Summary
Background
Around 70% of cutaneous malignant melanomas (MMs) develop de novo, and small‐diameter or ‘tiny’ lesions are expected to represent the earliest manifestation of most MMs.
Aim
To describe the clinical, histopathological and dermoscopic features of tiny MMs, and to investigate the impact of imaging tools, including total body photography (TBP) and sequential digital dermoscopy imaging (SDDI) in their detection.
Methods
Consecutive MMs diagnosed over 2 years in a referral centre were retrospectively included. Tiny MMs were defined as MMs with a diameter of ≤ 5 mm on dermoscopy. Dermoscopic features and the performance of four imaging methods were evaluated.
Results
Of the 312 MMs included, 86 (27.6%) measured ≤ 5 mm, and 44.2% of these were invasive. Tiny MMs were more frequently excised for being new and/or changing compared with nontiny MMs (77.9% vs. 50.9%; P < 0.001). Half of the tiny MMs would have been missed by the dermoscopic seven‐point checklist (48.2%) or the three‐point checklist (49.4%), while Menzies' method and the revised pattern analysis correctly identified respectively 65.9% and 63.5% of the tiny MMs. The most frequent positive features for tiny MMs were asymmetry in structure or colour (77.6%), brown dots (65.9%), irregular dots and globules (76.5%) and atypical pigment network (44.7%). Dermoscopic features predictive of invasion in tiny MMs were atypical vascular pattern (OR = 26.5, 95% CI 1.5–475.5, P < 0.01), shiny white lines (OR = 12.4, 95% CI 0.7–237.8, P = 0.04) and grey/blue structures (OR = 3.7, 95% CI 1.3–10.5, P = 0.01).
Conclusion
Tiny MMs are frequently invasive and represent a clinical, dermoscopic and histopathological challenge. Dermoscopy alone has suboptimal diagnostic accuracy. Early diagnosis relies on the detection of new or changing lesions aided by TBP and SDDI.
Small‐diameter melanomas are frequently invasive and represent a clinical, dermoscopic and histopathological diagnostic challenge. Most small‐diameter melanomas were detected for being a new or changing lesion and dermoscopy alone has suboptimal diagnostic accuracy. The most frequent positive dermoscopic features for small‐diameter melanomas are asymmetry in structure or colour, brown dots, irregular dots and globules and atypical pigment network. Atypical vascular pattern, shiny white lines and grey/blue structures on dermoscopy were associated with invasiveness and should prompt excision.
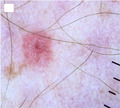
Introduction
Given that approximately 70% of cutaneous malignant melanomas (MMs) develop de novo, 1 small‐diameter or tiny lesions are expected to represent the earliest clinical manifestation of most MMs. 2 The proportion of MMs excised when still small varies considerably in the literature, ranging from 2.4% to 38.2%, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 depending on the measurement method and diameter cutoff used to define ‘small’. Although previous studies have shown a positive correlation between diameter and tumour thickness, 3 there is growing evidence demonstrating that small does not mean in situ, with up to 70% of these lesions reported as being invasive. 3 , 4 , 7 Tiny MMs also represent a diagnostic pitfall. Dermoscopy findings can be subtle due to incomplete development of specific MM features, leading to suboptimal diagnostic accuracy. 8 , 10 In some cases, the only clue may be evidence of a new or changing lesion based on total body photography (TBP) and sequential digital dermoscopy imaging (SDDI). 12 , 13
In this study, we aimed to describe the clinical, histopathological and dermoscopic features of tiny MMs, and to investigate the impact of imaging tools, particularly TBP and SDDI, in their detection.
Methods
The study was reviewed and approved by the Sydney Local Health District Ethics Committee (X15‐0311; 2019/ETH06854).
Study design
Consecutive primary MMs diagnosed over 2 years (2017–2019) at an Australian referral centre, the Sydney Melanoma Diagnostic Centre (SMDC), were retrospectively included. SMDC's screening strategy relies on regular full skin examination assessing all lesions with dermoscopy regardless of diameter. TBP is recommended for patients with naevus count > 100 and/or a personal history of MM. SDDI is used for equivocal lesions lacking obvious features for MM.
Dermoscopy
Dermoscopic images were obtained with a polarized or nonpolarized contact dermatoscope [DL4 (DermLite, San Juan Capistrano, CA, USA) or MoleScope II (MetaOptima Technology, Vancouver, BC, Canada)] or nonpolarized (Delta 20; Heine, Gilching, Germany) prior to biopsy or excision. Low‐quality images were excluded for the dermoscopy analysis. The built‐in scale of the dermatoscope visualized in all photographs enabled a precise diameter measurement. Tiny MMs were defined as those ≤ 5 mm in diameter. Lesions were classified as pigmented, hypomelanotic (pigmentation covering < 25% of the surface) or amelanotic (pigmentation completely absent). 14
Fifty dermoscopic features were documented for each lesion, including those of four methods used to diagnose MM (Menzies' method, 15 7‐point checklist, 16 3‐point checklist 17 and revised pattern analysis 18 , 19 ), those related to vascular morphology 20 and global dermoscopy patterns. 21 The accuracy of each method in diagnosing MMs was calculated.
Histopathology
Histopathological diagnosis was performed at a tertiary referral centre receiving high volumes of MMs. Borderline melanocytic lesions were reviewed by at least two pathologists for consensus. For this study, histopathology was further reviewed by a senior dermatopathologist who used strict histopathological criteria and a checklist of features to confirm the diagnosis. The MMs were then divided into two categories: (i) ‘clear‐cut’ MMs, which were lesions fulfilling unequivocal histopathological features of MM (confluent lentiginous growth with spread over suprapapillary plates, prominent pagetoid scatter and/or severe cytological atypia), and (ii) ‘borderline’ MMs, which were lesions presenting with ‘borderline to malignant’ features (focal severe cytological atypia, near‐confluent growth, central suprabasal scatter of clusters of melanocytes or occasional small melanocytes, increased atypical junctional melanocytes in severely sun‐damaged skin with extension down adnexae) but considered to be MMs (and managed as such) based on clinicopathological correlation (Fig. 1).
Figure 1.
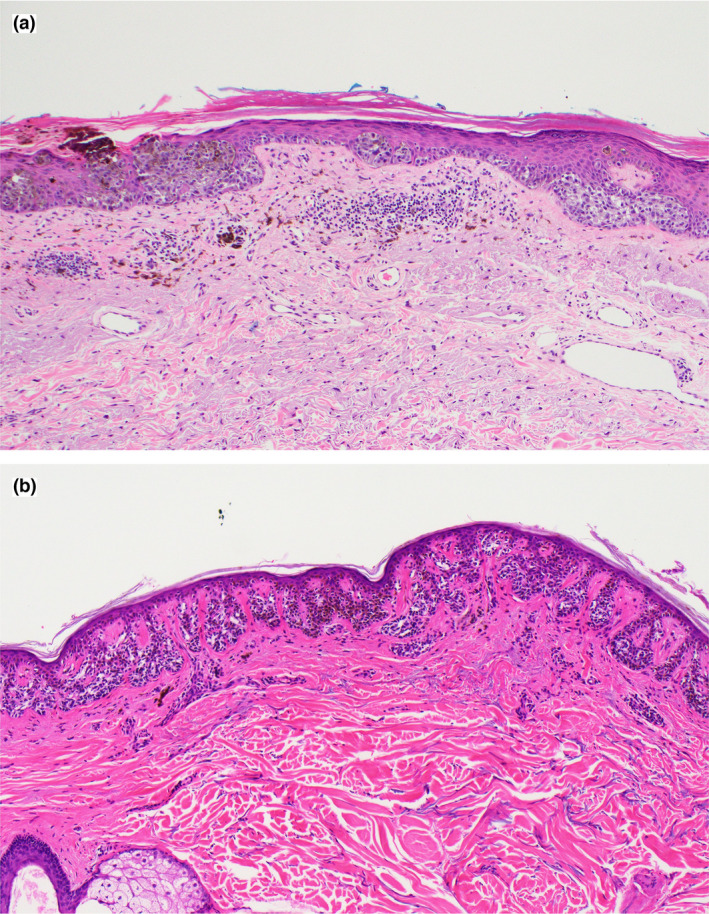
(a) ‘Clear‐cut’ melanoma in situ, showing large irregular nests, confluent lentiginous growth and pagetoid scatter of pleomorphic melanocytes; (b) ‘borderline’ melanoma in situ, showing extensive fusion of junctional melanocytic nests across rete pegs with some nonconfluent lentiginous spread over suprapapillary plates. Haematoxylin and eosin, original magnification (a,b) × 20. [Colour figure can be viewed at wileyonlinelibrary.com]
Statistical analyses
Statistical analyses were performed using SPSS (V26; IBM SPSS, Armonk, NY, USA). Categorical variables were summarized as numbers and percentages. Continuous variables were summarized as medians with interquartile range (IQR). Differences in proportions were analysed using Pearson χ2 test or Fisher exact test, while medians were compared with the Mann–Whitney test. Dermoscopic features of invasive and noninvasive tiny MMs were compared using univariable logistic regression.
Results
Lesions and histopathological results
In total, 316 MMs were diagnosed in 255 patients; after pathology review, 3 lesions were reclassified as benign and excluded, and 1 further lesion was excluded due to pathology slides missing. Approximately one‐third (86 of 312; 27.6%) of the included MMs measured ≤ 5 mm in diameter (median 4 mm; IQR 3.0–4.5; minimum 1.7 mm). The patient characteristics are presented in Table 1, and the clinicopathological features of the MMs and the detection methods used are presented in Table 2.
Table 1.
Clinical and epidemiological characteristics of the included patients.
| Characteristic a | Tiny (n = 71) b | Nontiny (n = 181) c | P |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 43 (60.6) | 75 (41.4) | < 0.01 |
| Male | 28 (39.4) | 106 (58.6) | |
| Age at diagnosis, years; median (IQR) | 53 (40–61) | 65 (54–73) | < 0.001 |
| MM history, n (%) | |||
| Personal | 39 (54.9) | 106 (58.6) | 0.60 |
| Family d | 34 (47.9) | 68 (37.8) | 0.14 |
| DNS, e n (%) | 41 (57.7) | 75 (43.1) | 0.04 |
| TBP available, n (%) | 44 (62.0) | 69 (38.1) | < 0.001 |
DNS, dysplastic naevus syndrome; IQR, interquartile range; MM, malignant melanoma; TBP, total body photography.
For patients presenting with multiple primary melanomas, the first melanoma diagnosed was considered as the reference category (tiny vs. nontiny).
Included 12 patients with multiple primary melanomas.
Included 29 patients with multiple primary melanomas.
Data missing for 1 patient diagnosed with a nontiny melanoma.
Data missing for 7 patients diagnosed with nontiny melanomas.
Table 2.
Clinicopathological features of the melanomas.
| Characteristic | Tiny (n = 86) | Nontiny (n = 226) | P |
|---|---|---|---|
| Invasive, n (%) | 38 (44.2) | 74 (32.7) | 0.06 |
| Breslow thickness, mm; median (IQR) [range] | |||
| All MMs |
in situ (in situ to 0.5) [in situ to 1.5] |
in situ (in situ to 0.43) [in situ to 3.4] |
0.09 |
| Invasive MMs |
0.5 (0.3–0.8) [0.2–1.5] |
0.5 (0.3–0.7) [0.15–3.4] |
0.62 |
| MM subtype, n (%) | |||
| Melanoma in situ, unspecified | 44 (51.2) | 105 (46.5) | 0.001 |
| Lentigo maligna | 4 (4.7) | 47 (20.8) | |
| Lentigo maligna melanoma | 1 (1.2) | 13 (5.8) | |
| Superficial spreading melanoma | 24 (27.9) | 50 (22.1) | |
| Nodular melanoma | 2 (2.3) | 2 (0.9) | |
| Naevoid melanoma | 4 (4.7) | 4 (1.8) | |
| Spitzoid melanoma | 2 (2.3) | 0 (0.0) | |
| Invasive melanoma, unspecified | 5 (5.8) | 5 (2.2) | |
| Other features, n (%) | |||
| Associated with a naevus | 19 (22.1) | 82 (36.3) | 0.02 |
| Ulceration present | 0 (0.0) | 1 (0.4) | 0.54 |
| Mitoses ≥ 1/mm2 | 11 (12.8) | 21 (9.3) | 0.36 |
| Borderline histopathology a | 43 (50) | 32 (14.2) | < 0.001 |
| Body site, n (%) | |||
| Head and neck | 5 (5.8) | 44 (19.5) | < 0.01 |
| Trunk | 26 (30.2) | 92 (40.7) | 0.09 |
| Upper limbs | 28 (32.6) | 58 (25.7) | 0.22 |
| Lower limbs | 27 (31.4) | 32 (14.2) | 0.01 |
| Colour, n (%) | |||
| Pigmented | 77 (89.5) | 192 (85.0) | < 0.05 |
| Hypomelanotic b | 2 (2.3) | 23 (10.2) | |
| Amelanotic c | 7 (8.1) | 11 (4.9) | |
| First noticed by, n (%) d | |||
| Patient | 10 (11.6) | 14 (6.6) | 0.14 |
| Doctor | 74 (86.0) | 197 (93.4) | |
| Appointment type on detection, n(%) | |||
| Initial | 12 (14) | 51 (22.6) | 0.09 |
| Follow‐up | 74 (86) | 175 (77.4) | |
| Main reason excision recommended, n (%) | |||
| Atypical baseline dermoscopy (exclusively) e | 18 (20.9) | 77 (34.1) | < 0.001 |
| Change (on SDDI and/or TBP) | 32 (37.2) | 75 (33.2) | |
| Atypical baseline dermoscopy + new lesion on TBP | 17 (19.8) | 12 (5.3) | |
| Atypical baseline dermoscopy + change (on SDDI and/or TBP) | 13 (15.1) | 22 (9.7) | |
| New lesion (according to TBP) | 5 (5.8) | 6 (2.7) | |
| Atypical findings on confocal microscopy | 1 (1.2) | 34 (15.0) | |
| Reason to recommend excision (combined), n (%) | |||
| Evidence of a new and/or changing lesion (SSDI and/or TBP) f | 67 (77.9) | 115 (50.9) | < 0.001 |
IQR, interquartile range; MM malignant melanoma; SDDI, sequential digital dermoscopy imaging; TBP, total body photography.
Focal severe cytological atypia, near‐confluent growth, central suprabasal scatter of clusters of melanocytes or occasional small melanocytes, increased atypical junctional melanocytes in severely sun‐damaged skin with extension down adnexae but regarded to be melanomas (and managed as such) based on clinicopathological correlation.
Pigmented areas representing < 25% of the total surface area.
Melanin pigmentation completely absent.
Data missing for 17 patients (2 patients with tiny melanomas and 15 with nontiny melanomas); these patients were excluded for P value calculation.
Excised due to an atypical baseline dermoscopy, with no other evidence of a new or changing lesion.
Combining the following categories: change on SDDI and/or TBP; atypical baseline dermoscopy + new on TBP; atypical baseline dermoscopy + change on SDDI and/or TBP; and new on TBP, regardless of atypical baseline dermoscopy.
Risk factors
Most patients (86.7%) had at least one strong risk factor for MM (personal history, family history and/or dysplastic naevus syndrome).
Invasiveness
Of the 86 tiny MMs, 38 (44.2%) were invasive. Breslow thickness did not differ between tiny MMs (in situ; in situ to 0.5 mm) and nontiny MMs (in situ; in situ to 0.43 mm) (P = 0.09), even when only invasive cases were considered: median 0.5 mm (IQR 0.3–0.8) vs. 0.5 mm (0.3–0.7); P = 0.62.
Reasons for excision
Tiny MMs were more frequently excised for being a new and/or changing lesion (77.9%, when the following four categories were combined: new; changing (on SDDI and/or TBP); new plus baseline dermoscopy atypical; changing plus baseline dermoscopy atypical), than nontiny MMs (50.9%), which were more often excised due to atypical baseline dermoscopy features alone (P < 0.001) (Fig. 2).
Figure 2.
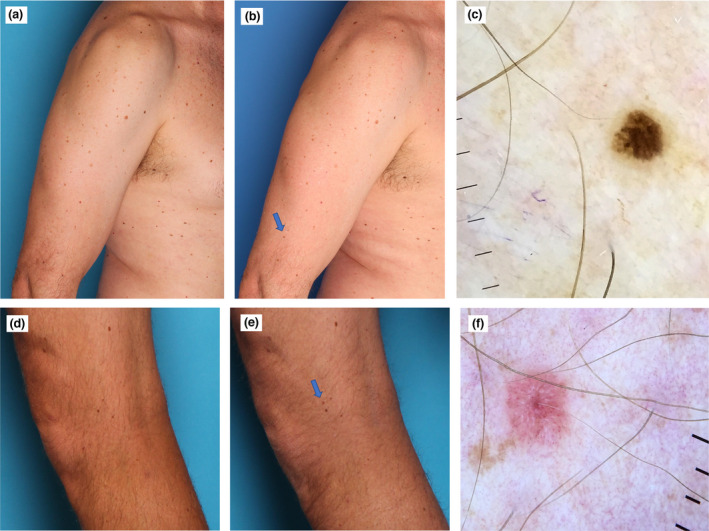
(a–c) Man in his 50s with dysplastic naevus syndrome. (a,b) A tiny melanoma 1.5 mm in diameter in situ detected on the comparison of the total body photographs (blue arrow) at (a) baseline and (b) at the 12‐month follow‐up, which revealed a completely new pigmented lesion. (c) Cross‐polarized dermoscopy (MoleScope II; MetaOptima Technology) findings were subtle, showing two colours and a few irregular peripheral brown dots (original magnification × 10). (d–f) Man in his 40s with a personal history of melanoma and dysplastic naevus syndrome. (d,e) Tiny invasive amelanotic melanoma (2.5 mm in diameter, Breslow thickness 0.2 mm, no ulceration, no mitoses) detected as a new lesion (blue arrow) on comparison of total body photographs at (d) baseline and (e) 12‐month follow‐up. (f) Cross‐polarized dermoscopy (Dermmlite DL4; 3Gen, Orange County, CA, USA) showed shiny white lines and dots and atypical vascular pattern with dotted vessels (cross‐polarized dermoscopy, original magnification × 10). [Colour figure can be viewed at wileyonlinelibrary.com]
Dermoscopy
The dermoscopic features and performance of the four diagnostic methods for diagnosing the MMs are presented in Table 3. The seven‐point and three‐point checklist would have missed 48.2% and 49.4%, respectively, of the tiny MMs, while the Menzies' method and the revised pattern analysis method correctly identified 65.9% and 63.5% of tiny MMs.
Table 3.
Overview of individual dermoscopy features present in tiny and nontiny melanomas. a
| Method and features | Tiny (n = 85) | Nontiny (n = 214) | P |
|---|---|---|---|
| Menzies method, n (%) | |||
| Asymmetry of pattern | 64 (75.3) | 189 (88.3) | < 0.01 |
| Number of colours, median (IQR) | 2 (2–3) | 3 (2–3) | < 0.001 |
| ≥ 5 colours | 0 (0.0) | 10 (4.7) | 0.04 |
| Blue–whitish veil | 1 (1.2) | 8 (3.7) | 0.24 |
| Brown dots | 56 (65.9) | 151 (70.6) | 0.43 |
| Pseudopods | 3 (3.4) | 0 (0.0) | 0.02 b |
| Radial streaming | 11 (12.9) | 2 (0.9) | < 0.001 |
| Scar‐like depigmentation | 5 (5.9) | 23 (10.7) | 0.19 |
| Peripheral black dots and globules | 15 (17.6) | 25 (11.7) | 0.17 |
| Multiple blue–grey dots (peppering) | 2 (2.4) | 39 (18.2) | < 0.001 |
| Broadened network | 27 (31.8) | 80 (37.4) | 0.36 |
| Correctly classified as MM | 56 (65.9) | 175 (81.8) | 0.003 |
| 7‐point checklist, n (%) | |||
| Atypical pigment network | 38 (44.7) | 129 (60.3) | 0.01 |
| Blue–whitish veil | 1 (1.2) | 8 (3.7) | 0.24 |
| Atypical vascular pattern | 8 (9.4) | 41 (19.2) | 0.04 |
| Irregular dots and globules | 65 (76.5) | 159 (74.3) | 0.70 |
| Irregular streaks | 12 (14.1) | 3 (1.4) | < 0.001 |
| Regression structures | 18 (21.2) | 81 (37.9) | < 0.01 |
| Irregular pigmentation | 31 (36.5) | 62 (29.0) | 0.21 |
| Correctly classified as MM | 44 (51.8) | 140 (65.4) | 0.04 |
| 3‐point checklist, n (%) | |||
| Asymmetry in structure or colour | 66 (77.6) | 196 (91.6) | 0.001 |
| Atypical network | 40 (47.1) | 129 (60.3) | 0.03 |
| Blue–white structures | 21 (24.7) | 83 (38.8) | 0.02 |
| Correctly classified as MM | 43 (50.6) | 144 (67.3) | 0.01 |
| Revised pattern analysis (chaos and clues), n (%) | |||
| Asymmetry of pattern | 63 (74.1) | 189 (88.3) | < 0.01 |
| Asymmetry of colour | 44 (51.8) | 172 (80.4) | < 0.001 |
| Grey or blue structures | 22 (25.9) | 81 (37.9) | 0.05 |
| Thick lines | 28 (32.9) | 72 (33.6) | 0.91 |
| White lines | 4 (4.7) | 22 (10.3) | 0.12 |
| Eccentric structureless areas | 17 (20.0) | 59 (27.6) | 0.18 |
| Peripheral black dots or clods | 15 (17.6) | 25 (11.7) | 0.17 |
| Radial lines or pseudopods | 12 (14.1) | 2 (0.9) | < 0.001 |
| Polymorphous vessels | 5 (5.9) | 24 (11.2) | 0.16 |
| Polygons | 1 (1.2) | 20 (9.3) | 0.01 |
| Correctly classified as MM | 54 (63.5) | 160 (74.8) | 0.07 |
| Atypical vessels, n (%) | |||
| Dotted | 6 (7.1) | 36 (16.9) | 0.03 |
| Linear | 6 (7.1) | 17 (7.9) | 0.80 |
| Coiled or glomerular | 2 (2.4) | 11 (5.1) | 0.29 |
| Looped | 0 (0.0) | 4 (1.9) | 0.58 b |
| Serpentine | 2 (2.4) | 5 (2.3) | 0.99 b |
| Comma‐like | 0 (0.0) | 3 (1.4) | 0.56 b |
| Corkscrew | 1 (1.2) | 4 (1.9) | 0.99 |
| Global pattern, n (%) | |||
| Homogeneous | 5 (6.0) | 3 (1.4) | 0.03 |
| Multicomponent | 8 (9.4) | 53 (24.8) | 0.003 |
| Starburst | 12 (14.1) | 0 (0.0) | < 0.001 |
| Reticular | 13 (15.3) | 52 (24.3) | 0.09 |
| Globular | 9 (10.6) | 2 (0.9) | < 0.001 |
| Unspecific | 38 (44.7) | 104 (48.6) | 0.54 |
| Additional features, n (%) | |||
| Chrysalis | 4 (4.7) | 22 (10.3) | 0.12 |
| Shiny white blotches | 2 (2.4) | 7 (3.3) | 0.68 |
| Rosettes | 1 (1.2) | 3 (1.4) | 0.99 b |
| Negative network | 3 (3.5) | 7 (3.3) | 0.91 |
| Milky‐red areas | 4 (4.7) | 20 (9.3) | 0.19 |
| Milky‐red globules | 1 (1.2) | 3 (1.4) | 0.99 b |
MM, malignant melanoma.
13 lesions were excluded for the dermoscopy analysis due to low‐quality images: these comprised 1 patient with a tiny MM and 12 patients with nontiny MMs.
P values were calculated with Fisher exact test as > 50% of cells had an expected count of < 5 individuals; all other P values were calculated with χ2 test.
Tiny MMs more frequently had a starburst (14.1%) or globular pattern (10.6%) compared with nontiny MMs (0% and 0.9%, respectively, P < 0.001 for both). Asymmetry of pattern was the most frequent feature for all MMs, but it was more common in nontiny MMs (88.3%) than in tiny MMs (75.3%) (P = 0.01). Radial streaming was more frequently seen in tiny MMs (12.9% vs. 0.9%; P < 0.0001).
Table 4 compares dermoscopic features of invasive and noninvasive tiny MMs. The main features predictive of invasiveness were: atypical vascular pattern (OR = 26.48, 95% CI 1.5–475.5; P = 0.01); shiny white lines/chrysalis (OR = 12.4, 95% CI 0.7–237.8; P = 0.04); and grey/blue structures (OR = 3.0, 95% CI 1.3–10.5; P = 0.01).
Table 4.
Prevalence of dermoscopy criteria for patients with invasive vs. in situ tiny melanomas and univariable logistic regression for dermoscopy features predicting invasiveness in patients with tiny melanomas. a
| Invasive (n = 38) | In situ (n = 47) | OR | 95% CI | P of 95% CI | |
|---|---|---|---|---|---|
| Menzies' method, n (%) | |||||
| Asymmetry of pattern | 28 (73.7) | 36 (76.6) | 0.86 | 0.32–2.30 | 0.76 |
| ≥ 5 colours | 0 (0.0) | 0 (0.0) | NA | NA | NA |
| Blue–whitish veil | 0 (0.0) | 1 (2.1) | 0.40 b | 0.02–10.17 | 0.57 |
| Brown dots | 23 (60.5) | 33 (70.2) | 0.65 | 0.26–1.60 | 0.35 |
| Pseudopods | 2 (5.3) | 1 (2.1) | 2.56 | 0.22–29.31 | 0.45 |
| Radial streaming | 6 (15.8) | 5 (10.6) | 1.58 | 0.44–5.42 | 0.48 |
| Scar‐like depigmentation | 2 (5.3) | 3 (6.4) | 0.82 | 0.13–5.14 | 0.83 |
| Peripheral black dots and globules | 5 (13.2) | 10 (21.3) | 0.56 | 0.17–1.81 | 0.33 |
| Multiple blue–grey dots (peppering) | 0 (0.0) | 2 (4.3) | 0.26 b | 0.02–5.51 | 0.51 |
| Broadened network | 11 (28.9) | 16 (34.0) | 0.79 | 0.31–1.99 | 0.62 |
| 7‐point checklist, n (%) | |||||
| Atypical pigment network | 16 (42.1) | 22 (46.8) | 0.83 | 0.35–1.96 | 0.67 |
| Blue–whitish veil | 0 (0.0) | 1 (2.1) | 0.43 b | 0.02–11.03 | 0.61 |
| Atypical vascular pattern | 8 (21.1) | 0 (0.0) | 26.48 b | 1.47–475.50 | < 0.01 |
| Irregular dots and globules | 26 (68.4) | 39 (83.0) | 0.44 | 0.16–1.24 | 0.12 |
| Irregular streaks | 6 (15.9) | 6 (12.8) | 1.28 | 0.38–4.35 | 0.69 |
| Regression structures | 11 (28.9) | 7 (14.9) | 2.32 | 0.80–6.76 | 0.12 |
| Irregular pigmentation | 17 (44.7) | 14 (29.8) | 1.91 | 0.78–4.67 | 0.16 |
| 3‐point checklist, n (%) | |||||
| Asymmetry in structure or colour | 30 (78.9) | 36 (76.6) | 1.15 | 0.41–3.22 | 0.80 |
| Atypical network | 17 (44.7) | 23 (48.9) | 0.85 | 0.36–1.99 | 0.70 |
| Blue–white structures | 12 (31.6) | 9 (19.1) | 1.95 | 0.72–5.29 | 0.19 |
| Revised pattern analysis (chaos and clues), n (%) | |||||
| Asymmetry of pattern | 28 (73.7) | 36 (76.6) | 0.75 | 0.28–1.99 | 0.56 |
| Asymmetry of colour | 20 (52.6) | 24 (51.1) | 1.07 | 0.45–2.51 | 0.89 |
| Grey or blue structures | 15 (39.5) | 7 (14.9) | 3.73 | 1.33–10.47 | 0.01 |
| Thick lines | 10 (26.3) | 18 (38.3) | 0.58 | 0.23–1.46 | 0.25 |
| White lines | 4 (10.5) | 0 (0.0) | 12.39 b | 0.65–237.80 | 0.04 |
| Eccentric structureless areas | 5 (13.2) | 12 (25.5) | 0.44 | 0.14–1.39 | 0.16 |
| Peripheral black dots or clods | 5 (13.2) | 10 (21.3) | 0.56 | 0.17–1.81 | 0.33 |
| Radial lines or pseudopods | 6 (15.8) | 6 (12.8) | 1.28 | 0.38–4.35 | 0.69 |
| Polymorphous vessels | 5 (13.2) | 0 (0.0) | 15.60 b | 0.83–291.70 | 0.02 |
| Polygons | 0 (0.0) | 1 (2.1) | 0.40 b | 0.02–10.17 | 0.57 |
| Atypical vessels, n (%) | |||||
| Dotted | 6 (15.9) | 0 (0.0) | 19.00 b | 1.03–349.10 | < 0.01 |
| Linear | 6 (15.9) | 0 (0.0) | 19.00 b | 1.03–349.10 | < 0.01 |
| Coiled or glomerular | 2 (5.3) | 0 (0.0) | 6.51 b | 0.31–139.71 | 0.18 |
| Serpentine | 2 (5.3) | 0 (0.0) | 6.51 b | 0.31–139.71 | 0.18 |
| Corkscrew | 1 (2.6) | 0 (0.0) | 3.8 b | 0.15–95.98 | 0.39 |
| Global pattern, n (%) | |||||
| Homogeneous | 2 (5.3) | 3 (6.5) | 0.80 | 0.13–5.03 | 0.81 |
| Multicomponent | 4 (10.5) | 4 (8.5) | 1.27 | 0.30–5.43 | 0.75 |
| Starburst | 6 (15.9) | 6 (12.8) | 1.28 | 0.38–4.35 | 0.69 |
| Reticular | 3 (7.9) | 10 (21.3) | 0.32 | 0.08–1.25 | 0.10 |
| Globular | 3 (7.9) | 6 (12.8) | 0.59 | 0.14–2.52 | 0.47 |
| Unspecific | 20 (52.6) | 18 (38.3) | 1.79 | 0.75–4.26 | 0.19 |
| Additional features, n (%) | |||||
| Chrysalis | 4 (10.5) | 0 (0.0) | 12.39 b | 0.65–237.80 | 0.04 |
| Shiny white blotches | 2 (5.3) | 0 (0.0) | 6.51 b | 0.31–139.71 | 0.18 |
| Rosettes | 1 (2.6) | 0 (0.0) | 3.8 b | 0.15–95.98 | 0.39 |
| Negative network | 2 (5.3) | 1 (2.1) | 2.56 | 0.22–29.31 | 0.45 |
| Milky‐red areas | 3 (7.9) | 1 (2.1) | 3.94 | 0.39–39.54 | 0.24 |
| Milky‐red globules | 0 (0.0) | 1 (2.1) | 0.40 b | 0.02–10.17 | 0.57 |
NA, not applicable.
One patient with a tiny melanoma was excluded from the analysis due to low quality image.
Haldane–Anscombe correction was used for cases with zero values.
Discussion
Small‐diameter MMs (≤ 5 mm) accounted for a significant proportion (27.6%) of the MMs diagnosed in our cohort, which is in accordance with rates reported previously (2.4%–38.2%). 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Nevertheless, the lack of consensus to define ‘small’ impairs the accurate comparison of different studies, with cutoff diameter varying from ≤ 6 mm to ≤ 3 mm. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 22
The proportion of small MMs may also have been overestimated in some case series by lesion measurement after histopathological processing, without considering the ex vivo tissue shrinkage of approximately 20%. 11 , 23 In vivo measurement, ideally guided by dermoscopy, is the most reliable method, as it includes peripheral areas not necessarily visible to the naked eye.
An important potential limitation of studies on tiny MMs is the relative subjectivity of the histopathological diagnosis. Despite being regarded as the gold‐standard diagnostic test, histopathology has consistently been shown to have low inter‐observer reproducibility, especially in the spectrum of borderline lesions ranging from severely dysplastic naevus to MM in situ to early invasive MM. 24 A tendency to ‘overcall’ dysplastic naevi as MMs has been observed over recent decades, possibly driven by asymmetrical financial incentives and medicolegal concerns. 25 Small‐diameter MMs, in particular, are more likely to show subtle and incomplete histological criteria for MM, as demonstrated in our series. 9 In this context, the documentation of a new and/or changing lesion must be taken into consideration, with clinicopathological correlation and pathology review for consensus considered to be best practice. 9 As the biological behaviour of tiny borderline melanocytic lesions is uncertain; however, in the context of a changing and clinically suspicious lesion with atypical histological features, particularly in high‐risk patients, our practice is to manage such lesions as MMs.
Patients diagnosed with a tiny MM were significantly younger than those with nontiny MMs. This could be explained by the larger proportion of lentigo maligna (LM) lesions among nontiny MMs, which are often diagnosed in elderly and heavily sun‐damaged individuals. LM lesions tend to grow slowly and horizontally before developing specific clinical and dermoscopic features, and is therefore usually larger than other subtypes on diagnosis. 26 For the same reason, related to MM subtype distribution, nontiny MMs were more often located on the head and neck area than tiny MMs, which predominated on the limbs.
The data presented support the hypothesis that small diameter does not mean in situ, as 44.2% of the tiny MMs we assessed were already invasive upon diagnosis. Tiny MMs frequently show a vertical growth phase and have similar prognosis to invasive nontiny lesions, with similar prognostic attributes. 7 Although invasive, most small‐diameter MMs we detected were early Stage 1 (T1a), with less impact on morbidity, mortality and healthcare costs. However, it is notable that median Breslow thickness was not deeper for lesions of larger diameter, even when only invasive MMs were considered, contrary to what would be intuitively expected. This may be explained by the group's heterogeneity in MM subtypes and the different potential of different subtypes to progress vertically, indicating that a profile of more ‘slow‐growing’ MMs predominated in the group of lesions detected only when > 5 mm. Based on this assumption, if the tiny MMs included in our series happened to be overlooked, by the time they reached the recently proposed biopsy threshold of 6 mm in diameter, 25 the final diameter of the wider excision would be larger, and the Breslow thickness would likely be greater, posing a higher risk for locoregional or distant metastasis.
A precursor melanocytic naevus was observed in only 22.1% of patients with tiny MMs, favouring the hypothesis that most of these lesions arise de novo. This is consistent with a recent metanalysis, which concluded that only 29.1% of MMs developed in conjunction with a pre‐existing naevus. 1
The screening strategy at SMDC (regular full skin examination, aided by TBP and SDDI), is recognized for facilitating early diagnosis, low benign‐to‐malignant ratios and optimal outcomes 27 and has been validated with a subset of our extremely high‐risk patients. 28
The recommendation for excision in our series was strongly influenced by TBP and/or SDDI, particularly for tiny lesions. Only 20.9% of such lesions were detected solely on the basis of an atypical baseline dermoscopy. For the remaining lesions, evidence of a new and/or changing lesion, associated or not with an atypical baseline dermoscopy, influenced the decision to excise. Our findings show that dermoscopy alone, without the aid of TBP and SDDI, is likely to miss a significant proportion of tiny MMs.
The most frequent positive features for tiny MMs were asymmetry in structure or colour (77.6%), brown dots (65.9%), irregular dots and globules (76.5%) and atypical pigment network (44.7%); the last two features were the most common features described in literature. 8 , 10 , 29 , 30
In their study, Seidenari et al. 10 included 79 small MMs (≤ 6 mm) and 403 nontiny MMs, and reported that dermoscopic scores increased proportionally to the diameter, with tiny lesions being more difficult to diagnose by dermoscopy.
The suboptimal performance of all four dermoscopy methods we tested emphasizes that small‐diameter MMs often do not reveal typical MM features on baseline dermoscopy, as previously reported. 10 , 22
However, Pupelli et al. 31 found a higher diagnostic accuracy of dermoscopy to diagnose small‐diameter MMs (≤ 5 mm, n = 24), with the seven‐point checklist correctly classifying 92% of them, compared with only 51.8% in our study. This could be partially explained by the subjectivity of some dermoscopic criteria, such as ‘irregular pigmentation’, which was much less prevalent in our sample, as we followed precisely the original description of this feature by Argenziano et al., 16 who defined it as irregular diffuse pigmentation (blotches). It could also be related to inherent differences in the studied population, with more amelanotic and light‐coloured MMs expected in Australian patients.
The presence of an atypical vascular pattern, polarizing‐specific lines (shiny white lines or chrysalis) and grey–blue structures were the dermoscopic features that were more strongly correlated with invasiveness. These dermoscopy features are associated with dermal malignant cells, regression, fibrosis and inflammation, justifying previous findings. 15 Therefore, if these features are present, excision instead of monitoring is advisable.
The strengths of the current study are its large sample size and the thorough pathological review of all included cases. The retrospective data collection and single‐centre design are limitations. The findings represent a high‐risk subset of Australian patients and may not necessarily be representative of MMs diagnosed in the general population or those from distinct geographical areas. Furthermore, the subjectivity of histopathological diagnosis is an inherent potential limitation of all studies that include early‐stage MMs.
Conclusion
Tiny MMs represent a clinical, dermoscopic and histopathological challenge. Despite their small size, these lesions are frequently invasive. Incomplete development of specific melanoma features on dermoscopy may lead to suboptimal diagnostic accuracy and therefore early diagnosis frequently relies on the detection of a new and changing lesion, aided by TBP and SDDI.
Conflict of interest
RAS has received fees for professional services from Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, NeraCare, AMGEN Inc., Bristol‐Myers Squibb, Myriad Genetics, GlaxoSmithKline, F. Hoffman‐La Roche Ltd., Evaxion and Merck Sharp & Dohme. The other authors declare that they have no conflicts of interest.
Acknowledgement
RAS is supported by a National Health and Medical Research Council of Australia (NHMRC) Practitioner Fellowship (no. APP1141295). Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians. WOA Institution: The University of Sydney.
What's already known about this topic?
Small‐diameter melanomas are frequently invasive and represent a clinical, dermoscopic and histopathological diagnostic challenge.
What does this study add?
Most small‐diameter melanomas were detected for being a new or changing lesion and dermoscopy alone has suboptimal diagnostic accuracy.
The most frequent positive dermoscopic features for small‐diameter melanomas were asymmetry in structure or colour, brown dots, irregular dots and globules, and atypical pigment network.
Atypical vascular pattern, shiny white lines and grey/blue structures on dermoscopy were associated with invasiveness and should prompt excision.
ARP and MC‐F contributed equally to this work and should be considered joint first authors.
References
- 1. Pampena R, Kyrgidis A, Lallas A et al. A meta‐analysis of nevus‐associated melanoma: prevalence and practical implications. J Am Acad Dermatol 2017; 77: 938–45.e4. [DOI] [PubMed] [Google Scholar]
- 2. Bono A, Tolomio E, Trincone S et al. Micro‐melanoma detection: a clinical study on 206 consecutive cases of pigmented skin lesions with a diameter < or = 3 mm. Br J Dermatol 2006; 155: 570–3. [DOI] [PubMed] [Google Scholar]
- 3. Shaw HM, McCarthy WH. Small‐diameter malignant melanoma: a common diagnosis in New South Wales, Australia. J Am Acad Dermatol 1992; 27: 679–82. [DOI] [PubMed] [Google Scholar]
- 4. Bono A, Bartoli C, Moglia D et al. Small melanomas: a clinical study on 270 consecutive cases of cutaneous melanoma. Melanoma Res 1999; 9: 583–6. [PubMed] [Google Scholar]
- 5. Fernandez EM, Helm KF. The diameter of melanomas. Dermatol Surg 2004; 30: 1219–22. [DOI] [PubMed] [Google Scholar]
- 6. Helsing P, Loeb M. Small diameter melanoma: a follow‐up of the Norwegian Melanoma Project. Br J Dermatol 2004; 151: 1081–3. [DOI] [PubMed] [Google Scholar]
- 7. Betti R, Crosti C, Vergani R et al. Invasive behavior of small diameter melanomas. J Dermatol 2012; 39: 870–1. [DOI] [PubMed] [Google Scholar]
- 8. de Giorgi V, Savarese I, Rossari S et al. Features of small melanocytic lesions: does small mean benign? A clinical‐dermoscopic study. Melanoma Res 2012; 22: 252–6. [DOI] [PubMed] [Google Scholar]
- 9. Ferrara G, Tomasini C, Argenziano G et al. Small‐diameter melanoma: toward a conceptual and practical reappraisal. J Cutan Pathol 2012; 39: 721–3. [DOI] [PubMed] [Google Scholar]
- 10. Seidenari S, Ferrari C, Borsari S et al. Dermoscopy of small melanomas: just miniaturized dermoscopy? Br J Dermatol 2014; 171: 1006–13. [DOI] [PubMed] [Google Scholar]
- 11. Abbasi NR, Shaw HM, Rigel DS et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA 2004; 292: 2771–6. [DOI] [PubMed] [Google Scholar]
- 12. Salerni G, Alonso C, Fernández‐Bussy R. Multiple primary invasive small‐diameter melanomas: importance of dermoscopy and digital follow‐up. Dermatol Pract Concept 2019; 9: 69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drugge ED, Volpicelli ER, Sarac RM et al. Micromelanomas identified with time‐lapse total body photography and dermoscopy. J Am Acad Dermatol 2018; 78: 182–3. [DOI] [PubMed] [Google Scholar]
- 14. Menzies SW, Kreusch J, Byth K et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol 2008; 144: 1120–7. [DOI] [PubMed] [Google Scholar]
- 15. Menzies S, Crotty K, Ingvar C, McCarthy W. Dermoscopy: An Atlas, 3rd edn. Sydney: McGraw‐Hill Australia, 2009. [Google Scholar]
- 16. Argenziano G, Fabbrocini G, Carli P et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7‐point checklist based on pattern analysis. Arch Dermatol 1998; 134: 1563–70. [DOI] [PubMed] [Google Scholar]
- 17. Soyer HP, Argenziano G, Zalaudek I et al. Three‐point checklist of dermoscopy. A new screening method for early detection of melanoma. Dermatology 2004; 208: 27–31. [DOI] [PubMed] [Google Scholar]
- 18. Kittler H, Rosendahl C, Cameron A, Tschandl P. Dermatoscopy – an algorithmic method based on pattern analysis. Facultas WUV, 2011.
- 19. Rosendahl C, Cameron A, McColl I, Wilkinson D. Dermatoscopy in routine practice – 'chaos and clues'. Aust Fam Physician 2012; 41: 482–7. [PubMed] [Google Scholar]
- 20. Zalaudek I, Kreusch J, Giacomel J et al. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. Melanocytic skin tumors. J Am Acad Dermatol 2010; 63: 361–74. quiz 375–6. [DOI] [PubMed] [Google Scholar]
- 21. Argenziano G, Soyer HP, Chimenti S et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the internet. J Am Acad Dermatol 2003; 48: 679–93. [DOI] [PubMed] [Google Scholar]
- 22. Carli P, De Giorgi V, Chiarugi A et al. Effect of lesion size on the diagnostic performance of dermoscopy in melanoma detection. Dermatology 2003; 206: 292–6. [DOI] [PubMed] [Google Scholar]
- 23. Silverman MK, Golomb FM, Kopf AW et al. Verification of a formula for determination of preexcision surgical margins from fixed‐tissue melanoma specimens. J Am Acad Dermatol 1992; 27: 214–19. [DOI] [PubMed] [Google Scholar]
- 24. Elmore JG, Barnhill RL, Elder DE et al. Pathologists' diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ 2017; 357: j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med 2021; 384: 72–9. [DOI] [PubMed] [Google Scholar]
- 26. Tiodorovic‐Zivkovic D, Argenziano G, Lallas A et al. Age, gender, and topography influence the clinical and dermoscopic appearance of lentigo maligna. J Am Acad Dermatol 2015; 72: 801–8. [DOI] [PubMed] [Google Scholar]
- 27. Salerni G, Carrera C, Lovatto L et al. Benefits of total body photography and digital dermatoscopy ("two‐step method of digital follow‐up") in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol 2012; 67: e17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moloney FJ, Guitera P, Coates E et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5‐year follow‐up study. JAMA Dermatol 2014; 150: 819–27. [DOI] [PubMed] [Google Scholar]
- 29. Megaris A, Lallas A, Bagolini LP et al. Dermoscopy features of melanomas with a diameter up to 5 mm (micromelanomas): a retrospective study. J Am Acad Dermatol 2020; 83: 1160–1. [DOI] [PubMed] [Google Scholar]
- 30. Salerni G, Alonso C, Fernández‐Bussy R. A series of small‐diameter melanomas on the legs: dermoscopic clues for early recognition. Dermatol Pract Concept 2015; 5: 31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pupelli G, Longo C, Veneziano L et al. Small‐diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol 2013; 168: 1027–33. [DOI] [PubMed] [Google Scholar]


