Abstract
Background and objectives
In patients with chronic kidney disease the risk of developing Tuberculosis is increased, while the presentation is often atypical making the diagnosis more difficult. The aim of this study is to describe the presentation of Tuberculosis in dialysis and kidney transplant patients, including the range of diagnostic approaches and the utility of different sample types.
Design, setting, participants, and measurements
In this retrospective study, case records of dialysis and kidney transplant patients were reviewed, including all those treated for Tuberculosis between January 2009 and December 2020.
Results
Over 12 years, there were 143 cases of Tuberculosis in 141 patients (aged 17–86, 50.4% male). Tuberculosis was most common in Asian patients (64%) and those receiving hemodialysis (82%), particularly during the first year after dialysis initiation (54% of dialysis cases). Non‐pleural/pulmonary disease accounted 40% of cases, and non‐organ‐specific presenting features were prominent including fever, lymphadenopathy, and weight loss. The diagnosis was confirmed microbiologically or histologically in 87 cases (61%), with low sensitivity observed for many types of samples including sputum (18%) and pleural fluid (12%). Higher sensitivity was observed with tissue samples including bronchoscopic lymph node aspiration (75%) and other lymph node sampling (92%). In the 52 cases where drug sensitivities were available, resistance to a first line treatment, most commonly isoniazid, was seen in 12 cases (23%). Furthermore, 1‐ and 5‐year survival from diagnosis were 78% and 61%, respectively. Baseline variables independently associated with poorer survival were age (OR 1.8 per decade, 95% CI 1.4–2.3), weight loss over 10% (OR 1.9, 95% CI 1.0–3.5), and a non‐confirmed diagnosis (OR 1.6, 95% CI 1.2–2.1).
Conclusions
Tuberculosis is common in dialysis and kidney transplant patients, particularly during the first year of dialysis. Short‐term mortality is high, but the diagnostic sensitivity of many types of samples is low, so that diagnosis is difficult, with treatment often initiated without confirmation. These data highlight the importance of judgment and clinical experience with this complex patient group.
Keywords: chronic kidney disease, dialysis, infections, kidney transplant, tuberculosis
INTRODUCTION
Unlike other diseases of antiquity, Tuberculosis (TB) remains a leading cause of infectious morbidity and mortality worldwide. The World Health Organization reports more than one third of the world's population to have been infected by TB, about 10% of whom will in their lifetime develop active disease. 1 Several chronic disease states confer particular susceptibility, including HIV, diabetes, and malnutrition.
Chronic kidney disease is increasing in prevalence in many countries, already affecting around 3% of the UK population, with patients receiving renal replacement therapy making up 0.13%. The link between TB and this latter group has been known since 1974, 2 , 3 with some studies suggesting a 50‐fold increase in risk, and with multiple possible explanations proposed, such as oxidative stress, malnutrition, disordered vitamin D metabolism, and impaired cell‐mediated immune responses. 1 , 2 , 4 In addition, many of the risk factors for TB, such as socioeconomic deprivation, are more common in patients with kidney disease.
The diagnosis of TB is notoriously difficult in this group. The same pathophysiology which confers susceptibility also alters host responses therefore interfering with clinical pattern recognition. Also, the features of TB in this group overlap with other common complications of kidney disease including fluid overload, malnutrition, and dialysis access infection. Though the clinical problem is well known, few studies provide guidance for TB diagnosis in this context. The aim of this study therefore is to use retrospective data to analyze the utility of diagnostic approaches, including the sensitivity of different sample types, to derive some guidance for a rational approach to the diagnosis of TB in dialysis and transplant patients.
METHODS
This was a retrospective study of electronic records at a mixed‐ethnicity renal center in London, UK. Patients with a kidney transplant, receiving dialysis, or pre‐dialysis with advanced kidney disease were eligible, and included if treated for tuberculosis between January 2009 and December 2020. The protocol for this retrospective analysis of healthcare records was reviewed and approved by institutional governance committee, with individual informed consent not required.
Demographic, clinical, and pathological data were collected, as well as patient survival until December 2020. Confirmed TB was defined as a microbiological (Auramine, Polymerase Chain Reaction or TB culture) or histological (granulomata) diagnosis, whereas cases were defined as non‐confirmed TB when the diagnosis was made on clinical suspicion without positive microbiological or histological evidence.
Samples were defined as positive if any test carried out was positive, on any sample of that type (if more than one sample was obtained), during the period of clinical illness. Sensitivity for each sample type was defined as the percentage of patients with a positive sample, out of all patients with this sample type analyzed during the illness, between symptom onset and treatment initiation. Confidence intervals for sensitivity were calculated using Wilson's binomial adjustment. The Kaplan‐Meier method and Cox proportional hazards model were used for analysis of patient survival. Analyses were performed using Excel v16 (Microsoft, Washington) and SPSS v25.0 (IBM, New York, NY).
RESULTS
Over the 12‐year study period, TB treatment was started on 145 occasions in 143 patients. Two of these were excluded since an alternative diagnosis was made during treatment (one post‐transplant lymphoma and one case of vertebral Mycobacterium avium‐intracellulare). Therefore 143 TB diagnoses in 141 patients (aged 17–86, 50.4% male) were included in the study. Characteristics of patients and TB diagnoses are provided in Table 1. TB was most common in Asian patients, who accounted for 64% of cases and least common in White patients (4%), though these groups account for similar proportions of the dialysis and transplant population. A history of prior treatment for active or latent TB was present in 21 cases (14.7%), including the two patients with both primary and recurrent cases during the study period.
TABLE 1.
Characteristics of patients (N = 141) and TB diagnoses (N = 143)
| All TB diagnoses | Confirmed TB only | |
|---|---|---|
| Patients (N) | 141 | 85 |
| Age (years) | 59 (43–72) | 57 (42–71) |
| Gender | ||
| Male | 71 (50.4%) | 42 (49.4) |
| Ethnicity | ||
| Black | 33 (23.4) | 24 (28.2) |
| White | 5 (3.5) | 2 (2.4) |
| Asian/other | 95 (67.4) | 59 (69.4) |
| Diagnoses (N) | 143 | 87 |
| Renal replacement | ||
| Hemodialysis | 118 (82.5) | 64 (73.6) |
| Peritoneal dialysis | 4 (2.8) | 4 (4.6) |
| Transplant | 16 (11.2) | 15 (17.2) |
| None | 5 (3.5) | 4 (4.6) |
| Treatment time (years) | ||
| Dialysis | 0.9 (0.2–2.7) | 0.6 (0.2–1.8) |
| Transplant | 4.3 (1.0–7.8) | 5.6 (0.9–7.9) |
| Prior treatment | ||
| Transplantation | 6 (4.2) | 5 (5.7) |
| Other immunosuppression | 12 (8.4) | 5 (5.7) |
| Tuberculosis | 21 (14.7) | 14 (16.1) |
| Clinical features | ||
| Fever | 83 (58.0) | 52 (62.5) |
| Weight loss >10% | 19 (29.2) | 14 (35.0) |
| Lymphadenopathy | 124 (86.7) | 77 (88.5) |
| Predominant organ involvement | ||
| Pulmonary/pleural | 86 (60.1) | 44 (50.6) |
| Superficial lymph node | 28 (19.6) | 23 (26.4) |
| GI/peritoneal | 16 (11.2) | 10 (11.5) |
| Genitourinary | 5 (3.5) | 4 (4.6) |
| Skeletal | 4 (2.8) | 4 (4.6) |
| Cutaneous | 2 (1.4) | 2 (2.3) |
| Neurological | 2 (1.4) | 0 (0.0) |
Note: Numbers are N (%) or median (IQR).
Abbreviation: GI, gastro‐intestinal.
Pulmonary and/or pleural was most common site of organ involvement, with lymphadenopathy, fever and weight loss being the most frequent non‐organ‐specific presenting features: at least one of these was present in 138 cases (96.5%), and Figure 1 provides the distribution of symptom combinations in all patients for whom 6‐month paired weight data were available (N = 102). Median(IQR) weight loss was 5.2(0.6–10.7)% during the 6 months prior to diagnosis, and sometimes severe with over 10% loss seen in 29%, and over 20% loss seen in 10%. Characteristic features of secondary hemophagocytosis were seen in 17 cases (12%), with bone marrow changes or significant cytopenias. Interferon gamma release assays (IGRA) were moderately helpful, being positive in 54 of the 86 cases in which they were measured (63%). With respect to clinical features, no differences between confirmed and non‐confirmed cases were found.
FIGURE 1.
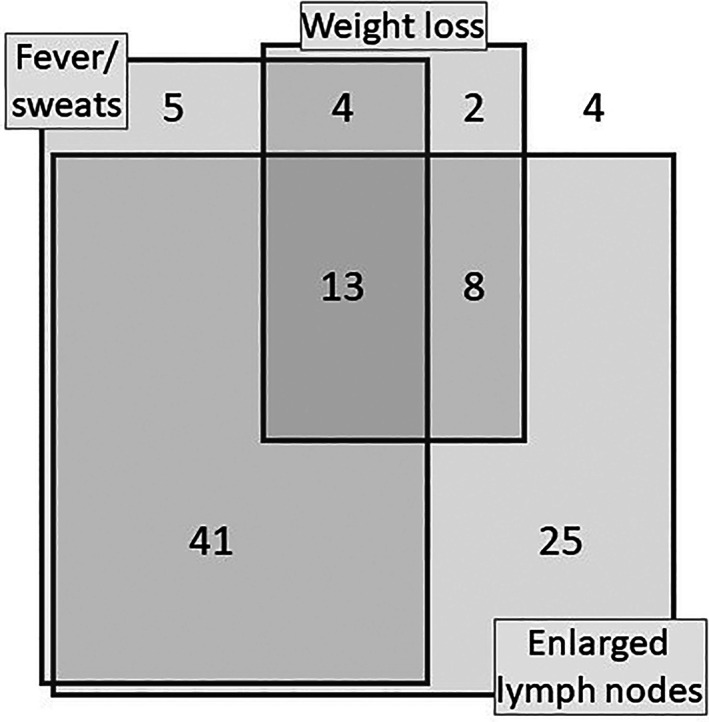
Non‐organ specific features at diagnosis. Number of cases with overlapping features in 102 patients with 6‐month paired weights available. Weight loss: Over 10%
Clinical illness in dialysis patients most often began soon after dialysis initiation, with 66 diagnoses (54%) made during the first year of dialysis. In contrast, illness in transplant patients was more dispersed in time, occurring more than 5 years after transplantation in 50% (Figure 2).
FIGURE 2.
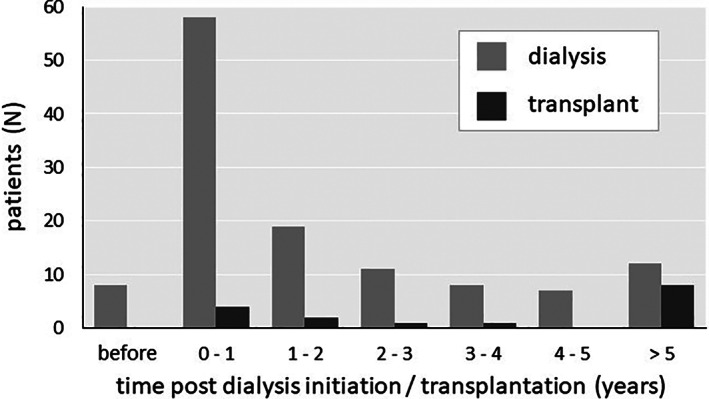
Timing of diagnosis relative to renal replacement therapy. Numbers of cases by time interval since dialysis initiation or transplantation
The incidence of TB remained similar in this population over the study period, though there was a sharp fall in diagnosis rate during the coronavirus epidemic. The diagnosis was confirmed (microbiologically or histologically) in 87 patients (61%), with an increase in invasive diagnostic sampling leading to an increase in the proportion of confirmed rather than non‐confirmed TB over time (Figure 3). Median(IQR) illness duration at the time of diagnosis, in the 128 (90%) cases in which it was known, was 2.0 (1.0–3.5) months, but compared to confirmed cases, tended toward longer durations in those with non‐confirmed TB (2.3 vs. 1.5 months, p = 0.13). Non‐pulmonary location (OR 2.8, 95% CI: 1.3–5.9) and transplant (vs. dialysis) modality were associated with a greater likelihood of TB confirmation.
FIGURE 3.

Diagnostic method trends. Proportion of cases identified by each diagnostic method over the study period. Apart from a reduction in cases identified during the coronavirus epidemic, the incidence of TB over this period remained constant
All diagnostic sampling performed during the clinical illness, prior to diagnosis, was analyzed. Since all such sampling was thought to be clinically indicated and potentially diagnostic, the proportion positive provides an estimate for the diagnostic sensitivity of each sample type. Since some of the non‐confirmed TB cases may have been incorrectly diagnosed, sensitivity may be under‐estimated if all TB diagnoses are included, but would be over‐estimated if restricting the analysis to only confirmed TB. Both analyses of sensitivity for each sample type are therefore provided in Figure 4, which highlights the generally low sensitivity compared to tissue samples of fluid samples, such as pleural fluid, which are not useful therefore in excluding a TB diagnosis when negative. Positive samples are further analyzed for the relative sensitivity of various laboratory techniques in Figure 5. Sensitivity is generally greater with histological than microbiological methods, and with TB culture rather than more rapid microbiological techniques.
FIGURE 4.

Sensitivity of diagnostic sampling. Each row shows a sample type, the number of cases for which this sample type was analyzed, and the diagnostic sensitivity. Left: All TB diagnoses. Right: Confirmed TB only. Other fluid types were: Ascites (12), joint (3), and pericardial (2)
FIGURE 5.

Laboratory tests in positive samples. Each row shows concordance or otherwise between two elements of laboratory analysis, for all samples where both were performed and at least one was positive. Absolute case numbers are provided in boxes. PCR, Polymerase chain reaction
First line treatments for dialysis patients during this period were isoniazid, rifampicin, pyrazinamide, and moxifloxacin. Drug sensitivity testing was available in 52 cases, of which resistance to a first line drug was seen in 12 patients (23%), including single‐agent resistance to isoniazid (10 cases) and rifampicin (one case) as well as multi‐agent resistance to both pyrazinamide and moxifloxacin (one case). Over a mean observation of 48 months (from first TB diagnosis of the study period), there were 63 deaths (45%). In the whole group, 1‐ and 5‐year survival from the time of diagnosis were 78.5% and 60.7%, respectively, but compared to confirmed cases, poorer survival was seen after a non‐confirmed diagnosis of TB (45.1 vs. 72.9% at 5 years, p = 0.002, Figure 6). In a Cox proportional hazards model, baseline variables independently associated with poorer survival were age (OR 1.8 per decade, 95% CI: 1.4–2.3), weight loss over 10% (OR 1.9, 95%CI: 1.0–3.5) and a non‐confirmed diagnosis (OR 1.6, 95% CI: 1.2–2.1, Table 2).
FIGURE 6.

Patient survival after diagnosis. Kaplan–Meier survival function separated by type of diagnosis. Patient numbers still at risk are provided at each timepoint in the panel
TABLE 2.
Predictors of reduced survival after TB diagnosis (N = 141)
| Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Patient | ||||||
| Age | ||||||
| Per decade | 2.02 | 1.63–2.52 | 0.000 | 1.78 | 1.38–2.29 | 0.000 |
| Gender | ||||||
| Female | 0.75 | 0.46–1.23 | 0.259 | 0.979 | ||
| Ethnicity a | ||||||
| Black | 0.48 | 0.26 – 0.87 | 0.017 | 0.288 | ||
| White | 1.74 | 0.78–3.85 | 0.176 | 0.386 | ||
| Modality | ||||||
| Transplant | 0.60 | 0.34–1.07 | 0.086 | 0.389 | ||
| Vintage | ||||||
| Per year | 1.06 | 0.99–1.14 | 0.091 | 0.733 | ||
| Comorbidity | ||||||
| Immunosuppression | 0.66 | 0.28–1.53 | 0.330 | 0.120 | ||
| Diagnosis | ||||||
| Location | ||||||
| Non‐pulmonary | 0.41 | 0.23–0.72 | 0.002 | 0.342 | ||
| Type | ||||||
| Non‐confirmed | 1.49 | 1.15–1.93 | 0.002 | 1.56 | 1.15–2.09 | 0.004 |
| Weight loss | ||||||
| Over 10% | 1.72 | 0.93–3.19 | 0.082 | 1.86 | 0.99–3.50 | 0.056 |
Abbreviation: OR, odds ratio.
Reference category Asian.
p values are provided without odds ratios for variables excluded from the model.
DISCUSSION
Tuberculosis is not uncommon in kidney patients born in endemic areas, with reactivation particularly seen after dialysis initiation. This study highlights the difficulty of accurate diagnosis in this group, with low sensitivity for some types of diagnostic sample, as well as the significant short‐term mortality.
Many studies report a high incidence of TB in kidney patients: compared to the general UK population, unadjusted rate ratios as high as 85 have been reported for dialysis, but ethnicity accounts for a sizable proportion of this risk. In an urban study only 13% of dialysis patients with TB were of White ethnicity, and in most studies country of birth is the strongest predictor of reactivation. 3 The 2020 Public Health England annual report noted that most TB cases were concentrated in major urban areas, with 74% occurring in patients born outside the United Kingdom, most commonly India, Pakistan, Bangladesh, Romania, and Eritrea. 5 A prior history of latent or active TB is established as a risk factor: the Joint Tuberculosis Committee of the British Thoracic Society notes that geographical risk associates with latent TB, and that risk is highest in those residing in United Kingdom for less than 5 years, though in dialysis studies most patients have been in the United Kingdom longer. 6
In this study, clinical illness was temporally associated dialysis initiation, with over half of cases diagnosed during the first dialysis year, similar to the 26/50 patients presenting during the first year reported elsewhere. 7 Although active tuberculosis may occasionally cause or hasten kidney disease (in this study five patients had genitourinary disease, and one had collapsing glomerulopathy associated with pulmonary disease) a more likely explanation is TB reactivation as part of an immune reconstitution syndrome after dialysis initiation. In contrast, transplantation involves prior selection for clinical stability, so that no such temporal trend is seen.
In keeping with published literature, pleuro‐pulmonary was the most common disease location, noted to account for 75% of cases in some studies, though several also highlight extra‐pulmonary manifestations, as well as the non‐specific nature of presentation. 3 , 8 , 9 Features of hemophagocytic lymphohistiocytosis, either in bone marrow samples or peripheral blood, were diagnostically helpful when present. Organ‐specific symptoms, such as cough, are often absent, emphasizing the need for clinical experience and the recognition of constitutional illness rather than organ‐specific features.
Few studies to date contain patient numbers sufficient for an assessment of the diagnostic utility of sample types in dialysis and transplant patients. Here, we observed low availability and low sensitivity for sputum, and generally lower sensitivity for fluid samples rather than tissue or cellular samples. In particular, pleural fluid was positive in only three of 25 cases, giving a sensitivity of 12% (95% CI: 4%–30%). The frequency of transudative effusion in dialysis patients may be an important factor, since weight loss without target weight adjustment is often associated with pleural effusion in dialysis patients. Limited test sensitivity is further highlighted by the frequent discordance between different components of laboratory analysis within the same sample. These difficulties explain the significant number of patients in whom treatment is initiated without confirmation, with the proportion in this study (39%) similar to that reported in kidney patients elsewhere. 7
Beyond diagnostic confidence, microbiological confirmation has the advantage of antibiotic sensitivity testing. There was a much higher frequency of drug resistance compared to the general population in this study, which is similar to the quarter seen in other urban kidney centers. 3 Unrecognized drug resistance resulting in non‐response to treatment is a possible explanation for the increased mortality seen in unconfirmed cases in this study. However, selection bias is also a possible mechanism, since frailer patients are more likely to be thought unsuitable for invasive sampling. The prognostic significance of weight loss at diagnosis adds support for timely treatment initiation, rather than extensive testing, in frailer patients.
Although IGRA tests are not usually advocated for diagnosis, they are often sought when treatment of non‐confirmed TB is considered, and this study allows some, albeit limited, assessment of their utility. The proportion of patients without active TB who have positive IGRA is highly dependent on the population studied, ranging, for example, from 9% in mixed‐ethnicity hospital patients without known TB exposure to 27% in recent migrants from endemic areas. 10 , 11 The median (range) estimated in these studies combined with these data suggest that for the diagnosis of active TB in patients with kidney failure, IGRA tests have a positive likelihood ratio of 3.5 (2.3–7.0) and negative likelihood ratio of 0.45 (0.40–0.50). Several authors have proposed routine IGRA testing for all patients beginning dialysis 12 , 13 , 14 , 15 though this approach is not widespread in clinical practice. 16 The UK PREDICT TB study concluded that either an IGRA or the Tuberculin Skin Test stratified by BCG status would be suitable for assessing risk of disease progression in high‐risk populations. 17 And the IDEA study concluded that second generation IGRA may be sufficiently sensitive to exclude tuberculosis in low‐risk settings. 18
This study has a number of important limitations: as a single‐center study, the population and clinical practice may be specific to this center rather than generalizable. Also, although the sample size is larger than many other studies, some of the diagnostic sampling subgroups are too small for firm conclusions to be drawn. The long period of observation incorporates trends in practice, some of which are apparent, such as the proportion of confirmed cases, but some of which may be unapparent leading to confounding. No control group without TB was included, therefore risk factors, which were not the main focus of study, are only analyzed descriptively.
Reactivation of tuberculosis is not uncommon in patients with kidney failure, especially during the first year of dialysis, and has a strong association with ethnicity. Short‐term mortality is high, but the diagnostic sensitivity of many types of sample is low, so that diagnosis is sometimes difficult, with treatment often initiated without confirmation, despite high rates of drug resistance. These data illustrate the value of microbiological sampling, while highlighting also the importance of judgment and clinical experience when caring for this complex patient group.
CONFLICT OF INTEREST
Authors have no potential conflict to declare.
AUTHOR CONTRIBUTIONS
Damien Ashby conceived the study informed by discussions with Richard Corbett, Rawya Charif, Neill Duncan, and Onn Min Kon. Mahrukh Ali, Dhriti Dosani, Rawya Charif, and Lina Johansson collected data, which were analyzed by Damien Ashby. Mahrukh Ali, Damien Ashby, and Richard Corbett wrote the manuscript, which was reviewed and approved by all authors.
ETHICS STATEMENT
The study was conducted according to the principles of the Declaration of Helsinki, with the protocol being reviewed and approved by institutional governance committee.
Ali M, Dosani D, Corbett R, Johansson L, Charif R, Kon OM, et al. Diagnosis of tuberculosis in dialysis and kidney transplant patients. Hemodialysis International. 2022;26:361–368. 10.1111/hdi.13010
REFERENCES
- 1. Holty J, Sista R. Mycobacterium tuberculosis infection in transplant recipients: early diagnosis and treatment of resistant tuberculosis. Curr Opin Organ Transplant. 2009;14(6):613–8. [DOI] [PubMed] [Google Scholar]
- 2. Romanowski K, Clark E, Levin A, Cook V, Johnston J. Tuberculosis and chronic kidney disease: an emerging global syndemic. Kidney Int. 2016;90(1):34–40. [DOI] [PubMed] [Google Scholar]
- 3. Ostermann M, Palchaudhuri P, Riding A, Begum P, Milburn H. Incidence of tuberculosis is high in chronic kidney disease patients in south East England and drug resistance common. Ren Fail. 2016;38(2):256–61. [DOI] [PubMed] [Google Scholar]
- 4. Vikrant S. Clinical profile of tuberculosis in patients with chronic kidney disease: a report from an endemic country. Saudi J Kidney Dis Transpl. 2019;30(2):470–7. [DOI] [PubMed] [Google Scholar]
- 5. UK Government , 2021. Tuberculosis in England: annual report. Available at https://www.gov.uk/government/publications/tuberculosis‐in‐england‐annual‐report. Accessed 13 June 2021.
- 6. Milburn, H. , Ashman, N. , Davies, P. , Doffman, S. , Drobniewski, F. , Khoo, S. , Ormerod, P. , Ostermann, M. and Snelson, C. , 2021. Guidelines for the prevention and management of mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. [DOI] [PubMed]
- 7. Shah K, Johnson E, Rahman A, McCaffferty K, Kunst H. Active tuberculosis in patients receiving renal replacement therapy for end stage renal disease. Am J Respir Crit Care Med. 2019;199:A5148. [Google Scholar]
- 8. Vikrant S. Tuberculosis in dialysis: clinical spectrum and outcome from an endemic region. Hemodial Int. 2018;23(1):88–92. [DOI] [PubMed] [Google Scholar]
- 9. Ram R, Swarnalatha G, Prasad N, Dakshinamurty KV. Tuberculosis in renal transplant recipients. Transpl Infect Dis. 2007;9(2):97–101. 10.1111/j.1399-3062.2006.00182.x [DOI] [PubMed] [Google Scholar]
- 10. Shah M, Miele K, Choi H, DiPietro D, Martins‐Evora M, Marsiglia V, et al. QuantiFERON‐TB gold in‐tube implementation for latent tuberculosis diagnosis in a public health clinic: a cost‐effectiveness analysis. BMC Infect Dis. 2012;12(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zenner D, Loutet M, Harris R, Wilson S, Ormerod L. Evaluating 17 years of latent tuberculosis infection screening in north‐West England: a retrospective cohort study of reactivation. Eur Respir J. 2017;50(1):1602505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pennington KM, Kennedy CC, Chandra S, Lauzardo M, Brito MO, Griffith DE, et al. Management and diagnosis of tuberculosis in solid organ transplant candidates and recipients: expert survey and updated review. J Clin Tuberc Other Mycobact Dis. 2018;11:37–46. 10.1016/j.jctube.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonkain F, De Clerck D, Dirix V, Singh M, Locht C, Mascart F, et al. Early diagnosis OF miliary tuberculosis in a hemodialysis patient by combining two interferon‐γ‐release assays: a case report. BMC Nephrol. 2020;21(1):214. 10.1186/s12882-020-01875-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shu C, Hsu C, Wei Y, Lee C, Liou H, Wu V, et al. Risk of tuberculosis among patients on dialysis. Medicine. 2016;95(22):e3813. 10.1097/md.0000000000003813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vikrant S, Agarwal S, Gupta S, Bhowmik D, Tiwari S, Dash S, et al. Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7(3–4):99–108. 10.1111/j.1399-3062.2005.00103.x [DOI] [PubMed] [Google Scholar]
- 16. Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, Khoo S, et al. Guidelines for the prevention and management of mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65(6):559–70. [DOI] [PubMed] [Google Scholar]
- 17. Abubakar I, Drobniewski F, Southern J, Sitch A, Jackson C, Lipman M, et al. Prognostic value of interferon‐γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UKPREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18(10):1077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitworth H, Badhan A, Boakye A, Takwoingi Y, Rees‐Roberts M, Partlett C, et al. Clinical utility of existing and second‐generation interferon‐γ release assays for diagnostic evaluation of tuberculosis: an observational cohort study. Lancet Infect Dis. 2019;19(2):193–202. [DOI] [PubMed] [Google Scholar]


