Abstract
Objective
To compare different antibiotic prophylaxis administered after preterm premature rupture of membranes to determine whether any were associated with differences in obstetric and/or neonatal outcomes and/or neurodevelopmental outcomes at 2 years of corrected age.
Design
Prospective, nationwide, population‐based EPIPAGE‐2 cohort study of preterm infants.
Setting
France, 2011.
Sample
We included 492 women with a singleton pregnancy and a diagnosis of preterm premature rupture of membranes at 24–31 weeks. Exclusion criteria were contraindication to expectant management or indication for antibiotic therapy other than preterm premature rupture of membranes. Antibiotic prophylaxis was categorised as amoxicillin (n = 345), macrolide (n = 30), third‐generation cephalosporin (n = 45) or any combinations covering Streptococcus agalactiae and >90% of Escherichia coli (n = 72), initiated within 24 hours after preterm premature rupture of membranes.
Methods
Population‐averaged robust Poisson models.
Main Outcome Measures
Survival at discharge without severe neonatal morbidity, 2‐year neurodevelopment.
Results
With amoxicillin, macrolide, third‐generation cephalosporin and combinations, 78.5%, 83.9%, 93.6% and 86.0% of neonates were discharged alive without severe morbidity. The administration of third‐generation cephalosporin or any E. coli‐targeting combinations was associated with improved survival without severe morbidity (adjusted risk ratio 1.25 [95% confidence interval 1.08–1.45] and 1.10 [95 % confidence interval 1.01–1.20], respectively) compared with amoxicillin. We evidenced no increase in neonatal sepsis related to third‐generation cephalosporin‐resistant pathogen.
Conclusion
In preterm premature rupture of membranes at 24–31 weeks, antibiotic prophylaxis based on third‐generation cephalosporin may be associated with improved survival without severe neonatal morbidity when compared with amoxicillin, with no evidence of increase in neonatal sepsis related to third‐generation cephalosporin‐resistant pathogen.
Tweetable Abstract
Antibiotic prophylaxis after PPROM at 24–31 weeks: 3rd‐generation cephalosporins associated with improved neonatal outcomes.
Keywords: amoxicillin, antenatal management, cephalosporins, latency, macrolides, neurodevelopment, obstetric intervention, perinatal outcome, prematurity, prophylactic antibiotics
Tweetable Abstract
Antibiotic prophylaxis after PPROM at 24–31 weeks: 3rd‐generation cephalosporins associated with improved neonatal outcomes.
1. INTRODUCTION
Preterm premature rupture of membranes (PPROM), defined as spontaneous rupture of fetal membranes before 37 weeks’ gestation and before labour, occurs in 3% of pregnancies and is one of the leading causes of preterm birth. 1 , 2 , 3 Intrauterine infection can be both a cause and a consequence of PPROM, exposing the mother, the fetus and subsequently the neonate to increased perinatal morbidity. 1 , 4 , 5 Antenatal management of women with PPROM thus aims to reduce the adverse consequences of intrauterine infection and prematurity.
The positive impact of antibiotic prophylaxis is now well evidenced, with significant reduction of neonatal and maternal morbidity (including neonatal infection, use of surfactant, oxygen therapy, abnormal cerebral ultrasound scan and clinically defined intrauterine infection) compared with placebo in large randomised controlled trials and meta‐analyses, 4 , 6 leading to strong recommendations and wide use in clinical practice. 3 , 7 , 8 , 9 , 10 , 11
However, treatment modalities (agent, route of administration and duration) are still being debated. As stated by Mercer et al., 12 a number of antibiotics and antimicrobial regimens have been studied to cover ‘the broad spectrum of aerobic and anaerobic bacteria and mycoplasmas that have been implicated as causative agents for intrauterine infection at the time of preterm delivery and PPROM’”. Indeed, a large meta‐analysis that compiled 22 randomised trials evaluating the benefits of antibiotics in PPROM (n = 6872), recorded 11 different antibiotic agents (mostly ampicillin/amoxicillin, broad spectrum penicillins, amoxicillin/clavulanate and macrolides), used as a single agent or in combination, intravenously, orally or both, for a duration ranging from 1 to 10 days, or until delivery. 4 Of them, amoxicillin/clavulanate has now been discarded as it was shown to be associated with an increased risk of necrotising enterocolitis (NEC) in preterm neonates. 13
The controversial role of mycoplasmas as ‘innocent bystanders’ or pathogens in intrauterine infections, 14 , 15 the evolution of bacterial ecology since the 1990s, the current questioning regarding the impact of antibiotic prescriptions on microbiota 16 and the scarce evaluation of antibiotics such as third‐generation cephalosporins (3GC) point out the urgent need for a re‐appreciation of available antibiotics. We aimed to compare different antibiotic prophylaxis administered after PPROM to determine whether any were associated with differences in obstetric and/or neonatal outcomes and/or neurodevelopmental outcomes at 2 years of age.
2. MATERIALS AND METHODS
2.1. Study design and participants
This is a secondary analysis of the EPIPAGE‐2 cohort, a prospective national population‐based cohort of preterm infants that included all live born or stillborn infants and all terminations of pregnancy at 22–34 completed weeks’ gestation in all maternity units from 25 regions in France in 2011. 17 Recruitment took place at birth, after families had received information and agreed to participate. Infants were included at three different periods by gestational age at birth: 8‐month recruitment for births at 22–26 weeks, 6‐month recruitment for 27–31 weeks, and 5‐week recruitment for 32–34 weeks. This recruitment strategy aimed to over‐represent extremely preterm births (22–26 weeks) because of their low incidence and to include only a sample of moderate preterm births (32–34 weeks). Maternal, obstetric and neonatal data were collected from medical records following a standardised protocol as previously reported. 17 At 2 years of corrected age, children participating in the follow‐up were assessed with a detailed neurological and sensory examination performed by their referring physician, and a standardised questionnaire about development was completed by the parents. 18 The cohort relies on several sources of funding through grants awarded after external peer review for scientific quality. Recruitment and follow‐ups were approved by the appropriate ethics committees, i.e. the advisory committee on the treatment of personal health data for research purposes (references 10‐626, 12‐109 and 16‐263) and the committee for the protection of people participating in biomedical research (reference n° 2011‐A00159‐32 and 2016‐A0033‐48). All the procedures were in accordance with the Declaration of Helsinki of the World Medical Association.
Patients were not involved in setting the research question or the outcome measures, nor were they involved in developing plans for design of the study. Parents showed overwhelming support for the study through high follow‐up rates. EPIPAGE‐2 maintains contact with parents in the cohort through letters, newsletters and its website. National parents’ associations assisted with results dissemination.
Our study population included all women with a singleton pregnancy, PPROM at 24–31 weeks and a non‐malformed fetus who was alive at PPROM diagnosis and born at 24–34 weeks (Figure 1). As recommended, the diagnosis of PPROM was based on maternal history and sterile speculum examination with a diagnostic test if necessary. We excluded women with contraindication to expectant management and/or indication for antibiotic therapy (such as overt intrauterine infection or fever) at diagnosis.
FIGURE 1.
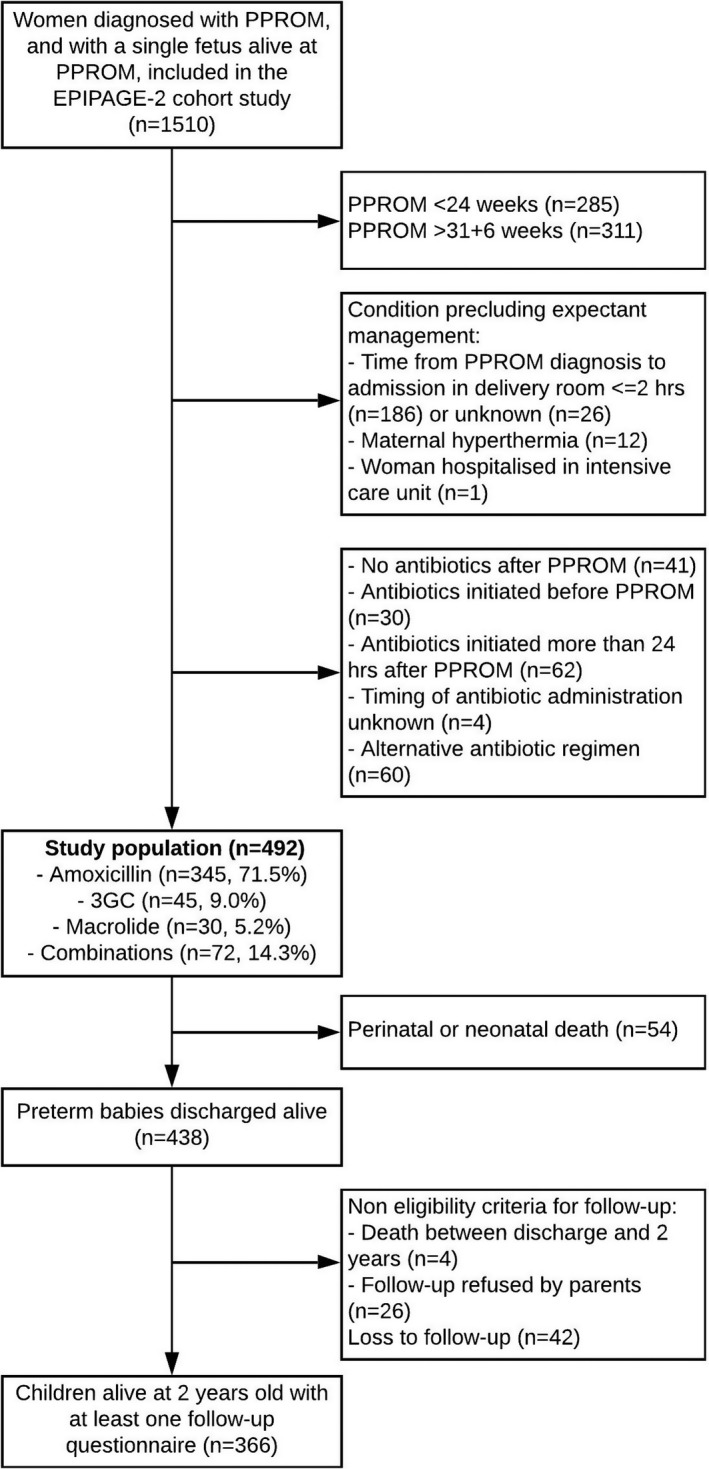
Flow chart. Summarises how sample size of analysis was obtained. PPROM, preterm premature rupture of membranes
2.2. Antibiotic prophylaxis
We excluded women who initiated antibiotics either before PPROM or >24 hours after PPROM and those who received amoxicillin/clavulanate because of the increased risk of NEC (Figure 1). Women who did not receive antibiotics at all received tocolysis and antenatal steroids less often and delivered more quickly, whereas women with an alternative antibiotic regimen overall displayed similar characteristics and outcomes to those analysed (Table S1). For analysis purposes, among the 30 different antibiotic prophylaxis regimens identified, we considered the main families of antibiotic prescribed with sufficient sample size, namely, amoxicillin (n = 345), macrolides/clindamycin (n = 30: erythromycin n = 16, spiramycin n = 1, and clindamycin n = 13), parenteral 3GC (n = 45: ceftriaxone, n = 32, cefotaxime, n = 13) and any combinations of antibiotics (n = 72) covering >90% of Escherichia coli (i.e. 3GC and/or an aminoglycoside) in addition to Streptococcus agalactiae (i.e. clindamycin, amoxicillin, 3GC or any other β‐lactam) (Table S2). The other antibiotic combinations (n = 27) were not investigated.
2.3. Outcomes
Perinatal outcomes included survival, i.e. the number of babies discharged alive relative to the number of fetuses alive at PPROM diagnosis, and survival without severe neonatal morbidity. 19 Severe neonatal morbidity was defined as any of the following: grades III–IV intraventricular haemorrhage, cystic periventricular leucomalacia, stages II–III NEC according to Bell’s staging, stage 3 or greater retinopathy of prematurity according to international classification and/or laser treatment, and severe bronchopulmonary dysplasia at 36 weeks of gestational age.
We further investigated prolongation of gestation and infectious morbidity for both the mother and the neonate. Prolongation of gestation was defined as latency duration (i.e. time from rupture of membranes to delivery) ≥48 hours and ≥7 days. Intrauterine infection was defined as a maternal temperature >37.8°C (100°F) with any two of the following criteria: uterine tenderness, purulent or foul‐smelling amniotic fluid, maternal tachycardia, fetal tachycardia, maternal leucocytosis >15 000 cells/mm3. 20 , 21 Among newborns, early‐onset sepsis (EOS) was diagnosed by positive bacteriology findings in blood or cerebrospinal fluid beginning in the first 72 hours of life. Late‐onset sepsis (LOS) was defined as a positive blood culture, occurring after 72 hours of life, associated with antibiotic administration for 5 days or more, or death within 5 days following positive blood culture. We were able to report bacterial documentation and resistances in EOS and LOS.
Finally, we studied survival at 2 years’ corrected age (CA) without severe or moderate neuromotor or sensory disabilities (i.e. without gross motor function classification system [GMFCS] level 2–5 cerebral palsy, 22 blindness and/or deafness), and parental‐reported neurodevelopment at 2 years’ corrected age (assessed with the second version of the 24 months’ Ages and Stages Questionnaire [ASQ]). 18 We presented both the overall total ASQ score (maximum = 300) and the ASQ score below threshold defined as a score <2 SD from the mean on any of the five domains, for children without cerebral palsy, blindness or deafness, whose parents completed the questionnaire between 22 and 26 months’ corrected age.
In the present study, we report 11 of 12 outcomes included in the core outcome set for neonatal research. 23 Quality of life was not collected as part of the 2‐year follow‐up evaluation.
2.4. Definition of other variables
Socio‐economic position was defined as the highest occupational status of the mother and father, or mother only if a single parent. Gestational age was determined as the best obstetrical estimate combining the last menstrual period and the first trimester ultrasonography assessment. Babies were considered small for gestational age if their birthweight was ≤10th percentile of the normalised Z‐score, calculated from French EPOPé intrauterine growth curves adjusted for fetal sex and gestational age. 24 Maternity units were considered as type 3 when associated with a neonatal intensive care unit (NICU).
2.5. Statistical analyses
Maternal and neonatal characteristics and outcomes were described as frequencies and percentages. Percentages were weighted according to the duration of the recruitment periods (in weeks) by gestational age: weights were 1.0 (35/35) for births at 24–26 weeks, 1.34 (35/26) at 27–31 weeks, and 7.0 (35/5) at 32–34 weeks. Weighting allowed us to account for the sampling scheme of the cohort and to ensure representativeness. We then compared characteristics and outcomes by antibiotic prophylaxis regimen using chi‐square or Fisher’s exact tests as appropriate for categorical variables, based on the weighted percentages, and nonparametric equality of medians test for quantitative variables. Survival curves of latency duration (considered as a continuous variable) by antibiotic prophylaxis regimen were plotted using the Kaplan–Meier method and compared with a log rank test.
The antibiotic prophylaxis regimen after PPROM is usually chosen at the unit‐level using a local guideline. Thus, the association between antibiotic prophylaxis regimens and outcomes was evaluated using population‐averaged Poisson regression models, a generalised linear model with a log link and a Poisson distribution, with robust variance estimation, which accounts for the clustering of women within maternity units. 25 , 26 Under the population‐averaged model, we obtained the risk of an average woman exposed to an antibiotic prophylaxis regimen presenting the outcome compared with the risk of an average woman exposed to another antibiotic prophylaxis regimen presenting the outcome. Multivariate models were minimally adjusted for gestational age at PPROM and the type of maternity unit, as these two variables were considered confounders. We assumed that other individual characteristics would not be taken into account in choosing a specific antibiotic, except antibiotic allergy but this information was not available. Results are reported as risk ratios (RRs) with 95% confidence intervals (95% CI).
Active antenatal management may differ by gestational age at PPROM, especially with extremely preterm rupture of membranes, and may result in poorer neonatal outcomes. We therefore performed a sensitivity analysis by stratifying on gestational age at PPROM (24–26 vs. 27–31 weeks), except for infectious morbidities, as there were too few of these to adequately run the models.
The proportion of missing data per covariate ranged from 0% to 7% for perinatal data, and reached 43% for the ASQ score because of attrition and further exclusion of ASQ results when parental questionnaires had been filled before 22 months or after 26 months of corrected age. Children who did not participate in the follow‐up at 2 years’ corrected age were born to younger mothers, more often after a complete course of antenatal steroids and in type 3 maternity units (Table S3). We performed multiple imputations with chained equations using baseline and outcomes variables and those potentially predicting nonresponse and/or outcomes (namely, type of maternity unit, maternal age, country of birth, socio‐economic position, parity, gestational age at PPROM, PPROM occurring during hospitalisation for another reason, antibiotic prophylaxis, tocolysis, antenatal steroids, in utero transfer, fetal presentation, fetal sex, small for gestational age, latency duration, mode of delivery, intrauterine infection, vital status at birth, survival at discharge, early‐onset sepsis, late‐onset sepsis, retinopathy of prematurity, severe neonatal morbidity, death between discharge and follow‐up at 2 years of corrected age, survival at 2 years without neurosensory impairment, ASQ score below threshold). Outcomes were estimated within each of the 50 imputed datasets generated with 20 iterations, and results were pooled according to Rubin rules. Statistical significance was set at a two‐tailed value of P < 0.05. No correction for multiple comparisons was made, as we aimed to generate hypotheses. Data were analysed using STATA/SE 13.0 (StataCorp LP).
3. RESULTS
A total of 492 women with PPROM from 85 maternity units met the inclusion criteria. Of them, 345, 30, 45 and 72 (weighted percentages, 71.5%, 5.2%, 9.0% and 14.3%) received amoxicillin, a macrolide, a 3GC or a combination targeting E. coli and S. agalactiae, respectively (Figure 1).
Overall, maternal, obstetric and neonatal characteristics were similar across the four groups (Table 1). Outcomes are described in Table 2 and their association with antibiotic prophylaxis is presented in Table 3. Latency duration after PPROM was reduced in the combinations group compared with the 3GC group (Tables 2 and 3, Figure S1). Altogether, 19 perinatal deaths were associated with an infection (n = 14/345, n = 1/30, n = 0/45 and n = 4/72 in the amoxicillin, macrolide, 3GC and combinations groups, respectively). Overall, there was no difference in survival and severe neonatal morbidities (Tables 2 and 3). However, differences in survival without severe morbidity were observed (78.5% with amoxicillin, 83.9% with macrolides, 93.6% with 3GC and 86.0% with combinations). After adjusting for gestational age at PPROM and type of unit, the use of a 3GC or a combination was associated with improved survival without severe neonatal morbidity (aRR 1.25, 95% CI 1.08–1.45 and aRR 1.10, 95% CI 1.01–1.20, respectively) when compared with amoxicillin, but not when compared with macrolides (Table 3).
TABLE 1.
Maternal, obstetric, neonatal and unit characteristics by antibiotic prophylaxis after PPROM
| Characteristics | Amoxicillin (n = 345) | Macrolide a (n = 30) | 3GC (n = 45) | Combinations b (n = 72) | Global P‐value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Maternal characteristics | |||||
| Age (years) (n = 492) | |||||
| <20 | 15 (3.4) | 3 (10.3) | 5 (9.4) | 2 (2.5) | 0.09 |
| 20–35 | 260 (77.0) | 22 (73.5) | 31 (74.2) | 61 (87.5) | |
| >35 | 70 (19.6) | 5 (16.2) | 9 (16.4) | 9 (10.0) | |
| Born in France or Europe (n = 478) | 258 (77.6) | 27 (92.9) | 36 (74.8) | 51 (70.3) | 0.31 |
| Married or living with a partner (n = 480) | 291 (89.1) | 25 (89.0) | 39 (88.6) | 67 (95.0) | 0.43 |
| Parents’ socio‐economic position (n = 458) | |||||
| Manager | 71 (20.3) | 5 (17.7) | 5 (10.9) | 10 (12.1) | 0.32 |
| Professional | 37 (12.9) | 4 (13.3) | 9 (19.6) | 18 (27.1) | |
| Intermediate c | 97 (30.2) | 11 (37.1) | 14 (39.0) | 20 (31.1) | |
| Sales and services worker | 52 (17.7) | 5 (17.7) | 6 (13.2) | 9 (16.3) | |
| Manual worker | 45 (14.6) | 4 (14.2) | 3 (6.9) | 8 (9.8) | |
| Unknown occupation | 17 (4.3) | 0 (0.0) | 5 (10.4) | 3 (3.6) | |
| Primiparity (n = 492) | 132 (40.4) | 16 (53.0) | 24 (53.0) | 38 (54.3) | 0.16 |
| Obstetric characteristics | |||||
| PPROM occurring during hospitalisation for another reason (n = 492) | 51 (12.3) | 1 (3.4) | 8 (14.4) | 5 (6.0) | 0.19 |
| Gestational age at PPROM (w) (n = 492), median (IQR) | 29.0 (26.4–30.9) | 29.1 (27.0–30.3) | 29.7 (26.3–30.4) | 29.3 (26.7–30.7) | 0.97 |
| Gestational age at PPROM (w) (n = 492) | |||||
| 24–26 | 128 (29.0) | 7 (21.3) | 19 (31.6) | 27 (27.7) | 0.58 |
| 27–29 | 122 (32.6) | 13 (44.5) | 10 (19.9) | 19 (29.1) | |
| 30–31 | 95 (38.4) | 10 (34.2) | 16 (48.5) | 26 (43.2) | |
| Positive vaginal swab at admission (n = 375) | 111 (40.9) | 9 (42.2) | 16 (35.4) | 22 (43.2) | 0.92 |
| Oligohydramnios (n = 441) | 147 (43.3) | 13 (44.9) | 21 (40.0) | 34 (56.0) | 0.38 |
| Gestational age at birth (w) (n = 492), median (IQR) | 30.6 (28.4–31.9) | 29.9 (28.3–31.0) | 30.6 (27.1–31.9) | 30.6 (28.3–31.9) | 0.82 |
| Gestational age at birth (w) (n = 492) | |||||
| 24–26 | 79 (14.7) | 3 (7.6) | 12 (17.7) | 19 (17.7) | 0.49 |
| 27–29 | 101 (25.2) | 13 (44.5) | 12 (23.8) | 21 (26.3) | |
| 30–31 | 147 (36.7) | 14 (47.9) | 19 (37.8) | 29 (36.4) | |
| 32–34 | 18 (23.4) | 0 (0.0) | 2 (20.7) | 3 (19.6) | |
| Obstetric management | |||||
| Type 3 maternity unit (with neonatal intensive care unit) (n = 492) | 321 (87.9) | 28 (93.2) | 44 (98.0) | 68 (89.7) | 0.40 |
| In utero transfer (n = 492) | 219 (61.0) | 16 (52.1) | 31 (56.5) | 59 (79.4) | 0.08 |
| Tocolysis (n = 492) | 271 (79.7) | 23 (76.9) | 34 (71.8) | 60 (85.9) | 0.43 |
| Tocolysis duration (n = 482) | |||||
| No tocolysis | 74 (22.0) | 7 (23.3) | 11 (25.0) | 12 (16.7) | 0.59 |
| <24 h | 56 (16.7) | 4 (13.3) | 6 (13.6) | 18 (25.0) | |
| 24–48 h | 126 (37.5) | 11 (36.7) | 21 (47.8) | 28 (38.9) | |
| >48 h | 80 (23.8) | 8 (26.7) | 6 (13.6) | 14 (19.4) | |
| Antenatal steroids (n = 486) | |||||
| None | 21 (9.0) | 3 (10.9) | 1 (1.5) | 4 (4.4) | 0.24 |
| Incomplete course | 38 (8.9) | 2 (7.3) | 4 (6.4) | 13 (14.2) | |
| Complete course | 283 (82.1) | 23 (81.8) | 40 (92.1) | 54 (81.3) | |
| Magnesium sulphate (n = 484) | 23 (5.6) | 2 (7.3) | 3 (5.0) | 1 (1.3) | 0.39 |
| Mode of delivery (n = 490) | |||||
| Vaginal delivery | 152 (43.7) | 13 (42.7) | 22 (57.4) | 34 (44.0) | 0.79 |
| Caesarean before labor | 135 (40.5) | 11 (37.6) | 16 (29.2) | 26 (37.1) | |
| Caesarean during labour | 57 (15.8) | 4 (19.7) | 7 (13.4) | 11 (19.0) | |
| Cephalic presentation (n = 483) | 220 (68.0) | 20 (68.1) | 37 (85.6) | 46 (58.8) | 0.06 |
| Neonatal characteristics | |||||
| Male fetus (n = 492) | 187 (51.2) | 21 (70.1) | 29 (70.2) | 37 (53.4) | 0.08 |
| Birthweight (g), median (IQR) (n = 491) | 1450 (1130–1785) | 1460 (1180–1580) | 1330 (1030–1710) | 1420 (1090–1850) | 0.45 |
| Small for gestational age (n = 491) | 66 (20.8) | 3 (10.3) | 11 (20.8) | 10 (11.9) | 0.24 |
All percentages were weighted to account for the sampling design of the EPIPAGE‐2 cohort.
Abbreviations: IQR, interquartile range; w, weeks’ gestation.
The macrolides group included 17 patients receiving macrolides and 13 patients receiving clindamycin.
Any combinations of antibiotics covering >90% of E. coli in addition to S. agalactiae.
Intermediate socio‐economic position included employees from administration and public services, self‐employed and students.
TABLE 2.
Outcomes by antibiotic prophylaxis after PPROM
| Outcome | Amoxicillin (n = 345) | Macrolide (n = 30) a | 3GC (n = 45) | Combinations b (n = 72) | Global P‐value |
|---|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | n/N (%) | ||
| Latency duration ≥48 h (n = 492) | 278/345 (83.2) | 24/30 (80.4) | 39/45 (89.1) | 49/72 (74.4) | 0.18 |
| Latency duration ≥7 days (n = 492) | 128/345 (42.5) | 10/30 (33.3) | 15/45 (44.0) | 19/72 (34.1) | 0.64 |
| Intrauterine infection (n = 487) | 28/340 (6.6) | 3/30 (10.3) | 2/45 (4.0) | 3/72 (3.4) | 0.49 |
| Vital status (n = 492) | |||||
| Termination of pregnancy | 1/345 (0.2) | 0/30 (0.0) | 0/45 (0.0) | 0/72 (0.0) | 0.95 |
| Antepartum stillbirth | 5/345 (0.9) | 0/30 (0.0) | 0/45 (0.0) | 0/72 (0.0) | |
| Per partum stillbirth | 4/345 (0.7) | 0/30 (0.0) | 0/45 (0.0) | 1/72 (0.9) | |
| Death in delivery room | 8/345 (1.6) | 1/30 (2.5) | 0/45 (0.0) | 3/72 (2.8) | |
| Death in NICU | 21/345 (4.3) | 2/30 (5.1) | 4/45 (5.9) | 4/72 (3.7) | |
| Discharged alive | 306/345 (92.3) | 27/30 (92.4) | 41/45 (94.1) | 64/72 (92.5) | |
| Severe neonatal morbidity | |||||
| Early‐onset sepsis (n = 453) c | 15/319 (3.7) | 1/28 (3.6) | 1/43 (2.1) | 0/63 (0.0) | 0.43 |
| Late‐onset sepsis (n = 453) c | 51/313 (11.8) | 4/29 (14.0) | 7/45 (11.9) | 9/66 (10.3) | 0.96 |
| Any sepsis (n = 442) c | 63/308 (15.2) | 5/28 (18.2) | 8/44 (14.1) | 9/62 (11.6) | 0.83 |
| Necrotising enterocolitis (n = 465) c | 12/324 (4.0) | 0/29 (0.0) | 0/44 (0.0) | 2/68 (2.3) | 0.47 |
| Severe cerebral lesion (n = 458) c | 24/316 (7.0) | 2/29 (6.1) | 2/45 (3.0) | 3/68 (3.2) | 0.43 |
| Severe bronchopulmonary dysplasia (n = 414) d | 19/287 (4.7) | 0/25 (0.0) | 0/39 (0.0) | 2/63 (2.4) | 0.30 |
| Retinopathy of prematurity (n = 469) c | 3/327 (0.6) | 1/29 (3.5) | 0/45 (0.0) | 0/72 (0.0) | 0.30 |
| Survival at discharge (n = 492) | 306/345 (92.3) | 27/30 (92.4) | 41/45 (94.1) | 64/72 (92.5) | 0.97 |
| Survival at discharge without severe neonatal morbidity e (n = 464) | 242/325 (78.5) | 22/27 (83.9) | 37/41 (93.6) | 58/71 (86.0) | 0.04 |
| Survival at 2 yo without neurosensory impairment f (among all fetuses, n = 383) | 213/266 (83.9) | 18/22 (84.8) | 31/37 (89.5) | 49/58 (88.8) | 0.61 |
| Total ASQ score med (IQR) (n = 242) | 236 (205–265) | 215 (200–250) | 216 (175–240) | 245 (225–280) | 0.42 |
| ASQ below threshold (n = 242) | 69/166 (43.5) | 11/15 (73.3) | 9/24 (46.7) | 14/37 (30.5) | 0.13 |
All percentages were weighted to account for the sampling design of the EPIPAGE‐2 cohort. Indications for termination of pregnancy: PPROM and intrauterine infection at 24 weeks. Causes of stillbirths: PPROM and intrauterine infection (n = 4), PPROM and placental abruption (n = 1), PPROM and cord prolapse (n = 1), unknown (n = 4). Causes of death in delivery room: infection and extremely preterm birth (n = 8), head entrapment and extremely preterm birth (n = 1), extremely preterm birth (n = 3). Causes of death in NICU: respiratory distress syndrome (n = 10), NEC (n = 1, in the Amoxicillin group), sepsis (n = 7), central nervous system injury (n = 6), other (n = 4), unknown (n = 3). P‐values in bold are statistically significant.
Abbreviations: ASQ, Ages and Stages Questionnaire; IQR, interquartile range; NICU, neonatal intensive care unit.
The macrolide group included 17 patients receiving macrolides and 13 patients receiving clindamycin.
Any combinations of antibiotics covering >90% of E. coli in addition to S. agalactiae.
Among 469 infants admitted to NICU.
Among 439 infants alive at 36 weeks.
Survival at discharge without any of the following: grades III–IV intraventricular haemorrhage, cystic periventricular leucomalacia, stages II–III NEC according to Bell’s staging, stage 3 or greater retinopathy of prematurity or severe bronchopulmonary dysplasia.
Survival at 2 years of corrected age without cerebral palsy GMFCS levels 2–5 or deafness or blindness.
TABLE 3.
Association between antibiotic prophylaxis after PPROM and outcomes
| Outcome | Cephalosporin vs. Amoxicillin (ref) | Amoxicillin vs. Macrolide (ref) | Cephalosporin vs. Macrolide (ref) |
|---|---|---|---|
| aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Latency prolonged by ≥48 h (n = 492) a | 1.07 (0.97–1.19) | 1.01 (0.86–1.19) | 1.09 (0.92–1.29) |
| Latency prolonged by ≥7 days (n = 492) a | 0.93 (0.64–1.36) | 0.99 (0.62–1.57) | 0.92 (0.51–1.65) |
| Intra‐uterine infection | |||
| CC (n = 487) | 0.54 (0.15–1.95) | 0.99 (0.34–2.86) | 0.53 (0.11–2.54) |
| MI (n = 492) | 0.53 (0.15–1.93) | 1.00 (0.35–2.90) | 0.53 (0.11–2.54) |
| Early‐onset sepsis (among infants admitted to NICU) | |||
| CC (n = 390) | 0.54 (0.07–4.17) | 1.16 (0.16–8.62) | 0.62 (0.04–9.42) |
| MI (n = 401) | 0.56 (0.07–4.18) | 0.98 (0.14–6.82) | 0.54 (0.04–7.85) |
| Late‐onset sepsis (among infants admitted to NICU) | |||
| CC (n = 453) | 0.76 (0.42–1.37) | 1.26 (0.66–2.39) | 0.96 (0.46–2.01) |
| MI (n = 469) | 0.81 (0.44–1.49) | 1.16 (0.61–2.22) | 0.94 (0.44–2.02) |
| Survival at discharge (n = 492) a | 1.04 (0.93–1.16) | 1.00 (0.93–1.07) | 1.04 (0.92–1.17) |
| Survival without severe morbidity b | |||
| CC (n = 464) | 1.26 (1.09–1.45) | 0.90 (0.75–1.08) | 1.13 (0.93–1.37) |
| MI (n = 492) | 1.25 (1.08–1.45) | 0.91 (0.76–1.09) | 1.14 (0.94–1.39) |
| Survival at 2 years without neurosensory impairment among all fetuses c | |||
| CC (n = 383) | 1.13 (0.97–1.31) | 0.98 (0.86–1.12) | 1.10 (0.93–1.31) |
| MI (n = 492) | 1.08 (0.94–1.23) | 1.00 (0.87–1.14) | 1.07 (0.91–1.27) |
| ASQ below threshold among infants alive at 2 years without neurosensory impairment | |||
| CC (n = 242) | 0.88 (0.54–1.42) | 0.61 (0.42–0.90) | 0.54 (0.30–0.96) |
| MI (n = 420) | 0.84 (0.51–1.37) | 0.77 (0.51–1.14) | 0.64 (0.35–1.18) |
| Combinations d vs. Amoxicillin (ref) | Combinations d vs. Macrolide (ref) | Combinations d vs. Cephalosporin (ref) | |
|---|---|---|---|
| aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Latency prolonged by ≥48 h (n = 492) a | 0.87 (0.72–1.05) | 0.88 (0.69–1.11) | 0.81 (0.66–0.99) |
| Latency prolonged by ≥7 days (n = 492) a | 0.78 (0.49–1.22) | 0.77 (0.40–1.45) | 0.83 (0.48–1.44) |
| Intrauterine infection | |||
| CC (n = 487) | 0.51 (0.18–1.41) | 0.50 (0.12–2.03) | 0.94 (0.17–5.10) |
| MI (n = 492) | 0.50 (0.18–1.39) | 0.50 (0.12–2.03) | 0.94 (0.17–5.11) |
| Late‐onset sepsis (among infants admitted to NICU) | |||
| CC (n = 453) | 0.88 (0.55–1.39) | 1.10 (0.53–2.27) | 1.15 (0.59–2.25) |
| MI (n = 469) | 0.85 (0.52–1.41) | 0.99 (0.48–2.07) | 1.06 (0.53–2.12) |
| Survival at discharge (n = 492) a | 1.00 (0.94–1.07) | 1.00 (0.91–1.10) | 0.96 (0.87–1.08) |
| Survival without severe morbidity b | |||
| CC (n = 464) | 1.11 (1.01–1.21) | 1.00 (0.84–1.18) | 0.88 (0.77–1.01) |
| MI (n = 492) | 1.10 (1.01–1.20) | 1.00 (0.85–1.18) | 0.88 (0.76–1.01) |
| Survival at 2 years without neurosensory impairment among all fetuses c | |||
| CC (n = 383) | 1.07 (0.97–1.17) | 1.04 (0.89–1.21) | 0.94 (0.81–1.11) |
| MI (n = 492) | 1.04 (0.95–1.14) | 1.04 (0.89–1.22) | 0.97 (0.84–1.12) |
| ASQ below threshold among infants alive at 2 years without neurosensory impairment | |||
| CC (n = 242) | 0.94 (0.57–1.55) | 0.58 (0.34–0.98) | 1.07 (0.58–1.97) |
| MI (n = 420) | 0.97 (0.63–1.48) | 0.74 (0.44–1.24) | 1.15 (0.65–2.04) |
Adjusted risk ratios (aRR) obtained from population‐averaged Poisson regression models with robust variance estimation, adjusted for gestational age at PPROM and the type of maternity unit (except for intrauterine infection adjusted only for gestational age at PPROM, as there were no intrauterine infections diagnosed with delivery in type 1 maternity units). There was no case of early‐onset sepsis in the combination group, hence this outcome is not reported in the second part of the table. P‐values in bold are statistically significant.
Abbreviations: aRR, adjusted risk ratios; ASQ, Ages and Stages Questionnaire; CC, complete cases; MI, multiple imputation; ref, reference.
No missing data.
Survival at discharge without any of the following: grades III–IV intraventricular haemorrhage, cystic periventricular leucomalacia, stages II–III NEC according to Bell’s staging, stage 3 or greater retinopathy of prematurity or severe bronchopulmonary dysplasia.
Survival at 2 years of corrected age without cerebral palsy GMFCS levels 2–5 or deafness or blindness.
Any combinations of antibiotics covering >90% of E. coli in addition to S. agalactiae.
With antibiotic prophylaxis using amoxicillin, a macrolide, a 3GC or an E. coli‐targeting combination, 83.9%, 84.8%, 89.5% and 88.8% of exposed fetuses were alive at 2 years’ corrected age without neurosensory impairment, respectively. Among the infants with a neurodevelopmental screening questionnaire between 22 and 26 months, 43.5%, 73.3%, 46.7% and 30.5% had an ASQ score below threshold (Table 2). Multivariate analyses did not reveal any association between antibiotic prophylaxis and neurosensory impairment. However, macrolides were associated with poorer neurodevelopmental outcome compared with the other groups in the complete cases analysis, but this association disappeared after multiple imputations (Table 3). A sensitivity analysis after stratification on gestational age at PPROM showed consistent results (Table S4).
We further investigated the episodes of neonatal sepsis. There were 17 cases of EOS, 15 in the amoxicillin group (10/15 caused by E. coli, of which six were ampicillin‐resistant and one was resistant to both ampicillin and 3GC, and 1/15 caused by ampicillin‐resistant S. agalactiae), one in the macrolide group (caused by Streptococcus agalactiae), one in the 3GC group (caused by Staphylococcus epidermidis) and none in the combinations group. Seventy‐one infants had a total of 98 episodes of LOS, 51 infants in the amoxicillin group (69 LOS), four in the macrolide group (four LOS), seven in the 3GC group (10 LOS) and nine in the combinations group (15 LOS). The most common pathogens in blood cultures collected during LOS were Gram‐positive organisms (coagulase negative staphylococci [53/98], Staphylococcus aureus [11/98], Enterococcus sp. [2/98] or another non‐specified pathogen [8/98]), followed by Gram‐negative organisms (E. coli [8/98], Acinetobacter baumanii [2/98], Enterobacter cloacae [3/98] or another pathogen [9/98]) and Candida sp. (2/98). LOS involved a pathogen resistant to the initial antibiotic prophylaxis regimen in 24 neonates: 21 from the amoxicillin group, one from the 3GC group and two from the E. coli‐ targeting combinations group.
4. DISCUSSION
4.1. Main findings
After PPROM at 24–31 weeks, antibiotic prophylaxis based on 3GC was associated with higher survival without severe neonatal morbidity than amoxicillin. To a weaker extent, the same pattern was found when comparing antibiotic combinations covering S. agalactiae and >90% of E. coli with amoxicillin. We evidenced no increase of EOS/LOS related to 3GC‐resistant pathogen among infants whose mothers received antenatal 3GC prophylaxis at 24–31 weeks.
4.2. Strengths and limitations
Strengths of our study include a population‐based sample of women with PPROM at a national level, with obstetrical management fitting the current clinical guidelines, and detailed high‐quality data covering pregnancy, neonatal hospitalisation (including bacterial documentation) and follow‐up at 2 years. Considering the paucity of evidence‐based data regarding the comparison of different antibiotic prophylaxis, such observational studies are instrumental in assessing routine clinical practices in a real‐life setting. We could evaluate the administration of amoxicillin and 3GC in this indication, which has commonly been overlooked in previous studies, 27 or has only been studied in combination with other antibiotics, which makes it challenging to determine their intrinsic effect. 28 , 29 , 30
The main limitations of our work should be kept in mind: a limited sample size and the large number of comparisons performed. The observed significant associations are biologically plausible but may be due to chance, although all outcomes were pre‐specified based on clinical relevance and previous studies on the topic. 31 Due to the right‐truncation of women with PPROM before 34 weeks who delivered from 35 weeks on (i.e. not eligible for the EPIPAGE‐2 cohort), we restricted our analyses to women with PPROM at 24–31 weeks and therefore likely missed a few births with the longest latency durations and best prognoses. Antibiotic treatments were compared irrespective of administration routes, dosages or durations, as these were beyond the scope of this study. As we focused on the initial management strategy implemented upon diagnosis of PPROM, and considering that most women gave birth within the first week after PPROM, we did not explore subsequent antibiotic administrations. Finally, bacterial ecology might have evolved since 2011, in particular with an increase in the frequency of extended‐spectrum beta‐lactamase‐producing bacteria. 32
4.3. Interpretation
The optimal antibiotic prophylaxis in the setting of PPROM should ideally combine good maternal and fetal tolerance profile and narrow spectrum focusing on the likely bacterial pathogens involved, namely, S. agalactiae and E. coli. Whether the antimicrobial spectrum should include genital mycoplasmas (Ureaplasma and other genital Mycoplasma species) remains unresolved: as ordinary colonisers of the lower genital tract, their frequent documentation in histologically confirmed intrauterine infection may be considered as by‐standing colonisation, a viewpoint reinforced by the lack of demonstrated benefit of any Mycoplasma‐targeting antibiotic in the management of intrauterine infection. 15 , 33 Conversely, some authors have provided evidence for potential pathogenicity in histologically confirmed intrauterine infections, including induction of local inflammatory response with deleterious consequences in animal models. 14 In this perspective, the optimal antibiotic choice is not straightforward, as all candidates exhibit pros and cons. Amoxicillin/ampicillin and penicillin are effective on 100% of S. agalactiae isolates but on no more than 60% of E. coli isolates, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) data. 34 Erythromycin, spiramycin and clindamycin are active on 75–80% of S. agalactiae but exhibit only marginal activity toward E. coli. 34 In addition, macrolides have limited transplacental passage, 35 which could limit the prevention of materno‐fetal infection. 27 3GC exhibit a broader spectrum that, beyond S. agalactiae, encompasses >90% of E. coli isolates, according to EUCAST data. 34 On the other hand, 3GC remain ineffective towards intracellular Mycoplasma sp., raising more concerns regarding the potential greater impact on maternal/neonatal gut microbiota. 16 , 28 Oral 3GC, because of their poor oral bioavailability, are not recommended in this setting. 36
In the present study, we could not evidence any superiority of amoxicillin, macrolides, 3GC or any combinations over the others regarding the reduction of intrauterine infection and neonatal sepsis. However, our results suggest a trend towards an overall beneficial effect of 3GC administration, with improved infant survival at discharge without severe neonatal morbidity. This was also found in the combinations group, which antibacterial spectrum could be considered roughly similar to the 3GC regimen, except for the subsets with additional anaerobic and mycoplasma coverage provided by clindamycin and macrolides, respectively. These differences did not translate into statistical differences in survival at 2 years without neurosensory impairment. This should be confirmed in a further study by studying complementary outcomes (e.g. minor morbidity) in the longer term, as some differences might become apparent later in life (as was the case in the ORACLE II trial 37 ).
We collected data regarding bacterial documentation in EOS and LOS that confirm the high involvement of E. coli in EOS and highlight the high frequency of amoxicillin‐resistance among E. coli isolates collected in infants after maternal exposure to amoxicillin. The latter observation is in line with previous publications that evidenced an increase in ampicillin‐resistant strains infections in preterm neonates whose mothers had received antenatal ampicillin, including in the setting of PPROM prophylaxis. 38 , 39 , 40
Our findings also suggested poorer neurodevelopment at 2 years’ corrected age in infants exposed to antenatal macrolides. This result should be interpreted very cautiously as (i) few women received this treatment, (ii) the trend was similar but no longer significant after multiple imputations, (iii) other factors, not taken into account in the analyses, could be associated with neurodevelopmental outcomes, and (iv) ASQ was estimated through a parental questionnaire, which results are to be confirmed based on the cognitive assessment by a neuropsychologist at the 5‐year follow‐up. No long‐term effect of erythromycin was identified in the ORACLE I trial (the largest randomised controlled trial comparing different broad‐spectrum antibiotics among 4826 women with PPROM). 41 However, in the ORACLE II trial (comparing antibiotics in women with spontaneous preterm labour and intact membranes), erythromycin was associated with an increase in functional impairment and cerebral palsy among children at 7 years of age. 37
4.4. Clinical and research implications
Whether the overall beneficial effect of 3GC or combination regimens could be attributed to the extended E. coli coverage and subsequent reduction of infection burden and related morbi‐mortalities is likely but should be further studied. Although no deleterious effect of neither 3GC or combinations treatments could be evidenced in this study, theoretical concerns remain regarding the impact of such broad spectrum antibiotics (3GC) or combinations on maternal and neonatal gut microbiota. 42 , 43 This should, however, be considered with the fact that most preterm infants born after PPROM will be exposed to antibiotics after birth. 44 , 45 It has also been recently emphasised that the clinical spectrum of one given antibiotic does not translate into ecological impact on the microbiota, the later depending on multiple factors including concentrations achieved in the colon. 46 Altogether, given these reservations and the lack of any additional benefit evidenced for combinations, at present such combined therapies should be discarded.
Finally, these exploratory findings based on observational data are not intended to lead to a change in clinical practice. The observed associations generate hypotheses that need to be confirmed or refuted in a subsequent study with a larger sample and pre‐planned hypotheses. The impact on maternal and neonatal microbiota, and resistances, optimal posology and duration of antibiotic prophylaxis remain to be determined.
5. CONCLUSION
Optimisation of the use of antibiotics by using the more restricted antibiotic spectrum with the most favourable clinical outcome, is a global priority to prevent antimicrobial resistance and preserve the efficacy of existing antibiotics. This is true as well for preterm babies, before and after birth. Evidence‐based data arising from new well‐conducted studies and randomised controlled trials, with long‐term follow‐up, are needed to better define which antibiotics are to be preferentially used after PPROM, and their modalities of administration.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interest forms are available to view online as supporting information.
AUTHOR CONTRIBUTIONS
Study concept and design of the present study: EL, CC, GK. Acquisition, analysis and interpretation of data: EL, ML, HT, LFLH, CG‐LG, VB, PB, CC, GK. Statistical analysis: EL. Drafting of the manuscript: EL, CC, GK. Critical revision of the manuscript for important intellectual content: EL, ML, HT, LFLH, CG‐LG, VB, PB, CC, GK. Final approval of the version to be published: EL, ML, HT, LFLH, CG‐LG, VB, PB, CC, GK. Agreement to be accountable for all aspects of the work: EL, ML, HT, LFLH, CG‐LG, VB, PB, CC, GK.
ETHICS APPROVAL
Recruitment in the EPIPAGE‐2 cohort study and follow‐up evaluations were approved by the appropriate ethics committees, i.e. the advisory committee on the treatment of personal health data for research purposes (references 10‐626, 12‐109 and 16‐263) and the committee for the protection of people participating in biomedical research (references 2011‐A00159‐32 and 2016‐A0033‐48). All the procedures are in accordance with the Declaration of Helsinki of the World Medical Association.
Supporting information
Fig S1
Table S1
Table S2
Table S3
Table S4
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the participating children and their families and to all maternity and neonatal units in France. The authors acknowledge the collaborators of the EPIPAGE‐2 Obstetric Writing Group: Pierre‐Yves Ancel, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Unité de Recherche Clinique – Centre d’Investigations Cliniques P1419, Département Hospitalo‐Universitaire Risks in Pregnancy, Cochin Hotel‐Dieu Hospital, Assistance Publique‐Hôpitaux de Paris, Paris F‐75014, France), Catherine Arnaud, MD, PhD (Research Unit on Perinatal Epidemiology, Childhood Disabilities and Adolescent Health, INSERM UMR 1027, Paul Sabatier University, Toulouse, France), Julie Blanc, MD (Department of Obstetrics and Gynaecology, Aix Marseille University, Marseille, France), Pascal Boileau, MD, PhD (Department of Neonatal Paediatrics, Poissy Saint Germain Hospital, France, Versailles Saint Quentin en Yvelines University, France), Thierry Debillon, MD, PhD (Department of Neonatal Paediatrics, University Hospital, Grenoble, France), Pierre Delorme, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Department of Obstetrics and Gynaecology, Cochin, Broca, Hôtel Dieu Hospital, AP‐HP, Paris, France), Claude D’Ercole, MD (Department of Obstetrics and Gynaecology, Nord Hospital, Assistance Publique des Hôpitaux de Marseille (AP‐HM), Aix Marseille Université, AMU, Marseille, France), Thomas Desplanches, RM, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France), Caroline Diguisto, MD, PhD (Université de Paris, Research Centrer for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Maternité Olympe de Gouges, University Francois Rabelais, Tours, France), Laurence Foix‐L’Helias, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Sorbonne Universités, UPMC Univ Paris 06, IFD, 4 Place Jussieu, 75252 PARIS cedex 05, Paris, France, Department of Neonatal Paediatrics, Trousseau Hospital, AP‐HP, Paris, France), Géraldine Gascoin, MD, PhD (Department of Neonatal Medicine, Toulouse University Hospital, Toulouse, France), Catherine Gire, MD (Department of Neonatology, North Hospital, Marseille, France), François Goffinet, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Department of Obstetrics and Gynaecology, Cochin, Broca, Hôtel Dieu Hospital, AP‐HP, Paris, France), Gilles Kayem, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Sorbonne Universités, UPMC Univ Paris 06, IFD, 4 Place Jussieu, 75252 PARIS cedex 05, Paris, France, Department of Obstetrics and Gynaecology, Trousseau Hospital, AP‐HP, Paris, France), Bruno Langer, MD (Department of Obstetrics and Gynaecology, Hautepierre Hospital, Strasbourg, France), Mathilde Letouzey, MD, MSc (Université de Paris, Research Center for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, Department of Neonatal Paediatrics, Poissy Saint Germain Hospital, France), Elsa Lorthe, RM, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France), Emeline Maisonneuve, MD, MSc (Department of Obstetrics and Gynaecology, Trousseau Hospital, APHP, Paris, France), Stéphane Marret, MD, PhD (Department of Neonatal Medicine, Rouen University Hospital and Région‐INSERM (ERI 28), Normandy University, Rouen, France), Isabelle Monier, RM, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France), Andrei Morgan, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France), Jean‐Christophe Rozé, MD, PhD (Department of Neonatal Medicine, Nantes University Hospital, Nantes, France, Epidémiologie Clinique, Centre d’Investigation Clinique (CIC004), Nantes University Hospital, Nantes, France), Thomas Schmitz, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Department of Obstetrics and Gynaecology, Robert Debré Hospital, Assistance Publique‐Hôpitaux de Paris, Paris, France), Loïc Sentilhes, MD, PhD (Depatment of Obstetrics and Gynaecology, Bordeaux University Hospital, Bordeaux, France), Damien Subtil, MD, PhD (Department of Obstetrics and Gynaecology, Jeanne de Flandre Hospital, Lille, France), Héloïse Torchin, MD, PhD (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France, Neonatal Medicine and Resuscitation Service in Port‐Royal, Cochin Hospital, Assistance Publique‐Hôpitaux de Paris, Paris, France), Barthélémy Tosello, MD, PhD (Department of Neonatology, Assistance Publique Hopitaux de Marseille, Marseille, France), Christophe Vayssière, MD, PhD (Department of Obstetrics and Gynaecology, University Hospital, Toulouse, France, Research Unit on Perinatal Epidemiology, Childhood Disabilities and Adolescent Health, INSERM UMR 1027, Paul Sabatier University, Toulouse, France), Norbert Winer, MD, PhD (Department of Obstetrics and Gynaecology, University Hospital, INRA, UMR 1280 Physiologie des adaptations nutritionnelles, Nantes, France), Jennifer Zeitlin (Université de Paris, Research Centre for Epidemiology and BioStatistics Sorbonne Paris Cité (CRESS), INRA, F‐75004 Paris, France).
The authors also acknowledge the collaborators of the EPIPAGE‐2 Study Group: Alsace: D Astruc, P Kuhn, B Langer, J Matis, C Ramousset; Aquitaine: X Hernandorena, P Chabanier, L Joly‐Pedespan, MJ Costedoat, A Leguen; Auvergne: B Lecomte, D Lemery, F Vendittelli; Basse‐Normandie: G Beucher, M Dreyfus, B Guillois, Y Toure; Bourgogne: A Burguet, S Couvreur, JB Gouyon, P Sagot, N Colas; Bretagne: J Sizun, A Beuchée, P Pladys, F Rouget, RP Dupuy, D Soupre, F Charlot, S Roudaut; Centre: A Favreau, E Saliba, L Reboul; Champagne‐Ardenne: N Bednarek, P Morville, V Verrière; Franche‐Comté: G Thiriez, C Balamou; Haute‐Normandie: L Marpeau, S Marret, C Barbier; Ile‐de‐France: G Kayem, X Durrmeyer, M Granier, M Ayoubi, O Baud, B Carbonne, L Foix L’Hélias, F Goffinet, PH Jarreau, D Mitanchez, P Boileau, C Duffaut, E Lorthe, L Cornu, R Moras; Languedoc‐Roussillon: P Boulot, G Cambonie, H Daudé, A Badessi, N Tsaoussis; Limousin: A Bédu, F Mons, C Bahans; Lorraine: MH Binet, J Fresson, JM Hascoët, A Milton, O Morel, R Vieux, L Hilpert; Midi‐Pyrénées: C Alberge, C Arnaud, C Vayssière, M Baron; Nord‐Pas‐de‐Calais: ML Charkaluk, V Pierrat, D Subtil, P Truffert, S Akowanou; PACA et Corse: C D’Ercole, C Gire, U Simeoni, A Bongain, M Deschamps; Pays de Loire: B Branger, JC Rozé, N Winer, V Rouger, C Dupont; Picardie: J Gondry, G Krim, B Baby; Rhône‐Alpes: M Debeir, O Claris, JC Picaud, S Rubio‐Gurung, C Cans, A Ego, T Debillon, H Patural, A Rannaud; Guadeloupe: E Janky, A Poulichet, JM Rosenthal, E Coliné; Guyane: A Favre, N Joly; Martinique: S Châlons, J Pignol, PL Laurence; La Réunion: PY Robillard, S Samperiz, D Ramful; Inserm UMR 1153: PY Ancel, V Benhammou, B Blondel, M Bonet, A Brinis, A Coquelin, M Durox, M Kaminski, K Khemache, B Khoshnood, C Lebeaux, L Marchand‐Martin, J Rousseau, MJ Saurel‐Cubizolles, D Tran, J Zeitlin. None of the collaborators of the EPIPAGE‐2 Obstetric Writing group and the EPIPAGE‐2 Study group have any conflict of interest or compensation in relation with this article to disclose. All of them consented to such an acknowledgement. Open access funding provided by Universite de Geneve.
Lorthe E, Letouzey M, Torchin H, Foix L'Helias L, Gras‐Le Guen C, Benhammou V, et al.; for the EPIPAGE‐2 Obstetric Writing Group . Antibiotic prophylaxis in preterm premature rupture of membranes at 24–31 weeks’ gestation: Perinatal and 2‐year outcomes in the EPIPAGE‐2 cohort. BJOG. 2022;129:1560–1573. 10.1111/1471-0528.17081
See Acknowledgements for the EPIPAGE‐2 Obstetric Writing Group.
Funding information
This work was partly supported by a postdoctoral grant from the Fondation des Treilles to EL. EPIPAGE‐2 was funded by the French Institute of Public Health Research (IRESP TGIR 2009‐01 programme)/Institute of Public Health and its partners: the French Health Ministry, the National Institute of Health and Medical Research (INSERM), the National Institute of Cancer, and the National Solidarity Fund for Autonomy (CNSA); the National Research Agency through the French EQUIPEX programme of investments for the future (grant number ANR‐11‐EQPX‐0038); and the PREMUP Foundation. Additional funding was obtained from Fondation pour la Recherche Medicale (grant number SPF 20160936356) and Fondation de France (grant numbers 00050329, Grand Prix R18202KK]). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Elsa Lorthe, Email: elsa.lorthe@gmail.com.
for the EPIPAGE‐2 Obstetric Writing Group:
Pierre‐Yves Ancel, Catherine Arnaud, Julie Blanc, Thierry Debillon, Pierre Delorme, Claude D’Ercole, Thomas Desplanches, Caroline Diguisto, Géraldine Gascoin, Catherine Gire, François Goffinet, Bruno Langer, Emeline Maisonneuve, Stéphane Marret, Isabelle Monier, Andrei Morgan, Jean‐Christophe Rozé, Thomas Schmitz, Loïc Sentilhes, Damien Subtil, Barthélémy Tosello, Christophe Vayssière, Norbert Winer, Jennifer Zeitlin, D Astruc, P Kuhn, J Matis, C Ramousset, X Hernandorena, P Chabanier, L Joly‐Pedespan, MJ Costedoat, A Leguen, B Lecomte, D Lemery, F Vendittelli, G Beucher, M Dreyfus, B Guillois, Y Toure, A Burguet, S Couvreur, JB Gouyon, P Sagot, N Colas, J Sizun, A Beuchée, P Pladys, F Rouget, RP Dupuy, D Soupre, F Charlot, S Roudaut, A Favreau, E Saliba, L Reboul, N Bednarek, P Morville, V Verrière, G Thiriez, C Balamou, L Marpeau, C Barbier, X Durrmeyer, M Granier, M Ayoubi, O Baud, B Carbonne, PH Jarreau, D Mitanchez, C Duffaut, L Cornu, R Moras, P Boulot, G Cambonie, H Daudé, A Badessi, N Tsaoussis, A Bédu, F Mons, C Bahans, MH Binet, J Fresson, JM Hascoët, A Milton, O Morel, R Vieux, L Hilpert, C Alberge, M Baron, ML Charkaluk, V Pierrat, P Truffert, S Akowanou, U Simeoni, A Bongain, M Deschamps, B Branger, V Rouger, C Dupont, J Gondry, G Krim, B Baby, M Debeir, O Claris, JC Picaud, S Rubio‐Gurung, C Cans, A Ego, H Patural, A Rannaud, E Janky, A Poulichet, JM Rosenthal, E Coliné, A Favre, N Joly, S Châlons, J Pignol, PL Laurence, PY Robillard, S Samperiz, D Ramful, B Blondel, M Bonet, A Brinis, A Coquelin, M Durox, M Kaminski, K Khemache, B Khoshnood, C Lebeaux, L Marchand‐Martin, J Rousseau, MJ Saurel‐Cubizolles, and D Tran
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101(1):178–93. [DOI] [PubMed] [Google Scholar]
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmitz T, Sentilhes L, Lorthe E, Gallot D, Madar H, Doret‐Dion M, et al. Preterm premature rupture of the membranes: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol. 2019;236:1–6. [DOI] [PubMed] [Google Scholar]
- 4. Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2013;12:CD001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menon R, Richardson LS. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hutzal CE, Boyle EM, Kenyon SL, Nash JV, Winsor S, Taylor DJ, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199(6):620.e1–8. [DOI] [PubMed] [Google Scholar]
- 7. Yudin MH, van Schalkwyk J, Van Eyk N. No. 233‐antibiotic therapy in preterm premature rupture of the membranes. J Obstet Gynaecol Can. 2017;39(9):e207–12. [DOI] [PubMed] [Google Scholar]
- 8. Prelabor rupture of membranes: ACOG practice bulletin, number 217. Obstet Gynecol. 2020;135(3):e80. [DOI] [PubMed] [Google Scholar]
- 9. Thomson AJ. Care of women presenting with suspected preterm prelabour rupture of membranes from 24+0 weeks of gestation. BJOG. 2019;126:e152–66. [DOI] [PubMed] [Google Scholar]
- 10. Ramsey PS, Nuthalapaty FS, Lu G, Ramin S, Nuthalapaty ES, Ramin KD. Contemporary management of preterm premature rupture of membranes (PPROM): a survey of maternal‐fetal medicine providers. Am J Obstet Gynecol. 2004;191(4):1497–502. [DOI] [PubMed] [Google Scholar]
- 11. Lorthe E, Ancel P‐Y, Torchin H, Kaminski M, Langer B, Subtil D, et al. Impact of latency duration on the prognosis of preterm infants after preterm premature rupture of membranes at 24 to 32 weeks’ gestation: a national population‐based cohort study. J Pediatr. 2017;182:47–52.e2. [DOI] [PubMed] [Google Scholar]
- 12. Mercer BM, Miodovnik M, Thurnau GR, Goldenberg RL, Das AF, Ramsey RD, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. JAMA. 1997;278(12):989–95. [PubMed] [Google Scholar]
- 13. Kenyon S, Taylor D, Tarnow‐Mordi W. Broad‐spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. Lancet. 2001;357(9261):979–88. [DOI] [PubMed] [Google Scholar]
- 14. Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev. 2017;30(1):349–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kayem G, Doloy A, Schmitz T, Chitrit Y, Bouhanna P, Carbonne B, et al. Antibiotics for amniotic‐fluid colonization by ureaplasma and/or Mycoplasma spp. to prevent preterm birth: a randomized trial. PLoS One. 2018;13(11):e0206290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woerther P‐L, Lepeule R, Burdet C, Decousser J‐W, Ruppé É, Barbier F. Carbapenems and alternative β‐lactams for the treatment of infections due to extended‐spectrum β‐lactamase‐producing Enterobacteriaceae: what impact on intestinal colonisation resistance? Int J Antimicrob Agents. 2018;52(6):762–70. [DOI] [PubMed] [Google Scholar]
- 17. Lorthe E, Benhammou V, Marchand‐Martin L, Pierrat V, Lebeaux C, Durox M, et al. Cohort profile: the Etude Epidémiologique sur les Petits Ages Gestationnels‐2 (EPIPAGE‐2) preterm birth cohort. Int J Epidemiol. 2021;50:1428–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pierrat V, Marchand‐Martin L, Arnaud C, Kaminski M, Resche‐Rigon M, Lebeaux C, et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: the EPIPAGE‐2 cohort study. BMJ. 2017;358:j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ancel P, Goffinet F, the EPIPAGE‐2 Writing Group . Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the epipage‐2 cohort study. JAMA Pediatr. 2015;169(3):230–8. [DOI] [PubMed] [Google Scholar]
- 20. Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol. 2012;206(2):119–23. [DOI] [PubMed] [Google Scholar]
- 21. Maisonneuve E, Lorthe E, Torchin H, Delorme P, Devisme L, L’Hélias LF, et al. Association of chorioamnionitis with cerebral palsy at two years after spontaneous very preterm birth: the EPIPAGE‐2 cohort study. J Pediatr. 2020;222:71–8.e6. [DOI] [PubMed] [Google Scholar]
- 22. Cans C. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42(12):816–24. [DOI] [PubMed] [Google Scholar]
- 23. Webbe JWH, Duffy JMN, Afonso E, Al‐Muzaffar I, Brunton G, Greenough A, et al. Core outcomes in neonatology: development of a core outcome set for neonatal research. Arch Dis Child Fetal Neonatal Ed. 2020;105(4):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ego A, Prunet C, Lebreton E, Blondel B, Kaminski M, Goffinet F, et al. Customized and non‐customized French intrauterine growth curves. I – Methodology. J Gynecol Obstet Biol Reprod (Paris). 2016;45(2):155–64. [DOI] [PubMed] [Google Scholar]
- 25. McNutt L‐A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 26. Chen W, Qian L, Shi J, Franklin M. Comparing performance between log‐binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatzakis C, Papatheodorou S, Sarafidis K, Dinas K, Makrydimas G, Sotiriadis A. Effect on perinatal outcome of prophylactic antibiotics in preterm prelabor rupture of membranes: network meta‐analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2020;55(1):20–31. [DOI] [PubMed] [Google Scholar]
- 28. Lee J, Romero R, Kim SM, Chaemsaithong P, Park C‐W, Park JS, et al. A new antimicrobial combination prolongs the latency period, reduces acute histologic chorioamnionitis and funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med. 2016;29(5):707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ehsanipoor RM, Chung JH, Clock CA, McNulty JA, Wing DA. A retrospective review of ampicillin‐sulbactam and amoxicillin + clavulanate vs cefazolin/cephalexin and erythromycin in the setting of preterm premature rupture of membranes: maternal and neonatal outcomes. Am J Obstet Gynecol. 2008;198(5):e54–6. [DOI] [PubMed] [Google Scholar]
- 30. Wolf MF, Sgayer I, Miron D, Krencel A, Sheffer VF, Idriss SS, et al. A novel extended prophylactic antibiotic regimen in preterm pre‐labor rupture of membranes: a randomized trial. Int J Infect Dis. 2020;96:254–9. [DOI] [PubMed] [Google Scholar]
- 31. Streiner DL. Best (but oft‐forgotten) practices: the multiple problems of multiplicity—whether and how to correct for many statistical tests. Am J Clin Nutr. 2015;102(4):721–8. [DOI] [PubMed] [Google Scholar]
- 32. Woerther P‐L, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended‐spectrum β‐lactamases in the community: toward the globalization of CTX‐M. Clin Microbiol Rev. 2013;26(4):744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Committee opinion no. 712: intrapartum management of intraamniotic infection. Obstet Gynecol. 2017;130(2):e95–101. [DOI] [PubMed] [Google Scholar]
- 34. European Committee on Antimicrobial Susceptibility Testing Data from the EUCAST MIC distribution website [cited 2021 Jul 01]. Available from: http://www.eucast.org
- 35. Viel‐Theriault I, Fell DB, Grynspan D, Redpath S, Thampi N. The transplacental passage of commonly used intrapartum antibiotics and its impact on the newborn management: a narrative review. Early Hum Dev. 2019;135:6–10. [DOI] [PubMed] [Google Scholar]
- 36. Doret Dion M, Cazanave C, Charlier C. Choix et durée de l’antibioprophylaxie en cas de rupture prématurée des membranes avant terme. RPC rupture prématurée des membranes avant terme CNGOF. Gynécol Obstét Fertil Sénol. 2018;46(12):1043–53. [DOI] [PubMed] [Google Scholar]
- 37. Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet. 2008;372(9646):1319–27. [DOI] [PubMed] [Google Scholar]
- 38. Didier C, Streicher M‐P, Chognot D, Campagni R, Schnebelen A, Messer J, et al. Late‐onset neonatal infections: incidences and pathogens in the era of antenatal antibiotics. Eur J Pediatr. 2011;171(4):681–7. [DOI] [PubMed] [Google Scholar]
- 39. Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin‐resistant, neonatal, early‐onset sepsis over 18 years. Pediatrics. 2010;125(5):e1031–8. [DOI] [PubMed] [Google Scholar]
- 40. Mercer BM, Carr TL, Beazley DD, Crouse DT, Sibai BM. Antibiotic use in pregnancy and drug‐resistant infant sepsis. Am J Obstet Gynecol. 1999;181(4):816–21. [DOI] [PubMed] [Google Scholar]
- 41. Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7‐year follow‐up of the ORACLE I trial. Lancet. 2008;372(9646):1310–8. [DOI] [PubMed] [Google Scholar]
- 42. Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez‐Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–93. [DOI] [PubMed] [Google Scholar]
- 44. Arboleya S, Sánchez B, Milani C, Duranti S, Solís G, Fernández N, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538–44. [DOI] [PubMed] [Google Scholar]
- 45. DiGiulio DB. Prematurity and perinatal antibiotics: a tale of two factors influencing development of the neonatal gut microbiota. J Pediatr. 2015;166(3):515–7. [DOI] [PubMed] [Google Scholar]
- 46. Ruppé E, Burdet C, Grall N, de Lastours V, Lescure F‐X, Andremont A, et al. Impact of antibiotics on the intestinal microbiota needs to be re‐defined to optimize antibiotic usage. Clin Microbiol Infect. 2018;24(1):3–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2
Table S3
Table S4
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


