ABSTRACT
Studies of biological soil crusts (biocrusts) have proliferated over the last few decades. The biocrust literature has broadened, with more studies assessing and describing the function of a variety of biocrust communities in a broad range of biomes and habitats and across a large spectrum of disciplines, and also by the incorporation of biocrusts into global perspectives and biogeochemical models. As the number of biocrust researchers increases, along with the scope of soil communities defined as ‘biocrust’, it is worth asking whether we all share a clear, universal, and fully articulated definition of what constitutes a biocrust. In this review, we synthesize the literature with the views of new and experienced biocrust researchers, to provide a refined and fully elaborated definition of biocrusts. In doing so, we illustrate the ecological relevance and ecosystem services provided by them. We demonstrate that biocrusts are defined by four distinct elements: physical structure, functional characteristics, habitat, and taxonomic composition. We describe outgroups, which have some, but not all, of the characteristics necessary to be fully consistent with our definition and thus would not be considered biocrusts. We also summarize the wide variety of different types of communities that fall under our definition of biocrusts, in the process of highlighting their global distribution. Finally, we suggest the universal use of the Belnap, Büdel & Lange definition, with minor modifications: Biological soil crusts (biocrusts) result from an intimate association between soil particles and differing proportions of photoautotrophic (e.g. cyanobacteria, algae, lichens, bryophytes) and heterotrophic (e.g. bacteria, fungi, archaea) organisms, which live within, or immediately on top of, the uppermost millimetres of soil. Soil particles are aggregated through the presence and activity of these often extremotolerant biota that desiccate regularly, and the resultant living crust covers the surface of the ground as a coherent layer. With this detailed definition of biocrusts, illustrating their ecological functions and widespread distribution, we hope to stimulate interest in biocrust research and inform various stakeholders (e.g. land managers, land users) on their overall importance to ecosystem and Earth system functioning.
Keywords: biological soil crust, biocrust, definition, taxonomy, habitat, physical structure, function, climate.
I. INTRODUCTION
Biological soil crusts (hereafter biocrusts) occur globally in ecosystems where limited vascular plant cover allows sunlight to reach the soil surface (Fig. 1); they are especially predominant in water‐limited ecosystems. Biocrusts’ relevance to ecosystem functioning is well documented: they stabilize the soil surface, thus effectively reducing erosion by both wind and water (Eldridge & Leys, 2003; Barger et al., 2006; Zhang et al., 2006; Bowker et al., 2008; Chaudhary et al., 2009; Belnap, Munson & Field, 2011; Faist et al., 2017); fix carbon and nitrogen, fertilizing nutrient‐poor dryland soils (Lange & Green, 2004; Veluci, Neher & Weicht, 2006; Elbert et al., 2012; Su, Wu & Zhang, 2012; Barger et al., 2016; Sancho et al., 2016); influence local and regional water cycling (Zhang et al., 2009; Bowker et al., 2013; Kidron & Büdel, 2014; Chamizo et al., 2016; Eldridge et al., 2020); and have multifaceted effects on plant germination and growth (Zhang et al., 2016; Ferrenberg et al., 2018; Havrilla et al., 2019). According to a recent estimate, biocrusts currently cover about 12% of Earth's terrestrial surface and about 30% of all dryland soils (Rodriguez‐Caballero et al., 2018a). Despite their prevalence, biocrusts are imperilled by global change factors, in particular the combined effects of land‐use intensification and climate change, which may cause a strong decrease in biocrust coverage at local, regional, and global scales (Reed et al., 2012; Maestre et al., 2013; Ferrenberg, Reed & Belnap, 2015; Rodriguez‐Caballero et al., 2018a). Because biocrusts are both imperilled and key to ecosystem functioning, their influence on ecosystems and consequences of their loss should be incorporated into analyses and models of global change (Elbert et al., 2012; Barger et al., 2016; Sancho et al., 2016; Ferrenberg, Tucker & Reed, 2017; Rodriguez‐Caballero et al., 2018b). Land managers, nature conservation organizations, policy makers, landscape architects, and the broader society need to consider and safeguard biocrusts to sustain the ecosystem services they provide (Lopez‐Rodriguez et al., 2020).
Fig. 1.
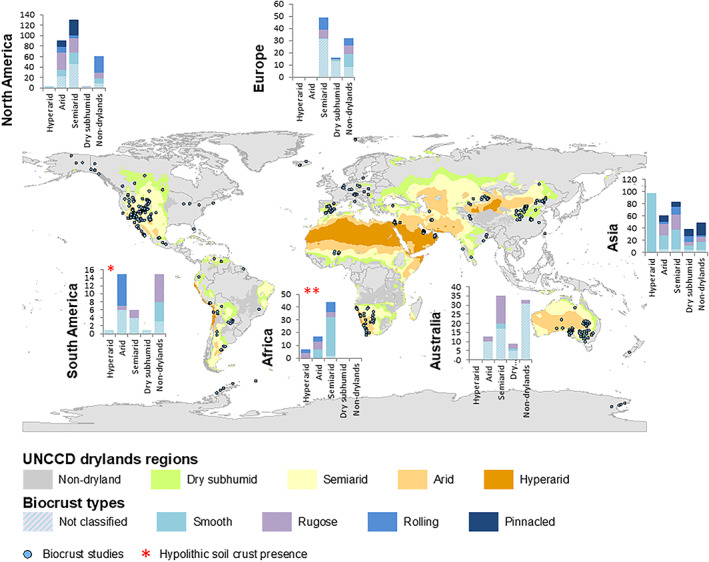
Sites of biocrust studies in dryland and non‐dryland regions. Biocrusts occur in all drylands around the world, but also in non‐dryland regions if microclimatic conditions are suitable. Composition of biocrust types varies depending both on continent and climatic region. Dryland regions shown according to UNCCD (UNEP‐WCMC, 2007). Biocrust study sites marked according to list in Rodríguez‐Caballero et al. (2018a). Biocrust types occurring in the different dryland and non‐dryland regions are presented in inset bar charts, with the number of described sites of occurrence on the y‐axis. Bar charts are presented for the different continents, excluding Antarctica.
Interest in biocrusts has experienced a dramatic increase over the past few decades. The number of publications dealing with biocrusts (or synonymous terms) in 2021 has increased threefold compared with all publications up to the year 2000 (252 compared to 86; search conducted on January 14, 2022; see online Supporting Information, Fig. S1), with research conducted on all continents (Weber, Belnap & Büdel, 2016a) (Fig. 1). Other disciplines, such as vegetation and soil science, animal ecology, physiology, remote sensing, and hydrology have also begun to appreciate the relevance of biocrusts, incorporating them into studies during recent years (Karnieli et al., 2003; Dumack et al., 2016; Havrilla et al., 2019; Eldridge et al., 2020). Given this increasing interest in biocrusts and acknowledgement of their importance, many researchers and land managers alike are frequently asked whether or not a specific community ‘qualifies’ as a biocrust or not, often noting that different perspectives and characterizations are used. These experiences suggest that a universal definition of biocrusts would be beneficial to ensure a more consistent usage of the term and clarify what sets biocrust communities apart from other biological communities with similar features.
Much of the early literature on biocrusts did not specifically use the term ‘crust’ (reviewed in Friedmann & Galun, 1974), instead referring to “terrestrial algae” (Fritsch, 1922), “soil algae” (Friedmann, Lipkin & Ocampo‐Paus, 1967), “cryptogamic covers” (Zhu, 1960; Kleiner & Harper, 1972; Jiang et al., 1995), “soil surface lichens” (Rogers, 1972), or “surface stratum” (Shields, Mitchell & Drouet, 1957). Fletcher & Martin (1948) was an exception, reporting them as “rain‐crusts.” A first attempt at a definition was made by Reiners, Worley & Lawrence (1971), who studied primary succession at Glacier Bay (USA). They coined the term “black crust”, describing it as a “cohesive, felt‐like crust on the soil surface” with successional roles in “soil stabilization, soil‐moisture retention, organic matter accumulation, and seed‐bed modification” (Reiners et al., 1971, p. 59). This early definition mentions structural and functional properties, and identified the soil surficial habitat. They described black crusts as “multi‐species colonies” comprising unrelated organisms of bryophytes, cyanobacteria, and lichens, adding a taxonomic element to the definition. West (1990, p. 180) in a global review defined “microphytic crusts” as “the complex of mosses, lichens, liverworts, algae, fungi and bacteria at the soil surface.” He went on to note that ferns and club mosses are not part of these communities, following Cameron (1978), again suggesting a taxonomic element to the emerging definition (Friedmann & Galun, 1974). For a detailed review on the recognition and past naming of biocrusts, see Lange & Belnap (2016).
The first attempt at a comprehensive definition of biocrusts was presented in the initial review volume on biocrusts (Belnap & Lange, 2003), where the authors focused on the taxonomy, habitat, physical structure and function of biocrusts. They stated “biological soil crusts result from an intimate association between soil particles and cyanobacteria, algae, microfungi, lichens, and bryophytes (in different proportions) which live within, or immediately on top of, the uppermost millimeters of soil. Soil particles are aggregated through the presence and activity of these biota, and the resultant living crust covers the surface of the ground as a coherent layer” (Belnap et al., 2003, p. 3). The ecological relevance of biocrusts was stressed in the second review volume (Weber, Büdel & Belnap, 2016b), explaining that biological soil crusts “consist of microscopic (cyanobacteria, algae, fungi, and bacteria) and macroscopic (lichens, bryophytes, and microarthropods) poikilohydric organisms that occur on or within the top few centimetres of the soil surface. In regions where water availability limits vascular plant cover, these communities are especially visible, creating an almost continuous living ‘skin’ that mediates most inputs, transfers, and losses across the soil surface boundary” (Belnap, Weber & Büdel, 2016, p. 3).
Thus, the definitions of the 21st century clarify that biocrusts are characterized simultaneously by their taxonomy, habitat, physical structure, and ecological functions, but from these short passages it still is not always clear what are the essential properties of biocrusts, what is the underlying rationale, or what is not a biocrust.
Based on these gaps in current biocrust definitions and as an aid to attract new interest in biocrusts from a scientific and practical perspective, this review will: (i) identify the key elements defining a biocrust, namely their habitat, physical structure, function, and taxonomy; (ii) distinguish other communities and features which exhibit some, but not all relevant biocrust features, and thus are not biocrusts; (iii) describe the variation within the bounds of our definition, including biocrust variants shaped by differing environments; (iv) propose a universal definition of biocrusts.
II. BIOCRUST‐DEFINING ELEMENTS
Biocrusts can be defined by elements related to habitat, function, physical structure, and taxonomy. The combination of these characteristics, summarized in Figs 2 and 3 and described in the following subsections, provides a thorough definition of biocrusts.
Fig. 2.
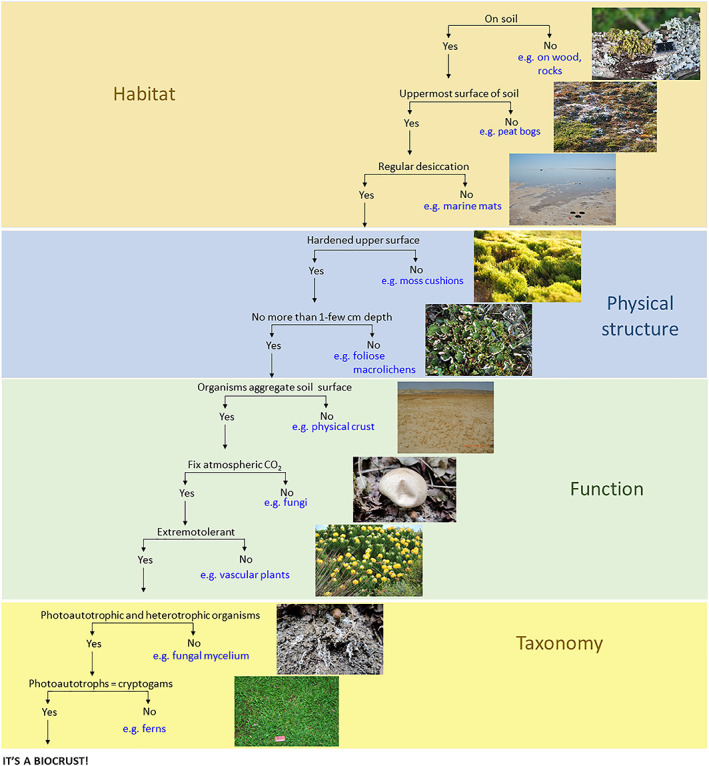
Biological soil crust (biocrust) definition based on a decision tree approach.
Fig. 3.
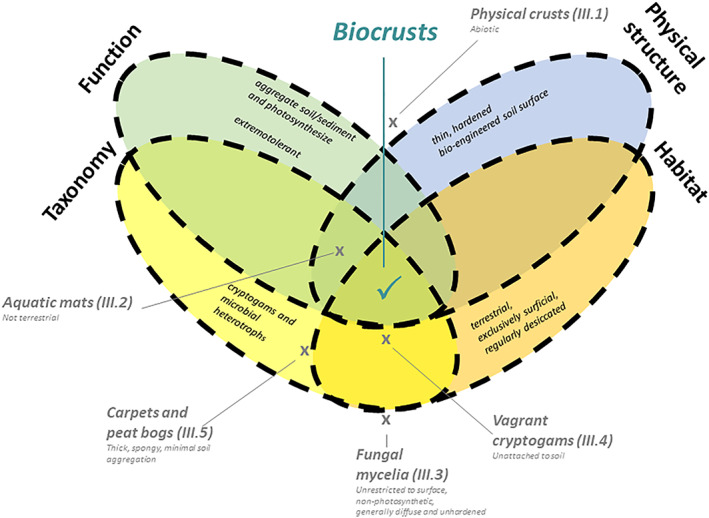
Biological soil crust (biocrust) definition illustrated in a Venn diagram. Ovals represent the four major elements of our biocrust definition. Biocrusts are consistent with the region where all four overlap. Other ‘outgroups’ are also mapped and labelled on the diagram, with the main reasons for their distinction from biocrusts listed. Parenthetical numbers indicate the relevant section of this review for each outgroup.
(1). Habitat characteristics
First, we define biocrusts as a community of organisms dwelling on the soil surface. This includes hypolithic biocrusts that colonize soils on the sides and beneath translucent rocks (Figs 2, 3; Pointing, 2016). We exclude organisms that predominantly grow in or on rocks, leaves, and wood (e.g. endolithic cyanobacteria, saxicolous lichens and bryophytes, and epiphytes), although we acknowledge there may be situations in which it is appropriate to study all such communities together (Elbert et al., 2012).
Most examples of biocrusts from the literature occur on and within the uppermost surface of the soil (usually an organo‐mineral A horizon) or, in some highly degraded situations, on the exposed underlying mineral horizon (Gretarsdottir et al., 2004). We do not rule out the possibility that biocrusts can grow on an O (organic layer) horizon, but generally communities in those habitats do not display other key biocrust properties (e.g. aggregating soil particles; for an expanded discussion, see Section III.3). Further, most biocrusts occupy terrestrial soils that desiccate regularly, and, generally speaking, are dry more often than not (Figs 2, 3; Raggio et al., 2014; Büdel, Williams & Reichenberger, 2018). We exclude any sediment‐associated communities that occur in freshwater, marine, or intertidal habitats (commonly referred to as ‘mats’ or ‘microbial mats’; Stal, 1994). We are also unaware of any examples of hydric soils supporting biocrusts; normally such soils either accumulate large amounts of organic matter or are characterized by high vascular plant productivity such that insufficient light reaches the soil surface to support the growth of biocrusts.
(2). Physical structural characteristics
Because of the soil aggregation conducted by biocrust organisms, and its location only at the soil surface, a detectable structural shift occurs at the soil surface: namely, a physically cohesive, thin and somewhat hardened upper surface layer (Figs 2, 3). This particular structural configuration is consistent with the general usage of the word ‘crust’: a hardened outer surface (e.g. as in a bread crust or the Earth's crust). Within the biocrust, or immediately below it, the amount of soil fine particles is often increased due to both dust entrapment and soil weathering processes, reinforcing the distinctiveness of the biocrust from underlying soil (Chen et al., 2009; Garcia‐Pichel et al., 2016). In some cases, biocrusts can contain several hardened layers on top of each other, resulting from recurring biocrust burial and new recolonization on top (Malam Issa et al., 2009; Drahorad & Felix‐Henningsen, 2013; Felde et al., 2014; Gao et al., 2017).
The thickness of biocrusts is a relative, rather than absolute, value, although we note that most examples from the literature describe communities that are no more than one to a few centimetres thick (Figs 2, 3; Belnap et al., 2003; Zhao et al., 2006; Belnap et al., 2016). Nonetheless, we are aware of communities where the biocrusts may be thicker, such as semi‐arid tall moss communities that have substantial above‐ground biomass up to several cm thick (Rosentreter, Bowker & Belnap, 2007). Biocrust thickness is relative to the soil horizon in which it grows; for example, a typical biocrust generally encompasses only a small fraction of the depth of an A horizon. Biocrusts alone do not generally constitute soil horizons. Because they are thin, biocrusts tend to break under pressure, rather than yielding and deforming.
(3). Functional characteristics
Perhaps the core functional element in the definition of a biocrust is that the component organisms and their exudates aggregate surface soil particles, increasing the stability of the soil surface above that of the underlying soil (Figs 2, 3, 4; Eldridge & Leys, 2003; Zhang et al., 2006; Chaudhary et al., 2009; Jimenez Aguilar et al., 2009; Belnap et al., 2014). A biocrust is thus different from a physical soil crust, because the aggregation is at least partially and often primarily engineered by living (i.e. biocrust) organisms. In particular, secretions of extracellular polymeric substances and filamentous biological structures (e.g. moss rhizoids, lichen rhizines, cyanobacterial filaments) are key to generating this aggregation (Fig. 4B; Hu et al., 2002; Neuman & Maxwell, 2002; Mager & Thomas, 2011; Rossi, Mugnai & Philippis, 2018).
Fig. 4.

Illustration of characteristics that define biocrusts (A, B) and of features that are not biocrusts (C–H). (A) Biocrusts aggregate surface soil particles, thus stabilizing soils; Sakaiká sclerophyllous shrubland, La Gran Sabana, Venezuela. (B) Filamentous biological structures (cyanobacterial filaments) cause a soil aggregation; Colorado Plateau, Southern Utah, USA. (C) Physical crust; Knersvlakte at Goedehoop farm, South Africa. (D) Microbial mat; Shannah, Oman. (E) Fungus of the genus Bovista with mycelium; Graz, Austria. (F) Vagrant Xanthoparmelia sp. (green) growing on top of a regular biocrust; Colorado Plateau, Southeast Utah (photographs: courtesy of Kyle Doherty). (G) Cyanobacterial macrocolonies; former limestone quarry, Aschfeld, Germany. (H) Lichen and bryophyte carpet, Illulisat, Greenland.
Biocrusts contain photosynthetic organisms. These photoautotrophs fix atmospheric carbon dioxide and thus require presence at the soil surface.
Biocrusts are extremotolerant (Fig. 2). They can withstand extreme temperatures and low precipitation through desiccation tolerance (Lange, Belnap & Reichenberger, 1998; Raggio et al., 2014; Green & Proctor, 2016). Once dry, biocrust organisms are in a largely inactive physiological stage characterized by a wide tolerance of extreme environmental conditions (Mazor et al., 1996; Karsten, Herburger & Holzinger, 2016). Biocrust organisms withstand high levels of UV radiation by manufacturing sunscreen pigments (e.g. scytonemin; Soule et al., 2009; Karsten & Holzinger, 2014), and many components (e.g. the cyanobacterium Microcoleus and related genera) can also tolerate high salinity levels (Kakeh et al., 2021).
(4). Taxonomic composition
A taxonomic definition of biocrusts is fraught with difficulty because biocrusts are comprised of an assortment of photoautotrophic and heterotrophic organisms that span domains, kingdoms, and phyla (Figs 2, 3). The photoautotrophic component includes multiple lineages, namely cyanobacteria, algae, lichens, and bryophytes, but excludes ferns, fern allies, and vascular seed plants (Cameron, 1978; West, 1990). Cyanobacteria, algae, lichens and bryophytes all belong to the historical grouping of ‘cryptogams’ (meaning ‘hidden reproduction’), which are organisms reproducing by spores rather than seeds, all lack highly developed vascular tissue and many, if not most, of them are able to desiccate regularly. Thus, the photoautotrophic component of biocrusts is composed of non‐vascular cryptogams. Despite this grouping not being phylogenetically based, it is useful, and some recent literature has returned to using this term to describe the majority of the photoautotrophic biocrust components, because they tend to co‐occur in similar habitat types where they engineer physically and functionally similar communities (e.g. Elbert et al., 2012; Deane‐Coe & Stanton, 2017). As the dominating photoautotroph is one of the important determinants of biocrust type, this descriptor is often used in the literature [i.e. cyanobacteria‐, lichen‐, or bryophyte‐dominated biocrusts (Lazaro et al., 2008; Büdel et al., 2009)].
In addition to the photoautotrophic cryptogams, biocrusts contain a great diversity of microbial heterotrophs, including fungi, bacteria, and archaea (Maier et al., 2018, 2022; Abed et al., 2019; Pombubpa et al., 2020). These organisms can consume the carbon compounds released by the photoautotrophs during rainfall events (Beraldi‐Campesi et al., 2009). Biocrusts create a habitat that is occupied by microfauna such as protozoa, nematodes, tardigrades, rotifers, and microarthropods (Neher et al., 2009; Liu et al., 2011). Thus, biocrusts form entire foodwebs/ecosystems, made up of photoautotrophic producers and heterotrophic consumers (Fig. 5).
Fig. 5.
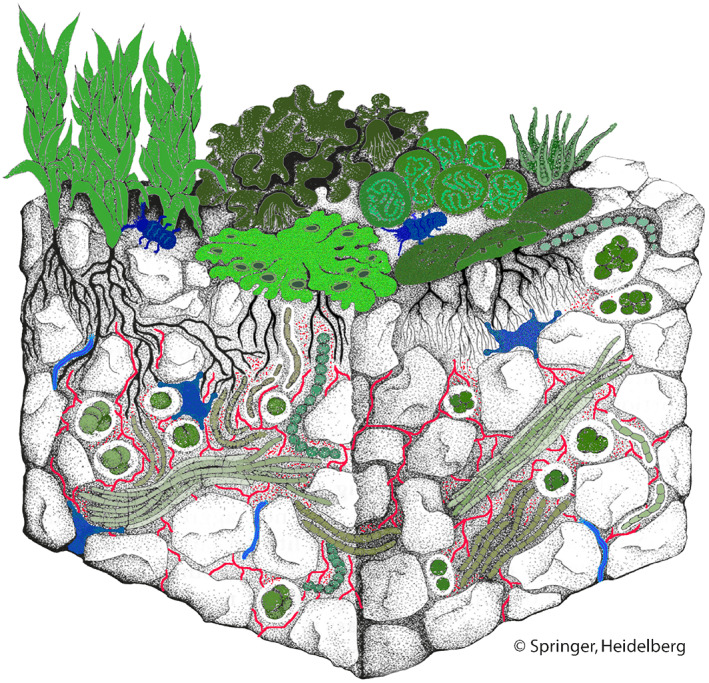
Biological soil crusts (biocrusts) form miniature ecosystems. They are composed of photoautotrophic producers (i.e. cyanobacteria, algae, lichens, and bryophytes; shown in green), microfauna acting as consumers (i.e. protozoa, nematodes, tardigrades, rotifers, and microarthropods; shown in blue), and decomposers (i.e. fungi, bacteria, and archaea; shown in red). Colouration of drawing by Renate Klein‐Rödder, originally published in Belnap & Lange (2003); courtesy of Springer.
III. WHAT IS NOT A BIOCRUST?
There are features, structures, organisms, or community types on soil and other substrates that share some, but not all, biocrust characteristics. The boundary between what is, and what is not, a biocrust is often not clearly defined and intermediate forms may be found (Figs 2, 3). We do not view this as problematic, but rather typical of most commonly recognized types of biological communities (e.g. ecotonal communities). Below, we present several instructive examples of ‘outgroups’ that may be encountered and that cannot be considered biocrusts (see Fig. 2).
(1). Physical crusts
Physical crusts are created by abiotic processes (Figs 2, 3). Compressional and shear forces, such as raindrops, runoff, hooves and vehicles, disrupt soil aggregates; with additional rain, the individual soil particles then reform into a hard‐packed layer (Valentin & Bresson, 1992; Pagliai & Stoops, 2010). Alternatively, precipitated salts in the soil that form through evaporation can aggregate surface particles into a physical crust (Fang et al., 2007; Bu, Wu & Yang, 2014). Physical crusts are particularly common in hot deserts and disturbed lands (Fig. 4C). Over time, they may be colonized by biocrust biota and transform into biocrusts. Thus, physical crusts and biocrusts often co‐occur, especially in dryland settings (Malam Issa et al., 2011; Beaugendre et al., 2017).
(2). Freshwater, intertidal, and marine mats
In shallow freshwater, marine, intertidal, and other non‐ephemeral aquatic settings, the sediment within the water is often colonized by ‘microbial mats’ (Stal, 1994). These microbial mats consist of both photoautotrophic and heterotrophic organisms which occur as layers, giving the community a ‘striped’ appearance (Schulz, 1937). As in biocrusts, heterotrophic anaerobes are found in the deeper strata, whereas the photoautotrophs occur in the uppermost layers (Figs 2, 3, 4). These communities are also extremotolerant, as they are subjected to rapid changes of osmotic pressure, regular desiccation, and high ultraviolet (UV) load (Bolhuis, Cretoiu & Stal, 2014; Prieto‐Barajas, Valencia‐Cantero & Santoyo, 2018). While there are many similarities between microbial mats and biocrusts, they are excluded because they are not clearly terrestrial and are often inundated by water.
(3). Fungal mycelial mats
Non‐flooded soils with substantial organic matter, such as forest floors, are dominated by fungi with varying functional roles from saprobic decomposers to pathogens to mycorrhizal heterotrophs (Griffiths, Castellano & Caldwell, 1991). Fungi create underground networks of hyphae, or mycelia, often near the soil surface where organic matter is concentrated, but they may densely or diffusely permeate an entire soil horizon (Figs 2, 3, 4). While mycelia clearly contribute to soil aggregation and fungal hyphae are generally found within biocrusts, fungal mycelial mats are excluded from biocrusts for two reasons: there are no photosynthetic organisms present and they are not purely a soil surface phenomenon.
(4). Vagrant lichens, moss balls, and detached cyanobacterial colonies
Both mosses and lichens may become detached from the soil surface in some environments, including some of the drylands occupied by biocrusts (Perez, 1991; Rosentreter, 1993). These may be taxa that sometimes are part of a biocrust when they are within or attached to the soil surface (e.g. Xanthoparmelia spp.; Eldridge & Leys, 1999). In the detached or vagrant state, they are transportable by wind, water, and gravity (Figs 3, 4). Despite habitat overlap and some functional and taxonomic similarity, we consider vagrant organisms to be distinct from biocrusts because they do not aggregate soil particles, as most of their biomass rests on top of it.
In a variety of habitats around the world, from the tropics to the Arctic and Antarctic, large (up to several centimetres in diameter) foliose or stringy colonies of cyanobacteria (notably Nostoc) may periodically develop rapidly and cover substantial proportions of either soil, rock, or the built environment (Fig. 4G). They may persist on the soil surface or in the soil. Although the genus Nostoc is an important component of biocrusts around the world, the detached macrocolonies of Nostoc commune and Nostoc flagelliforme are distinguished from biocrusts, because they occur above the soil rather than being attached to it, and they do not contribute to the aggregation of soil particles. Further, like vagrant lichens and moss balls, the dry colonies may be distributed by the wind. Thus, we suggest that in the detached, vagrant state, these colonies do not constitute biocrusts.
(5). Lichen/bryophyte carpets and peat bogs
In regions where water is seldom limiting, lichens and bryophytes can be a dominant component of the ecosystem, as in the ‘lichen meadows’ and ‘bryophyte carpets’ found in tundra and taiga ecosystems (Figs 2, 3, 4). Such carpets can also occur in some temperate regions in habitats where vascular plants are limited in growth, for example, by low light conditions inside a forest (e.g. forest ‘bryoid layers’). Peat bogs form in frequently flooded soils; in these communities, the growing front of Sphagnum mosses is near the surface, whereas beneath there may be substantial accumulations of dead biomass.
All these communities may accumulate substantial living and dead biomass, lending them a thickness usually of several centimetres or much more. Most of the moss or lichen biomass occurs above the mineral soil surface, and most of the live photoautotrophic tissue generally does not make contact with the mineral soil surface nor does it aggregate it. When subjected to compressional forces, their soft and sponge‐like structure can bend and yield, rather than be crushed and broken like biocrusts.
IV. VARIATIONS IN FORM AND FUNCTION WITHIN BIOCRUSTS
Globally, biocrusts take on a wide variety of forms, as their external appearance is determined by many factors such as species composition, biomass of biocrust organisms, internal physical structure, (micro‐)climate, soils, and disturbance history. These factors, combined with the resultant external morphology, then determine the functions the biocrust will play in a given ecosystem. Here, we highlight both common and rare biocrust forms to illustrate the wide variation in physical morphology and composition found in different ecological settings. In general, lichen and bryophyte cover and rugosity increase as potential evapo‐transpiration (PET) declines; this is accompanied by a decline in cyanobacterial cover (Bowker et al., 2016). However, other drivers such as disturbance and soil type can override generalizations based on climate (Bowker et al., 2016).
(1). Hyper‐arid regions (AI < 0.05)
Hyper‐arid regions (e.g. portions of the Negev, Namib, Sahara, Mojave, Atacama, Taklamakan deserts; Fig. 1) have very low precipitation and very high summer temperatures; this results in a very low aridity index (AI), which is the ratio of long‐term water supply or precipitation (P) and potential evapotranspiration (PET; i.e. the ‘drying power’ of the atmosphere to remove water from terrestrial surfaces by evaporation and by plant transpiration). Given the very low availability of soil moisture, biocrusts are generally composed of a relatively low biomass of cyanobacteria and/or algae, although small pockets of bryophytes and lichens can be found in wetter microhabitats (Romero et al., 2020). These organisms reduce erosional features that roughen surfaces and there is no frost‐heaving which would normally create microtopographic features. As a result, biocrusts in hyper‐arid regions often have a smooth surface (Fig. 6A). The smooth surface topography facilitates movement of materials (e.g. litter, seeds, sediment) across the soil surface in contrast to most other biocrust types. Due to their low biomass, carbon and nitrogen inputs from these biocrusts are comparatively low, but often important due to the largely absent vascular vegetation (Abed et al., 2019).
Fig. 6.
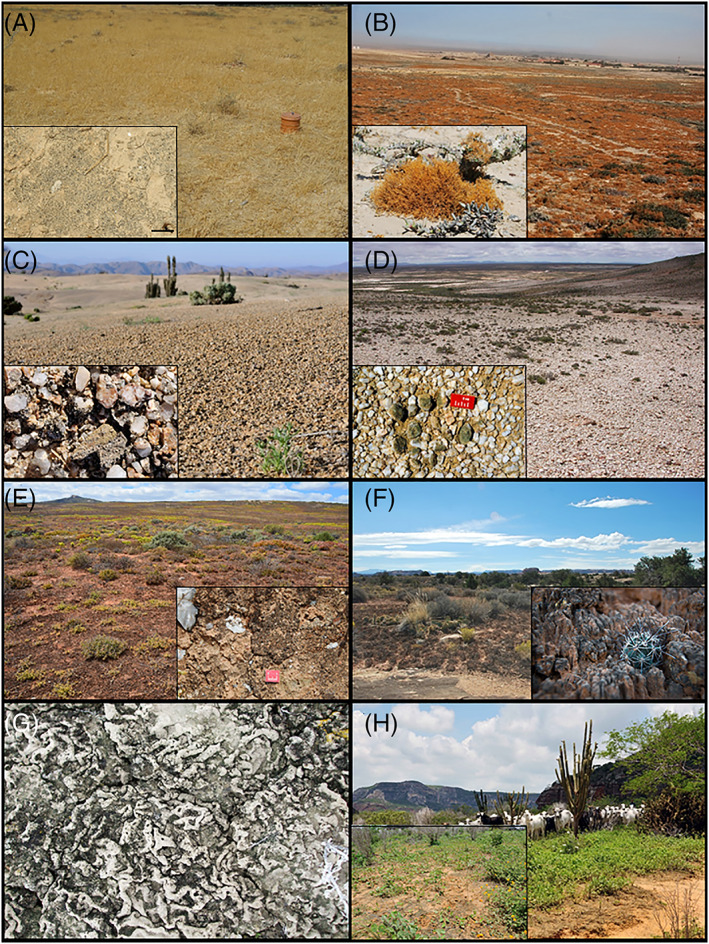
Biocrusts varying in form and function within different climatic settings. (A) Smooth cyanobacteria‐dominated crust near Beersheba, Israel. (B) Lichen fields with Teloschistes sp. as the prominent genus; Namib Desert, Alexanderbay, South Africa. (C) Grit crust with weathered granite pebbles, enveloped and aggregated by several species of chlorolichens, accompanied by fungi and cyanobacteria; Atacama Desert, Chile. (D) Hypolithic biocrust with cyanobacteria growing on the sides and underside of translucent quartz pebbles; Goedehoop farm, Knersvlakte, South Africa. (E) Cyanobacteria‐dominated biocrust with cyano‐ and chlorolichens; Soebatsfontein region, Succulent Karoo, South Africa. (F) Cyanobacterially dominated pinnacled crust with mosses and lichens; Canyonlands National Park, Colorado Plateau region, USA; Cactacea occurring in‐between pinnacles. (G) ‘Wrinkled’ biocrust in semiarid regions; Great Basin region, northwest Utah, USA. (H) Cyanobacteria‐dominated biocrust with mosses and liverworts, impacted by trampling; Fazenda Brejo, Caatinga, Brazil.
Hyper‐arid regions can host three other distinctive biocrust or biocrust‐like communities. In fog deserts (e.g. portions of the Namib, Atacama deserts), where most precipitation results from regularly occurring fog, lichen fields may occur (Schieferstein & Loris, 1992; Büdel et al., 2009; Fig. 6B). In these communities, soil‐adherent lichens and cyanobacteria are joined by erect, relatively tall fruticose lichens with strongly branched thalli that ‘comb’ the fog for water.
The recently described ‘grit‐crust’, known only from the Atacama Desert, is unique in that it develops on the upper weathered bedrock layer, a nascent ‘soil’. This surface is mostly composed of grit‐sized (~6 mm) pebbles, enveloped and aggregated by several species of green algal lichens (chlorolichens), accompanied by fungi and cyanobacteria (Fig. 6C; Jung et al., 2020). Whether or not this community is considered a biocrust according to our definition depends on whether the habitat is interpreted as soil or rock; physical structural, functional, taxonomic, and some habitat characteristics suggest at least a very close affinity to biocrusts.
In regions where annual precipitation is too low even for cyanobacteria to live unsheltered on the surface, hypolithic biocrusts dominated by cyanobacteria can often be found (Fig. 6D). The constituent organisms attach to soil beneath rocks; hypoliths attached only to rock would not be consistent with our definition. Their dry limit corresponds to <5 mm precipitation of rainfall per year or even decadal periods without rain (Warren‐Rhodes et al., 2006). The translucent rocks allow light to penetrate to the photosynthetic organisms and also ameliorate water stress by reducing evaporation, increasing the surface area that harvests water and facilitating nightly condensation (Weber et al., 2013).
(2). Arid regions (0.05 ≤ AI < 0.2)
Similar to biocrusts found in hyper‐arid regions, those in arid deserts (e.g. parts of the Mojave, Sahel, and Karoo deserts; Fig. 1) are generally dominated by cyanobacteria or lichens, with patches of bryophytes commonly found in wetter microsites (Malam Issa et al., 1999). The presence of lichens and bryophytes commonly generates rugosity, in which these components are elevated by about 1–2 cm above an otherwise flat surface (Figs 1, 6). This higher cover of lichens and bryophytes and longer residence times of soil moisture, and thus biocrust activity, result in more inputs of carbon and nitrogen and greater soil stability (Malam Issa et al., 2001). Rugosity may reduce the propensity for a biocrust to accelerate the movement of materials across the soil surface, perhaps even allowing the biocrust to function more as a mobile resource accumulator rather than resource shedder (Williams, Buck & Beyene, 2012). Arid regions also support biocrusts with a smooth topography (Fig. 1); these function similarly to those of hyperarid regions. If translucent rock pebbles are present, hypolithic cyanobacteria, bryophytes, and/or lichens might be found (Ekwealor & Fisher, 2020).
(3). Semi‐arid regions (0.2 ≤ AI < 0.5)
As PET decreases relative to arid regions in parts of North America (e.g. the Colorado Plateau), several deserts of Northern China and central Asia and across large areas of South America (e.g. Patagonia), Africa (e.g. Kalahari), Australia, and the Mediterranean Basin (Fig. 1), the number of biocrust species and cover of lichens and bryophytes increases (Maestre et al., 2011; Bowker et al., 2017). In Mediterranean ecosystems, temperature and precipitation were observed not only to determine lichen and bryophyte cover, but also to affect cyanobacterial composition (Munoz‐Martin et al., 2019). In addition, some semi‐arid regions commonly experience freezing winter temperatures. Frost‐heaving uplifts the soil surface with its mosaic of cyanobacteria, lichens, and bryophytes, resulting in a differentially eroding surface. This may create striking castle‐like pinnacles that are up to 15 cm high and with delicate tips <4 mm across (Figs 1, 6). Because of the increased surface area created by the pinnacles and the lower PET, the biomass and nutrient input of biocrust organisms can be quite high and soil stability greatly increased by their presence (Pérez, 2021). Due to the strong preservation of surface roughness, they are often strong sinks for mobile resources such as water, seeds, and sediment. This crust type is generally the most vulnerable to soil surface disturbance, as the frost‐heaved surface is easily broken and churned, burying the biocrust organisms. Hypoliths are also found in semi‐arid regions, with cyanobacteria, lichens and bryophytes colonizing the hypolithic environment (Büdel & Schultz, 2003).
As PET decreases further in semi‐arid regions (e.g. steppe regions such as the North American Great Basin, central Mongolia, Europe, Australia), biocrusts generally become heavily dominated by a wide variety and high biomass of lichens and bryophytes, which may attain a thickness of centimetres, and many green algal species (Samolov et al., 2019). Here, biocrusts can be so cohesive that they completely eliminate erosion (Eldridge & Kinnell, 1997; Leys & Eldridge, 1998; Gao et al., 2017). The microtopography may resemble rolling, gentle micro‐hills with a few centimetres of relief, especially when lichens or bryophytes dominate. The very high biomass, compared to biocrusts in more arid regions, results in a large contribution of stability, nitrogen, and carbon to these soils (Chamizo et al., 2012). In other areas (probably determined by soil properties or increasing summer rainfall), cyanobacteria retain dominance (Williams, Büdel & Williams, 2018), and their morphology may take the appearance of a wrinkled skin, perhaps superimposed over desiccation cracks (Fig. 6G).
(4). Dry sub‐humid regions (0.5 ≤ AI < 0.65)
Drier sub‐humid regions have sufficiently high PET to limit vascular plant cover and thus support biocrusts (e.g. southern Serengeti savannah in Tanzania, Africa; short‐grass prairie in North America, Caatinga in South America; Fig. 1). These regions are traditionally heavily grazed by wildlife and domestic livestock (Szyja et al., 2019), and are often affected by periodic fires (Siebert & Dreber, 2019). Plant litter can have an inhibitory effect on biocrust growth and development (Ding & Eldridge, 2020). Biocrusts in this region are often dominated by cyanobacteria, with a limited bryophyte and lichen component, likely due to the trampling impact of grazers (Fig. 6H), periodic fires, and vascular plant coverage or litter (Szyja et al., 2019; Palmer, Hernandez & Lipson, 2020; Ding & Eldridge, 2020). The biocrust morphology is usually smooth in purely cyanobacterial biocrusts and varies to rugose when bryophytes and lichens are present (Szyja et al., 2019; Fig. 1). Because plant cover is relatively high and bryophyte/lichen cover relatively low, these biocrusts play a lesser functional role than in other regions where their cover, biomass, and species diversity is higher.
(5). Alpine and polar regions
Biocrusts are also found in cold climates of polar and high elevation regions, where water may be limited due to aridity or because of frequent freezing, and seed plant cover may be sparse or absent (Fig. 1). Patchy or continuous covers of biocrusts (Fig. 7A) dominated by bryophytes, lichens, cyanobacteria, or mixtures have variously been described from scattered locations such as the Canadian Arctic (Gold & Bliss, 1995; Hogg et al., 2018), Svalbard (Williams et al., 2017), Antarctica (Colesie et al., 2014a), Icelandic highlands (Arnalds, 2015), the high Andes (Perez, 1997), and the Austrian Alps (Büdel et al., 2014; Jiang et al., 2018), among others. Unlike in the drylands, a universal driver of biocrust form and function at high latitudes/elevations has not been identified, nor has a general classification emerged. However, several locally or regionally important factors have been found to affect biocrust form and function. Across 10 high‐latitude sites, Williams et al. (2017) classified biocrust habitat types dictated by altitudinal gradients and differing land forms (e.g. scree slopes, hillocks, plains, etc.). Altitudinal gradients and land forms influence such factors as particle size and rock content of soils, snow melt timing, soil moisture content, and cryogenic processes, all of which may shape the biocrusts. Wetter, warmer low‐lying sites tend to support greater moss abundance, thick mats of cyanobacteria, or mixtures, whereas drier uplands tend to favour lichens (Stewart et al., 2011; Williams et al., 2017).
Fig. 7.
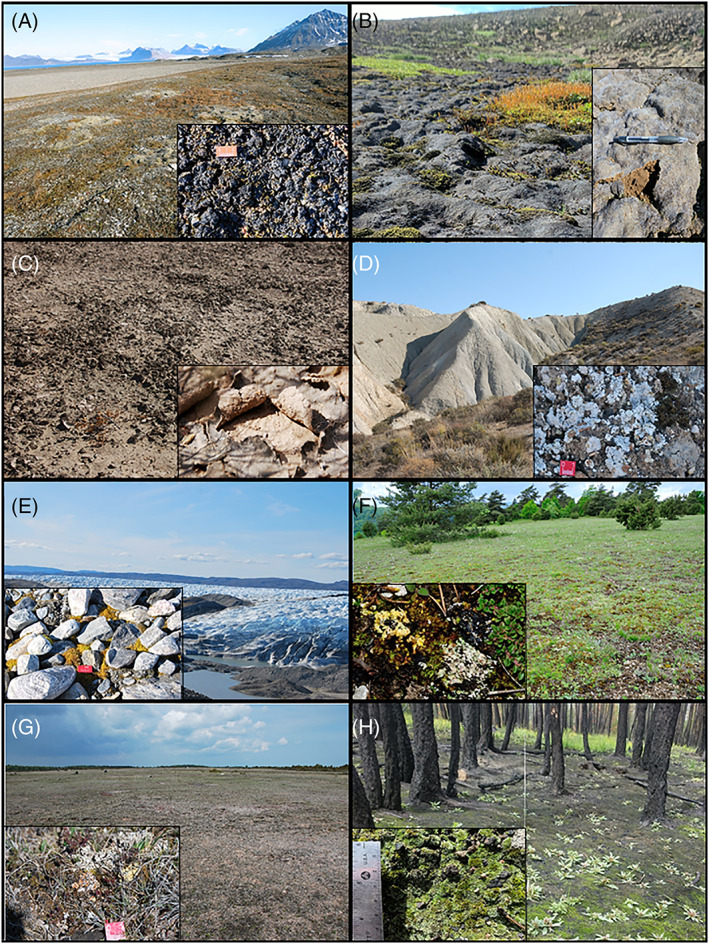
Biocrusts varying in form and function within different climatic, edaphic, and land‐use settings. (A) Polar region with low cover of vascular vegetation, but a dense cover of cyanobacteria‐dominated biocrusts with bryophytes; Zeppelinhamna, Ny Alesund, Spitsbergen. (B) Liverwort‐dominated biocrusts; because of extreme frost‐heaving, biocrusts are smoother overall than uncrusted soils; Icelandic Highlands. (C) ‘Peeling’ biocrust, dominated by cyanobacteria; Central Chihuahuan desert, Mexico. (D) Biocrust on gypsiferous soil, with particularly high coverage of chlorolichens; Tabernas Badlands, Spain. (E) Postglacial biocrust, dominated by mosses and cyanobacteria; Kangerlussuaq region, Greenland. (F) Biocrusts of temperate dry meadows (‘Trockenrasen’) with typical lichen community comprising Fulgensia fulgens, Toninia caerulionigricans, Cladonia convoluta, and Diploschistes muscorum; Ruine Homburg, Aschfeld, Germany. (G) Stora Alvaret (barren limestone terrace) on the island of Öland, Sweden; biocrusts with lichens of the genera Fulgensia, Psora, and Cladonia. (H) Biocrusts after fire in former forests; main photograph: Lolo Fire, western Montana, USA; insert: Cajete fire, northwestern New Mexico, USA (photographs courtesy of Henry Grover).
Latitude may also influence cold‐climate biocrust characteristics, for example in Antarctica. At higher latitudes, snowfall only generates available water if temperature allows thawing within a few hours. Otherwise snow sublimates with no effect on biocrusts. Activity periods are thus reduced to a few hours or days per year, leading to biocrusts dominated by chlorolichens and, more rarely, green algae (Colesie et al., 2014b, 2016b), whereas cyanobacteria are constrained to the hypolithic habitat. Biocrusts formed by cyanobacteria, green algae, lichens or mosses, or mixtures are mostly reported from the somewhat wetter lower latitudes of Antarctica.
High‐latitude and alpine biocrust morphologies are also highly variable and have not been documented thoroughly on a global scale. Smooth to rolling morphologies can be observed (Büdel & Colesie, 2014), but because of extreme frost‐heaving, biocrusts may be smoother overall than uncrusted soil (Fig. 7B). Other cryogenically generated features, such as patterned ground or polygonal rock nets, may confer unique morphologies to superimposed biocrusts (Williams et al., 2017). In addition to functional properties common to most biocrusts, in these cold environments they are also notable for facilitating vascular plant colonization because of their moderation of frost heaving and warming of the surface, and because of their role providing nutrients and promoting water retention (Bliss & Gold, 1999; Arnalds, 2015; Benavent‐González et al., 2018).
(6). Special edaphic settings
While general characteristics of biocrusts in drylands can be predicted by climate, many edaphic factors, such as soil texture, chemistry, fertility, and parent material can have an overriding influence on biocrust species composition. These factors may shift the type of biocrust one might expect to find within a given climate. Here, we discuss some of these unique situations.
Fine‐textured soils often support a high cover of lichens and mosses (Anderson, Harper & Holmgren, 1982). Biocrusts on fine‐textured clay‐rich soils, dominated by cyanobacteria and/or lichens can exhibit a cracking or peeling surface (Fig. 7C). Fine soils with a high content of shrink‐swell clays often form highly eroded badlands in semi‐arid and drier regions, which tend to support far fewer species and much less biocrust cover than other soils in an area (Bowker et al., 2006; Bowker & Belnap, 2008). On the other hand, in clay badlands in sub‐humid climates, biocrusts may fill niches too stressful for seed plants (Loppi, Boscagli & Dominicis, 2004).
Gypsiferous soils, including gypsiferous badlands (e.g. the Tabernas Desert), are well known to support a high diversity of mosses and lichens relative to other soils in a given area, including species found only on gypsum (Lazaro et al., 2008; Bowker et al., 2017). Especially in semi‐arid regions, a very high cover of crustose, squamulose, and foliose lichens, with a rugose to pinnacled morphology, can be found (Fig. 7D).
Special parent materials such as grussy granites (granitoids that weather granularly into angular gravels) may lead to an unusually high preponderance of hypolithic and lichen/bryophyte biocrusts (Pietrasiak, Johansen & Drenovsky, 2011). This likely occurs because the relatively small translucent quartz crystals have a high dust‐trapping capacity. In the high antarctic climate of the transantarctic mountain range there are snow‐free areas, where true soils are not developed. The upper ground layer consists of grussy granites harbouring chlorolichen biocrusts with a surface structure somewhat smoother than the non‐colonized grussy granites (Colesie et al., 2014a).
Depending on their origin, mine tailings can vary widely in texture, nutrient availability and chemistry. The sum of these factors often influence the taxonomic biocrust composition, whereas climatic factors are of minor relevance (Sun et al., 2004; Purvis & Pawlik‐Skowrońska, 2008).
Naturally unstable surfaces, such as sand dunes, also can support biocrusts, regardless of the climatic conditions, and the degree of biocrust development is often dependent on the stability of the dune (Corbin & Thiet, 2020). Semi‐fixed dunes can support a relatively high cover of mosses and lichens (e.g. in the Gurbantunngut Desert, China, and in the temperate USA), whereas more mobile dunes support, at most, only a low cover of pioneers like cyanobacteria or algae. Inter‐dunal areas are often stable and generally support whatever biocrust type is allowed by the climate zone, assuming the area is left uncovered by sand for a sufficient time (Hagemann et al., 2017).
(7). Biocrusts in early successional mesic environments
In addition to climatic and edaphic properties of the habitat, disturbance has a strong effect on biocrust growth and the types that may occur (Steven et al., 2015). Disturbance and subsequent succession provide an opportunity for biocrusts to develop in high‐precipitation areas that would normally not host these communities due to high cover of vascular plants and plant litter (Büdel et al., 2014; Corbin & Thiet, 2020). These biocrusts are generally transient in nature, becoming greatly diminished in abundance, or supplanted entirely as they are replaced by vascular plant vegetation over months to several years. If, however, disturbance persists alongside soil conditions stressful to vascular plants (acidity, excessive drainage, infertility, shallow depth), biocrusts can form a long‐lasting feature in temperate environments (Corbin & Thiet, 2020).
Transient biocrusts occur in different climatic and disturbance regimes and on soils of variable texture and nutrient content. Thus, they vary substantially in form and composition among distinct settings. Post‐glacial environments are a notable example, in which cyanobacteria alone or in combination with lichens, liverworts, or mosses can completely cover and alter the new soil surfaces (Reiners et al., 1971; Schmidt et al., 2009; Raggio et al., 2012; Nascimbene et al., 2017; Fig. 7E).
Not all examples are primary successional settings, as more common anthropogenic disturbances can also allow for secondary succession and can facilitate biocrusts to colonize. Examples of such transient biocrusts include those of temperate dry meadows (‘Trockenrasen’), dominated by lichens and some bryophytes, where the soils are shallow, poor in nutrients, and, due to grazing disturbance (or the artificial removal of vegetation), shrubs do not encroach into the meadow (Fig. 7F). Alvar communities are exposed to similar conditions of shallow nutrient‐deprived soils and regular disturbance by grazing, which facilitates dominance by lichens and bryophytes (Fig. 7G; Büdel et al., 2014; Corbin & Thiet, 2020). Another example occurs in African Miombo woodlands, where the residual soils from abandoned termite mounds support high lichen cover (Belnap, Sanford & Lungu, 1996). Tree fall, mechanical vegetation removal, and fire can produce unvegetated soil surfaces that are quickly colonized by moss‐dominated biocrusts. Under these conditions, biocrusts may form a valuable ecosystem component (Seitz et al., 2017). For example, a community of ruderal bryophytes, or ‘fire mosses’ is common on burned soil in the months or years following forest fire (Grover, Bowker & Fule, 2019; Fig. 7H). In some cases, a combination of different disturbances keeps vegetation low and facilitates the growth of biocrusts. In sand plains and pine barrens, a wealth of moss and lichen biocrusts are provided with a niche by combinations of periodic fire, and fossorial mammal and agricultural activity (Corbin & Thiet, 2020). In coastal dune systems, disturbances in the form of coastal storms, sand saltation, and salt spray act as stressors for the vegetation and thus facilitate long‐lasting algal or cyanobacterial biocrust occurrence (Mikhailyuk et al., 2019; Corbin & Thiet, 2020).
V. A UNIVERSAL BIOCRUST DEFINITION
After an extensive review of the literature and discussions with other biocrust researchers, it is clear that the definition put forth by Belnap et al. (2003) applies to the majority of biocrust communities. However, as interest in biocrusts grows and expands, this definition requires refinement to cover all biocrust communities. Therefore, we propose the following refinement of the Belnap et al. (2003) definition: Biological soil crusts (biocrusts) result from an intimate association between soil particles and differing proportions of photoautotrophic (e.g. cyanobacteria, algae, lichens, bryophytes) and heterotrophic (e.g. bacteria, fungi, archaea) organisms, which live within, or immediately on top of, the uppermost millimetres of soil. Soil particles are aggregated through the presence and activity of these often extremotolerant biota that desiccate regularly, and the resultant living crust covers the surface of the ground as a coherent layer.
VI. CONCLUSIONS
We propose a clarified definition of biocrusts that clearly distinguishes them from several other community types (Fig. 2), yet allows for the inclusion of a wide variety of very different assemblages and morphologies. The proposed set of criteria in the decision tree (Fig. 2) helps define the limits to biocrusts and simultaneously opens a broad climatic, edaphic, taxonomic, and functional spectrum for studying biocrust communities; this is a useful advance, given the multiplicity of habitats and regions where biocrusts occur and have important ecological roles. We have not attempted to produce a definitive classification of different biocrust types, but rather provide a description of the wide range of biocrusts found around the world. Others have provided potential schemes that might be adapted globally (Rosentreter & Belnap, 2003; Büdel et al., 2009; Colesie, Felde & Büdel, 2016a; Williams et al., 2017).
Adoption of this refined definition of biocrusts could result in multiple positive outcomes. Much unnecessary confusion arises when scientists apply the same term to different phenomena, or vice‐versa. With the revised biocrust definition we aim to reduce this problem. In particular, we hope that this unified definition will expand the already multi‐disciplinary biocrust research community by drawing in new researchers and land managers, who may not be aware that their focal systems comprise a type of biocrust. We also hope that this review will improve our ability to synthesize biocrust ecology and uncover linkages across studies and study systems. Finally, we hope this review stimulates the use of biocrusts as a model system (Faist et al., 2021) to teach scientific concepts in the classroom, to improve scientific literacy and connection to nature in the general public, and we encourage the incorporation of biocrusts into land‐management decision making.
Supporting information
Fig. S1. Number of studies on biological soil crusts per year, covering the time span from 1989 to 2021.
VII. ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to Otto L. Lange, who openly shared his ideas on biocrusts and worked with us on an earlier version of this review. This work was conducted as part of the “Completing the dryland puzzle: creating a predictive framework for biological soil crust function and response to climate change” Working Group supported by the John Wesley Powell Center for Analysis and Synthesis, funded by the U.S. Geological Survey. V.B.C. is funded by the National Science Foundation (DEB‐1844531). F.T.M. acknowledges support from the European Research Council (ERC Grant Agreement 647038 [BIODESERT]) and Generalitat Valenciana (CIDEGENT/2018/041). K.E.Y. and A.D.‐N. were supported by the National Science Foundation (#1557162 & #1557135). E.R.‐C. was supported by the Ramon y Cajal fellowship (RYC2020‐030762‐I) and the REBIOARID Project (2018‐101921‐B‐I00) founded by FEDER/Ministerio de Ciencia e Inovacion‐Agencia estatal de investigacion, and the BIOCOST project (Conservación debiocostras como estrategia de adaptación al cambio climático: alineando avances científicos con la gestión y sociedad) supported by the Biodiversity Foundation of the Ministry for the Ecological Transition and the Demographic Challenge. S.C.R. was supported by USGS Ecosystems Mission Area. E.H.‐S. acknowledges financial support from CONACYT grant SEP‐CB‐2015‐01‐251388. We are grateful to the Fort Collins, Colorado, Whole Foods salad bar for providing considerable fodder, both figurative and literal, that supported many lengthy discussions leading to this review. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Contributor Information
Bettina Weber, Email: bettina.weber@uni-graz.at.
Matthew A. Bowker, Email: matthew.bowker@nau.edu.
REFERENCES
- Abed, R. M. M. , Tamm, A. , Hassenrück, C. , Al‐Rawahi, A. N. , Rodríguez‐Caballero, E. , Fiedler, S. , Maier, S. & Weber, B. (2019). Habitat‐dependent composition of bacterial and fungal communities in biological soil crusts from Oman. Scientific Reports 9, 6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, D. C. , Harper, K. T. & Holmgren, R. C. (1982). Factors influencing development of cryptogamic soil crusts in Utah deserts. Journal of Range Management 35, 180–185. [Google Scholar]
- Arnalds, O. (2015). The Soils of Iceland. Springer, Heidelberg, Berlin. [Google Scholar]
- Barger, N. N. , Herrick, J. E. , van Zee, J. & Belnap, J. (2006). Impacts of biological soil crust disturbance and composition on C and N loss from water erosion. Biogeochemistry 77, 247–263. [Google Scholar]
- Barger, N. N. , Weber, B. , Garcia‐Pichel, F. , Zaady, E. & Belnap, J. (2016). Patterns and controls on nitrogen cycling of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 257–285. Springer, Cham. [Google Scholar]
- Beaugendre, N. , Malam Issa, O. , Choné, A. , Cerdan, O. , Desprats, J.‐F. , Rajot, J. L. , Sannier, C. & Valentin, C. (2017). Developing a predictive environment‐based model for mapping biological soil crust patterns at the local scale in the Sahel. Catena 158, 250–265. [Google Scholar]
- Belnap, J. , Büdel, B. & Lange, O. L. (2003). Biological soil crusts: characteristics and distribution. In Biological Soil Crusts: Structure, Function, and Management (Volume 150, eds Belnap J. and Lange O. L.), pp. 3–30. Springer, Berlin and Heidelberg. [Google Scholar]
- Belnap, J. & Lange, O. L. (2003). Biological Soil Crusts: Structure, Function, and Management. Springer, Heidelberg, Berlin. [Google Scholar]
- Belnap, J. , Munson, S. M. & Field, J. P. (2011). Aeolian and fluvial processes in dryland regions: the need for integrated studies. Ecohydrology 4, 615–622. [Google Scholar]
- Belnap, J. , Sanford, R. L. & Lungu, L. (1996). Biological soil crusts: ecological roles and response to fire in Miombo woodlands of Zimbabwe. Transactions of the Zimbabwe Scientific Association 70, 14–20. [Google Scholar]
- Belnap, J. , Walker, B. J. , Munson, S. M. & Gill, R. A. (2014). Controls on sediment production in two US deserts. Aeolian Research 14, 15–24. [Google Scholar]
- Belnap, J. , Weber, B. & Büdel, B. (2016). Biological soil crusts as an organizing principle in drylands. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 3–13. Springer, Cham. [Google Scholar]
- Benavent‐González, A. , Delgado‐Baquerizo, M. , Fernández‐Brun, L. , Singh, B. K. , Maestre, F. T. & Sancho, L. G. (2018). Identity of plant, lichen and moss species connects with microbial abundance and soil functioning in Maritime Antarctica. Plant and Soil 429, 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraldi‐Campesi, H. , Hartnett, H. E. , Anbar, A. , Gordon, G. W. & Garcia‐Pichel, F. (2009). Effect of biological soil crusts on soil elemental concentrations: implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 7, 348–359. [DOI] [PubMed] [Google Scholar]
- Bliss, L. C. & Gold, W. G. (1999). Vascular plant reproduction, establishment, and growth and the effects of cryptogamic crusts within a polar desert ecosystem, Devon Island, NWT, Canada. Canadian Journal of Botany‐Revue Canadienne De Botanique 77, 623–636. [Google Scholar]
- Bolhuis, H. , Cretoiu, M. S. & Stal, L. J. (2014). Molecular ecology of microbial mats. FEMS Microbiology Ecology 90, 335–350. [DOI] [PubMed] [Google Scholar]
- Bowker, M. A. & Belnap, J. (2008). A simple classification of soil types as habitats of biological soil crusts on the Colorado Plateau, USA. Journal of Vegetation Science 19, 831–840. [Google Scholar]
- Bowker, M. A. , Belnap, J. , Büdel, B. , Sannier, C. , Pietrasiak, N. , Eldridge, D. J. & Rivera‐Aguilar, V. (2016). Controls on distribution patterns of biological soil crusts at micro‐ to global scales. In Biological Soil Crusts: An Organizing Principle in Drylands, Ecological Studies (eds Weber B., Büdel B. and Belnap J.), pp. 173–197. Springer International Publishing, Cham. [Google Scholar]
- Bowker, M. A. , Belnap, J. , Chaudhary, V. B. & Johnson, N. C. (2008). Revisiting classic water erosion models in drylands: the strong impact of biological soil crusts. Soil Biology & Biochemistry 40, 2309–2316. [Google Scholar]
- Bowker, M. A. , Belnap, J. , Davidson, D. W. & Harland, G. (2006). Correlates of biological soil crust abundance across a continuum of spatial scales: support for a hierarchical conceptual model. Journal of Applied Ecology 43, 152–163. [Google Scholar]
- Bowker, M. A. , Büdel, B. , Maestre, F. T. , Antoninka, A. J. & Eldridge, D. J. (2017). Bryophyte and lichen diversity on arid soils: determinants and consequences. In The Biology of Arid Soils (ed. Steven B.), pp. 73–96. De Gruyter, Berlin. [Google Scholar]
- Bowker, M. A. , Eldridge, D. J. , Val, J. & Soliveres, S. (2013). Hydrology in a patterned landscape is co‐engineered by soil‐disturbing animals and biological crusts. Soil Biology & Biochemistry 61, 14–22. [Google Scholar]
- Bu, C. F. , Wu, S. F. & Yang, K. B. (2014). Effects of physical soil crusts on infiltration and splash erosion in three typical Chinese soils. International Journal of Sediment Research 29, 491–500. [Google Scholar]
- Büdel, B. & Colesie, C. (2014). Biological soil crusts. In Antarctic Terrestrial Microbiology (ed. Cowan D. A.), pp. 131–161. Springer, Berlin, Heidelberg. [Google Scholar]
- Büdel, B. , Colesie, C. , Green, T. G. A. , Grube, M. , Suau, R. L. , Loewen‐Schneider, K. , Maier, S. , Peer, T. , Pintado, A. , Raggio, J. , Ruprecht, U. , Sancho, L. , Schroeter, B. , Turk, R. , Weber, B. , et al. (2014). Improved appreciation of the functioning and importance of biological soil crusts in Europe: the Soil Crust International Project (SCIN). Biodiversity and Conservation 23, 1639–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdel, B. , Darienko, T. , Deutschewitz, K. , Dojani, S. , Friedl, T. , Mohr, K. , Salisch, M. , Reisser, W. & Weber, B. (2009). Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microbial Ecology 57, 229–247. [DOI] [PubMed] [Google Scholar]
- Büdel, B. & Schultz, M. (2003). A way to cope with high irradiance and drought: inverted morphology of a new cyanobacterial lichen, Peltula inversa sp. nov., from the Nama Karoo, Namibia. Bibliotheca Lichenologica 86, 225–232. [Google Scholar]
- Büdel, B. , Williams, W. J. & Reichenberger, H. (2018). Annual net primary productivity of a cyanobacteria‐dominated biological soil crust in the Gulf Savannah, Queensland, Australia. Biogeosciences 15, 491–505. [Google Scholar]
- Cameron, R. E. (1978). The perplexity of desert preservation in a threatening world. In Earthcare: Global Protection of Natural Areas (ed. Schofield E. A.), pp. 411–443. Westview Press, Boulder. [Google Scholar]
- Chamizo, S. , Belnap, J. , Eldridge, D. J. , Cantón, Y. & Issa, O. M. (2016). The role of biocrusts in arid land hydrology. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 321–346. Springer, Cham. [Google Scholar]
- Chamizo, S. , Cantón, Y. , Miralles, I. & Domingo, F. (2012). Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biology & Biochemistry 49, 96–105. [Google Scholar]
- Chaudhary, V. B. , Bowker, M. A. , O'Dell, T. E. , Grace, J. B. , Redman, A. E. , Rillig, M. C. & Johnson, N. C. (2009). Untangling the biological contributions to soil stability in semiarid shrublands. Ecological Applications 19, 110–122. [DOI] [PubMed] [Google Scholar]
- Chen, R. Y. , Zhang, Y. M. , Li, Y. , Wei, W. S. , Zhang, J. & Wu, N. (2009). The variation of morphological features and mineralogical components of biological soil crusts in the Gurbantunggut Desert of Northwestern China. Environmental Geology 57, 1135–1143. [Google Scholar]
- Colesie, C. , Felde, V. J. M. N. L. & Büdel, B. (2016a). Composition and macrostructure of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 159–172. Springer, Cham. [Google Scholar]
- Colesie, C. , Gommeaux, M. , Green, T. G. A. & Büdel, B. (2014a). Biological soil crusts in continental Antarctica: Garwood Valley, southern Victoria Land, and Diamond Hill, Darwin Mountains region. Antarctic Science 26, 115–123. [Google Scholar]
- Colesie, C. , Green, T. G. A. , Raggio, J. & Büdel, B. (2016b). Summer activity patterns of Antarctic and high alpine lichen‐dominated biological soil crusts‐Similar but different? Arctic Antarctic and Alpine Research 48, 449–460. [Google Scholar]
- Colesie, C. , Green, T. G. A. , Türk, R. , Hogg, I. D. , Sancho, L. G. & Büdel, B. (2014b). Terrestrial biodiversity along the Ross Sea coastline, Antarctica: lack of a latitudinal gradient and potential limits of bioclimatic modeling. Polar Biology 37, 1197–1208. [Google Scholar]
- Corbin, J. D. & Thiet, R. K. (2020). Temperate biocrusts: mesic counterparts to their better‐known dryland cousins. Frontiers in Ecology and the Environment 18, 456–464. [Google Scholar]
- Deane‐Coe, K. K. & Stanton, D. (2017). Functional ecology of cryptogams: scaling from bryophyte, lichen, and soil crust traits to ecosystem processes. New Phytologist 213, 993–995. [DOI] [PubMed] [Google Scholar]
- Ding, J. & Eldridge, D. J. (2020). Biotic and abiotic effects on biocrust cover vary with microsite along an extensive aridity gradient. Plant and Soil 450, 429–441. [Google Scholar]
- Drahorad, S. L. & Felix‐Henningsen, P. (2013). Application of an electronic micropenetrometer to assess mechanical stability of biological soil crusts. Journal of Plant Nutrition and Soil Science 176, 904–909. [Google Scholar]
- Dumack, K. , Koller, R. , Weber, B. & Bonkowski, M. (2016). Estimated abundance and diversity of heterotrophic protists in South African biocrusts. South African Journal of Science 112, 56–60. [Google Scholar]
- Ekwealor, J. T. B. & Fisher, K. M. (2020). Life under quartz: hypolithic mosses in the Mojave Desert. PLoS One 15(7), e0235928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert, W. , Weber, B. , Burrows, S. , Steinkamp, J. , Büdel, B. , Andreae, M. O. & Pöschl, U. (2012). Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience 5, 459–462. [Google Scholar]
- Eldridge, D. J. & Kinnell, P. I. A. (1997). Assessment of erosion rates from microphyte‐dominated calcareous soils under rain‐impacted flow. Soil Research 35, 475–489. [Google Scholar]
- Eldridge, D. J. & Leys, J. F. (1999). Wind dispersal of the vagant lichen Chondropsis semiviridis in Semi‐arid Eastern Australia. Australian Journal of Botany 47, 157–164. [Google Scholar]
- Eldridge, D. J. & Leys, J. F. (2003). Exploring some relationships between biological soil crusts, soil aggregation and wind erosion. Journal of Arid Environments 53, 457–466. [Google Scholar]
- Eldridge, D. J. , Reed, S. , Travers, S. K. , Bowker, M. A. , Maestre, F. T. , Ding, J. Y. , Havrilla, C. , Rodriguez‐Caballero, E. , Barger, N. , Weber, B. , Antoninka, A. , Belnap, J. , Chaudhary, B. , Faist, A. , Ferrenberg, S. , et al. (2020). The pervasive and multifaceted influence of biocrusts on water in the world's drylands. Global Change Biology 26, 6003–6014. [DOI] [PubMed] [Google Scholar]
- Faist, A. M. , Antoninka, A. J. , Barger, N. N. , Bowker, M. A. , Chaudhary, V. B. , Havrilla, C. A. , Huber‐Sannwald, E. , Reed, S. C. & Weber, B. (2021). Broader impacts for ecologists: biological soil crust as a model system for education. Frontiers in Microbiology 11, 577922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faist, A. M. , Herrick, J. E. , Belnap, J. , van Zee, J. W. & Barger, N. N. (2017). Biological soil crust and disturbance controls on surface hydrology in a semi‐arid ecosystem. Ecosphere 8, e01691. [Google Scholar]
- Fang, H. Y. , Cai, Q. G. , Chen, H. & Li, Q. Y. (2007). Mechanism of formation of physical soil crust in desert soils treated with straw checkerboards. Soil & Tillage Research 93, 222–230. [Google Scholar]
- Felde, V. J. M. N. L. , Peth, S. , Uteau‐Puschmann, D. , Drahorad, S. & Felix‐Henningsen, P. (2014). Soil microstructure as an under‐explored feature of biological soil crust hydrological properties: case study from the NW Negev Desert. Biodiversity and Conservation 23, 1687–1708. [Google Scholar]
- Ferrenberg, S. , Faist, A. M. , Howell, A. & Reed, S. C. (2018). Biocrusts enhance soil fertility and Bromus tectorum growth, and interact with warming to influence germination. Plant and Soil 429, 77–90. [Google Scholar]
- Ferrenberg, S. , Reed, S. C. & Belnap, J. (2015). Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proceedings of the National Academy of Sciences of the United States of America 112, 12116–12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrenberg, S. , Tucker, C. L. & Reed, S. C. (2017). Biological soil crusts: diminutive communities of potential global importance. Frontiers in Ecology and the Environment 15, 160–167. [Google Scholar]
- Fletcher, J. E. & Martin, W. P. (1948). Some effects of algae and molds in the rain‐crust of desert soils. Ecology 29, 95–100. [Google Scholar]
- Friedmann, E. I. & Galun, M. (1974). Desert algae, lichens, and fungi. In Desert Biology (ed. Brown G. W. Jr.), pp. 165–212. Academic Press, New York. [Google Scholar]
- Friedmann, I. , Lipkin, Y. & Ocampo‐Paus, R. (1967). Desert algae of the Negev. Phycologia 6, 185–195. [Google Scholar]
- Fritsch, F. E. (1922). The terrestrial alga. Journal of Ecology 10, 220–236. [Google Scholar]
- Gao, L. , Bowker, M. A. , Xu, M. , Sun, H. , Tuo, D. & Zhao, Y. (2017). Biological soil crusts decrease erodibility by modifying inherent soil properties on the Loess Plateau, China. Soil Biology & Biochemistry 105, 49–58. [Google Scholar]
- Garcia‐Pichel, F. , Felde, V. J. M. N. L. , Drahorad, S. L. & Weber, B. (2016). Microstructure and weathering processes within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 237–255. Springer, Cham. [Google Scholar]
- Gold, W. G. & Bliss, L. C. (1995). Water limitations and plant community‐development in a polar desert. Ecology 76, 1558–1568. [Google Scholar]
- Green, T. G. A. & Proctor, M. C. F. (2016). Physiology of photosynthetic organisms within biological soil crusts: their adaptation, flexibility, and plasticity. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 347–381. Springer, Cham. [Google Scholar]
- Gretarsdottir, J. , Aradottir, A. L. , Vandvik, V. , Heegaard, E. & Birks, H. J. B. (2004). Long‐term effects of reclamation treatments on plant succession in Iceland. Restoration Ecology 12, 268–278. [Google Scholar]
- Griffiths, R. P. , Castellano, M. A. & Caldwell, B. A. (1991). Hyphal mats formed by two ectomycorrhizal fungi and their association with Douglas‐fir seedlings: a case study. Plant and Soil 134, 255–259. [Google Scholar]
- Grover, H. S. , Bowker, M. A. & Fule, P. Z. (2019). Improved, scalable techniques to cultivate fire mosses for rehabilitation. Restoration Ecology 28(S2), S17–S24. [Google Scholar]
- Hagemann, M. , Henneberg, M. , Felde, V. J. M. N. L. , Berkowicz, S. M. , Raanan, H. , Pade, N. , Felix‐Henningsen, P. & Kaplan, A. (2017). Cyanobacterial populations in biological soil crusts of the northwest Negev Desert, Israel‐effects of local conditions and disturbance. Fems Microbiology Ecology 93, fiw228. [DOI] [PubMed] [Google Scholar]
- Havrilla, C. A. , Chaudhary, V. B. , Ferrenberg, S. , Antoninka, A. J. , Belnap, J. , Bowker, M. A. , Eldridge, D. J. , Faist, A. M. , Huber‐Sannwald, E. , Leslie, A. D. , Rodriguez‐Caballero, E. , Zhang, Y. M. & Barger, N. N. (2019). Towards a predictive framework for biocrust mediation of plant performance: a meta‐analysis. Journal of Ecology 107, 2789–2807. [Google Scholar]
- Hogg, I. D. , Sancho, L. G. , Türk, R. , Cowan, D. A. & Green, T. G. A. (2018). “Lichens in High Arctic ecosystems: recommended research directions for assessing diversity and function near the Canadian High Arctic Research Station, Cambridge Bay, Nunavut.” Polar Knowledge: Aqhaliat 2018, Polar Knowledge Canada, pp. 1–8.
- Hu, C. X. , Liu, Y. D. , Song, L. R. & Zhang, D. K. (2002). Effect of desert soil algae on the stabilization of fine sands. Journal of Applied Phycology 14, 281–292. [Google Scholar]
- Jiang, D. S. , Fan, X. K. , Li, X. H. & Zhao, H. L. (1995). Study on horizontal and vertical regulation of soil anti‐scourability in area with serious soil erosion on Loess Plateau. Chinese Journal of Soil and Water Conservation 1995, 1–8. [Google Scholar]
- Jiang, Z.‐Y. , Li, X.‐Y. , Wei, J.‐Q. , Chen, H.‐Y. , Li, Z.‐C. , Liu, L. & Hu, X. (2018). Contrasting surface soil hydrology regulated by biological and physical soil crusts for patchy grass in the high‐altitude alpine steppe ecosystem. Geoderma 326, 201–209. [Google Scholar]
- Jimenez Aguilar, A. , Huber‐Sannwald, E. , Belnap, J. , Smart, D. R. & Arredondo Moreno, J. T. (2009). Biological soil crusts exhibit a dynamic response to seasonal rain and release from grazing with implications for soil stability. Journal of Arid Environments 73, 1158–1169. [Google Scholar]
- Jung, P. , Baumann, K. , Lehnert, L. W. , Samolov, E. , Achilles, S. , Schermer, M. , Wraase, L. M. , Eckhardt, K. U. , Bader, M. Y. , Leinweber, P. , Karsten, U. , Bendix, J. & Büdel, B. (2020). Desert breath‐How fog promotes a novel type of soil biocenosis, forming the coastal Atacama Desert's living skin. Geobiology 18(1), 113–124. [DOI] [PubMed] [Google Scholar]
- Kakeh, J. , Gorji, M. , Mohammadi, M. H. , Asadi, H. , Khormali, F. , Sohrabi, M. & Eldridge, D. J. (2021). Biocrust islands enhance infiltration, and reduce runoff and sediment yield on a heavily salinized dryland soil. Geoderma 404, 115329. [Google Scholar]
- Karnieli, A. , Kokaly, R. F. , West, N. E. & Clark, R. N. (2003). Remote sensing of biological soil crusts. In Biological Soil Crusts: Structure, Function, and Management (Volume 150, eds Belnap J. and Lange O. L.), pp. 431–455. Springer, Berlin and Heidelberg. [Google Scholar]
- Karsten, U. , Herburger, K. & Holzinger, A. (2016). Living in biological soil crust communities of African deserts – physiological traits of green algal Klebsormidium species (Streptophyta) to cope with desiccation, light and temperature gradients. Journal of Plant Physiology 194, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten, U. & Holzinger, A. (2014). Green algae in alpine biological soil crust communities: acclimation strategies against ultraviolet radiation and dehydration. Biodiversity and Conservation 23, 1845–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidron, G. J. & Büdel, B. (2014). Contrasting hydrological response of coastal and desert biocrusts. Hydrological Processes 28, 361–371. [Google Scholar]
- Kleiner, E. F. & Harper, K. T. (1972). Environment and community organization in grasslands of Canyonlands National Park. Ecology 53, 299–309. [Google Scholar]
- Lange, O. L. & Belnap, J. (2016). How biological soil crusts became recognized as a functional unit: a selective history. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 15–33. Springer, Cham. [Google Scholar]
- Lange, O. L. , Belnap, J. & Reichenberger, H. (1998). Photosynthesis of the Cyanobacterial soil‐crust lichen Collema tenax from arid lands in southern Utah, USA: role of water content on light and temperature responses of CO(2) exchange. Functional Ecology 12, 195–202. [Google Scholar]
- Lange, O. L. & Green, T. G. A. (2004). Photosynthetic performance of the squamulose‐soil crust lichen Squamarina lentigera: laboratory measurements and long term monitoring of CO2 exchange in the field. Bibliotheca Lichenologica 88, 363–390. [Google Scholar]
- Lazaro, R. , Canton, Y. , Sole‐Benet, A. , Bevan, J. , Alexander, R. , Sancho, L. G. & Puigdefabregas, J. (2008). The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the Tabernas badlands (SE Spain) and its landscape effects. Geomorphology 102, 252–266. [Google Scholar]
- Leys, J. F. & Eldridge, D. J. (1998). Influence of cryptogamic crust disturbance to wind erosion on sand and loam rangeland soils. Earth Surface Processes and Landforms 23, 963–974. [Google Scholar]
- Liu, Y. , Li, X. , Jia, R. , Huang, L. , Zhou, Y. & Gao, Y. (2011). Effects of biological soil crusts on soil nematode communities following dune stabilization in the Tengger Desert, Northern China. Applied Soil Ecology 49, 118–124. [Google Scholar]
- Lopez‐Rodriguez, M. D. , Chamizo, S. , Canton, Y. & Rodriguez‐Caballero, E. (2020). Identifying social‐ecological gaps to promote biocrust conservation action. Web Ecology 20, 117–132. [Google Scholar]
- Loppi, S. , Boscagli, A. & Dominicis, V. d. (2004). Ecology of soil lichens from Pliocene clay badlands of central Italy in relation to geomorphology and vascular vegetation. Catena 55, 1–15. [Google Scholar]
- Maestre, F. T. , Bowker, M. A. , Canton, Y. , Castillo‐Monroy, A. P. , Cortina, J. , Escolar, C. , Escudero, A. , Lazaro, R. & Martinez, I. (2011). Ecology and functional roles of biological soil crusts in semi‐arid ecosystems of Spain. Journal of Arid Environments 75, 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre, F. T. , Escolar, C. , Guevara, M. L. d. , Quero, J. L. , Lazaro, R. , Delgado‐Baquerizo, M. , Ochoa, V. , Berdugo, M. , Gozalo, B. & Gallardo, A. (2013). Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Global Change Biology 19, 3835–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager, D. M. & Thomas, A. D. (2011). Extracellular polysaccharides from cyanobacterial soil crusts: a review of their role in dryland soil processes. Journal of Arid Environments 75, 91–97. [Google Scholar]
- Maier, S. , Kratz, A. M. , Weber, J. , Prass, M. , Liu, F. , Clark, A. T. , Abed, R. M. M. , Su, H. , Cheng, Y. , Eickhorst, T. , Fiedler, S. , Pöschl, U. & Weber, B. (2022). Water‐driven microbial nitrogen transformations in biological soil crusts causing atmospheric nitrous acid and nitric oxide emissions. The ISME Journal 16, 1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, S. , Tamm, A. , Wu, D. , Caesar, J. , Grube, M. & Weber, B. (2018). Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. The ISME Journal 12, 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malam Issa, O. , Defarge, C. , Trichet, J. , Valentin, C. & Rajot, J. L. (2009). Microbiotic soil crusts in the Sahel of Western Niger and their influence on soil porosity and water dynamics. Catena 77, 48–55. [Google Scholar]
- Malam Issa, O. , Le Bissonnais, Y. , Défarge, C. & Trichet, J. (2001). Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 101, 15–30. [Google Scholar]
- Malam Issa, O. , Trichet, J. , Défarge, C. , Couté, A. & Valentin, C. (1999). Morphology and microstructure of microbiotic soil crusts on a tiger bush sequence (Niger, Sahel). Catena 37, 175–196. [Google Scholar]
- Malam Issa, O. , Valentin, C. , Rajot, J. L. , Cerdan, O. , Desprats, J. F. & Bouchet, T. (2011). Runoff generation fostered by physical and biological crusts in semi‐arid sandy soils. Geoderma 167–168, 22–29. [Google Scholar]
- Mazor, G. , Kidron, G. J. , Vonshak, A. & Abeliovich, A. (1996). The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiology Ecology 21, 121–130. [Google Scholar]
- Mikhailyuk, T. , Glaser, K. , Tsarenko, P. , Demchenko, E. & Karsten, U. (2019). Composition of biological soil crusts from sand dunes of the Baltic Sea coast in the context of an integrative approach to the taxonomy of microalgae and cyanobacteria. European Journal of Phycology 54, 263–290. [Google Scholar]
- Munoz‐Martin, M. A. , Becerra‐Absalon, I. , Perona, E. , Fernandez‐Valbuena, L. , Garcia‐Pichel, F. & Mateo, P. (2019). Cyanobacterial biocrust diversity in Mediterranean ecosystems along a latitudinal and climatic gradient. New Phytologist 221, 123–141. [DOI] [PubMed] [Google Scholar]
- Nascimbene, J. , Mayrhofer, H. , Dainese, M. & Bilowitz, P. O. (2017). Assembly patterns of soil‐dwelling lichens after glacier retreat in the European Alps. Journal of Biogeography 44, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, D. A. , Lewins, S. A. , Weicht, T. R. & Darby, B. J. (2009). Microarthropod communities associated with biological soil crusts in the Colorado Plateau and Chihuahuan deserts. Journal of Arid Environments 73, 672–677. [Google Scholar]
- Neuman, C. M. & Maxwell, C. (2002). Temporal aspects of the abrasion of microphytic crusts under grain impact. Earth Surface Processes and Landforms 27, 891–908. [Google Scholar]
- Pagliai, M. & Stoops, G. (2010). Physical and biological surface crusts and seals. In Interpretation of Micromorphological Features of Soils and Regoliths (eds Stoops G., Marcelino V. and Mees F.), pp. 419–440. Amsterdam: Elsevier. [Google Scholar]
- Palmer, B. , Hernandez, R. & Lipson, D. (2020). The fate of biological soil crusts after fire: a meta‐analysis. Global Ecology and Conservation 24, e01380. [Google Scholar]
- Perez, F. L. (1991). Ecology and morphology of globular mosses of Grimmia longirostris in the Paramo De Piedras Blancas, Venezuelan Andes. Arctic and Alpine Research 23, 133–148. [Google Scholar]
- Perez, F. L. (1997). Microbiotic crusts in the high equatorial Andes, and their influence on paramo soils. Catena 31, 173–198. [Google Scholar]
- Pérez, F. L. (2021). Cryptogams build up a living microcosm: geoecological effects of biocrusts on volcanic tephra (Haleakalā, Maui, Hawai'i). Catena 203, 105320. [Google Scholar]
- Pietrasiak, N. , Johansen, J. R. & Drenovsky, R. E. (2011). Geologic composition influences distribution of microbiotic crusts in the Mojave and Colorado Deserts at the regional scale. Soil Biology & Biochemistry 43, 967–974. [Google Scholar]
- Pointing, S. B. (2016). Hypolithic communities. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 199–213. Springer, Cham. [Google Scholar]
- Pombubpa, N. , Pietrasiak, N. , Ley, P. d. & Stajich, J. E. (2020). Insights into dryland biocrust microbiome: geography, soil depth and crust type affect biocrust microbial communities and networks in Mojave Desert, USA. FEMS Microbiology Ecology 96, fiaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto‐Barajas, C. M. , Valencia‐Cantero, E. & Santoyo, G. (2018). Microbial mat ecosystems: structure types, functional diversity, and biotechnological application. Electronic Journal of Biotechnology 31, 48–56. [Google Scholar]
- Purvis, O. W. & Pawlik‐Skowrońska, B. (2008). Chapter 12 lichens and metals. In British Mycological Society Symposia Series (eds Avery S. V., Stratford M. and van West P.), pp. 175–200. Academic Press, London. [Google Scholar]
- Raggio, J. , Pintado, A. , Vivas, M. , Sancho, L. G. , Budel, B. , Colesie, C. , Weber, B. , Schroeter, B. , Lazaro, R. & Green, T. G. A. (2014). Continuous chlorophyll fluorescence, gas exchange and microclimate monitoring in a natural soil crust habitat in Tabernas badlands, Almeria, Spain: progressing towards a model to understand productivity. Biodiversity and Conservation 23, 1809–1826. [Google Scholar]
- Raggio, J. , Green, T. G. A. , Crittenden, P. D. , Pintado, A. , Vivas, M. , Pérez‐Ortega, S. , de los Rios, A. & Sancho, L. G. (2012). Comparative ecophysiology of three Placopsis species, pioneer lichens in recently exposed Chilean glacial forelands. Symbiosis 56, 55–66. [Google Scholar]
- Reed, S. C. , Coe, K. K. , Sparks, J. P. , Housman, D. C. , Zelikova, T. J. & Belnap, J. (2012). Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nature Climate Change 2, 752–755. [Google Scholar]
- Reiners, W. A. , Worley, I. A. & Lawrence, D. B. (1971). Plant diversity in a chronosequence at Glacier‐Bay, Alaska. Ecology 52, 55–69. [Google Scholar]
- Rodriguez‐Caballero, E. , Belnap, J. , Büdel, B. , Crutzen, P. J. , Andreae, M. O. , Pöschl, U. & Weber, B. (2018a). Dryland photoautotrophic soil surface communities endangered by global change. Nature Geoscience 11, 185–189. [Google Scholar]
- Rodriguez‐Caballero, E. , Castro, A. J. , Chamizo, S. , Quintas‐Soriano, C. , Garcia‐Llorente, M. , Canton, Y. & Weber, B. (2018b). Ecosystem services provided by biocrusts: from ecosystem functions to social values. Journal of Arid Environments 159, 45–53. [Google Scholar]
- Rogers, R. W. (1972). Soil surface lichens in arid and subarid south‐eastern Australia III. Australian Journal of Botany 20, 301–316. [Google Scholar]
- Romero, A. L. N. , Moratta, M. A. H. , Carretero, E. M. , Rodriguez, R. A. & Vento, B. (2020). Spatial distribution of biological soil crusts along an aridity gradient in the central‐west of Argentina. Journal of Arid Environments 176, 104099. [Google Scholar]
- Rosentreter, R. (1993). Vagrant lichens in North‐America. Bryologist 96, 333–338. [Google Scholar]
- Rosentreter, R. & Belnap, J. (2003). Biological soil crusts of North America. In Biological Soil Crusts: Structure, Function, and Management (Volume 150, eds Belnap J. and Lange O. L.), pp. 31–50. Springer, Berlin and Heidelberg. [Google Scholar]
- Rosentreter, R. , Bowker, M. A. & Belnap, J. (2007). A Field Guide to Biological Soil Crusts of Western U.S. Drylands. U.S. Government Printing Office, Denver. [Google Scholar]
- Rossi, F. , Mugnai, G. & Philippis, R. d. (2018). Complex role of the polymeric matrix in biological soil crusts. Plant and Soil 429, 19–34. [Google Scholar]
- Samolov, E. , Mikhailyuk, T. , Lukešová, A. , Glaser, K. , Büdel, B. & Karsten, U. (2019). Usual alga from unusual habitats: biodiversity of Klebsormidium (Klebsormidiophyceae, Streptophyta) from the phylogenetic superclade G isolated from biological soil crusts. Molecular Phylogenetics and Evolution 133, 236–255. [DOI] [PubMed] [Google Scholar]
- Sancho, L. G. , Belnap, J. , Colesie, C. , Raggio, J. & Weber, B. (2016). Carbon budgets of biological soil crusts at micro‐, meso‐, and global scales. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 287–304. Springer, Cham. [Google Scholar]
- Schieferstein, B. & Loris, K. (1992). Ecological investigations on lichen fields of the Central Namib. 1. Distribution patterns and habitat conditions. Vegetatio 98, 113–128. [Google Scholar]
- Schmidt, S. K. , Nemergut, D. R. , Miller, A. E. , Freeman, K. R. , King, A. J. & Seimon, A. (2009). Microbial activity and diversity during extreme freeze‐thaw cycles in periglacial soils, 5400 m elevation, Cordillera Vilcanota, PerA(0). Extremophiles 13, 807–816. [DOI] [PubMed] [Google Scholar]
- Schulz, E. (1937). Das Farbstreifen‐Sandwatt und seine Fauna, eine ökologische‐biozönotische Untersuchung an der Nordsee. Kieler Meeresforschung 1, 359–378. [Google Scholar]
- Seitz, S. , Nebel, M. , Goebes, P. , Käppeler, K. , Schmidt, K. , Song, Z. , Webber, C. L. , Weber, B. & Scholten, T. (2017). Bryophyte‐dominated biological soil crusts mitigate soil erosion in an early successional Chinese subtropical forest. Biogeosciences 14, 5775–5788. [Google Scholar]
- Shields, L. M. , Mitchell, C. & Drouet, F. (1957). Alga‐stabilized and lichen‐stabilized surface crusts as soil nitrogen sources. American Journal of Botany 44, 489–498. [Google Scholar]
- Siebert, F. & Dreber, N. (2019). Forb ecology research in dry African savannas: knowledge, gaps, and future perspectives. Ecology and Evolution 9, 7875–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule, T. , Palmer, K. , Gao, Q. , Potrafka, R. M. , Stout, V. & Garcia‐Pichel, F. (2009). A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genomics 10, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal, L. J. (ed.) (1994). Microbial Mats: Structure, Development and Environmental Significance. Springer, Berlin, Heidelberg. [Google Scholar]
- Steven, B. , Kuske, C. R. , La Gallegos‐Graves, V. , Reed, S. C. & Belnap, J. (2015). Climate change and physical disturbance manipulations result in distinct biological soil crust communities. Applied and Environmental Microbiology 81, 7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, K. J. , Lamb, E. G. , Coxson, D. S. & Siciliano, S. D. (2011). Bryophyte‐cyanobacterial associations as a key factor in N‐2‐fixation across the Canadian Arctic. Plant and Soil 344, 335–346. [Google Scholar]
- Su, Y. G. , Wu, L. & Zhang, Y. M. (2012). Characteristics of carbon flux in two biologically crusted soils in the Gurbantunggut Desert, Northwestern China. Catena 96, 41–48. [Google Scholar]
- Sun, Q. Y. , An, S. Q. , Yang, L. Z. & Wang, Z. S. (2004). Chemical properties of the upper tailings beneath biotic crusts. Ecological Engineering 23, 47–53. [Google Scholar]
- Szyja, M. , Souza Menezes, A. G. , Oliveira, F. D. A. , Leal, I. , Tabarelli, M. , Büdel, B. & Wirth, R. (2019). Neglected but potent dry forest players: ecological role and ecosystem service provision of biological soil crusts in the human‐modified Caatinga. Frontiers in Ecology and Evolution 7, 482. [Google Scholar]
- UNEP‐WCMC (2007). A spatial analysis approach to the global delineation of dryland areas of relevance to the CBD Programme of Work on Dry and Subhumid Lands. Dataset based on spatial analysis between WWF terrestrial ecoregions (WWF‐US, 2004) and aridity zones (CRU/UEA; UNEPGRID, 1991). Dataset checked and refined to remove many gaps, overlaps and slivers (July 2014).
- Valentin, C. & Bresson, L.‐M. (1992). Morphology, genesis and classification of surface crusts in loamy and sandy soils. Geoderma 55, 225–245. [Google Scholar]
- Veluci, R. M. , Neher, D. A. & Weicht, T. R. (2006). Nitrogen fixation and leaching of biological soil crust communities in mesic temperate soils. Microbial Ecology 51, 189–196. [DOI] [PubMed] [Google Scholar]
- Warren‐Rhodes, K. A. , Rhodes, K. L. , Pointing, S. B. , Ewing, S. A. , Lacap, D. C. , Gomez‐Silva, B. , Amundson, R. , Friedmann, E. I. & McKay, C. P. (2006). Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microbial Ecology 52, 389–398. [DOI] [PubMed] [Google Scholar]
- Weber, B. , Belnap, J. & Büdel, B. (2016a). Synthesis on biological soil crust research. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 527–534. Springer, Cham. [Google Scholar]
- Weber, B. , Büdel, B. & Belnap, J. (2016b). Biological Soil Crusts: An Organizing Principle in Drylands. Springer, Heidelberg, Berlin. [Google Scholar]
- Weber, B. , Wessels, D. C. J. , Deutschewitz, K. , Dojani, S. , Reichenberger, H. & Büdel, B. (2013). Ecological characterization of soil‐inhabiting and hypolithic soil crusts within the Knersvlakte, South Africa. Ecological Processes 2, 8. [Google Scholar]
- West, N. E. (1990). Structure and function of microphytic soil crusts in wildland ecosystems of arid to semi‐arid ecosystems. Advances in Ecological Research 20, 179–223. [Google Scholar]
- Williams, A. J. , Buck, B. J. & Beyene, M. A. (2012). Biological soil crusts in the Mojave Desert, USA: micromorphology and pedogenesis. Soil Science Society of America Journal 76, 1685–1695. [Google Scholar]
- Williams, L. , Borchhardt, N. , Colesie, C. , Baum, C. , Komsic‐Buchmann, K. , Rippin, M. , Becker, B. , Karsten, U. & Büdel, B. (2017). Biological soil crusts of Arctic Svalbard and of Livingston Island, Antarctica. Polar Biology 40, 399–411. [Google Scholar]
- Williams, W. , Büdel, B. & Williams, S. (2018). Wet season cyanobacterial N enrichment highly correlated with species richness and Nostoc in the northern Australian savannah. Biogeosciences 15, 2149–2159. [Google Scholar]
- Zhang, J. , Zhang, Y.‐M. , Downing, A. , Cheng, J.‐H. , Zhou, X.‐B. & Zhang, B.‐C. (2009). The influence of biological soil crusts on dew deposition in Gurbantunggut Desert, Northwestern China. Journal of Hydrology 379, 220–228. [Google Scholar]
- Zhang, Y. , Aradottir, A. L. , Serpe, M. & Boeken, B. (2016). Interactions of biological soil crusts with vascular plants. In Biological Soil Crusts: An Organizing Principle in Drylands (eds Weber B., Büdel B. and Belnap J.), pp. 385–406. Springer, Cham. [Google Scholar]
- Zhang, Y. M. , Wang, H. L. , Wang, X. Q. , Yang, W. K. & Zhang, D. Y. (2006). The microstructure of microbiotic crust and its influence on wind erosion for a sandy soil surface in the Gurbantunggut Desert of Northwestern China. Geoderma 132, 441–449. [Google Scholar]
- Zhao, Y. G. , Xu, M. X. , Wang, Q. J. & Shao, M. A. (2006). Physical and chemical properties of biological soil crust on rehabilitated grassland in hilly Loess Plateau of China. Chinese Journal of Applied Ecology 17(8), 1429–1434. [PubMed] [Google Scholar]
- Zhu, X. M. (1960). Effect of vegetation on soil and water loss in the Loess region. Acta Pedologica Sinica 1960, 110–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Number of studies on biological soil crusts per year, covering the time span from 1989 to 2021.


