Abstract
In Finland, a systematic public health programme was implemented from 2008 to 2018 to mitigate the burden of allergic disorders by revisiting the prevention strategy. Allergy health and contacts with natural environment were emphasized to promote immunological and psychological resilience instead of poorly justified avoidance. Allergy management practices were improved and low‐valued recommendations for care, for example for food allergy, were revised. Patients and families were empowered to use guided self‐management to proactively stop symptom exacerbations. A professional non‐governmental organization implemented the nationwide education for healthcare and patient NGOs for patients, families and lay public. In healthcare, the work supporting allergic patients and families was organized towards common goals and integrated into everyday work without extra costs. Reaching the predefined goals was followed by employing the national healthcare registers and questionnaire surveys. Governmental bodies contributed with kick‐off funding, which was supplemented by private funding. International collaboration, for example with the European patient organization (EFA), increased awareness of the Finnish action and predisposed it for peer review. The 10‐year results are favourable, patients are less disabled, practices and attitudes in healthcare have changed, and major cost savings have been obtained. Views of the lay public and patients are slow to move, however. Local multidisciplinary allergy teams were set up to continue the activities also after the Programme. Changes in environment and lifestyle in the last 50 years are the main reasons for the allergy rise. The Finnish experience may help to manage allergic diseases, improve nature relatedness in the fast‐urbanizing world, combat nature loss and reduce the disease burden.
Keywords: allergy campaign, allergy epidemic, allergy prevention, allergy programme, biodiversity, public health
1. INTRODUCTION
1.1. Why to start?
The fast health crisis, global communicable disease pandemic, COVID‐19 locked down societies to avoid the immediate hazards. In longer term, vaccinations are relied on to induce immunological, psychological and societal resilience, and to re‐open societies. Allergic diseases and asthma are indicators of non‐communicable diseases (NCDs) causing slow crisis, the increase of which appears only over decades. The burden they give rise to, healthcare need, disability and costs, has cumulated in the highly urbanized societies. NCDs seem to share common underlying factors—microbial imbalance, immune dysfunction and low‐grade inflammation—related to changes in living environment and lifestyle. Again, the immunological resilience of the human population is at stake and needs to be restored (Box 1).
BOX 1. Change of strategy.
| Avoidance strategy has not reduced prevalence of allergic diseases, healthcare need and disability of patients or costs in the society. Justified avoidance has helped individual patients to avoid severe symptoms. |
| The biodiversity hypothesis implies that contact with natural environment is necessary to obtain and keep up balanced immunoregulatory circuits. |
| A public health programme was taken to educate healthcare professionals to promote allergy health and reduce medicalization and encourage patients and families to live normal life even with allergies. |
| Low‐value recommendations and guidelines for allergic disorders were revised and removed. |
| The results of the 10‐year systematic approach tell that a marked change is achievable. |
In 1873, Charles Blackley, a general practitioner, wrote: ‘Hay‐fever is said to be an aristocratic disease, and there can be no doubt that, if it is not almost wholly confined to the upper classes of society, it is rarely, if ever, met with but among the educated’. 1 Those experienced first the increase of standard of living and credits of modern, urban societies. Now we know that the environmental and lifestyle factors mostly explain the rise of allergic disorders, 2 , 3 and the management and disease prevention are in change. 4 , 5 The biodiversity hypothesis states that contact with natural environment enriches the human microbiome promotes immune balance, and protects from disease. 6 , 7 , 8 , 9 , 10 Although the evidence is still mainly associative rather than showing cause and effect, the results are supported by a few controlled interventions, both in a mouse model 11 and in young children. 12 Consequently, the dominating avoidance strategy has been supplemented and turned to pursue for immunological and psychological tolerance or resilience. For that, immunotherapy has been an important part of allergy praxis ever since 1911. 13 Poorly justified avoidance, on the other hand—just to be on the safe side—has caused medicalization, especially in food allergy. Both over‐ and under‐diagnostics should be reduced, and limited resources targeted wisely.
The Finnish healthcare adopted the new approach and implemented a nationwide, systematic programme from 2008 to 2018 to promote allergy health and mitigate the overall allergy burden. Here, we present the flow of the Programme—from the original ideas to implementation, main outcomes, lessons and a short prescription for others. How was the campaign designed? What were the key elements to get it going and keep alive? What were the sources of relevant data for follow‐up? Part of the information presented here has been published previously in different contexts. 14 , 15 , 16 , 17 , 18 , 19
Hints for the practical and operational measures are useful while planning a local or nationwide programme or campaign. The Finnish experience may help others to combat the burden of allergic diseases or even the NCDs in general. However, the Finnish healthcare is comprehensive which is not the case everywhere (Ref. [20], The Finnish healthcare, Data S1). Programmes and campaigns must always be tailored to meet local needs and fit the unique healthcare environment.
2. PROGRAMME DESIGN
2.1. To get going
The need for a change was recognized already in 1998 in a consensus meeting of opinion leaders and healthcare professionals. 21 In 2006, four opinion leaders from the Helsinki University Hospital and a patient organization met the Minister of Social Affairs and Health for political support. As a result, the governmental Institute for Health and Welfare nominated a multidisciplinary counselling group to evaluate the recent scientific data on allergy management and prevention. A smaller working group prepared the 10‐year implementation programme (Box 2). It was based not only on the scientific data but also on long clinical experience, which is important in pursuing change in attitudes and management practices.
BOX 2. Seek for consensus and political support.
| Reasonable consensus among opinion leaders is needed for an action plan. Clinical allergology and immunology are not a speciality/subspeciality in all European countries. |
| The representatives of medical disciplines involved in allergy practice—mainly paediatrics, pulmonary medicine, dermatology, ENT and general medicine—should agree on priorities and leadership. |
| To be successful in long‐term, find political support and if possible, financial contribution from some of the governmental bodies in question. |
| Allergic disorders are multifaceted. The foci of a programme or campaign had to target the central problems and must be plausible, pragmatic and achievable. |
Prospective planning is based on retrospective evidence but cannot fully predict the future. Despite the uncertainties, strategies were chosen, goals set, tools agreed upon and evaluation methods defined (Figure 1) (Table S1). The programme was launched in April 2008 ( 22 , 24 ).
FIGURE 1.
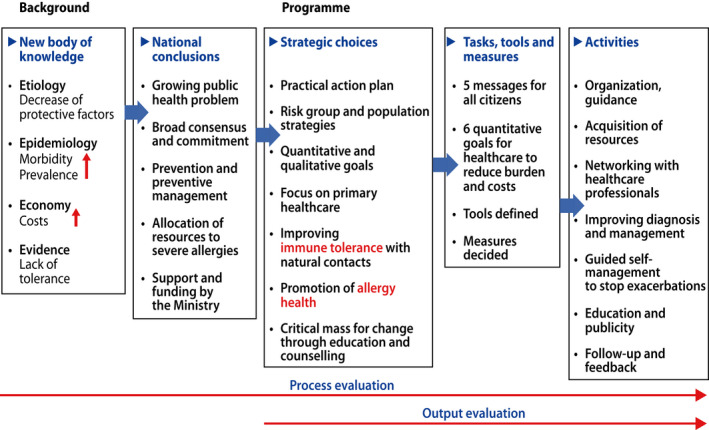
The strategic planning of the programme (Ref. [17,19,22], modified)
The working body continued as a multidisciplinary Programme Steering Group, which planned and supervised the implementation. A professional non‐governmental organization (NGO), the Finnish Lung Health Association (Filha) organized the education for healthcare. Two patient NGOs, Allergy, Skin and Asthma Federation and the Organization for Respiratory Health in Finland ran together the campaign for patients, their families and lay public. The structure of the programme steering was kept simple and flat (Figure 2).
FIGURE 2.
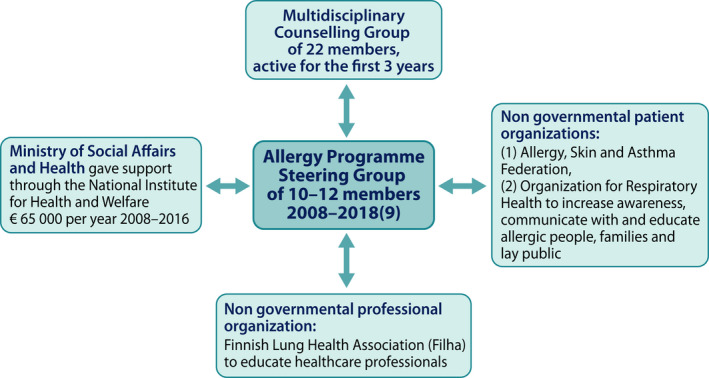
Steering of the programme was kept simple
3. GOALS AND MESSAGES
The necessary steps for a credible programme or campaign include predefined goals, tasks, tools and outcomes (Figure 3). The specific goals and indicators for healthcare professionals were quantitative, such as allergy diets should drop by 50% and asthma emergency visits by 40% within 10 years. Each of the six goals had its specific tasks, tools and evaluation methods. Tasks were the activities or targets in pursuing the goal. Tools were the means by which the tasks were carried out. Evaluation methods were the verification of outcomes. The specific goal was reached if the indicator actualized.
FIGURE 3.
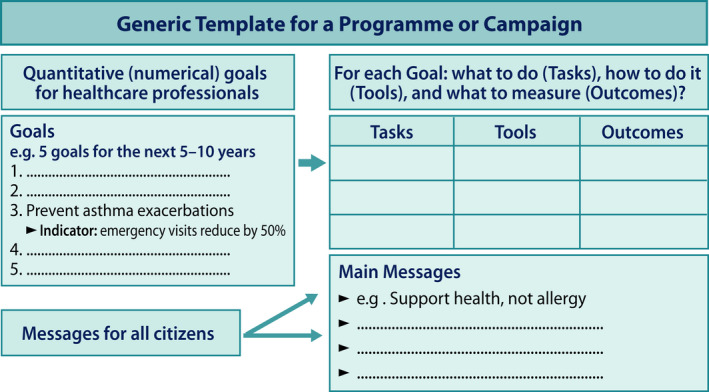
Goals for healthcare with specific tasks, tools and outcomes for follow‐up. Indicators for goals were quantitative. Key messages were set up for all citizens (Ref. [25], modified)
3.1. Messages both for professionals and lay public
Healthcare professionals were introduced to new information to diagnose, treat and advice better their patients but the common message was the same for them, patients, families and lay public. Contacts with natural environment should be increased in everyday life: it matters, what you eat, 26 drink, breathe and touch (Figure 4). Interaction of human body with environmental biodiversity with micro‐organisms and biogenic compounds is essential for health in preventing dysregulation of the immune system. 5 Simple guidance to support immune tolerance/resilience instead of poorly justified avoidance of allergens was provided. 15 , 22 , 27
FIGURE 4.
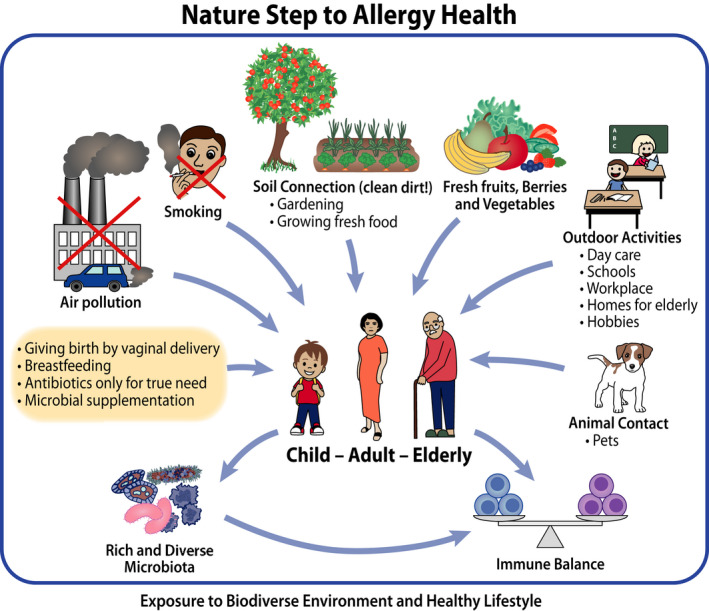
Practical advice was given for building and improving tolerance/resilience (primary prevention) as well as preventing symptoms and exacerbations (secondary and tertiary prevention) (Ref. [27], modified, Table S2)
The key messages targeted all citizens, opinion leaders and authorities and the patients, families and lay public. 22 The relevance and acceptance of these messages were tested in 2008 in an email survey among 744 asthma contact persons. 15 The messages were well received. For example, general practitioners scored strengthen tolerance as 9.1 (scale 4–10) (Table 1). Allergy management practice left, however, much room for improvement, for example availability of allergen immunotherapy was poor (score 5.4). 28
TABLE 1.
Key messages and their acceptance by the healthcare professionals (scale 4‒10)
| Key messages to all citizens | Acceptance by healthcare professionals | |
|---|---|---|
| Nurses | Doctors | |
| Support health, not allergy | 8.8 | 9.2 |
| Strengthen tolerance/resilience | 8.7 | 9.1 |
| Avoid allergens only if mandatory | 8.7 | 9.3 |
| Focus on severe allergies, treat them early | 9.2 | 9.5 |
| Improve air quality. Stop smoking | 9.6 | 9.6 |
4. PROGRAMME IN ACTION (Box 3)
BOX 3. Set up goals and messages, motivate and organize.
| Goals for healthcare should be quantitative, if all possible. We should not ‘develop’ anything but be specific and tell what we are going to do, by what means and how to measure the outcome. |
|---|
| Messages for all citizens must be short, readily understandable and should speak directly to people. |
| To keep up motivation, healthcare professionals need evidence‐based data, encouragement, feedback and information on the process. |
| The keywords are motivate and organize. |
4.1. Educating professionals
The Finnish Lung Health Association (Filha) was responsible for the systematic action plan educating healthcare workers (doctors, nurses, pharmacists, medical and nursing students, nutritionists). During the programme years, the 365 educational sessions gathered more than 23 000 participants (Figure 5). The education was held during working hours and was free of charge.
FIGURE 5.
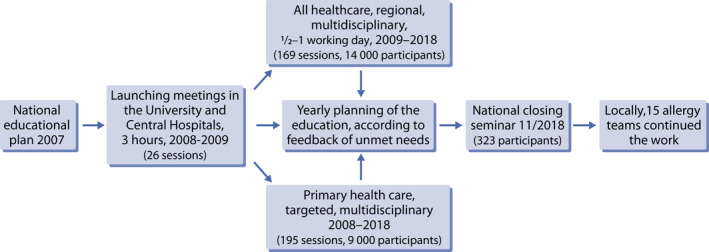
The stepwise education of healthcare professionals
The education took advantage of the contact person network created already during the previous Asthma Programme 1994–2004. In each municipal health centre, there were asthma contact persons (in 2008, 200 physicians and 580 nurses specifically trained in asthma). Similarly, in pharmacies, 695 pharmacists had been educated as asthma contact persons (94% coverage of the pharmacies in Finland). These networks were strengthened for the allergy campaign, and a new allergy contact network, with some 200 nurses, was created for the local maternity and child health clinics and schools.
In 2015, of those 2207 healthcare professionals taking part in the educational sessions 61% were nurses and 26% doctors (Figure 6A). The main aim was to improve diagnostics and treatment of allergic disorders in everyday practice.
FIGURE 6.
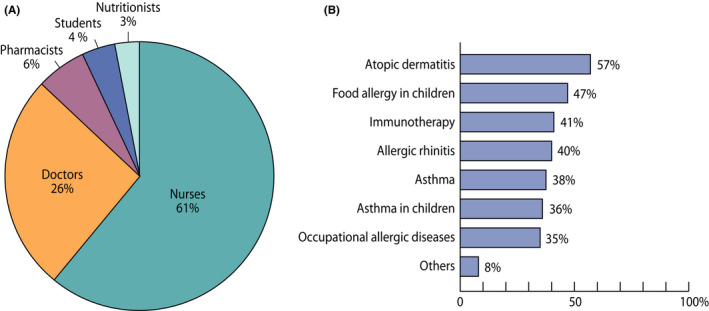
(A) Healthcare professionals taking part in the educational sessions in 2015. (B) Unmet educational needs of 557 responders, who responded to an email survey in 2018. Other topics included indoor air problems, anaphylaxis, asthma diagnostics, reimbursement of medication, allergy to pollens, animals and moulds, food allergy in adults and allergens in cosmetics
At the end of the programme, in 2018, unmet educational needs were surveyed by email among those, who had taken part in the sessions during that year. Five hundred fifty‐seven of them responded (20%). Atopic dermatitis, food allergy and immunotherapy were the top priorities (Figure 6B). The relatively low priority of asthma probably indicated success of the long‐term efforts for asthma education starting already during the Asthma Programme. 29
Special attention was paid to guided self‐management for which instructions for asthma had paved the way. 30 Now, also other allergic conditions were included in 13 booklets, also available on internet, from allergic rhinitis to anaphylaxis and to tips for parents of babies (Table S3). The patients were guided: (1) to note the early signs of symptom escalation, (2) proactively start or increase the symptom‐specific medication or management and (3) take a contact with the doctor or nurse in due time for further advice.
4.2. Informing patients, families and lay public
Lay public was approached by two NGOs for patients: (1) the Allergy, Skin and Asthma Federation, and (2) the Organization for Respiratory Health, with circa 60,000 members together. They arranged regional education for their personnel and peer workers, which had a major impact on direct patient counselling and distribution of educational material.
NGOs organized a joint project between 2011 and 2015 to produce media material to support the programme. In practice, two persons were responsible for the project focusing on the digital media. A new website (Allergy Health) was developed and optimized for search engines. By the end of the programme, more than 600 000 web pages had been browsed. Several banner campaigns were put into practice, including the biggest social media service (www. Suomi24.fi) and the largest health and welfare online service in Finland (www.terve.fi). Both included the Q&A section for allergic people. A new website (Healthy at work) supporting young allergic people in choosing education and occupation was launched in co‐operation with the Finnish Institute of Occupational Health.
During the five‐year project, altogether 12 media campaigns were executed on the Internet and Radio. The coverage of the project information was monitored by attention value research; in total, 2.3 million Finns (>40% of the population) were reached.
Besides allergy risk groups, the project was targeted to inform lay public, and especially children and their parents. A healthy natural contact with biodiverse environment was the key element of all campaigns, posters, leaflets, interviews and lectures. It was also the basis for the co‐operation with the kindergartens. In 2014, altogether 768 kindergartens (response rate 20%) participated in a national inquiry, indicating strong nature‐related practice in the Finnish day‐care routine.
4.3. Changing allergy practice in children
At the beginning of the programme, we relied on study data but also on clinical experience in managing young children with early signs of atopic disease. Despite the controversies in the scientific and professional communities, our recommendations mostly proved to be valid in later clinical trials from around the world. Some examples include early feeding with putatively allergic foods instead of allergen avoidance in infancy 31 and ignoring pet ownership as a risk factor for asthma in childhood. 32
4.3.1. Food allergy
Inquiring the unmet needs among healthcare professionals, food allergy was mentioned by 78% of the responders (Figure 7).
FIGURE 7.
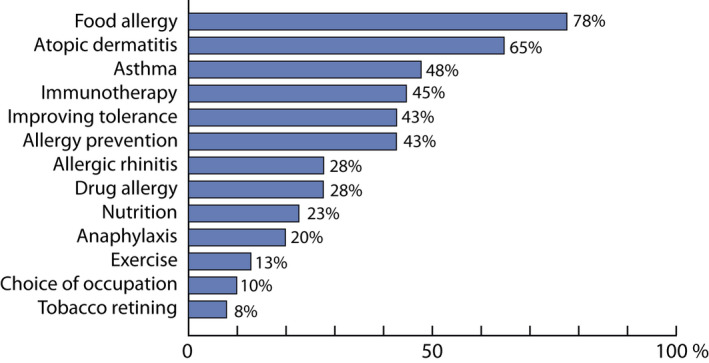
Allergy in children. Unmet needs for education as reported by 40 healthcare professionals working in the Western part of Finland in 2012
We advocated for all stakeholders to use emollients, and if necessary, hydrocortisone creams in babies with dry skin showing even mild signs of eczema. It became obvious that sensitization to foods commonly takes place through inflamed skin, thus we educated healthcare professionals to aim for normalizing the infant's skin. Mothers were encouraged to start feeding solid foods, while breastfeeding, at the age of 4–6 months, and discouraged to use avoidance diets without proven need. Breastfeeding mothers were not put on special diets even though the infant was having food allergy. This has been the practice for 10 years in the whole country with only a few exceptions. Diversity of foods served for infants by 6 months of age was emphasized. All these factors may have contributed to the documented 50% decrease in the milk allergy incidence. 33 The same has happened with wheat allergy (unpublished).
Increasing tolerance has been the main principle throughout the programme, both in changing psychological attitudes, and through desensitization. Burden of allergy diets particularly in day‐care has been reduced significantly by simple change in practices. 33 We were one of the first European countries to investigate and use oral immunotherapy (OIT) for food allergies. 34 , 35 , 36 , 37 , 38
To avoid medicalization and slow down the increasing outpatient visits, we discouraged healthcare professionals to do IgE testing unless the child had rapid reactions against food allergens. The concept of food introductory clinics was never adopted in Finland. Adequate use of both skin prick tests and specific IgE measurements was guided by education. During the latter part of the programme, component diagnostics, for example for peanut and egg, has been recommended. The cut‐off values have been studied among Finnish children. 39 , 40 , 41 , 42 IgG food antibody testing has been discouraged.
4.3.2. Asthma
Diagnostic criteria of asthma, particularly among young children, varied a lot in the country. The criteria in different age groups were unified and asthma guidelines updated twice during the programme. In small children below 3 years of age, wheezing episodes or symptomatic periods were evaluated by defining asthma predictive index (API). 43 After adopting these simple recommendations, use of asthma drugs in smallest children decreased by 40% without an increase in hospitalizations or acute visits.
For children between 3–7 years of age, impulse oscillometry has been validated and combined with free field running with bronchodilatation test. 44 , 46 Asthma drug reimbursement is entitled to a child having both clinical symptoms and variable airflow limitation shown by lung function measurements. For school‐aged children, particularly those above 12 years of age, diagnostic requirements are the same as for adults. Hospitalization or acute visits for children above 4–5 years of age are rare in Finland demonstrating the long‐term validity of the diagnostic policy. Paediatric asthma deaths are almost non‐existent in Finland. 47
In primary care and in low‐ and middle‐income countries, measures like the allergen component diagnostics, OIT or impulse oscillometry are not readily available or usable, but they are mentioned here to keep up interest also to specialists and allergists being in key position to promote the programme goals.
The practical measures for food allergy, wheezing and asthma are listed in Table 2.
TABLE 2.
Choosing wisely for managing common allergic manifestations in children
| Sign/disease | Action |
|---|---|
| Dry skin, early sign of eczema | Apply daily emollients and episodic hydrocortisone |
| Solid food introduction | Individually at 4–6 months of age, preferably together with breastfeeding continuing as long as possible. No restrictive feeding recommendations. |
| Breastfeeding mother's diet with food allergic child | Preferably no diets or restrictions for mother |
| Food allergy testing | Only on solid medical grounds, use either specific IgE or skin prick testing for screening. If necessary, component diagnostics with individual food‐specific cut‐off values. National guidelines for food challenges. No food introductory clinics. |
| Asthmatic symptoms in children <3 years of age | Clinical diagnosis with 3–4 wheezing episodes, start of controller medication utilizing asthma predictive index |
| Asthmatic symptoms in children 3–7 years of age | Evaluate symptoms, bronchodilator responsiveness and define lung function by impulse oscillometry, preferably combined to field running test and bronchodilatation |
| Asthmatic symptoms in school‐aged children | Evaluate symptoms, bronchodilator responsiveness and define lung function by spirometry, preferably combined to field running test and bronchodilatation. Children above 12 years are addressed like adults (PEF, methacholine responsiveness) |
5. DATA SOURCES AND OUTCOMES
5.1. Public health registers
The Finnish healthcare registers provided invaluable data sources for the outcome evaluation: (1) the hospital admission register of the National Institute for Health and Welfare, (2) the registers of the Social Insurance Institute (SII) for drug reimbursements, sick‐leave allowances, disability pensions and rehabilitation, (3) the drug consumption and sales register of the Finnish Medicines Agency, (4) the register of the National Institute of Occupational Health for occupational diseases verified by insurance companies and (5) the Finnish Anaphylaxis Register at the Skin and Allergy Hospital of the Helsinki University Central Hospital. 48 , 49 Physicians (mostly allergists) from the whole country voluntarily report severe allergic reactions independent of the causative agent. A one‐page questionnaire for medical professionals is available on the Internet.
5.2. Did we reach the main goals?
For outcome evaluation, the baseline was 2007–2010, depending on survey, source, or method. The indicators of the goals were closely followed, and results reported at 5 and 10 years, both for the Finnish healthcare and internationally. The results have been published elsewhere in more detail, 15 , 17 , 28 but the main outcomes, meeting the original goals and indicators, are summarized in Table 3.
TABLE 3.
The programme goals, indicators for follow‐up and outcomes at 10 years
| 1. Prevent allergy |
| Indicator: Asthma, rhinitis and atopic eczema prevalence reduces by 20% |
| Result: The prevalence of these 3 conditions levelled off |
| 2. Improve tolerance |
| Indicator: Food allergy diets reduce by 50% |
| Result: The diets reduced around 50%, in Helsinki capital area by 43% |
| 3. Improve allergy diagnostics |
| Indicator: Patients are skin prick tested in certified testing centres |
| Result: Around 90% of patients are tested in certified centres |
| 4. Reduce work‐related allergies |
| Indicator: Occupational allergies reduce by 50% |
| Result: Cases accepted by insurance companies reduced by 45% |
| 5. Focus on severe allergies and treat in time |
| Indicator: Good allergy practice works; asthma emergency visits reduce by 40% |
| Result: Emergency visits reduced by 6%, in children 53%. Hospital days reduced by 50% |
| 6. Reduce allergy & asthma costs |
| Indicator: Allergy costs reduce by 20% |
| Result: Healthcare and disability costs reduced by 30% (€195 million in 2018 vs. 2007) |
Programme goals were mostly achieved but not fully. The first goal to turn down allergy prevalence by 20% was too ambitious. Nevertheless, in military conscripts the prevalence of asthma and allergic conditions levelled off in 2020s. 50 From 2006 to 2016, postal surveys of random adult populations indicated a decrease in allergic rhinoconjunctivitis, and asthma symptoms decreased markedly. 51 The Finnish society has tackled smoking in many ways, and stop smoking was also one of the programme messages. Indeed, from 2008 to 2018, smoking decreased from 20% to 14%, 52 which may have mitigated asthma symptoms.
The second goal, reduction of food allergy diets was achieved, which is probably a sum of different factors. 33 Re‐visiting the diet guidelines and drawing attention to medicalization certainly had an effect. Improvement of biological tolerance needs a study of its own.
The fifth goal was reached in children but not in adults. Asthma emergency visits dropped by 46% at 5 years but only 6% at 10 years. The primary care in the public health system has suffered from shortage of doctors during the last 5–10 years affecting also on asthma care, but reliable analysis is lacking.
Although anaphylaxis was not mentioned in the main goals, it was a central topic of education and information. Awareness improved considerably, and from 2008 to 2019, 1048 cases were reported in the anaphylaxis register. In children, foods caused most (78%) of the severe reactions, in adults, drugs (44%). 49 In 17 years, from 1996 to 2013, anaphylaxis caused 56 deaths, all in adults. 53 Both in Finland and Sweden, anaphylaxis in children was a slightly increasing cause of hospitalizations between 1999 and 2011 but the incidence stayed lower in Finland. 54
5.3. Notes for cost analyses
Allergy and asthma costs were analysed from all data sources in collaboration with government officials. 18 , 55 , 56 Direct cost analyses included outpatient visits, hospital days, travel expenses, rehabilitation and drugs. Additional cost of allergy diets in school, preschool and kindergarten were also included. Reimbursement statistics were used to estimate the costs of cow's milk allergy in infants.
Indirect costs comprised of premature reduced working capacity (presenteeism), sickness absences (absenteeism) and disability pensions. Short‐term absences and reduced working capacity, not recorded in any databases, were based on two population‐based questionnaire surveys in 2013 and 2019. To improve reliability, the results were analysed together. The trend of longer‐term sickness absences can be followed by the statistics of the Social Insurance Institute (SII), which records all sickness allowances of absences lasting nine or more days.
Cost studies are usually based on questionnaire surveys of various patient or population cohorts. 57 , 58 Surveys can distort the results, for example overestimate drug costs or need for medical care. 59 If a survey includes only patients seeking medical care, it creates bias as in the general population most patients have a mild disease. 60
In 2018, the total costs of allergic diseases and asthma were estimated euro 1.5–1.8 billion. Major part of them was indirect cost, €1.2–1.5 billion. Reduced working capacity accounted for 65%, sickness absences 25% and disability pensions 10% of the indirect costs. The direct healthcare costs were €331 million: for asthma €203, allergic rhinitis €48 and atopic eczema €35 million. The comparable costs of allergic diseases and asthma in 2018 vs. 2007 decreased by almost a third (€195 M), mainly because of marked improvement in working ability and decreasing number of people on disability pensions. The cumulative cost savings during the whole programme were €1.2 billion (Figure 8).
FIGURE 8.
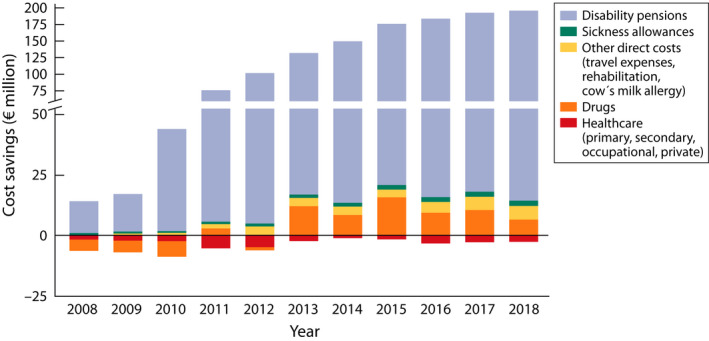
Annual cost savings during the programme 2008‒2018 (€ million) as compared to the year 2007. The direct (outpatient visits, hospital days, drugs, other) and indirect costs (sickness allowances, disability pensions, according to SII) (Ref. [18], modified)
Statistics from several sources supported each other. Decrease in disability pensions, sickness absences and hospital days indicate that asthma and allergy have become less severe during the programme. The same conclusion was reached in the Finnish Pharmacy study 61 , 62 and in the surveys of the City of Helsinki in 1996, 2006 and 2016. 51 Estimating improved quality of life (e.g. in food allergy or asthma) by cost‐utility or cost‐effectiveness analyses 63 , 64 would have even increased the savings.
Cost savings in healthcare are achieved by improving treatment and rationalizing practice. Hospital days in Finland have been decreasing in general, but more in asthma and allergy. 65 Drug use for allergic diseases and asthma is in slow increase, but costs have even decreased during the last years due to price regulation. 66
Comparing health economics between countries is difficult due to heterogeneity in design, methods, measures, outcomes and national differences in healthcare systems, 58 , 67 , 68 , 69 but follow‐up in one country is quite feasible.
5.4. Feedback from professionals, patients and lay public
5.4.1. Methods
Eight surveys were mostly conducted online with Zef and Surveypal programmes. 70 Three of the surveys were conducted among healthcare professionals (2008–2009 and 2018), two among the members of the patient NGOs (2015 and 2018), and three among the Finnish population (2011, 2015 and 2019).
5.4.2. Professionals
Allergy training improved markedly. In the baseline survey, 52% of the responders had participated in allergy or asthma training in the previous year. In 2018, almost everyone (96%) had attended some form of training, and nearly a third (31%) had attended Allergy Programme events 4–9 times. Three‐fourths of the healthcare professionals reported that their allergy practice had improved. Only 4.5% did not find any change. For example, food avoidance was significantly less recommended for pregnant women to prevent allergies (15% in 2008–2009, 3% in 2018). As many as 86% of the responders had actively worked in their area to break down unnecessary diets in schools and daycare centres. Finally, 70% concurred that the programme had reached its goals, 0.4% did not.
5.4.3. Patients and lay public
There was still much room for improvement in allergy knowledge. The patient NGO member and wider population surveys indicated a slow change in attitudes and practice. In 2018, careful allergen avoidance at home was still important for every fifth of the respondents. The changes in lifestyle and environment (urbanization) influencing on allergies were insufficiently understood; only 38% of the respondents agreed in 2019. Almost one in three thought there is no way to protect themselves from allergies. Still half of the allergic population controlled their food, and allergy also played a marked role in outdoor activities, having a pet, eating outside home and shopping daily consumer goods. More than every fifth experienced stress and anxiety due to allergies.
6. FINANCE
From 2008 to 2016, the Ministry of Welfare and Health allocated annually €60 000–65 000 for educational and coordinating work. The Finnish Lung Health Association (Filha), organizing the education, also raised private funds of €50 000‒100 000 per year to carry out the various learning activities. A full‐time nurse worked in the field. The medical educator (paediatrician) received fees for lectures and educational sessions.
From 2011 to 2014, two patient organizations (NGOs), the Skin, Allergy and Asthma Federation and the Organization for Respiratory Health received an annual payment of €200 000 for patient education and public communication from the Funding Centre for Social Welfare and Health Organizations (STEA). Two full‐time employees launched and implemented the information campaign. After four years, the campaign was included as part of everyday activities of the patient NGOs. The Väinö and Laina Kivi Foundation also supported the programme.
The members of the Steering group or other opinion leaders were not paid for their extra work. In the field, the education was integrated into the everyday work of the healthcare professionals in maternity clinics, schools, daycare, primary care, occupational care, hospitals and pharmacies. The allergy work was re‐organized towards common goals. After 2018, the multidisciplinary educational activities were continued by the 15 regional expert teams.
7. COMMENTS AND LESSONS
The questions to ask when setting up a programme are numerous. Where are we heading for, in what time frame, what are the goals and the practical steps to achieve them, how we evaluate whether we are on the right track? Should we focus more on children? 14 , 71 How do we communicate with professionals and lay public to convince that we work for them as well as for common good? What is our evidence to assure others? 72 Most importantly, who is doing and what? Can we truly measure objective changes induced by the campaign? And who is paying this all?
First, we should also ask ourselves the fundamental question: do we really want to ‘turn down the allergy epidemic’? Practising doctors work patient by patient and do their best to treat them well. However, when asthma patients in Finland started to disappear from hospitals along with the effective national asthma programme, 73 worries were raised of the resource reduction and even of the future of the pulmonary speciality. So far, the national or local allergy and asthma actions in other countries have mostly been awareness campaigns (Table S4). Obviously, these attitudes are not unknown in the pharmaceutical industry, not mentioning the patient organizations, the existence of which depends on having the patients. 74
Healthcare, allergy not being an exception, is full of recommendations and guidelines, which may not be relevant but rather source of poorly justified restrictions and costs. 75 A novel approach, the Choosing Wisely De‐Implementation Framework (CWDIF), has been taken to systematically reduce low‐value care and advance the science of de‐implementation. 76 In Finland, it was rational to engage the patient organizations in the programme from the very beginning. They worked actively, although some of the fundamentalist members also gave the heaviest criticism: ‘The Programme has been set up just to save money!’ Healthcare is not free from the paradigm of continuous growth, labelled in the selfish human genome to compete, benefit and survive. 77
Doctors witness epidemics of both communicable (fast) and non‐communicable (slow) diseases, with unpredictable outcomes. 78 Microbe‒immune system interplay is decisive for maintenance of the immunoregulatory circuits and tolerance. If the crosstalk is not versatile enough, dysregulation arises. Reduced contact to environmental microbial diversity 2 , 3 , 80 , 79 and airborne biogenic chemicals 80 , 81 as well as everyday use of epithelial barrier‐damaging agents 82 are probably the main reasons for the compromised immunological resilience of urban populations. In epidemiological analyses, the logistic regression models should identify not only the conventional determinants increasing disease risk but also the factors lowering the risk, that is mediating protection.
Allergy is not an isolated case but concurrent with the increase of both type I and II diabetes, obesity, cardiovascular, inflammatory bowel and neurological diseases, mental disorders and even cancer. 5 , 10 It is intriguing to think, that preventing allergic disorders may pave the way to reduce the burden of non‐communicable diseases in general, and, at the same time, meet the major environmental challenges like nature loss. 83 This may be especially important in low‐ and middle‐income countries, 84 and in developing economies outside Europe. 85 , 86 The COVID‐19 pandemic has shown the societal priorities: in an acute situation health safety comes first. The medical discipline should also use its significant societal power more effectively for disease prevention.
The Allergy Programme was not an academic effort, but as it questioned many practices, it stimulated research both in laboratory and clinical settings, also in other disciplines like ecology. A programme of this kind may serve as a useful impulse and frame for initiatives, innovations and academic qualifications.
The Finnish initiative is far from perfect, but the successes and failures guide others to create better models. From the public health perspective, a comprehensive approach to allergic disorders seems useful. In Portugal, a media campaign has commenced to encourage people with allergy to make positive changes in their life. 87 Overall, the digital revolution in healthcare along with social media has opened exciting options for change of management. 88 Different ‘light versions’ of the Finnish Programme could also be applicable. The international dimensions provide platforms to change experience and learn from others (Data S1, Collaborators). The low‐ and middle‐income countries are in a critical position to restrict the ‘allergy epidemic’ (Box 4).
BOX 4. To sum up.
| In low‐ and middle‐income countries with rapid urbanization, asthma and allergic disorders are on the rise along with changes in the living environment and lifestyle. Awareness must be improved both among healthcare professionals and lay public. Treatment needs should be met by organizing education. |
|---|
| Asthma causes roughly 60% and the other allergic conditions 40% of the total societal costs. Indirect disability costs comprise most of the costs and can be markedly reduced in a relatively short period of time. ‘The patient has the disease but lives a normal life’. |
| In prevention, not all the factors protecting from allergy are fully evidence‐based, but it is reasonable to promote: (1) traditional diet including fresh fruits and vegetables, (2) physical exercise and mobility, (3) healthy housing (e.g. avoiding biomass smoke in cooking) and (4) contacts with wider nature. |
| Smoking is a major risk for asthma control and should be restricted by education, legislation and prizing. Air pollution in big cities must be mitigated by governmental and other societal actions. |
| In urban settings, green infrastructure and nature‐based solutions should be in focus. |
| Allergy and Asthma Programme or Campaign is a systematic approach to lessen the disease burden. It is also an inspiring frame for education, research and academic qualifications among young people. |
8. A SHORT PRESCRIPTION
In Finland, a systematic public health programme has been implemented to mitigate allergy burden in the society (Table 4). It was based on a paradigm shift from poorly justified avoidance of allergens and other factors to endorsing immune tolerance/resilience. Major educational efforts of healthcare professionals took place, and patients, families and lay public were targeted by an information campaign. Steering of the nationwide action was simple and straightforward. Funding was primarily received from national health authorities but supplemented also from private sources. Changing the management practices and prevention, with a modest investment, helped the patients to do better, and the society to save resources. The Finnish experience scaled up to European level would be a step to better health and moving towards economic and environmental sustainability.
TABLE 4.
Short prescription of an Allergy Programme. In practice, implementation means education and dissemination of the new knowledge for (1) better management, (2) prevention, (3) immune tolerance/resilience and (4) allergy health (Ref. [17, 19], modified)
| 1. Practical steps to start |
| Define the community (population) for which the programme will be addressed (e.g. city or hospital district, region, province, national level). |
| Organize a local consensus meeting to agree on action to improve management and reduce allergy & asthma burden. Contact local administration. Find support from opinion leaders, decision‐makers and politicians. |
| Set up a steering group of experts, opinion leaders and members of patient organizations (9–12 members) to plan and implement the campaign in detail. |
| Apply funding to commence the campaign. Raise some public funding, which can be supplemented with private funding. Funding for the first year means that you get started. |
| Get the campaign going. Seek for support also on administrative and political level. |
| 2. Set up key messages for all citizens. Set up goals for healthcare. Each goal has specific tasks, tools and evaluation methods. Goals and their indicators should preferably be quantitative. |
| 3. Set up a plan for the educational process with two edges, healthcare and lay public |
| Education of the healthcare professionals is the key to success. Decide the organization responsible for organizing the education. It can be hospital or healthcare‐based, or a non‐governmental organization (NGO). |
| The education is integrated into everyday work of professionals. A part‐time educator contributing to the fieldwork and a part‐time assistant/secretary is employed. The local experts are consulted to set the content and feedback for the educational sessions. |
| Information of the lay public and communication via internet and social media is planned and needs a part‐time worker (at least at the beginning of the programme). |
| The process of education and information is also a learning process for the steering group! |
| 4. Explore public healthcare registers and other data sources to measure outcomes |
| For example, emergency visits, hospitalizations, drug use, days off work, pensions, food allergy diets, cost estimates, etc. |
| The register information is supplemented by targeted opinion surveys and questionnaires. |
| Important! Integrate practical actions and systematic follow‐up. Is the programme on the right track, reaching the goals? Motivate actions for research and follow‐up surveys. |
| 5. Set up timelines |
| Planning the campaign takes a year. Two keywords: Motivate and Organize! |
CONFLICT OF INTEREST
Tari Haahtela has received personal lecturing fees from GSK, Mundipharma, Orion Pharma, Sanofi, Erkka Valovirta from ALK‐Abello, AstraZeneca, Mylan, Nestle, Nigaard, Orion Pharma, Stallergenes Greer, ThermoFisher, TEVA, Tuula Vasankari from AstraZeneca, MSD, Boehringer Ingelheim, and Mika J. Mäkelä from GSK and Orion Pharma, outside the submitted work. The other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
All the authors participated in writing the article. TH wrote the first draft of the paper with input from the co‐authors. All authors decided to publish the paper.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We thank the members of the Allergy Programme Steering Group (Data S1, names and professional profiles). We also thank our international collaborators Eric Bateman, Jean Bousquet, Kristin Carson‐Chahhoud, Yoon‐Seok Chang, Alvaro Cruz, Piotr Kuna, Kristina Ljungros, Karin Lødrup‐Carlsen, Andre Moreira, Susanna Palkonen, Harald Renz, Arunas Valiulis and Torsten Zuberbier for the information of national initiatives for allergic conditions in their country or organization.
Haahtela T, Jantunen J, Saarinen K, et al. Managing the allergy and asthma epidemic in 2020s—Lessons from the Finnish experience. Allergy. 2022;77:2367–2380. doi: 10.1111/all.15266
Funding information
None
REFERENCES
- 1. Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus Aestivus. Tindall & Cox; 1873. [Google Scholar]
- 2. Riedler J, Braun‐Fahrländer C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross‐sectional survey. Lancet. 2001;358:1129‐1133. [DOI] [PubMed] [Google Scholar]
- 3. Hanski I, von Hertzen L, Fyhrquist N, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA. 2012;109:8334‐8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Mutius E, Smits HH. Primary prevention of asthma: from risk and protective factors to targeted strategies for prevention. Lancet. 2020;396:854‐866. [DOI] [PubMed] [Google Scholar]
- 5. Haahtela T, Alenius H, Lehtimäki J, et al. Immunological resilience and biodiversity for prevention of allergic diseases and asthma. Allergy. 2021;76:3613‐3626. [DOI] [PubMed] [Google Scholar]
- 6. von Hertzen L, Hanski I, Haahtela T. Natural immunity. Biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12:1089‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haahtela T, Holgate S, Pawankar R, et al. The biodiversity hypothesis for allergic disease: World Allergy Organization position statement. WAO J. 2013;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential for health. Proc Natl Acad Sci USA. 2013;110:18360‐18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruokolainen L, Lehtimäki J, Karkman A, Haahtela T, von Hertzen L, Fyhrquist N. Holistic view of health: two protective layers of biodiversity. Ann Zool Fennici. 2017;54:39‐49. [Google Scholar]
- 10. Haahtela T. A biodiversity hypothesis. Allergy. 2019;74:1455‐1456. [DOI] [PubMed] [Google Scholar]
- 11. Ottman N, Ruokolainen L, Suomalainen A, et al. Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model. J Allergy Clin Immunol. 2019;143:1198‐1206. [DOI] [PubMed] [Google Scholar]
- 12. Roslund M, Puhakka R, Grönroos M, et al. Biodiversity intervention enhances immune regulation and health‐associated commensal microbiota among daycare children. Sci Adv. 2020;6(42):eaba2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfaar O, Bousquet J, Durham SR, et al. One hundred and ten years of allergen immunotherapy: a journey from empiric observation to evidence. Allergy. 2022;77:454‐468. [DOI] [PubMed] [Google Scholar]
- 14. Pelkonen AS, Kuitunen M, Dunder T, Reijonen T, Valovirta E, Mäkelä MJ; Finnish Allergy Programme . Allergy in children: practical recommendations of the Finnish Allergy Programme 2008–2018 for prevention, diagnosis, and treatment. Pediatr Allergy Immunol. 2012;23:103‐116. [DOI] [PubMed] [Google Scholar]
- 15. Haahtela T, Valovirta E, Bousquet J, Mäkelä M; the Allergy Programme Steering Group . The Finnish Allergy Programme 2008–2018 works. Eur Respir J. 2017;49(6):1700470. [DOI] [PubMed] [Google Scholar]
- 16. Valovirta E, Haahtela T, Saarinen K, Tommila E, Vasankari T, Mäkelä MJ. Provision of care—implementation of the Finnish Allergy Programme 2008–2018. Chapter 25. In: Agache I, Hellings P, eds. Implementing Precision Medicine in Best Practices of Chronic Airway Diseases. Academic Press; 2019:177‐187. [Google Scholar]
- 17. Haahtela T, Valovirta E, Saarinen K, et al. The Finnish Allergy Program 2008–2018: society‐wide proactive program for change of management to mitigate allergy burden. J Allergy Clin Immunol. 2021;148:319‐326. [DOI] [PubMed] [Google Scholar]
- 18. Jantunen J, Kauppi P, Linna M, Mäkelä M, Pelkonen A, Haahtela T. Real‐world evidence of reduced disability costs during the Finnish Allergy Programme 2008–2018. Allergy. 2021;76:3817‐3819. [DOI] [PubMed] [Google Scholar]
- 19. Haahtela T. Clinical application of the biodiversity hypothesis in the management of allergic disorders. In: Rook GAW, Lowry CA, eds. Evolution, biodiversity, and a reassessment of the hygiene hypothesis. Springer Nature; 2022. in press. [Google Scholar]
- 20. Saltman RB, Teperi J. Health Statistics for the Nordic countries. Nordic Medico‐Statistical Committee. 2016;104:1‐242. [Google Scholar]
- 21. Haahtela T, Björksten F, eds. Allergic Population – a consensus statement 1998. Duodecim, the Finnish Academy; 1998:1‐25 (in Finnish). [Google Scholar]
- 22. Haahtela T, von Hertzen L, Mäkelä M, et al. Finnish Allergy Programme 2008–2018 – time to act and change the course. Allergy. 2008;63:634‐645. [DOI] [PubMed] [Google Scholar]
- 23. von Hertzen LC, Savolainen J, Hannuksela M, et al. Scientific rationale for the Finnish Allergy Programme 2008–2018: emphasis on prevention and endorsing tolerance. Allergy. 2009;64:678‐701. [DOI] [PubMed] [Google Scholar]
- 24. Bousquet J, Bieber T, Fokkens W, et al. In Allergy, ‘A new day has begun’. Allergy. 2008;63:631‐633. [DOI] [PubMed] [Google Scholar]
- 25. Haahtela T. Evidence for asthma control ‒ zero tolerance to asthma with the Finnish Programmes. In: Akdis CA, Agache I, eds. Global atlas of asthma. EAACI; 2013:135‐137. [Google Scholar]
- 26. Garcia‐Larsen V, Del Giacco SR, Moreira A, et al. Asthma and dietary intake: an overview of systematic reviews. Allergy. 2016;71:433‐442. [DOI] [PubMed] [Google Scholar]
- 27. Haahtela T, von Hertzen L, Anto JM, et al. Helsinki by nature: the nature step to respiratory health. Clin Transl Allergy. 2019;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kauppi P, Kämäräinen J, Haahtela T. Kansallinen allergiaohjelma vaatii koulutusta ja työkaluja (The National Allergy Programme needs education and tools). Finn Med J. 2010;65:3515‐3520. (in Finnish, Abstract in English). [Google Scholar]
- 29. Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10‐year asthma programme in Finland: major change for the better. Thorax. 2006;61:663‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lahdensuo A, Haahtela T, Herrala J, et al. Randomised comparison of self‐management and traditional treatment of asthma over one year. BMJ. 1996;312:748‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du Toit G, Roberts G, Sayre PH, et al. Randomozed trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lødrup Carlsen KC, Roll S, Carlsen KH, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European cohorts. PLoS One. 2012;7:e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erkkola M, Saloheimo T, Hauta‐alus H, et al.; LILLA study group . Burden of allergy diets in Finnish day care reduced by change in practices. Allergy. 2016;71:1453‐1460. [DOI] [PubMed] [Google Scholar]
- 34. Kuitunen M, Englund H, Remes S, et al. High IgE levels to α‐lactalbumin, β‐lactoglobulin and casein predict less successful cow´s milk oral immunotherapy. Allergy. 2015;70:955‐962. [DOI] [PubMed] [Google Scholar]
- 35. Kukkonen AK, Uotila R, Malmberg LP, Pelkonen AS, Mäkelä MJ. Double‐blind placebo‐controlled challenge showed that peanut oral immunotherapy was effective for severe allergies without negative effects on airway inflammation. Acta Paediatr. 2017;106:274‐281. [DOI] [PubMed] [Google Scholar]
- 36. Uotila R, Kukkonen AK, Greco D, Pelkonen AS, Mäkelä MJ. Peanut oral immunotherapy decreases IgE to Ara h 2 and Ara h 6 but does not enhance sensitization to cross‐reactive allergens. J Allergy Clin Immunol. 2017;139:1393‐1396. [DOI] [PubMed] [Google Scholar]
- 37. Kulmala P, Pelkonen AS, Kuitunen M, et al. Wheat oral immunotherapy was moderately successful but was associated with very frequent adverse events in children aged 6–18 years. Acta Paediatr. 2018;107:861‐870. [DOI] [PubMed] [Google Scholar]
- 38. Palosuo K, Karisola P, Savinko T, Fyhrquist N, Alenius H, Mäkelä MJ. A randomized, open‐label trial of Hen's egg oral immunotherapy: efficacy and humoral immune responses in 50 children. J Allergy Clin Immunol Pract. 2021;S2213‐S2198. [DOI] [PubMed] [Google Scholar]
- 39. Kukkonen AK, Pelkonen AS, Mäkinen‐Kiljunen S, Voutilainen H, Mäkelä MJ. Ara h 2 and Ara 6 are the best the best predictors of severe peanut allergy: a double‐blind placebo‐controlled study. Allergy. 2015;70:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 40. Uotila R, Kukkonen AK, Pelkonen A, Mäkelä MJ. Cross‐sensitization profiles of edible nuts in a birch‐endemic area. Allergy. 2016;71:514‐521. [DOI] [PubMed] [Google Scholar]
- 41. Palosuo K, Kukkonen AK, Pelkonen AS, Mäkelä MJ. Gal d 1‐specific IgE predicts allergy to heated egg in Finnish children. Pediatr Allergy Immunol. 2018;29:637‐643. [DOI] [PubMed] [Google Scholar]
- 42. Uotila R, Röntynen P, Pelkonen AS, Voutilainen H, Kukkonen AK, Mäkelä MJ. For hazelnut allergy, component testing of Cor a 9 and Cor a 14 is relevant also in birch‐endemic areas. Allergy. 2020;75:2977‐2980. [DOI] [PubMed] [Google Scholar]
- 43. Csonka P, Tapiainen T, Mäkelä MJ, Lehtimäki L. Heterogeneity of emergency treatment practices in wheezing preschool children. Acta Paediatr. 2021;110:2448‐2454. [DOI] [PubMed] [Google Scholar]
- 44. Kalliola S, Malmberg LP, Kajosaari M, Mattila PS, Pelkonen AS, Mäkelä MJ. Assessing direct and indirect airway hyperresponsiveness in children using impulse oscillometry. Ann Allergy Asthma Immunol. 2014;113:166‐172. [DOI] [PubMed] [Google Scholar]
- 45. Kalliola S, Malmberg LP, Malmström K, Pelkonen A, Mäkelä MJ. Airway hyperresponsiveness in young children with respiratory symptoms: a five‐year follow‐up. Ann Allergy Asthma Immunol. 2019;122:492‐497. [DOI] [PubMed] [Google Scholar]
- 46. Burman J, Malmberg LP, Remes S, Jartti T, Pelkonen AS, Mäkelä MJ. Impulse oscillometry and free‐running tests for diagnosing asthma and monitoring lung function in young children. Ann Allergy Asthma Immunol. 2021;127:326‐333. [DOI] [PubMed] [Google Scholar]
- 47. Kivistö JE, Karjalainen J, Kivelä L, Huhtala H, Protudjer JLP. Very low asthma death incidence among Finnish children from 1999 to 2015. Pediatr Pulmonol. 2018;53:1009‐1013. [DOI] [PubMed] [Google Scholar]
- 48. Mäkinen‐Kiljunen S, Haahtela T. Eight years of severe allergic reactions in Finland. A register‐based report. WAO J. 2008;1:184‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edelman SM, Kukkonen AK, Mäkelä MJ. Eliciting allergens and treatment of anaphylaxis: report of the Finnish national anaphylaxis registry. Allergy. 2019;74:2010‐2013. [DOI] [PubMed] [Google Scholar]
- 50. Reijula J, Latvala J, Mäkelä M, Siitonen S, Saario M, Haahtela T. Long‐term trends of asthma, allergic rhinitis and atopic eczema in Finnish young men: a retrospective analysis, 1926‒2017. Eur Respir J 2020;56:1902144. [DOI] [PubMed] [Google Scholar]
- 51. Hisinger‐Mölkänen H, Pallasaho P, Haahtela T, Lindqvist A, Sovijärvi A, Piirilä P. The increase of asthma prevalence has levelled off and symptoms decreased in adults during 20 years from 1966 to 2016 in Helsinki, Finland. Respir Dis. 2019;155:121‐126. [DOI] [PubMed] [Google Scholar]
- 52. Jääskeläinen M, Virtanen S. Tupakkatilasto 2020 (Statistical Report of Smoking 2020). Finn Inst Health Welfare. 2021;38:1‐20. [Google Scholar]
- 53. Kivistö JE, Dunder T, Protudjer JLP, Karjalainen J, Huhtala H, Mäkelä MJ. Adult but no paediatric anaphylaxis‐related deaths in the Finnish population from 1996 to 2013. J Allergy Clin Immunol. 2016;138:630‐631. [DOI] [PubMed] [Google Scholar]
- 54. Kivistö JE, Protudjer JL, Karjalainen J, Wickman M, Bergström A, Mattila VM. Hospitalizations due to allergic reactions in Finnish and Swedish children during 1999–2011. Allergy. 2016;71:677‐683. [DOI] [PubMed] [Google Scholar]
- 55. Jantunen J, Kauppi P, Linna M, et al. Astman ja allergian kustannukset ovat suuret mutta laskussa. (Asthma and allergy costs are high but reducing). Finn Med J. 2014;69:641‐647. (In Finnish, abstract in English). [Google Scholar]
- 56. Jantunen J, Kauppi P, Linna M, Mäkelä M, Pelkonen A, Haahtela T. Astman ja allergian kustannusten myönteinen kehitys jatkui (Positive trend in asthma and allergy costs continues). Finn Med J. 2021;76:797‐804. (In Finnish, abstract in English). [Google Scholar]
- 57. Bahadori K, Doyle‐Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dierick BJH, van der Molen T, Flokstra‐de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437‐453. [DOI] [PubMed] [Google Scholar]
- 59. Ehteshami‐Afshar S, FitzGerald JM, Doyle‐Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11‐23. [DOI] [PubMed] [Google Scholar]
- 60. Avdeeva KS, Reitsma S, Fokkens WJ. Direct and indirect costs of allergic and non‐allergic rhinitis in the Netherlands. Allergy. 2020;75:2993‐2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kauppi P, Peura S, Salimäki J, Järvenpää S, Linna M, Haahtela T. Reduced severity and improved control of self‐reported asthma in Finland during 2001–2010. Asia Pac Allergy. 2015;5:32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jantunen J, Haahtela T, Salimäki J, Pelkonen A, Mäkelä M, Kauppi P. Astma ja allergia lievenevät Suomessa ‒ apteekkien allergiabarometri 2010‒2016. (Asthma and allergy are becoming less severe in Finland – pharmacy barometer surveys 2010‒2016). Finn Med J. 2018;73:367‐371. (In Finnish, abstract in English). [Google Scholar]
- 63. de Silva D, Rodriguez Del Rio P, de Jong NW, et al. Allergen immunotherapy and/or biologicals for IgE‐mediated food allergy: a systematic review and meta‐analysis. Allergy. 2022;77(6):1852‐1862. doi: 10.1111/all.15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Greenhawt M, Oppenheimer J, Codispoti CD. A practical guide to understanding cost‐effectiveness analyses. J Allergy Clin Immunol Pract. 2021;12:4200‐4207. [DOI] [PubMed] [Google Scholar]
- 65. Martikainen V, Järvelin J. Non‐psychiatric specialized health care 2018. Statistical report 51/2019. Finnish Institute for Health and Welfare. 23 p. (In Finnish, abstract in English).
- 66. Koskinen H. Pharmaceutical Expenditures, the Reference Price System and Competition in the Pharmaceutical Market. A Register Study. Studies in Social Security and Health. The Social Insurance Institution of Finland. 2018;150:1‐80. [Google Scholar]
- 67. Zuberbier T, Lotvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2)LEN review. Allergy. 2014;69:1275‐1279. [DOI] [PubMed] [Google Scholar]
- 68. Nunes C, Pereira AM, Morais‐Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of Rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1274‐1286. [DOI] [PubMed] [Google Scholar]
- 70. Saarinen K, Jantunen J. A Survey of Attitudes and Opinions Among Allergic People 2011–2015. The Finnish Allergy Programme 2008–2018. South‐Karelia Allergy and Environment Institute; 2015:1‐30. (In Finnish) http://www.allergiaterveys.fi/upload/vaestokyselyt‐2015 [Google Scholar]
- 71. Sampath V, Abrams EM, Adlou B, et al. Food allergy across the globe. J Allergy Clin Immunol. 2021;148:1347‐1364. [DOI] [PubMed] [Google Scholar]
- 72. Haahtela T, Laatikainen T, Alenius H, et al. Hunt for the origin of allergy—comparing the Finnish and Russian Karelia. Clin Exp Allergy. 2015;45:891‐901. [DOI] [PubMed] [Google Scholar]
- 73. Haahtela T, Klaukka T, Koskela K, Erhola M, Laitinen LA; Working Group of the Asthma Programme in Finland 1994–2004 . Asthma programme in Finland: a community problem needs community solutions. Thorax. 2001;56:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Conrad P. The shifting engines of medicalization. J Health Soc Behav. 2005;46:3‐14. [DOI] [PubMed] [Google Scholar]
- 75. Markovitz AA, Hofer TP, Froehlich W, et al. Identifying recommendations for stopping or scaling back unnecessary routine services in primary care. JAMA Intern Med. 2020;180:414‐416. [DOI] [PubMed] [Google Scholar]
- 76. Grimshaw JM, Patey AM, Kirkham KR, et al. De‐implementing wisely: developing the evidence base to reduce low‐value care. BMJ Qual Saf. 2020;29:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dawkins R. The Selfish Gene. Oxford University Press; 1976. [Google Scholar]
- 78. Haahtela T, Anto JM, Bousquet J. Fast and slow crises of Homo urbanicus: loss of resilience in communicable diseases, like COVID‐19, and non‐communicable diseases. Porto Biomed J. 2020;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moore MN. Do airborne biogenic chemicals interact with the PI3K/Akt/mTOR cell signalling pathway to benefit human health and wellbeing in rural and coastal environments? Environ Res. 2015;140:65‐75. [DOI] [PubMed] [Google Scholar]
- 81. Antonelli M, Donelli D, Barbieri G, Valussi M, Maggini V, Firenzuoli F. Forest volatile organic compounds and their effects on human health: a state‐of‐the‐art review. Int J Environ Res Public Health. 2020;17:6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Akdis C. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other conditions? Nat Rev Immunol. 2021;21:739‐751. [DOI] [PubMed] [Google Scholar]
- 83. Haahtela T, Vuori A, Laine M, et al. Nature step to health ‐ Lahti regional health and environment programme 2022–2032. GARD Annual Meeting. ePoster 2021.
- 84. Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low‐income and middle‐income countries: from challenges to solutions. Lancet. 2021;397:928‐940. [DOI] [PubMed] [Google Scholar]
- 85. Asher I, Haahtela T, Selroos O, Ellwood P, Ellwood E; Global Asthma Network Study Group . Global Asthma Network survey suggests more national asthma strategies could reduce burden of asthma. Allergol Immunopathol. 2017;45:105‐114. [DOI] [PubMed] [Google Scholar]
- 86. Pawankar R, Wang JY, Wang IJ, et al. Asia Pacific association of allergy asthma and clinical immunology white paper 2020 on climate change, air pollution, and biodiversity in Asia‐Pacific and impact on allergic diseases. Asia Pac Allergy. 2020;10:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moreira A, de Castro F, Rama T, et al. AlergiaPT: a Portuguese media campaign to inspire people with allergies to make a positive change in their life. Porto Biomed J. 2022;7(1):e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bousquet J, Anto JM, Bachert C, et al. ARIA digital anamorphosis: digital transformation of health and care in airway diseases from research to practice. Allergy. 2021;76:168‐190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


