Abstract
Objective
This study aimed to assess whether non‐invasive brain stimulation with transcranial alternating current stimulation at gamma‐frequency (γ‐tACS) applied over the precuneus can improve episodic memory and modulate cholinergic transmission by modulating cerebral rhythms in early Alzheimer's disease (AD).
Methods
In this randomized, double‐blind, sham controlled, crossover study, 60 AD patients underwent a clinical and neurophysiological evaluation including assessment of episodic memory and cholinergic transmission pre and post 60 minutes treatment with γ‐tACS targeting the precuneus or sham tACS. In a subset of 10 patients, EEG analysis and individualized modelling of electric field distribution were carried out. Predictors to γ‐tACS efficacy were evaluated.
Results
We observed a significant improvement in the Rey Auditory Verbal Learning (RAVL) test immediate recall (p < 0.001) and delayed recall scores (p < 0.001) after γ‐tACS but not after sham tACS. Face‐name associations scores improved with γ‐tACS (p < 0.001) but not after sham tACS. Short latency afferent inhibition, an indirect measure of cholinergic transmission, increased only after γ‐tACS (p < 0.001). ApoE genotype and baseline cognitive impairment were the best predictors of response to γ‐tACS. Clinical improvement correlated with the increase in gamma frequencies in posterior regions and with the amount of predicted electric field distribution in the precuneus.
Interpretation
Precuneus γ‐tACS, able to increase γ‐power activity on the posterior brain regions, showed a significant improvement of episodic memory performances, along with restoration of intracortical excitability measures of cholinergic transmission. Response to γ‐tACS was dependent on genetic factors and disease stage. ANN NEUROL 2022;92:322–334
The World Health Organization has declared Alzheimer's disease (AD) a priority health problem, due to its increasing incidence and high societal impact, and there is an urgent need for the identification of novel therapeutic targets. 1
Cholinergic enhancement has been the mainstay of AD therapeutics from 1996 up to now, 2 and very recently, aducanumab, which reduces beta amyloid plaques deposition, 3 has received Food and Drug Administration approval. 4
Along with cholinergic deficits and amyloid deposition as pathological hallmarks of AD, recent literature has highlighted gamma desynchronization as an early occurrence, thus holding the potential to be used as an additional therapeutic target. In particular, AD is characterized by a prominent disruption of oscillations in the gamma frequency band (30–80 Hz), which is proportional to disease severity and progression. 5 , 6
The clinical potential of restoring gamma oscillations via noninvasive brain stimulation has recently gained attention. Indeed, restoration of gamma oscillations by neural entrainment in animal models of AD induces a remarkable decrease in the pathological burden of amyloid and significantly improves cognitive performance. 7 , 8 , 9
In this context, transcranial alternating current stimulation (tACS) is a unique noninvasive tool which allows the modulation of brain rhythms at specific frequencies in pre‐defined cerebral regions, 10 and that can result in improvement of cognitive processes in healthy subjects. 11 , 12 , 13 , 14 tACS is easy to apply, safe, painless, inexpensive and deliverable in at‐home settings. 15 In a pilot study carried out in patients with mild dementia due to AD, we demonstrated that exposure to γ‐tACS targeting the precuneus led to a significant improvement in performance in several memory tasks, along with the restoration of intracortical excitability measures of cholinergic neurotransmission, compared to sham‐tACS. 16 Despite the promising results, several issues needed still to be addressed, and were the objective of the present study: (a) to confirm and extend in an independent larger sample of subjects the efficacy of γ‐tACS over the precuneus on episodic memory performances and cholinergic neurotransmission; (b) to demonstrate that γ‐tACS can modulate brain activity and that the predicted enhancement of gamma activity accounted for (or at least was associated with) the memory effects; and (c) to assess possible predictors of response to the γ‐tACS intervention, such as apolipoprotein E (ApoE) genotype, the major recognized genetic risk factor for late‐onset AD, 17 and brain derived neurotrophic factor (BDNF) genotype, previously shown to affect transcranial stimulation effectiveness. 18 , 19
Methods
Participants
Participants fulfilling current criteria for AD 20 were recruited at the Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
At enrollment, each patient underwent a standardized neuropsychological assessment, as previously published, 21 a structural imaging study, and a blood sampling for genotype analyses. Cognitive reserve was evaluated with the Italian version of the Cognitive Reserve Index (CRIq) which is based on education levels, working activities, and leisure time. 22
AD diagnosis was corroborated by either cerebrospinal fluid (CSF) analysis supporting an AD pathological process (Aβ1−42 ≤ 600 ng/L and tau≥400 ng/L) or positive amyloid‐positron emission tomography (PET) scan. 23
The following exclusion criteria were applied: (a) cerebrovascular disorders, hydrocephalus, and intracranial mass documented by magnetic resonance imaging (MRI); (b) history of traumatic brain injury; (c) serious medical illness other than AD; (d) history of seizures; (e) metal implants in the head; (f) electronic implants (i.e., pacemaker).
Participants who were already on a pharmacologic regimen were allowed to continue it provided it had been unchanged for 6 weeks prior to the intervention, but initiation of drugs after the start of the observation period was not allowed.
Full written informed consent was obtained from all participants according to the Declaration of Helsinki. The study protocol was approved by the local ethics committee (Brescia Hospital, #NP4479). The trial was registered at ClinicalTrials.gov (NCT04842955).
Study Design
For the main study, participants were randomized into two groups in a 1:1 ratio and each group received a single session of exposure to γ‐tACS targeting the precuneus or a single session of sham‐tACS first and, after 1 week, stimulation was inverted (crossover phase) (see Supporting Information Figure S1, which is available online). In each session, a set of tasks assessing episodic memory was tested twice, at baseline (pre‐stimulation) and after tACS (post‐stimulation). Moreover, a memory task assessing associative memory was carried out during the last 20 minutes of tACS stimulation (see neuropsychological assessment below). In each session, a transcranial magnetic stimulation (TMS) protocol assessing short‐latency afferent inhibition (SAI), an indirect measure of cholinergic transmission, was tested twice in all subjects, at baseline (pre‐stimulation) and after tACS (post‐stimulation) (see TMS assessment below). In a subset of 10 participants, an electroencephalogram (EEG) was recorded twice in each session, at baseline (pre‐stimulation) and immediately after tACS (post‐stimulation), before TMS assessment (see EEG assessment below).
We performed two supporting information studies in order to assess the specific effects of γ‐tACS on other cognitive domains (executive functions, verbal fluency and visuospatial abilities ‐ supporting information study 1) and site (regarding the specific stimulated brain region ‐ supporting information study 2) (see Supporting Information Figure S1).
For supporting information study 1, 12 participants were randomized into two groups in a 1:1 ratio and each group received a single session of exposure to γ‐tACS targeting the precuneus or a single session of sham‐tACS first and, after 1 week, stimulation was inverted (crossover phase). In each session, a set of tasks assessing executive functions, verbal fluency and visuospatial abilities (digit span backward, phonemic and semantic fluencies, trail making test part A and B, clock drawing test) was tested twice, at baseline (pre‐stimulation) and after tACS (post‐stimulation).
For supporting information study 2, 12 participants were randomized into two groups in a 1:1 ratio and each group received a single session of exposure to γ‐tACS targeting the right dorsolateral prefrontal cortex (rDLPFC) or a single session of sham‐tACS first and, after 1 week, stimulation was inverted (crossover phase). In each session, the same set of memory tasks performed in the main study was tested twice, at baseline (pre‐stimulation) and after tACS (post‐stimulation).
Sample size for both supporting information studies was calculated based on results of the main study, to obtain a minimum set of participants in whom significant effects could be observed by assessing each specific cognitive test.
The participants and the examiners performing clinical ratings, tACS, EEG and TMS protocols were blinded to the type of stimulation. B.B. was responsible for random allocation sequences, enrollment of participants, allocation concealment, and assignment of participants to specific interventions. Computer‐assisted block randomization was used to randomize subjects into groups that resulted in equal sample sizes.
According to literature data, the effects of a single session of tACS are expected to last for 30–70 minutes. 24 Hence, participants were expected to return to their initial clinical status between the two stimulation sessions which were separated by at least a week.
Outcome Measures
The primary endpoints were a priori defined as: (a) the change from baseline in episodic and associative memory scores after γ‐tACS, compared to after sham stimulation; and (b) change from the baseline in gamma brain activity after γ‐tACS, compared to after sham stimulation.
The secondary endpoints were defined as: (a) changes from baseline in cholinergic transmission, evaluated indirectly with TMS, and (b) differences in γ‐tACS effects on memory performance according to potential predictors, such as ApoE and BDNF genotypes, along with demographic and clinical covariates.
Cognitive Assessment
To assess the effect of tACS on episodic memory, at each time‐point (pre‐stimulation and post‐stimulation) of γ‐tACS targeting the precuneus and sham‐tACS, the Rey Auditory Verbal Learning (RAVL) test was carried out, and total recall and long delayed recall were considered. 25 Different lists were randomized and used during pre‐ and post‐stimulation to avoid learning effects.
The Face‐Name Association memory Task (FNAT) was used to assess the patient's associative memory and was composed of encoding and retrieval phases. 16 , 26 Subjects were seated in a dimly lit room, facing a computer monitor that was placed 60 cm from the subject. The stimuli were presented using Presentation software (Version 14.9, www.neurobs.com) running on a personal computer with a 27‐inch screen. During the encoding phase, the patient was shown a gray‐scale picture of a face on a monitor together with a proper name, and the patient was required to tell the researcher whether the face belonged to a woman or a man and was required to encode the face‐name association. A set of 20 unfamiliar faces was associated to a set of 20 unfamiliar proper names (10 male, 10 female). During the retrieval phase, the patient was shown a face together with four proper names (the correct name, two previously presented names and one new name), and the patient was asked to associate the correct name with each face. Responses were collected via a response‐box, and the stimuli remained on the screen until the response was made.
EEG Recordings
EEG was performed using a NicoletOne EEG system and was continuously recorded from 19 scalp sites positioned according to the 10–20 International System. The ground electrode was positioned in Fpz, while the reference one was positioned at Cz. The electrode for delivering tACS (Pz) was kept on site during the entire recording, in order to avoid reapplication of the EEG cap after tACS and was thus excluded from the EEG recording. The EEG signals were band‐pass filtered at 0–500 Hz and digitized at a sampling rate of 1,024 Hz. Skin/electrode impedance was maintained below 5 kΩ. Resting EEG was recorded for 10 min with eyes closed before and after sham and γ‐tACS in two separate sessions at least 1 week apart.
EEG Analysis
EEG was analyzed using the freely available academic software Cartool (https://sites.google.com/site/cartoolcommunity). As a first step, data were re‐referenced to Fp1, downsampled to 256 Hz and band‐pass filtered between 1 and 100 Hz (Non‐causal Butterworth filter). Then, independent component analysis (ICA) was applied to remove eye‐movement (eye blinks and saccades). 27 Artifact‐free epochs of 2 second duration were then visually selected. A fast Fourier transform (FFT) was then calculated for each channel and each 2 second epoch using a Hanning window and averaged across all epochs. The resulting power values were then averaged across four bands: theta (3–6 Hz), alpha (6–12 Hz), beta (12–20 Hz), and gamma (20–40 Hz).
TMS Assessment
A TMS figure‐of‐eight coil (each loop diameter 70 mm) connected to a monophasic Magstim Bistim 2 system (Magstim Company, Oxford, UK) was used. 28 Motor evoked potentials (MEPs) were recorded from the right first dorsal interosseous muscle through surface Ag/AgCl electrodes placed in a belly‐tendon montage and acquired using a Biopac MP‐150 electromyograph (BIOPAC Systems Inc., Santa Barbara, USA). The TMS coil was held tangentially over the scalp region corresponding to the primary hand motor area contralateral to the target muscle, with the coil handle pointed 45° posteriorly and laterally to the sagittal plane. The “hot spot” was defined as the scalp location from which magnetic stimulation resulted in motor evoked potentials (MEPs) of greatest amplitude with the minimum stimulation intensity, as previously reported. 29
SAI was studied using a paired‐pulse protocol, employing a conditioning‐test design. 30 The test stimulus (TS) was adjusted to evoke an MEP of approximately 1 mV peak‐to‐peak amplitude, while the conditioning stimulus (CS) consisted of a single pulse (200 μs) of electrical stimulation to the right median nerve at the wrist, using a bipolar electrode with the cathode positioned proximally, at an intensity sufficient to evoke a visible twitch of the thenar muscles. Different interstimulus intervals (ISIs) were assessed (0, +4 ms), which were fixed relative to the peak latency of the N20 component of the somatosensory evoked potential of the median nerve. For each ISI, 10 different paired CS‐TS and control TS were delivered in all participants in a pseudo randomized sequence, with an inter‐trial interval of 5 seconds (±10%).
Audio‐visual feedback was provided to ensure muscle relaxation during the entire experiment and trials were discarded if EMG activity exceeded 100 μV prior to TMS stimulus delivery. Less than 5% of trials were discarded for each protocol. All of the participants were capable of following instructions and reaching complete muscle relaxation.
Computational Modeling of Electric Field Distribution
Brain images were collected using a 3 T MRI scanner (Siemens Skyra, Erlangen, Germany), equipped with a circularly polarized transmit–receive coil to acquire 3D magnetization‐prepared rapid gradient echo (MPRAGE) T1‐weighted scans (repetition time 3,200 ms, echo time 402 ms, matrix 256 × 256, field of view 282 mm, slice thickness 1.10 mm, flip angle 8°). This dataset was fused with a model derived from the Visible Human Project to extend the field of volume to the level of the shoulders to mimic the exact experimental montage. The standard Laplace equation was applied considering volume conduction. While the current direction reverses at regular intervals, with the Pz electrode serving as the anode in one cycle and cathode in the other, the cortical electric field (EF) magnitude plot, which indicates where the current is flowing, does not change. The region corresponding to the precuneus was segmented and individually analyzed.
ApoE and BDNF Genotyping
Genomic DNA was extracted from whole peripheral blood using Maxwell® 16 Blood DNA Purification Kit with Maxwell® 16 Instrument (both Promega). The regions encompassing both APOE rs429358 and rs7412 and BDNF rs6265 polymorphisms were amplified by polymerase chain reaction (PCR) using GoTaq® Hot Start Polymerase (Promega) or Optimase® Polymerase (ADS Biotech). PCR products were purified with Amicon® Ultra 0.5 mL Centrifugal Filters (Merck Millipore). Cycle sequencing was performed with the AB Prism Big Dye Terminator Sequencing kit 3.1 (Life Technologies), following the manufacturer's instructions. Sequences were subsequently purified using MicroSEQ™ ID Sequencing Clean‐up Cartridges (Life Technologies) and then loaded on a 3,500 Genetic Analyzer (Life Technologies). All sequences were analyzed using the Chromas software (Technelysium Pty Ltd).
γ‐tACS
A single session of tACS was delivered by a battery‐driven current stimulator (BrainStim, EMS, Italy) through a pair of saline‐soaked (0.9% NaCl) surface sponge electrodes (5.5 × 6 cm). One electrode was placed on the scalp over the precuneus (with the center over Pz position according to the 10–20 international EEG coordinates) and the other over the right deltoid muscle. This particular montage was chosen after performing computational modelling of electric field distribution and considering a previous study with similar montages, showing that tACS with an extracephalic electrode led to significant entrainment of brain oscillations (reported as phase stability) compared to other cephalic montages. 31 For supporting information study 1, we adopted the same montage, while for supporting information study 2, one electrode was placed on the scalp over the rDLPFC (with the center over F4 position according to the 10–20 international EEG coordinates) and the other over the right deltoid muscle.
The electrodes were secured using elastic gauzes, and the electroconductive gel was applied to electrodes to reduce contact impedance (<5 kΩ for all sessions).
During single session real stimulation, an alternating sinusoidal current of 1.5 mA peak‐to‐baseline (3.0 mA peak‐to‐peak, current density: 0.09 mA/cm2) at a frequency of 40 Hz was applied for 60 minutes. For the sham condition, the electrode placement was the same, but the electric current was ramped down 60 seconds after the beginning of the stimulation to make this condition indistinguishable from the experimental stimulation. To detect differences in the perception of the stimulation, participants were asked whether they thought they received real or sham stimulation at the end of each session, and if they perceived tingling cutaneous sensations or phosphenes/light flickering. Sensations were rated on a scale from 0 to 4, with 0 = no sensations reported, 1 = mild, 2 = moderate, 3 = strong, 4 = very strong sensations reported.
During stimulation (both real and sham), participants were sitting in a comfortable chair in a well‐lit and quiet room, keeping their eyes open and asked not to speak or move significantly.
Statistical Analyses
Data are expressed as mean ± standard deviation, unless otherwise stated. Baseline demographic and clinical variables were compared across groups using Student's t‐test or Fisher's tests, as appropriate. Cohen's Kappa was run to determine if there was an agreement between the type of sensation perceived and the type of stimulation received. A Wilcoxon signed‐rank test was used to evaluate differences in perception of cutaneous sensation during real and sham stimulation.
To assess the effect of exposure to γ‐tACS (main study and supporting information studies 1 and 2), the cross‐over design was analyzed with a linear mixed‐effect model by restricted maximum likelihood (REML), considering baseline values, treatment (sham vs. real), block (first session of exposure vs. second session of exposure after 1 week) and randomization (sham‐real vs. real‐sham) as fixed effects, and patients as random effects. This was adopted to avoid any potential carry‐over effects of stimulation on clinical outcomes.
Changes in γ‐tACS efficacy associated with potential predictors were evaluated with linear regression models using improvement of cognitive scores after γ‐tACS stimulation, or γ‐tACS vs. sham tACS, as response variables, and ApoE genotype (coded as 0 = no ε4 alleles, 1 = one ε4 allele, 2 = two ε4 alleles) and BDNF genotype (coded as 0 = no M alleles, 1 = one or two M alleles), along with demographic and clinical characteristics as covariates.
With regard to EEG analyses, paired t‐tests were used to compare post vs. pre γ‐tACS or sham stimulation. As exploratory analysis, Spearman rank‐order correlations were used to assess associations between the improvement in memory scores and the increase in gamma frequencies at specific brain regions. A two‐sided p‐value<0.05 was considered significant.
Statistical analyses were performed using SPSS version 21 (SPSS, Inc., Chicago, USA).
Results
Participants
Seventy participants were initially screened, with two participants not meeting inclusion criteria because they were carriers of an electronic implant (pacemaker) and two more because of a diagnosis of epilepsy; six were excluded because negative to AD biomarkers (see Supporting Information Figure S2). Sixty participants (mean age ± SD = 72.3 ± 7.0 years; female = 51.7%, mean Mini‐Mental State Examination (MMSE) score ± SD = 23.9 ± 4.2; mean disease duration±SD = 3.1 ± 2.4 years) were enrolled and randomized to receive γ‐tACS or sham stimulation first in a 1:1 ratio (see Supporting Information Table S1 for demographic and clinical characteristics).
All participants completed the study and were included in the final analysis. No tACS‐related side effects were observed, and tACS was well tolerated by all participants. Regarding the differences in the participants' perception of the stimulation, there was no statistically significant association between type of stimulation, as assessed by Cohen's Kappa (κ = −0.10, p = 0.432). Moreover, tingling cutaneous sensations were equally perceived in both real and sham conditions (z = 0.852, p = 0.394 by Wilcoxon signed‐rank test), and none of the participants reported phosphenes/light flickering, suggesting that exposure to γ‐tACS targeting the precuneus could not be distinguished from sham stimulation.
Effects on Episodic Memory
Results for RAVLT immediate and delayed recall, and FNAT scores, pre‐ and post‐stimulation are reported in Table 1 and Figure 1.
TABLE 1.
Memory Scores and TMS Measures Before and After Precuneus γ‐tACS or Sham Stimulation
| Variable | Sham‐tACS | γ‐tACS Targeting the Precuneus | ||
|---|---|---|---|---|
| Baseline | Post tACS | Baseline | Post tACS | |
| Memory tasks | ||||
| RAVL, immediate recall | 20.3 ± 6.5 | 18.7 ± 6.3 | 18.4 ± 6.9 | 25.6 ± 8.4 b , c |
| RAVL, delayed recall | 1.4 ± 1.5 | 1.0 ± 1.2 | 1.5 ± 1.4 | 2.5 ± 2.1 b , c |
| FNAT a | 5.5 ± 2.4 | 8.2 ± 3.1 b | ||
| TMS assessment | ||||
| Mean SAI (0, +4 ms) | 0.83 ± 0.15 | 0.83 ± 0.14 | 0.86 ± 0.11 | 0.50 ± 0.13 b , c |
Results are expressed as mean ± standard deviation.
For FNAT, results are reported during stimulation.
Significant difference compared to sham stimulation.
Significant difference compared to baseline.
tACS = transcranial alternating current stimulation; RAVL = Rey Auditory Verbal Learning test; FNAT = face–name associations task; TMS = transcranial magnetic stimulation; SAI = short‐latency afferent inhibition.
FIGURE 1.
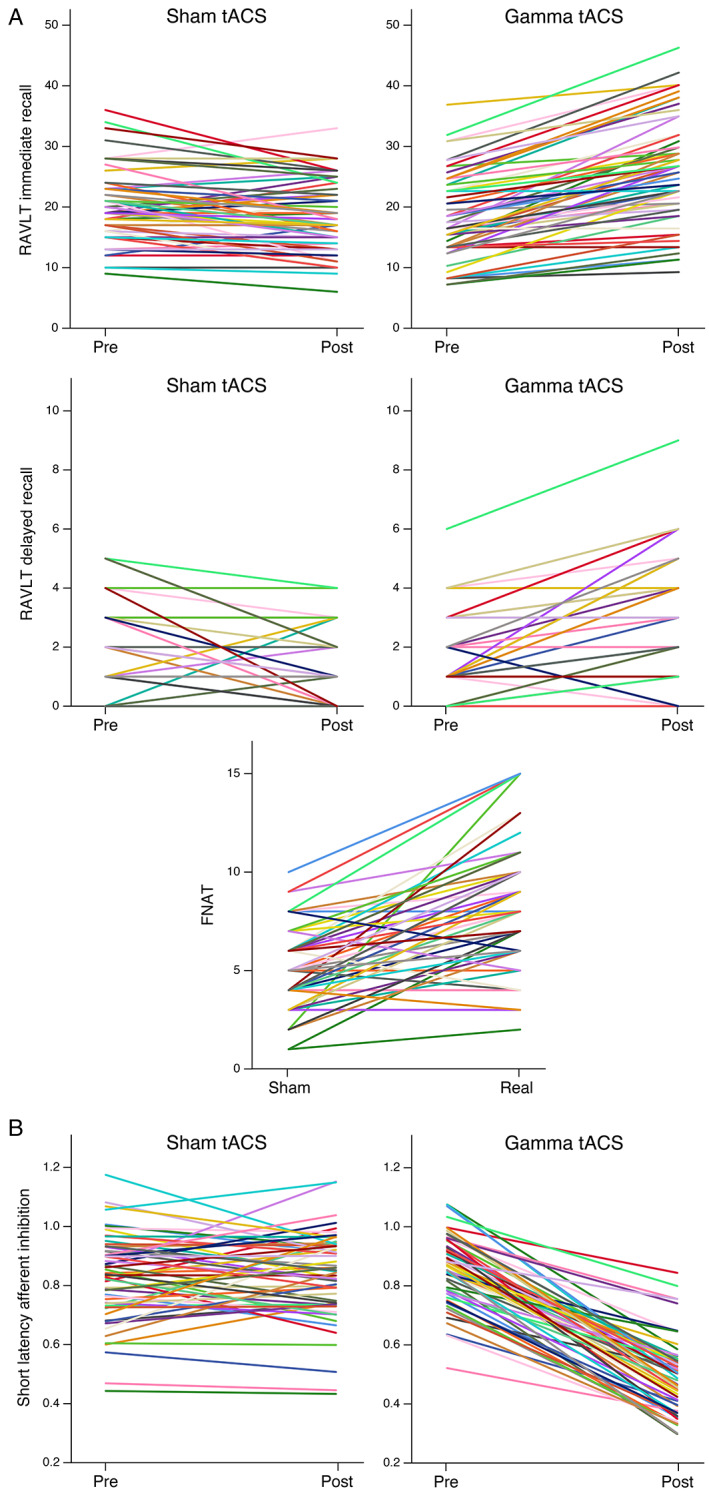
Neuropsychological and neurophysiological scores pre and post sham or γ‐tACS. (A) Spaghetti plots of RAVL total recall, RAVL long delayed recall, FNAT scores. (B) Spaghetti plots of SAI measures. Legend: RAVL = Rey Auditory Verbal Learning test; FNAT = face–name associations task; tACS = transcranial alternating current stimulation; SAI = short‐latency afferent inhibition. *For FNAT, results are reported during stimulation.
By applying mixed‐effect models, we observed a significant effect of treatment (γ‐tACS vs sham‐tACS) on the RAVLT immediate recall (p < 0.001), with an estimate difference of −7.0 (90% confidence interval [CI] = −8.2 to −5.8) points, and on the RAVLT delayed recall (p < 0.001), with an estimate difference of −1.6 (90%CI = −2.0 to −1.2) points between treatments. In the FNAT, we observed a significant effect of treatment (p < 0.001), with an estimate difference of −2.9 (90%CI = −3.6 to −2.3) points between treatments (see Table 2).
TABLE 2.
Linear Mixed‐Effects Models Output of the Cross‐Over Design
| Variable | RAVLT Immediate | RAVLT Delayed | FNAT | SAI | ||||
|---|---|---|---|---|---|---|---|---|
| β ± SE | p‐Value | β ± SE | p‐Value | β ± SE | p‐Value | β ± SE | p‐Value | |
| (Intercept) | 10.2 ± 1.4 | <0.001 | 1.45 ± 0.28 | <0.001 | 8.20 ± 0.48 | <0.001 | 0.13 ± 0.08 | 0.109 |
| Pre‐treatment test score | 0.87 ± 0.07 | <0.001 | 0.72 ± 0.09 | <0.001 | ‐ | ‐ | 0.43 ± 0.09 | <0.001 |
| Treatment (real vs. sham) | −7.02 ± 0.70 | <0.001 | −1.61 ± 0.25 | <0.001 | −2.95 ± 0.42 | <0.001 | 0.35 ± 0.02 | <0.001 |
| Block (T1 vs. T2) | −1.89 ± 0.72 | 0.011 | 0.28 ± 0.25 | 0.271 | 0.018 ± 0.42 | 0.965 | −0.01 ± 0.02 | 0.890 |
| Randomization (SR vs RS) | −1.10 ± 0.88 | 0.213 | −0.12 ± 0.25 | 0.635 | 0.17 ± 0.563 | 0.767 | −0.01 ± 0.02 | 0.691 |
RAVL = Rey Auditory Verbal Learning test; FNAT = face–name associations task; SAI = short‐latency afferent inhibition; T1 = first session of exposure; T2 = second session of exposure after 1 week; SR = block 1 sham and block 2 real; RS = block 1 real and block 2 sham (see Supporting Information Figure 1 for details). β ± SE = regression coefficient estimate ± standard error.
Effects on Executive Functions, Verbal Fluency and Visuospatial Abilities
In supporting information study 1, by applying mixed‐effect models, we did not observe any significant effect of treatment on digit span backward (p = 0.600), phonemic fluencies (p = 0.439), semantic fluencies (p = 0.814), clock drawing (p = 0.984), or trail making test part A (p = 0.500) and B (p = 0.499).
Effects of γ‐tACS on Stimulation Site
In supporting information study 2, we evaluated if the effects of γ‐tACS on cognition were site‐specific, and applied rDLPFC γ‐tACS compared to sham tACS. Contrary to what observed for precuneus γ‐tACS, we did not observe significant effects of treatment (γ‐tACS vs sham‐tACS) on the RAVLT immediate recall (p = 0.942), RAVLT delayed recall (p = 0.983), and on FNAT scores (p = 0.588).
Effect on Cholinergic Dysfunction
TMS measures of cholinergic inhibition, evaluated with SAI, are reported in Table 1 and Figure 1 at each time point. We observed a significant effect of treatment (p < 0.001), with an estimate difference of +0.35 (90%CI = +0.31 to +0.39) points between treatments (see Table 2).
SAI restoration (i.e., the difference between post γ‐tACS and pre γ‐tACS), corrected for donepezil intake, directly correlated with improvement of RAVL delayed recall scores after γ‐tACS (i.e., the difference between post γ‐tACS and pre γ‐tACS scores, r = 0.271, p = 0.038) as well as with the difference of FNAT scores (i.e., the difference between the score during γ‐tACS and during sham‐tACS, r = 0.307, p = 0.018). No significant correlations between SAI restoration and improvement of RAVL immediate recall scores was found (see Supporting Information Figure S3).
Predictors of γ‐tACS Efficacy
As an exploratory analysis, we examined the predictors of improvement after γ‐tACS (i.e., the difference between post γ‐tACS and pre γ‐tACS scores, or γ‐tACS vs. sham tACS).
Of the considered predictors of RAVL immediate recall improvement, ApoE genotype and MMSE scores were statistically significant (p < 0.001, see Table 3).
TABLE 3.
Predictors of Memory Improvement After Real γ‐tACS Stimulation
| Variable | RAVLT Immediate Improvement° | RAVLT Delayed Improvement° | FNAT Difference^ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 90%CI | p‐Value | β | 90%CI | p‐Value | β | 90%CI | p‐Value | |
| Age at onset, year | −0.10 | −0.23 to 0.02 | 0.17 | −0.06 | −0.10 to − 0.01 | 0.03 | −0.04 | −0.13 to 0.05 | 0.47 |
| Sex, male | 1.15 | −0.54 to 2.84 | 0.27 | −0.17 | −0.76 to 0.42 | 0.63 | 0.83 | −0.37 to 2.05 | 0.26 |
| Education, years | 0.14 | −0.16 to 0.44 | 0.14 | −0.02 | −0.12 to 0.09 | 0.80 | −0.31 | −0.5 to − 0.10 | 0.02 |
| Cognitive reserve | −0.01 | −0.07 to 0.07 | 0.93 | 0.02 | −0.01 to 0.04 | 0.32 | 0.03 | −0.02 to 0.08 | 0.37 |
| MMSE score | 0.57 | 0.35 to 0.79 | <0.001 | 0.13 | 0.05 to 0.21 | 0.01 | 0.10 | −0.06 to 0.26 | 0.33 |
| BADL score | 2.32 | 0.02 to 4.62 | 0.10 | 1.08 | 0.28 to 1.89 | 0.04 | −1.6 | −3.57 to 0.44 | 0.21 |
| NPI score | 0.01 | −0.12 to 0.15 | 0.86 | 0.02 | −0.03 to 0.06 | 0.56 | −0.04 | −0.14 to 0.06 | 0.55 |
| ApoE (ε4 carriers) | −3.58 | −4.86 to − 2.30 | <0.001 | −0.92 | −1.37 to − 0.47 | 0.002 | −1.22 | −2.1 to − 0.30 | 0.04 |
| BDNF (M carriers) | 0.44 | −1.39 to 2.26 | 0.70 | −0.33 | −0.97 to 0.30 | 0.39 | −0.76 | −2.05 to 0.54 | 0.35 |
RAVL = Rey Auditory Verbal Learning test; FNAT = face–name associations task; CI = confidence intervals; MMSE = Mini‐Mental State Examination; BADL = basic activities of daily living; NPI = Neuropsychiatry Inventory; ApoE = aaaapolipoprotein E; BDNF = brain derived neurotrophic factor.
°Improvement = difference between scores after γ‐ACS and scores before γ‐tACS; ^ difference = difference between FNAT score during γ‐tACS and FNAT score during sham‐tACS.β = regression coefficient estimate; 90%CI = 90% confidence interval of β. Significant p‐values are reported in boldface.
In particular, the greatest improvement was observed in ApoE ε4 non‐carriers (9.6 ± 4.2 points), with a progressive loss of RAVL immediate recall improvement in heterozygous ApoE ε4 carriers (−3.58, 90%CI = −4.86 to −2.30) and further in ApoE ε4/ε4 carriers as compared to ApoE ε4 non‐carriers (−7.16, 90%CI = −9.72 to −4.60). Moreover, the milder the disease stage (as measured by MMSE), the greater the improvement in the RAVL immediate recall (see Table 3).
When predictors of RAVL delayed recall improvement were considered, comparable results were obtained. ApoE genotype was found statistically significant (p = 0.001), with a progressive loss of RAVL delayed recall improvement from ApoE ε4 non‐carriers (1.73 ± 1.3 points) to ApoE ε4 heterozygous carriers (−0.92, 90%CI = −1.37 to −0.47), and to ApoE ε4/ε4 carriers (−1.84, 90%CI = −2.74 to −4.60). Milder disease stage, preserved functional activities of daily living score, and younger disease onset were associated to greater RAVL delayed recall improvement (all p < 0.05, see Table 3).
When the difference of FNAT scores between γ‐tACS and sham‐tACS was considered, ApoE genotype was statistically significant (p = 0.034), the greatest difference being observed in ApoE ε4 non‐carriers (3.6 ± 2.2 points), with a progressive score reduction in ApoE ε4 heterozygous carriers (−1.21, 90%CI = −2.13 to −0.30), and further in ApoE ε4/ε4 carriers (−2.42, 90%CI = −4.26 to −0.60). In addition, a significant direct correlation between education and FNAT improvement was found (p = 0.019) (see Table 3).
We did not observe any significant associations between the improvement in cognitive scores after γ‐tACS and sex, cognitive reserve index, NPI scores or BDNF genotype (all p > 0.05).
EEG Analysis
EEG recordings were acquired in a subset of randomly selected 10 participants (age = 74.8 ± 3.7 years, female = 50%). Compared to pre‐stimulation, immediately after γ‐tACS we observed a significant relative gamma power increase (20–40 Hz) on electrodes T4 and O2, a significant decrease in theta power (3–6 Hz) at electrodes F3, T3, and T4, and a significant increase in beta power (12–20 Hz) on electrodes T5, P3, T6, and O2 (all p < 0.05). Relative alpha frequencies were not significantly modulated after γ‐tACS (see Figure 2 and Supporting Information Figure S4).
FIGURE 2.
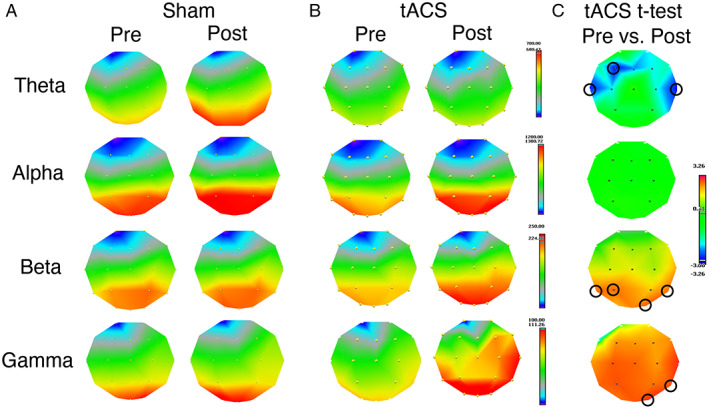
Result of the EEG frequency analysis. Frequency bands: theta (3–6 Hz), alpha (6–12 Hz), beta (12–20 Hz), gamma (20–40 Hz). (A) Power maps pre and post sham stimulation. (B) Power maps pre and post γ‐tACS. (C) t‐Maps of the paired t‐test post vs. pre γ‐tACS. Blue areas indicate a power decrease after γ‐tACS, red areas a power increase. Significant effects at p < 0.05 were found on electrodes F3, T3, and T4 for theta (decrease after γ‐tACS), on electrodes P3, T5, T6, and O2 for beta (increase after γ‐tACS), and on electrodes T4 and O2 for gamma. No effect was found in the alpha band after γ‐tACS and no effect was found on any bands when comparing pre vs. post sham stimulation. Legend: EEG = electroencephalography; tACS = transcranial alternating current stimulation.
We did not observe significant modulation of any frequency ranges when comparing pre to post sham stimulation.
We observed a significant positive correlation between the increase in gamma frequencies in the parietal lobes (average P3 and P4) and the improvement at the RAVL delayed recall (i.e., the difference between post γ‐tACS and pre γ‐tACS scores; r s = 0.724, p = 0.018) and FNAT scores (i.e., the difference between the score during γ‐tACS and during sham‐tACS; r s = 0.815, p = 0.005), but not in frontal, temporal or occipital regions (see Supporting Information Figure S5).
Computational Modelling of Electric Field Distribution
We performed individualized computation modelling of electric field distribution in a subset of thirteen participants. After precuneus segmentation, which was the hypothesized target of γ‐tACS, the maximum value of the induced electric field (IEF) was 0.35 ± 0.15 V/m, whereas the mean IEF was to be 0.09 ± 0.02 V/m.
We observed a significant positive correlation between IEF electric field values and the improvement at the RAVL immediate (maximum IEF r s = 0.693, p = 0.009; mean IEF r s = 0.523, p = 0.067) and delayed recall (maximum IEF r s = 0.712, p = 0.006; mean IEF r s = 0.697, p = 0.008).
Discussion
The first goal of any treatment in AD is to improve memory functions, hopefully reverting the ongoing pathological process. Brain oscillations, which arise from synchronized interactions between neuronal populations, are essential for cognitive performances, and gamma oscillations have been shown to be associated with long‐term memory‐related synaptic changes in the hippocampus. 32 , 33 Accordingly, AD patients present dysregulation of gamma activity, which represents an early event and might trigger clinical onset. 34 , 35 , 36
In patients with mild dementia due to AD, a small number of recent studies has suggested the potential therapeutic benefits of γ‐tACS, 15 , 37 , 38 , 39 able to modulate brain activity and entrain gamma rhythms by low‐amplitude alternating sinusoidal currents. 40 , 41
The present study confirms and extends previous findings suggesting a potential beneficial effect of precuneus γ‐tACS on memory in AD patients. Notably, we found a significant correlation between enhancement of episodic memory after γ‐tACS and the betterment of indirect measures of cholinergic neurotransmission, consistent with the link between acetylcholine levels and gamma oscillations in AD. 42 , 43 , 44
The aforementioned cognitive benefits were corroborated by electrophysiological changes observed after γ‐tACS stimulation in patients with AD, resulting in entrainment of gamma frequency, but also in increased beta power activity on the posterior brain regions and decreased theta power activity on the anterior brain regions. The improvement in long‐term memory correlated with the increase of gamma activity over posterior regions, suggesting a site‐specific effect of γ‐tACS, involving structures as the posterior parietal cortices and the precuneus. Moreover, computational modelling based on individual patients' MRIs showed a positive correlation between the current that effectively reached the precuneus and the improvement after γ‐tACS at the RAVL immediate and delayed recall.
It is difficult to compare the present study to recently published investigations using γ‐tACS in AD, 15 , 39 as we targeted the precuneus because it is one of the first regions to be affected in AD and deeply involved in associative and episodic memory. 45
Moreover, compared to most of the studies in the literature, we employed tACS rather than other non‐invasive stimulation methods. 46 Indeed, as compared to transcranial direct current stimulation (tDCS), tACS is able to enhance synchronization of cortical oscillations beyond restoring brain plasticity, 47 and as compared to repetitive transcranial magnetic stimulation (rTMS), tACS may be easily delivered in at‐home settings. 48 , 49 In this view, a recent pilot study carried out on two AD patients demonstrated the safety of home‐based, remotely‐monitored and caregiver‐administered tACS intervention. 15
We selected the precuneus as the target area to be stimulated, because it is one of the first regions to be affected in prodromal AD and the hub of the default mode network (DMN), functionally connected to mesial temporal regions, while being easily accessible by transcranial stimulation. Alterations of the DMN have been identified as responsible of memory impairment 50 , 51 , 52 and the precuneus has been described as a key node for memory functioning. 53 , 54 Recently, high‐frequency rTMS over the precuneus has demonstrated an improvement in episodic memory associated to brain connectivity changes in AD patients, suggesting that precuneus stimulation might represent a useful technique in order to enhance episodic memory. 55 The improvement observed at the FNAT, which has been shown to depend primarily on hippocampal structures, 56 could be explained by the entrainment of large‐scale cortical network activity by network resonance, functionally connected to the precuneus via the DMN 57 consistent with previous studies showing that parietal cortical stimulation modulates neural activity in a hippocampal‐cortical network that supports episodic memory processing. 58 , 59
Interestingly, we also identified the predictors of tACS efficacy. Our study clearly indicated that an increased response pattern was influenced by ApoE genotype, with greater cognitive improvement in ApoE ε4 non‐carriers, and with a progressive loss of stimulation efficacy in subjects with at least one ApoE ε4 allele, and even more in subjects with two ApoE ε4 alleles. This is in agreement with previous studies emphasizing that interventions designed to modify cholinergic transmission 60 or to alter amyloid load in AD may interact with ApoE genotype, resulting in differential efficacy and outcome. 61 This observation may be an important factor to consider in future trial design. We did not observe a significant effect of BDNF genotype nor for cognitive reserve on clinical improvement after γ‐tACS. Furthermore, as reported in previous AD pharmacological treatments, 62 we found that milder disease stage was associated with greater improvement of episodic memory performances after stimulation.
The observed effects on the primary outcomes, i.e., the improvement in RAVL immediate and delayed recall and FNAT scores, after γ‐tACS, although small, were still large enough to meet the criteria for minimal clinically important difference (MCID), that is defined as the smallest change in a treatment outcome that an individual patient would identify as important and which would indicate a change in the patient's management. 63 Moreover, we demonstrated that these effects were site specific (i.e., γ‐tACS over the dorsolateral prefrontal cortex did not improve memory performances) and specifically restored memory functions (i.e., γ‐tACS over the precuneus did not improve other cognitive domains).
We acknowledge that this study entails some limits. First, we evaluated the effects of a single session of γ‐tACS over the precuneus, but long‐term effects need to be assessed in multisession trials. Second, a larger, multicenter sample of subjects may further strengthen the results and account for possible confounders. However, we applied a crossover trial, which is statistically efficient and requires fewe subjects than non‐crossover designs; further, the influence of confounding covariates is reduced because each subject serves as his or her own control. 64 Third, the change in RAVL delayed recall scores, although statistically significant, was minimal, particularly considering that patients at this stage have virtually absent delayed recall on the RAVL. Fourth, we did not assess long‐term effects after γ‐tACS, which are probably unrealistic to see after a single 60‐minute stimulation, and should be ideally evaluated after multiple repeated sessions of γ‐tACS. Finally, learning effects should be considered, even though we administered different sets of memory tests during the experimental neuropsychological assessment.
In conclusion, γ‐tACS over the precuneus can safely and efficiently induce entrainment of neural oscillations in patients with AD, improving memory functions and ameliorating cholinergic deficits. The refinement of predictors of outcome may best identify patients who may benefit most from γ‐tACS stimulation.
These findings suggest that γ‐tACS stimulation over the precuneus may represent a novel therapeutic approach in AD. Future studies with multisession γ‐tACS and with at‐home setting design are warranted.
Author Contributions
A.B. and B.B. contributed to the conception and design of the study; A.B., V.C., M.G., L.B., C.M.M., A.D., C.T., S.G., M.S.C., M.B., E.P., Y.G., M.C., M.P., F.P., M.S., S.A., E.S., A.P., A.P.L., and B.B. contributed to the acquisition and analysis of data; A.B., L.B., and B.B. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
A.B. and B.B. have pending patent on the use of non‐invasive brain stimulation to increase memory functions in patients with Alzheimer Disease.
Supporting information
Appendix S1. Supporting Information.
Acknowledgments
The authors thank all patients for their participation in this research, and Ilenia Libri and Jasmine Rivolta for the great support in neurophysiological and clinical evaluations. The present work was supported by the Airalzh‐AGYR2020 grant issued to AB and by the Italian Ministry of Health (Ricerca Corrente), issued to MC. Open Access Funding provided by Universita degli Studi di Brescia within the CRUI‐CARE Agreement.
Data Availability
All data, includeing outcome measure results, study protocol, and statistical analysis plan, will be shared through ClinicalTrials.gov via public access (https://clinicaltrials.gov/ct2/show/NCT04842955).
References
- 1. Cummings J, Lee G, Ritter A, et al. Alzheimer's disease drug development pipeline: 2020. Alzheimer's Dement. Transl Res Clin Interv 2020;6:e12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hampel H, Mesulam MM, Cuello AC, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain 2018;141:1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease [internet]. Nature 2016;537:50–56. Available from: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 4. FDA Grants Accelerated Approval for Alzheimer's Drug [Internet] . 2021. [cited 2021 Jul 6 ] Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- 5. Koenig T, Prichep L, Dierks T, et al. Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment [internet]. Neurobiol Aging 2005;26:165–171.[cited 2019 Oct 9 ] Available from:. https://www.sciencedirect.com/science/article/pii/S0197458004001538?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- 6. Babiloni C, Lizio R, Marzano N, et al. Brain neural synchronization and functional coupling in Alzheimer's disease as revealed by resting state EEG rhythms [internet]. Int J Psychophysiol 2016;103:88–102. Available from. 10.1016/j.ijpsycho.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 7. Iaccarino HF, Singer AC, Martorell AJ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia [internet]. Nature 2016;540:230–235. Available from. 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martorell AJ, Paulson AL, Suk H, et al. Multi‐sensory gamma stimulation ameliorates Alzheimer's‐associated pathology and improves cognition. [internet]. Cell 2019;177:256–271.e22. Available from. 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adaikkan C, Middleton SJ, Marco A, et al. Gamma entrainment binds higher‐order brain regions and offers neuroprotection [internet]. Neuron 2019;102:1–15. Available from. https://linkinghub.elsevier.com/retrieve/pii/S0896627319303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrmann CS, Murray MM, Ionta S, et al. Shaping intrinsic neural oscillations with periodic stimulation. J Neurosci 2016;36:5328–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci 2013;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fröhlich F, Sellers KK, Cordle AL. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev Neurother 2014;15:145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vosskuhl J, Strüber D, Herrmann CS. Non‐invasive brain stimulation: a paradigm shift in understanding brain oscillations. Front Hum Neurosci 2018;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrmann CS, Rach S, Neuling T, Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. [Internet]. Front Hum Neurosci 2013;7:279. Available from. http://www.ncbi.nlm.nih.gov/pubmed/23785325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bréchet L, Yu W, Biagi MC, et al. Patient‐tailored, home‐based non‐invasive brain stimulation for memory deficits in dementia due to Alzheimer's disease. Front Neurol 2021;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benussi A, Cantoni V, Cotelli MS, et al. Exposure to gamma tACS in Alzheimer's disease: a randomized, double‐blind, sham‐controlled, crossover, pilot study [internet]. Brain Stimul 2021;14:531‐540. https://linkinghub.elsevier.com/retrieve/pii/S1935861X21000589. [DOI] [PubMed] [Google Scholar]
- 17. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. [internet]. Science 1993;261:921–923. Available from. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8346443&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 18. Riddle J, McPherson T, Atkins AK, et al. Brain‐derived neurotrophic factor (BDNF) polymorphism may influence the efficacy of tACS to modulate neural oscillations [internet]. Brain Stimul 2020;13:998–999. 10.1016/j.brs.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guerra A, Asci F, Zampogna A, et al. Gamma‐transcranial alternating current stimulation and theta‐burst stimulation: inter‐subject variability and the role of BDNF [internet]. Clin Neurophysiol 2020;131:2691–2699. 10.1016/j.clinph.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 20. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease [Internet]. Alzheimers Dement 2018;14:535–562.Available from. https://linkinghub.elsevier.com/retrieve/pii/S1552526018300724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benussi A, Dell'Era V, Cantoni V, et al. TMS for staging and predicting functional decline in frontotemporal dementia. [Internet]. Brain Stimul 2020;13:386–392. Available from:. http://www.ncbi.nlm.nih.gov/pubmed/31787557. [DOI] [PubMed] [Google Scholar]
- 22. Nucci M, Mapelli D, Mondini S. Cognitive reserve index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res 2012;24:218–226. [DOI] [PubMed] [Google Scholar]
- 23. Padovani A, Benussi A, Cantoni V, et al. Diagnosis of mild cognitive impairment due to Alzheimer's disease with transcranial magnetic stimulation. [Internet] J Alzheimers Dis 2018;65:221–230. Available from. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=30010131&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 24. Kasten FH, Dowsett J, Herrmann CS. Sustained aftereffect of α‐tACS lasts up to 70 min after stimulation. Front Hum Neurosci 2016;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rey A. L'Examen Clinique en Psychologie [clinical examination in psychology]. 1964.
- 26. Rentz DM, Amariglio RE, Becker JA, et al. Face‐name associative memory performance is related to amyloid burden in normal elderly [internet]. Neuropsychologia 2011;49:2776–2783. Available from. 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000;37:163–178. [PubMed] [Google Scholar]
- 28. Benussi A, Cosseddu M, Filareto I, et al. Impaired long‐term potentiation‐like cortical plasticity in presymptomatic genetic frontotemporal dementia. [internet]. Ann Neurol 2016;80:472–476. 10.1002/ana.24731. [DOI] [PubMed] [Google Scholar]
- 29. Benussi A, Grassi M, Palluzzi F, et al. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. [internet]. Ann Neurol 2020;87:394–404. Available from. http://www.ncbi.nlm.nih.gov/pubmed/31925823. [DOI] [PubMed] [Google Scholar]
- 30. Tokimura H, Di Lazzaro V, Tokimura Y, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. [internet]. J Physiol 2000;523:503–513. 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta AR, Pogosyan A, Brown P, Brittain JS. Montage matters: the influence of transcranial alternating current stimulation on human physiological tremor [internet]. Brain Stimul 2015;8:260–268. 10.1016/j.brs.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernández‐Ruiz A, Oliva A, Soula M, et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 2021;372:eabf3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high‐frequency gamma oscillations [internet]. Cell 2014;157:845–857. 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 34. Scott L, Feng J, Kiss T, et al. Age‐dependent disruption in hippocampal theta oscillation in amyloid‐β overproducing transgenic mice [internet]. Neurobiol Aging 2012;33:1481.e13–1481.e23. 10.1016/j.neurobiolaging.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 35. Başar E, Emek‐Savaş DD, Güntekin B, Yener GG. Delay of cognitive gamma responses in Alzheimer's disease. NeuroImage Clin 2016;11:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grunwald M, Busse F, Hensel A, et al. Correlation between cortical θ activity and hippocampal volumes in health, mild cognitive impairment, and mild dementia. J Clin Neurophysiol 2001;18:178–184. [DOI] [PubMed] [Google Scholar]
- 37. Naro A, Corallo F, De Salvo S, et al. Promising role of Neuromodulation in predicting the progression of mild cognitive impairment to dementia. J Alzheimers Dis 2016;53:1375–1388. [DOI] [PubMed] [Google Scholar]
- 38. Xing Y, Wei P, Wang C, et al. TRanscranial AlterNating current stimulation FOR patients with mild Alzheimer's disease (TRANSFORM‐AD study): protocol for a randomized controlled clinical trial. Alzheimer's Dement Transl Res Clin Interv 2020;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J, Kim H, Jeong H, et al. tACS as a promising therapeutic option for improving cognitive function in mild cognitive impairment: a direct comparison between tACS and tDCS [internet]. J Psychiatr Res 2021;141:248–256. 10.1016/j.jpsychires.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 40. Johnson L, Alekseichuk I, Krieg J, et al. Dose‐dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates [Internet]. Sci.Adv 2020;6:eaaz2747. Available from. 10.1126/sciadv.aaz2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beliaeva V, Polania R. Can low‐intensity tACS genuinely entrain neural activity in vivo? Brain Stimul 2020;13:1796–1799. [DOI] [PubMed] [Google Scholar]
- 42. Amat‐Foraster M, Leiser SC, Herrik KF, et al. The 5‐HT6 receptor antagonist idalopirdine potentiates the effects of donepezil on gamma oscillations in the frontal cortex of anesthetized and awake rats without affecting sleep‐wake architecture [internet]. Neuropharmacology 2017;113:45–59. 10.1016/j.neuropharm.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 43. Spencer JP, Middleton LJ, Davies CH. Investigation into the efficacy of the acetylcholinesterase inhibitor, donepezil, and novel procognitive agents to induce gamma oscillations in rat hippocampal slices [internet]. Neuropharmacology 2010;59:437–443. 10.1016/j.neuropharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 44. Babiloni C, Del Percio C, Bordet R, et al. Effects of acetylcholinesterase inhibitors and memantine on resting‐state electroencephalographic rhythms in Alzheimer's disease patients [internet]. Clin Neurophysiol 2013;124:837–850. 10.1016/j.clinph.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 45. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–583. [DOI] [PubMed] [Google Scholar]
- 46. Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non‐invasive brain stimulation tools [internet]. Clin Neurophysiol 2016;127:1031–1048. Available from. http://linkinghub.elsevier.com/retrieve/pii/S1388245715010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inukai Y, Saito K, Sasaki R, et al. Comparison of three non‐invasive transcranial electrical stimulation methods for increasing cortical excitability. [internet]. Front Hum Neurosci 2016;10:668. Available from. http://www.journal.frontiersin.org/article/10.3389/fnhum.2016.00668/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sivaramakrishnan A, Datta A, Bikson M, Madhavan S. Remotely supervised transcranial direct current stimulation: a feasibility study for amyotrophic lateral sclerosis. NeuroRehabilitation 2019;45:369–378. [DOI] [PubMed] [Google Scholar]
- 49. Charvet LE, Shaw MT, Bikson M, et al. Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice [internet]. Brain Stimul 2020;13:686–693. Available from. 10.1016/j.brs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 50. Huo L, Li R, Wang P, et al. The default mode network supports episodic memory in cognitively unimpaired elderly individuals: different contributions to immediate recall and delayed recall. Front Aging Neurosci 2018;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whitfield‐Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 2012;8:49–76. [DOI] [PubMed] [Google Scholar]
- 52. Small GW, Ercoli LM, Silverman DHS, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A 2000;97:6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lundstrom BN, Petersson KM, Andersson J, et al. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage 2003;20:1934–1943. [DOI] [PubMed] [Google Scholar]
- 54. Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 2005;9:445–453. [DOI] [PubMed] [Google Scholar]
- 55. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease [internet]. Neuroimage 2018;169:302–311. 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 56. Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face‐name pairs. Hippocampus 2004;14:919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ali MM, Sellers KK, Fröhlich F. Transcranial alternating current stimulation modulates large‐scale cortical network activity by network resonance. J Neurosci 2013;33:11262–11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meng A, Kaiser M, de Graaf TA, et al. Transcranial alternating current stimulation at theta frequency to left parietal cortex impairs associative, but not perceptual, memory encoding [internet]. Neurobiol. Learn. Mem 2021;182:107444. 10.1016/j.nlm.2021.107444. [DOI] [PubMed] [Google Scholar]
- 59. Freedberg M, Reeves JA, Toader AC, et al. Persistent enhancement of hippocampal network connectivity by parietal rTMS is reproducible. eNeuro 2019;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cacabelos R. Pharmacogenetic considerations when prescribing cholinesterase inhibitors for the treatment of Alzheimer's disease [internet]. Expert Opin Drug Metab Toxicol 2020;16:673–701. 10.1080/17425255.2020.1779700. [DOI] [PubMed] [Google Scholar]
- 61. Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders [internet]. Lancet Neurol 2011;10:241–252. 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andrieu S, Coley N, Lovestone S, et al. Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions [internet]. Lancet Neurol 2015;14:926–944. Available from. http://linkinghub.elsevier.com/retrieve/pii/S1474442215001532. [DOI] [PubMed] [Google Scholar]
- 63. Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther 2012;20:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Honnorat J, Antoine J‐C, Saiz A, et al. Cerebellar ataxia with anti‐glutamic acid decarboxylase antibodies: study of 14 patients. [internet]. Arch Neurol 2001;58:225–230. Available from. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11176960&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
All data, includeing outcome measure results, study protocol, and statistical analysis plan, will be shared through ClinicalTrials.gov via public access (https://clinicaltrials.gov/ct2/show/NCT04842955).


