Key points
What's already known about this topic?
Discordant NIPT results can rarely unravel maternal malignancies, especially when multiple chromosomal imbalances are reported. Both solid and hematological neoplasms have been described.
What does this study add?
This is the first case of a discordant NIPT result due to Chronic Lymphocytic Leukemia associated with trisomy of the chromosome 12. Putative maternal malignancy should be considered and investigated through sensitive techniques even in presence of a single chromosomal anomaly. This must be considered especially when the imbalance is known to recur in hematological neoplasms.
1. MAIN TEXT
Since its implementation in 2011, Non‐Invasive Prenatal Test (NIPT) has become a reliable and sensitive method of screening to evaluate the risk for fetal chromosomal imbalances. As the number of pregnancies screened with NIPT increases, so does the number of sporadic reports of discordant results between NIPT and invasive prenatal diagnostic procedures performed to confirm pathological results. The cause of discordancy is strictly related to the nature of the sample under analysis in NIPT, that is cell free DNA (cfDNA) circulating in maternal blood. Total cfDNA is a mixture of cell‐free fetal DNA (cffDNA), originating from trophoblast cells, and maternal cfDNA (mcfDNA), mostly originating from apoptotic hematopoietic cells, particularly from white‐cell lineage (70%). 1 The ratio between cffDNA and total cfDNA is known as fetal fraction (FF). Among maternal causes of discordancy, maternal neoplasms have been reported, due to mcfDNA originating from tumor cells. In the present report, the diagnosis of a tumor specific single chromosomal anomaly, after a discordant NIPT result, helped uncover the rare occurrence of chronic lymphatic leukemia (CLL) during pregnancy.
A 37‐years‐old healthy pregnant woman performed NIPT at 11 gestational weeks as a first‐tier screening for age‐related risk of fetal aneuploidies. NIPT was performed using a whole genome sequencing approach with the IONA NIPT Workflow (Yourgene Health) on S5XL sequencer (ThermoFisher) according to manufacturer's instructions. Results indicated a high risk for a female fetus with an aneuploidy of chromosome 12 (F F = 13%). At genetic counseling, the familial and personal histories of the woman and her partner were unremarkable for intellectual disability, congenital malformations, known genetic conditions, and consanguinity. Ultrasound examinations at 6 and 12 gestational weeks showed a single viable intrauterine pregnancy, biometry appropriate for gestational age, and absence of gross fetal anomalies. The only relevant clinical finding was a maternal blood count with increased lymphocytes (6.37 × 103, normal value <5 × 103). Toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and Human Immunodeficiency Virus infections were excluded.
To confirm the NIPT result, standard karyotype was performed on amniotic cells collected at 16 gestational weeks. In addition, standard karyotype analysis on peripheral blood of the couple (on 100 mitoses, to exclude low‐level mosaicism) and array‐CGH for both the parents and the fetus identified no numerical nor structural chromosomal alterations. In the fetus, array‐CGH reported two microduplications of chromosome X inherited from the healthy father, hence considered likely benign.
The patient was referred for a fetal morphological evaluation at our obstetrics unit. Ultrasound scans at 16 and 20 gestational weeks reported no fetal anomalies. However, maternal blood lymphocytes count still increased, reaching the value of 10.0 × 103. Based on these findings and the NIPT result, a Chronic Lymphocytic Leukemia (CLL) associated with trisomy 12 was suspected. The woman was then referred to our onco‐hematological unit. Subsequent cytofluorimetric analysis disclosed the presence of a B‐lymphocyte clone [CD5+, CD19+, CD20+, CD200+ CD38+, CD23+(60%) FMC7+(47%), immunoglobulin light chain restricted] representing 87% of total lymphocytes, thus confirming the suspected CLL. Onco‐hematological cytogenetic characterization with a different protocol based on ChromoLympho‐B Proliferation MIX (BIOZOL) was used to specifically stimulate cell proliferation of B‐lymphocytes. Fluorescent in situ hybridization (FISH) on interphase nuclei of 48h cultivated cells detected three signals for chromosome 12 in 30 out of 200 cells (15%), indicating a trisomy 12. Results and other details of FISH are reported in Figure 1A.
FIGURE 1.
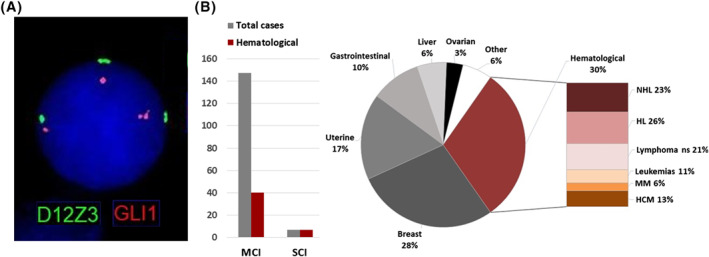
(A) Fluorescent in situ hybridization (FISH) on the interphase nucleus of a patient's B lymphocyte using Kreatech GLI1 (12q13)/SE 12 FISH probes showing three fluorescent signals for the analyzed regions of chromosome 12. The result is compatible with trisomy of chromosome 12. Other analyzed regions were 11q22 (ATM locus, probe LSI ATM/(11q22)/CEP11, Vysis), 17p13 (TP53 locus, probe TP53 (17p13)/SE17, Kreatech), 13q14.3 (D13S25, Vysis): none of these probes showed the presence of imbalances. (B) Types of pre‐malignant or malignant conditions and types of imbalances reported in discordant cfDNA‐NIPT cases (total number of cases = 154). Reported single chromosomal anomalies: trisomy 12 (n = 1), trisomy 8 (n = 3), 5q deletion (n = 1), 20q deletion (n = 2). “Other” includes one case of the following malignancies: neuroendocrine tumor, adenocarcinoma, osteosarcoma, nasopharyngeal carcinoma, lung cancer, cervical neoplasia, and retroperitoneal sarcoma. References of literature review 1 , 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 : HCM, hemopoietic clonal mosaicism HL, Hodgkin Lymphoma; MM, Multiple Myeloma; NHL, Non‐Hodgkin Lymphoma; ns, not specified
At diagnosis, the patient did not present with either enlarged lymphnodes/spleen/liver or anemia or thrombocytopenia. CLL was then classified at stage 0 according to Rai and stage A according to Binet, both corresponding to an estimated overall survival >10 years. Onco‐hematological surveillance was started with serial blood counts during pregnancy (Table 1) and continued after delivery. At 40 gestational weeks labor was inducted with cervical ripening balloon allowing the birth of a healthy female baby not requiring any intensive care. Follow up after 12 months demonstrated stable hematological parameters (WBC 13,5 × 103/µl; Lymphocytes absolute value 7,1 × 103/µl; Platelet absolute value 308 × 103/µl) with no need for therapy.
TABLE 1.
Complete blood counts of the patient during the whole pregnancy
| Unit | Reference values | Gestational age (weeks) | ||||||
|---|---|---|---|---|---|---|---|---|
| 12 | 20 | 23 | 26 | 30 | 36 | |||
| WBC | x103/µl | 4–10 | 14,9↑ | 20,2↑ | 20,26↑ | 22,1↑ | 22,4↑ | 19,4↑ |
| LYM# | x103/µl | 1–4,5 | 6,37↑ | 10↑ | 11,63↑ a | 11,6↑ | 11,26↑ | 9,3↑ |
| LYM% | % | 20–45 | 42,7 | 49,4 | 58,0↑ | 52,4↑ | 50,3↑ | 47,8 ↑ |
| RBC | x106/µl | 3,8–5,2 | 4,58 | 3,71↓ | 3,55↓ | 3,65↓ | 3,83 | 3,85 |
| HGB | g/dL | 12–16 | 12,9 | 11,2↓ | 10,3↓ | 10,5↓ | 11,2↓ | 11,2↓ |
| PLT | x103/µl | 150–450 | 334 | 336 | 324 | 376 | 372 | 273 |
Flow cytometry (lymphocyte subsets): T‐cells 5,8% (range 59,4–84,6); B‐cells 87% (range 5–15) CD19+, CD20+, CD5+, CD38+, CD200+, CD23+ (60%), FMC7+ (47%).
Abbreviations: HGB, Hemoglobin concentration; LYM#, Lymphocytes absolute value; LYM%, Lymphocytes relative value; PLT, Platelet absolute value; RBC, Red Blood Count; WBC, White Blood Count; ↑: increased respect to reference value; ↓: decreased respect to reference value.
To the best of our knowledge, this is the first report of CLL in pregnancy revealed after discordant NIPT result. Since some imbalances of chromosome 12 can be compatible with fetal development (e.g. tetrasomy 12p, trisomy 12p and monosomy 12p), our first concern was to exclude the fetal origin of the abnormal result: those conditions, indeed, have been described either as confined placental mosaicism (CPM) or true fetal mosaicism. Although CPMs are a major reason for NIPT discordancy, 2 in the present case maternal lymphocytosis drove our attention to look for other causes besides fetal and placental ones. The whole diagnostic pathway following NIPT definitely helped the woman to get answers on questions about the health of both herself and her fetus. Firstly, the finding of a maternal cause for the imbalance reassured her about the prognosis for the future child; secondly, she received relief from the early inclusion in regular hematological follow up for her condition.
The onset of CLL is rare in women of childbearing age, representing only 2% of total cases: during pregnancy most cases are diagnosed after incidental asymptomatic lymphocytosis (Rai stage 0 or Binet stage A), allowing a watch and wait approach with serial blood counts. 3 However, pregnant women are at increased risk for CLL complications (e.g. infections, cytopenia, autoimmune pathologies) that need to be monitored. Moreover, during follow up, young patients require pharmacological therapies more often than those with onset at a later age. For this reason, early diagnosis can help establish a timely treatment, whenever it becomes necessary. 3 As in other hematological neoplasms, the prognosis and treatment of CLL are additionally better defined after the identification of specific recurrent chromosomal imbalances. Trisomy 12 is the second most frequent cytogenetic aberration, being found in 10%–25% CLLs at diagnosis, and is considered an intermediate prognostic factor, with median values for first treatment and overall survival of 33 and 114 months respectively. Other features of trisomy 12 in CLL are an increased risk for cytopenia and Richter syndrome, a complication characterized by sudden transformation of CLL in a more aggressive disease, generally a diffuse large B‐cell lymphoma. 4 Due to the low spontaneous proliferation of B‐lymphocytes, dedicated cytogenetic protocols based on a culture supplemented with CpG oligonucleotides and interleukin 12 followed by FISH on interphase nuclei are used as first line test for aneuploidies in CLL. 4 This is important to consider because standard cytogenetic karyotyping protocols, generally used to investigate constitutional chromosomal rearrangements on peripheral blood, would probably fail to detect these somatically acquired imbalances due to preferential proliferation of T‐lymphocytes.
Maternal neoplasms causing discordant NIPT results are a complex matter in prenatal diagnosis that has been receiving growing attention in recent years. In most reported cases of discordancy, using a genome wide sequencing approach, NIPT revealed multiple chromosomal imbalances (MCI) due to the presence of cfDNA originating from maternal neoplasm, thus perturbing the risk estimate for fetal aneuploidies. 5 , 6 , 7 Dedicated bioinformatics pipelines followed by multidisciplinary diagnostic pathways have been proposed to interpret these results and search for a putative neoplastic condition, with some studies estimating a positive predictive value of 73%–75%. 5 , 6 , 7 The present case indicates that maternal neoplasms should be considered also in presence of NIPT discordant results for single chromosomal imbalances (SCI). We reviewed the literature cases in which SCI and MCI were indicated by NIPT and subsequently demonstrated to derive from maternal malignant or premalignant conditions (Figure 1B). These were seen more frequently in MCI cases (n = 147) compared to SCI (n = 7). Interestingly, while MCI are generally not specific for the underlying type of neoplasia, the rare SCI were all associated with conditions of hematological origin that overall account for 30% of neoplasms reported in cases of discordant NIPT results (Figure 1B). This could be explained by the peculiarity of hematological neoplasms, often associated with specific SCI. Excluding the present case of trisomy 12, the other reported SCI were trisomy 8 and deletions of 5q and 20q chromosomal regions. They are aberrations known to recur in myeloid neoplasms and in such cases, the resulting maternal clonal mosaicisms can lead to discordant NIPT results. 2 , 5 Since these pre‐malignancies are often subclinical and asymptomatic, they might be overlooked if sensitive techniques such as flow cytometric immunophenotyping and/or dedicated onco‐hematological cytogenetics protocols are not performed. Moreover, in three such cases the clonal mosaicism was demonstrated only after a bone marrow biopsy. 2 , 5
In conclusion, when discordant cfDNA‐NIPT results are due to SCI, maternal malignant or premalignant conditions are worth considering in the differential diagnosis, even though CPM remains the most likely explanation. The frequency of these secondary findings appears to be quite rare, although its real occurrence may be underestimated since maternal investigations were not systematically performed in all studies reporting discordant NIPT results. Differently from MCI, SCI may sometimes indicate a specific type of maternal clonality, especially those already known to recur in hematological neoplasms. In these cases, careful collection of clinical data, including whole blood count and serum protein electrophoresis, need to be considered in the diagnostic workflow after discordant NIPT results. If appropriate, the workflow should also consider morphological analysis, flow cytometric immunophenotyping, and dedicated onco‐hematological cytogenetics protocols on peripheral blood or bone marrow.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENT
The Authors would thank the patient and all the personnel of San Camillo‐Forlanini Hospital who contributed in the makings of this report. The authors did not receive any funding for the present work.
Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement.
Di Giosaffatte N, Bottillo I, Laino L, et al. Discordant cfDNA‐NIPT result unraveling a trisomy 12 chronic lymphocytic leukemia in a 37 years old pregnant woman. Prenat Diagn. 2022;42(8):1000‐1003. 10.1002/pd.6158
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bianchi DW. Cherchez la femme: maternal incidental findings can explain discordant prenatal cell‐free DNA sequencing results. Genet Med. 2018;20(9):910‐917. 10.1038/gim.2017.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van der Meij KRM, Sistermans EA, Macville MVE, et al. TRIDENT‐2: national implementation of genome‐wide non‐invasive prenatal testing as a first‐tier screening test in the Netherlands. Am J Hum Genet. 2019;105(6):1091‐1101. 10.1016/j.ajhg.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamad N, Kliman D, Best OG, et al. Chronic lymphocytic leukaemia, monoclonal B‐lymphocytosis and pregnancy: five cases, a literature review and discussion of management. Br J Haematol. 2015;168(3):350‐360. 10.1111/bjh.13134 [DOI] [PubMed] [Google Scholar]
- 4. Autore F, Strati P, Laurenti L, Ferrajoli A. Morphological, immunophenotypic, and genetic features of chronic lymphocytic leukemia with trisomy 12: a comprehensive review. Haematologica. 2018;103(6):931‐938. 10.3324/haematol.2017.186684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lenaerts L, Brison N, Maggen C, et al. Comprehensive genome‐wide analysis of routine non‐invasive test data allows cancer prediction: a single‐center retrospective analysis of over 85,000 pregnancies. EClinicalMedicine. 2021;35:100856. 10.1016/j.eclinm.2021.100856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dow E, Freimund A, Smith K, et al. Cancer diagnoses following abnormal noninvasive prenatal testing: a case series, literature review, and proposed management model. JCO Precis Oncol. 2021;5:1001‐1012. 10.1200/po.20.00429 [DOI] [PubMed] [Google Scholar]
- 7. Ji X, Li J, Huang Y, et al. Identifying occult maternal malignancies from 1.93 million pregnant women undergoing noninvasive prenatal screening tests. Genet Med. 2019;21(10):2293‐2302. 10.1038/s41436-019-0510-5 [DOI] [PubMed] [Google Scholar]
- 8. Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162‐169. 10.1001/jama.2015.7120 [DOI] [PubMed] [Google Scholar]
- 9. Dharajiya NG, Grosu DS, Farkas DH, et al. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem. 2018;64(2):329‐335. 10.1373/clinchem.2017.277517 [DOI] [PubMed] [Google Scholar]
- 10. Janssens K, Deiteren K, Verlinden A, et al. Detection of a case of chronic myeloid leukaemia with deletions at the t(9;22) translocation breakpoints by a genome‐wide non‐invasive prenatal test. Prenat Diagn. 2016;36(8):760‐765. 10.1002/pd.4857 [DOI] [PubMed] [Google Scholar]
- 11. Lenaerts L, Van Calsteren K, Che H, Vermeesch JR, Amant F. Pregnant women with confirmed neoplasms should not have noninvasive prenatal testing. Prenat Diagn. 2019;39(12):1162‐1165. 10.1002/pd.5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imbert‐Bouteille M, Chiesa J, Gaillard JB, et al. An incidental finding of maternal multiple myeloma by non invasive prenatal testing. Prenat Diagn. 2017;37(12):1257‐1260. 10.1002/pd.5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith J, Kean V, Bianchi DW, et al. Cell‐free DNA results lead to unexpected diagnosis. Clin Case Rep. 2017;5(8):1323‐1326. PMID: 28781851; PMCID: PMC5538058. 10.1002/ccr3.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


