Abstract
Metastasis is a leading cause of cancer‐related death and consists of a sequence of events including tumor expansion, intravasation of cancer cells into the circulation, survival in the bloodstream, extravasation at distant sites, and subsequent organ colonization. Particularly, intravasation is a process whereby cancer cells transverse the endothelium and leave the primary tumor site, pioneering the metastatic cascade. The identification of those mechanisms that trigger the entry of cancer cells into the bloodstream may reveal fundamentally novel ways to block metastasis at its start. Multiple factors have been implicated in cancer progression, yet, signals that unequivocally provoke the detachment of cancer cells from the primary tumor are still under investigation. Here, we discuss the role of intrinsic properties of cancer cells, tumor microenvironment, and mechanical cues in the intravasation process, outlining studies that suggest the involvement of various factors and highlighting current understanding and open questions in the field.
Keywords: cancer, circulating tumor cells, intravasation, metastasis
Metastasis is a leading cause of cancer‐related death. Particularly, intravasation is a process whereby cancer cells transverse the endothelium and leave the primary tumor site, pioneering the metastatic cascade. Here, we discuss the role of intrinsic properties of cancer cells, tumor microenvironment, and mechanical cues in the intravasation process, highlighting current understanding and open questions in the field.
Abbreviations
- CAF
cancer‐associated fibroblast
- CTC
circulating tumor cell
- ECM
extracellular matrix
- EMT
epithelial‐to‐mesenchymal transition
- i.v
intravenous injection assay
- IFP
interstitial fluid pressure
- Mena
actin‐regulatory protein mammalian enabled
- mtDNA
mitochondrial DNA
- NETs
neutrophil extracellular traps
- ROS
reactive oxygen species
- TAM
tumor‐associated macrophage
- WES
whole exome sequencing
Introduction
Metastasis is the leading cause of cancer‐related deaths [1]. It proceeds via a sequence of events, beginning with tumor expansion and progressing with the intravasation of cancer cells into the circulation, their subsequent survival within the blood stream, extravasation at distant sites, and finally metastatic colonization and expansion. Each of these steps is considered to be extremely inefficient, estimating that < 1% of cells that intravasate into the circulation ultimately succeed to establish a distant metastasis [2]. However, tumors are composed of billions of cells and, as a consequence, it is assumed that millions of individual cells may be shed into circulation from 1 g of cancerous tissue in rodents each day [3]. Whether intravasation is a passive or actively regulated phenomenon is debated, yet, unarguably, the identification of those mechanisms that trigger the entry of cancer cells into the bloodstream may reveal fundamentally novel ways to block metastasis right where it begins (Fig. 1).
Fig. 1.
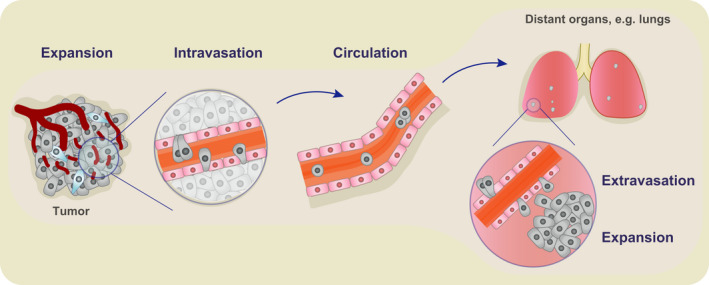
Metastasis consists of a series of steps including tumor expansion, intravasation of cancer cells into the circulation, their survival in the circulation, followed by extravasation and expansion at distant sites.
Intravasation is a process whereby cancer cells detach from the tumor mass and penetrate through the endothelial walls of blood vessels to reach the blood stream, where they are referred to as circulating tumor cells (CTCs). Intravital microscopy studies have shown that cancer cells generate protrusions that are first aligned along endothelial cell–cell contacts and then inserted in between endothelial cells [4, 5]. Both single cells and clusters of cancer cells together with partnering cells from the microenvironment can intravasate into blood [6, 7, 8, 9, 10], with cluster presence in the circulation associated with a more aggressive disease progression [6, 7].
It has been discussed whether the process of intravasation of cancer cells and shedding of CTCs into circulation is occurring randomly or is predetermined by specific factors. While no definitive proof is present at the moment, and likely more than one mechanism is involved, it is hard to imagine that intravasation would be neutral with respect to all the environmental and intrinsic factors such as proximity to blood vessels, mechanical forces, presence of immune cells, or the aggressiveness of a particular subclone of tumor cells (Fig. 2). To verify this, there is a need for rigorous studies that would prove causality between different factors and intravasation.
Fig. 2.
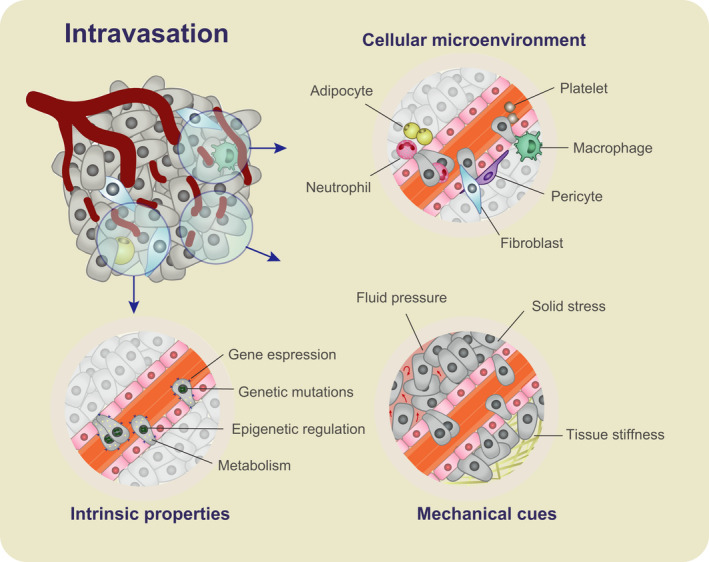
The intravasation process is regulated by intrinsic, microenvironmental, and mechanical factors. Intrinsic properties include genetic mutations, epigenetic changes, gene expression, and metabolic profile alterations that give cells an intravasation advantage. The cellular microenvironment consists of fibroblasts, adipocytes, pericytes, platelets, and immune cells such as macrophages and neutrophils. These cells may regulate the intravasation of cancer cells by cell‐to‐cell signaling, altering the tumor microenvironment and direct participation in the invasion process. Mechanical cues such as tissue stiffness, stress, and interstitial fluid pressure may also affect cancer cell dissemination into the circulation.
Multiple elements have been implicated in cancer progression in general, ranging from genetic, transcriptional, immune cell involvement, and others [11]. However, it is a challenge to disentangle the processes that directly contribute to individual steps of the metastatic cascade, given the complexity of growing tumors and their associated microenvironments. With that regard, the goal of this review is to outline those studies that suggest a linkage of various factors with the intravasation process itself, highlighting the current understanding and open questions for metastasis research. These factors include intrinsic properties of cancer cells, signals from the tumor microenvironment, and mechanical cues.
Intrinsic properties of cancer cells
Cell‐intrinsic properties may play a key role in promoting intravasation of cells into the circulation. Particularly, here we discuss how genetic mutations, gene expression profiles, epigenetic profiles, and metabolism of cells may affect their proclivity to intravasate. These factors have been discussed to be essential in various aspects of tumor development and progression. However, much information comes from correlative studies that analyze tumor profiles and disease outcome or, alternatively, that focus on the overall disease outcome without distinction into individual steps of the metastatic cascade. A useful approach to dissect the role of various factors in the intravasation process is to focus on CTCs—given their short half‐life in circulation [6, 12]—considering them as a ‘snapshot’ image of those features that cancer cells have acquired in order to leave the tumor site, for example, when compared to cells that did not intravasate.
Gene expression profiles
Though the expression of several genes has been implicated in cancer metastasis [13, 14, 15, 16], their role in specific steps of the metastatic cascade remains to be fully understood. The expression of some genes may predispose cancer cells to intravasate into circulation: for instance, the role of certain signaling pathways has been emphasized in this context, in particular the Wnt pathway and epithelial‐to‐mesenchymal transition (EMT) program. The expression of genes involved in Wnt signaling is enriched in pancreatic cancer mouse model CTCs [17], and a study using mouse orthotopic models of glioblastoma and CTC samples from glioblastoma patients has suggested that Wnt promotes the expression of stemness markers such as Sox2, Oct4, and Nanog, with upregulated levels in CTCs compared with the primary tumor counterpart. Functionally, those cells showed enhanced proliferation and contribution to tumor growth in vivo [18]. Recently, in an effort to group and unify the sequencing data in relation to metastasis in patients, a database of published CTC and primary tumor RNA expression profiles has been generated [19]. Furthermore, a study analyzing comparative expression of genes between primary and secondary tumors in patients identified the involvement of genes including Wnt8A, Fgf8, pik3CB, ESR2, and others, as well as ORH5221, CATSPER4, and CLRN1 that were not previously known in the context of breast cancer [20]. ‘Stemness’ genes, in turn, were implicated in CTCs of PDAC—Lin28B and Klf4, as well as Wnt5a, and LGALS3. Lin28B knockout resulted in a reduced cell aggressiveness in vitro and in vivo [21]. Altogether, the Wnt pathway emerges as a strong candidate in cancer invasiveness, since its components appear to be overrepresented in CTCs. However, more studies will be needed in this area to fully dissect the gene expression changes of CTCs compared with primary tumor cells in various cancer types, and beyond correlative efforts, to conclusively demonstrate a functional role for specific genes in dictating intravasation dynamics.
Epithelial‐to‐mesenchymal transition has been implicated in cancer progression [11] and is a process whereby junctions of cells are disrupted, epithelial‐like cells acquire a mesenchymal phenotype, resulting in invasive properties and detachment of single cells. While EMT has an effect on invasive properties in vitro and in vivo, it is not fully understood whether it plays a role in the spontaneous intravasation of cancer cells into the circulation and, as a consequence, enhanced colonization of distant organs [22, 23]. In mouse models, the ectopic activation of EMT transcription factors induced invasive phenotype and intravasation into circulation [24, 25, 26], although contrary reports, whereby more epithelial cells were migratory, also exist [27, 28]. In studies without overexpression systems, more complex hybrid phenotypes have been observed [29, 30], arguing in favor of cellular plasticity rather than a full, rigid EMT transition. Some of the conclusions from the analysis of EMT mouse models may be in conflict with the data from patient tumor histology; for example, primary breast tumors are overwhelmingly epithelial [31]. Recently, a study showed that in breast cancer xenograft models, E‐cadherin expression is essential for metastasis formation [32]. On the other side, E‐cadherin negative cells have been seen shown to be more migratory in an intravital study of aggressive breast cancer mouse model; however, intravasation events themselves were not recorded [33]. In patient samples, CTCs with a broad spectrum of EMT features have been detected, although some of these studies used microfluidic enrichment without unequivocal validation of their putative cancer identity, for example, by means of genomic analysis [34, 35]. A recent study, on the other hand, found that there was no correlation between EMT and the clinical stage of hepatocellular carcinoma [36]. At the same time, collective cell migration and detection of CTC clusters in the circulation with evident epithelial features has been reported [6, 7, 8, 10, 32]. Altogether, while occurring at different degrees during cancer progression and in a model‐dependent fashion, recent studies argue that EMT may be dispensable for intravasation in human disease. In our opinion, more focus should be paid to cellular programs and plasticity, and should be studied out of the box of EMT dogma, though not necessarily excluding it.
Genetic mutations
Similarly to gene expression profiles, it is conceivable that the acquisition of a particular set of mutations may confer a high propensity to intravasate, consistent with the emergence of mutationally defined, metastasis‐prone tumor subclones. A few studies have investigated the mutational profile of CTCs compared with primary tumor cells in patients and identified private mutations specific to CTCs [37, 38, 39, 40, 41]. In prostate cancer, whole exome sequencing (WES) of matched tumor and CTC samples from two patients and subsequent SNV analysis revealed mutations in DNAH8 encoding a dynein heavy chain and in receptor tyrosine kinase EPHB1 [37]. Another WES analysis revealed genes involved in cytoskeleton modeling (e.g., MACF1) or invasion (NEDD9) as exclusively mutated in prostate cancer CTCs [39]. In breast cancer, loss of chromosome 1p was observed in CTCs and absent in the primary tumor counterpart [40]. However, it is important to note that some of these CTC‐only mutations may emerge due to low depth of sequencing of the tumor or its incomplete sampling and may actually still be present subclonally within primary tumor cells [42]. Of note, several studies have identified genes promoting metastasis to defined organs [14, 15, 16]; however, the associated profiles seem reflective of the adaptation of cells to specific environments following extravasation. Nevertheless, some genes such as Nedd9, which was identified as a melanoma metastasis gene [13], have been found privately mutated in CTCs relative to tumor [39]. A higher depth of sequencing combined with an increase in the number of sampled CTCs could potentially reveal more genes simultaneously mutated or expressed in CTCs and metastases. Together, while it is likely that certain mutations may increase CTCs’ proclivity to intravasate, additional sequencing efforts and functional studies will be needed for a better understanding of this phenomenon.
Epigenetic factors
DNA methylation, histone modifications, and chromatin remodeling have been characterized in various tumors [43, 44], yet the effect of those epigenetic changes on metastasis are only beginning to be resolved.
Comparative analysis of the methylation landscape of mouse primary tumor and metastatic cells [45], as well as of primary tumor and metastasis patient‐derived cell lines [46], has indicated epigenetic factors that may drive metastasis. Focusing on CTCs, the hypomethylation of tumor suppressor and metastasis suppressor genes including CST6, Sox17, and BRMS1 have been observed in breast cancer patients [47]. Recently, a genome‐wide methylation profile was reported for CTCs in breast cancer patients and mouse models [48]. The study revealed differential methylation pattern between single and clustered CTCs, with CTC clusters showing DNA hypomethylation at binding sites of transcription factors that are typically involved in the regulation of pluripotency and stemness in embryonic stem cells, such as OCT4, NANOG, SOX2, and SIN3A. Analysis of The Cancer Genome Atlas revealed that the hypomethylation pattern of CTC clusters was also detected in primary tumors and correlated with a poor prognosis of patients [48]. This highlights the possibility that epigenetic signatures present at the primary tumor level may predispose toward intravasation. Further comparative analysis of epigenetic patterns in CTCs and primary tumor samples may help to identify epigenetic marks with an impact on cancer cells intravasation.
Outlook
Molecular analysis of single and clustered CTCs and their comparison with primary tumor cells has begun to highlight some of the cell‐intrinsic factors that could play a role in the intravasation. These include metastasis‐promoting shifts in gene expression, acquisition of mutational profiles, and epigenetic patterns that favor the metastatic process. A major challenge in this regard is to compare these profiles to an adequate number of matched primary tumor cells and to minimize technical biases related to sample characteristics (e.g., solid vs liquid biopsy) and procedures (e.g., single cell vs bulk sequencing). It will also be important to focus these analyses on a high number of patient samples, that is, progressively stepping away from model systems that may not fully recapitulate the complexity of the intravasation process in cancer patients. Integration of multi‐omics methods along with dedicated computational pipelines will be instrumental in this context, promoting the identification of cell‐intrinsic features with an impact on the intravasation process.
Metabolism of cancer cells
Metabolism emerges as an uneven characteristic of tumor cells with a potential impact on various steps of tumor progression, as cancer cells display a significant extent of metabolic plasticity and heterogeneity. They can rewire their metabolic profiles to ensure a variety of features, including survival, proliferation, and invasion [49, 50]. However, it is likely that metabolic pathways involved in the expansion of the primary tumor are distinct from those that give advantage for metastatic progression. For instance, the tissue of origin of the primary tumor may influence the type of metabolic processes in action [51], given the variability in parameters such as organ vascularization and oxygen availability. Therefore, finding a pattern between cellular metabolism and metastatic intravasation in different cancer types is a challenge as well as an opportunity for future studies.
Glycolysis
One of the key players in cancer cell survival is glucose metabolism. Glucose serves as the main energy source in mammalian cells, by feeding into the glycolytic pathway. Indeed, cancer cells are commonly characterized by the Warburg effect [52]—their metabolism heavily relies on glycolysis, with the omission of the TCA cycle even in the high abundance of oxygen. However, how glucose metabolism affects metastasis, and intravasation specifically, needs to be addressed. Hexokinase 2 and pyruvate kinase 2 are the enzymes involved at the beginning and at the end of glycolysis, respectively. Their expression levels are correlated with motility and invasiveness as measured in in vitro and in vivo transplantation assays, and there is also a correlation between their levels in cancer tissue and disease aggressiveness in patients [53, 54, 55, 56, 57, 58, 59]. Furthermore, a byproduct of glycolysis, methylglyoxal, may be capable of inducing YAP‐mediated metastasis, as shown in vitro and in in vivo transplantation assays [60]. Additionally, heavy reliance on glycolysis results in the accumulation of lactate. In a breast cancer mouse transplantation model, L‐lactate did not affect tumor growth, but significantly increased metastatic potential [61]. Lactate production may be involved in pH change that could stimulate activation of cathepsins and mmp9, which degrade ECM and promote invasion [62]. Indeed, inhibition of MCT1 lactate transporter could be a good therapeutic target as shown in vitro and in vivo transplantation model of breast cancer [63]. Interestingly, a recent study showed that pyruvate uptake induces the production of α‐ketoglutarate, which remodels collagen via increasing the activity of the enzyme collagen prolyl‐4‐hydroxylase (P4HA). Inhibition of pyruvate metabolism was sufficient to impair collagen hydroxylation and decrease the metastatic burden of breast cancer in different mouse models [64].
Mitochondrial metabolism
Parallel with the reports of reliance of cancer cells on glycolysis, oxidative phosphorylation still plays a significant role, though the extent of this process may be variable in different cancer types [65]. Using isotope tracing, glucose oxidation was found to be low in clear renal cell carcinoma as studied in patients [66], while both glucose oxidation and glycolysis were high in lung cancer in patients, and glioblastoma models in mice [67, 68, 69]. Importantly, one of those studies revealed metabolic heterogeneity within tumor cells with respect to both glycolysis and glucose oxidation [68]. The question arises about the extent of cellular metabolic heterogeneity within human tumors, and more specifically, whether enhanced oxidation is of an advantage/disadvantage for metastatic intravasation.
In general, reports about the effect of mitochondria function on cancer cell invasiveness have been conflicting. Mitochondrial biogenesis and oxidative metabolism have been shown to promote metastasis in breast cancer in vivo models [70, 71, 72] with enhanced oxidative phosphorylation observed in breast cancer CTCs in patients [70], as well as to suppress it based on a study of prostate and melanoma models [73, 74]. These studies relied on knockdown and overexpression of PGC1α that mediates oxidative phosphorylation, and therefore, the discrepancy may result from tissue specific differences in endogenous levels of PGC1α. It is possible that there is an optimal range of PGC1α levels that promotes metastasis. It is also likely that cancer cells display a significant level of metabolic flexibility during disease progression and respond to other metabolic cues: For example, even though the Warburg effect is very common in cancer cells, alternative pathways could be activated when glycolysis is switched off. Indeed, oxidative phosphorylation was increased in cancer cell lines upon inhibition of glycolysis [75], provision of low glucose concentration in the medium [76], and following induction of lactic acidosis [77]. Vice versa, suppression of PGC1α‐dependent mitochondrial oxidative metabolism brought up glycolysis in a melanoma cell line [78]. These findings emphasize the importance of single‐cell resolution metabolic profiling of tumor cells along with matched circulating tumor cells to identify metabolic processes that may confer intravasation benefit.
Oxidative stress
Oxidative stress arises as a consequence of an imbalance between production and detoxification of reactive oxygen species (ROS), which are normally generated as by‐products of oxygen metabolism. However, environmental stressors, which are prevalent during carcinogenesis, also contribute to the production of ROS [79]. ROS has been recognized as a metastasis promoting factor [71, 80, 81], and it could do so by promoting DNA damage or through the activation of prometastatic signaling pathways within cells [82, 83]. For example, pharmacological scavenging of mitochondrial superoxide prevented metastatic spread from melanoma models in mice [71]. One study showed that mitochondrial DNA (mtDNA) of cells with high metastatic potential contained G13997A and 13885insC mutations in the gene encoding NADH (reduced form of nicotinamide adenine dinucleotide) dehydrogenase subunit 6 (ND6). These mutations resulted in a deficiency in respiratory complex I activity and consequently overproduction of ROS. When the endogenous mtDNA of poorly metastatic cells was replaced with that of a highly metastatic cell line of Lewis lung carcinoma, those recipient cells gave rise to more lung metastases as determined by intravenous (i.v.) injection assay [81]. Another study confirmed that the metastatic potential of melanoma cell lines, as revealed by i.v. assay, correlated with ROS levels [80]. However, both those studies examined the metastatic potential of cells without considering intravasation requirements, as the cells were injected directly into the circulation [80, 81].
More recently, however, oxidative stress has been shown to inhibit metastasis and intravasation specifically, as blocking folate pathway and antioxidant treatment resulted in a higher number of CTCs and a higher incidence of metastases in melanoma models [84]. This antioxidative stress protection may happen via Nur77 and TGFB signaling as shown in vitro and in transplantation mouse models of melanoma [85]. Other studies have supported this notion of the negative effect of oxidative stress on intravasation in the context of lung cancer [86, 87, 88] and melanoma [89]. It needs to be further defined if this factor could have its effect on metastasis via rendering the cells fit for intravasation and/or survival in the blood circulation. It may be that antioxidant metabolism facilitates cell survival by counteracting stress signals arising as a consequence of matrix detachment during intravasation. Altogether, while oxidative stress is generally considered to be metastasis‐promoting, controversy is present and its specific role in the spontaneous intravasation of cancer cells in vivo remains to be fully dissected. The apparent disparity could, at least partially, result from different experimental approaches, using i.v. assays and tumor models to address this question [80, 81, 84, 85, 86, 87, 88, 89].
Hypoxia and tumor vascularization
Hypoxia and vascularization, though apparently of opposite effect, are key features involved in cancer progression [11]. There is vast evidence for the role of hypoxia in metastasis promotion [90, 91]. For example, induction of hypoxia in mouse tumor models using oxygen chambers resulted in increased metastasis to the lymph nodes in cervical carcinoma [92] and to distant organs in sarcoma [93]. Furthermore, other studies show a positive correlation between the extent of hypoxia in the primary tumor and metastatic rate as observed for mouse xenograft models of pancreatic cancer [94] and in patients with sarcoma [95]. High expression levels of hypoxia master regulators, Hif1α and Hif2α, correlate with a worse patient prognosis and a higher metastatic incidence in various cancers [90, 96, 97, 98, 99].
Focusing on intravasation, it has been shown in vitro that Hif1α and Hif2α reduce endothelial cell–cell adhesion via angiopoietin‐like 4 (ANGPTL‐4) signaling and increase endothelial‐cancer cell adhesion via induction of L1CAM on cancer cell surface [100], thus providing a possible mechanism of hypoxia‐driven intravasation. Another study suggested that hypoxia could drive intravasation via signaling through CXCR4, which results in adhesion of cancer cells to endothelial cells and trans‐endothelial migration in in vitro assays [101]. Hypoxia also induces secretion of VEGF, rendering blood vessels more permeable [90]. A study using a fibrosarcoma cell line found increased hypoxia, enhanced blood vessel permeability, and elevated intravasation rate at the core in a chick embryo mesodermal model of tumor growth [102]. These findings should also be verified with more standard approaches of in vivo orthotopic transplantation models. A recent study on breast tumor xenograft models suggested that both central/core as well as scattered hypoxia areas are present in a model‐dependent fashion. More importantly, however, the study provided a direct link between intravasation and hypoxia, by showing that CTC clusters arise from hypoxic areas. Interestingly, the authors show that in vivo, hypoxia results in cell–cell junction upregulation and intravasation of clustered CTCs with an elevated metastatic ability [103].
Fatty acid uptake
Interestingly, fatty acid metabolism has also been shown to have an effect on metastasis. A population of cells expressing high levels of CD36, a fatty acid translocase associated with stem cell‐like features of some cancer cells [104, 105], showed high metastatic potential, while stimulation of cells with palmitic acid increased their metastatic ability in orthotopic mouse models of human oral cancer, in a CD36‐dependent manner [106]. Furthermore, an analysis of published patient datasets suggested that mutations in the pathways responsible for the uptake of free fatty acids correlated with invasiveness [107]. A possible mechanism of fatty acid action could be via their incorporation into membrane structure to induce oncogenic signaling [108], or via their oxidation and subsequent signaling [109]. These reports are very interesting and may suggest a more prominent role of fatty acid metabolism in the metastatic process than previously anticipated.
Outlook
While cancer cell metabolism has been extensively studied, highlighting intriguing roles for glycolysis, mitochondrial metabolism, antioxidant metabolism, tumor hypoxia, and fatty acid uptake, it is becoming increasingly clear that cancer cells are also heterogeneous in the context of their metabolic status, raising the intriguing question of whether or not altered metabolism can be intravasation‐promoting in individual tumor ecosystems. Clearly, an answer to this question will require metabolic profiling of matched primary tumor cells, CTCs and metastatic deposits, possibly at the single‐cell level. We speculate that such analyses should also be extended to cells that are part of the tumor microenvironment, as their metabolic activity could influence intravasation dynamics.
Cells in the microenvironment
Tumor microenvironment consists of a variety of cells including immune cells, endothelial cells, activated fibroblasts, and pericytes. Here, we outline their potential involvement in the intravasation process. Their mechanisms of action include modification of cancer cell properties via signaling pathways, altering the tumor microenvironment or direct participation in the intravasation process. While the effects of individual groups of cells are discussed below, future research efforts should also focus on their cooperative activity.
Cancer‐associated Fibroblasts
Fibroblasts are residents of many tissues, and during cancer development they can become activated or recruited to the tumor site, and as such they are referred to as cancer associated fibroblasts (CAFs) [110]. This population of cells has been associated with various aspects of tumor progression including tumor expansion, extracellular matrix (ECM) remodeling, angiogenesis, invasiveness, and metastasis [111]. Both progression‐promoting and inhibitory roles in metastasis have been reported [112, 113]. It is therefore crucial to identify subpopulations of CAFs and their respective functions.
CAFs were observed to be part of CTC clusters in efferent blood from tumors, and partial depletion of CAFs in mice resulted in reduced metastatic burden to the lungs [114]. Additionally, an observation was made that lung carcinoma as well as brain metastases in patients contained CAF populations within their microenvironment, while no fibroblasts were detected in healthy brain tissue or in primary brain cancers [114]. In line with the above, CAFs have also been detected as part of CTC clusters in the peripheral blood circulation in patients [115] and may confer shear resistance to CTCs as studied in in vitro systems [115]. Altogether, these studies suggest that CAFs may be associated with CTCs and potentially involved in their intravasation. Further evidence for CAF involvement in this process comes from the analysis of primary tumors. Fibroblasts were observed at the leading edge of invasive cancers, surrounding cancer cells in ‘collective invasion packs’ in vivo [116]. The cells at the leading edge have been shown to invade together by means of heterotypic N‐cad/E‐cad‐based adhesion between CAFs and cancer cells in 3D/2D migration assays and supported by protein expression analysis in patient tumor samples [117]. Altogether, these results suggest a potential role for CAFs in the intravasation process, as well as, more broadly, in metastatic cancer progression, and their full characterization in this regard is likely to reveal important mechanisms of cancer spread.
Neutrophils
Neutrophils are cells of the innate immune system that abundantly infiltrate the developing tumor. For long, they have been considered as bystanders; however, the past decade has provided evidence that in fact they play a very important role in tumor development and progression [118, 119, 120]. Neutrophils may indeed promote metastasis, especially by creating a premetastatic niche in the distant organs [121, 122]. One way in which the neutrophils could exert their prometastatic function is via release of neutrophil extracellular traps (NETs), which are commonly produced to trap microorganisms and consist of chromatin DNA filaments coated with granule proteins. Indeed, recently, NETs have been detected in metastatic lesions and to a lesser extent in primary tumors of breast cancer patients [123]. NETs have been shown to be implicated in the formation of a premetastatic niche in the liver and their removal impaired metastasis formation in vivo [123], while the role of NETs at other stages of the metastatic cascade and, particularly during intravasation, needs to be investigated. More generally, the presence of neutrophils has been previously correlated with increased intravasation rates in vivo [124], but recently it has been directly shown that these cells participate in the intravasation of CTC clusters in breast cancer patients and mouse models, particularly by partnering with cancer cells and sustaining their cell cycle progression in circulation, leading to increase metastatic propensity [7].
Macrophages
Macrophages are the cells of the innate immune system, and when recruited to the cancer site, they are referred to as tumor associated macrophages (TAMs). Macrophages have been shown to display both pro‐ and antitumorigenic functions [125].
Direct insight into the role of macrophages in intravasation was given by the discovery of the Tumor Microenvironment of Metastasis (TMEM) Doorway, composed of a Tie2‐high VEGFA‐high perivascular macrophage, a tumor cell expressing high levels of the actin‐regulatory protein mammalian enabled (Mena), and an endothelial cell as functional ‘doorway’ for hematogenous dissemination. Tie2‐high VEGFA‐high perivascular macrophages cause transient vascular permeability and result in intravasation of tumor cells as shown in the MMTV‐PyMT breast cancer model and in the PDX TN1 xenograft model [126, 127, 128]. Moreover, macrophages enhance the performance of cancer cells in in vitro transmigration assays [4] and promote invadopodia formation in a Rho‐A signaling‐dependent fashion [4] or via Notch1/Mena signaling pathway [129]. Macrophages have also been implicated in promoting early dissemination of cancer cells in breast cancer model [130]. In contrast, some other studies have shown a tumor‐inhibitory function for macrophages. This dual nature of macrophages (pro‐ vs antitumor) in cancer progression may arise from the existence of different functional subtypes, including the M1 and M2 macrophages that are described to be of proinflammatory and immunosuppressive function, respectively [131, 132]. Altogether, there is strong evidence that macrophages may play a significant role in the intravasation of cancer cells. The involvement of specific subpopulations of macrophages needs to be determined for designing efficient antimetastatic therapies that act at the macrophage level. Furthermore, it will be essential to understand fully the involvement of macrophages in concert with other tumor‐associated cell types [131], while considering macrophage heterogeneity and the possibility that different macrophage subtypes may intervene at different stages of the metastatic progression.
T cells
T lymphocytes are cells of the adaptive immune cells that commonly infiltrate the tumor environment during disease progression [133, 134]. While cytotoxic CD8+ T cells are believed to exhibit antitumor activity, helper CD4+ T cells have been suggested to promote cancer progression in some contexts. The link between these cell types and intravasation is beginning to be resolved.
CD4+ T lymphocytes have appeared as cells contributing to enhanced intravasation via direct signaling to cancer cells [135] or via indirect effect through regulation of the TAMs [136]. These studies have used mouse models of mammary cancer combined with depletion and inactivation of lymphocyte subpopulations, followed by analysis of metastasis to the lungs and count of CTCs. However, a more recent study has suggested that CD4+ T lymphocytes may be involved in vasculature normalization, and therefore, via this mechanism they could impede cancer cell intravasation [137]. The role of CD8+ T cells in intravasation has been investigated to a lesser extent; however, a recent study has demonstrated that tumors in CD8 knockout mice and mice with CD8 inactivation give rise to more metastases and increased numbers of CTCs [138]. This suggests that CD8+ cells play a safeguard role to intravasation. To corroborate these findings, it would be essential to understand the role of various subpopulations of CD4+ and CD8+ T cells in different contexts and stages of metastatic progression.
Platelets
Platelets have been implicated in angiogenesis and metastasis promotion [139, 140]. One study suggested that platelets induce EMT in cancer cells in vitro and improve extravasation by means of i.v. injection in mice [141]. While these studies highlight the involvement of platelets in the later stages of the metastatic cascade, it is possible that platelets also play an important role in the early events. For example, their involvement at the intravascular transit should be considered, both from the blood stream as well as from the tumor site [140]. Notably, platelet signatures have been consistently found in both mouse and human CTC clusters [6, 7].
Pericytes
Pericytes are commonly associated with endothelial cells, and are considered to be vital regulators of angiogenesis and vascular stability in both healthy and pathological conditions. Recruitment of pericytes to newly formed vessels in tumors has been shown to impede their sprouting, enhance vessel maturation, and decrease their leakiness [142, 143]. As such, they may be considered as gatekeepers of metastasis.
This notion is supported by the analysis of patient samples, which showed that low pericyte coverage of blood vessels is associated with a drop in survival in invasive breast cancer patients [144, 145, 146], and it is further corroborated by various mouse model studies. For instance, mice deficient in endosialin, a pericyte and myofibroblast marker [147, 148, 149], showed an increased metastatic rate with no effect on tumor growth and no effect on extravasation as studied by i.v. injection in mice [150]. Additionally, enhanced metastasis accompanied by overall tumor growth reduction in mice was observed following depletion of Ng2+ pericytes [151], and in PDGFR‐β ret/ret mouse model (bearing a truncating mutation in PDGFR‐β, a putative pericyte marker) [152]. Conditional inactivation of Shb—an adapter protein downstream of tyrosine kinases such as VEGFR2 and PDGFR—in pericytes with the PDGFR‐β‐CreERT2 reporter system resulted in a decreased pericyte coverage of small tumor vessels, increased leakage, and higher metastatic rate without affecting tumor growth [153]. This reinforces the notion that pericytes may be involved in intravasation by influencing blood vessel wall leakage. It should be noted, however, that part of the effect observed with the PDGFR‐β‐CreERT2 reporter system may be due to the fact that PDGFR‐β promoter may also be active in other cell types such as CAFs [154, 155, 156] or tumor cells themselves [157, 158]. Another study supports the hypothesis that pericytes influence endothelial wall leakiness, which was observed in response to the deletion of the A6‐integrin subunit of A6β1 integrin. Surprisingly though, while reduced pericyte coverage of tumor vessels and enhanced leakiness were observed, other parameters such as tumor growth, blood vessel density, and metastases were not affected [159].
On the opposing side, a study has suggested that orthotopic colon cancer grown in endosialin‐negative context resulted in a reduced tumor growth, invasion, and metastatic burden compared with wild‐type mice [160]. Another article showed that tumor cells may influence pericytes to convert into fibroblast‐like cells, thereby enhancing the invasiveness and intravasation of cancer cells [161]. Altogether, it is possible that the role of pericytes, and ultimately their subpopulations, may be context‐dependent. Indeed, the extent of pericyte coverage, and consequently their collective effect, could be variable with respect to tumor type and its location [162], as well as tumor progression stage [163]. Furthermore, the functional heterogeneity of pericytes based on different marker expression [143] should be carefully examined in future studies, as this could potentially reveal differential effects of pericyte subpopulations.
Adipocytes
Adipocytes are the main constituents of adipose tissue, and their function is to store fat as well as to secrete lipid and protein factors [164]. They have drawn attention in recent times in the context of growing obesity rates, and the observed correlation between obesity and cancer incidence [165]. Additionally, they are the major cellular component of breast tissue and get activated during cancer tumorigenesis, that is, become cancer associated adipocytes [166]. They are also a prevailing cell type in melanoma [167]. Cancer‐associated adipocytes have been implicated in cancer development and metastasis [166, 168, 169]. In vitro studies suggest that adipocytes may indeed promote cancer cell migration and invasion, and several mechanisms have been proposed. Coculture of ovarian cancer cells with adipocytes increases the proliferation of cells in vitro and the growth of those cells in transplantation assays in vivo and may promote their metastasis by inducing Feb4 expression in cancer cells [169, 170]. Similarly, the proliferation, invasion, and migration of melanoma and colon cancer cell lines in vitro were increased due to adipocyte influence [171]. Adipocytes may also act on cancer cells via the transfer of vesicles that contain enzymes involved in fatty acid oxidation [172], or via lipid transfer from adipocytes to melanoma cell lines in vitro and in Zebrafish transplantation model [173]. Finally, adipocytes may act to remodel collagen and in this way enhance migration and intravasation of cells [168]. More in vivo mouse models and analyses of patient samples will be necessary to assess whether and how adipocytes directly affect intravasation.
Outlook
In recent years, significant progress has been made in the understanding of the effect that different cell types within the tumor microenvironment have on metastatic progression, including intravasation. It will be interesting to gain a high‐resolution insight into the involvement of various microenvironmental cell types in different steps of intravasation—their contribution to the initial migration or detachment of cancer cells, cancer cell interaction with the endothelial wall, and during endothelial transit. Interactions between different cell types and their collective effect on intravasation should also be considered.
Mechanical cues
Cancer is confined by the tissue in which it resides ‘with limited inlets and outlets for cells, fluids and waste’ [174]. When cancer invades the surrounding tissue, it causes a buildup of pressure. Biomechanical abnormalities in tumors consist in elevated solid stress, elevated interstitial fluid pressure, increased stiffness, altered material properties, and altered tissue microarchitecture [175]. These mechanical cues have an effect on other cancer‐associated features, for instance, influencing microenvironment components, inducing inflammation, and promoting abnormal signaling.
There is evidence that mechanical cues may affect metastasis, but how precisely, and by which mechanical, cellular and molecular mechanisms, remains to be discovered. YAP and TAZ, transcriptional coactivators that respond to various stimuli including the hippo pathway or mechanical signals, are commonly hyperactivated in human cancers. However, no activating mutations have been detected, and hippo pathway mutations are rare [176]. Therefore, it is possible that YAP/TAZ activation is induced by mechano‐stimuli within the tumor. For example, cytoskeleton arrangements could be involved in the activation of YAP/TAZ [176]. Because of their role in mechano‐transduction [176, 177], YAP‐TAZ involvement in different steps of the metastatic cascade—including intravasation—should be studied mechanistically and functionally.
Aggregation of ECM proteins may result in stiffness, which is correlated with poor patient prognosis [178]. ECM stiffening may be due to activity of cancer cells and cells of the microenvironment such as macrophages and CAFs [179]. For example, stiff ECM may induce CAF activation in a YAP/TAZ‐dependent manner, which then further contributes to ECM stiffening in a positive feedback loop [180]. Reports have shown that stiffness of matrices is linked to cancer cell invasiveness [181, 182, 183] and their metastatic potential in vivo [184], though some other studies suggest that the migration velocity of cells of higher metastatic potential is independent of matrix stiffness [185] or may rather depend on the matrix molecular composition [186]. How exactly stiffness can affect cancer cell intravasation remains to be clarified.
Interstitial fluid pressure (IFP) buildup is another occurrence in cancer. In healthy tissue, influx of plasma from the blood vasculature with nutrients and oxygen is used for metabolism of cells and is then reabsorbed by post‐capillary venules, while a fraction drains through the lymphatic system to reach the blood stream. In cancer, the amount of fluid is very high and not drained well due to various abnormalities in the vasculature, lymphatic co‐option, and increase in cell number and density [174]. IFP may have an effect on metastasis: for example, reducing interstitial fluid pressure with gelatin modified cationic lipid particles leads to decreased pulmonary metastasis in an orthotopic breast cancer model [187]. Xenografts of cervical and melanoma cell lines and patient tumors show a correlation between IFP level and metastasis [188, 189]. It would be crucial to understand how these mechanical signals translate into molecular changes within cancer and stromal cells and feed into the enhancement of the intravasation process. Contradictory with the mentioned observations, another study suggests a lack of correlation between IFP of tumor or lymph node metastasis and lungs in mouse models [190]; however, the dissemination of cells from minority high‐IFP areas, that are not evident in the average IFP measurement, could take place.
Outlook
The effect of mechano‐stimuli on cancer progression is a developing field and the link between mechanical stimuli and intravasation should be rigorously investigated, possibly using spontaneous metastasis models in vivo as well as dedicated reporter systems to measure the activity of mechano‐sensing pathways during cancer progression.
Concluding remarks
Several processes and factors have been highlighted in the context of metastatic intravasation (Fig. 3). Collectively, intravasation is mostly referred to and generally associated with the process in which cancer cells enter the bloodstream from a primary tumor site. However, it is important to highlight that intravasation could also occur from established metastatic deposits and be the key event in metastasis‐to‐metastasis dissemination of cancer. Clearly, intravasation biology could be organ‐dependent, with different mechanisms being involved at distant sites as a consequence of varying tissue properties. Generally, in recent years, various studies have suggested a role for immune cells or genetic and gene expression profiles in the intravasation process. At the same time, other areas, such as epigenetic, metabolic, and mechanical inputs, are being investigated. Focusing future research efforts on elucidating the drivers of the intravasation process, individually and in combination, in an organ‐specific fashion and using spontaneous metastasis models in vivo and patient samples will be instrumental in dissecting the dynamics of metastasis and more importantly, in identifying new treatment opportunities for patients with aggressive cancers.
Fig. 3.
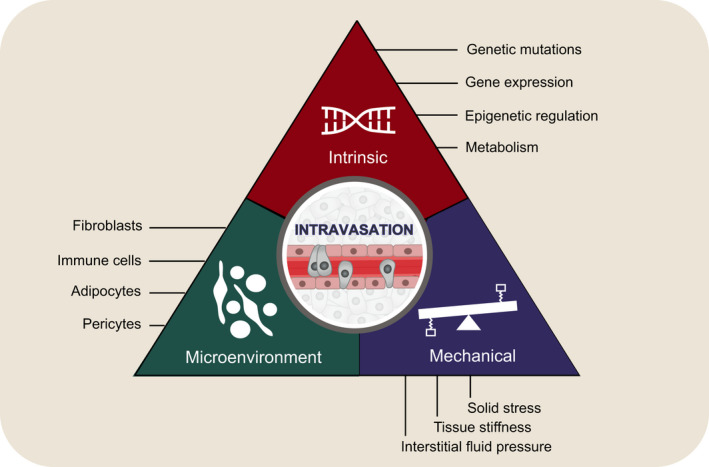
Summary of factors involved in the regulation of intravasation process: intrinsic cancer cell properties, cellular microenvironment, and mechanical properties.
Conflict of interest
N. Aceto is listed as inventor in patent applications related to CTCs and is a paid consultant for companies with an interest in liquid biopsy. M.K. Sznurkowska declares no competing financial interests.
Author contributions
M.K. Sznurkowska and N. Aceto wrote the manuscript and created the figures.
Acknowledgements
We thank all members and collaborators of the Aceto laboratory for scientific feedback and discussions. Research in the Aceto laboratory is supported by the European Research Council (grants 678834 and 840636), the Future and Emerging Technologies programme of the European Commission (grant 801159‐B2B), the Swiss National Science Foundation (grants PP0P3_163938, PP00P3_190077, and IZLIZ3_182962), the Swiss Cancer League (grants KFS‐3811‐02‐2016, KLS‐4222‐08‐2017, and KLS‐4834‐08‐2019), the Basel Cancer League (grants KLbB‐4173‐03‐2017 and KLbB‐4763‐02‐2019), the two Cantons of Basel through the ETH Zürich (grant PMB‐01‐16), and the University of Basel. M.K. Sznurkowska is a H2020 Marie Skłodowska‐Curie Actions (847012) and EMBO Long Term Fellow. Open Access Funding provided by Eidgenossische Technische Hochschule Zurich.
References
- 1. Dillekås H, Rogers MS & Straume O (2019) Are 90% of deaths from cancer caused by metastases? Cancer Med 8, 5574–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 1251‐S‐lodo‐2'‐deoxyuridine. J Natl Cancer Inst 45, 773–782. [PubMed] [Google Scholar]
- 3. Butler TP & Gullino PM (1975) Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res 35, 512–516. [PubMed] [Google Scholar]
- 4. Roh‐Johnson M, Bravo‐Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L & Condeelis J (2014) Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33, 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wyckoff JB, Jones JG, Condeelis JS & Segall JE (2000) A critical step in metastasis: In vivo analysis of intravasation at the primary tumor. Cancer Res 60, 2504–2511. [PubMed] [Google Scholar]
- 6. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H et al. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szczerba BM, Castro‐Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J et al. (2019) Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557. [DOI] [PubMed] [Google Scholar]
- 8. Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, Takahashi T, Murakami H, Nakamura Y, Tsuya A et al. (2013) Size‐based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One 8, e67466 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo X, Mitra D, Sullivan RJ, Wittner BS, Kimura AM, Pan S, Hoang MP, Brannigan BW, Lawrence DP, Flaherty KT et al. (2014) Isolation and molecular characterization of circulating melanoma cells. Cell Rep 7, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molnar B, Ladanyi A, Tanko L, Sréter L & Tulassay Z (2001) Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res 7, 4080–4085. [PubMed] [Google Scholar]
- 11. Hanahan D & Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 12. Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D et al. (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10, 8152–8162. [DOI] [PubMed] [Google Scholar]
- 13. Kim M, Gans JD, Nogueira C, Wang A, Paik JH, Feng B, Brennan C, Hahn WC, Cordon‐Cardo C, Wagner SN et al. (2006) Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell 125, 1269–1281. [DOI] [PubMed] [Google Scholar]
- 14. Massagué J & Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri D, Viale A, Olshen AB, Gerald WL & Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, Van De Vijver MJ, Gerald WL, Foekens JA et al. (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ et al. (2012) RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 487, 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu T, Xu H, Huang M, Ma W, Saxena D, Lustig RA, Alonso‐Basanta M, Zhang Z, O’Rourke DM, Zhang L et al. (2018) Circulating glioma cells exhibit stem cell‐like properties. Cancer Res 78, 6632–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao L, Wu X, Li T, Luo J & Dong D (2020) ctcRbase: the gene expression database of circulating tumor cells and microemboli. Database (Oxford) 2020, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang JE, Ring A, Porras T, Kaur P, Forte VA, Mineyev N, Tripathy D, Press MF & Campo D (2018) RNA‐Seq of circulating tumor cells in stage II–III breast cancer. Ann Surg Oncol 25, 2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franses JW, Philipp J, Missios P, Bhan I, Liu A, Yashaswini C, Tai E, Zhu H, Ligorio M, Nicholson B et al. (2020) Pancreatic circulating tumor cell profiling identifies LIN28B as a metastasis driver and drug target. Nat Commun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bill R & Christofori G (2015) The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS Lett 589, 1577–1587. [DOI] [PubMed] [Google Scholar]
- 23. Diepenbruck M & Christofori G (2016) Epithelial‐mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 43, 7–13. [DOI] [PubMed] [Google Scholar]
- 24. Tsai JH, Donaher JL, Murphy DA, Chau S & Yang J (2012) Spatiotemporal regulation of epithelial‐mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ocaña OH, Córcoles R, Fabra Á, Moreno‐Bueno G, Acloque H, Vega S, Barrallo‐Gimeno A, Cano A & Nieto MA (2012) Metastatic colonization requires the repression of the epithelial‐mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724. [DOI] [PubMed] [Google Scholar]
- 26. Tran HD, Luitel K, Kim M, Zhang K, Longmore GD & Tran DD (2014) Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res 74, 6330–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Celià‐Terrassa T, Meca‐Cortés Ó, Mateo F, De Paz AM, Rubio N, Arnal‐Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra‐Rebollo M et al. (2012) Epithelial‐mesenchymal transition can suppress major attributes of human epithelial tumor‐initiating cells. J Clin Invest 122, 1849–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt JM, Panzilius E, Bartsch HS, Irmler M, Beckers J, Kari V, Linnemann JR, Dragoi D, Hirschi B, Kloos UJ et al. (2015) Stem‐cell‐like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell Rep 10, 131–139. [DOI] [PubMed] [Google Scholar]
- 29. Saitoh M (2018) Involvement of partial EMT in cancer progression. J Biochem 164, 257–264. [DOI] [PubMed] [Google Scholar]
- 30. Saxena K, Jolly MK & Balamurugan K (2020) Hypoxia, partial EMT and collective migration: emerging culprits in metastasis. Transl Oncol 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li CI, Anderson BO, Daling JR & Moe RE (2003) Trends in Incidence rates of invasive lobular and ductal breast carcinoma. J Am Med Assoc 289, 1421–1424. [DOI] [PubMed] [Google Scholar]
- 32. Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS & Ewald AJ (2019) E‐cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beerling E, Seinstra D, de Wit E , Kester L, van der Velden D , Maynard C, Schäfer R, van Diest P , Voest E, van Oudenaarden A et al. (2016) Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis‐enhancing stem cell capacity. Cell Rep 14, 2281–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM et al. (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ting DT, Wittner BS, Ligorio M, Vincent Jordan N, Shah AM, Miyamoto DT, Aceto N, Bersani F, Brannigan BW, Xega K et al. (2014) Single‐cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep 8, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Y, Li S, Li W, Yang R, Zhang X, Ye Y, Yu J, Ye L & Tang W (2019) Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci Rep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz‐Gordillo P, Francis JM, Zhang CZ, Shalek AK, Satija R et al. (2014) Whole‐exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol 32, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Liping GUO, Feng L, Zhang W, Xiao T, Xuebing DI, Chen G & Zhang K (2018) Single nucleotide variant profiles of viable single circulating tumour cells reveal CTC behaviours in breast cancer. Oncol Rep 39, 2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faugeroux V, Lefebvre C, Pailler E, Pierron V, Marcaillou C, Tourlet S, Billiot F, Dogan S, Oulhen M, Vielh P et al. (2020) An accessible and unique insight into metastasis mutational content through whole‐exome sequencing of circulating tumor cells in metastatic prostate cancer. Eur Urol Oncol 3, 498–508. [DOI] [PubMed] [Google Scholar]
- 40. Heidary M, Auer M, Ulz P, Heitzer E, Petru E, Gasch C, Riethdorf S, Mauermann O, Lafer I, Pristauz G et al. (2014) The dynamic range of circulating tumor DNA in metastatic breast cancer. Breast Cancer Res 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ni X, Zhuo M, Su Z, Duan J, Gao Y, Wang Z, Zong C, Bai H, Chapman AR, Zhao J et al. (2013) Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci USA 110, 21083–21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl‐Geigl J, Mauermann O, Lackner C et al. (2013) Complex tumor genomes inferred from single circulating tumor cells by array‐CGH and next‐generation sequencing. Cancer Res 73, 2965–2975. [DOI] [PubMed] [Google Scholar]
- 43. Okugawa Y, Grady WM & Goel A (2015) Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 149, 1204–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Y, Sarkissyan M & Vadgama JV (2015) Epigenetics in breast and prostate cancer. Methods Mol Biol 1238, 425–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vizoso M, Ferreira HJ, Lopez‐Serra P, Carmona FJ, Martínez‐Cardús A, Girotti MR, Villanueva A, Guil S, Moutinho C, Liz J et al. (2015) Epigenetic activation of a cryptic TBC1D16 transcript enhances melanoma progression by targeting EGFR. Nat Med 21, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chatterjee A, Stockwell PA, Ahn A, Rodger EJ, Leichter AL & Eccles MR (2017) Genome‐wide methylation sequencing of paired primary and metastatic cell lines identifies common DNA methylation changes and a role for EBF3 as a candidate epigenetic driver of melanoma metastasis. Oncotarget 8, 6085–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chimonidou M, Strati A, Tzitzira A, Sotiropoulou G, Malamos N, Georgoulias V & Lianidou ES (2011) DNA methylation of tumor suppressor and metastasis suppressor genes in circulating tumor cells. Clin Chem 57, 1169–1177. [DOI] [PubMed] [Google Scholar]
- 48. Gkountela S, Castro‐Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C, Stirnimann CU et al. (2019) Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei Q, Qian Y, Yu J & Wong CC (2020) Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications. Oncogene 39, 6139–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Faubert B, Solmonson A & DeBerardinis RJ (2020) Metabolic reprogramming and cancer progression. Science 368, eaaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elia I, Schmieder R, Christen S & Fendt SM (2016) Organ‐specific cancer metabolism and its potential for therapy. Handb Exp Pharmacol 233, 321–353. [DOI] [PubMed] [Google Scholar]
- 52. Lu J (2019) The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev 38, 157–164. [DOI] [PubMed] [Google Scholar]
- 53. Botzer LE, Maman S, Sagi‐Assif O, Meshel T, Nevo I, Yron I & Witz IP (2016) Hexokinase 2 is a determinant of neuroblastoma metastasis. Br J Cancer 114, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson M, Marayati R, Moffitt R & Yeh JJ (2017) Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget 8, 56081–56094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun H, Zhu A, Zhang L, Zhang J, Zhong Z & Wang F (2015) Knockdown of PKM2 suppresses tumor growth and invasion in lung adenocarcinoma. Int J Mol Sci 16, 24574–24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu WR, Tian MX, Yang LX, Lin YL, Jin L, Bin DZ, Shen YH, Peng YF, Gao DM, Zhou J et al. (2015) PKM2 promotes metastasis by recruiting myeloid‐derived suppressor cells and indicates poor prognosis for hepatocellular carcinoma. Oncotarget 6, 846–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lu W, Cao Y, Zhang Y, Li S, Gao J, Wang XA, Mu J, Hu YP, Jiang L, Dong P et al. (2016) Up‐regulation of PKM2 promote malignancy and related to adverse prognostic risk factor in human gallbladder cancer. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu G, Yu W, Jin G, Xu D, Chen Y, Xia T, Yu A, Fang W, Zhang X, Li Z et al. (2015) PKM2 regulates neural invasion of and predicts poor prognosis for human hilar cholangiocarcinoma. Mol Cancer 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou CF, Li XB, Sun H, Zhang B, Han YS, Jiang Y, Zhuang QL, Fang J & Wu GH (2012) Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life 64, 775–782. [DOI] [PubMed] [Google Scholar]
- 60. Nokin MJ, Durieux F, Peixoto P, Chiavarina B, Peulen O, Blomme A, Turtoi A, Costanza B, Smargiasso N, Baiwir D et al. (2016) Methylglyoxal, a glycolysis side‐product, induces Hsp90 glycation and YAP‐ mediated tumor growth and metastasis. eLife 5, e19375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bonuccelli G, Tsirigos A, Whitaker‐Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez‐Outschoorn UE et al. (2010) Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 9, 3506–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Payen VL, Porporato PE, Baselet B & Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci 73, 1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Payen VL, Hsu MY, Rädecke KS, Wyart E, Vazeille T, Bouzin C, Porporato PE & Sonveaux P (2017) Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Res 77, 5591–5601. [DOI] [PubMed] [Google Scholar]
- 64. Elia I, Rossi M, Stegen S, Broekaert D, Doglioni G, van Gorsel M , Boon R, Escalona‐Noguero C, Torrekens S, Verfaillie C et al. (2019) Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu J, Locasale JW, Bielas JH, O’Sullivan J, Sheahan K, Cantley LC, Heiden MGV & Vitkup D (2013) Heterogeneity of tumor‐induced gene expression changes in the human metabolic network. Nat Biotechnol 31, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Courtney KD, Bezwada D, Mashimo T, Pichumani K, Vemireddy V, Funk AM, Wimberly J, McNeil SS, Kapur P, Lotan Y et al. (2018) Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab 28, 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fan TWM, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M & Miller DM (2009) Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope‐resolved metabolomics (SIRM). Mol Cancer 8, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hensley CT, Faubert B, Yuan Q, Lev‐Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L et al. (2016) Metabolic heterogeneity in human lung tumors. Cell 164, 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marin‐Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z et al. (2012) Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab 15, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lebleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, De Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM et al. (2014) PGC‐1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16, 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Porporato PE, Payen VL, Pérez‐Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T, Bouzin C et al. (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8, 754–766. [DOI] [PubMed] [Google Scholar]
- 72. Andrzejewski S, Klimcakova E, Johnson RM, Tabariès S, Annis MG, McGuirk S, Northey JJ, Chénard V, Sriram U, Papadopoli DJ et al. (2017) PGC‐1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metab 26, 778–787. [DOI] [PubMed] [Google Scholar]
- 73. Torrano V, Valcarcel‐Jimenez L, Cortazar AR, Liu X, Castillo‐Martin M, Fernández‐Ruiz S, Morciano G, Guiu M, Zúñiga‐García P, Graupera M et al. (2016) The metabolic co‐regulator PGC1α suppresses prostate cancer metastasis. Nat Cell Biol 18, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Luo C, Lim JH, Lee Y, Granter SR, Thomas A, Vazquez F, Widlund HR & Puigserver P (2016) A PGC1α‐mediated transcriptional axis suppresses melanoma metastasis. Nature 537, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fantin VR, St‐Pierre J & Leder P (2006) Attenuation of LDH‐A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434. [DOI] [PubMed] [Google Scholar]
- 76. Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB & Sabatini DM (2014) Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D, Ji B, Luo Y & Hu X (2014) Beyond Warburg effect ‐ dual metabolic nature of cancer cells. Sci Rep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lim JH, Luo C, Vazquez F & Puigserver P (2014) Targeting mitochondrial oxidative metabolism in melanoma causes metabolic compensation through glucose and glutamine utilization. Cancer Res 74, 3535–3545. [DOI] [PubMed] [Google Scholar]
- 79. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D & Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, Giordano S, Pani G & Galeotti T (2006) Pro‐metastatic signaling by c‐Met through RAC‐1 and reactive oxygen species (ROS). Oncogene 25, 3689–3698. [DOI] [PubMed] [Google Scholar]
- 81. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y & Hayashi J‐I (2008) ROS‐generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664. [DOI] [PubMed] [Google Scholar]
- 82. Reczek CR & Chandel NS (2017) The two faces of reactive oxygen species in cancer. Annu Rev Cancer Biol 1, 79–98. [Google Scholar]
- 83. Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA & Sethi G (2019) Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ & Morrison SJ (2015) Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li XX, Wang ZJ, Zheng Y, Guan YF, Yang PB, Chen X, Peng C, He JP, Ai YL, Wu SF et al. (2018) Nuclear receptor Nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol Cell 69, 480–492. [DOI] [PubMed] [Google Scholar]
- 86. Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B et al. (2019) Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell 178, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, Ziegler DV, Xu X, Ghosh T, Mondal T et al. (2019) BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178, 330–345. [DOI] [PubMed] [Google Scholar]
- 88. Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P & Bergo MO (2014) Cancer: Antioxidants accelerate lung cancer progression in mice. Sci Transl Med 6. [DOI] [PubMed] [Google Scholar]
- 89. Le Gal K, Ibrahim MX, Wiel C, Sayin VI, Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J et al. (2015) Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7, 1–8. [DOI] [PubMed] [Google Scholar]
- 90. Araos J, Sleeman JP & Garvalov BK (2018) The role of hypoxic signalling in metastasis: towards translating knowledge of basic biology into novel anti‐tumour strategies. Clin Exp Metastasis 35, 563–599. [DOI] [PubMed] [Google Scholar]
- 91. Rankin EB, Nam JM & Giaccia AJ (2016) Hypoxia: signaling the metastatic cascade. Trends Cancer 2, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cairns RA & Hill RP (2004) Acute hypoxia enhances spontaneous lymph node metastasis in an orthotopic murine model of human cervical carcinoma. Cancer Res 64, 2054–2061. [DOI] [PubMed] [Google Scholar]
- 93. Cairns RA, Kalliomaki T & Hill RP (2001) Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res 61, 8903–8908. [PubMed] [Google Scholar]
- 94. Chang Q, Jurisica I, Do T & Hedley DW (2011) Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res 71, 3110–3120. [DOI] [PubMed] [Google Scholar]
- 95. Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR & Dewhirst MW (1996) Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 56, 941–943. [PubMed] [Google Scholar]
- 96. Wang S, Ren T, Huang Y, Bao X, Sun K, Shen D & Guo W (2017) BMPR2 and HIF1‐α overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chinese J Cancer Res 29, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tong WW, Tong GH, Chen XX, Zheng HC & Wang YZ (2015) HIF2α is associated with poor prognosis and affects the expression levels of survivin and cyclin D1 in gastric carcinoma. Int J Oncol 46, 233–242. [DOI] [PubMed] [Google Scholar]
- 98. Chen WT, Huang CJ, Wu MT, Yang SF, Su YC & Chai CY (2005) Hypoxia‐inducible factor‐1α is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol 35, 207–213. [DOI] [PubMed] [Google Scholar]
- 99. Klaus A, Fathi O, Tatjana TW, Bruno N & Oskar K (2018) Expression of hypoxia‐associated protein HIF‐1α in follicular thyroid cancer is associated with distant metastasis. Pathol Oncol Res 24, 289–296. [DOI] [PubMed] [Google Scholar]
- 100. Zhang H, Wong CCL, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT et al. (2012) HIF‐1‐dependent expression of angiopoietin‐like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene 31, 1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101. Jin F, Brockmeier U, Otterbach F & Metzen E (2012) New insight into the SDF‐1/CXCR4 axis in a breast carcinoma model: Hypoxia‐induced endothelial SDF‐1 and tumor cell CXCR4 are required for tumor cell intravasation. Mol Cancer Res 10, 1021–1031. [DOI] [PubMed] [Google Scholar]
- 102. Deryugina EI & Kiosses WB (2017) Intratumoral cancer cell intravasation can occur independent of invasion into the adjacent stroma. Cell Rep 19, 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Donato C, Kunz L, Castro‐Giner F, Paasinen‐Sohns A, Strittmatter K, Szczerba BM, Scherrer R, Di Maggio N, Heusermann W, Biehlmaier O et al. (2020) Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hale JS, Otvos B, Sinyuk M, Alvarado AG, Hitomi M, Stoltz K, Wu Q, Flavahan W, Levison B, Johansen ML et al. (2014) Cancer stem cell‐specific scavenger receptor 36 drives glioblastoma progression. Stem Cells 32, 1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liang Y, Han H, Liu L, Duan Y, Yang X, Ma C, Zhu Y, Han J, Li X & Chen Y (2018) CD36 plays a critical role in proliferation, migration and tamoxifen‐inhibited growth of ER‐positive breast cancer cells. Oncogenesis 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CSO, Berenguer A, Prats N, Toll A, Hueto JA et al. (2017) Targeting metastasis‐initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. [DOI] [PubMed] [Google Scholar]
- 107. Nath A & Chan C (2016) Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Louie SM, Roberts LS, Mulvihill MM, Luo K & Nomura DK (2013) Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta 1831, 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Park JH, Vithayathil S, Kumar S, Sung P‐L, Dobrolecki LE, Putluri V, Bhat VB, Bhowmik SK, Gupta V, Arora K et al. (2016) Fatty acid oxidation‐driven Src links mitochondrial energy reprogramming and regulation of oncogenic properties in triple negative breast cancer. Cell Rep 14, 2154–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H & Takeyama H (2015) Cancer‐associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 7, 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, Tan P & Ishimoto T (2019) Biological heterogeneity and versatility of cancer‐associated fibroblasts in the tumor microenvironment. Oncogene 38, 4887–4901. [DOI] [PubMed] [Google Scholar]
- 112. O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB et al. (2011) VEGF‐A and Tenascin‐C produced by S100A4 + stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108, 16002–16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Özdemir BC, Pentcheva‐Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV et al. (2014) Depletion of carcinoma‐associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Duda DG, Duyverman AMMJ, Kohno M, Snuderl M, Steller EJA, Fukumura D & Jain RK (2010) Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 107, 21677–21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ortiz‐Otero N, Marshall JR, Lash B & King MR (2020) Chemotherapy‐induced release of circulating‐tumor cells into the bloodstream in collective migration units with cancer‐associated fibroblasts in metastatic cancer patients. BMC Cancer 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Richardson AM, Havel LS, Koyen AE, Konen JM, Shupe J, Wiles WG, David Martin W, Grossniklaus HE, Sica G, Gilbert‐Ross M et al. (2018) Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell‐cancer‐associated fibroblast interactions during collective invasion. Clin Cancer Res 24, 420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Labernadie A, Kato T, Brugués A, Serra‐Picamal X, Derzsi S, Arwert E, Weston A, González‐Tarragó V, Elosegui‐Artola A, Albertazzi L et al. (2017) A mechanically active heterotypic E‐cadherin/N‐cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol 19, 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hurt B, Schulick R, Edil B, El Kasmi KC & Barnett C (2017) Cancer‐promoting mechanisms of tumor‐associated neutrophils. Am J Surg 214, 938–944. [DOI] [PubMed] [Google Scholar]
- 119. Granot Z (2019) Neutrophils as a therapeutic target in cancer. Front Immunol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shaul ME & Fridlender ZG (2019) Tumour‐associated neutrophils in patients with cancer. Nat Rev Clin Oncol 16, 601–620. [DOI] [PubMed] [Google Scholar]
- 121. Wculek SK & Malanchi I (2015) Neutrophils support lung colonization of metastasis‐initiating breast cancer cells. Nature 528, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tüting T & De Visser K (2016) How neutrophils promote metastasis. Science 352, 9–11. [DOI] [PubMed] [Google Scholar]
- 123. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F et al. (2020) DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138. [DOI] [PubMed] [Google Scholar]
- 124. Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP & Deryugina EI (2011) Tumor‐recruited neutrophils and neutrophil TIMP‐free MMP‐9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179, 1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhou J, Tang Z, Gao S, Li C, Feng Y & Zhou X (2020) Tumor‐associated macrophages: recent insights and therapies. Front Oncol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG & Condeelis JS (2015) Real‐time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage‐derived VEGFA. Cancer Discov 5, 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ginter PS, Karagiannis GS, Entenberg D, Lin Y, Condeelis J, Jones JG & Oktay MH (2019) Tumor microenvironment of metastasis (TMEM) doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW & Condeelis J (2007) Direct visualization of macrophage‐assisted tumor cell intravasation in mammary tumors. Cancer Res 67, 2649–2656. [DOI] [PubMed] [Google Scholar]
- 129. Pignatelli J, Bravo‐Cordero JJ, Roh‐Johnson M, Gandhi SJ, Wang Y, Chen X, Eddy RJ, Xue A, Singer RH, Hodgson L et al. (2016) Macrophage‐dependent tumor cell transendothelial migration is mediated by Notch1/Mena INV‐initiated invadopodium formation. Sci Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Linde N, Casanova‐Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J et al. (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lin Y, Xu J & Lan H (2019) Tumor‐associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J Hematol Oncol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC, Chen YY, Liu YC, Hong TH, Yu SL, Chen JJW et al. (2015) Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. De Visser KE, Eichten A & Coussens LM (2006) Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 6, 24–37. [DOI] [PubMed] [Google Scholar]
- 134. Mantovani A, Allavena P, Sica A & Balkwill F (2008) Cancer‐related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- 135. Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM & Karin M (2011) Tumour‐infiltrating regulatory T cells stimulate mammary cancermetastasis through RANKL‐RANK signalling. Nature 470, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N & Coussens LM (2009) CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tian L, Goldstein A, Wang H, Lo HC, Kim IS, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N et al. (2017) Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Joseph R, Soundararajan R, Vasaikar S, Yang F, Allton KL, Tian L, Den Hollander P, Rodriguez‐canales J, Gelovani J, Chang JT et al. (2021) CD8 + T cells inhibit metastasis and CXCL4 regulates its function. Br J Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yan MJ & Jurasz P (2016) The role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochim Biophys Acta 1863, 392–400. [DOI] [PubMed] [Google Scholar]
- 140. Lou XL, Sun J, Gong SQ, Yu XF, Gong R & Deng H (2015) Interaction between circulating cancer cells and platelets: clinical implication. Chinese J Cancer Res 27, 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Labelle M, Begum S & Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell 20, 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Von Tell D, Armulik A & Betsholtz C (2006) Pericytes and vascular stability. Exp Cell Res 312, 623–629. [DOI] [PubMed] [Google Scholar]
- 143. Bergers G & Song S (2005) The role of pericytes in blood‐vessel formation and maintenance. Neuro Oncol 7, 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. O’Keeffe MB, Devlin AH, Burns AJ, Gardiner TA, Logan ID, Hirst DG & McKeown SR (2008) Investigation of pericytes, hypoxia, and vascularity in bladder tumors: association with clinical outcomes. Oncol Res 17, 93–101. [DOI] [PubMed] [Google Scholar]
- 145. Stefansson IM, Salvesen HB & Akslen LA (2006) Vascular proliferation is important for clinical progress of endometrial cancer. Cancer Res 66, 3303–3309. [DOI] [PubMed] [Google Scholar]
- 146. Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T & Imamura M (2005) Absence of smooth muscle actin‐positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology 69, 159–166. [DOI] [PubMed] [Google Scholar]
- 147. Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D & Augustin HG (2008) Endosialin (Tem1) is a marker of tumor‐associated myofibroblasts and tumor vessel‐associated mural cells. Am J Pathol 172, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Simonavicius N, Robertson D, Bax DA, Jones C, Huijbers IJ & Isacke CM (2008) Endosialin (CD248) is a marker of tumor‐associated pericytes in high‐grade glioma. Mod Pathol 21, 308–315. [DOI] [PubMed] [Google Scholar]
- 149. MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, Sutton‐Smith M, Dell A, Van Der Geer P et al. (2005) Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett 579, 2569–2575. [DOI] [PubMed] [Google Scholar]
- 150. Viski C, König C, Kijewska M, Mogler C, Isacke CM & Augustin HG (2016) Endosialin‐expressing pericytes promote metastatic dissemination. Cancer Res 76, 5313–5325. [DOI] [PubMed] [Google Scholar]
- 151. Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S et al. (2012) Pericyte depletion results in hypoxia‐associated epithelial‐to‐mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21, 66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Xian X, Håkansson J, Ståhlberg A, Lindblom P, Betsholtz C, Gerhardt H & Semb H (2006) Pericytes limit tumor cell metastasis. J Clin Invest 116, 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. He Q, Li X, He L, Li Y, Betsholtz C & Welsh M (2020) Pericyte dysfunction due to Shb gene deficiency increases B16F10 melanoma lung metastasis. Int J Cancer 147, 2634–2644. [DOI] [PubMed] [Google Scholar]
- 154. Costa A, Kieffer Y, Scholer‐Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C et al. (2018) Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479. [DOI] [PubMed] [Google Scholar]
- 155. Nishishita R, Morohashi S, Seino H, Wu Y, Yoshizawa T, Haga T, Saito K, Hakamada K, Fukuda S & Kijima H (2018) Expression of cancer‐associated fibroblast markers in advanced colorectal cancer. Oncol Lett 15, 6195–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP, Cox TR & Timpson P (2019) CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer 5, 724–741. [DOI] [PubMed] [Google Scholar]
- 157. Chang KK, Yoon C, Yi BC, Tap WD, Simon MC & Yoon SS (2018) Platelet‐derived growth factor receptor‐α and ‐β promote cancer stem cell phenotypes in sarcomas. Oncogenesis 7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158. Kim Y, Kim E, Wu Q, Guryanova O, Hitomi M, Lathia JD, Serwanski D, Sloan AE, Weil RJ, Lee J et al. (2012) Platelet‐derived growth factor receptors differentially inform intertumoral and intratumoral heterogeneity. Genes Dev 26, 1247–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Reynolds LE, D’Amico G, Lechertier T, Papachristodoulou A, Muñoz‐Félix JM, DeArcangelis A, Baker M, Serrels B & Hodivala‐Dilke KM (2017) Dual role of pericyte α6β1‐integrin in tumour blood vessels. J Cell Sci 130, 1583–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nanda A, Karim B, Peng Z, Liu G, Qiu W, Gan C, Vogelstein B, Croix BS, Kinzler KW & Huso DL (2006) Tumor endothelial marker 1 (Tem1) functions in the growth and progression of abdominal tumors. Proc Natl Acad Sci USA 103, 3351–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y et al. (2016) Pericyte‐fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA 113, 5618–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH & Augustin HG (2000) Heterogeneity of angiogenesis and blood vessel maturation in human tumors: Implications for antiangiogenic tumor therapies. Cancer Res 60, 1388–1393. [PubMed] [Google Scholar]
- 163. Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto H, Gu C, De Palma M, Kalluri R & LeBleu VS (2015) Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin‐2. Cell Rep 10, 1066–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ali AT, Hochfeld WE, Myburgh R & Pepper MS (2013) Adipocyte and adipogenesis. Eur J Cell Biol 92, 229–236. [DOI] [PubMed] [Google Scholar]
- 165. Avgerinos KI, Spyrou N, Mantzoros CS & Dalamaga M (2019) Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 92, 121–135. [DOI] [PubMed] [Google Scholar]
- 166. Zhao C, Wu M, Zeng N, Xiong M, Hu W, Lv W, Yi Y, Zhang Q & Wu Y (2020) Cancer‐associated adipocytes: emerging supporters in breast cancer. J Exp Clin Cancer Res 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Clement E, Lazar I, Muller C & Nieto L (2017) Obesity and melanoma: could fat be fueling malignancy? Pigment Cell Melanoma Res 30, 294–306. [DOI] [PubMed] [Google Scholar]
- 168. Wei X, Li S, He J, Du H, Liu Y, Yu W, Hu H, Han L, Wang C, Li H et al. (2019) Tumor‐secreted PAI‐1 promotes breast cancer metastasis via the induction of adipocyte‐derived collagen remodeling. Cell Commun Signal 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell‐Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS et al. (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17, 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mukherjee A, Chiang CY, Daifotis HA, Nieman KM, Fahrmann JF, Lastra RR, Romero IL, Fiehn O & Lengyel E (2020) Adipocyte‐induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res 80, 1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ko J, Bhagwat N, Black T, Yee SS, Na YJ, Fisher S, Kim J, Carpenter EL, Stanger BZ & Issadore D (2018) MiRNA profiling of magnetic nanopore‐isolated extracellular vesicles for the diagnosis of pancreatic cancer. Cancer Res 78, 3688–3697. [DOI] [PubMed] [Google Scholar]
- 172. Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux‐Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S et al. (2016) Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res 76, 4051–4057. [DOI] [PubMed] [Google Scholar]
- 173. Zhang M, Di Martino JS, Bowman RL, Campbell NR, Baksh SC, Simon‐Vermot T, Kim IS, Haldeman P, Mondal C, Yong‐Gonzales V et al. (2018) Adipocyte‐derived lipids mediate melanoma progression via FATP proteins. Cancer Discov 8, 1006–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Munson JM & Shieh AC (2014) Interstitial fluid flow in cancer: Implications for disease progression and treatment. Cancer Manag Res 6, 317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nia HT, Munn LL & Jain RK (2020) Physical traits of cancer. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Panciera T, Azzolin L, Cordenonsi M & Piccolo S (2017) Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol 18, 758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Martinez B, Yang Y, Harker DMR, Farrar C, Mukundan H, Nath P & Mascareñas D (2019) YAP/TAZ related biomechano signal transduction and cancer metastasis. Front Cell Dev Biol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wei B, Zhou X, Liang C, Zheng X, Lei P, Fang J, Han X, Wang L, Qi C & Wei H (2017) Human colorectal cancer progression correlates with LOX‐induced ECM stiffening. Int J Biol Sci 13, 1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Emon B, Bauer J, Jain Y, Jung B & Saif T (2018) Biophysics of tumor microenvironment and cancer metastasis – a mini review. Comput Struct Biotechnol J 16, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Calvo F, Ege N, Grande‐Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G et al. (2013) Mechanotransduction and YAP‐dependent matrix remodelling is required for the generation and maintenance of cancer‐associated fibroblasts. Nat Cell Biol 15, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Grasset EM, Bertero T, Bozec A, Friard J, Bourget I, Pisano S, Lecacheur M, Maiel M, Bailleux C, Emelyanov A et al. (2018) Matrix Stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res 78, 5229–5242. [DOI] [PubMed] [Google Scholar]
- 182. Berger AJ, Renner CM, Hale I, Yang X, Ponik SM, Weisman PS, Masters KS & Kreeger PK (2020) Scaffold stiffness influences breast cancer cell invasion via EGFR‐linked Mena upregulation and matrix remodeling. Matrix Biol 85–86, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Pathak A & Kumar S (2012) Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA 109, 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dong Y, Zheng Q, Wang Z, Lin X, You Y, Wu S, Wang Y, Hu C, Xie X, Chen J et al. (2019) Higher matrix stiffness as an independent initiator triggers epithelial‐mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Li Y, Fanous MJ, Kilian KA & Popescu G (2019) Quantitative phase imaging reveals matrix stiffness‐dependent growth and migration of cancer cells. Sci Rep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Anguiano M, Morales X, Castilla C, Pena AR, Ederra C, Martínez M, Ariz M, Esparza M, Amaveda H, Mora M et al. (2020) The use of mixed collagen‐Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS One 15, e0220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gao X, Zhang J, Huang Z, Zuo T, Lu Q, Wu G & Shen Q (2017) Reducing interstitial fluid pressure and inhibiting pulmonary metastasis of breast cancer by gelatin modified cationic lipid nanoparticles. ACS Appl Mater Interfaces 9, 29457–29468. [DOI] [PubMed] [Google Scholar]
- 188. Hompland T, Ellingsen C, Øvrebø KM & Rofstad EK (2012) Interstitial fluid pressure and associated lymph node metastasis revealed in tumors by dynamic contrast‐enhanced MRI. Cancer Res 72, 4899–4908. [DOI] [PubMed] [Google Scholar]
- 189. Rofstad EK, Galappathi K & Mathiesen BS (2014) Tumor interstitial fluid pressure‐a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia 16, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lunt SJ, Kalliomaki TMK, Brown A, Yang VX, Milosevic M & Hill RP (2008) Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


