Abstract
Blood glucose levels exceeding 8 mM are shown to increase glucose levels in airway surface in cystic fibrosis (CF). Moreover, high levels of endobronchial glucose are proposed to increase the growth of common CF bacteria and feed the neutrophil‐driven inflammation. In the infected airways, glucose may be metabolized by glycolysis to lactate by both bacteria and neutrophils. Therefore, we aimed to investigate whether increased blood glucose may fuel the glycolytic pathways of the lung inflammation by determining sputum glucose and lactate during an oral glucose tolerance test (OGTT). Sputum from 27 CF patients was collected during an OGTT. Sputum was collected at fasting and one and two hours following the intake of 75 g of glucose. Only participants able to expectorate more than one sputum sample were included. Glucose levels in venous blood and lactate and glucose content in sputum were analyzed using a regular blood gas analyzer. We collected 62 sputum samples: 20 at baseline, 22 after 1 h, and 20 after 2 h. Lactate and glucose were detectable in 30 (48.4%) and 43 (69.4%) sputum samples, respectively. The sputum lactate increased significantly at 2 h in the OGTT (p = 0.024), but sputum glucose was not changed. As expected, plasma glucose level significantly increased during the OGTT (p < 0.001). In CF patients, sputum lactate increased during an OGTT, while the sputum glucose did not reflect the increased plasma glucose. The increase in sputum lactate suggests that glucose spills over from plasma to sputum where glucose may enhance the inflammation by fueling the anaerobic metabolism in neutrophils or bacteria.
Keywords: Sputum, lactate, cystic fibrosis, OGTT
INTRODUCTION
Cystic fibrosis (CF) is a deadly autosomal genetic disease affecting 70,000–100,000 people worldwide [1, 2]. CF is caused by variations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which was first described in 1989 by Riordan et al. [3]. Most CF patients are experiencing recurrent and chronic lung infections, which constitutes major lethal complications [4, 5]. In addition, many CF patients develop impaired glucose tolerance or cystic fibrosis‐related diabetes (CFRD) [6, 7]. CFRD is a comorbidity characterized by elevated levels of blood glucose caused by a delayed and insufficient insulin secretion [8, 9, 10]. In CF, increased plasma glucose is correlated with an accelerated decline in lung function [11], but the underlying mechanism has so far not been fully explained. It has, however, been proposed that increased plasma glucose may reach the endobronchial zones, where the glucose could serve at nutrients for pathogens [12]. In addition to promoting growth of pathogens, we speculated that the increased plasma glucose may reinforce lung inflammation by serving as substrate for glycolysis in the endobronchial neutrophils, which are directly correlated to decreased lung function [13]. Since the product of glycolysis by neutrophils is lactate [14], which is related to the lung function in CF patients [15], we tested whether the increasing levels of plasma glucose during an oral glucose tolerance test (OGTT) may increase the levels of lactate in expectorated sputum samples from CF patients.
METHODS
CF patients (>18 years) were enrolled from the adult department at the Copenhagen CF Centre during a cross‐sectional study in 2017. From 27 patients, we collected a total of 81 blood samples and 62 non‐induced sputum samples during an OGTT with the intake of 75 g of glucose. We collected 20 sputum samples at baseline (fasting), 22 samples after 1 h, and 20 samples after 2 h. Plasma glucose in venous blood samples was analyzed with a Cobas 8000, c702 module. The supernatant from the sputum samples was isolated after centrifugation (15 min, 21,000 g, 4 °C) (Heraeus Fresco 21, Thermo Fisher, Copenhagen, Denmark) and stored at −80°C before analyzing. The content of lactate and glucose in sputum supernatants was analyzed using a regular blood gas analyzer (BGA) (ABL90 FLEX, Radiometer, Copenhagen, Denmark). Sputum samples with insufficient amount of material were excluded. The glycemic states of the patients were determined according to recent recommendations [16].
Statement of ethics
The study was approved by the Regional Committee on Health Research Ethics (Region H: H16037693), and all enrolled patients signed a consent form.
Statistics
Data were tested for normality and compared by one‐way ANOVA test followed by Bonferroni's multiple comparisons correction using Graphpad prism 9 (GraphPad Software Inc., La Jolla, CA, USA). p < 0.05 was considered significant.
RESULTS
Demographics
Twenty‐seven CF patients were included in the study and the median age (range) was 33 years (18–54). Eleven patients (41%) were female. The median percent predicted forced expiratory volume in one second (FEV1%) (range) was 56% (14–104), and the median percent predicted forced vital capacity (FVC%) (range) was 86% (47–108). Twenty‐five of the patients (93%) had chronic lung infection, and Pseudomonas aeruginosa was the most prevalent pathogen (Table 1).
Table 1.
Baseline characteristics in the CF study population
| Characteristics | |
| Participants, n (%) | 27 (100) |
| Age (years), median (range) | 33 (18–54) |
| Female, n (%) | 11 (41) |
| Chronic lung infection, n (%) | 25 (93) |
| Pseudomonas aeruginosa | 13 (48) |
| Staphylococcus aureus | 5 (19) |
| Other pathogens | 11 (41) |
| Lung function (%) | |
| FEV1, median (range) | 56 (14–104) |
| FVC, median (range) | 86 (47–108) |
| Glucose tolerance, n (%) | |
| Normal 1 | 7 (29) |
| Indeterminate glucose tolerance 2 | 4 (15) |
| Impaired glucose tolerance 1 | 7 (29) |
| Cystic fibrosis‐related diabetes 1 | 9 (33) |
Normal <7.8, impaired glucose tolerance ≥7.8, and <11.1 and cystic fibrosis‐related diabetes >11.1.
Glucose tolerance is based on 2‐h plasma glucose (mM) in an OGTT.
Indeterminate glucose tolerance: 1‐h plasma glucose (mM) >11.0 but 2‐h <7.8 in an OGTT.
Levels of glucose and lactate
As expected, glucose levels in plasma increased after one and two hours of the OGTT (p < 0.001) (Fig. 1A). Using the blood gas analyzer, glucose was detectable in 28 (45%) of the sputum samples, whereas lactate could be determined in 41 (66%) of the sputum samples. The sputum glucose level did not change during the OGTT (Fig. 1B). However, the concentration of sputum lactate increased significantly between fasting and 2 h after glucose exposure (p = 0.023) (Fig. 1C). The number of samples at all time points was stable throughout the experiment indicating that the fraction of inconclusive samples did not change. In addition, we were not able to relate the levels of lactate to any of the detected pathogens in the sputum samples (data not shown).
Fig. 1.
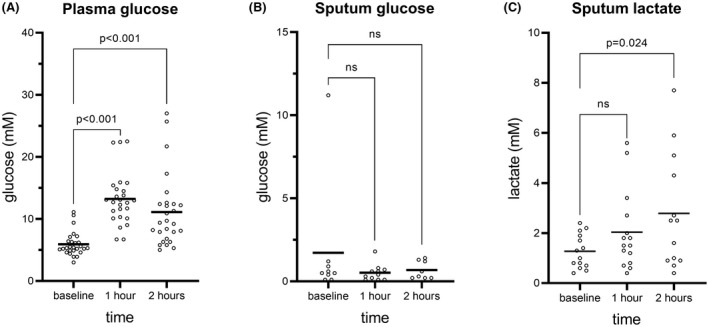
Glucose levels in plasma and sputum and lactate levels in sputum during fasting and 1 and 2 h after oral intake of glucose. The number of samples in A was 27 at baseline, 26 at 1 h, and 27 at 2 h. The number of samples in B was 9 at baseline, 11 at 1 h, and 8 at 2 h. The number of samples in C was 14 at baseline, 15 at 1 h, and 13 at 2 h. The means are marked by black lines. Data were analyzed using one‐way ANOVA test followed by Bonferroni's multiple comparisons correction. p < 0.05 was considered significant.
To compare the effects of the glycemic states on sputum lactate and plasma glucose, the patients were stratified in cystic fibrosis–related diabetes (CFRD), impaired glucose tolerance (IGT), indeterminate glucose tolerance (INDET) and normal glucose tolerance (NGT) (Fig. 2). The plasma glucose was significantly higher in CFRD patients at 2 h (p < 0.05), but no significant differences in the levels of sputum lactate or glucose between the glycemic states were observed at any time point.
Fig. 2.
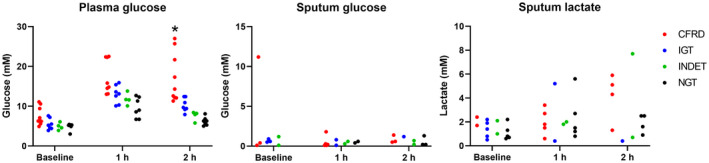
Glycemic states and glucose levels in plasma and sputum and lactate levels in sputum during fasting and 1 and 2 h after oral intake of glucose. The patients were divided in cystic fibrosis‐related diabetes (CFRD), impaired glucose tolerance (IGT), indeterminate glucose tolerance (INDET), and normal glucose tolerance (NGT). Data were analyzed using one‐way ANOVA test followed by Bonferroni's multiple comparisons correction. p < 0.05 was considered significant.
DISCUSSION AND CONCLUSIONS
To our knowledge, increased levels of endobronchial lactate during an OGTT in CF patients have not been reported before. Increased levels of lactate during addition of glucose are suggestive of glucose being oxidized by glycolytic conversion to lactate, which is the process providing more than 85% of the adenosine triphosphate needed for the vital neutrophil host defense activities such as phagocytosis [14]. The neutrophils in sputum from chronically infected CF patients are actively engaged in bactericidal activities as evidenced by the ongoing production of reactive oxidative species and nitric oxide [17, 18], and accordingly, the level of lactate correlates to the density of neutrophils in sputum from chronically infected CF patients [19]. Moreover, the presence of glucose may enhance inflammatory activities, such as the release of NETs, and the secretion of lactate by activated neutrophils [20]. The entry route of lactate into the sputum could not be determined in the present study, but we propose that glucose leaves the blood and enters the endobronchial space before being converted into lactate. This proposition is based on the modest increase in the low levels of blood lactate during OGTT in individuals without CF [21] and CF patients having lower levels of lactate in blood (1.3 ± 0.4 mM) [22] than we found in sputum (2.8 ± 2.3 mM) (Fig. 1), which minimize passive diffusion of lactate from the blood to the endobronchial mucus. The suggested glucose pathway is also supported by the absence of apical secretion of lactate by CF airway epithelial cells when blood glucose rises [23] and by the leaking of glucose through the paracellular junctions between lung epithelial cells from the blood into the airway surface liquid during inflammation [12]. The latter is possibly driven in part by the concentration gradient created by high glucose levels in blood and low levels in sputum (Fig. 1). Furthermore, upon arrival in the endobronchial mucus, glucose is ingested by neutrophils as evidenced by the intracellular accumulation of [18F] fluorodeoxyglucose ([18F] FDG) in endobronchial neutrophils after intravenous supply of [18F] FDG [24]. Meanwhile, glucose might also be converted to lactate during the glycolysis in other active inflammatory cells such as M1 macrophages and Th17 cells [25]. Apart from the glycolytic conversion of glucose to lactate, depletion of glucose in the sputum may potentially also result from other metabolic pathways such as the tricarboxylic acid cycle and the generation of lipids [26]. Even though the mechanisms of lactate removal and non‐glycolytic consumption of glucose are not within the primary scope of this study, we speculate as for how long the levels of lactate will increase in the sputum to evaluate the duration of the potential inflammatory effects linked to increased sputum lactate resulting from peaks of plasma glucose.
It may be expected that parts of the sputum lactate have microbial origin. However, several CF‐pathogens do not secrete lactate in significant amounts except for S. aureus, that may secrete substantial amounts of lactate, but only at aerobic conditions, which are rare in large parts of the CF sputum [15, 27]. In addition, the bacterial metabolic activity is very low in the endobronchial secretions of CF patients with chronic lung infection [28]. Finally, it has never been studied whether insulin affects the endobronchial mucus in CF which could potentially stimulate glucose uptake and glycolysis in leukocytes during an OGTT [29].
The ability of the levels of plasma glucose during an OGTT to serve as a determinant of the glycemic state was confirmed for CFRD in the present study. We were, however, unable to relate the levels of sputum lactate to the glycemic states, which is possibly due to the limited number of sputum samples. Thus, our study was not structured to adequately address whether sputum lactate dynamics may provide additional information to the clinical relevance of other glycemic stages, such as INDET, which has recently been associated with beta‐cell dysfunctionalities resembling CFRD and IGT [30]. To effectively assess the relation between sputum lactate and glycemic stages in CF, we propose to examine larger groups of patients and to detect lactate using methods with a higher sensitivity.
A limitation of this study is that the blood gas analyzer has never been validated for the purpose of analyzing supernatant from sputum, where, for example, glucose levels are below physiological levels in blood. Moreover, we can only speculate that the samples with inconclusive results were predominantly caused by the metabolite levels being below the threshold of the device rather than insufficient sample volumes.
In conclusion, our results support the hypothesis that high glucose levels may increase the levels of lactate in expectorated sputum samples from CF patients. This link may provide important information for explaining the association of high blood glucose with accelerated lung function decline by increased neutrophil‐mediated lung inflammation resulting from endobronchial influx of glucose fueling glycolysis of the neutrophils. Consequently, controlling the levels of blood glucose is important for stabilizing the lung function of CF patients.
We would like to thank the Cystic Fibrosis Centre Copenhagen for their excellent assistance and Christine Råbjerg Mikkelsen M.D., for her excellent contribution to the collection of sputum samples.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
POJ, BUN, DF‐J, and IHMM were involved in the study conception and design. BUN, DF‐J, and IHMM were involved in inclusion of patients. POJ, MK, and IHMM Performed the experiments. POJ, BUN, and DF‐J analyzed the results. POJ, BUN, DF‐J, MK, TP, and IHMM have interpreted data, drafted the manuscript, and substantively revised it.
REFERENCES
- 1. Kelly J. Environmental scan of cystic fibrosis research worldwide. J Cyst Fibros. 2017;16:367–70. [DOI] [PubMed] [Google Scholar]
- 2. Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519–31. [DOI] [PubMed] [Google Scholar]
- 3. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. [DOI] [PubMed] [Google Scholar]
- 4. Mayer‐Hamblett N, Ramsey BW, Kulasekara HD, Wolter DJ, Houston LS, Pope CE, et al. Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin Infect Dis. 2014;59:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skov M, Hansen CR, Pressler T. Cystic fibrosis – an example of personalized and precision medicine. APMIS. 2019;127:352–60. [DOI] [PubMed] [Google Scholar]
- 6. Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, et al. Diabetes‐related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis‐related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul‐Karim FW, Dahms BB, Velasco ME, Rodman HM. Islets of Langerhans in adolescents and adults with cystic fibrosis. A quantitative study. Arch Pathol Lab Med. 1986;110:602–6. [PubMed] [Google Scholar]
- 9. Granados A, Chan CL, Ode KL, Moheet A, Moran A, Holl R. Cystic fibrosis related diabetes: pathophysiology, screening and diagnosis. J Cyst Fibros 2019;18:S3‐S9. [DOI] [PubMed] [Google Scholar]
- 10. Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, et al. Diabetes mellitus associated with cystic fibrosis. J Pediatr. 2018;112:373–7. [DOI] [PubMed] [Google Scholar]
- 11. Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162:891–5. [DOI] [PubMed] [Google Scholar]
- 12. Prentice BJ, Jaffe A, Hameed S, Verge CF, Waters S, Widger J. Cystic fibrosis‐related diabetes and lung disease: an update. Eur Respir Rev. 2021;30:200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer‐Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Investig. 1982;70:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bensel T, Stotz M, Borneff‐Lipp M, Wollschläger B, Wienke A, Taccetti G, et al. Lactate in cystic fibrosis sputum. J Cyst Fibros. 2011;10:37–44. [DOI] [PubMed] [Google Scholar]
- 16. Kelly A, Moran A. Update on cystic fibrosis‐related diabetes. J Cyst Fibros. 2013;12:318–31. [DOI] [PubMed] [Google Scholar]
- 17. Kolpen M, Hansen CR, Bjarnsholt T, Moser C, Christensen LD, van Gennip M, et al. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax. 2010;65:57–62. [DOI] [PubMed] [Google Scholar]
- 18. Kolpen M, Bjarnsholt T, Moser C, Hansen C, Rickelt L, Kuhl M, et al. Nitric oxide production by polymorphonuclear leukocytes in infected cystic fibrosis sputum. Clin Exp Immunol. 2014;177:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen BU, Kolpen M, Jensen PØ, Katzenstein T, Pressler T, Ritz C, et al. Neutrophil count in sputum is associated with increased sputum glucose and sputum L‐lactate in cystic fibrosis. PLoS One. 2020;15(9):e0238524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodríguez‐Espinosa O, Rojas‐Espinosa O, Moreno‐Altamirano MM, López‐Villegas EO, Sánchez‐García FJ. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145:213–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prando R, Cheli V, Buzzo P, Melga P, Ansaldi E, Accoto S. Blood lactate behavior after glucose load in diabetes mellitus. Acta Diabetol Lat. 1988;3:247–56. [DOI] [PubMed] [Google Scholar]
- 22. Nikolaizik WH, Knöpfli B, Leister E, de Boer P, Sievers B, Schöni MH. The anaerobic threshold in cystic fibrosis: comparison of V‐slope method, lactate turn points, and Conconi test. Pediatr Pulmonol. 1998;25:147–53. [DOI] [PubMed] [Google Scholar]
- 23. Garnett JP, Kalsi KK, Sobotta M, Bearham J, Carr G, Powell J, et al. Hyperglycaemia and Pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate‐H + secretion. Sci Rep. 2016;6:37955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen DL, Ferkol TW, Mintun MA, Pittman JE, Rosenbluth DB, Schuster DP. Quantifying pulmonary inflammation in cystic fibrosis with positron emission tomography. Am J Respir Crit Care Med. 2006;173:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soto‐Heredero G, de Las G, Heras MM, Gabandé‐Rodríguez E, Oller J, Mittelbrunn M. Glycolysis – a key player in the inflammatory response. FEBS J. 2020;287(16):3350–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28(5):514–24. [DOI] [PubMed] [Google Scholar]
- 27. Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolpen M, Kragh KN, Enciso JB, Faurholt‐Jepsen D, Lindegaard B, Egelund GB, et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax. 2022. doi: 10.1136/thoraxjnl-2021-217576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz‐Pineda WD, Parra‐Rojas I, Rodríguez‐Ruíz HA, Illades‐Aguiar B, Matia‐García I, Garibay‐Cerdenares OL. The regulatory role of insulin in energy metabolism and leukocyte functions. J Leukoc Biol. 2022;111:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piona C, Volpi S, Zusi C, Mozzillo E, Tosco A, Franzese A, et al. Glucose tolerance stages in cystic fibrosis are identified by a unique pattern of defects of Beta‐cell function. J Clin Endocrinol Metab. 2021;106:e1793–802. [DOI] [PubMed] [Google Scholar]


