Abstract
Burning candles at home emit small particles and gases that pollute indoor air. Exposure to fine particles in outdoor air has been convincingly linked to cardiovascular and respiratory events, while the associations with fine and ultrafine particles from candle burning remain unexplored. We examined the association between the use of candles and incident cardiovascular and respiratory events. We collected data on 6757 participants of the Copenhagen Aging and Midlife Biobank cohort recruited in 2009 and followed them up for the first hospital contact for incident cardiovascular and respiratory events until 2018. We investigated an association between the self‐reported frequency of candle use in wintertime and cardiovascular and respiratory events, using Cox regression models adjusting for potential confounders. During follow‐up, 1462 and 834 were admitted for cardiovascular and respiratory events, respectively. We found null associations between candle use and a hospital contact due to cardiovascular and respiratory events, with hazard ratios (HRs) and 95% confidence intervals (CI) of 0.97 (95% CI: 0.84, 1.11) and 0.98 (95% CI: 0.81, 1.18), respectively, among those using candles >4 times/week compared with <1 time/week. For cause‐specific cardiovascular diseases, HRs were 1.10 (95% CI: 0.85, 1.43) for ischemic heart disease and 1.18 (95% CI: 0.77, 1.81) for myocardial infarction. For chronic obstructive pulmonary disease, HR was 1.26 (95% CI: 0.81, 1.97). We found no statistically significant associations between candle use and the risk of cardiovascular and respiratory events. Studies with improved exposure assessments are warranted.
Keywords: candle, cardiovascular disease, cohort, environmental exposure, indoor air pollution, respiratory disease
Practical Implications.
Frequent candle users were more physically active and had a higher socioeconomic status than non‐users and those who used candles rarely.
Candle use was not associated with the occurrence of cardiovascular and respiratory events.
Studies with more detailed information on candle use are warranted to investigate the adverse effects of exposure to the candle‐burning particles on cardiovascular and respiratory events.
1. INTRODUCTION
Exposure to outdoor air pollution is a major environmental stressor responsible for 790 000 deaths in Europe, the majority of which are due to ischemic heart disease (40%) and stroke (8%). 1 Long‐term exposure to outdoor particulate matter (PM) with a diameter less than 2.5 μm (PM2.5), black carbon (BC), and nitrogen dioxide (NO2) has been convincingly linked to the increased risk of cardiovascular and respiratory events 2 , 3 , 4 , 5 and mortality. 6 , 7 Likewise, household air pollution due to the combustion of polluting fuels for heating and cooking is responsible for 1.8 million deaths and 61 million disability‐adjusted life years globally. 8 This burden is by far greatest in low‐ and middle‐income countries. However, the association between indoor sources of fine and ultrafine particles (PM with a diameter less than 0.1 μm, UFP) in high‐income countries, such as candle use, and cardiovascular and respiratory events, is much less studied.
In Nordic countries, indoor candle use is an inherent part of the lifestyle, especially in dark winter months. Denmark has some of the highest at‐home candle use, with more than 60% of Danes reporting burning candles more than twice a week in wintertime. 9 Combustion of candles emits toxic substances, including PM2.5, NO2, varieties of volatile organic compounds, and polycyclic aromatic hydrocarbons, which are similar to emissions from traffic and other unclean fuels emissions. 10 , 11 , 12 , 13 , 14 , 15 A large proportion of the PM emitted by candles is ultrafine and accumulation mode particles. 14
Experimental animal studies showed that exposure to candle emissions leads to similar health effects as those found with ambient air pollution and diesel exhaust particles: pulmonary inflammation, 16 impaired endothelial function, 17 shortening of telomere lengths in the lungs and spleen, and progression of atherosclerotic plaques in the aorta. 18 Human exposure studies found that candle emissions caused a transient decrease in lung function 19 and cognitive function, 20 as well as increases in arterial stiffness and high‐frequency heart rate variability. 21 However, epidemiological studies on long‐term at‐home candle use are scarce. A Danish cross‐sectional study found no association between self‐reported use of candles and lung function or diagnosis of asthma or chronic obstructive pulmonary disease (COPD), 22 while another Danish cross‐sectional study found lower lung function and higher HbA1c (prediabetic biomarker) and leukocyte counts associated with UFP counts, mainly attributed to the candle burning in 48‐h indoor home measurements. 23
Here, we aimed to examine the association of candle use with cardiovascular and respiratory events and examine potential effect modification of these associations by lifestyles and pre‐existing health conditions using a cohort study following the participants prospectively over up to 10 years.
2. METHODS
2.1. Study population
The Copenhagen Aging and Midlife Biobank (CAMB) is a cohort and biobank established between 2009–2011. The purpose was twofold: to research age‐associated changes in health and to research the life course (biological, cognitive, physical, and social factors in childhood, adolescence, and early adulthood) on early indicators of aging. 24 CAMB is based on three established cohorts: the Metropolit 1953 Danish Male Birth Cohort (MP), the Copenhagen Perinatal Cohort (CPC), and the Danish Longitudinal Study on Work, Unemployment, and Health (DALWUH). The MP cohort included 11 532 boys born in 1953 in the Copenhagen Metropolitan area, including the counties of Copenhagen, Frederiksberg, Gentofte, and Roskilde. 25 The CPC cohort included all children born at the National University Hospital in Copenhagen between 1959 and 1961 (9125 children). 26 The DALWUH cohort consisted of a random sample of 10% of Danish men and women born in either 1949 or 1959, resulting in 7588 individuals included in the cohort. 27
Among the three existing cohorts from which we pulled data, participants were aged 48–60 years, and a total number of 17 938 people living in the eastern parts of Denmark (Zealand) less than 100 km from the study clinic were invited to participate in the study (7750 from the MP cohort, 5282 from the CPC cohort, and 4906 from the DALWUH cohort). 28 Recruitment to the study included both a self‐administrated questionnaire and an invitation to clinical tests, cognitive tests, and blood samples. The questionnaire consisted of 96 questions on health, major life events, and working and family life. Of the invited, 7189 answered the postal questionnaire between April 20, 2009, and March 2, 2011. 24 All participants were linked to the Danish Civil Registration System (DCRS) 29 and followed up until either date of death, emigration, or disappearance from the DCRS, or the end of the study (December 31, 2018), whichever came first.
The local ethical committee and the Danish Data Protection Agency have approved the CAMB as a database combining three cohorts: approval No. H‐A‐2008‐126 and No. 2013‐41‐1814, respectively.
2.2. Candle Use Definition
The use of candles was assessed with the following question at baseline: “In winter, how often do you have candles lit in the evenings?” (Never, <1 time/week, 1–2 times/week, 3–4 times/week, or >4 times/week) (Table S1). The use of candles was categorized into three levels: never or <1 time/week, 1–4 times/week, and >4 times/week for an assessment of exposure.
2.3. Outcome
We obtained information on the participants' hospital contacts (in‐ and outpatient and emergency room visits) from the Danish National Patient Register that contains information on nationwide historical data when a person has been in contact with Danish hospital service as part of examinations or treatment since 1977. 30 We defined cardiovascular events (the International classification of diseases [ICD]: 40×, 41×, 42×, 43×, 44×, and 45× for the 8th revision [ICD‐8] before 1994 and I00–I99 for the 10th revision [ICD‐10]) and respiratory events (ICD‐8: 46×, 47×, 48×, 49×, 50×, and 51×; ICD‐10: J00–J99) when the participants had the hospital contacts during follow‐up. In addition to all cardiovascular events, we defined specific cardiovascular events that might be linked to exposure to ambient air pollution: ischemic heart disease 31 (ICD‐8: 410–414; ICD‐10: I20–I25), cerebrovascular disease (ICD‐8: 430–438; ICD‐10: I60–I69), 32 myocardial infarction (ICD‐8: 410; ICD‐10: I21), and other cardiovascular diseases (CVD events other than ischemic heart disease, cerebrovascular disease, and myocardial infarction). 5 , 33 , 34 We also examined specific respiratory diseases, including asthma (ICD‐8: 493; ICD‐10: J45–46), COPD (ICD‐8: 490, 491, 492; ICD‐10: J40–J44), pneumonia (ICD‐8: 480–486; ICD‐10: J12–J18), and other respiratory diseases (respiratory disease other than asthma, COPD, and pneumonia). We defined a previous history of cardiovascular or respiratory disease when the first cardiovascular or respiratory admission, respectively, occurred before the enrollment of the study.
2.4. Covariates
To identify the minimal sufficient covariate adjustment set, we used a directed acyclic graph (DAG). We evaluated all possible covariates for associations with both exposure and health outcomes to determine potential confounders: cohort, baseline year, age, sex, smoking status, alcohol consumption, physical activity, body mass index (BMI), income, marital status, previous history of the disease, and ambient PM2.5 and NO2. As there appear to be no published studies on associations between candle use and most covariates, we identified the direct paths between exposure and potential confounders from empirical evidence in the present study. Similarly, cohort and baseline year were also empirically identified as confounders in the present study. A literature review was conducted in PubMed to identify authoritative guidelines or systematic reviews for the associations between health outcomes and potential confounders, as indicated in Table S2. The DAG model identified air pollutants (PM2.5 and NO2) as the ancestors of the outcome; however, we did not consider the air pollutants as confounders because the pollutants were not associated with candle use in the previously published study based on 5199 participants in the present cohort. 35 We included the potential confounders in the final model: cohort, baseline year, age, sex, smoking status, alcohol consumption, physical activity, BMI, income, marital status, and previous history of the disease (Figure S1). Code in the DAGitty (http://www.dagitty.net/) is also available in Text S1.
At baseline, information on age, sex, smoking status, alcohol consumption, physical activity, and BMI was obtained from the questionnaire. The variable on smoking status was derived from the question on smoking and categorized into three groups: current (daily or occasional), former, and never smokers. The variable regarding alcohol consumption was based on the Danish Health Authority's current recommendation at that time of collection on the maximum weekly consumption of any type of alcoholic beverage per week: 21 units for men and 14 units for women. 36 The variable on alcohol consumption was divided into four levels: never (0 unit/week), low risk (<14 units/week for women and <21 for men), elevated risk (more than 14–35 units/week for women and 21–35 for men), and high risk (>35 units/week). Physical activity was defined as the total time spent on physical activity, including sports, exercise, housework, gardening, and walks and cycling trips between home and work, on a weekly basis: ≤2 h/week, 3–6 h/week, and ≥7 h/week. BMI was calculated based on self‐reported weight (kg) and height (cm) and used to define obesity (BMI > 30 kg/m2).
We additionally linked the CAMB participants to multiple national registers 37 by the Danish individual unique identification number to obtain annual disposable household income (amount in Danish Kroner) and marital status (widowed or divorced, married or had a registered partner, and single) at baseline.
2.5. Statistical analysis
Analyses were performed using Cox proportional hazard models, with age as the underlying time scale, and two models were defined a priori. In Model 1, we examined the associations between candle use (<1 [reference], 1–4, and >4 times/week) and cardiovascular events (all, ischemic heart disease, cerebrovascular disease, myocardial infarction, and other cardiovascular) and respiratory events (all, asthma, COPD, pneumonia, and other respiratory) after adjusting for age (time scale), cohort indicator, sex, and calendar year at baseline. In Model 2, we additionally adjusted for a minimum set of covariates that were determined by the DAG: obesity status, smoking status, alcohol consumption, physical activity, marital status, and household income. Estimated associations were expressed as hazard ratios (HRs) with 95% confidence intervals (CI). Cox proportional hazard assumptions for the use of candles (<1, 1–4, and >4 times/week) and covariates (Model 2) were met in a statistical test based on the scaled Schoenfeld residuals. We conducted several sensitivity analyses. First, we estimated HRs of incidence of the outcome of interest after excluding any events of the respective disease before baseline. Second, instead of excluding participants with prevalence, we adjusted for the previous history of the disease in the model. Third, we estimated the Fine and Gray's sub‐distribution HR after considering a competing risk of all‐cause mortality on outcomes (cardiovascular and respiratory events). 38 Fourth, we examined the associations between candle use (never‐ and ever‐candle users) and cardiovascular and respiratory events.
To explore potential effect modifiers of the association between candle use and cardiovascular and respiratory events, we stratified the data and estimated the associations by characteristics known or suspected to modify the effects of ambient air pollution on cardiovascular and respiratory events, including sex, obesity status, alcohol consumption, smoking status, physical activity, hypertension, previous histories of cardiovascular, and respiratory disease. 39 , 40 We used the chi‐squared test to examine the statistically significant difference of the associations by subgroups (e.g., male vs. female) by including an interaction term (e.g., candle use × sex) in the model. Separate models were run for each interaction term after adjusting for the same covariates as the main model. All analyses were conducted using SAS version 9.4 (SAS Institute Inc.) and the R 4.0.3 (R Foundation for Statistical Computing).
3. RESULTS
A total of 6757 participants were analyzed after excluding those with missing information on exposure (n = 35) and covariates, including obesity status, smoking status, alcohol consumption, physical activity, marital status, and household income (n = 397). Of 6757 participants, 31% were female (n = 2097), mean follow‐up for study populations was 8.7 years, and mean age of participants at baseline was 54.4 years (SD: 4.0 years). Among the participants, 40% (n = 2733) lit candles >4 times/week during wintertime (Table 1). Those using candles >4 times/week were more likely to be female, obese, former smokers, more physically active, and to have reported higher household income and higher alcohol consumption, compared with those using candles less frequently (<1 or 1–4 times/week). Those using candles moderately (1–4 times/week) were more likely to have hypertension before baseline, compared with other candle‐use groups (<1 or >4 times/week).
TABLE 1.
Characteristics of the study participants at baseline (n = 6757)
| Variables | All | Candle use | |||
|---|---|---|---|---|---|
| (n = 6757) | <1/week (n = 1418) | 1–4/week (n = 2606) | >4/week (n = 2733) | p‐value a | |
| Follow‐up years, mean (sum) | 8.7 (46 887.9) | 8.7 (9605.7) | 8.7 (18 267.6) | 8.7 (19 014.5) | 0.146 |
| Sex, n (%) | |||||
| Female | 2097 (31.0) | 373 (26.3) | 785 (30.1) | 939 (34.4) | <0.001 |
| Male | 4660 (69.0) | 1045 (73.7) | 1821 (69.9) | 1794 (65.6) | |
| Age (years, mean ± SD) | 54.4 ± 4.0 | 54.7 ± 3.9 | 54.3 ± 4.0 | 54.3 ± 4.0 | 0.002 |
| Obesity, n (%) | |||||
| BMI >30 kg/m2 | 1035 (15.3) | 254 (17.9) | 398 (15.3) | 383 (14.0) | 0.004 |
| BMI ≤30 kg/m2 | 5722 (84.7) | 1164 (82.1) | 2208 (84.7) | 2350 (86.0) | |
| Alcohol consumption, n (%) | |||||
| None | 822 (12.2) | 266 (18.8) | 259 (9.9) | 297 (10.9) | <0.001 |
| Low risk | 3379 (50.0) | 708 (49.9) | 1424 (54.6) | 1247 (45.6) | |
| Elevated risk | 1302 (19.3) | 196 (13.8) | 493 (18.9) | 613 (22.4) | |
| High risk | 1254 (18.6) | 248 (17.5) | 430 (16.5) | 576 (21.1) | |
| Smoking status, n (%) | |||||
| Current | 1754 (26.0) | 396 (27.9) | 1044 (40.1) | 681 (24.9) | 0.001 |
| Former | 2684 (39.7) | 497 (35.1) | 885 (34.0) | 1143 (41.8) | |
| Never | 2319 (34.3) | 525 (37.0) | 396 (27.9) | 909 (33.3) | |
| Physical activity, n (%) | |||||
| ≥7 h+/week | 3292 (48.7) | 671 (47.3) | 1029 (39.5) | 1413 (51.7) | <0.001 |
| 3–6 h/week | 2505 (37.1) | 508 (35.8) | 369 (14.2) | 968 (35.4) | |
| ≤2 h/week | 960 (14.2) | 239 (16.9) | 671 (47.3) | 352 (12.9) | |
| Household income (1000 DKK, mean ± SD) | 319.5 ± 238.4 | 290.8 ± 144.8 | 323.5 ± 268.3 | 330.5 ± 246.0 | <0.001 |
| Marital status, n (%) | |||||
| Widowed/divorced | 1025 (15.2) | 267 (18.8) | 367 (14.1) | 391 (14.3) | <0.001 |
| Married/registered partner | 4840 (71.6) | 818 (57.7) | 1916 (73.5) | 2106 (77.1) | |
| Single | 892 (13.2) | 333 (23.5) | 323 (12.4) | 236 (8.6) | |
| Previous history of hypertension, n (%) | |||||
| Yes | 1785 (26.4) | 399 (28.1) | 638 (24.5) | 748 (27.4) | 0.015 |
| No | 4972 (73.6) | 1019 (71.9) | 1968 (75.5) | 1985 (72.6) | |
| Previous history of cardiovascular disease, n (%) | |||||
| With | 1226 (18.1) | 265 (18.7) | 451 (17.3) | 510 (18.7) | 0.367 |
| Without | 5531 (81.9) | 1153 (81.3) | 2155 (82.7) | 2223 (81.3) | |
| Previous history of respiratory disease, n (%) | |||||
| With | 1088 (16.1) | 233 (16.4) | 411 (15.8) | 444 (16.3) | 0.832 |
| Without | 5669 (83.9) | 1185 (83.6) | 2195 (84.2) | 2289 (83.8) | |
Abbreviations: BMI, body mass index; SD, standard deviation.
p‐value for the difference in continuous or categorical variables by candle use.
Among the participants, 1462 and 834 suffered a new cardiovascular and respiratory event, respectively, during follow‐up, and of those, 512 and 243 had a previous history of cardiovascular and respiratory disease, respectively, before baseline. Those who had either of these events were more likely to be older, more overweight, and current smokers, and have less household income than those who did not suffer a cardiovascular or respiratory event during the follow‐up (Table 2).
TABLE 2.
Characteristics of the study participants who contacted hospitals due to a cardiovascular or respiratory event during follow‐up (n = 6757)
| Variables | Participants with cardiovascular event | Participants with respiratory event | ||||
|---|---|---|---|---|---|---|
| Yes (n = 1462) | No (n = 5295) | p‐value a | Yes (n = 834) | No (n = 5923) | p‐value a | |
| Sex, n (%) | ||||||
| Female | 319 (21.8) | 1778 (33.6) | <0.001 | 240 (28.8) | 1857 (31.4) | 0.132 |
| Male | 1143 (78.2) | 3517 (66.4) | 594 (71.2) | 4066 (68.7) | ||
| Age at baseline (years, mean ± SD) | 55.4 ± 3.7 | 54.1 ± 4.0 | <0.001 | 54.7 ± 4.0 | 54.3 ± 4.0 | 0.008 |
| Obesity, n (%) | ||||||
| BMI >30 kg/m2 | 288 (19.7) | 747 (14.1) | <0.001 | 163 (19.5) | 872 (14.7) | <0.001 |
| BMI ≤30 kg/m2 | 1174 (80.3) | 4548 (85.9) | 671 (80.5) | 5051 (85.3) | ||
| Alcohol consumption, n (%) | ||||||
| None | 191 (13.1) | 631 (11.9) | 0.351 | 147 (17.6) | 675 (11.4) | <0.001 |
| Low risk | 735 (50.3) | 2644 (49.9) | 358 (42.9) | 3021 (51.0) | ||
| Elevated risk | 261 (17.9) | 1041 (19.7) | 151 (18.1) | 1151 (19.4) | ||
| High risk | 275 (18.8) | 979 (18.5) | 178 (21.3) | 1076 (18.2) | ||
| Smoking status, n (%) | ||||||
| Current | 452 (30.9) | 1302 (24.6) | <0.001 | 278 (33.3) | 1476 (24.9) | <0.001 |
| Former | 578 (39.5) | 2106 (39.8) | 335 (40.2) | 2349 (39.7) | ||
| Never | 432 (29.6) | 1887 (35.6) | 221 (26.5) | 2098 (35.4) | ||
| Physical activity, n (%) | ||||||
| ≥7 h+/week | 722 (49.4) | 2570 (48.5) | 0.060 | 377 (45.2) | 2915 (49.2) | 0.013 |
| 3–6 h/week | 510 (34.9) | 1995 (37.7) | 313 (37.5) | 2192 (37.0) | ||
| ≤2 h/week | 230 (15.7) | 730 (13.8) | 144 (17.3) | 816 (13.8) | ||
| Household income (1000 DKK, mean ± SD) | 308.4 ± 220.5 | 322.5 ± 243.1 | 0.045 | 302.4 ± 248.8 | 321.9 ± 236.9 | 0.027 |
| Marital status, n (%) | ||||||
| Widowed/divorced | 229 (15.7) | 796 (15.0) | 0.835 | 148 (17.8) | 877 (14.8) | 0.057 |
| Married/registered partner | 1042 (71.3) | 3798 (71.7) | 571 (68.5) | 4269 (72.1) | ||
| Single | 191 (13.1) | 701 (13.2) | 115 (13.8) | 777 (13.1) | ||
| Previous history of hypertension, n (%) | ||||||
| Yes | 579 (39.6) | 1206 (22.8) | <0.001 | 277 (33.2) | 1508 (25.5) | <0.001 |
| No | 883 (60.4) | 4089 (77.2) | 557 (66.8) | 4415 (74.5) | ||
| Previous history of cardiovascular disease, n (%) | ||||||
| With | 512 (35.0) | 714 (13.5) | <0.001 | 207 (24.8) | 1019 (17.2) | <0.001 |
| Without | 950 (65.0) | 4581 (86.5) | 627 (75.2) | 4904 (82.8) | ||
| Previous history of respiratory disease, n (%) | ||||||
| With | 269 (18.4) | 819 (15.5) | 0.007 | 243 (29.1) | 845 (14.3) | <0.001 |
| Without | 1193 (81.6) | 4476 (84.5) | 591 (70.9) | 5078 (85.7) | ||
Abbreviations: BMI, body mass index; SD, standard deviation.
p‐value for the difference in continuous or categorical variables by cardiovascular and respiratory events.
The HRs of all cardiovascular events for those using candles 1–4 and >4 times/week were 0.91 (95% CI: 0.80, 1.05) and 0.97 (95% CI: 0.84, 1.11), respectively, compared with those using candles <1 time/week in the adjusted model (Table 3). The HRs of cause‐specific cardiovascular events also showed null associations. For example, the risk of ischemic heart disease was not significantly different by candle use: HR: 1.10 (95% CI: 0.85, 1.43) for those using candles >4 times/week compared with those using candles <1 time/week. The HR of myocardial infarction was 1.18 (95% CI: 0.77, 1.81) for those with candle use at >4 times/week compared with those using candles rarely. For all respiratory events, we found HRs close to 1 related to the use of candles. We also observed non‐significant associations between candle use and COPD, asthma, and pneumonia incidence (HR: 1.26 [95% CI: 0.81, 1.97], 0.85 [95% CI: 0.52, 1.37], and 0.83 [95% CI: 0.62–1.12], respectively) for those using candles >4 times/week compared with those <1 time/week (Table 3). The associations remained null when we excluded participants with a previous history of the respective disease or adjusted for the previous history in the model (Tables S3 and S4). The Fine and Gray's sub‐division HRs, considering the competing risk of all‐cause mortality on cause‐specific outcomes, were similar to the main results (Table S5). When we compared the risk of cardiovascular or respiratory events among never‐ and ever‐candle users, we observed no significant differences (Table S6).
TABLE 3.
Hazard ratios of hospital contacts due to cardiovascular and respiratory events associated with candle use (reference: <1/week) (n = 6757)
| Events (n) | Hazard ratio (95% confidence intervals) | ||
|---|---|---|---|
| <1/week | 1–4/week | >4/week | |
| All cardiovascular disease (1462) | 1 | 0.91 (0.80, 1.05) | 0.97 (0.84, 1.11) |
| Ischemic heart disease (433) | 1 | 1.03 (0.80, 1.33) | 1.10 (0.85, 1.43) |
| Cerebrovascular disease (190) | 1 | 0.99 (0.68, 1.44) | 1.04 (0.71, 1.52) |
| Myocardial infarction (153) | 1 | 0.85 (0.55, 1.31) | 1.18 (0.77, 1.81) |
| Other cardiovascular diseases (854) | 1 | 0.85 (0.71, 1.02) | 0.90 (0.75, 1.07) |
| All respiratory disease (834) | 1 | 0.96 (0.80, 1.16) | 0.98 (0.81, 1.18) |
| Asthma (115) | 1 | 0.85 (0.52, 1.37) | 0.85 (0.52, 1.37) |
| Chronic obstructive pulmonary disease (136) | 1 | 0.84 (0.52, 1.36) | 1.26 (0.81, 1.97) |
| Pneumonia (328) | 1 | 0.94 (0.71, 1.24) | 0.83 (0.62, 1.12) |
| Other respiratory diseases (329) | 1 | 1.06 (0.78, 1.44) | 1.08 (0.79, 1.47) |
Note: Model adjusted for age (time scale), sex, baseline year, cohort indicator, marital status, household income, obesity status, alcohol consumption, smoking status, and physical activity.
Stratified analyses showed minor differences with respect to HRs, although none of these modifiers or any of the interaction terms were statistically significant (Figure 1; Table S7). The previous history of cardiovascular and respiratory disease did not modify the associations between candle use and incident cardiovascular and respiratory events.
FIGURE 1.
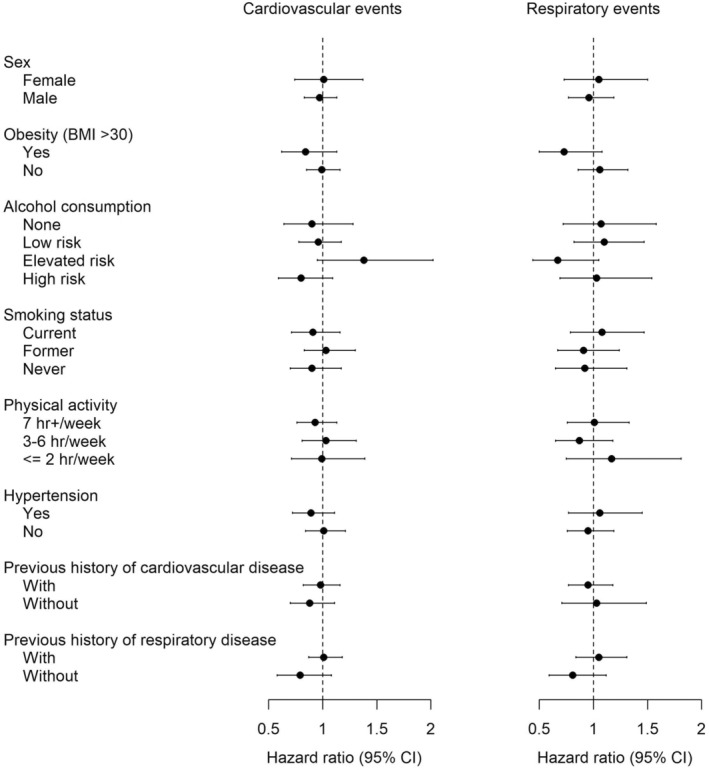
Associations between candle use (>4/week vs. <1/week) and cardiovascular and respiratory events by potential effect modifiers. Model adjusted for age (time scale), sex, baseline year, cohort indicator, marital status, household income, obesity status, alcohol consumption, smoking status, and physical activity.
4. DISCUSSION
Based on a Danish cohort of 6757 participants, we found that candle use was not associated with the occurrence of cardiovascular and respiratory events. We also observed that frequent candle users were more physically active and had a higher socioeconomic status (SES) than non‐users and those who used candles rarely.
The exact level of exposure to PM2.5 derived from candle emissions cannot be estimated from our data. However, we can roughly estimate the level based on a study, which predicted the contribution from candle use to total personal exposure to PM2.5 and black smoke based on data on 48 h of monitoring during each of the four seasons in Copenhagen, Denmark. Extrapolating regression estimates from that study would indicate that candle use for 1 h per day would be equivalent to 5.8 and 4.0 μg/m3 personal PM2.5 exposure from candle emission in the cold and warm parts of the year, respectively. Thus, candle use more than 4 times per week could easily contribute more than 5.0 μg/m3 PM2.5 as average exposure. One could argue that long‐term exposure to candle use emitting non‐trivial concentrations of PM2.5 may have similar adverse effects as ambient PM2.5 on cardiovascular and respiratory events. A recent meta‐analysis found an overall HR of 1.19 (95% CI: 1.09, 1.30) for ischemic heart disease and 1.12 (95% CI: 1.05, 1.19) for stroke per each 10 μg/m3 increase in long‐term exposure to PM2.5. 41 However, our study found non‐significant HRs of 0.97, 1.09, and 1.16 for overall cardiovascular disease, ischemic heart disease, and myocardial infarction, respectively, comparing candle use of >4/week to <1 time/week.
Biological effects which could mechanistically lead to ischemic heart disease and myocardial infarction related to burning candles were found in previous animal and human experimental studies. 16 , 17 , 18 , 42 Soppa et al. 42 suggested that short‐term exposure to candle flames may increase arterial stiffness, attributable to the UFPs from using candles at home. Long‐term exposure to UFPs in outdoor air was associated with cardiovascular disease risk. 43 , 44 Given the evidence from the previous studies, it is plausible that exposure to UFPs from a candle burning in indoor air may also contribute to the development of cardiovascular disease. However, we did not observe any significant associations between overall and cause‐specific cardiovascular hospital contacts and candle use in this study population.
We found no evidence of any particular subgroup being susceptible to candle emissions with respect to the risk of cardiovascular events, although in particular, those with overweight, obesity, or hypertension may be more vulnerable to ambient air pollution. 39 , 45 , 46 , 47 , 48 , 49
We found a null association of candle use with the risk of COPD, although long‐term exposure to ambient air pollution is associated with an HR estimate for COPD of 1.18 (95% CI: 1.13–1.23) based on a meta‐analysis. 50 Furthermore, for asthma, the association with candle use we found was inverse, in contrast to several cohort studies indicating associations between long‐term exposure to ambient air pollution and asthma incidence. 4 , 51 Indeed, lung function may be affected by short‐term exposure to candle emissions, as shown in a controlled exposure study with human volunteers. 19 Moreover, in a cross‐sectional study of 78 healthy middle‐aged Danes, high levels of UFP derived from candle use at home were associated with reduced lung function. 23 However, in another cross‐sectional study with 3471 participants, lung function and self‐reported respiratory symptoms were not associated with candle use. 22 A possible explanation for a potential inverse association between asthma and the use of candles could be that the latter results in the emission of NO2 and PM, which might elicit overt symptoms in people with asthma who then might refrain from candle use, although this has yet to be addressed in the literature.
The main strength of this study is the availability of the data on exposure to candle use in the CAMB cohort as well as detailed information on risk factors of cardiovascular and respiratory disease, including SES (e.g. household income) and lifestyle (smoking, alcohol consumption, and physical activity). Second, this study benefited from the objective and validated definitions of cardiovascular and respiratory events 30 obtained through a 10‐year‐follow‐up of the participants in internationally unique population‐based nationwide Danish health registries. Furthermore, this outcome assessment method is likely to capture nearly all relevant outcomes.
The present study also has several limitations. First, the exposure was defined by the self‐reported frequency of candle use in the wintertime only at the cohort baseline, without information on the historical use of candles or variations in exposure during the follow‐up time. Furthermore, we lacked information on the detailed intensity of candle use, such as the number of candles, hours of using candles, types of candles, and ventilation (e.g., window opening and use of air purifiers at home). Therefore, the weekly frequency of candle use may not be sufficient to capture the exposed group to the emissions while burning candles. The non‐differential exposure misclassification due to the inability to include time‐varying exposure data and more detailed exposure measures can bias the results toward the null (Rothman, 2012). 52 Second, we lacked information on changes in the covariates (lifestyle and SES) during the follow‐up time, leading to migration bias. Moreover, some of the individual characteristics collected at baseline, including physical activity and BMI, were based on self‐reported questionnaires, which may cause misclassification of covariates and insufficient adjustment for confounding. Third, there is a possibility for spatial confounding if candle use is associated with ambient air pollution, which we did not consider in the present study. However, we recently reported that 2‐year NO2 levels modeled at the residential address were not correlated with candle use using a subset of the present cohort. 35 Fourth, the CAMB study was limited to the participants living in eastern parts of Denmark, and 40% of eligible participants responded to the questionnaires, which may give rise to selection bias in the study. 24 Although the CAMB participants had similar characteristics as non‐participants in terms of educational level, more participants were employed at follow‐up than the non‐participants, suggesting that the CAMB study participants represented a somewhat socially selected group. 28 Furthermore, frequent candle users were more physically active than those who used candles rarely. Although we tried to address this issue in stratified analyses and inclusion as a confounder, we might not have been able to control sufficiently for a “healthy candle user effect,” which may influence our results toward the null, and possibly explain the weak associations observed between candle use and cardiovascular and respiratory health. Fifth, the study used health outcomes from both outpatient clinics and hospital admission. However, those patients who were only treated by their general physician and not referred to a hospital physician were not included in the study. This may have underestimated the incidence of clinical events. However, there is a reasonable assumption that this is not different for those who burn candles and those who do not, making bias unlikely. Finally, we made a large number of comparisons (10 overall and cause‐specific cardiovascular and respiratory events and nine potential effect modifiers), which could lead the associations to be identified as significant due to chance. However, we observed no associations between candle use and cardiovascular and respiratory events.
5. CONCLUSION
In a prospective cohort study of middle‐aged men and women, we did not find evidence of an increased risk of cardiovascular and respiratory events associated with candle use. We also found that candle users were more physically active and had a higher socioeconomic status than non‐users, which may explain null associations in this study. Studies with more detailed information on candle use are warranted to investigate the adverse effects of exposure to the candle‐burning particles on cardiovascular and respiratory events.
AUTHOR CONTRIBUTIONS
Steffen Loft involved in funding acquisition, conceptualization, and writing—original draft preparation. Zorana J. Andersen, Rudi G.J. Westendorp and Rikke Lund involved in conceptualization and writing—review and editing. Jeanette Therming Jørgensen involved in writing—review and editing (equal). Amalie Darling Kristiansen and Julie Kamstrup Dam involved in data curation. Johannah Cramer involved in formal analysis and writing—review and editing. Youn‐Hee Lim involved in writing—original draft preparation and formal analysis.
FUNDING INFORMATION
This work was funded by RealDania (PRJ‐2018‐00211) and the Novo Nordisk Foundation Challenge Programme (NNF17OC0027812). The Copenhagen Aging and Midlife Biobank was funded by generous grants from the VELUX FOUNDATION (VELUX 26145 and 31539).
CONFLICT OF INTEREST
None to declare.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
This manuscript was prepared in collaboration with members of the CAMB steering committee. The list of the CAMB steering committee and those responsible for the collection of historical data can be found at http://www.camb.ku.dk/.
Loft S, Andersen ZJ, Jørgensen JT, et al. Use of candles and risk of cardiovascular and respiratory events in a Danish cohort study. Indoor Air. 2022;32:e13086. doi: 10.1111/ina.13086
As of September 1, 2020, Johannah Cramer entered employment with Novo Nordisk A/S. The author's contributions to this study were made prior to the start of this employment.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Lelieveld J, Klingmüller K, Pozzer A, et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J. 2019;40:1590‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta‐analysis in 11 European cohorts from the ESCAPE project. BMJ. 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang X‐Q, Mei X‐D, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis. 2016;8:E31‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu S, Jørgensen JT, Ljungman P, et al. Long‐term exposure to low‐level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. 2021;57:2003099. [DOI] [PubMed] [Google Scholar]
- 5. Newby DE, Mannucci PM, Tell GS, et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Hoek G. Long‐term exposure to PM and all‐cause and cause‐specific mortality: a systematic review and meta‐analysis. Environ Int. 2020;143:105974. [DOI] [PubMed] [Google Scholar]
- 7. So R, Jørgensen JT, Lim YH, et al. Long‐term exposure to low levels of air pollution and mortality adjusting for road traffic noise: a Danish Nurse Cohort study. Environ Int. 2020;143:105983. [DOI] [PubMed] [Google Scholar]
- 8. Lee KK, Bing R, Kiang J, et al. Adverse health effects associated with household air pollution: a systematic review, meta‐analysis, and burden estimation study. Lancet Glob Health. 2020;8:e1427‐e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregersen K. Forskere Advarer: Danskerne Tænder Alt for Mange Stearinlys. Bolius; 2018. [Google Scholar]
- 10. Derudi M, Gelosa S, Sliepcevich A, et al. Emission of air pollutants from burning candles with different composition in indoor environments. Environ Sci Pollut Res. 2014;21:4320‐4330. [DOI] [PubMed] [Google Scholar]
- 11. Fan C‐W, Zhang JJ. Characterization of emissions from portable household combustion devices: particle size distributions, emission rates and factors, and potential exposures. Atmos Environ. 2001;35:1281‐1290. [Google Scholar]
- 12. Fine PM, Cass GR, Simoneit BR. Characterization of fine particle emissions from burning church candles. Environ Sci Technol. 1999;33:2352‐2362. [Google Scholar]
- 13. Klosterköther A, Kurtenbach R, Wiesen P, Kleffmann J. Determination of the emission indices for NO, NO2, HONO, HCHO, CO, and particles emitted from candles. Indoor Air. 2020;31:116‐127. [DOI] [PubMed] [Google Scholar]
- 14. Pagels J, Wierzbicka A, Nilsson E, et al. Chemical composition and mass emission factors of candle smoke particles. J Aerosol Sci. 2009;40:193‐208. [Google Scholar]
- 15. Zai S, Zhen H, Jia‐song W. Studies on the size distribution, number and mass emission factors of candle particles characterized by modes of burning. J Aerosol Sci. 2006;37:1484‐1496. [Google Scholar]
- 16. Skovmand A, Gouveia ACD, Koponen IK, Møller P, Loft S, Roursgaard M. Lung inflammation and genotoxicity in mice lungs after pulmonary exposure to candle light combustion particles. Toxicol Lett. 2017;276:31‐38. [DOI] [PubMed] [Google Scholar]
- 17. Weber LP, Al‐Dissi A, Marit JS, German TN, Terletski SD. Role of carbon monoxide in impaired endothelial function mediated by acute second‐hand tobacco, incense, and candle smoke exposures. Environ Toxicol Pharmacol. 2011;31:453‐459. [DOI] [PubMed] [Google Scholar]
- 18. Damiao Gouveia AC, Skovman A, Jensen A, et al. Telomere shortening and aortic plaque progression in Apoliprotein E knockout mice after pulmonary exposure to candle light combustion particles. Mutagenesis. 2018;33:253‐261. [DOI] [PubMed] [Google Scholar]
- 19. Soppa VJ, Schins RP, Hennig F, et al. Respiratory effects of fine and ultrafine particles from indoor sources—a randomized sham‐controlled exposure study of healthy volunteers. Int J Environ Res Public Health. 2014;11:6871‐6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shehab M, Pope F. Effects of short‐term exposure to particulate matter air pollution on cognitive performance. Sci Rep. 2019;9:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagerman I, Isaxon C, Gudmundsson A, et al. Effects on heart rate variability by artificially generated indoor nano‐sized particles in a chamber study. Atmos Environ. 2014;88:165‐171. [Google Scholar]
- 22. Hersoug LG, Husemoen LL, Sigsgaard T, Madsen F, Linneberg A. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993‐1000. [DOI] [PubMed] [Google Scholar]
- 23. Karottki DG, Bekö G, Clausen G, et al. Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle‐aged subjects. Environ Int. 2014;73:372‐381. [DOI] [PubMed] [Google Scholar]
- 24. Lund R, Mortensen EL, Christensen U, et al. Cohort profile: the Copenhagen Aging and Midlife Biobank (CAMB). Int J Epidemiol. 2016;45:1044‐1053. [DOI] [PubMed] [Google Scholar]
- 25. Osler M, Lund R, Kriegbaum M, Christensen U, Andersen A‐MN. Cohort profile: the Metropolit 1953 Danish male birth cohort. Int J Epidemiol. 2006;35:541‐545. [DOI] [PubMed] [Google Scholar]
- 26. Mortensen EL. The Copenhagen Perinatal Cohort and the prenatal development project. Int J Risk Saf Med. 1997;10:199‐202. [DOI] [PubMed] [Google Scholar]
- 27. Christensen U, Lund R, Damsgaard MT, et al. Cynical hostility, socioeconomic position, health behaviors, and symptom load: a cross‐sectional analysis in a Danish population‐based study. Psychosom Med. 2004;66:572‐577. [DOI] [PubMed] [Google Scholar]
- 28. Avlund K, Osler M, Mortensen EL, et al. Copenhagen Aging and Midlife Biobank (CAMB) an introduction. J Aging Health. 2014;26:5‐20. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22‐25. [DOI] [PubMed] [Google Scholar]
- 30. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ljungman PL, Andersson N, Stockfelt L, et al. Long‐term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect. 2019;127:107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stafoggia M, Cesaroni G, Peters A, et al. Long‐term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. 2014;122:919‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al‐Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17:656‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yue C, Yang F, Li F, Chen Y. Association between air pollutants and atrial fibrillation in general population: a systematic review and meta‐analysis. Ecotoxicol Environ Saf. 2021;208:111508. [DOI] [PubMed] [Google Scholar]
- 35. Lim Y‐H, Hersoug L‐G, Lund R, et al. Inflammatory markers and lung function in relation to indoor and ambient air pollution. Int J Hyg Environ Health. 2022;241:113944. [DOI] [PubMed] [Google Scholar]
- 36. Danish Health Authority . Sundhedsstyrelsens Udmeldinger Om Alkohol. Danish Health Authority. Accessed January 2, 2021. https://www.sst.dk/da/Viden/Alkohol/Alkoholforebyggelse/Sundhedsstyrelsens‐udmeldinger‐om‐alkohol [Google Scholar]
- 37. Statistics Denmark . Registers in Forskningssservice's basic database. 2020. Accessed December 6, 2020. http://www.dst.dk/extranet/forskningvariabellister/Oversigt%20over%20registre.html
- 38. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. Effect modification of long‐term air pollution exposures and the risk of incident cardiovascular disease in US women. J Am Heart Assoc. 2015;4:e002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li N, Chen G, Liu F, et al. Associations between long‐term exposure to air pollution and blood pressure and effect modifications by behavioral factors. Environ Res. 2020;182:109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang H, Li S, Sun L, et al. Smog and risk of overall and type‐specific cardiovascular diseases: a pooled analysis of 53 cohort studies with 21.09 million participants. Environ Res. 2019;172:375‐383. [DOI] [PubMed] [Google Scholar]
- 42. Soppa VJ, Shinnawi S, Hennig F, et al. Effects of short‐term exposure to fine and ultrafine particles from indoor sources on arterial stiffness—a randomized sham‐controlled exposure study. Int J Hyg Environ Health. 2019;222:1115‐1132. [DOI] [PubMed] [Google Scholar]
- 43. Corlin L, Woodin M, Hart JE, et al. Longitudinal associations of long‐term exposure to ultrafine particles with blood pressure and systemic inflammation in Puerto Rican adults. Environ Health. 2018;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Downward GS, van Nunen EJ, Kerckhoffs J, et al. Long‐term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect. 2018;126:127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brook RD, Newby DE, Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens. 2018;31:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim H, Byun G, Choi Y, Kim S, Kim S‐Y, Lee J‐T. Effects of long‐term exposure to air pollution on all‐cause mortality and cause‐specific mortality in seven major cities of South Korea: Korean national health and nutritional examination surveys with mortality follow‐up. Environ Res. 2021;192:110290. [DOI] [PubMed] [Google Scholar]
- 47. Li J, Liu F, Liang F, et al. Long‐term effects of high exposure to ambient fine particulate matter on coronary heart disease incidence: a population‐based Chinese cohort study. Environ Sci Technol. 2020;54:6812‐6821. [DOI] [PubMed] [Google Scholar]
- 48. Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity. 2014;22:1580‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang B‐Y, Guo Y, Markevych I, et al. Association of long‐term exposure to ambient air pollutants with risk factors for cardiovascular disease in China. JAMA Netw Open. 2019;2:e190318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park J, Kim HJ, Lee CH, Lee CH, Lee HW. Impact of long‐term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Environ Res. 2021;194:110703. [DOI] [PubMed] [Google Scholar]
- 51. Lee D‐W, Han C‐W, Hong Y‐C, et al. Long‐term exposure to fine particulate matter and incident asthma among elderly adults. Chemosphere. 2021;272:129619. [DOI] [PubMed] [Google Scholar]
- 52. Rothman KJ. Epidemiology: an introduction. Oxford university press; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


