Abstract
Introduction
The platelet function analyzer (PFA) is a popular platelet function screening instrument, highly sensitive to von Willebrand disease (VWD) and to aspirin therapy, with moderate sensitivity to defects in platelet function and/or deficiencies in platelet number. There are two models, the original PFA‐100 and the contemporary PFA‐200. Normal reference ranges (NRRs) provided by the manufacturer are the same for both models, instead being based on the type of test cartridge, for which there are two main ones: collagen/epinephrine (C/Epi) and collagen/adenosine diphosphate (C/ADP).
Methods
Comparative evaluations of PFA testing and reporting in six different sites of a large pathology network, aiming to harmonize NRRs and test reporting across all network sites. A separate comparative study of testing a range of samples (n > 150) on a PFA‐100 versus that on a PFA‐200. Review of contemporary literature.
Results
Each site was identified to have a different reporting NRR, which after consolidating data permitted establishment of an agreed harmonized NRR for use across the network (C/Epi: 90–160; C/ADP: 70–124; based on n > 180). Similarly, each site reported and interpreted results in different ways, and after discussion and consolidation, a harmonized approach to interpretation and reporting was achieved. The separate comparative study of PFA‐100 versus PFA‐200 testing confirmed instrument equivalence.
Conclusion
We achieved harmonized NRRs and reporting for PFA testing across a large pathology network. Our approach may be useful for other laboratory networks wishing to harmonize PFA testing.
Keywords: harmonization, PFA‐100, PFA‐200, platelet function analyzer
1. INTRODUCTION
The platelet function analyzer (PFA) is a popular platelet function screening instrument, originally described over 25 years ago, in 1995. 1 , 2 The PFA was based on the technology of an earlier instrument, the Thrombostat 4000, which was otherwise identified as an “in vitro bleeding time.” 3 The originally released model of the PFA was called the PFA‐100, and likewise became known as an “in vitro bleeding time.” 4 , 5 The PFA‐100 is widely distributed internationally, including Europe, Asia Pacific, and North America. A newer model of the PFA, called the PFA‐200, has been released in some countries, but this currently excludes the United States. Nevertheless, the PFA‐200 will become the dominant instrument over time, as the manufacturer (Siemens Healthineers) has an active program of replacing the existing PFA‐100 intruments with their newer PFA‐200, and ceasing service contracts with the PFA‐100, at least in Australia.
The PFA‐100 and PFA‐200 both report test results as a “closure time” (CT), which is the time (in seconds, s) in which an aperture in the test cartridge is blocked, after whole blood is introduced into the cartridge, flows through the cartridge under sheer pressure, and finally makes contact with the test membrane coated with the various platelet agonists. Platelets adhere to the membrane, then become activated and aggregate, eventually blocking the aperture. There are three different test cartridge types, according to the platelet agonists coated onto a membrane within the cartridge, and namely collagen/epinephrine (C/Epi), collagen/adenosine diphosphate (C/ADP), and the so‐called Innovance PFA P2Y cartridge coated with ADP, prostaglandin E1 and calcium chloride. Like the PFA‐200, the Innovance PFA P2Y, marketed as sensitive to P2Y12 antagonist therapy, such as clopidogrel, is not available in all geographies, including the United States. Moreover, the C/Epi and C/ADP test cartridges are those primarily used within most laboratories, and are the subject of the current report.
The PFA is sensitive to disturbances in the primary haemostasis, as well to a variety of drugs, supplements and foods. 6 , 7 , 8 , 9 , 10 In particular, the PFA is highly sensitive to deficiency or defect in von Willebrand factor (VWF), and thus to the presence of von Willebrand disease (VWD). 6 , 7 , 8 , 9 , 10 Indeed, a normal PFA test result using the C/Epi test cartridge represents a highly effective negative exclusion for VWD, in some laboratories even better than individual tests for VWF level and activity. 6 , 7 , 8 The PFA is also highly sensitive to aspirin anti‐platelet therapy, in particular using the C/Epi test cartridge, 9 , 10 but only moderately sensitive to deficiency of platelets or the presence of platelet dysfunction. 6 , 9 , 10 The PFA is also sensitive to a plethora of other drugs and supplements, as well as certain foods (including high garlic, chocolate or fish oil intake). 10 The PFA is also sensitive to haematocrit levels. 10 Overall, then, prolongation in PFA CTs may reflect one or more of a variety of events, and thus is not specific for any particular defect, and normal CTs, for example to exclude VWD, seem to have better clinical utility.
Notably, the results of testing via the PFA‐100 and the PFA‐200 are considered “identical,” in so far as the manufacturer provided CT normal reference ranges (NRRs) for the two instrument platforms are identical. That is, the CT NRRs are not instrument‐centric, but rather are based on the test cartridge type used by the instruments. The manufacturer CT NRRs for the C/Epi and C/ADP test cartridges are, respectively, 82–150 and 62–100 s, for use on both the PFA‐100 and PFA‐200. However, the previous extensive review of the literature shows an extraordinary range of reported NRRs in actual use in different laboratories. 10 Indeed, we confirmed the use of different NRRs at each site in which a PFA‐100/200 was in use even in our laboratory network, NSW Health Pathology (NSWHP). Moreover, each site in our network interpreted and reported differently in terms of associated test result comments for requesting clinicians. The current study therefore reports on our harmonization of CT NRRs and test reporting for PFA‐100/200 for all sites in NSWHP.
2. MATERIALS AND METHODS
2.1. Overview of setting and study design
This evaluation was undertaken by NSWHP personnel, and intended to achieve harmonization of PFA‐100/200 NRRs and reporting in all NSWHP laboratories performing PFA tests. In Australia, PFA testing is used by laboratories as a screen of platelet function and VWD. Such testing is regulated by several Australian agencies. 11 First, laboratory instruments and reagents (“in vitro diagnostics”; IVDs) require regulatory approval by the Therapeutic Goods Administration (TGA). Laboratory testing practice also requires accreditation status, which is effected by the National Association of Testing Authorities (NATA), in part using guidance from the National Pathology Accreditation Advisory Council (NPAAC). In relation to PFA testing, laboratories are required to establish or verify NRRs for CTs for use. The simplest approach is verification, which can employ straightforward methods as per Clinical and Laboratory Standards Institute (CLSI) guidance. 12 In addition, there is a requirement for ongoing quality control and participation in external quality assessment (EQA), which in Australia is undertaken by the Royal College of Pathologists of Australasia Quality Assurance Program (RCPAQAP). 13 , 14 , 15
Given PFA testing is only performed within the larger sites of our organization, six major sites (Supplementary Table S1) partook in this study. Specifically, each site provided historical data for normal individual samples assessed at each site as part of their internal validation studies for their instrument installation, and as potentially used to derive local CT NRRs. Subsequent to historical PFA installation, all sites have been accredited for PFA testing, and participate in ongoing EQA for these tests. The historical data were assessed separately and in composite to eventually establish a harmonized NRR for the entire network. The second process was undertaken to establish harmonized reporting of associated interpretive comments, which are used to explain PFA test results to requesting clinicians. This was undertaken by discussion and agreement of lead scientists and haematologists. The third evaluation was undertaken at the Institute of Clinical Pathology and Medical Research (ICPMR) site to confirm comparability of PFA‐100 and PFA‐200 instrumentation. In this substudy, approximately 20–30 samples (representing a variety of CT values; both normal and prolonged) were co‐tested per year from 2014 (when the PFA‐200 was acquired) onward, as part of a quality control process to assess and confirm ongoing equivalence for patient reporting purposes (laboratory accreditation requirement). This process is summarized in Supplementary Figure S1.
The instruments in place at the six sites comprised both PFA‐100 and PFA‐200 instruments (Supplementary Table S1). All samples utilized were based on sodium citrate (3.2%) anticoagulation, employing whole blood collected and processed according to the PFA manufacturer instructions, with testing completed within 4 h of blood collection.
2.2. Verification/establishment of CT NRRs and Statistical analysis
We performed several procedures to establish, or verify manufacturer recommended, CT NRRs for both C/Epi and C/ADP test cartridges, in part utilizing the CLSI guidance document “Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory”. 12 This study primarily used historical data as collected and utilized at each site at implementation of PFA testing at that site, as well as normal individual data potentially collected since implementation at some sites. The normal individuals most typically comprised laboratory or other staff from our organization. Both male and female adults (>18 yr) were included, and as there is no gender or adult age‐related differences in PFA CTs, gender and age was not always documented. All testing was performed within 4 h of sample collection. Although we attempted to exclude individuals who were on antiplatelet medication, this was assessed inconsistently between sites, and it is possible that some individuals had recently consumed undisclosed agents capable of affecting platelet function (e.g., agents used to treat headaches and other aches and pains; supplements or foods). Such undisclosed intake may have led to occasional high‐outlier CT data. Not all sites assessed these collected samples for platelet count, haematocrit, and/or VWF level and activity, all of which are also known to affect PFA CTs. However, data from several sites where this did occur is available and confirmed normal platelet count, normal haematocrit, and normal levels of VWF for these data subsets in almost all cases. Nevertheless, an undetected low platelet count, haematocrit of VWF level or activity may occasionally have also yielded high‐outlier CT data. We assessed agreement (or lack thereof) of NRRs between individual sites, and also evaluated site data as well as composite data for normality by several statistical methods using the GraphPad Prism software (La Jolla, CA, USA). The four normality tests available in this software are the Anderson–Darling, D'Agostino and Pearson, Shapiro–Wilk and Kolmogorov–Smirnov tests. We also calculated normal ranges based on various procedures, ultimately deciding on 2.5–97.5th percentiles, because the arising data did not consistently show a normal distribution. We also ultimately decided on 2.5–97.5th percentiles for composite data after the removal of visual outliers. Comparative data using separate processes of statistical outlier removal was also performed (using the Prism recommended Rout test, and a common Tukey inter‐quarter range‐based test) and results of these are shown for comparison. Data are otherwise presented numerically or in a qualitative synthesis.
2.3. Ethical considerations
According to the guidance from local Human Research Ethics Committees, formal ethical approval for this study was not sought, as the evaluation represents a Quality Assurance project of method verification.
2.4. Literature search
A PubMed search of ((PFA 100) or (PFA 200) or “PFA”) was also undertaken (on March 10, 2022) without time restriction to primarily identify contemporary literature, in particular those published studies reporting PFA‐100/200 CT NRRs. The search generated 1202 reports. We selected the most recent 100 of these reports in English language, and thus representing studies conducted in the last 3 years, and searched these to identify any study reporting a NRR for either C/Epi and/or C/ADP. This permitted identification of what we felt was a selection of the most recent “contemporary” NRRs reported in the literature. This “contemporary” dataset was compared to NRRs previously identified in an earlier literature review 10 as used to identify a “historical” dataset. Furthermore, the PubMed search generating 1202 reports was additionally searched by adding the word “harmoni*” to attempt to capture any relevant articles reflecting harmonization (or harmonization) of PFA CTs. This resulted in the capture of two papers, neither of which discussed harmonization of PFA CTs.
3. RESULTS
3.1. Individual site and composite data for NRR determination
Individual site data are shown in Figure 1, and summarized in Table 1. Each site performed testing using between 22–48 individual normal samples. Some data points could be visually observed to be clear outlier points (identified in Figure 1 using red circles), with some sites showing a greater proportion of visual outliers. These outliers could represent undisclosed use of an anti‐platelet agent or other compound/supplement affecting PFA CTs, or unrecognized low platelet counts, haematocrits or VWF. However, where data was available, platelet counts, haematocrits and VWF level and activity were generally normal for samples collected from these normal individuals (Supplementary Figure S3). Also, there was limited correlation between PFA CTs and platelet count or haematocrit for this normal data set; however, there was some correlation with VWF level and activity, especially for C/ADP (Supplementary Figure S3).
FIGURE 1.
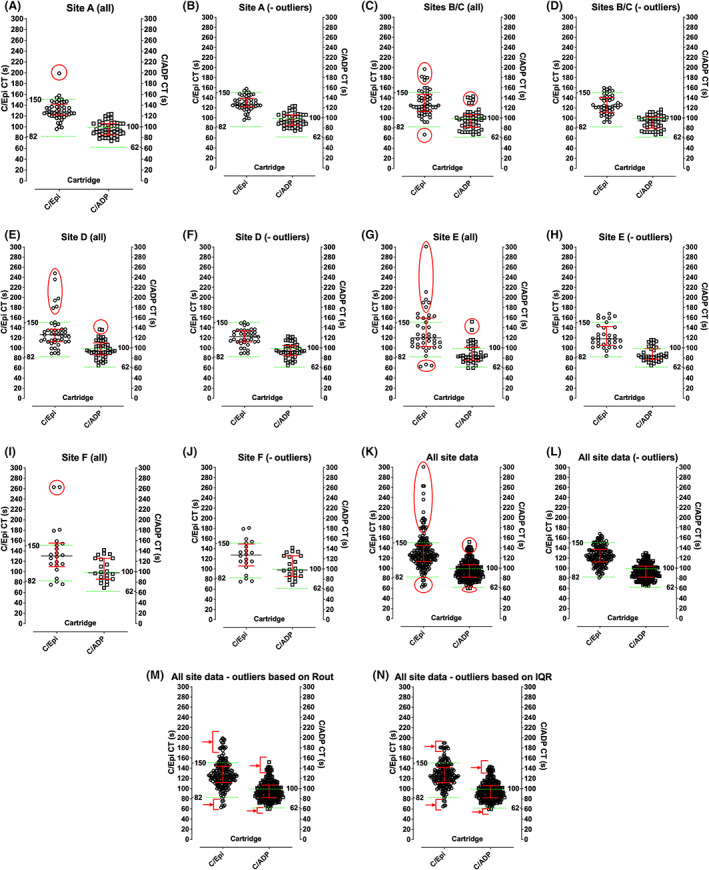
Normal reference range data sets from each site plus composite data. Figures A, C, E, G, and I show individual site data with visual outliers identified in red circles. Figures B, D, F, H, and J show individual site data with visual outliers removed. Figures K and L show composite data with visual outliers identified (K) and removed (L). Figures M and N show composite data with statistical outliers removed according to two separate procedures; some visual outliers are still evident in these (arrowed). Left y‐axis in each figure shows C/Epi CT in seconds; right y‐axis shows C/ADP CT in seconds. The Siemens product information CT NRRs are shown by the green horizontal dashed lines.
TABLE 1.
Individual site and composite data for NRR determination
| Data set from site | No. donors Col/ADP, Col/Epi | Col/ADP | Col/Epi |
|---|---|---|---|
| Original Manufacturer NRR (PFA‐100 and PFA‐200) | 309 | 62–100 | 82–150 |
| Current NRRs | |||
| A. ICPMR | 64–127 | 94–162 | |
| B/C. JHH/RNSH (harmonized; manufacturer) | 62–100 | 82–150 | |
| C. RNSH (historical; pre‐harmonization) | 73–127 | 94–162 | |
| D/E. RPA/Liverpool (harmonized) | 60–120 | 80–170 | |
| E. Liverpool (historical; pre‐harmonization) | 73–127 | 81–146 | |
| F. ISLHD | 71–125 | 94–193 | |
| Calculated NRRs (− visual outliers; 2.5th–97.5th percentiles) | |||
| A. ICPMR | 48, 47 | 75–124 | 97–157 |
| B/C. JHH/RNSH | 46, 46 | 67–116 | 92–160 |
| D. RPA | 42, 38 | 65–123 | 89–151 |
| E. Liverpool | 38, 33 | 65–116 | 84–168 |
| F. ISLHD | 22, 20 | 69–142 | 75–181 |
| Composite NSWHP NRR (− visual outliers) | 194, 184 | 69–124 | 89–160 |
| Composite NSWHP NRR (− statistical outliers a ) | 208, 202 | 67–139 | 75–194 |
| Composite NSWHP NRR (− statistical outliers b ) | 207, 196 | 67–137 | 76–181 |
| Composite NSWHP NRR (− visual outliers) (minor rounding). Ranges to be adopted for harmonization | 194, 184 | 70–124 | 90–160 |
Using the Prism recommended Rout method and selecting 5% as Q. Selecting 10% as Q did not exclude any more outliers; selecting 2% as Q resulted in 1 less outlier for C/Epi.
Using the Tukey interquartile range (IQR) method.
Removal of visual outlier data points was undertaken to calculate “site‐specific” and overall composite NRRs. Using CLSI guidance, 12 manufacturer recommended CT NRRs for C/Epi could theoretically be adopted at some sites, with >90% of data points within the manufacturer ranges, but not at other sites (where <90% data points within the manufacturer ranges). In general, the manufacturer recommended ranges could not be adopted for C/ADP at any site, with <90% data points within the manufacturer ranges. Interestingly, data passed several tests of normality for some sites, but not at other sites (Supplementary Table S2). Data from all sites were combined, as also shown in Figure 1, and summarized in Table 1. Again, visual outlier data points, potentially representing undisclosed anti‐platelet agents/supplements or low platelet count/haematocrit/VWF were removed as a comparative exercise. Composite data largely agreed with the manufacturer recommended range for C/Epi, but not for C/ADP. This composite data passed tests for normality for C/Epi, but not for C/ADP (Supplementary Table S2). Accordingly, it was decided to calculate NRRs based on 2.5–97.5th percentiles, as shown in Figure 2 and Table 1. Table 1 also shows NRRs that were currently in place at each site prior to this study. Notably, several sites had already “harmonized” NRRs with each other (Table 1). Nevertheless, the NRRs in use at different sites, or otherwise calculated based on 2.5–97.5th percentiles, differed from one another. In addition to the removal of visual outliers, composite data were also evaluated for outlier removal using several statistical tests, as shown in Figure 1 and Table 1. These statistical tests removed far fewer outliers than those visually identified, with some residual CTs still appearing as visual outliers (Figure 1). The final composite ranges agreed by the team are also shown in Table 1, these being 70–124 (C/ADP) and 90–160 (C/Epi).
FIGURE 2.
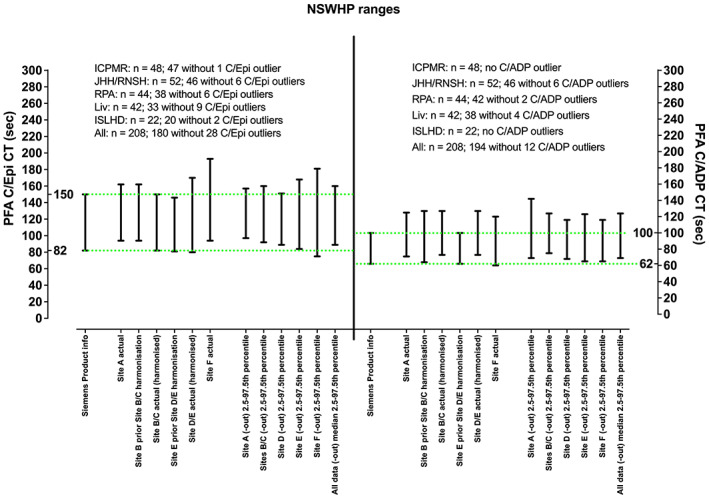
Normal reference ranges calculated from data sets in Figure 1 (with outliers removed), as well as those active prior to harmonization. Left y‐axis shows C/Epi CT in seconds; right y‐axis shows C/ADP CT in seconds. The Siemens product information CT NRRs are shown by the green horizontal dashed lines.
3.2. Literature‐reported NRRs
For comparison, some CT NRRs reported in the contemporary 6 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 and original literature 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 are shown in Figure 3. In most instances, these NRRs differ from each other and also from the manufacturer ranges, especially for C/ADP.
FIGURE 3.
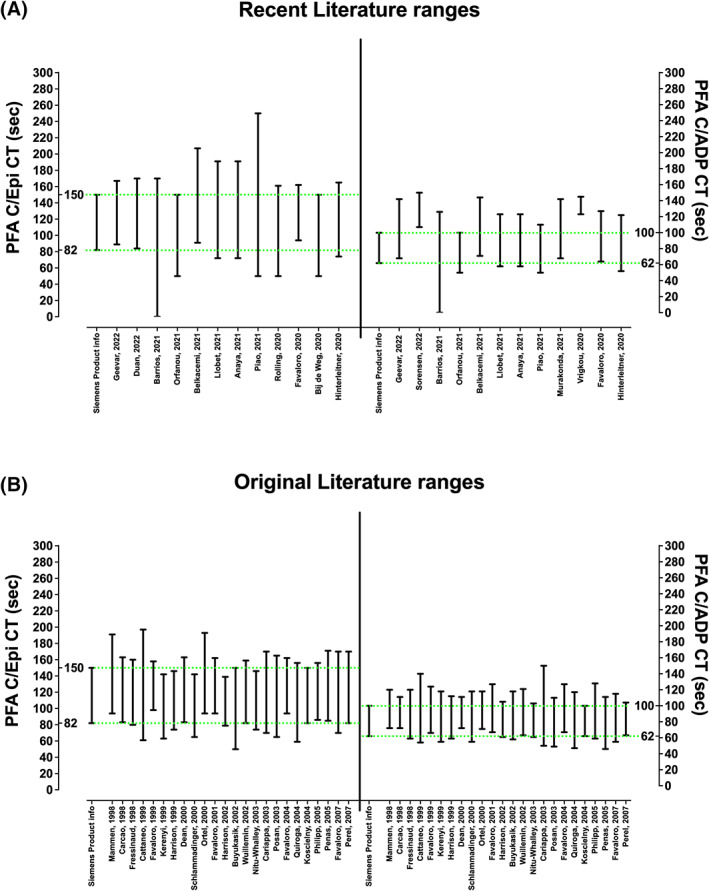
A selection of CT NRRs from published literature. Left y‐axes show C/Epi CT in seconds; right y‐axes show C/ADP CT in seconds. The Siemens product information CT NRRs are shown by the green horizontal dashed lines. Figure A shows NRRs from some contemporary literature as obtained from the recent literature search (see Methods), and reflecting information from the past 3 years. 6 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Figure B shows NRRs from some early literature as otherwise reviewed in Reference 10. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 Reference numbers identify their place in the reference list. In recent literature, many publications only provide an upper cut‐off value, since they were only interested in CT prolongations. These data have arbitrarily been given a lower limit value of 50s to enable a range to be shown. One publication (Reference 18) indicated NRRs for both C/Epi and C/ADP starting at 0, which is not feasible.
3.3. PFA‐100 versus PFA‐200 testing
One site, the ICPMR, had both a PFA‐100 and PFA‐200 on site, and had performed parallel testing over several years (since 2014) in order to show ongoing comparability as part of a quality control process. This data is summarized in Supplementary Figure S2, and largely confirms that CTs from these instruments were comparable.
3.4. Interpretive reporting
After discussion and agreement, the final harmonized interpretive comments as associated to PFA test results, and for the benefit of requesting clinicians, is shown in Figure 4. Discussion and agreement involved scientific representatives of all participant sites, as well as lead clinical haematologists/pathologists. In brief, there are three possibilities for each cartridge, being below, within, and above the NRR limits. Thus, using both C/Epi and C/ADP test cartridges, there are 3 × 3 (=9) possible scenarios, with some scenarios being more common than others, and some scenarios being rare.
FIGURE 4.
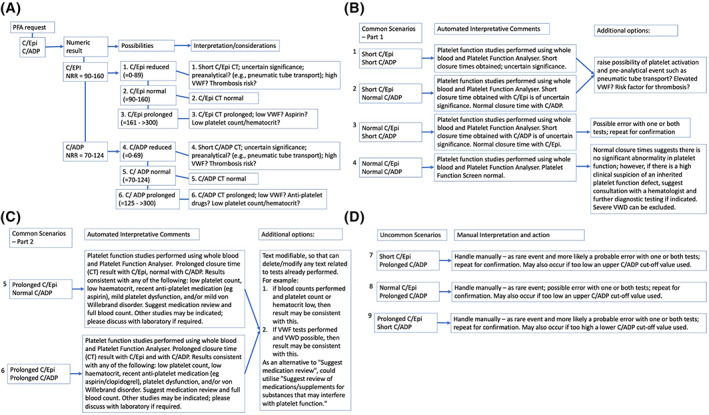
NSWHP harmonized interpretive comments to accompany numeric test results. Figure A shows an algorithm with representative strategy according to different groups of data for C/Epi and C/ADP CTs. In total, with three possible outcomes for C/Epi (short, normal or prolonged CT) and for C/ADP, nine different scenarios are possible. Figure B shows four common scenarios for combining short and normal C/Epi and C/ADP CT data, together with the harmonized automated interpretative comments, plus additional options for investigation. Figure C shows two common scenarios for combining normal and prolonged C/Epi and C/ADP CT data, together with the harmonized automated interpretative comments. Figure D shows three uncommon scenarios combining unexpected C/Epi and C/ADP CT test patterns, together with manual interpretation and suggested follow‐up action
4. DISCUSSION
To our knowledge, this is the only report of multi‐site harmonized PFA CT NRRs and test interpretation/reporting in the literature. The harmonized C/Epi NRR is similar to the manufacturer recommended NRR, but the harmonized C/ADP NRR is quite different (essentially much wider). A similar picture is actually observed in the literature, with C/Epi NRRs being more similar to manufacturer ranges than C/ADP (Figure 3). However, what is more striking is the dissimilarity in NRRs in use from laboratory‐to‐laboratory (Figure 3), including within the same laboratory network (Table 1). To some extent, this may be due to the use of small groups of normal individuals for generation of “less accurate” NRRs (see, e.g., different NRRs calculated for each site (Table 1, Figure 2). Moreover, high‐outlier CTs could potentially be due to undisclosed antiplatelet agents or other compounds/supplements known to affect PFA CTs, as well unrecognized low platelet counts/haematocrits/VWF levels, and low‐outlier CTs potentially due to high levels of VWF or inflammation. 9 , 10 In some cases, from the literature, this may be compounded by the statistical method used to generate NRRs, be it +/− 2 standard deviations (SDs) as is often done, 5‐95th percentiles, or 2.5–97.5th percentiles as in our case. Interestingly, most papers in the literature do not disclose the number of normal individuals used to generate NRRs, nor the statistical method used. Critically, the “correct” upper cut‐off value is important to optimize detection or exclusion of primary haemostasis defects, such as VWD. As an example, the ICPMR laboratory recently published its extensive validation of the PFA in terms of excluding VWD should normal PFA CTs be found. 6 In this study, the ICPMR NRRs of 64–127 (C/ADP) and 94–162 (C/Epi) were employed, thereby “validating” these NRRs for such purpose. An earlier but the similar study from France showed the similar utility for the PFA in VWD exclusion. 8
Notably, too high a CT cut‐off, especially for C/Epi, may miss cases of primary haemostasis defects, and too low a cut‐off may over‐call the presence of primary haemostasis defects. Many papers from the literature only cite a high‐level cut‐off CT value, since this is seen as important for recognition of bleeding risk, due to VWD, platelet dysfunction, low platelet count, anti‐platelet medication (etc). Although low‐level cut‐off values seem to have lower utility, especially for identification/exclusion of primary haemostasis defects, they may identify other pertinent issues, such as pre‐analytical problems (e.g., platelet activation due to pneumatic tube transport often results in short CTs). Moreover, short CTs have also been associated with increased thrombotic risk, in part reflective of high levels of VWF activity. 6 , 54 Thus, we feel it important to identify the full range to include both lower and upper thresholds.
We chose to use visual identification of outliers for removal, and as potentially reflective of undisclosed or unrecognized confounders, and for which there are many possibilities. 9 , 10 In particular, aspirin and non‐steroid anti‐inflammatory agents such as ibuprofen are in widespread use in a range of compounds, including for acute analgesic pain relief. Moreover, use of statistical tools to assess outlier removal identified much fewer outliers and generate much wider NRRs (Table 1), which reflected even higher discordance to both manufacturer ranges and most literature data. Finally, visual outlier removal resulted in NRRs that were close to that verified as clinically useful for VWD exclusion, 6 whereas statistical outlier removal retained many data points that visually seemed to be outliers, resulting in much wider NRRs (Table 1). As there is no gold standard process for outlier removal, and as visual outlier removal remains a tool used by many in haemostasis, we feel that this provided the best outcome in our study.
Our study also confirmed the essential equivalence of CTs from PFA‐100 or PFA‐200 (Supplementary Figure S2). We are not aware of any similar study in the literature. Importantly, the equivalence of PFA‐100 and PFA‐200 CTs has also been shown using EQA data. 13 , 14 , 15 Regarding the interpretive comments, we hope that these are comprehensive enough to enable laboratories to choose the best options for their own diagnostic practice.
We acknowledge several limitations in our study. This study was a laboratory‐based evaluation, and did not further assess the “utility” of these ranges to identify or exclude primary haemostasis defects, based on clinical criteria. Further, we did not specifically assess the influence of all variables, such as platelet count or haematocrit, on PFA test results, and undisclosed antiplatelet compounds could have led to high CTs in the NRR data set. One the other hand, the ICPMR lab has published some clinical utility data, 6 and using similar NRRs to those ultimately adopted by the group. 6 As a strength, our composite study data comprised in excess of 180 normal individuals, and is likely one of the larger datasets used by laboratories attempting to establish CT NRRs for PFA testing. Ultimately, the cut‐offs defined for the PFA reflect a trade‐off between sensitivity and specificity, with too low a cut‐off potentially capturing false positives, and too high a cut‐off potentially missing true positives.
5. CONCLUSIONS
We essentially harmonized NRRs and interpreted comments for PFA testing in our large pathology network, NSWHP, representing the largest public pathology organization in Australia. Harmonization has a number of advantages, as partly explored elsewhere. 55 We propose our evaluation will be useful for other organizations wishing to harmonize PFA testing in their networks. Moreover, we propose that our NRRs may provide a useful comparator for individual laboratories that perform such testing. In particular, NRRs that greatly differ from those shown here may yield relatively poor performance of PFA for its intended purpose, as a screen of primary haemostasis including VWD. We also propose that the C/ADP CT NRRs recommended by the manufacturer should be revised in line with our data and also that of the vast majority of studies reported in the literature, which show wider ranges with higher upper limit values. Finally, we suspect that the narrow C/ADP ranges provided by the manufacturer, with cut‐off of 100 s, will likely over‐call PFA CTs as “abnormal,” with a cut‐off closer to 120 s likely showing better utility.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Supporting information
Supplementary Table S1 Study sites participating in this evaluation*
Supplementary Table S2. Summary of normality tests “passed” using reference range (NRR) data from current report.*
Supplementary Figure S1 Study summary. A summary of the main evaluations undertaken as part of this study.
Supplementary Figure S2 Comparison of closure time (CT) data obtained using PFA‐100 versus PFA‐200 at Site A. Figure A shows data for C/Epi (n = 168 samples), and B shows data for C/ADP (n = 153 samples), co‐tested over a period of 5 years. Data shown as Box and Whiskers with 10‐90th percentiles. There was no statistically significant difference in test results (p values shown, generated using the two‐tailed Mann–Whitney test).
Supplementary Figure S3 A, B. Comparison of CTs (C/Epi [A] and C/ADP [B]; sec) and platelet count (x109/L) and haematocrit values for normal individuals where combined testing was done. Data from 3 participant laboratory sites. Several high‐outlier CTs obtained for C/Epi shown on right portion of figure; these outliers could not be generally linked to low platelet counts or low haematocrit. C, D. Comparison of CTs (C/Epi [C] and C/ADP [D]; sec) and VWF level (VWF:Ag) and activity (VWF:CB or VWF:RCo) where combined testing was done. Data from 1 participant laboratory site. Several high‐outlier CTs obtained for C/Epi shown on right of figure; these outliers could not be generally linked to low levels of VWF or VWF activity. However, there was a statistically significant relationship between C/Epi and C/ADP (all figures), as well as between C/ADP and VWF level and activity (Figure D), which was not evident for any other comparison. Low cut‐off values for other parameters are as follows: platelet count (150 × 109/L), haematocrit (females; 0.355), VWF level (VWF:Ag, 50 U/dL) or activity (50 U/dL VWF:CB; 40 U/dL VWF:RCo).
ACKNOWLEDGEMENTS
NSW Health Pathology is acknowledged for providing in‐kind support. The views expressed herein are those of the authors and are not necessarily those of NSW Health Pathology.
Favaloro EJ, Mohammed S, Vong R, et al. Harmonizing platelet function analyzer testing and reporting in a large laboratory network. Int J Lab Hematol. 2022;44(5):934‐944. doi: 10.1111/ijlh.13907
Funding information NSW Health Pathology
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kundu SK, Heilmann EJ, Sio R, Garcia C, Davidson RM, Ostgaard RA. Description of an in vitro platelet function analyzer‐‐PFA‐100. Semin Thromb Hemost. 1995;21(Suppl 2):106‐112. doi: 10.1055/s-0032-1313612 [DOI] [PubMed] [Google Scholar]
- 2. Mammen EF, Alshameeri RS, Comp PC. Preliminary data from a field trial of the PFA‐100 system. Semin Thromb Hemost. 1995;21(Suppl 2):113‐121. doi: 10.1055/s-0032-1313613 [DOI] [PubMed] [Google Scholar]
- 3. Högman CF, Eriksson L, Kristensen J. Leukocyte‐depleted platelets prepared from pooled buffy coat post‐transfusion increment and "in vitro bleeding time" using the Thrombostat 4000/2. Transfus Sci. 1993;14(1):35‐39. doi: 10.1016/0955-3886(93)90051-U [DOI] [PubMed] [Google Scholar]
- 4. Van der Planken MG, Claeys MJ, Vertessen FJ, et al. Comparison of turbidimetric aggregation and in vitro bleeding time (PFA‐100) for monitoring the platelet inhibitory profile of antiplatelet agents in patients undergoing stent implantation. Thromb Res. 2003;111(3):159‐164. doi: 10.1016/j.thromres.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 5. Sap F, Kavaklı T, Kavaklı K, Dizdarer C. The prevalence of von Willebrand disease and significance of in vitro bleeding time (PFA‐100) in von Willebrand disease screening in the İzmir region. Turk J Haematol. 2013;30(1):40‐47. doi: 10.4274/tjh.2011.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Favaloro EJ. Utility of the platelet function analyser (PFA‐100/200) for exclusion or detection of von Willebrand disease: a study 22 years in the making. Thromb Res. 2020;1(188):17‐24. doi: 10.1016/j.thromres.2020.01.029 [DOI] [PubMed] [Google Scholar]
- 7. Favaloro EJ. Commentary: the platelet function Analyser (PFA)‐100 and von Willebrand disease: a story well over 16 years in the making. Haemophilia. 2015;21(5):642‐645. doi: 10.1111/hae.12710 [DOI] [PubMed] [Google Scholar]
- 8. Ardillon L, Ternisien C, Fouassier M, et al. Platelet function analyser (PFA‐100) results and von Willebrand factor deficiency: a 16‐year 'real‐world' experience. Haemophilia. 2015;21(5):646‐652. doi: 10.1111/hae.12653 [DOI] [PubMed] [Google Scholar]
- 9. Favaloro EJ. Clinical utility of closure times using the platelet function analyzer (PFA)‐100/200. Am J Hematol. 2017;92(4):398‐404. doi: 10.1002/ajh.24620 [DOI] [PubMed] [Google Scholar]
- 10. Favaloro EJ. Clinical utility of the PFA‐100. Semin Thromb Hemost. 2008;34:709‐733. doi: 10.1055/s-0029-1145254 [DOI] [PubMed] [Google Scholar]
- 11. Favaloro EJ. Standardisation, regulation, quality assurance and emerging Technologies in Hemostasis: issues, controversies, benefits and limitations. Semin Thromb Hemost. 2007;33:290‐297. doi: 10.1055/s-2007-971816 [DOI] [PubMed] [Google Scholar]
- 12. CLSI . Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline – Third Edition. CLSI EPC28‐A3c. Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 13. Favaloro EJ, Bonar R. External quality assessment/proficiency testing and internal quality control for the PFA‐100 and PFA‐200: an update. Semin Thromb Hemost. 2014;40(2):239‐253. doi: 10.1055/s-0034-1365844 [DOI] [PubMed] [Google Scholar]
- 14. Favaloro EJ, Bonar R. An update on quality control for the PFA‐100/PFA‐200. Platelets. 2018;29(6):622‐627. doi: 10.1080/09537104.2018.1475636 [DOI] [PubMed] [Google Scholar]
- 15. Favaloro EJ. Novel approaches to quality control and external quality assessment for platelet function testing with a focus on the platelet function analyser (PFA‐100 and PFA‐200). Ann Blood. 2019;4:3. doi: 10.21037/aob.2019.01.02 [DOI] [Google Scholar]
- 16. Geevar T, Dave RG, Mathews NS, et al. Laboratory characterization of obligate carriers of type 3 von Willebrand disease with a potential role for platelet function analyzer (PFA‐200). Int J Lab Hematol. 2022;44:603‐609. doi: 10.1111/ijlh.13787 [DOI] [PubMed] [Google Scholar]
- 17. Duan R, Goldmann L, Brandl R, et al. Effects of the Btk‐inhibitors Remibrutinib (LOU064) and Rilzabrutinib (PRN1008) with varying Btk selectivity over Tec on platelet aggregation and in vitro bleeding time. Front Cardiovasc Med. 2021;24(8):749022. doi: 10.3389/fcvm.2021.749022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrios RHS, Burguera Vion V, Álvarez Nadal M, et al. Safety of renal biopsy bleeding prophylaxis with desmopressin. J Int Med Res. 2021;49(9):3000605211040764. doi: 10.1177/03000605211040764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orfanou C, Koutalas I, Valsami S, Staikou C. Uneventful epidural catheter removal in a patient with postoperative acute coronary syndrome receiving emergency triple antithrombotic therapy: a case report. Braz J Anesthesiol. 2021;71(4):454‐457. doi: 10.1016/j.bjane.2021.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belkacemi M, Merad Y, Merbouh MA. Establishment of reference intervals for platelet function analyzer −100 closure time in Algerian adults. PLoS One. 2021;16(4):e0249402. doi: 10.1371/journal.pone.0249402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llobet D, Vallvé C, Tirado I, et al. Platelet hyperaggregability and venous thrombosis risk: results from the RETROVE project. Blood Coagul Fibrinolysis. 2021;32(2):122‐131. doi: 10.1097/MBC.0000000000001006 [DOI] [PubMed] [Google Scholar]
- 22. Anaya R, Rodriguez M, Gil JM, et al. Correlation between PlateletWorks® and PFA‐100® for measuring platelet function before urgent surgery in patients with chronic antiplatelet therapy. J Clin Med. 2021;10(2):255. doi: 10.3390/jcm10020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piao J, Yoo C, Kim S, Whang YW, Choi CU, Shin S. Performance comparison of the PFA‐200 and Anysis‐200: assessment of bleeding risk screening in cardiology patients. Clin Hemorheol Microcirc. 2021;79(3):445‐454. doi: 10.3233/CH-211185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vrigkou E, Tsantes AE, Kopterides P, et al. Coagulation profiles of pulmonary arterial hypertension patients, assessed by non‐conventional hemostatic tests and markers of platelet activation and endothelial dysfunction. Diagnostics (Basel). 2020;10(10):758. doi: 10.3390/diagnostics10100758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rolling CC, Tomada J, Frölich AM, et al. Comparison of acetylsalicylic acid and clopidogrel non‐responsiveness assessed by light transmittance aggregometry and PFA‐100® in patients undergoing neuroendovascular procedures. Clin Chem Lab Med. 2020;59(2):383‐392. doi: 10.1515/cclm-2020-0737 [DOI] [PubMed] [Google Scholar]
- 26. Bij de Weg JM, Abheiden CNH, Fuijkschot WW, et al. Resistance of aspirin during and after pregnancy: a longitudinal cohort study. Pregnancy Hypertens. 2020;19(25–30):25‐30. doi: 10.1016/j.preghy.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 27. Hinterleitner C, Pecher AC, Kreißelmeier KP, et al. Disease progression and defects in primary hemostasis as major cause of bleeding in multiple myeloma. Eur J Haematol. 2020;104(1):26‐35. doi: 10.1111/ejh.13331 [DOI] [PubMed] [Google Scholar]
- 28. Sorensen EN, Plazak ME, Dees LM, et al. Comparison of two individualized antithrombotic protocols in HeartWare HVAD recipients. Artif Organs. 2022;46(1):117‐127. doi: 10.1111/aor.14055 [DOI] [PubMed] [Google Scholar]
- 29. Murakonda VB, Mohapatra A, Geevar T, et al. Does hemodialysis need to be initiated to improve platelet function in CKD G5 patients? A pilot prospective, observational cohort study. Indian. J Nephrol. 2021;31(1):43‐49. doi: 10.4103/ijn.IJN_232_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mammen EF, Comp PC, Gosselin R, et al. PFA‐100 system: a new method for assessment of platelet dysfunction. Semin Thromb Hemost. 1998;24:195‐202. [DOI] [PubMed] [Google Scholar]
- 31. Carcao MD, Blanchette VS, Dean JA, et al. The platelet function analyzer (PFA‐100): a novel in‐vitro system for evaluation of primary haemostasis in children. Br J Haematol. 1998;101:70‐73. [DOI] [PubMed] [Google Scholar]
- 32. Fressinaud E, Veyradier A, Truchaud F, et al. Screening for von Willebrand disease with a new analyzer using high shear stress: a study of 60 cases. Blood. 1998;91:1325‐1331. [PubMed] [Google Scholar]
- 33. Cattaneo M, Federici AB, Lecchi A, et al. Evaluation of the PFA‐100 system in the diagnosis and therapeutic monitoring of patients with von Willebrand disease. Thromb Haemost. 1999;82:35‐39. [PubMed] [Google Scholar]
- 34. Favaloro EJ, Facey D, Henniker A. Use of a novel platelet function analyzer (PFA‐100) with high sensitivity to disturbances in von Willebrand factor to screen for von Willebrand's disease and other disorders. Am J Hematol. 1999;62:165‐174. [DOI] [PubMed] [Google Scholar]
- 35. Kerenyi A, Schlammadinger A, Ajzner E, et al. Comparison of PFA‐100 closure time and template bleeding time of patients with inherited disorders causing defective platelet function. Thromb Res. 1999;96:487‐492. [DOI] [PubMed] [Google Scholar]
- 36. Harrison P, Robinson MS, Mackie IJ, et al. Performance of the platelet function analyser PFA‐100 in testing abnormalities of primary haemostasis. Blood Coagul Fibrinolysis. 1999;10:25‐31. [DOI] [PubMed] [Google Scholar]
- 37. Dean JA, Blanchette VS, Carcao MD, et al. von Willebrand disease in a paediatric‐based population ‐ comparison of type 1 diagnostic criteria and use of the PFA‐100® and a von Willebrand factor/collagen‐bindiing assay. Thromb Haemost. 2000;84:401‐409. [PubMed] [Google Scholar]
- 38. Schlammadinger A, Kerenyi A, Muszbek L, Boda Z. Comparison of the O'Brien filter test and the PFA‐100 platelet analyser in the laboratory diagnosis of von Willebrands disease. Thromb Haemost. 2000;84:88‐92. [PubMed] [Google Scholar]
- 39. Ortel TL, James AH, Thames EH, Moore KD, Greenberg CS. Assessment of primary hemostasis by PFA‐100 analysis in a tertiary care center. Thromb Haemost. 2000;84:93‐97. [PubMed] [Google Scholar]
- 40. Favaloro EJ, Kershaw G, Bukuya M, Hertzberg M, Koutts J. Laboratory diagnosis of von Willebrand disorder (VWD) and monitoring of DDAVP therapy: efficacy of the PFA‐100® and VWF:CBA as combined diagnostic strategies. Haemophilia. 2001;7:180‐189. [DOI] [PubMed] [Google Scholar]
- 41. Harrison P, Robinson M, Liesner R, et al. The PFA‐100: a potential rapid screening tool for the assessment of platelet dysfunction. Clin Lab Haematol. 2002;24:225‐232. [DOI] [PubMed] [Google Scholar]
- 42. Buyukasik Y, Karakus S, Goker H, et al. Rational use of the PFA‐100 device for screening of platelet function disorders and von Willebrand disease. Blood Coagul Fibrinolysis. 2002;13:349‐353. [DOI] [PubMed] [Google Scholar]
- 43. Wuillemin WA, Gasser KM, Zeerleder SS, Lämmle B. Evaluation of a platelet function Analyser (PFA‐100) in patients with a bleeding tendency. Swiss Med Wkly. 2002;132:443‐448. [DOI] [PubMed] [Google Scholar]
- 44. Nitu‐Whalley IC, Lee CA, Brown SA, Riddell A, Hermans C. The role of the platelet function analyser (PFA‐100) in the characterization of patients with von Willebrand's disease and its relationships with von Willebrand factor and the ABO blood group. Haemophilia. 2003;9:298‐302. [DOI] [PubMed] [Google Scholar]
- 45. Cariappa R, Wilhite TR, Parvin CA, Luchtman‐Jones L. Comparison of PFA‐100 and bleeding time testing in pediatric patients with suspected hemorrhagic problems. J Pediatr Hematol Oncol. 2003;25:474‐479. [DOI] [PubMed] [Google Scholar]
- 46. Posan E, McBane RD, Grill DE, Motsko CL, Nichols WL. Comparison of PFA‐100 testing and bleeding time for detecting platelet hypofunction and von Willebrand disease in clinical practice. Thromb Haemost. 2003;90:483‐490. [DOI] [PubMed] [Google Scholar]
- 47. Favaloro EJ. Template bleeding time and PFA‐100 have low sensitivity to screen patients with hereditary mucocutaneous hemorrhages: comparative study of 148 patients‐‐a rebuttal. J Thromb Haemost. 2004;2:2280‐2282. [DOI] [PubMed] [Google Scholar]
- 48. Quiroga T, Goycoolea M, Muñoz B, et al. Template bleeding time and PFA‐100 have low sensitivity to screen patients with hereditary mucocutaneous hemorrhages: comparative study in 148 patients. Thromb Haemost. 2004;2:892‐898. [DOI] [PubMed] [Google Scholar]
- 49. Koscielny J, Ziemer S, Radtke H, et al. A practical concept for preoperative identification of patients with impaired primary hemostasis. Clin Appl Thromb Hemost. 2004;10:195‐204. [DOI] [PubMed] [Google Scholar]
- 50. Philipp CS, Miller CH, Faiz A, et al. Screening women with menorrhagia for underlying bleeding disorders: the utility of the platelet function analyser and bleeding time. Haemophilia. 2005;11:497‐503. [DOI] [PubMed] [Google Scholar]
- 51. Penas N, Pérez‐Rodríguez A, Torea JH, et al. von Willebrand disease R1374C: type 2A or 2M? A challenge to the revised classification. High frequency in the northwest of Spain (Galicia). Am J Hematol. 2005;80:188‐196. [DOI] [PubMed] [Google Scholar]
- 52. Favaloro EJ, Lloyd J, Rowell J, et al. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [biostate and AHF (high purity)] in people with von Willebrand disorder. A randomised cross‐over, multi‐Centre study. Thromb Haemost. 2007;97:922‐930. [PubMed] [Google Scholar]
- 53. Perel JM, Just S, Rowell J, Williams B, Kennedy GA. Utility of the PFA‐100 analyser in the evaluation of primary haemostasis in a paediatric population. Int J Lab Hematol. 2007;29:480‐481. [DOI] [PubMed] [Google Scholar]
- 54. Vazquez‐Santiago M, Vilalta N, Cuevas B, et al. Short closure time values in PFA‐100 are related to venous thrombotic risk. Results from the RETROVE study. Thromb Res. 2018;169:57‐63. [DOI] [PubMed] [Google Scholar]
- 55. Favaloro EJ, Gosselin R, Olson J, Jennings I, Lippi G. Recent initiatives in harmonization of hemostasis practice. Clin Chem Lab Med. 2018;56(10):1608‐1619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Study sites participating in this evaluation*
Supplementary Table S2. Summary of normality tests “passed” using reference range (NRR) data from current report.*
Supplementary Figure S1 Study summary. A summary of the main evaluations undertaken as part of this study.
Supplementary Figure S2 Comparison of closure time (CT) data obtained using PFA‐100 versus PFA‐200 at Site A. Figure A shows data for C/Epi (n = 168 samples), and B shows data for C/ADP (n = 153 samples), co‐tested over a period of 5 years. Data shown as Box and Whiskers with 10‐90th percentiles. There was no statistically significant difference in test results (p values shown, generated using the two‐tailed Mann–Whitney test).
Supplementary Figure S3 A, B. Comparison of CTs (C/Epi [A] and C/ADP [B]; sec) and platelet count (x109/L) and haematocrit values for normal individuals where combined testing was done. Data from 3 participant laboratory sites. Several high‐outlier CTs obtained for C/Epi shown on right portion of figure; these outliers could not be generally linked to low platelet counts or low haematocrit. C, D. Comparison of CTs (C/Epi [C] and C/ADP [D]; sec) and VWF level (VWF:Ag) and activity (VWF:CB or VWF:RCo) where combined testing was done. Data from 1 participant laboratory site. Several high‐outlier CTs obtained for C/Epi shown on right of figure; these outliers could not be generally linked to low levels of VWF or VWF activity. However, there was a statistically significant relationship between C/Epi and C/ADP (all figures), as well as between C/ADP and VWF level and activity (Figure D), which was not evident for any other comparison. Low cut‐off values for other parameters are as follows: platelet count (150 × 109/L), haematocrit (females; 0.355), VWF level (VWF:Ag, 50 U/dL) or activity (50 U/dL VWF:CB; 40 U/dL VWF:RCo).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


