Abstract
Background
Patients with psoriasis have a high risk for multiple comorbid conditions. However, few studies have examined the association between psoriasis and severe and rare infections. This study reports the incidence of severe and rare infections (considered as rare in Denmark) among Danish patients with psoriasis, compared with the general population.
Objectives
The objectives of this study were to assess the incidence and risk of severe and rare infections in Danish patients with psoriasis and the matched general population, and to compare this risk for patients with severe or mild psoriasis with that of the general population.
Methods
Data for individuals aged ≥18 years who were alive and resident in the source population were collected from the Danish National Patient Register between 1 January 1997 and 31 December 2018. Individuals with any of the investigated chronic infections prior to inclusion were excluded. Patients with psoriasis were matched (1 : 6) for age and sex with general population controls. Severe infections were defined as infections requiring treatment in a hospital setting and rare infections included HIV, hepatitis B and C, and tuberculosis infections. Incidence rates (IRs) were reported per 100 000 person‐years of exposure. Severe psoriasis was defined according to previous or active use of systemic or biological treatment. Patients who never received biological and/or systemic treatment were categorized as having mild psoriasis.
Results
A total of 94 450 patients with psoriasis were matched with 566 700 controls. The respective IRs were higher for patients with any psoriasis compared with controls; IR 3104·9 [95% confidence interval (CI) 3066·6 to 3143·7] and IR 2381·1 (95% CI 2367·6 to 2394·6) for any infection, IR 3080·6 (95% CI 3042·5 to 3119·3) and IR 2364·4 (95% CI 2350·9 to 2377·9) for severe infections, and IR 42·9 (95% CI 38·89 to 47·4) and IR 31·8 (95% CI 30·34 to 33·3) for rare infections, respectively. Patients with severe psoriasis had higher IRs of severe or rare infections (IR 3847·7, 95% CI 3754·3 to 3943·4) compared with patients with mild psoriasis and controls.
Conclusions
As the severity of psoriasis increases, so does the risk of severe and rare infections. Therefore, clinicians should be aware of the increased risk of severe and rare infections in patients with severe psoriasis so that early investigation and treatment can be initiated.
What is already known about this topic?
Few studies have looked at the incidence and prevalence of serious infections (associated with hospitalization) and rare infections including tuberculosis, hepatitis B and C, and HIV among patients with different severities of psoriasis.
What does this study add?
Patients with psoriasis have an increased risk of severe and rare infections.
Clinicians should be aware of the increased risk of severe and rare infections in patients with severe psoriasis so that early investigation and treatment can be initiated.
This Danish nationwide population‐based cohort study revealed an increased incidence of severe and rare infections among patients with severe and mild psoriasis compared with the general population.

Psoriasis is a common chronic, immune‐mediated inflammatory disease that affects 2 to 4% of the population in Western countries. 1 , 2 Psoriasis is characterized by lesional skin exhibiting red plaques with silver or white scales and disease pathogenesis is driven by a network of leucocytes and cytokines. 3 The global burden of psoriasis is large, both in terms of impact on quality of life for patients 4 and healthcare costs. 5 , 6 , 7
Patients with psoriasis are known to be at higher risk for multiple comorbid conditions, including psoriatic arthritis, nonalcoholic steatohepatitis, cardiovascular disease and certain types of cancer. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Previous studies have also found that patients with psoriasis have an increased risk of serious infections (associated with hospitalization, pneumonia and herpes zoster). 18 , 19 , 20 A UK population‐based cohort study from 2020 describes the risk of hospitalization and death resulting from infection in people with psoriasis and found associations with small increases in risk for any infection, respiratory infections and soft tissue and skin infections. 21 Additionally, patients with psoriasis are often treated with immunosuppressive therapies, which may lead to or aggravate infections. Thus, the potential association between psoriasis and risk of incidence of infections is important. However, few studies have assessed the incidence and risk of severe infections and rare infections, the latter of which, in Denmark, includes tuberculosis (TB), hepatitis B (HBV) and hepatitis C (HCV) and HIV, among patients with psoriasis with different disease severities. 17 , 18 , 19 , 20
There is currently limited real‐world evidence regarding the occurrence and risk of severe and rare infections among patients with psoriasis. Furthermore, certain treatments for psoriasis may aggravate infections. Therefore, the current nationwide population‐based cohort study aimed to investigate Danish nationwide administrative longitudinal registries for descriptive data on the incidence of severe infections and infections considered rare in Denmark among individuals with different psoriasis disease severities and matched individuals from the general population. Additionally, the risk for all patients with mild or severe psoriasis was compared with that of the general population.
Materials and methods
Study design and setting
In this population‐based cohort study, descriptive data were obtained from Danish administrative registries for residents in Denmark and were linked using the Civil Registration System. 22 Collected data were linked using a unique numerical identification assigned to all Danish residents since 1968. 22 National data on drug use in Denmark were extracted from the Danish National Prescription Database. 23 This registry contains complete information, from 1 January 1995 onwards, on all prescriptions dispensed to Danish residents at community pharmacies. Registered drugs are categorized according to the Anatomical Therapeutic Chemical Classification System, a hierarchical classification developed by the World Health Organization for purposes of drug‐use statistics. The Danish National Prescription Database is reported to have a high level of completeness and validity. 24 Medical data from inpatient and outpatient hospital clinics, including medication and treatment procedures (e.g. medication given during hospitalization or given directly from the outpatient clinics, or phototherapy treatments) were extracted from the Danish National Patient Register, 25 which contains nationwide data on hospital admissions since 1977 and outpatient contacts since 1995. In this registry, discharge and contact diagnoses are coded according to the International Classification of Diseases (ICD) 8th revision (ICD‐8) from 1977 to 1993, and according to the ICD 10th revision (ICD‐10) since 1994, noting that ICD‐9 was never utilized.
Participants
All individuals in the entire Danish population aged ≥18 years between 1 January 1997 and 31 December 2018 were identified. To be eligible for inclusion in the study, individuals had to be alive and resident in the source population and have at least 1 day of follow‐up. Individuals with any of the investigated chronic infections prior to inclusion were excluded. As study cases, we identified all individuals with at least one diagnosis of psoriasis (ICD‐10 diagnosis code L40·0 or L40·9) in the Danish National Patient Register during the study period or those who had filled a minimum of two prescriptions of calcipotriol (Anatomical Therapeutic Chemical code D05AX02 or D05AX52). Patients were followed from the date of first recorded psoriasis diagnosis or second calcipotriol prescription during the study period until the first of either death, migration, the occurrence of an endpoint, or 31 December 2018. Each patient with psoriasis was matched for sex and exact date of birth with six individuals from the general population in Denmark, using incidence density sampling. Furthermore, patients were stratified by psoriasis severity according to treatment with systemics, including biologics, for psoriasis (Table S1; see Supporting Information). The category of patients with ‘any’ type of psoriasis included all cases irrespective of severity. Patients with severe psoriasis were patients treated with systemic agents for psoriasis, and patients with mild psoriasis were those who were not treated with any of these agents. Psoriasis severity was included as a time‐dependent variable and patients with severe psoriasis were considered as having severe psoriasis from the prescription date of the first prescribed systemic agent. This was in accordance with the newly proposed categorization of psoriasis severity from the International Psoriasis Council (IPC). 26 The diagnosis of psoriasis has been validated in the Danish National Patient Register, with an overall positive predictive value (PPV) of 97·1% (98·0% in adults and 94·6% in children) based on medical chart reviews. 27 The diagnosis of psoriasis based on prescription of calcipotriol has been validated with a PPV of 83·3% in adults. 28
Study outcomes
The outcomes of the current study were occurrences of severe and/or rare infections. Rare infections included HIV, TB, HBV and HCV. Severe infections were defined as infections requiring assessment at a hospital department (both inpatients and outpatients); the full list of infections is provided in Table S2 (see Supporting Information). The list of infections was based on previous studies in atopic dermatitis and inflammatory bowel diseases (IBDs). 29 , 30 In a subanalysis, infections were limited to infections leading to hospitalization (inpatients only).
Statistical analysis
Population statistics were obtained and linked by Statistics Denmark, a governmental institution that collects and maintains data. Descriptive data were tabulated for patients with psoriasis and the matched general population. Incidence rates (IRs) are reported per 100 000 person‐years and presented with 95% confidence intervals (CIs). Summary statistics were generated and expressed as mean for normally distributed variables, median and interquartile range (IQR) for nonnormally distributed continuous variables, and frequency for categorical variables. For age, both mean (SD) and median (IQRs) were presented, to give a more detailed impression of the subgroup distribution. Analyses were carried out for all patients with psoriasis, stratified by severity (mild and severe), and in the general population. Cox proportional hazards regression models with calendar time as the underlying timescale were used to estimate hazard ratios (HRs) for the association between psoriasis and the infections. In the adjusted model, the adjusted HRs (aHRs) were adjusted for sex, age, ethnicity, socioeconomic status, alcohol‐related conditions and Charlson Comorbidity Index. Cox proportional hazard regressions were used to compare all severities of psoriasis with the matched general population. Subanalyses were conducted according to psoriasis severity, comparing those with mild or severe psoriasis with the matched general population.
Results
Participants
A total of 94 450 patients with psoriasis and 566 700 general population reference individuals matched for age and sex were included in this study. General study cohort characteristics are shown in Table 1. The median age was 52·3 years (IQR 38·3 to 64·1), and 50·2% of the study cohort were female. Several comorbidities were more prevalent in patients with psoriasis compared with the general population (Table 1).
Table 1.
Characteristics of study cohort
| Reference (N = 566 700) | Psoriasis (N = 94 450) | |
|---|---|---|
| Female sex | 284 340 (50·2) | 47 390 (50·2) |
| Age, years, median (IQR) | 52·3 (38·3–64·1) | 52·3 (38·3–64·1) |
| Mean (SD) | 51·5 (17·0) | 51·5 (17·0) |
| Mild psoriasis | – | 85 389 (90·4%) |
| Severe infections | 118 059 (20·8) | 24 742 (26·2) |
| Rare infections | 1761 (0·3) | 396 (0·4) |
| Socioeconomic status | ||
| Lowest | 114 515 (20·2) | 17 716 (18·8) |
| Below average | 113 138 (20·0) | 19 091 (20·2) |
| Average | 113 164 (20·0) | 19067 (20·2) |
| Above average | 113 321 (20·0) | 18 908 (20·0) |
| Highest | 112 562 (19·9) | 19 668 (20·8) |
| Alcohol‐related conditions | 24 676 (4·4) | 5468 (5·8) |
| Charlson Comorbidity Index | ||
| None | 532 049 (93·9) | 86 120 (91·2) |
| One comorbidity | 24 014 (4·2) | 5482 (5·8) |
| Two comorbidities | 5116 (0·9) | 1317 (1·4) |
| Three or more comorbidities | 5521 (1·0) | 1531 (1·6) |
| Specific comorbidities | ||
| COPD | 12 731 (2·3) | 3111 (3·3) |
| Hypertension | 69 951 (12·3) | 14 622 (15·5) |
| Hyperlipidaemia | 66 008 (11·7) | 13 313 (14·1) |
| Ischaemic heart disease | 30 731 (5·4) | 6603 (7·0) |
| DM2 | 24 655 (4·4) | 5855 (6·2) |
COPD, chronic obstructive pulmonary disease; DM2, type 2 diabetes mellitus; IQR, interquartile range. Data are presented as n (%) unless otherwise stated.
Incidence rates of any infections (severe and rare)
Patients with any type of psoriasis
Among patients with any type of psoriasis, the IR of any infection (severe and rare) per 100 000 person‐years was higher at 3104·9 (95% CI 3066·6 to 3143·7), compared with 2381·1 (95% CI 2367·6 to 2394·6) for the control group (Table 2). The crude HR for patients with psoriasis and the age‐ and sex‐matched general population (control group) was 1·31 (95% CI 1·29 to 1·33) and the HR adjusted for sex, age, ethnicity, socioeconomic status, alcohol‐related conditions and Charlson Comorbidity Index (aHR) was 1·29 (95% CI 1·27 to1·31) (Table 3 and Figure 1). When infections were limited to only those infections leading to hospitalization (inpatients only), it resulted in a higher IR of 2005·1 (95% CI 1973·0 to 2037·7) among patients with any psoriasis, compared with 1531·8 (95% CI 1520·6 to 1543·0) among the control group (Table 4), and demonstrated similar HRs (Figure 2 and Table S3; see Supporting Information).
Table 2.
Infections among the general population and patients with any psoriasis
| IR per 100 000 person‐years among the general population (95% CI) | IR per 100 000 person‐years among patients with any psoriasis (95% CI) | IR differences per 100 000 person‐years (95% CI) | |
|---|---|---|---|
| Any infections (rare or severe) | 2381·1 (2367·6–2394·6) | 3104·9 (3066·6–3143·7) | 723·8 (699·0–749·1) |
| Severe infections | |||
| Any severe infections | 2364·4 (2350·9–2377·9) | 3080·6 (3042·5–3119·3) | 716·2 (691·6–741·4) |
| CNS infections | 21·29 (20·12–22·55) | 29·65 (26·34–33·38) | 8·36 (6·23–10·84) |
| URTI | 127·20 (124·26–130·21) | 173·68 (165·36–182·42) | 46·48 (41·10–52·21) |
| Pulmonary infections | 772·53 (765·16–779·98) | 984·21 (963·90–1005·01) | 211·7 (198·7–227·0) |
| Heart infections | 34·72 (32·00–36·30) | 45·05 (40·92–49·59) | 10·33 (8·92–13·29) |
| GI infections | 565·04 (558·73–571·42) | 718·21 (700·84–736·01) | 153·2 (142·1–164·6) |
| UTI | 456·33 (450·69–462·03) | 564·98 (549·70–580·67) | 108·7 (99·0–118·6) |
| Gynaecological infections | 44·01 (41·59–46·57) | 53·97 (47·63–61·16) | 9·96 (6·04–14·58) |
| Musculoskeletal infections | 55·94 (53·99–57·94) | 82·96 (77·28–89·05) | 27·02 (23·28–31·11) |
| Skin and subcutaneous tissue infections | 337·18 (332·33–342·09) | 502·94 (488·48–517·82) | 165·8 (156·2–175·7) |
| Opportunistic infections | 86·91 (84·48–89·39) | 115·33 (108·59–122·48) | 28·42 (24·11–33·08) |
| Other infections | 280·60 (276·20–285·06) | 381·85 (369·38–394·74) | 101·3 (93·2–109·7) |
| Sepsis | 240·74 (236·68–244·87) | 335·40 (323·75–347·46) | 94·7 (87·1–102·6) |
| Rare | |||
| Any rare infections | 31·79 (30·34–33·31) | 42·91 (38·89–47·35) | 11·13 (8·55–14·05) |
| HIV | 3·86 (3·37–4·41) | 5·95 (4·57–7·74) | 2·09 (1·19–3·34) |
| Hepatitis (B and C) | 21·23 (20·05–22·48) | 29·89 (26·56–33·63) | 8·65 (6·51–11·15) |
| Tuberculosis | 7·05 (6·38–7·78) | 7·46 (5·89–9·44) | 0·41 (−0·49–1·66) |
CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; IR, incidence rate; URTI, upper respiratory tract infections; UTI, urinary tract infections.
Table 3.
Infections among the general population and patients with any psoriasis
| Patient‐years at risk | Number of events | HR (95% CI) crude | P‐values | HR (95% CI) adjusteda | P‐values | |
|---|---|---|---|---|---|---|
| Any infections (rare or severe) | ||||||
| Reference | 4 987 540 | 118 756 | – | – | – | – |
| Psoriasis | 801 860 | 24 897 | 1·31 (1·29–1·33) | < 0·0001 | 1·29 (1·27–1·31) | < 0·0001 |
| Severe infections | ||||||
| Any severe infections | ||||||
| Reference | 4 993 240 | 118 059 | – | – | – | – |
| Psoriasis | 803 150 | 24 742 | 1·31 (1·29–1·32) | < 0·0001 | 1·29 (1·27–1·31) | < 0·0001 |
| CNS infections | ||||||
| Reference | 5 545 840 | 1181 | – | – | – | – |
| Psoriasis | 924 040 | 274 | 1·39 (1·22–1·59) | < 0·0001 | 1·37 (1·20–1·56) | < 0·0001 |
| URTI | ||||||
| Reference | 5 518 200 | 7019 | – | – | – | – |
| Psoriasis | 917 770 | 1594 | 1·37 (1·30–1·45) | < 0·0001 | 1·36 (1·29–1·43) | < 0·0001 |
| Pulmonary infections | ||||||
| Reference | 5 404 440 | 41 751 | – | – | – | – |
| Psoriasis | 892 900 | 8788 | 1·28 (1·25–1·31) | < 0·0001 | 1·25 (1·22–1·28) | < 0·0001 |
| Heart infections | ||||||
| Reference | 5 542 010 | 1924 | – | – | – | – |
| Psoriasis | 923 450 | 416 | 1·30 (1·17–1·44) | < 0·0001 | 1·26 (1·13–1·40) | < 0·0001 |
| GI infections | ||||||
| Reference | 5 393 260 | 30 474 | – | – | – | – |
| Psoriasis | 892 640 | 6411 | 1·27 (1·24–1·31) | < 0·0001 | 1·25 (1·22–1·29) | < 0·0001 |
| UTI | ||||||
| Reference | 5 452 470 | 24 881 | – | – | – | – |
| Psoriasis | 905 170 | 5114 | 1·24 (1·20–1·28) | < 0·0001 | 1·23 (1·19–1·26) | < 0·0001 |
| Gynaecological infections | ||||||
| Reference | 2 730 940 | 1202 | – | – | – | – |
| Psoriasis | 455 790 | 246 | 1·23 (1·07–1·41) | 0·004 | 1·20 (1·04–1·37) | 0·01 |
| Musculoskeletal infections | ||||||
| Reference | 5 533 190 | 3095 | – | – | – | – |
| Psoriasis | 920 980 | 764 | 1·48 (1·37–1·61) | < 0·0001 | 1·42 (1·31–1·54) | < 0·0001 |
| Skin and subcutaneous infections | ||||||
| Reference | 5 442 850 | 18 352 | – | – | – | – |
| Psoriasis | 897 730 | 4515 | 1·49 (1·44–1·54) | < 0·0001 | 1·45 (1·41–1·50) | < 0·0001 |
| Opportunistic infections | ||||||
| Reference | 5 531 270 | 4807 | – | – | – | – |
| Psoriasis | 920 870 | 1062 | 1·33 (1·24–1·42) | < 0·0001 | 1·30 (1·21–1·38) | < 0·0001 |
| Other infections | ||||||
| Reference | 5 498 270 | 15 428 | – | – | – | – |
| Psoriasis | 912 930 | 3486 | 1·37 (1·32–1·42) | < 0·0001 | 1·34 (1·29–1·39) | < 0·0001 |
| Sepsis | ||||||
| Reference | 5 519 210 | 13 287 | – | – | – | – |
| Psoriasis | 917 420 | 3077 | 1·39 (1·34–1·45) | < 0·0001 | 1·35 (1·29–1·40) | < 0·0001 |
| Rare | ||||||
| Any rare infections | ||||||
| Reference | 5 540 140 | 1761 | – | – | – | – |
| Psoriasis | 922 800 | 396 | 1·35 (1·21–1·51) | < 0·0001 | 1·34 (1·20–1·50) | < 0·0001 |
| HIV | ||||||
| Reference | 5 549 580 | 214 | – | – | – | – |
| Psoriasis | 924 980 | 55 | 1·54 (1·14–2·07) | 0·0044 | 1·58 (1·17–2·13) | 0·0025 |
| Hepatitis | ||||||
| Reference | 5 543 860 | 1177 | – | – | – | – |
| Psoriasis | 923 550 | 276 | 1·41 (1·23–1·60) | < 0·0001 | 1·39 (1·22–1·59) | < 0·0001 |
| Tuberculosis | ||||||
| Reference | 5 549 360 | 391 | – | – | – | – |
| Psoriasis | 925 030 | 69 | 1·06 (0·82–1·37) | 0·66 | 1·05 (0·81–1·36) | 0·72 |
CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; HR, hazard ratio; URTI, upper respiratory tract infections; UTI, urinary tract infections. aAdjusted for sex, age, ethnicity, socioeconomic status, alcohol‐related conditions and Charlson Comorbidity Index.
Figure 1.
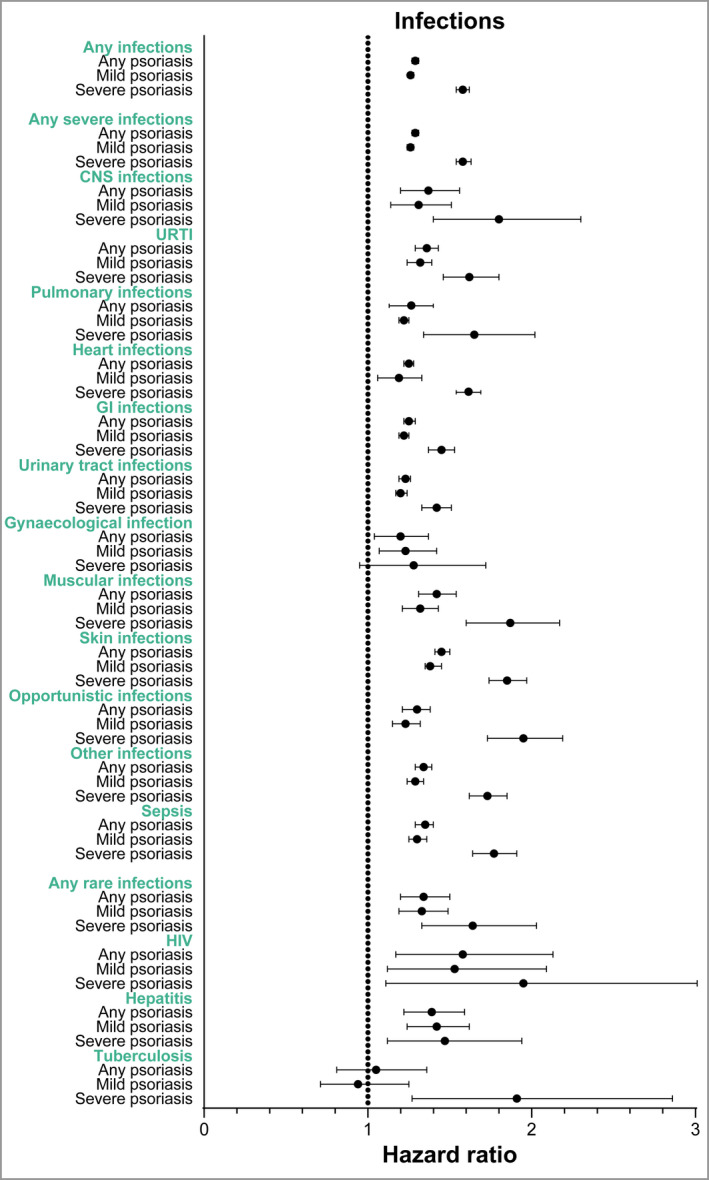
Hazard ratios (adjusted for sex, age, ethnicity, socioeconomic status, alcohol‐related conditions and Charlson Comorbidity Index) for infections in patients with any psoriasis, patients with mild psoriasis and patients with severe psoriasis compared with the general population. CNS, central nervous system; GI, gastrointestinal; URTI, upper respiratory tract infections. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 4.
Infections leading to hospitalization (inpatients only) among the general population and patients with any psoriasis
| IR per 100 000 person‐years among the general population (95% CI) | IR per 100 000 person‐years among patients with any psoriasis (95% CI) | IR differences per 100 000 person‐years (95% CI) | |
|---|---|---|---|
| Any infections (rare or severe) | 1531·8 (1520·6–1543·0) | 2005·1 (1973·0–2037·7) | 473·3 (452·4–494·7) |
| Serious infections | |||
| Any severe infections | 1529·7 (1518·5–1540·9) | 2001·8 (1969·7–2034·3) | 472·1 (451·2–493·4) |
| CNS infections | 17·32 (16·25–18·45) | 22·95 (20·06–26·26) | 5·64 (3·81–7·81) |
| URTI | 27·01 (25·67–28·42) | 35·99 (32·29–40·11) | 8·98 (6·63–11·69) |
| Pulmonary infections | 620·59 (613·94–627·31) | 780·45 (762·20–799·15) | 159·9 (148·3–171·8) |
| Heart infections | 29·04 (27·66–30·50) | 36·62 (32·92–40·74) | 7·58 (5·26–10·24) |
| GI infections | 354·08 (349·05–359·18) | 450·42 (436·57–464·72) | 96·34 (87·52–105·5) |
| UTI | 297·49 (292·91–302·13) | 379·82 (367·25–392·83) | 82·33 (74·34–90·70) |
| Gynaecological infections | 23·13 (21·39–25·01) | 27·03 (22·65–32·26) | 3·90 (1·26–7·25) |
| Musculoskeletal infections | 27·58 (26·23–29·00) | 43·11 (39·06–47·57) | 15·53 (12·84–18·57) |
| Skin and subcutaneous tissue infections | 107·76 (105·01–110·57) | 170·76 (162·34–179·63) | 63·00 (57·33–69·06) |
| Opportunistic infections | 46·34 (44·58–48·17) | 63·25 (58·30–68·61) | 16·91 (13·73–20·44) |
| Other infections | 140·97 (137·85–144·16) | 193·81 (184·92–203·13) | 52·84 (47·07–58·97) |
| Sepsis | 218·98 (215·11–222·93) | 302·6 (291·53–314·08) | 83·62 (76·42–91·15) |
| Rare | |||
| Any rare infections | 6·47 (5·83–7·18) | 8·47 (6·78–10·57) | 2·00 (0·95–3·39) |
| HIV | 0·85 (0·64–1·13) | 0·97 (0·51–1·87) | 0·13 (−0·13–0·74) |
| Hepatitis | 2·74 (2·34–3·22) | 4·34 (3·18–5·91) | 1·59 (0·84–2·69) |
| Tuberculosis | 3·01 (2·59–3·50) | 3·35 (2·36–4·77) | 0·34 (−0·23–1·26) |
CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; IR, incidence rate; URTI, upper respiratory tract infections; UTI, urinary tract infections.
Figure 2.
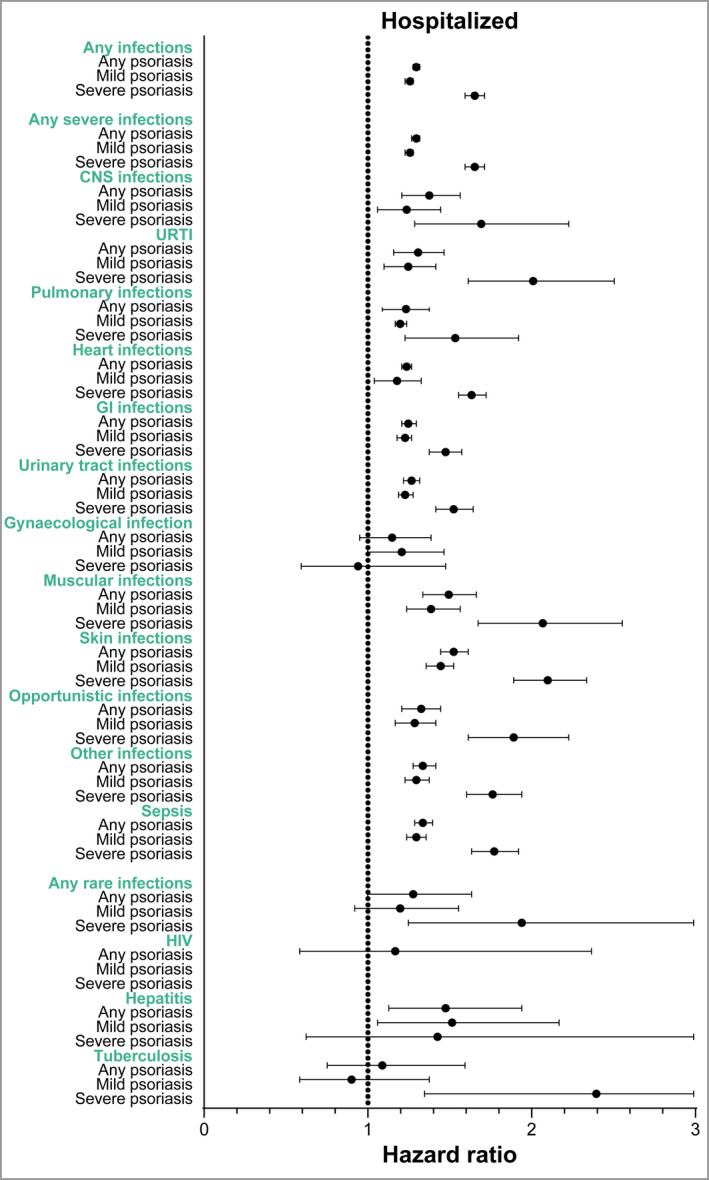
Hazard ratios (adjusted for sex, age, ethnicity, socioeconomic status, alcohol‐related conditions and Charlson Comorbidity Index) for infections leading to hospitalization (inpatients only) in patients with any psoriasis, patients with mild psoriasis and patients with severe psoriasis compared with the general population. CNS, central nervous system; GI, gastrointestinal; URTI, upper respiratory tract infections. [Colour figure can be viewed at wileyonlinelibrary.com]
Patients with mild psoriasis
The IR of any infections among patients with mild psoriasis (IR 3003·5, 95% CI 2964·1 to 3043·4) was higher than in the control group (Table S4), and the aHR for patients with mild psoriasis compared with the matched general population was 1·26 (95% CI 1·25 to 1·28) (Figure 1 and Table S5; see Supporting Information). When infections were limited to only those infections leading to hospitalization, it resulted in an IR of 1925·7 (95% CI 1892·8 to 1959·1) among patients with mild psoriasis, with similar HRs for infections overall (Table S6; see Supporting Information).
Patients with severe psoriasis
The IR among patients with severe psoriasis (IR 3847·7, 95% CI 3754·3 to 3943·4) was higher than that found in the control group (Table 5); and the aHR for patients with severe psoriasis compared with the matched general population was 1·58 (95% CI 1·54–1·62) (Figure 1 and Table S7; see Supporting Information). When infections were limited to only those infections leading to hospitalization, it resulted in an IR of 2535·2 (95% CI 2456·2 to 2616·8) among patients with severe psoriasis (Table S8; see Supporting Information), with similar HRs for infections overall (Figure 2 and Table S9; see Supporting Information).
Table 5.
Infections among the general population and patients with severe psoriasis
| IR per 100 000 person‐years among the general population (95% CI) | IR per 100 000 person‐years among patients with severe psoriasis (95% CI) | IR differences per 100 000 person‐years (95% CI) | |
|---|---|---|---|
| Any infections (rare or severe) | 2351·9 (2322·9–2381·2) | 3847·7 (3754·3–3943·4) | 1495·8 (1431·4–1562·2) |
| Severe infections | |||
| Any severe infections | 2335·0 (2306·1–2364·1) | 3824·6 (3731·6–3919·9) | 1489·6 (1425·5–1555·8) |
| CNS infections | 22·03 (19·53–24·84) | 40·32 (32·47–50·06) | 18·29 (12·94–25·22) |
| URTI | 137·40 (130·92–144·21) | 226·99 (207·11–248·79) | 89·59 (76·19–104·6) |
| Pulmonary infections | 732·82 (717·46–748·50) | 1248·2 (1199·32–1299·0) | 515·3 (481·9–550·5) |
| Heart infections | 34·44 (31·28–37·92) | 59·56 (49·84–71·18) | 25·12 (18·56–33·26) |
| GI infections | 581·99 (568·30–569·01) | 872·69 (832·10–915·25) | 290·7 (263·8–346·24) |
| UTI | 445·53 (433·65–457·73) | 663·59 (628·63–700·48) | 218·1 (194·9–242·8) |
| Gynaecological infections | 40·33 (35·52–45·79) | 54·174 (41·49–70·73) | 13·84 (5·98–24·94) |
| Musculoskeletal infections | 55·23 (51·19–59·60) | 110·88 (97·27–126·39) | 55·64 (46·08–66·78) |
| Skin and subcutaneous tissue infections | 342·69 (332·28–353·44) | 671·88 (636·41–709·32) | 329·19 (304·13–355·88) |
| Opportunistic infections | 88·37 (83·20–93·85) | 181·09 (163·46–200·63) | 92·72 (80·25–106·8) |
| Other infections | 298·21 (288·56–308·18) | 542·26 (510·90–575·56) | 244·1 (222·3–267·4) |
| Sepsis | 235·25 (226·72–244·1) | 450·33 (421·94–480·63) | 215·1 (195·2–236·5) |
| Rare | |||
| Any rare infections | 31·45 (28·44–34·79) | 55·69 (46·31–66·97) | 24·24 (17·87–32·18) |
| HIV | 4·15 (3·15–5·48) | 7·86 (4·81–12·82) | 3·70 (1·67–7·34) |
| Hepatitis | 20·04 (17·66–22·73) | 32·48 (25·52–41·34) | 12·44 (7·86–18·61) |
| Tuberculosis | 7·56 (6·15–9·28) | 15·72 (11·11–22·22) | 8·16 (4·96–12·94) |
CI, confidence interval; CNS, central nervous system; GI, gastrointestinal; IR, incidence rate; URTI, upper respiratory tract infections; UTI, urinary tract infections.
Incidence rates of any severe infections
Patients with any psoriasis
Among patients with any psoriasis, the IR of any severe infections was higher at 3080·6 (95% CI 3042·5 to 3119·3), compared with 2364·4 (95% CI 2350·9 to 2377·9) for the control group (Table 2). Pulmonary, gastrointestinal and urinary tract infections showed the highest IRs (Table 2). The HR for any severe infections for patients with psoriasis compared with the control group was 1·31 (95% CI 1·29 to 1·32) and the aHR was 1·29 (95% CI 1·27 to 1·31) (Table 3). Psoriasis was associated with the development of all the assessed infections, compared with the control group (Figure 1). The highest observed HR was seen for musculoskeletal, skin and subcutaneous tissue infections (aHR 1·42, 95% CI 1·31 to 1·54 and aHR 1·45, 95% CI 1·41 to 1·50, respectively). The HR for central nervous system infections was 1·39 (95% CI 1·22 to 1·59) and the HR for opportunistic infections was 1·33 (95% CI 1·24 to 1·42). When infections were limited to only those infections leading to hospitalization, it resulted in an IR of 2001·8 (95% CI 1969·7 to 2034·3) among patients with psoriasis, compared with 1529·7 (95% CI 1518·5 to 1540·9) among the control group (Table 4), with similar HRs for all severe infections (Figure 2 and Table S3; see Supporting Information).
Patients with mild psoriasis
The IR among patients with mild psoriasis (IR 2979·1, 95% CI 2939·0 to 3018·8) was higher than the IR in the control group (Table S4), and the HR for patients with mild psoriasis compared with the matched general population was 1·26 (95% CI 1·24 to 1·28) (Table S5). When infections were limited to only those infections leading to hospitalization, it resulted in an IR of 1922·3 (95% CI 1899·5 to 1955·6) among patients with mild psoriasis, with similar HRs for infections overall (Table S6).
Patients with severe psoriasis
The IR of any severe infections among patients with severe psoriasis (IR 3824·6, 95% CI 3731·6 to 3919·9) was higher than that of the control group (Table 5) and the aHR for patients with severe psoriasis compared with the matched general population was 1·58 (95% CI 1·54 to 1·63) (Figure 1 and Table S7). For any severe infections leading to hospitalization (inpatients only) the IR was higher at 2537·7 (95% CI 2458·8 to 2619·2) among patients with severe psoriasis compared with 1460·9 (95% CI 1437·4 to 1484·7) among the control group (Table S8).
Incidence rates of any rare infections
Patients with any psoriasis
Among patients with any psoriasis, the IR of any rare infections was higher at 42·91 (95% CI 38·89 to 47·35), compared with 31·79 (95% CI 30·34 to 33·31) for the control group (Table 2). The IR was 5·95 (95% CI 4·57 to 7·74) for HIV, 7·46 (95% CI 5·89 to 9·44) for TB, and 29·89 (95% CI 26·56 to 33·63) for hepatitis (Table 2). Psoriasis was associated with an increased risk of any rare infections (aHR 1·34, 95% CI 1·20 to 1·50), which was attributed to higher incidences of HIV and hepatitis but not TB (Table 3). For any rare infections leading to hospitalization (inpatients only), the IR was higher at 8·47 (95% CI 6·78 to 10·57) among patients with psoriasis compared with 6·47 (95% CI 5·83 to 7·18) among the control group (Table 4). Only the association for hepatitis remained significant when infections were limited to those leading to hospitalization (Figure 2 and Table S3).
Patients with mild psoriasis
The IR among patients with mild psoriasis (IR 42·30, 95% CI 38·13 to 46·93) was higher than that found in the control group (Table S4); the HR was also higher than that found in the control group (Table S5). Restricting the definition of infections to only those leading to hospitalization resulted in an IR of 7·86 (95% CI 6·17 to 10·00) among patients with mild psoriasis.
Patients with severe psoriasis
The IR of any rare infections among patients with severe psoriasis (IR 55·69, 95% CI 46·31to 66·97) was higher than that of the control group (Table 5) and the aHR for patients with severe psoriasis compared with the matched general population was 1·64 (95% CI 1·33 to 2·03) (Figure 1 and Table S7). In particular, the IR of TB was higher among patients with severe psoriasis (Table S8) and an association between TB and severe psoriasis was observed (Figure 1 and Table S9; see Supporting Information). For any severe infections leading to hospitalization (inpatients only), the IR was 12·84 (95% CI 8·74–18·86) among patients with severe psoriasis (Table S8). The association for TB remained significant for patients with severe psoriasis when the definition of infections was restricted to only those leading to hospitalization (Figure 2 and Table S9).
Discussion
This Danish nationwide population‐based cohort study revealed an increased incidence of severe and rare infections among patients with severe and mild psoriasis compared with the general population. The risk was higher for patients with severe psoriasis compared with those with mild psoriasis. To date, this is the first study to report the IRs of severe infections, rare infections and infections leading to hospitalization for patients with psoriasis in Denmark.
In Denmark, patients with psoriasis initiating biological medications are screened for TB and rare viral infections including HBV, HCV and HIV prior to starting such therapies. 17 , 18 , 19 , 20 The resulting data suggest that among patients with psoriasis overall, there were significantly higher rates of any infection (severe or rare). Patients with severe psoriasis had an increased risk of any rare infections, which was attributed to higher incidences of HIV and hepatitis, but not TB, indicating that susceptibility to infection rates may differ depending on psoriasis disease severity. In this patient group, there were higher rates of infections leading to hospitalization. For any rare infections, the association for hepatitis remained significant when infections were limited to only those leading to hospitalization, which supports a recent systematic review showing that patients with psoriasis have an increased prevalence of HVC. 31 The current subanalysis showed a variability of infection risk based on severity of disease; however, this might be a result of the methodology used to screen patients. Similar to the previous findings, the current subanalysis also reported significantly higher rates of any, severe, and rare infections among patients with severe psoriasis. The IR of TB was higher among patients with severe psoriasis and an association between TB and severe psoriasis was observed, most likely owing to screening of TB prior to initiation of biologics. Also, there were higher rates of infections leading to hospitalization in this patient group. For any rare infections, the association for TB remained significant when infections were limited to only those leading to hospitalization.
Factors that may explain the increased risk of infection include the altered immune environment in patients with psoriasis, which involves a network of leucocytes and proinflammatory cytokines in disease pathogenesis. 2 , 3 Patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biological. Therefore, the increased risk may be a consequence of treatment and not the severity of psoriasis. In a nationwide cohort study of 190 694 patients with IBD in France, the risks of serious and opportunistic infections were higher with immunosuppressive regimens. 29
In contrast, a Dutch study revealed a greater risk for serious infection, independent of treatment, in patients with severe psoriasis. 17 Furthermore, a recently published investigation on 44 239 new users of biologics in France found that the risk of serious infections was higher for new users of adalimumab or infliximab vs. etanercept, whereas the risk of serious infections was not increased for users of secukinumab. 32 Certain treatments for psoriasis may aggravate existing infections and, as population‐based studies are limited and the evidence is conflicting, the risk of rare infections in patients with psoriasis needs to be continually explored. 33 , 34
Major limitations of the study include the absence of data describing confounding factors, such as weight, body mass index and smoking status. This study could potentially have been affected by surveillance bias, as the increased risk of TB in those with severe psoriasis may be due to screening for TB in this population rather than due to psoriasis or psoriasis treatments. Also, psoriasis severity indexes such as the Psoriasis Area and Severity Index, were not used in this study; however, the disease severity was based on prescription information. This is in agreement with the recent proposed severity categorization from the IPC. 26
If clinicians are aware of the increased risk of severe infection in patients with severe psoriasis who are being treated with a systemic agent, the surveillance of these patients could be increased for signs of infection.
Author contributions
Nikolai Dyrberg Loft: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (lead); supervision (equal); writing – review and editing (equal). Lone Skov: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); supervision (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Craig Richardson: Conceptualization (equal); data curation (equal); funding acquisition (equal); project administration (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Vivek Trivedi: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); project administration (equal); software (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Ivette Alarcon: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Alexander Egeberg: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
Supporting information
Table S1 Treatments used to identify patients with severe psoriasis.
Table S2 List of severe infections and their corresponding International Classification of Diseases 10th revision codes.
Table S3 Infections leading to hospitalization (inpatients only) among the general population and patients with any type of psoriasis.
Table S4 Infections among the general population and patients with mild psoriasis.
Table S5 Infections among the general population and patients with mild psoriasis.
Table S6 Infections leading to hospitalization (inpatients only) among the general population and patients with mild psoriasis.
Table S7 Infections among the general population and patients with severe psoriasis.
Table S8 Infections leading to hospitalization (inpatients only) among the general population and patients with severe psoriasis.
Table S9 Infections leading to hospitalization (inpatients only) among the general population and patients with severe psoriasis.
Acknowledgments
All authors collaborated on writing the manuscript (with the assistance of a professional medical writer funded by Novartis) and made the decision to submit the manuscript for publication. The authors thank Hayley Furlong and Linda Hassanali of Novartis Ireland Ltd. for providing medical writing/editorial assistance in accordance with Good Publication Practice guidelines (www.ismpp.org/gpp3).
Funding sources Novartis funded and participated in the study design and interpretation of data, in the writing of the manuscript and in the decision to submit the paper for publication. Novartis was not involved in data collection or analysis and did not have access to the raw data.
Conflicts of interest N.L. has been a paid speaker for Eli Lilly and Janssen Cilag. L.S. has received honoraria as consultant and/or speaker from AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, Bristol Myers Squibb and Sanofi. She has served as an investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novartis, Regeneron and LEO Pharma, and has received research grants from Novartis, Sanofi, Bristol Myers Squibb, Janssen Cilag and LEO Pharma. A.E. has received research funding from Pfizer, Eli Lilly, Novartis, Bristol Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation and the Kongelig Hofbundtmager Aage Bang Foundation, in addition to honoraria as a consultant and/or speaker from AbbVie, Almirall, LEO Pharma, Zuellig Pharma Ltd., Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol Myers Squibb and Janssen Pharmaceuticals. V.T. is an employee of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA and C.R. and I.A. are employees of Novartis Pharma AG, Basel, Switzerland.
Data availability Novartis is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the study, in accordance with applicable laws and regulations.
Ethics statement Approval from an ethics committee is not required for register studies in Denmark.
Plain language summary available online
Data availability statement
Novartis is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymised to respect the privacy of patients who have participated in the study in line with applicable laws and regulations.
References
- 1. Parisi R, Symmons DP, Griffiths CEM et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–85. [DOI] [PubMed] [Google Scholar]
- 2. Smith CH, Barker JN. Psoriasis and its management. BMJ 2006; 333:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson‐Huang LM, Lowes MA, Krueger JG. Putting together the psoriasis puzzle: an update on developing targeted therapies. Dis Model Mech 2012; 5:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geale K, Henriksson M, Schmitt‐Egenolf M. How is disease severity associated with quality of life in psoriasis patients? Evidence from a longitudinal population‐based study in Sweden. Health Qual Life Outcomes 2017; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fowler JF, Duh MS, Rovba L et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol 2008; 59:772–80. [DOI] [PubMed] [Google Scholar]
- 6. Norlin JM, Steen Carlsson K, Persson U et al. Resource use in patients with psoriasis after the introduction of biologics in Sweden. Acta Derm Venereol 2015; 95:156–61. [DOI] [PubMed] [Google Scholar]
- 7. Yu AP, Tang J, Xie J et al. Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin 2009; 25:2429–38. [DOI] [PubMed] [Google Scholar]
- 8. Loft ND, Vaengebjerg S, Skov L. Cancer risk in patients with psoriasis: should we be paying more attention? Expert Review Clin Immunol 2020; 16:479–92. [DOI] [PubMed] [Google Scholar]
- 9. Egeberg A. Psoriasis and comorbidities. Epidemiological studies. Dan Med J 2016; 63:B5201. [PubMed] [Google Scholar]
- 10. Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta‐analysis. JAMA Dermatol 2020; 156:421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radtke MA, Schäfer I, Glaeske G et al. Prevalence and comorbidities in adults with psoriasis compared to atopic eczema. J Eur Acad Dermatol Venereol 2017; 31:151–7. [DOI] [PubMed] [Google Scholar]
- 12. Feldman SR, Hur P, Zhao Y et al. Incidence rates of comorbidities among patients with psoriasis in the United States. Dermatol Online J 2018; 24:13030/qt2m18n6vj. [PubMed] [Google Scholar]
- 13. Feldman SR, Zhao Y, Shi L, Tran MH. Economic and comorbidity burden among patients with moderate‐to‐severe psoriasis. J Manag Care Spec Pharm 2015; 21:874–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population‐based study. Br J Dermatol 2011; 165:1037–43. [DOI] [PubMed] [Google Scholar]
- 15. Richard MA, Barnetche T, Horreau C et al. Psoriasis, cardiovascular events, cancer risk and alcohol use: evidence‐based recommendations based on systematic review and expert opinion. J Eur Acad Dermatol Venereol 2013; 27 (Suppl. 3):2–11. [DOI] [PubMed] [Google Scholar]
- 16. Miele L, Vallone S, Cefalo C et al. Prevalence, characteristics and severity of non‐alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol 2009; 51:778–86. [DOI] [PubMed] [Google Scholar]
- 17. Wakkee M, de Vries E, van den Haak P, Nijsten T. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population‐based cohort. J Am Acad Dermatol 2011; 65:1135–44. [DOI] [PubMed] [Google Scholar]
- 18. Kanada K, Schupp C, Armstrong A. Association between psoriasis and viral infections in the United States: focusing on hepatitis B, hepatitis C and human immunodeficiency virus. J Eur Acad Dermatol Venereol 2013; 27:1312–16. [DOI] [PubMed] [Google Scholar]
- 19. Shalom G, Zisman D, Bitterman H et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500 000 person‐year study. JAMA Dermatol 2015; 151:533–8. [DOI] [PubMed] [Google Scholar]
- 20. Kao L‐T, Lee C‐Z, Liu S‐P et al. Psoriasis and the risk of pneumonia: a population‐based study. PLOS ONE 2014; 9:e116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yiu ZZN, Parisi R, Lunt M et al. Risk of hospitalization and death due to infection in people with psoriasis: a population‐based cohort study using the Clinical Practice Research Datalink. Br J Dermatol 2021; 184:78–86. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29:541–9. [DOI] [PubMed] [Google Scholar]
- 23. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017; 46:798–8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011; 39 (7 Suppl.):38–41. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SAJ, Sandegaard JL et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strober B, Ryan C, van de Kerkhof P et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol 2020; 82:117–22. [DOI] [PubMed] [Google Scholar]
- 27. Loft ND, Andersen CH, Halling‐Overgaard AS et al. Validation of psoriasis diagnoses in the Danish National Patient Register. Acta Dermato Venereol 2019; 99:1037–8. [DOI] [PubMed] [Google Scholar]
- 28. Egeberg A, Andersen YMF. Use of topical calcipotriol for identification of patients with psoriasis in administrative healthcare data‐a validation study. J Eur Dermatol Venereol 2020; 34:e90–1. [DOI] [PubMed] [Google Scholar]
- 29. Kirchgesner J, Lemaitre M, Carrat F et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018; 155: 337–46.e10. [DOI] [PubMed] [Google Scholar]
- 30. Droitcourt C, Vittrup I, Kerbrat S et al. Risk of systemic infections in adults with atopic dermatitis: a nationwide cohort study. J Am Acad Dermatol 2021; 84:290–99. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Cui SN, Duan MY et al. Is there a relationship between psoriasis and hepatitis C? A meta‐analysis and bioinformatics investigation. Virology J 2021; 18:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penso L, Dray‐Spira R, Weill A et al. Association between biologics use and risk of serious infection in patients with psoriasis. JAMA Dermatol 2021; 157:1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garcia‐Doval I, Cohen AD, Cazzaniga S et al. Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti‐tumor necrosis factor agents versus classic therapies: prospective meta‐analysis of Psonet registries. J Am Acad Dermatol 2017; 76:299–308.e16. [DOI] [PubMed] [Google Scholar]
- 34. Kalb RE, Fiorentino DF, Lebwohl MG et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol 2015; 151:961–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Treatments used to identify patients with severe psoriasis.
Table S2 List of severe infections and their corresponding International Classification of Diseases 10th revision codes.
Table S3 Infections leading to hospitalization (inpatients only) among the general population and patients with any type of psoriasis.
Table S4 Infections among the general population and patients with mild psoriasis.
Table S5 Infections among the general population and patients with mild psoriasis.
Table S6 Infections leading to hospitalization (inpatients only) among the general population and patients with mild psoriasis.
Table S7 Infections among the general population and patients with severe psoriasis.
Table S8 Infections leading to hospitalization (inpatients only) among the general population and patients with severe psoriasis.
Table S9 Infections leading to hospitalization (inpatients only) among the general population and patients with severe psoriasis.
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymised to respect the privacy of patients who have participated in the study in line with applicable laws and regulations.


