Abstract
Introduction
Decades of inherited bleeding disorders (BD) research transformed severe haemophilia from a childhood killer to a disorder managed across a full lifespan for many in economically developed countries. Health equity, a life unimpaired by disease complications, however, remains unimaginable for most people with an inherited BD (PWIBD).
Aim
The National Hemophilia Foundation (NHF) and American Thrombosis and Hemostasis Network (ATHN) undertook the development of a community‐driven United States (US) National Blueprint for Inherited Bleeding Disorders Research to transform the experience of all PWIBD and those who care for them.
Methods
Extensive community consultations were conducted to identify the issues most important to PWIBD and those who love and care for them. Expert multidisciplinary teams distilled these key areas of need into prioritised research questions, and identified the resources and infrastructure required to pursue them. A summit was held to gather feedback and inform the detailed blueprint.
Results
Community‐prioritised research areas fell into three broad categories: issues common across inherited BDs, those specific to individual disorders, and issues of infrastructure and capacity. NHF State of the Science Research Summit discussions of the research questions derived from the community priorities by six working groups provided important input for the drafting of the research blueprint for the coming decades.
Conclusion
The inherited BD community came together to develop the US National Blueprint for Inherited Bleeding Disorders Research dedicated to transforming the lives of all PWIBD including innovating solutions for the rarest disorders and under‐represented populations.
Keywords: advocacy, blueprint, community, health equity, inherited bleeding disorders, research
1. INTRODUCTION
Historically, inherited bleeding disorders (BD) research priorities have been defined by researchers and industry. Thirty years ago, focused almost exclusively on men with haemophilia A (clotting factor [F] VIII deficiency) and B (FIX deficiency), inherited BD research sought a safe source of replacement factor to control bleeding and provide a comparable life expectancy to that of people without an inherited BD. 1 , 2 , 3 Today, these goals have been attained. In economically developed countries, people with haemophilia (PWH) who achieve haemostatic control through prophylactic factor replacement and multidisciplinary team‐based care enjoy a life expectancy similar to the average male population and a very low annualised bleeding rate (ABR), 1 , 4 , 5 , 6 accompanied by better joint outcomes for children 7 , 8 and adults. 9 As gene therapy appears close to offering a ‘functional cure’ for these two factor deficiencies, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 we must ask if the needs of all people with an inherited BD (PWIBD) are being met.
For decades, narrowly focused clinical trial designs missed opportunities to generate robust data supporting some of the most effective treatment/diagnostic practices and the outcomes most important to PWIBD. For example, despite numerous haemophilia prophylaxis clinical trials, in 2015 the German Institute for Quality and Efficiency in Health Care (IQWiG) reported that, due to the overall inadequate quality of data for the comparison one could only conclude that prophylaxis versus on‐demand treatment of children with FVIII offered a hint of an added benefit with regard to severe bleeding but no hint of an added benefit with regard to state of health and pain, all‐cause mortality and life‐threatening bleeding, and joint function and health‐related quality of life (QoL). 11 Healthcare budget constraints mean an increased focus on optimising resource allocation, therefore robust data demonstrating the value of interventions that affect outcomes of importance to PWH are critical to ensuring (continued) access. 12 Such data are also required to inform clinical practice guidelines that support education, advocacy, and decision‐making related to treatment and the delivery of care. 13 Those designing and conducting clinical trials have a responsibility to optimise the collection and sharing of data on outcomes that matter most to people with the disorder, especially in rare disorders with their inherent scarcity of potential trial participants. 14 , 15
Equity, defined by the World Health Organization (WHO) as the ‘absence of avoidable or remediable differences among groups of people,’ 16 remains out of reach for all but the most fortunate PWIBD. Simply surviving or not bleeding is far from attaining a life unimpaired by disease complications. 1 Progress towards equity is measured through patient‐important, often patient‐centred, impacts which include clinical/medical outcomes but also how a disease and/or its treatments may impact the life of a patient or their family (e.g. caregiver/family stresses, economic burden, etc). 12 , 17 , 18 Significant strides remain to be made in fundamental areas such as eliminating morbidity, disability, and pain. The ability to participate fully in normal family, career, and social activities, and to live an unrestricted lifestyle are key milestones on the path to optimal wellness, just as freedom from spontaneous bleeding events and the need for additional intervention in the context of minor trauma or surgery characterise a clinical progression towards normal haemostasis. 1
Recent and ongoing developments in haemophilia therapeutics present great promise, however, the vast majority of PWIBD cannot currently achieve health equity. Von Willebrand disease (VWD), the most common inherited BD, inherited equally by men and women, is clinically complex and technically challenging to diagnose, 19 yet far fewer resources have been devoted to it than to haemophilia. Rare and ultra‐rare inherited BDs which cause significant morbidity remain difficult to diagnose, and are largely underdiagnosed globally despite technical advances. 20 , 21 Evidence‐based guidelines for the treatment of most inherited BDs are largely absent, due to the lack of supporting aggregate data. 22 Sexism has been an issue in BD for centuries. Women and girls with bleeding disorders (WGBD) experience lengthy delays in diagnosis and frequently encounter ignorance, stigmatisation, and dismissal of their symptoms by healthcare professionals (HCP) and society. 23 Health equity is also limited by greater socio‐historical constraints. It cannot be achieved through health‐specific projects alone, particularly for individuals who embody the cumulative effects of historic and current marginalisation, if the mechanisms generating inequalities are not addressed. 24
It is an exciting time in inherited BD research. New technologies and analytical tools, infusions of research funding, and blossoming international interest present an opportunity to dramatically improve the experiences of PWIBD. So, how should the research priorities for the next several decades be set? The National Hemophilia Foundation (NHF) and the American Thrombosis and Hemostasis Network (ATHN) propose a radically novel approach: people who live with inherited BDs every day of their lives are subject matter experts (SME) possessing unique and important expertise about their disorders and they should set the research agenda, aligned with what is important to them. Priorities must be driven by the community, the individuals and family members, specialists, and allied HCPs most invested in minimising the burden of inherited BDs and innovating solutions for the rarest disorders and under‐represented populations.
In 2020, NHF launched a transformative community initiative to shape research priorities seeking to ensure every PWIBD has access to safe, effective, convenient therapeutics, diagnostics, and digital technologies to deliver optimal health outcomes at the lowest total cost of care (Figure 1). Extensive inclusive community consultations identified the issues most important to PWIBD and those who love and care for them. Multidisciplinary teams of HCPs, researchers, and SMEs distilled these key areas of need into prioritised research questions, and identified the resources and infrastructure required to pursue them. Their findings were reviewed by the community at a State of the Science Research Summit (SOSRS), reports of which are currently in preparation for publication as components of a research agenda. Herein, the authors detail the process of building a community‐driven United States (US) National Blueprint for Inherited Bleeding Disorders Research. The resulting blueprint will embrace people‐centric principles and holistically address the priorities of the inherited BD community, especially its under‐represented populations. This report offers insights relevant to the international inherited BD community, and the approach can be applied to any area of health research, anywhere.
FIGURE 1.
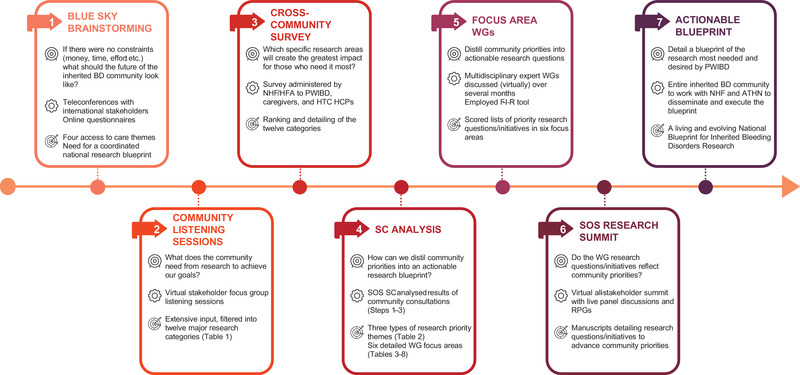
Objective, working, and output of the seven steps in the process of building a community‐generated national research blueprint. Abbreviations: ATHN, American Thrombosis and Hemostasis Network; BD, bleeding disorders; F‐I‐R, feasibility‐impact‐risk; HCP, healthcare professional; HFA, Hemophilia Federation of America; HTC, haemophilia treatment centre; NHF, National Hemophilia Foundation; PWIBD, people with inherited bleeding disorders; RPG, remote participation group; SC, steering committee; SOS, State of the Science; WG, working group
2. COMMUNITY CONSULTATIONS
2.1. ‘Blue Sky’ brainstorming
In June and July 2020, NHF conducted a series of ‘blue sky’ teleconferences asking what the future of the inherited BD community should look like, if neither money, time, nor effort were limiting. The responses of the 101 participants from across the international inherited BD community, including physicians, nurses, other HCPs, SMEs, patient organisation leaders (national and regional), and NHF staff and board members, coalesced into four themes, all centred‐on access to care:
Sustaining and expanding the comprehensive care model to strive towards health equity.
Harnessing/leveraging technology to expand access to care.
Ensuring sustainability of the comprehensive care model thereby promoting access to care.
Envisioning the NHF in 2030.
An overarching conclusion of these discussions, and the 130 responses to a follow‐up online community questionnaire, was the need for a coordinated national research plan. Research will be key in transforming the future of the inherited BD community, and SMEs must be central in defining the agenda for the coming decades.
The details of the Blue Sky project will be reported separately.
2.2. Virtual community listening sessions
Having established the primacy of access to care and the need to develop a coordinated national research blueprint, NHF, in collaboration with the Centre for Information and Study on Clinical Research Participation (CISCRP) and Tufts University Centre of Study of Drug Development, conducted a series of focus group listening sessions from June to November 2020. They asked the inherited BD community what they need from research to achieve their life goals. Due to pandemic restrictions, all listening sessions were conducted virtually. Input was solicited from diverse stakeholders including PWIBD, parents and caregivers, patient organisation representatives, local chapter and member organisation directors, HCPs, and industry representatives (Table S1). Participants included representation of the following inherited BDs: haemophilia A, haemophilia B, VWD, platelet dysfunction, Glanzmann thrombasthenia, and deficiencies of FVII, FX, FXI, and FXIII. Their expressed needs fell into twelve major research categories (Table 1).
TABLE 1.
Major categories of community‐prioritised research
| Access to specialised care |
| Access to research and mechanisms (ways) to conduct research |
| Continuation of the HTC model of care (training future HCPs and their financial sustainability) |
| Health care differences in various communities (based on race, ethnicity, gender, education, income, etc.) |
| Joint disease and management |
| Mental health (depression, anxiety, emotional impact, substance misuse, etc.) |
| Pain management |
| Treatment for all BD (gene therapy, non‐factor replacements, etc.) |
| Treatment for, specifically, other RBDs (non‐haemophilia, non‐VWD) |
| Treatment of other chronic diseases and issues affecting those with BDs – such as heart disease or diabetes, etc. |
| Treatment of the ageing population with BD |
| Women's health and care |
Abbreviations: BD, bleeding disorder; HCP, healthcare provider; HTC, haemophilia treatment centre; RBD, rare bleeding disorder; VWD, von Willebrand disease.
2.3. Cross‐community survey
In order to better understand which specific research areas offer the greatest potential to transform the lives of PWIBD, especially those who need it the most, NHF created a survey to invite a wider community to rank and comment on the twelve major research categories identified above (Table 1). The survey was administered through NHF's Chapters and Haemophilia Federation of America (HFA) member organisations to the PWIBD and caregiver community, and by NHF to haemophilia treatment centre (HTC) HCPs, in January 2021.
The 335 survey participants included 125 PWIBD and 112 HCPs. The top‐ranked focus was sustaining the HTC care model, including ensuring training of future HCPs with expertise in inherited BDs and the financial stability of the centres. Many respondents emphasised the need for more research into new therapies for all inherited BDs, not just severe haemophilia. Access to care, across diverse communities and including specialised care (e.g. ultra‐rare disorders), were priorities shared across stakeholder groups. Mental health, joint disease, ageing with inherited BDs, and pain management were the top daily living concerns.
3. DISTILLING COMMUNITY PRIORITIES INTO ACTIONABLE RESEARCH QUESTIONS
Early in the blueprint development, NHF engaged independent consultant, Donna DiMichele, MD, for guidance patterning the process on her previous work with the National Heart, Lung, and Blood Institute (NHLBI) State of the Science on FVIII inhibitors in 2018. 25 , 26 NHF and ATHN completed the SOSRS Executive Committee (EC), co‐chaired by the NHF President and Chief Executive Officer and ATHN's Chief Science Officer. Together they recruited inherited BD community experts to the Steering Committee (SC) with partner federal agency representatives in an advisory role (Table S2).
3.1. Focus area working groups
The SC studied all of the input gathered through community consultations. They formulated a number of research themes that they categorised into three types: those pertaining to all inherited BDs, those specific to individual inherited BDs, and infrastructure and capacity opportunities (Table 2).
TABLE 2.
Three types of community‐prioritised inherited BD research themes
| Category | Prioritised themes |
|---|---|
| Themes applicable across inherited BDs |
|
| Priorities specific to individual inherited BDs |
|
| Infrastructure and capacity opportunities |
|
Abbreviations: BD, bleeding disorder; HTC, haemophilia treatment centre; LGBTQ, lesbian, gay, bisexual, transgender, queer; NHF, National Hemophilia Foundation; POC, point of care; PWIBD, people with an inherited bleeding disorder; QoL, quality of life; RBD, rare bleeding disorder; VWD, von Willebrand disease.
The SC then organised the community‐identified priorities into six focus areas and recruited expert Working Groups (WG) (Table S3) to distil each into research questions (Tables 3, 4, 5, 6, 7, 8). WGs featured diverse voices from across the inherited BD community including those of under‐represented groups (e.g. WGBD, the lesbian, gay bisexual, transgender, and queer [LGBTQ] community, minority ethnic populations), at least one SME, an NHF Board member, an NHF staff member, HCPs from multiple disciplines (nursing, social work, physiotherapy, haematology, obstetrics, gynaecology, dentistry, etc. as appropriate), and industry professionals. SMEs were actively encouraged by WG co‐chairs to contribute to all discussions and their input frequently solicited. An NHF support person met with them periodically, individually and in small groups, throughout the process to accompany and empower them to contribute confidently. Members were recruited primarily from the US with a few international invitees. Expertise external to the inherited BD community was also sought where necessary to address the full breadth of community‐identified priorities.
TABLE 3.
WG 1: research priorities for haemophilia A and B
| Co‐chairs |
Duc Quang Tran, Jr., MD, MSc Annette von Drygalski, MD, PharmD, RMSK |
| Key question | How can we use new technologies to discover therapies to improve life with haemophilia while working to deliver a safe and meaningful cure? |
| Community‐identified priorities |
|
Abbreviation: WG, working group.
TABLE 4.
WG 2: research priorities for VWD, platelet dysfunction and other mucocutaneous inherited BDs
| Co‐chairs |
Veronica H. Flood, MD Robert F. Sidonio, Jr., MD, MSc |
| Key question | What is needed to engender more targeted and accessible diagnostics and therapies for all people with these disorders? |
| Community‐identified priorities |
|
Abbreviations: BD, bleeding disorder; PRO, patient‐reported outcome; VWD, von Willebrand disease; WG, working group.
TABLE 5.
WG 3: research priorities for ultra‐rare inherited BDs
| Co‐chairs |
Suchitra S. Acharya, MD Diane Nugent, MD Amy D. Shapiro, MD |
| Key questions |
How can we better understand the biology of these rare disorders? How can we stimulate research and optimise the regulatory process to vastly improve diagnosis and targeted treatment? |
| Community‐identified priorities |
|
Abbreviations: BD, bleeding disorder; WG, working group.
TABLE 6.
WG 4: research priorities for the health of women, girls, and people with the potential to menstruate
| Co‐chairs |
Maureen K. Baldwin, MD, MPH Angela C. Weyand, MD |
| Key questions | How can we improve care for this group of PWIBD through a better understanding of the sex and gender biology of bleeding, as well as through new tools like non‐invasive prenatal testing or therapies for reproductive system bleeding? |
| Community‐identified priorities |
|
Abbreviations: BD, bleeding disorder; PWIBD, person with an inherited bleeding disorder; WG, working group.
TABLE 7.
WG 5: diversity, equity and inclusion, health services research, and implementation science
| Co‐chairs |
Judith R. Baker, DrPH, MHSA Tyler Buckner, MD, MSc Vanessa R. Byams, DrPH, MPH |
| Key questions |
How can we make the greatest impact on equal access to care and more inclusive coverage? What digital tools could be implemented to reach this goal? |
| Community‐identified priorities |
|
Abbreviations: BD, bleeding disorder; PRO, patient‐reported outcome; WG, working group.
[Correction added on 15 July 2022, after first online publication: The PhD degree was changed to DrPH for “Judith R. Baker” and “Vanessa R. Byams”.]
TABLE 8.
WG 6: facilitating priority research in the inherited BD community
| Co‐chairs |
Margaret V. Ragni, MD, MPH (Resources and Funding) Jordan Shavit, MD, PhD (Workforce) Guy Young, MD (Infrastructure) |
| Key questions |
How can we build and fund a research network that is centred on care delivery and designed to reduce the burden of participation? How can we encourage more trainees to join our professional community so that PWIBD are assured of having care providers well into the future? |
| Community‐identified research priorities |
|
Abbreviations: BD, bleeding disorder; PWIBD, person with an inherited bleeding disorder; WG, working group.
Under the guidance of the SC, and led by their co‐chairs, the WGs met (virtually) frequently throughout the first half of 2021 to study their community‐prioritised themes. They consulted the literature and existing resources, convened expert speakers, debated internally, and engaged in inter‐group cross‐talk. They asked whether enough data exist to determine the most important hypothesis‐based research questions in their focus area. If so, the WG defined specific actionable research questions to drive meaningful and lasting change in the evidence‐based care of PWIBD. If not, they were challenged to identify how a better dataset could be established through, for example, observational data collection, pilot clinical studies, or mechanistic or point of care (POC) research.
3.2. Feasibility, impact, and risk
The WG deliberations yielded many more excellent research questions than can realistically be immediately pursued, therefore, one of the authors (DDM) devised a prioritisation matrix (Figure 2). This framework of scored questions guided WGs 1‐5 in evaluating the feasibility, impact, and risk associated with each question (Table S4). Feasibility assessed the difficulty in answering the proposed question, including required expertise, infrastructure, and resources. Impact estimated the change to be affected (e.g. to standards of care or access to care), whether it might change a therapeutic paradigm or be applicable to other areas. Risk, scored with increasingly negative values, assessed the challenges facing each question, such as the risk/benefit ratio for novel therapies or ethical considerations. WG 6 scored on the same three dimensions, using pre‐specified criteria to evaluate infrastructure, resources/funding, and future workforce development models rather than research questions. Summing across the three dimensions permitted relative prioritisation of very different initiatives in very different contexts. While an ‘easy’ question mightscore well on feasibility criteria, a more challenging question with greater potential impact could yield an equally high sum result.
FIGURE 2.
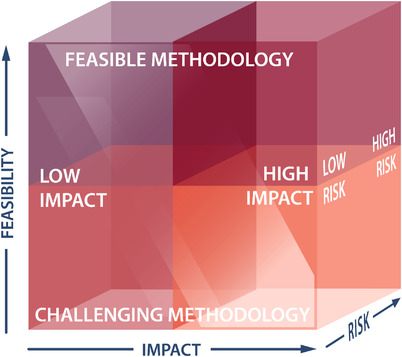
Ranking research questions on three dimensions: feasibility, impact, and risk.
3.3. Roles for NHF and ATHN
WGs were tasked with identifying how NHF might support or facilitate their research priorities. As the largest inherited BD patient organisation, NHF is committed to a world without inherited BDs, a vision that starts with research. 27 Applying a people‐centred approach to all initiatives, it has the capacity and expertise to support research in myriad ways including advocacy, education, policy change, liaising with regulators, and funding.
The WGs were also charged with identifying opportunities to leverage existing infrastructure and resources to pursue research priorities. ATHN constitutes one such opportunity. ATHN partners with 146 US HTCs to build a safe, secure national database—the ATHNdataset. 28 , 29 The network's goal is to secure data, advance knowledge, transform care and ultimately improve the lives of people with bleeding and clotting disorders. Their standardised integrated systems, data, and processes offer a unique opportunity to efficiently initiate novel collaborative inclusive research within an established network, decreasing the time and cost involved.
4. STATE OF THE SCIENCE RESEARCH SUMMIT
12–15 September 2021, NHF hosted the State of the Science (virtual) Research Summit (SOSRS), an opportunity for all community members to weigh in on the research questions and infrastructure initiatives prioritised by the WGs. The Summit, supported entirely by NHF, with no funding from industry or commercial partners, was open to all without charge. NHF, ATHN, and partner social media and email campaigns featured SME and HCP testimonials emphasising the opportunity to direct inherited BD research towards PWIBD priorities (Figure S1). The NHF SOSRS was well attended with 441 unique attendees from all stakeholder groups (Table S5).
The co‐chairs presented the research questions derived by each WG in response to the community‐identified priorities, in the context of expert plenary presentations and SME vignettes illustrating current unmet needs (Table S6). A live 90‐min panel discussion engaged co‐chairs, several WG members including SMEs, and the plenary speaker with real‐time input from attendees, to validate whether the WG conclusions reflected their goals. A final 2‐h panel discussion between the SC, WG co‐chairs, and SMEs, with live community input, synthesised the learnings from the entire Summit. Recordings of all SOSRS sessions are available on NHF's YouTube channel: https://www.youtube.com/user/NHFvideo.
4.1. Remote participation
The development of a research blueprint advancing equity for all PWIBD must recognise that the broader social milieu of inequity, that has been inscribed on the very bodies of those facing disparity, also erects barriers to their participation in consultative initiatives. 24 NHF obtained an NHLBI R13 grant (R13HL158209) to increase outreach to, and reduce the burden of participation on, under‐represented populations through the organisation and facilitation of Remote Participation Groups (RPG). This option was offered to groups of Black/African Americans, LatinX Americans, Asian Americans, Indigenous Americans, the LGBTQ+ community, the ageing community, and those living rurally or geographically challenged. Five RPGs with 3–15 participants each, for a total of 40, amplified input from: Black/African Americans, ageing men, the LGBTQ+ community, women, and Hispanic women (RPG conducted in Spanish). RPGs met virtually or gathered locally, per preferences and pandemic restrictions, once, to view their choice of SOSRS sessions (live or recorded) with one or two facilitators. Facilitators offered explanations to empower RPG members’ understanding of the content and confidence in responding to it. Participants shared real‐time perspectives and comments to which the SOSRS panels were invited to respond (see also below). 30 Facilitator reports summarising RPG discussions, particularly whether the research priorities presented resonated with participants, whether any important priorities had been omitted, what their hopes for Summit outcomes were, and how NHF can engage the SME community to continue to advance the research priorities, were shared with WG co‐chairs and informed blueprint component manuscripts (currently in preparation).
4.2. Building the blueprint
With the conclusion of the NHF SOSRS the next phase in the formulation of a community‐generated national research plan began. Each WG, lead by their co‐chairs, will now refine their conclusions with the insights from the Summit panel discussions and RPGs into individual manuscripts. Their compiled final reports will be offered back to the community, published as the National Blueprint for Inherited Bleeding Disorders Research.
5. EXECUTING THE BLUEPRINT
NHF and ATHN undertook a transformative community‐driven initiative to shape the inherited BD research priorities of the future, to build upon the exciting progress made in recent years and to dramatically accelerate initiatives making the greatest difference to the individuals and families living with these disorders. They started with wide‐open community consultation, enlisted the expertise of SMEs, HCPs and researchers from within and beyond the inherited BD community, and brought the conclusions full circle for community scrutiny. The culmination of this extensive inclusive process, the publication of the National Blueprint for Inherited Bleeding Disorders Research, will mark the beginning of the most important work. This Blueprint must not become yet another report relegated to gather dust on a shelf. As a blueprint it must be executed to serve its function: to build the research most needed and desired by PWIBD.
NHF and ATHN commit to actively and transparently ensuring the execution of the Blueprint through communications, education, and implementation volets. They will map out how the Blueprint will move the whole community towards transformational change, defining stakeholder roles and identifying resources required, where and how best to deploy those resources, action timelines, and mechanisms for follow‐up, evaluation, and evolution.
NHF and ATHN call upon the entire inherited BD community to engage with them in ensuring the execution of the Blueprint. Academic and industry investigators and management should look to the Blueprint for guidance as they design their research. Funding agencies have a responsibility to accelerate research progress in the areas of greatest need for affected individuals and their families. Advocates, policy makers, and regulators must bring about the changes that will facilitate a more efficient and affordable research to care pipeline delivering safe, effective, and convenient diagnostics, therapeutics, and digital technologies for all inherited BDs. Everyone must address health disparities, access barriers, and gaps in care and proactively advance equity for all PWIBD. And SMEs, and those who care for them, must continue to provide input and insights, to engage with and participate in research, and to hold all of the preceding stakeholders accountable to bring the National Blueprint for Inherited Bleeding Disorders Research to life.
CONFLICT OF INTEREST
LAV, MES, and MLW state that they have no interests which might be perceived as posing a conflict or bias. The opinions expressed in this manuscript by LAV are his own and not necessarily reflective of those of the National Hemophilia Foundation or its Board of Directors. DDM acted as a paid consultant to the National Hemophilia Foundation in the design, organisation, and execution of the State of the Science Research Summit on Inherited Bleeding Disorders. MR's employers have received research support from Bayer, BioMarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark, Takeda, and uniQure. MR has acted as a paid consultant to Catalyst Biosciences, CSL Behring, Genentech, Hema Biologics, Kedrion, Novo Nordisk, Octapharma, Pfizer, Sanofi, Takeda, and uniQure. He is on the Board of Directors of the Foundation for Women and Girls with Blood Disorders and Partners in Bleeding Disorders.
AUTHOR CONTRIBUTIONS
Leonard A. Valentino conceived of the development of the national research blueprint, contributed to the development and organisation of the SOSRS, and had input into all aspects of writing of this manuscript. Michelle L. Witkop and Maria E. Santaella contributed to SOSRS development and organisation, data collection and analysis, and had input into all aspects of writing and editing this manuscript. Donna DiMichele contributed to the development and organisation of the SOSRS, data collection and analysis, and manuscript editing. Michael Recht contributed to the development and organisation of the SOSRS and had input into all aspects of writing this manuscript.
[Correction added on 15 July 2022, after first online publication: Table S3 was replaced with a revised version.]
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R13HL158209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Fiona Robinson, PhD provided professional medical writing support during manuscript development. The entire State of the Science Research Summit initiative and this manuscript were funded by National Hemophilia Foundation.
Valentino LA, Witkop ML, Santaella ME, DiMichele D, Recht M. Building the blueprint: Formulating a community‐generated national plan for future research in inherited bleeding disorders. Haemophilia. 2022;28:760–768. 10.1111/hae.14588
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Skinner MW, Nugent D, Wilton P, et al. Achieving the unimaginable: health equity in haemophilia. Haemophilia. 2020;26(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evatt BL. The tragic history of AIDS in the hemophilia population, 1982‐1984. J Thromb Haemost. 2006;4(11):2295‐2301. [DOI] [PubMed] [Google Scholar]
- 3. Pierce GF. Uncertainty in an era of transformative therapy for haemophilia: addressing the unknowns. Haemophilia. 2021;27(3):103‐113. [DOI] [PubMed] [Google Scholar]
- 4. Oldenburg J, Dolan G, Lemm G. Haemophilia care then, now and in the future. Haemophilia. 2009;15(1):2‐7. [DOI] [PubMed] [Google Scholar]
- 5. Young G. Management of children with hemophilia A: how emicizumab has changed the landscape. J Thromb Haemost. 2021;19(7):1629‐1637. [DOI] [PubMed] [Google Scholar]
- 6. Valentino L, Baker J, Butler R, et al. Integrated hemophilia patient care via a national network of care centers in the United States: a model for rare coagulation disorders. J Blood Med. 2021;12:897‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manco‐Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535‐544. [DOI] [PubMed] [Google Scholar]
- 8. Warren BB, Thornhill D, Stein J, et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the Joint Outcome Continuation Study. Blood Adv. 2020;4(11):2451‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manco‐Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115‐2124. [DOI] [PubMed] [Google Scholar]
- 10. Thornburg CD. Prepare the way for hemophilia a gene therapy. N Engl J Med. 2022;386(11):1081‐1082. [DOI] [PubMed] [Google Scholar]
- 11. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen . Executive Summary of Rapid Report: Treatment of People with Haemophilia. Federal Ministry of Health; 2015. [Google Scholar]
- 12. O'Mahony B, Dolan G, Nugent D, Goodman C, International haemophilia access strategy C. Patient‐centred value framework for haemophilia. Haemophilia. 2018;24(6):873‐879. [DOI] [PubMed] [Google Scholar]
- 13. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd ed. Haemophilia. 2020;26(6):1‐158. [DOI] [PubMed] [Google Scholar]
- 14. O'Mahony B, Wong O, Eichler H, Neumann P, Carlsson KS, Noone D. Preparing for tomorrow: defining a future agenda. Haemophilia. 2022;28(2):35‐41. [DOI] [PubMed] [Google Scholar]
- 15. Skinner MW, Dolan G, Eichler H, O'Mahony B, International haemophilia access strategy C. A preliminary application of a haemophilia value framework to emerging therapies in haemophilia. Haemophilia. 2022;28(Suppl 2):9‐18. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Health equity [Aug 2, 2021]. Available from: https://www.who.int/westernpacific/health‐topics/equity
- 17. Konkle BA, Skinner M, Iorio A. Hemophilia trials in the twenty‐first century: defining patient important outcomes. Res Pract Thromb Haemost. 2019;3(2):184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perfetto EM, Kennedy A, Birght J. Contributor: A Vision for Patient‐Centered Core Impact Sets—A Unifying Approach to Patient Centricity 2021 [Dec 13, 2021]. Available from: https://www.ajmc.com/view/contributor‐a‐vision‐for‐patient‐centered‐core‐impact‐sets‐a‐unifying‐approach‐to‐patient‐centricity
- 19. Laffan M, Sathar J, Johnsen JM. von Willebrand disease: diagnosis and treatment, treatment of women, and genomic approach to diagnosis. Haemophilia. 2021;27(Suppl 3):66‐74. [DOI] [PubMed] [Google Scholar]
- 20. Meijer K, van Heerde W, Gomez K. Diagnosis of rare bleeding disorders. Haemophilia. 2021;27(3):60‐65. [DOI] [PubMed] [Google Scholar]
- 21. Batsuli G, Kouides P. Rare coagulation factor deficiencies (Factors VII, X, V, and II). Hematol Oncol Clin North Am. 2021. [DOI] [PubMed] [Google Scholar]
- 22. Shapiro A. The use of prophylaxis in the treatment of rare bleeding disorders. Thromb Res. 2020;196:590‐602. [DOI] [PubMed] [Google Scholar]
- 23. Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2021;5(1):51‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Creary MS. Bounded justice and the limits of health equity. J Law Med Ethics. 2021;49(2):241‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pipe SW, Sabatino DE, Nugent DJ, et al. Executive summary of the NHLBI State of the Science (SOS) Workshop: overview and next steps in generating a national blueprint for future research on factor VIII inhibitors. Haemophilia. 2019;25(4):610‐615. [DOI] [PubMed] [Google Scholar]
- 26. Sabatino DE, Pipe SW, Nugent DJ, et al. Origins and organization of the NHLBI State of the Science Workshop: generating a national blueprint for future research on factor VIII inhibitors. Haemophilia. 2019;25(4):575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Hemophilia Foundation. Mission & History [Jan 5, 2022]. Available from: https://www.hemophilia.org/who‐we‐are/our‐story/mission‐history
- 28. American Thrombosis and Hemostasis Network. What We Do Overview [Jan 5, 2022]. Available from: https://athn.org/what‐we‐do/overview.html
- 29. American Thrombosis and Hemostasis Network. Advancing research with better data through the ATHNdataset [Nov 12, 2021]. Available from: https://athn.org/what‐we‐do/national‐projects/athndataset.html
- 30. Santaella ME, Witkop ML, Mills K, Recht M, DiMichele D, Valentino LA. National Hemophilia Foundation Enlists Diverse Patient Voices to Inform a National Research Blueprint for Inherited Bleeding Disorders. 63rd American Society of Hematology (ASH) Annual Meeting and Exposition; Dec 10‐14, 2021; Atlanta, GA (and virtual): Blood. 2021;138(Suppl 1):1904. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
Data available on request from the authors.


