Abstract
Aims
To describe paracetamol dosing and liver function test (LFT) monitoring in older hospital inpatients who are frail or have low body weight.
Methods
Retrospective observational study, at a 790‐bed metropolitan public health service in Australia. Patients aged ≥70 years, with body weight <50 kg or frailty index based on laboratory data (FI‐Lab) score ≥0.3, who were administered paracetamol during an admission with length‐of‐stay >72 hours, were included. Data were extracted from electronic medical records. Paracetamol doses administered in hospital, and doses prescribed on discharge, were compared against consensus guidelines that recommended ≤60 mg/kg/d for older people weighing <50 kg, and ≤3000 mg/d for frail older people.
Results
In total, 240 admissions (n = 229 patients, mean age 84.7 years) were analysed. During 150 (62.5%) admissions, higher than recommended paracetamol doses were prescribed. On 138 (57.5%) occasions, patients were prescribed paracetamol on discharge, and 112/138 (81.2%) doses were higher than recommended. Most discharge prescriptions (97/138, 70.3%) were for regular administration. The median daily dose on discharge for patients <50 kg was 83.7 mg/kg (interquartile range 73.6–90.9 mg/kg). For frail patients ≥50 kg, the median daily discharge dose was 3990 mg (interquartile range 3000–4000 mg). LFTs were measured in hospital for 151/200 (75.5%) and 93/166 (56.0%) patients who received paracetamol for >48 hours and >5 days, respectively.
Conclusion
Majority of paracetamol doses prescribed for frail or low‐weight older patients in hospital and on discharge were higher than recommended in consensus guidelines. LFTs were not measured for 44% patients who received paracetamol regularly for >5 days. Further studies are needed to explore long‐term outcomes of this practice.
Keywords: acetaminophen (paracetamol), aged, frail elderly, inpatients, prescriptions
What is already known about this subject
Paracetamol is commonly prescribed for older people in hospital.
Although supporting evidence is limited, some guidelines recommend lower doses for patients who are frail or weigh <50 kg, to reduce the risk of paracetamol toxicity.
There are few published data describing paracetamol dosing practices for this patient population.
What this study adds
Nearly 2/3 of frail or low‐weight older patients received higher than recommended paracetamol doses in hospital.
More than half of these patients were prescribed paracetamol on discharge, mostly for regular administration, and usually at higher than recommended doses.
Further studies are needed to explore the long‐term outcomes of this practice.
1. INTRODUCTION
Paracetamol is widely used in the management of acute and chronic pain. 1 , 2 It is generally safe at recommended doses, but is hepatotoxic in overdose. 3 Older people, especially those who are underweight or frail, may be at increased risk. 4 , 5 Physiological and pathological changes that occur with ageing and frailty influence the pharmacokinetics of paracetamol. 6 , 7 , 8 Pharmacokinetic studies have reported that paracetamol's volume of distribution and clearance are reduced by approximately 20 and 38%, respectively, in frail older people compared with younger adults, leading to higher paracetamol serum levels. 9 , 10 Frail older people may also accumulate active metabolites. 11 A retrospective case series of outcomes associated with paracetamol overdose reported that fulminant hepatic failure and death was more likely in older people. 6 Although paracetamol‐induced hepatotoxicity at standard therapeutic doses (up to 4 g/d) is rare, cases have been reported. 4 , 12 , 13
Despite a plausible rationale for increased risk of hepatotoxicity in frail and low‐weight older people at standard therapeutic doses, and isolated case reports of toxicity with repeated therapeutic dosing, there is no conclusive evidence that low body weight and frailty are independent risk factors for paracetamol‐induced hepatotoxicity. 3 , 5 , 8 , 9 , 10 , 14 , 15 Nevertheless, some clinical guidelines and expert opinions recommend that the dose of paracetamol should be reduced in patients who may be more susceptible to paracetamol‐induced liver injury, including older people who are frail or have low body weight (<50 kg). 10 , 14 , 15
Studies from the UK and Australia indicate that the prevalence of paracetamol use in hospital patients is high (62–72% patients). 2 , 16 , 17 Furthermore, unintentional paracetamol overdosing is a common medication error in hospitals. 16 , 18 Internationally, concerns have been raised about potentially excessive paracetamol dosing in frail older and low‐weight individuals. 13 , 19 For example, England's Healthcare Safety Investigation Branch, an organisation responsible for the investigating patient safety concerns, is conducting an investigation titled Unintentional overdose of paracetamol in adults with low body weight. 19 This was prompted by reports of paracetamol overdose in patients with low body weight at therapeutic doses. 19 In New Zealand, the Health Quality and Safety Commission's Medication Safety Watch bulletin advised “for adults <50kg (the frail elderly or those with eating disorders/chronic disease), reduce the maximum total daily dose from the usual 4g/day”. 13 This was partly triggered by 2 deaths that highlighted the risk of usual adult doses in the frail elderly. 13
Some Australian jurisdictions have published guidelines recommending lower paracetamol doses in selected patient groups. The New South Wales Therapeutic Advisory Group paracetamol guideline recommends a maximum dose of 60 mg/kg/d for older people weighing <50 kg. 14 The Queensland Department of Health paracetamol guideline recommends a maximum of 3000 mg/d for adults with risk factors such as being older and frail. 15 Both guidelines recommend liver function tests (LFTs) if paracetamol is continued for >48 hours. 14 , 15
Despite concerns about potentially excessive dosing in frail and low‐weight older people in hospital, there are few published data describing paracetamol prescribing and monitoring practices for these groups. Therefore, the aim of this study was to describe paracetamol dosing and LFT monitoring in older hospitalised patients who are frail or have low body weight.
2. METHODS
2.1. Study design and setting
This was a retrospective observational study, conducted at a major metropolitan public health service in Melbourne, Australia comprising 3 hospitals (790 inpatient beds total) with electronic prescribing and medical records.
2.2. Patient selection
A list of potentially eligible patients was generated from the hospital's clinical research data warehouse (a database that captures routinely collected electronic patient data from clinical systems) in March 2019. The data were reviewed and cleaned before analysis to remove ineligible patients and identify data anomalies.
Eligible patients were those aged ≥70 years, admitted to hospital between August and December 2018, length‐of‐stay >72 hours, administered paracetamol in hospital, and body weight <50 kg or frail. Frailty was identified using a frailty index based on physiological and laboratory test data (FI‐Lab). 20 FI‐Lab is a validated frailty index, with higher scores being associated with increased risk of death and other adverse outcomes. 20 , 21 , 22 The FI‐Lab used in our study comprised 27 variables, including 25 routine blood tests plus measured systolic and diastolic blood pressure (Table S1). The FI‐Lab score is calculated by coding each variable as 0 or 1, where 0 indicates that value is within the normal range and 1 indicates that value is above or below the normal range. Consistent with validation studies, a score was calculated only if >70% variables were available. An FI‐Lab score ≥0.3 was used to indicate frailty. 23 Patients were excluded if they were admitted for an elective procedure, admitted under palliative care or psychiatry, or did not have weight recorded. Patients who weighed >50 kg but had insufficient data available to calculate FI‐Lab were also excluded.
Patients were categorised as low body weight (<50 kg) or weight ≥50 kg and frail (FI‐Lab ≥0.3). All low‐weight patients were included in the study regardless of frailty status. The same number of patients who weighed ≥50 kg and were frail were selected using random numbers generated in Microsoft Excel.
2.3. Data collection
The data warehouse report provided demographic data, length of stay, treating unit, body weight, FI‐Lab score and paracetamol dosing data. For inpatient orders, total paracetamol dose received each day, duration of paracetamol administration and whether orders were for regular administration or when needed (PRN) was manually extracted from electronic records. For discharge orders, paracetamol doses were determined from prescription records and discharge summaries. When a patient had regular and PRN orders, the total daily dose was calculated from the sum of the regular dose and the maximum PRN dose that the patient could use according to the prescription.
Data regarding paracetamol use and dosing prior to admission were obtained from patients' Medication History on Admission forms (best‐possible medication history verified by a hospital pharmacist using 2 or more sources). For PRN orders, we used the maximum dose that the patient could use according to the documented regimen.
Data were collected by 2 intern pharmacists (O.R. and J.N., 120 records each), with training and supervision from the primary investigator (R.A.E.). Ten percent of records were reviewed by R.A.E. for accuracy.
Paracetamol dosing was compared to consensus guidelines that recommended a maximum of 60 mg/kg/d for patients weighing <50 kg, and 3000 mg daily for patients who weigh >50 kg but are frail (FI‐Lab ≥0.3). 14 , 15 We determined from patients' electronic records whether LFTs were measured during the admission in concordance with the guidelines. 14 , 15
The primary endpoint was the percentage of patients prescribed a paracetamol dose higher than recommended in consensus guidelines. Secondary endpoints were:
Median paracetamol dose prescribed (in mg/kg/d for older patients <50 kg, and in mg/d for frail older patients >50 kg);
Percentage of patients whose higher than recommended paracetamol dose was initiated in hospital;
Percentage of patients who were discharged on a higher than recommended dose of paracetamol;
Median number of days of paracetamol administration in hospital; and
The percentage of patients with LFTs measured after 48 hours and after 5 days of continuous paracetamol treatment.
2.4. Sample size and data analysis
A pragmatic target sample size of at least 200 patients was planned, comprising at least 100 low‐weight and 100 frail older patients, as this was considered enough to identify prescribing patterns. Data were analysed using Microsoft Excel. Data were reported as proportions, mean and standard deviation for normally distributed data and median and interquartile range (IQR) for non‐normally distributed data.
The study was approved by the hospital's Human Research Ethics Committee (approval LNR/18/Austin/155).
3. RESULTS
There were 2414 hospital admissions for potentially eligible older patients who received paracetamol in hospital over the study period. From these, 240 admissions were analysed: 120 for low‐weight patients (<50 kg) and 120 for patients who weighed ≥50 kg but were frail (Figure 1, Table 1). Median admission length was 11.0 days (IQR 6.3–20.8). There were 229 unique patients (mean age 84.7 y). Compared to admissions that were not included (n = 2174), the study cohort had a similar number of medications charted (mean 10.1 vs. 10.2) and paracetamol dose administered (median 3000 mg/d both cohorts) in hospital, but more study patients were female (69.2 vs. 53.6%) and older (mean age 84.7 vs. 81.7 y; Table S2).
FIGURE 1.
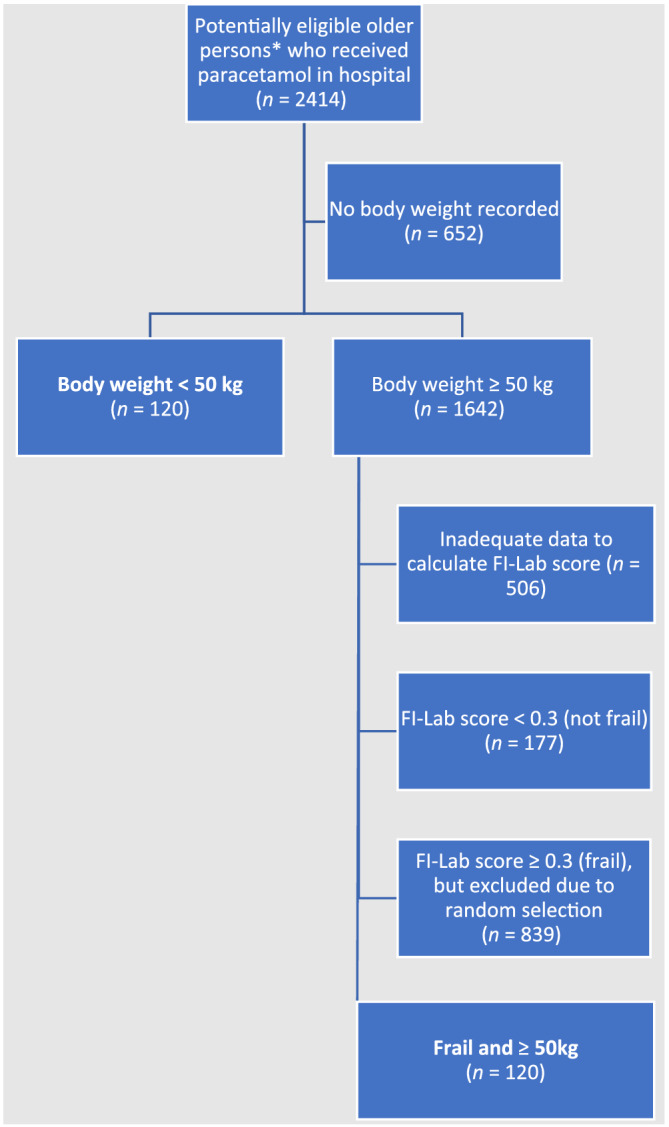
Patient selection. FI‐Lab = Frailty Index based on laboratory and physiological test data. *Aged >70 years, not admitted under palliative care or psychiatry units or for an elective procedure, length‐of‐stay >72 hours
TABLE 1.
Patient demographics
| <50 kg (n = 120 admissions) | Frail and >50 kg (n = 120 admissions) | Combined (n = 240 admissions) | |
|---|---|---|---|
| Patient age (y), mean (SD) | 85.5 (7.3) | 83.8 (6.4) | 84.7 (6.9) |
| Sex, n (%) | F = 109 (90.8) | F = 57 (47.5) | F = 166 (69.2) |
| M = 11 (9.2) | M = 63 (52.5) | M = 74 (30.8) | |
| Patient weight (kg), median (IQR, range) | 45.0 (41.2–47.2, 29.5–49.9) | 73.8 (64.7–84.2, 50.0–141.0) | 50.0 (45.0–73.7, 29.5–141.0) |
| Number of medications charted on admission, mean (SD, range) | 11.5 (3.7, 1–20) | 10.8 (4.2, 2–23) | 10.1 (4.1, 1–23) |
| Admitting team, n (%) a | Surgical: 24 (20.0) | Surgical: 21 (17.5) | Surgical: 45 (18.8) |
| Medical: 96 (80.0) | Medical: 99 (82.5) | Medical: 195 (81.3) | |
| FI‐Lab score, mean (SD, range) | 0.2 (0.1, 0.0–0.5) b | 0.4 (0.1, 0.3–0.7) | 0.3 (0.1, 0–0.7) b |
FI‐Lab, Frailty Index based on laboratory and physiological test data; IQR, interquartile range; SD, standard deviation.
Surgical includes: cardiac surgery, general surgery, neurosurgery, orthopaedics, vascular surgery, ear, nose and throat/head and neck unit, plastic surgery, thoracic surgery; medical includes: general medicine, cardiology, geriatrics, haematology, oncology, rehabilitation, renal, respiratory, stroke, urology, rheumatology, clinical pharmacology/toxicology, gastroenterology.
26 patients with body weight <50 kg did not have sufficient data to calculate an FI‐Lab score.
3.1. Inpatient paracetamol prescribing
In 85/240 (35.4%) admissions, paracetamol was newly started in hospital. Of the 155 patients who were using paracetamol at home prior to admission, 45 (29.0%) had their dose increased in hospital. For most admissions (204/240, 85.0%), the inpatient paracetamol order was for regular administration (Table 2).
TABLE 2.
Inpatient paracetamol administration
| <50 kg (n = 120 admissions) | Frail and >50 kg (n = 120 admissions) | Combined (n = 240 admissions) | |
|---|---|---|---|
| Paracetamol prescribed for regular administration, n (%) | 100/120 (83.3) | 104/120 (86.7) | 204/240 (85.0) |
| Higher than recommended dose a administered, n (%) | 77/120 (64.2) b | 73/120 (60.8) | 150/240 (62.5) |
| Higher than recommended dose was initiated in hospital, n (%) | 48/77 (62.3) | 39/73 (53.4) | 87/150 (58.0) |
| Number of full inpatient days^ on which paracetamol was administered, median; (IQR, range) | 6.0 (3.0–14.5, 0–51) | 7.0 (2.8–14.0, 0–69) | 6.0 (3.0–14.0, 0–69) |
| Number of days patient received higher than recommended paracetamol dose, median (IQR, range) | 3.0 (IQR: 0.0–6.0, 0.0–46.0) | 3.0 (0.0–8.0, 0.0–29.0) | 3.0 (0.0–8.0, 0.0–46.0) |
IQR, interquartile range.
^ Excludes the day of admission and the day of discharge, when a full daily paracetamol dose could not have been administered in hospital.
Higher than recommended paracetamol dose ≥60 mg/kg/d for patients <50 kg or >3000 mg/d for frail older patients.
53/120 (44.2%) patients <50 kg received a paracetamol dose >75 mg/kg/d.
During 200/240 (83.3%) admissions, paracetamol was administered for >48 hours, and in 166/240 (69.2%) admissions it was administered for >5 days. During 150/240 (62.5%) admissions, the patient received a paracetamol dose higher than recommended in consensus guidelines. The higher than recommended dose was initiated in hospital in 87/150 (58.0%) cases. The median number of days of higher than recommended dosing in hospital was 3.0 (IQR 0–8.0, range 0–46; Table 2).
The median daily inpatient dose for patients weighing <50 kg was 69.7 mg/kg (IQR 42.9–85.1 mg/kg, range 14.5–121.2 mg/kg). For frail patients weighing >50 kg the median daily inpatient dose was 3990 mg (IQR 2660–4000 mg, range 1000–5000 mg; Table 2).
LFTs were measured in hospital for 151/200 (75.5%) and 93/166 (56.0%) patients who received >48 hours and >5 days of paracetamol treatment, respectively (Table 3).
TABLE 3.
Liver function test monitoring
| <50 kg (n = 120 admissions) | Frail and >50 kg (n = 120 admissions) | Combined (n = 240 admissions) | |
|---|---|---|---|
| ≥48 h of continuous paracetamol administration, n (%) | 98/120 (81.7) | 103/120 (85.8) | 201/240 (83.8) |
| LFT measured after 48 h of continuous paracetamol administration, n (%) | 65/98 (66.3) | 86/102 (84.3) | 151/200 (75.5) |
| ≥5 d of continuous paracetamol administration, n (%) | 80/120 (66.7) | 87/120 (72.5) | 167/240 (69.6) |
| LFT measured after 5 d of continuous paracetamol administration, n (%) | 38/80 (47.5) | 55/86 (64.0) | 93/166 (56.0) |
LFT, liver function test.
3.2. Discharge paracetamol prescribing
For 138/240 (57.5%) admissions, the patient was prescribed paracetamol on discharge, and 112/138 (81.2%) doses were higher than recommended. The median daily dose on discharge for patients <50 kg was 83.7 mg/kg (IQR 73.6–90.9 mg/kg, range 28.3–126.0 mg/kg). For frail patients >50 kg, the median daily dose on discharge was 3990 mg (IQR 3000–4000 mg, range: 1995–4000 mg). The majority of discharge orders (97/138, 70.3%) were for regular administration (Table 4).
TABLE 4.
Discharge paracetamol prescribing
| <50 kg (n = 120 admissions) | Frail and >50 kg (n = 120 admissions) | Combined (n = 240 admissions) | |
|---|---|---|---|
| Patient discharged on paracetamol, n (%) | 71/120 (59.2) | 67/120 (55.8) | 138/240 (57.5) |
| Paracetamol prescription was for regular administration, n (%) | 49/71 (69.0) | 48/67 (71.6) | 97/138 (70.3) |
| Paracetamol dose was higher than recommended a , n (%) | 60/71 (84.5) b | 52/67 (77.6) | 112/138 (81.2) |
Higher than recommended dose ≥60 mg/kg/d for patients <50 kg or >3000 mg/d for frail older patients.
52/71 (73.2%) patients <50 kg received a paracetamol dose >75 mg/kg/d.
4. DISCUSSION
There are few published data describing paracetamol dosing and LFT monitoring for older frail and low‐weight hospital inpatients, a group who may be at increased risk of liver injury. 10 No previous study has explored paracetamol dosing on discharge from hospital. Our study addressed these gaps in the literature.
We found that nearly 2/3 of older frail and low‐weight inpatients who were prescribed paracetamol in hospital received doses higher than recommended by consensus guidelines. 14 , 15 When paracetamol was prescribed on discharge, >80% of doses were higher than recommended. Even when assessed against a more conservative maximum daily dose (75 mg/kg/d, which is considered to be the maximum safe dose for adults without additional risk‐factors for paracetamol toxicity), 10 , 24 3/4 of patients who weighed <50 kg and were discharged on paracetamol had an excessive dose prescribed.
Previous studies that explored paracetamol dosing in older hospital inpatients reported lower levels of potentially excessive dosing; however, most did not focus on frail and low‐weight patients. 2 , 16 , 17 For example, a study at 9 hospitals in Victoria, Australia reported that 36% (357/993) patients aged ≥65 years who were prescribed paracetamol had regimens that would allow >4 g/d to be administered, but dosing in patients who were frail or with low body weight was not investigated. 16 A study conducted in New South Wales, Australia, where there was a reduced‐dose policy for paracetamol dosing in older patients, found that 28% (196/274) patients aged ≥65 years received >3 g/d of paracetamol, but patients' frailty status and body weight was not reported. 17 A French study reported that 50% (302/606) patients aged >75 years with 1 or more additional risk factor for toxicity (including low body weight or malnutrition) did not have their paracetamol dose reduced. 25 A recent study at a Swiss hospital reported that 1/3 (68/206) of patients aged >75 years with body weight <50 kg received >60 mg/kg/d. 26
In our study, in a majority (58.0%) of instances, the higher than recommended dose was initiated in hospital (either newly commenced in hospital or dose‐increased after admission). This may reflect lack of clinician awareness of guidelines for dose‐reduction in older people, or the limited evidence for increased risk with unadjusted dosing in frail and low‐weight older patients. Three quarters of patients had LFTs measured after 48 hours of paracetamol treatment in line with Australian guidelines, 14 , 15 but only 56% had LFTs measured after 5 days of treatment. Only 1 previous study explored LFT monitoring in older recipients of paracetamol in hospital. 17 Mitchell et al. found that around 50% of older patients had LFTs measured within 72 hours of starting paracetamol in hospital. 17 Neither our study nor Mitchell's explored clinical outcomes or followed patients beyond discharge.
4.1. Strengths and limitations
Selection bias was avoided by including all patients with low body weight recorded in their medical record, and randomly selecting patients from the eligible frail cohort. Twenty‐seven percent of potentially eligible patients were excluded from the study due to having no weight recorded, and 21% due to insufficient data to calculate FI‐Lab. Given the very high frequency of excessive dosing, and the fact that excluded patients received the same median daily dose of paracetamol in hospital as the study cohort, it is unlikely that exclusions had a substantial impact on generalisability of our findings. The study patients were slightly older and more likely to be female than the rest of the potentially eligible cohort, which is probably due to the inclusion of low‐weight patients, who were predominantly female. As this was a single‐centre study at a large metropolitan public health service, our findings may not be generalisable to other settings including private and rural hospitals. Some of the biological markers used in the FI‐Lab may be impacted by acute illness, and this may lead to over‐estimation of frailty. However, there is evidence that FI‐Lab scores on admission to hospital are associated with short and long‐term adverse outcomes. 22 , 27 Additionally, we used a more conservative cut‐point (0.3) to define frailty than the commonly used frailty index cut‐point of 0.25. 28 The mix of variables included in our FI‐Lab differed slightly from validation studies; however, we selected variables a priori that were consistent with validation studies and commonly measured in our hospital. Our FI‐Lab included 27 variables. In comparison, the number of FI‐Lab variables used in validation studies varied from 21 to 32, and all FI‐Lab versions were associated with mortality and other outcomes, 20 , 21 , 22 , 23 , 27 , 29 , 30 therefore we expect that our version would perform similarly. Our study did not assess clinical outcomes (paracetamol effectiveness and adverse effects including liver injury), as there was no follow‐up of patients after hospital discharge.
Reasons why paracetamol doses were not routinely reduced for older frail and low‐weight people at the study hospital were not explored. Contributing factors may include: (i) no local institutional guideline for paracetamol prescribing in older people; (ii) no decision‐support related to paracetamol dosing embedded within the electronic medication management system; and (iii) mixed expert opinion on the need to use lower doses in this population. 9 The Australian national analgesic consensus guidelines at the time of this study recommended dose reduction in “people who have significant liver disease, are of small size, are malnourished, or are frail older patients”, but provided no specific guidance for how to reduce the dose. 31 The current version of these guidelines provide less guidance on dosing in older people, citing concerns that under‐dosing may lead to use of stronger analgesics such as opioids. 32 Lack of clear guidance for dose‐reductions may contribute to variable practice and potentially excessive dosing.
Although there is limited evidence that the standard maximum therapeutic dose (4 g/d) leads to significant rates of liver injury in frail and low‐weight older people, 3 we believe that reduced doses should be considered for 3 reasons. Firstly, pharmacokinetic studies indicate that these patients may have lower volumes of distribution, higher plasma concentrations and lower paracetamol clearance with standard doses compared to younger adults. 9 , 10 Therefore, it is likely that slightly lower doses (e.g. 3 g/d instead of 4 g/d), or weight‐based dosing for people <50 kg, will be effective for many of these patients while potentially reducing risk of toxicity. Although evidence for effectiveness of reduced doses is scant, randomised controlled studies in nursing home residents (a population that is often frail and/or under‐weight) investigated whether regular administration of paracetamol (up to 1 g 3 times daily) could reduce the sequelae of untreated pain and improve functioning in patients with dementia; they reported reductions in behavioural symptoms of dementia. 33 , 34 Secondly, older hospitalised patients usually have a high pill‐burden and medication regimen complexity, 35 , 36 and taking 2 500‐mg paracetamol tablets 4 times a day contributes significantly to this. Even with sustained release dose‐forms (665 mg), to achieve 4 g/d requires 2 tablets 3 times a day. Reducing regimen complexity and pill‐burden may improve medication adherence. 37 We believe the benefits of using lower doses are therefore likely to outweigh the risk of under‐treatment in this population. However, further studies exploring the comparative effective and safety of standard dosing versus reduced dosing would be helpful to provide a stronger evidence‐base for this practice. 9
There has been little research focusing on ways to reduce potentially excessive paracetamol dosing in older people. A recent study at a Swiss hospital described the development and implementation of an electronic tool with algorithm‐based alerts for excessive paracetamol. 26 The tool identified >70% of instances of excessive dosing. Pharmacists used the alerts to make recommendations to prescribers, of which >70% were accepted. For alerts related to body weight and age >75 years, the acceptance rate was 80%. 26 Sustainability of this intervention is questionable, as each alert had to be validated by a pharmacist who then made recommendations to prescribers. Alerts that trigger at the time of prescribing may be more efficient; however, caution is needed to avoid excessive alerts, which can lead to alert fatigue and thus poor prescriber compliance with alert recommendations. 38 , 39
At the study hospital, it has been proposed that order sentences for lower dose regimens and prescriber alerts within the electronic prescribing system could encourage prescribers to consider dose‐reduction in patients with low body weight or frailty. An education campaign targeting, prescribers, pharmacists and nursing staff could be used to communicate the need to consider patients' risk‐factors for paracetamol‐induced hepatotoxicity and the impact of regular paracetamol at 4 g/d on medication regimen complexity.
In conclusion, approximately 2/3 of frail or low‐weight older patients were prescribed paracetamol doses that were higher than recommended by consensus guidelines. The majority of higher than recommended doses were initiated in hospital and continued on discharge. LFTs were checked at least once for most patients, but when paracetamol was continued for more than 5 days almost half of the patients did not have LFTs re‐measured. Given the frequency of higher than recommended paracetamol dosing, and the lack of high‐quality evidence to guide dosing in this population, further research is urgently needed to establish safe and effective doses and assess the risk of adverse outcomes associated with potentially excessive dosing in frail and low‐weight older people.
COMPETING INTEREST
The authors have no conflicts of interest to declare.
CONTRIBUTORS
R.A.E. conceived, designed and led the study, contributed to data collection and analysis, drafted the manuscript with O.R. and edited the manuscript. O.R. collected data, led the data analysis and drafted the manuscript with R.E. J.N. assisted with data collection and analysis. E.S. assisted with data extraction from the clinical research data warehouse and data analysis and reviewed and edited drafts of the manuscript. S.L. designed and led the data extraction from the clinical research data warehouse and reviewed and edited drafts of the manuscript. All authors contributed to data interpretation and approved the final manuscript.
Supporting information
TABLE S1 FI‐Lab variables and reference ranges
TABLE S2 All patient demographics (included and excluded patients)
ACKNOWLEDGEMENTS
Nil.
No funding was received for this study. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Reid O, Ngo J, Lalic S, Su E, Elliott RA. Paracetamol dosing in hospital and on discharge for older people who are frail or have low body weight. Br J Clin Pharmacol. 2022;88(10):4565‐4572. doi: 10.1111/bcp.15394
The authors confirm that the Principal Investigator for this paper is Rohan A. Elliott and that he did not have direct clinical responsibility for patients in this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Abdel Shaheed C, Ferreira GE, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. Med J Aust. 2021;214(7):324‐331. doi: 10.5694/mja2.50992 [DOI] [PubMed] [Google Scholar]
- 2. Manyemba J, Batty GM, Grant RR, et al. Prescription of paracetamol‐containing medications as indicator of quality of prescribing. Age Ageing. 2007;36(5):582‐584. doi: 10.1093/ageing/afm090 [DOI] [PubMed] [Google Scholar]
- 3. Caparrotta TM, Antoine DJ, Dear JW. Are some people at increased risk of paracetamol‐induced liver injury? A critical review of the literature. Eur J Clin Pharmacol. 2018;74(2):147‐160. doi: 10.1007/s00228-017-2356-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ging P, Mikulich O, O'Reilly KMA. Unexpected paracetamol (acetaminophen) hepatotoxicity at standard dosage in two older patients: time to rethink 1 g 4 times daily? Age Ageing. 2016;45(4):566‐567. doi: 10.1093/ageing/afw067 [DOI] [PubMed] [Google Scholar]
- 5. Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen‐induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol. 2016;4(2):131‐142. doi: 10.14218/jcth.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt LE. Age and paracetamol self‐poisoning. Gut. 2005;54(5):686‐690. doi: 10.1136/gut.2004.054619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mangoni AA, Jackson SHD. Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2003;57(1):6‐14. doi: 10.1046/j.1365-2125.2003.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLachlan AJ, Bath S, Naganathan V, et al. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment. Br J Clin Pharmacol. 2011;71(3):351‐364. doi: 10.1111/j.1365-2125.2010.03847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mian P, Allegaert K, Spriet I, Tibboel D, Petrovic M. Paracetamol in older people: towards evidence‐based dosing? Drugs Aging. 2018;35(7):603‐624. doi: 10.1007/s40266-018-0559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. What dose of paracetamol for older people? Drug Ther Bull. 2018;56(6):69‐72. doi: 10.1136/dtb.2018.6.0636 [DOI] [PubMed] [Google Scholar]
- 11. Mitchell SJ, Hilmer SN, Murnion BP, Matthews S. Hepatotoxicity of therapeutic short‐course paracetamol in hospital inpatients: impact of ageing and frailty. J Clin Pharm Ther. 2011;36(3):327‐335. doi: 10.1111/j.1365-2710.2010.01193.x [DOI] [PubMed] [Google Scholar]
- 12. Nash E, Sabih AH, Chetwood J, et al. Drug‐induced liver injury in Australia, 2009–2020: the increasing proportion of non‐paracetamol cases linked with herbal and dietary supplements. Med J Aust. 2021;215(6):261‐268. doi: 10.5694/mja2.51173 [DOI] [PubMed] [Google Scholar]
- 13. Health Quality & Safety Commission New Zealand . Medication Safety Watch, Issue 16. 2016. Accessed December 30, 2021. https://www.hqsc.govt.nz/assets/Medication-Safety/Watch-Updates/Medication-Safety-Watch-16-Jan-2016.pdf
- 14. NSW TAG . Paracetamol use: a position statement of the New South Wales Therapeutic Advisory Group Inc. 2008. Accessed December 30, 2021. http://www.nswtag.org.au/wp-content/uploads/2017/07/paracetamol-use-dec-2008.pdf
- 15. Queensland Health . Safe paracetamol use guideline. 2014. Accessed December 30, 2021. https://www.health.qld.gov.au/__data/assets/pdf_file/0030/147666/qh-gdl-415.pdf
- 16. Elliott RA, Woodward MC, Oborne CA. Quality of prescribing for elderly inpatients at nine hospitals in Victoria, Australia. J Pharm Pract Res. 2003;33(2):101‐105. doi: 10.1002/jppr2003332101 [DOI] [Google Scholar]
- 17. Mitchell S, Murnion B, Matthews S, Hilmer S. Compliance with paracetamol prescribing policies at a Sydney hospital. J Pharm Pract Res. 2009;39(2):122‐128. doi: 10.1002/j.2055-2335.2009.tb00435.x [DOI] [Google Scholar]
- 18. Niedrig DF, Bucklar G, Fetzer M, Mächler S, Gött C, Russmann S. Paracetamol overdosing in a tertiary care hospital: implementation and outcome analysis of a preventive alert programme. J Clin Pharm Ther. 2016;41(5):515‐518. doi: 10.1111/jcpt.12427 [DOI] [PubMed] [Google Scholar]
- 19. Healthcare safety investigation branch (HSIB) . Unintentional overdose of paracetamol in adults with low bodyweight. 2021. Accessed December 30, 2021. https://www.hsib.org.uk/investigations-cases/unintentional-overdose-paracetamol-adults-low-bodyweight/
- 20. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12(1):171. doi: 10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. Geroscience. 2017;39(4):447‐455. doi: 10.1007/s11357-017-9993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis HL, Wan B, Yeung M, et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI‐Laboratory) comprising routine blood test results. CMAJ. 2020;192(1):E3‐E8. doi: 10.1503/cmaj.190952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang M, Zhuo Y, Hu X, Xie L. Predictive validity of two frailty tools for mortality in Chinese nursing home residents: frailty index based on common laboratory tests (FI‐Lab) versus FRAIL‐NH. Aging Clin Exp Res. 2018;30(12):1445‐1452. doi: 10.1007/s40520-018-1041-7 [DOI] [PubMed] [Google Scholar]
- 24. UK National Poisons Information Service (NPIS) . TOXBASE [website]. NPIS Edinburgh. Accessed December 23, 2021. https://www.toxbase.org/
- 25. Bacle A, Pronier C, Gilardi H, Polard E, Potin S, Scailteux L‐M. Hepatotoxicity risk factors and acetaminophen dose adjustment, do prescribers give this issue adequate consideration? A French university hospital study. Eur J Clin Pharmacol. 2019;75(8):1143‐1151. doi: 10.1007/s00228-019-02674-5 [DOI] [PubMed] [Google Scholar]
- 26. Cabrera‐Diaz F, Zaugg C, Lim S, Blum K, Salili AR. Implementation and outcome of an electronic tool for detection of paracetamol overdose in a tertiary care hospital. Int J Clin Pharmacol. 2021;43(3):681‐688. doi: 10.1007/s11096-020-01182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jäger J, Sieber CC, Gaßmann K‐G, Ritt M. Changes of a frailty index based on common blood and urine tests during a hospital stay on geriatric wards predict 6‐month and 1‐year mortality in older people. Clin Interv Aging. 2019;14:473‐484. doi: 10.2147/cia.s191117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol a Biol Sci Med Sci. 2007;62(7):738‐743. doi: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 29. Ritt M, Jäger J, Ritt J, Sieber C, Gaßmann K. Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging. 2017;12:1029‐1040. doi: 10.2147/cia.s131987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitnitski A, Collerton J, Martin‐Ruiz C, et al. Age‐related frailty and its association with biological markers of ageing. BMC Med. 2015;13(1):161. doi: 10.1186/s12916-015-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Therapeutic Guidelines: Analgesic, Version 6. Melbourne: Therapeutic Guidelines Limited; 2012.
- 32.Paracetamol in pain management [published Dec 2020]. In: Therapeutic Guidelines [digital]. Melbourne: Therapeutic Guidelines Limited; Mar 2021. https://www.tg.org.au.acs.hcn.com.au
- 33. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well‐being, and psychotropic medication use in nursing home residents with moderate‐to‐severe dementia. J Am Geriatr Soc. 2005;53(11):1921‐1929. doi: 10.1111/j.1532-5415.2005.53572.x [DOI] [PubMed] [Google Scholar]
- 34. Husebø B, Ballard C, Sandvik R, Nilsen O, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343(jul15 1):d4065. doi: 10.1136/bmj.d4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elliott RA, O'Callaghan CJ. Impact of hospitalisation on the complexity of older patients' medication regimens and potential for regimen simplification. J Pharm Pract Res. 2011;41(1):21‐25. doi: 10.1002/j.2055-2335.2011.tb00060.x [DOI] [Google Scholar]
- 36. Hubbard RE, Peel NM, Scott IA, et al. Polypharmacy among inpatients aged 70 years or older in Australia. Med J Aust. 2015;202(7):373‐377. doi: 10.5694/mja13.00172 [DOI] [PubMed] [Google Scholar]
- 37. Cross AJ, Elliott RA, Petrie K, Kuruvilla L, George J. Interventions for improving medication‐taking ability and adherence in older adults prescribed multiple medications. Cochrane Database Syst Rev. 2020;5(5):CD012419. doi: 10.1002/14651858.CD012419.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36. doi: 10.1186/s12911-017-0430-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of Drug Safety Alerts in Computerized Physician Order Entry. J Am Med Inform Assoc. 2006;13(2):138‐147. doi: 10.1197/jamia.m1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 FI‐Lab variables and reference ranges
TABLE S2 All patient demographics (included and excluded patients)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


