubMed] [Google Scholar]
- 21. Ponsonby AL, Pezic A, Cameron FJ, et al. Higher parental occupational social contact is associated with a reduced risk of incident pediatric type 1 diabetes: mediation through molecular enteroviral indices. PLoS One. 2018;13(4):e0193992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherriff A, Golding J, Team AS. Factors associated with different hygiene practices in the homes of 15 month old infants. Archives of Disease in Childhood. 2002;87(1):30‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gray LE, Ponsonby AL, Collier F, et al. Deserters on the atopic march: Risk factors, immune profile, and clinical outcomes of food‐sensitized‐tolerant infants. Allergy. 2020;75(6):1404‐1413. [DOI] [PubMed] [Google Scholar]
- 24. Little RJ, Rubin DB. Statistical Analysis with Missing Data, vol. 793. John Wiley & Sons; 2019. [Google Scholar]
- 25. Ponsonby A‐L. Reflection on modern methods: building causal evidence within high‐dimensional molecular epidemiological studies of moderate size. Int J Epidemiol. 2021;50(3):1016‐1029. [DOI] [PubMed] [Google Scholar]
- 26. Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied logistic regression, vol. 398. John Wiley & Sons; 2013. [Google Scholar]
- 27. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458‐472. [DOI] [PubMed] [Google Scholar]
- 28. Hicks R, Tingley D. Causal mediation analysis. Stata J. 2011;11(4):605‐619. [Google Scholar]
- 29. VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. 2016;37:17‐32. [DOI] [PubMed] [Google Scholar]
- 30. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E‐values. Epidemiology (Cambridge, Mass). 2018;29(5):e45‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 32. Olin A, Henckel E, Chen Y, et al. Stereotypic immune system development in newborn children. Cell. 2018;174(5):1277‐1292.e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alduraywish S, Lodge C, Campbell B, et al. The march from early life food sensitization to allergic disease: a systematic review and meta‐analyses of birth cohort studies. Allergy. 2016;71(1):77‐89. [DOI] [PubMed] [Google Scholar]
- 34. Hinz D, Simon JC, Maier‐Simon C, et al. Reduced maternal regulatory T cell numbers and increased T helper type 2 cytokine production are associated with elevated levels of immunoglobulin E in cord blood. Clin Exp Allergy. 2010;40(3):419‐426. [DOI] [PubMed] [Google Scholar]
- 35. Schaub B, Liu J, Höppler S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123(4):774‐782 e775. [DOI] [PubMed] [Google Scholar]
- 36. Holt P, Clough J, Holt B, et al. Genetic ‘risk’ for atopy is associated with delayed postnatal maturation of T‐cell competence. Clin Exp Allergy. 1992;22(12):1093‐1099. [DOI] [PubMed] [Google Scholar]
- 37. Martino D, Neeland M, Dang T, et al. Epigenetic dysregulation of naive CD4+ T‐cell activation genes in childhood food allergy. Nat Commun. 2018;9(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng S‐S, Gao R, Ren J, et al. Maternal allergic disease history affects childhood allergy development through impairment of neonatal regulatory T‐cells. Respir Res. 2016;17(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schaub B, Liu J, Höppler S, et al. Impairment of T‐regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol. 2008;121(6):1491‐1499.e1413. [DOI] [PubMed] [Google Scholar]
- 40. Wood H, Acharjee A, Pearce H, et al. Breastfeeding promotes early neonatal regulatory T‐cell expansion and immune tolerance of non‐inherited maternal antigens. Allergy. 2021;76(8):2447‐2460. [DOI] [PubMed] [Google Scholar]
- 41. Omidakhsh N, Hansen J, Ritz B, Olsen J, Heck JE. High parental occupational social contact and risk of childhood hematopoietic, brain and bone cancers. Cancer Epidemiol. 2019;62:101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruohtula T, de Goffau MC, Nieminen JK, et al. Maturation of gut microbiota and circulating regulatory T cells and development of IgE sensitization in early life. Front Immunol. 2019;10:2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Östman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ‐free mice. Eur J Immunol. 2006;36(9):2336‐2346. [DOI] [PubMed] [Google Scholar]
- 44. Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. 2014;133(2):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller JE, Carter KW, de Klerk N, Burgner DP. The familial risk of infection‐related hospitalization in children: a population‐based sibling study. PLoS One. 2021;16(4):e0250181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Purewal R, Christley R, Kordas K, et al. Socio‐demographic factors associated with pet ownership amongst adolescents from a UK birth cohort. BMC Vet Res. 2019;15(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karmaus W, Arshad S, Sadeghnejad A, Twiselton R. Does maternal immunoglobulin E decrease with increasing order of live offspring? Investigation into maternal immune tolerance. Clin Exp Allergy. 2004;34(6):853‐859. [DOI] [PubMed] [Google Scholar]
- 49. Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 50. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ishikawa H, Tanaka K, Maeda Y, et al. Effect of intestinal microbiota on the induction of regulatory CD25+ CD4+ T cells. Clin Exp Immunol. 2008;153(1):127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T‐cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci. 2010;107(27):12204‐12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holt PG, Strickland DH, Custovic A. Targeting maternal immune function during pregnancy for asthma prevention in offspring: harnessing the “farm effect”? J Allergy Clin Immunol. 2020;146(2):270‐272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


