Abstract
Mycoplasma bovis, the most important etiological agent of bovine mycoplasmosis, undergoes extensive antigenic variation of major and highly immunogenic surface lipoprotein antigens (Vsps). A family of 13 related but divergent vsp genes, which occur as single chromosomal copies, was recently found in the chromosome of M. bovis. In the present study, the molecular mechanism mediating the high-frequency phase variation of two Vsps (VspA and VspC) as representatives of the Vsp family was investigated. Analysis of clonal isolates exhibiting phase transitions of VspA or of VspC (i.e., ON→OFF→ON) has shown that DNA inversions occur during Vsp phase variation. The upstream region of each vsp gene contains two sequence cassettes. The first (cassette no. 1), a 71-bp region upstream of the ATG initiation codon, exhibits 98% homology among all vsp genes, while the second (cassette no. 2), upstream of cassette no. 1, ranges in size from 50 to 180 bp and is more divergent. Examination of the ends of the inverted fragments during VspA or VspC phase variation revealed that in both cases, a change in the organization of vsp upstream cassettes involving three vsp genes had occurred. Primer extension and Northern blot analysis have shown that a specific cassette no. 2, designated A2, is an active promoter and that juxtaposition of this regulatory element to a silent vsp gene by DNA inversions allows transcription initiation of the recipient gene. Further genetic analysis revealed that phase variation of VspA or of VspC involves two site-specific DNA inversions occurring between inverted copies of a specific 35-bp sequence present within the conserved cassette no. 1. A model for the control of Vsp phase variation is proposed.
Over 180 species are now assigned to the genus Mycoplasma (25, 26). Most have been identified as infectious agents of humans or other animals (36, 38). Mycoplasma infections are rarely of the fulminant type but rather follow a chronic course, indicating a frequent failure of the host defense mechanisms to eradicate the parasites. Although the molecular basis for mycoplasma pathogenicity and chronicity remains largely elusive, it is well appreciated that variation of surface components plays a central role in establishing the chronic nature of mycoplasma infections and is an important parameter in the interaction of these small wall-less prokaryotes with their host (9, 26, 41, 42).
A common theme among pathogenic bacteria for maintaining surface variability is the utilization of clusters of variable genes undergoing random and spontaneous ON/OFF switching at a high frequency using diverse genetic mechanisms (3, 8, 14, 21, 28, 33, 34). One mechanism by which phenotypic diversity is generated involves chromosomal rearrangements that reassort coding and regulatory regions in order to activate silent genes or pseudogenes or to generate new coding sequences by chimeric gene fusions (1, 4, 5, 7, 8, 19, 22). As a result, the bacterial population is heterogeneous, displaying different antigenic phenotypes which enable efficient avoidance of host defense mechanisms (8, 33, 34, 42). Adaptive surface variation through error-prone mutational systems linked to multigene families has been proposed as a general mycoplasma strategy for generating high-frequency size and phase variations in coat proteins (3, 11, 23, 35, 43).
Lipoproteins in mycoplasma have attracted much attention in recent years due to their abundance in the single mycoplasma membrane in contrast to the limited number of lipoproteins in membranes of other eubacteria (25, 26). Furthermore, lipoproteins are the most dominant antigens in mollicutes, many of them were shown to undergo phase variation and to possess repetitive domains (26), a motif that is found in surface antigens of bacterial pathogens and is thought to be a ligand-binding domain (40).
One pathogenic mycoplasma species that extensively changes the antigenic characteristics of its surface lipoproteins is Mycoplasma bovis, recognized as the most important etiological agent of bovine mycoplasmosis in Europe and North America. M. bovis is capable of producing subacute to acute inflammation of various organs, including the udder, joints, and the respiratory or genital tracts (13, 24). Variation in the antigenic repertoire of the M. bovis cell surface is achieved by high-frequency phase as well as size variation of major lipoprotein antigens known as variable membrane surface lipoproteins (Vsps) (2, 29).
In a previous paper, we reported the identification and characterization of the vsp genomic locus of M. bovis (18). This locus of about 23 kb contains 13 single-copy vsp genes, each of which exists as a complete open reading frame (ORF) encoding a putative surface lipoprotein (18). All vsp genes encode highly conserved N-terminal domains for membrane insertion and lipoprotein processing (12), while the rest of the mature Vsp molecules display sequence divergence (18). A major portion of the vsp coding sequence is composed of in-frame tandem repeats that create a periodicity in the polypeptide structure. Eighteen distinct repetitive domains of different length and amino acid sequences were found within the various vsp genes. The repeat domain is subjected to size variation by spontaneous expansion or contraction of these repeating units (18). Each vsp structural gene is linked to highly homologous upstream regions composed of two cassettes.
Phenotypic switching of the Vsp proteins was shown to involve rearrangement events occurring at high frequencies of about 10−2 to 10−3 per cell per generation within the vsp locus (17). The presence of multiple copies of high sequence similarity (18) allows for the modulation of the Vsp antigenic repertoire by recombination among vsp genes.
Recently, we have also shown that in addition to variations in the expression of individual Vsps, the vsp genomic repertoire is subject to changes. An intergenic recombination between closely related vsp genes (vspA and vspO) has led to the generation of a chimeric and functional vsp gene, namely, the vspC gene (19). The VspC product was shown to be accessible on the cell surface and to be a highly immunogenic antigen and to undergo an independent high-frequency phase as well as size variation (2).
In the present study, the molecular basis of VspA and of VspC phase variation, as representatives of the Vsp lipoprotein family, was investigated. We provide experimental evidence demonstrating that site-specific DNA inversions mediate VspA phase variation by fusion of an active promoter to the upstream region of a silent vsp gene. We propose a model for Vsp phase variation that involves site-specific DNA inversions between specific 35-bp sequences, designated vis (vsp inversion sequence), present within the conserved upstream region of all known vsp genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, chemicals, and growth conditions.
The M. bovis strain used in this study was the PG45 type strain. Its origin and growth conditions have been described elsewhere (29). Escherichia coli strain DH5αMCR (Gibco BRL Life Technologies, Inc., Gaithersburg, Md.) was used as a host. Recombinant clones were constructed in the plasmid vector pKS (Strategene, La Jolla, Calif.). E. coli cultures for plasmid isolation were grown in Luria-Bertani broth (31). Restriction enzymes, T4 ligase, and T4 polynucleotide kinase were purchased from MBI Fermentas, Amherst, N.Y. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), isopropyl-β-d-thiogalactopyranoside (IPTG), and ampicillin were purchased from Sigma Chemicals (St. Louis, Mo.). [γ-32P]ATP, [α-32P]CTP, and [α-33P]deoxynucleoside triphosphate. ([α-33P]dNTP) were purchased from Amersham, Little Chalfont, United Kingdom.
Electrophoresis and Western immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed by the method of Laemmli (16). Samples were prepared by heating at 100°C for 5 min in sample buffer (2% SDS, 5% [vol/vol] 2-mercaptoethanol, 10% [vol/vol] glycerol and 62.5 mM Tris, pH 6.8). Proteins were separated in α 9% acrylamide gel and were transferred to nitrocellulose membrane filters (0.45-μm pore size; Schleicher & Schuell, Dassel, Germany) by the method of Towbin et al. (37). Blot contents were incubated for 1 h at room temperature with phosphate-buffered saline (PBS) buffer containing 3% bovine serum albumin (Sigma, St. Louis, Mo.) and were then incubated overnight at 4°C with the primary antibodies diluted in phosphate-buffered saline buffer containing 20% (vol/vol) fetal calf serum. After three washes in PBS buffer, blots were incubated for 2 h at room temperature in peroxidase-conjugated goat antiserum to mouse immunoglobulin M or to mouse immunoglobulin G (Jackson ImmunoResearch Laboratories, West Grove, Pa., and Nordic, Tilburg, The Netherlands). For detection, the enzyme substrate o-dianisidine (Sigma) was used as previously described (2, 29).
DNA preparation and manipulation.
Genomic DNAs from M. bovis PG45 clonal populations were extracted and purified by the method of Marmur (20). Plasmid isolation, restriction endonuclease digestions, and gel electrophoresis of DNA or proteins were performed as previously described (17, 44).
Oligonucleotides, labeling, and hybridization conditions.
Synthetic oligonucleotides were synthesized on a model 380B DNA synthesizer (Applied Biosystems, Inc., Foster City, Calif.). The sequence of the oligonucleotides that target unique sequences of cassette no. 2 of the vspA, vspO, and vspL genes (designated A2, O2, and L2, respectively) are as follows: 5′-GCTTTTATTTAGTTCTTAATACTTCATATAATAAA-3′ (A2), 5′-CCTGGGTAACAGATGCAA-3′, (O2), and 5′-GCTTCTTAAGTGCAATAT-3′ (L2). The EX-1 oligonucleotide (5′-AATTTATGCCTTTTTGCA-3′) was used as for primer extension analysis. The RA-1 (5′-CGCCAGGTGTTTTATTTT-3′), the RA-4 (5′-GTTAGTTCCTGCACCTTGTT-3′), and the RF-2 (5′-TGGTGCTTTAGGTGCTCC-3′) oligonucleotides were used as probes in Northern blot analysis. The conditions of oligonucleotide labeling or DNA labeling and Southern blot hybridization were described elsewhere (17).
RNA isolation and Northern blot analysis.
Total cellular RNA was extracted from mid-logarithmic-phase cultures of M. bovis PG45 clonal variants using the RNA isolation RNeasy kit (Qiagen, Hilden, Germany). Total RNAs (2 μg) were denatured for 10 min at 65°C in the presence of 65% formamide and 8% formaldehyde. RNA was fractionated by electrophoresis in a 1% agarose gel containing 6% of formaldehyde (vol/vol) in morpholinepropanesulfonic acid buffer (31). After electrophoresis, the RNA was stained with ethidium bromide and was visualized under UV light. RNA marker I (Boehringer Mannheim, Mannheim, Germany) was included. The RNA was transferred onto a nylon membrane (Schleicher & Schuell) and was baked for 2 h at 80°C. The membrane was prehybridized at 42°C for 2 h in 50% formamide, 5× Denhardt's reagent, 0.1% SDS, 200 μg of salmon sperm DNA/ml, and 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2 PO4, and 1 mM EDTA [pH 7.7] (31). Hybridization was performed at 42°C in 50% formamide, 2.5× Denhardt's reagent, 0.1% SDS, 100 μg of salmon sperm DNA/ml, and 5× SSPE. The membranes were washed twice for 15 min each at room temperature in 6× SSPE and 0.1% SDS and once for 15 min at 37°C in 1× SSPE and 0.1% SDS. The membranes were dried and autoradiographed using Super RX Fuji X-Ray Film (Tokyo, Japan).
Primer extension.
Primer EX-1 was end labeled using T4 polynucleotide kinase and [γ32P]ATP and was then purified on a G-50 minicolumn (Boehringer Mannheim). The primer extension reaction contained 2 μg of total RNA, 100 ng of labeled primer, 3.9 μl of reaction buffer (0.1 M Tris-HCl, pH 8.3, 0.14 M KCl, 10 mM MgCl2, and 10 mM dithiothreital) in a final volume of 15 μl. The reaction mixture was incubated for 10 min at 65°C, followed by 5 min at room temperature. After annealing, a mixture of dNTPs (2.5 mM each) (Boehringer Mannheim) and 5 U of avian myeloblastosis virus reverse transcriptase (Promega) were added. The reaction mixture was incubated at 42°C for 60 min. RNAse (66 μg/ml) was then added, and the reaction mixture was incubated for 30 min at 37°C, incubation was then terminated by heat inactivation (10 min; 75°C). Primer extension products were mixed with loading buffer and were resolved on a 6% polyacrylamide sequencing gel. DNA sequence analysis was performed by the dideoxy chain termination method (32) using the Thermo Sequenase Radiolabeled Terminator Cycle Sequencing Kit (Amersham).
PCR.
Reactions were carried out in 100 μl containing 100 ng of template DNA, 5 U of Vent DNA polymerase in its 1× ThermoPol Buffer (New England BioLabs), a 0.2 mM mixture of dNTPs, and 500 ng of each primer. PCR amplifications were performed using the PC-Personal cycler (Biometra, Gottingen, Germany) programmed for 31 cycles as follows: one cycle of 3 min at 95°C, 1 min at 54°C, and 90 at 72°C, followed by 30 cycles of 30 at 95°C, 30 at 54°C, and 1 min at 72°C. The reaction mixtures were then incubated for 10 min at 72°C and allowed to cool to 4°C. The amplicons were purified using High Pure Filter Columns (Boehringer Mannheim GmbH, Indianapolis, Ind.) and were directly sequenced. Synthetic oligonucleotides used as primers for the VspA variants were 5′-TGAATCTGATTCTCCCCC-3′, 5′-TTTACATAGTGTTATTGTGC-3′, and 5′-GCTTGTTCTCTTTGACCCAC-3′ (designated P1, P2, and P3, respectively). PCR primers for the VspC variants were 5′-TCATCTGCTGGTTTGTCAG-3′, 5′-CACCAGAAGAGGAAAATGCTG-3′, and 5′-GCCTTGATCTGTATTTTCGC-3′ (designated Pe-3, Pi-3, and nt-2, respectively).
Nucleotide sequence accession number.
Sequence data were analysed using MacVector 6.5.10 software. The nucleotide sequences of the vsp genomic regions reported in this study have been assigned the following GenBank accession numbers: the vspA ON and the vspA OFF configurations (see Fig. 3A and B), AF36969 and AF36970, respectively, and the vspC ON and the vspC OFF configurations (see Fig. 4A and B), AF36971 and AF36972, respectively.
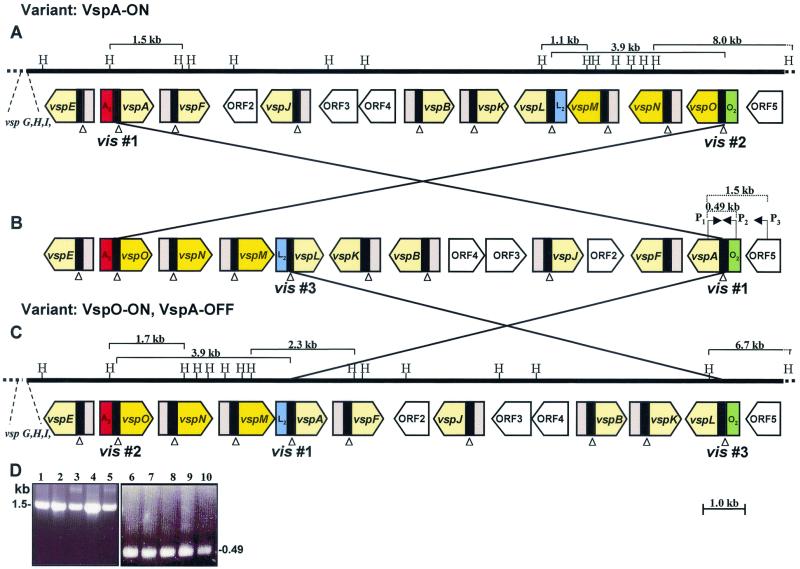
FIG. 3.
Schematic representation of DNA inversion events in the M. bovis vsp locus during VspA phase variation. (A to C) The solid line represents about 17 kb of the vsp locus that underwent inversion during the transition from VspA ON to VspA OFF (A and C, respectively). The positions of the HindIII (H) restriction sites are marked. Large, yellow, open arrows indicate the location and direction of the vsp genes. The vspM, vspN, and vspO genes are shown in darker yellow. The location of other vsp genes (vspG, vspH, and vspl) is marked. The location of four non-vsp ORFs (ORF2 to ORF5) is shown by open-labeled arrows. A black block 5′ to each vsp gene represents homologous cassette no. 1, while cassettes no. 2 are shown by pink blocks. Cassettes no. 2 of the vspA, vspL, and vspO genes (A2, L2, and O2, respectively) are differently colored. Several relevant HindIII fragments are indicated by parentheses with the corresponding size. Open triangles indicate the locations of the vis sites within conserved cassette no. 1. The postulated DNA inversions yielding three distinct vsp configurations (A to C) are indicated by crossed lines, and the involved vis sites (vis nos. 1 to 3) are indicated. The locations of the PCR primers (P1, P2, and P3) and their amplicons are marked by parentheses. (D) PCR amplification of M. bovis VspA variants and of the PG45-type strain. PCR primer pairs P1 and P3 (lanes 1 to 5) or P1 and P2 (lanes 6 to 10) were used to amplify a 1,500-or 490-bp region, respectively. Isolates included a PG45-type strain (lanes 1 and 6), VspA ON isolate No. 1 (lanes 2 and 7), VspA OFF no. 2 (lanes 3 and 8), VspA ON no. 3 (lanes 4 and 9), and VspA OFF no. 4 (lanes 5 and 10). The size of the PCR products is shown.
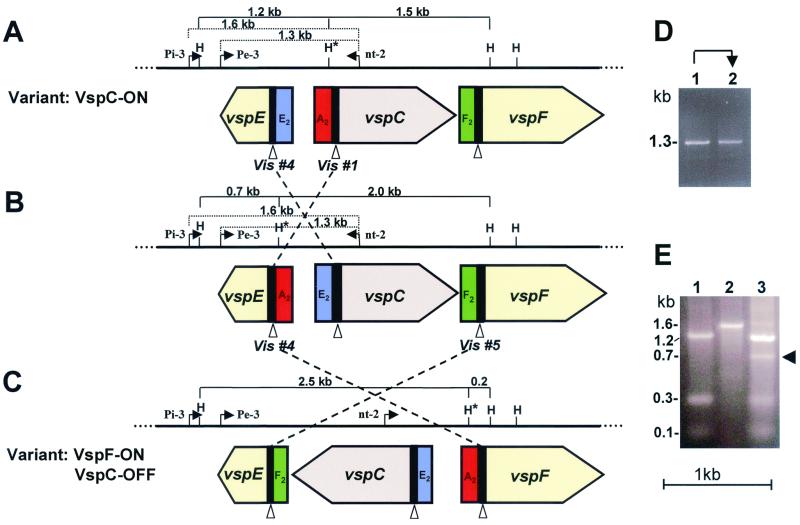
FIG. 4.
Schematic representation of DNA inversion events in the M. bovis vsp locus during VspC phase variation. (A to C) The solid line represents the portion of the vsp locus that underwent inversion during the transition from VspC ON to VspC OFF (A and C, respectively). The positions of the HindIII (H) restriction sites are marked. The HindIII site that underwent a change in its position is marked by an asterisk. Large open-labeled arrows indicate the location and direction of three vsp genes. A black block 5′ to each vsp gene represents homologous cassette no. 1. The A2, E2, and F2 cassettes no. 2 are differently colored. Several relevant HindIII fragments are indicated by parentheses with the corresponding size. Open triangles indicate the locations of the vis sites within conserved cassette no. 1. The postulated DNA inversions yielding three distinct vsp configurations (A to C) are indicated by crossed and broken lines, and the involved vis sites (vis nos. 1, 4, and 5) are indicated. The locations of the PCR primers (Pi-3, Pe-3, and nt-2) and their amplicons are marked by broken parentheses. (D) PCR amplification of M. bovis VspC phase variants. PCR primers Pe-3 and nt-2 were used to amplify a 1.3-bp region from VspC ON and VspC OFF variants (lanes 1 and 2, respectively). (E) Identification of a PCR amplicon corresponds to the middle VspC configuration. PCR primers Pi-3 and nt-2 were used to amplify a 1.6-bp region of the VspC ON or VspC OFF variant (the 1.6-kb amplicon of the VspC ON is shown in lane 2). An authentic 0.7-kb HindIII genomic fragment obtained after HindIII digestion of the 1.6-kb PCR product of the VspC OFF variant is shown (lane 3, indicated by arrowhead).
RESULTS
Phase variation of VspA or of VspC involves rearrangement of vsp locus.
Two Vsps (VspA and VspC) were chosen as representatives of the Vsp family to study the molecular mechanisms that mediate Vsp phenotypic switching. The genetic analysis was done using a linage of clonal isolates of M. bovis strain PG45 from successive generations of phase transition (i.e., ON→OFF→ON) of only the VspA or of the VspC product. VspA phase variants expressing a 63-kDa product (Fig. 1A) were isolated using monoclonal antibody (MAb) 1E5 as previously described (2, 17). Isolation of VspC phase variants expressing a 75-kDa product (Fig. 1B) was done by immunostaining colonies of a VspC clonal isolate expressing the VspC product with anti-VspC MAb 2A8 to monitor variations in VspC expression (30). Individual colonies were isolated, and their progenies were plated and in turn were subjected to immunostaining. Continued switching (ON to OFF and vice versa) was confirmed by Western blot analysis.
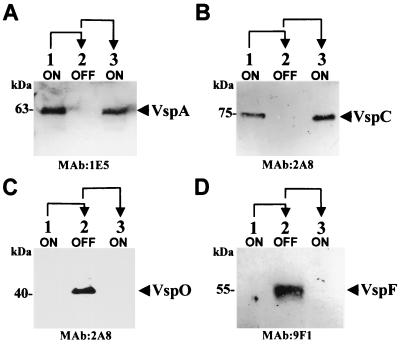
FIG. 1.
Western blot analysis of M. bovis PG45 clonal isolates. Total cell proteins of clonal isolates representing successive generations and phase transitions of VspA (A and C) or of VspC (B and D) were subjected to SDS-polyacrylamide gel electrophoresis and were immunoblotted with the following MAbs: 1E5 (A), 2A8 (B and C), and 9F1 (D). The 63-kDa VspA, the 75-kDa VspC, the 40-kDa VspO, and the 55-kDa VspF proteins are indicated. Phenotypic transitions (ON→OFF→ON, lanes 1 to 3, respectively) are indicated at the top with arrows.
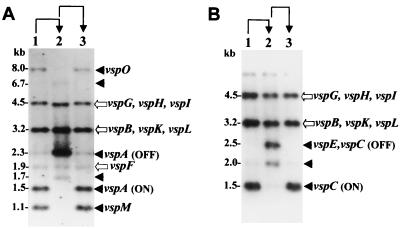
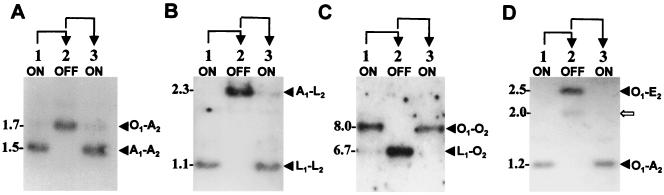
Genomic DNAs of the VspA and of the VspC phase variants were digested with the restriction enzyme HindIII and were subjected to Southern blot hybridization. The VspA variants were hybridized with a 2.3-kb HindIII genomic fragment, previously shown to carry the vspA gene in the OFF expression state (17) (Fig. 2A), and the VspC variants were hybridized with a 1.5-kb HindIII genomic fragment carrying the vspC gene (19) (Fig. 2B). The resulting hybridization patterns showed that phase variation of both Vsps occurs via reversible rearrangements of vsp-related fragments. Three HindIII fragments of 1.1, 1.5, and 8.0 kb present in the VspA ON variants (Fig. 2A, lanes 1 and 3) were missing in the VspA OFF variant. The VspA OFF variant, however, possessed other fragments of 1.7 kb, a 6.7-kb fragment that exhibits a weak signal, and a strongly hybridizing fragment of 2.3 kb (Fig. 2A, lane 2). Three invariant fragments of 1.9, 3.2, and 4.5 kb were also observed regardless of the VspA expression state (Fig. 2A). Similarly, the 1.5-kb HindIII fragment carrying the vspC gene in the variants expressing the VspC protein (Fig. 2B, lanes 1 and 3) was missing in the VspC OFF variant, which possesses two new HindIII fragments of 2.0 and of 2.5 kb (Fig. 2B, lane 2). Other vsp-related fragments remained unaltered (Fig. 2B).
FIG. 2.
Identification of vsp-related genomic fragments undergoing rearrangements during VspA or VspC phase variation. Chromosomal DNAs of the VspA variants (A) or of the VspC variants (B) were restricted with the HindIII restriction enzyme, subjected to Southern blot hybridization, and probed with a 2.3-kb HindIII fragment carrying the vspA gene in the OFF state (A) or with a 1.5-kb HindIII fragment carrying the vspC ON gene (B). Variable HindIII fragments present only in the VspA ON or in the VspC ON variants (A and B, lanes 1 and 3) are indicated by solid arrows. Invariant HindIII fragments (A and B) are indicated by open arrows. The names of the vsp genes corresponding to the HindIII genomic fragments are shown. Phenotypic transitions (ON→OFF→ON, lanes 1 to 3, respectively) are indicated at the top with arrows.
To determine the precise nature of the observed rearrangement events, the vsp locus from each of the VspA as well as of the VspC variants was cloned and sequenced. Comparison of the nucleotide sequences of the vsp loci of the VspA phase variants revealed that during VspA phase transition, a major rearrangement event occurred that affected the vsp gene configuration. In the VspA ON variants, a 3.9-kb genomic fragment carrying three vsp genes (vspM, vspN, and vspO) that was located 8.6 kb downstream of the vspA gene (Fig. 3A) underwent rearrangement. This fragment was found in the VspA OFF variant in an inverted orientation between the vspA and the vspE genes (Fig. 3C). The position of the other vsp genes remained unaltered. Notably, regaining expression of the VspA product in a variant representing a third generation in which a direct switch of the VspA protein from OFF to ON had occurred was accompanied by a reversible rearrangement event. In that clonal isolate the 3.9-kb fragment was repositioned to its original location within the vsp locus as shown in Fig. 3A.
Similar comparison of the vsp loci of the VspC variants revealed that during VspC phase variation, an inversion of the vspC gene had occurred. The position and orientation of the rest of the vsp genes remained unaltered (18, 19). The vsp genomic region undergoing inversion during the transition from VspC ON to VspC OFF is shown in Fig. 4A and C.
DNA inversions within vsp locus change organization of vsp upstream regions.
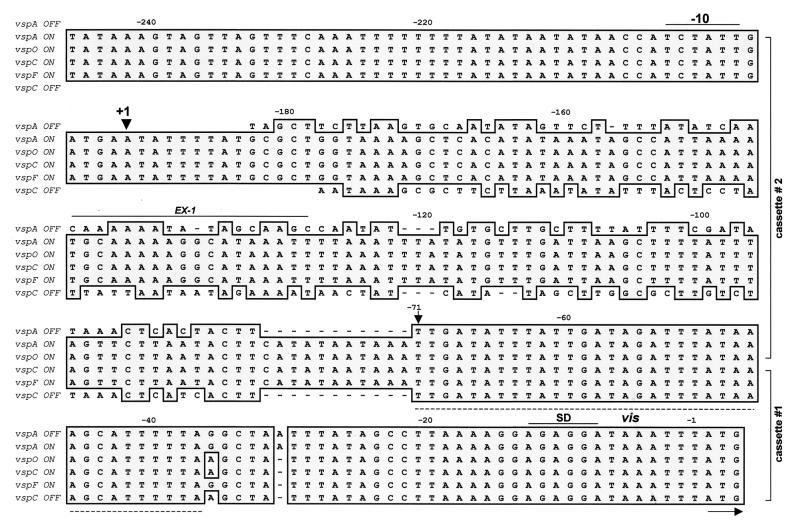
Each vsp gene member possesses a conserved 5′ noncoding sequence divided into two cassettes (18). The first (cassette no. 1) is a 71-bp region 5′ upstream of the ATG initiation codon and contains a putative ribosome binding site and exhibits 98% homology among all known vsp genes. The second (cassette no. 2), ranges in size from 50 to 180 bp (depending on the gene) and is more divergent.
Interestingly, examination of the inverted genomic fragments during VspA phase variation revealed that the upstream cassette region of three distinct vsp genes, vspA, vspO, and vspL, underwent rearrangement (Fig. 3 and 5). First, vspA cassette no. 2 (A2) (Fig. 3, shown in red) of the VspA ON variant was found upstream of cassette no. 1 (O1) of the vspO gene, generating the A2-to-O1 region in the VspA OFF variant. Second, vspL cassette no. 2 (L2) (Fig. 3, shown in blue) of the VspA ON variant was fused to vspA cassette no. 1 (A1), generating the L2-to-A1 region upstream of the vspA gene in the VspA OFF variant. Third, vspO cassette no. 2 (O2) (Fig. 3, shown in green) was fused to vspL cassette no. 1 (L1), generating the O2-to-L1 region upstream of the vspL gene in the VspA OFF variant.
FIG. 5.
Nucleotide sequence alignment of the upstream regions of the vspA OFF, vspA ON, vspO ON, vspC ON, vspF ON, and vspC OFF genes. Identical nucleotides are shown by dark, shaded boxes. The name of each vsp gene and its expression state (ON or OFF) are shown on the left. Numbers above sequences indicate nucleotide position relative to the initiation codon. The position of the putative transcriptional start sites (+1) is indicated by an arrow. A prokaryotic ς70 -dependent consensus sequence (−10) and a ribosome binding site (SD) are overlined. The positions of the two-vsp cassettes (nos. 1 and 2) are bracketed on the right. The arrow at position −71 marks the boundary between these two vsp cassettes. The position of the 35-bp region vis is indicated by a broken line. The binding site for the EX-I is marked by a solid line.
Similarly, in the VspC variants, inversion of the vspC gene led to rearrangement of the vsp upstream region of three vsp genes (vspC, vspE, and vspF) (Fig. 4 and 5). First, cassette no. 2 of the vspC ON gene (A2, shown in red) was found upstream of the vspF gene in the VspC OFF variant. Second, cassette no. 2 of the vspF gene (F2, shown in green) in the VspC ON variant was found upstream of the vspE gene in the VspC OFF variant. Third, cassette no.2 of the vspE gene (E2, shown in blue) was found upstream of the inverted vspC gene in the OFF expression state.
Additional experimental evidence demonstrating the change in the organization of the vsp upstream cassettes during Vsp phase variation was obtained by monitoring the presence and size of fragments bearing the corresponding cassettes in the chromosome of the Vsp phase variants. Three oligonucleotides complementary to unique sequences of cassettes no. 2 of the vspA, vspO, and vspL genes (designated A2, O2, and L2, respectively) were used in Southern blot hybridization against HindIII-digested genomic DNAs of the VspA variants (Fig. 6A to C, respectively). The A2 probe identified the A2 cassette on a 1.5-kb fragment carrying the vspA ON gene (Fig. 6A, lanes 1 and 3; see also Fig. 3A). In the VspA OFF phase variant, however, this probe identified the A2 cassette on a 1.7-kb fragment which was shown to carry the vspO gene and part of the vspN gene (Fig. 6A, lane 2; see also Fig. 3C). Similarly, the L2 probe identified a 1.1-kb fragment carrying the L2 cassette in the VspA ON variants or a 2.3-kb fragment bearing the L2-to-A1 fusion region in the VspA OFF variant (Fig. 6B, see also Fig. 3A and C, respectively). The O2 probe recognized an 8.0-kb fragment carrying the vspO gene in the VspA ON phase variants (Fig. 6C, lanes 1 and 3; see also Fig. 3A). In the VspA OFF variant, however, this probe identified a 6.7-kb fragment bearing the O2-to-L1 cassette region upstream of the vspL structural gene (Fig. 6C, lane 2; see also Fig. 3C).
FIG. 6.
Detection of the change in the organization of the vsp cassette no. 2 during VspA or VspC phase variation. Chromosomal DNAs of the VspA (A to C) or of the VspC (D) phase variants were digested with the HindIII restriction enzyme and were subjected to Southern blot hybridization. Three oligonucleotides complementary to unique sequences of cassette no. 2 of the vspA and of the vspC (A2), vspL (L2), and vspO (O2) genes were used as probes as follows: A2 (A and D), L2 (B), and O2 (C). The HindIII genomic fragments carrying the corresponding cassettes are marked by labeled arrows. Molecular-size markers (in kilobases) are shown on the left. Phenotypic transitions (ON→OFF→ON, lanes 1 to 3, respectively) are indicated at the top with arrows.
In the VspC variants, changes in the vsp upstream regions during ON/OFF switching were monitored using an oligonucleotide representing unique sequences of the vspC A2 cassette as a probe in Southern blot hybridization with HindIII-restricted genomic DNAs of VspC phase variants (Fig. 6D). Notably, since the vspC gene is a fusion of the vspA and vspO genes, it retains the vspA A2 cassette (19). A single fragment of 1.2 kb carrying the A2 cassette was identified in the VspC ON variants (Fig. 6D, lanes 1 and 3), while in the VspC OFF variant, the A2 cassette was identified on a 2.5-kb fragment (Fig. 6D, lane 2). These results were consistent with the position and size of the restriction genomic fragments carrying the corresponding cassettes obtained from the sequence analysis of the VspC variants (Fig. 4A and C). A faint band of 2 kb in size (Fig. 6D, lane 2, indicated by an open arrow) was also observed in the VspC OFF variant and will be discussed in the following section.
Site-specific DNA inversions occur during Vsp phase variation.
Since cassette no. 1 exhibits 98% homology among vsp genes (18, 19), the exact boundary between the two cassettes in which the inversion event took place cannot be precisely determined. However, differences of a few nucleotides within cassette no. 1 of the involved vsp genes (vspA, vspC, vspF, and vspO) on the one hand and the divergent sequence of cassette no. 2 on the other hand, allow us to use these differences as distinctive fingerprints and to identify a 35-bp sequence, located at nucleotides −37 to −71 of cassette no. 1 of all known vsp genes, as the potential sequence for the DNA inversion events. This site was designated vis (vsp inversion sequence) (Fig. 5, indicated by a broken line; see also Fig. 3 and 4).
The identification of the vis sequence at the ends of the inverted fragments during VspA and VspC phase variation suggests a site-specific DNA inversion as a possible mechanism for the control of Vsp phase variation. It should be noted that vis site-mediated DNA inversion can occur between vis copies that are oppositely oriented in the chromosome but not between copies that are oriented in the same direction. Nonetheless, the transition from the vspA ON gene configuration (Fig. 3A) to the vspA OFF configuration (Fig. 3C) or the transition from the vspC ON (Fig. 4A) to the vspC OFF configuration (Fig. 4C) could not be obtained by a single DNA inversion event. A possible model suggests that in these cases, two DNA inversions were required.
In the case of the vspA gene, the first inversion occurred between two vis's of the vspA and the vspO genes (Fig. 3A, designated vis no. 1 and 2, respectively, in figure), giving rise to a DNA inversion of a 13.4-kb fragment and to the generation of the vsp configuration as illustrated in Fig. 3B. A second DNA inversion of a 9.5-kb fragment could then occur between the former vis site of the vspA gene (vis no. 1) and the vis site of the vspL gene (vis no. 3), generating the vsp configuration identified in the VspA OFF variant (Fig. 3C).
In the case of the vspC gene, the first inversion of a 545-bp fragment could have occurred between two inverted vis sites of the vspE and the vspC genes (Fig. 4A, designated vis no. 4 and no. 1, respectively), yielding the vsp configuration shown in Fig. 4B. This configuration is then subjected to a second site-specific DNA inversion of a 1,820-bp fragment that is postulated to occur between the former vis no. 4 site of the vspE gene and the vis no. 5 site of the vspF gene (Fig. 4B) to generate the vspC OFF configuration shown in Fig. 4C.
To verify the proposed model, it was necessary to identify the middle vsp configurations of both Vsps (Fig. 3B and 4B). Since the vsp configuration shown in Fig. 3B resulted from an inversion of a large region of 13.4 kb, restriction fragments corresponding to this region comigrate with their counterparts from the VspA ON configuration and thus did not differ in gels. We therefore utilized PCR to amplify the middle configuration. Notably, in the middle vsp configuration, the vspA structural gene is linked to cassette no. 2 (O2) of the vspO gene and is located upstream of ORF5, generating a region unique to the middle configuration (Fig. 3B). Three specific PCR primers spanning this region were used to amplify the corresponding genomic region from the VspA phase variants as well as from the original M. bovis PG45 type strain. The first primer is located within the vspA structural gene, the second within the O2 cassette, and the third within ORF5 (Fig. 3B, designated P1, P2 and P3, respectively). A single PCR product of 1,500 or 490 bp was obtained using the primer pairs P1 and P3 or P1 and P2, respectively, in the VspA variants as well as from the M. bovis PG45 type strain (Fig. 3D), suggesting that the middle configuration does exist. Sequence analysis of the two PCR amplicons confirmed the existence of the vspA-O2-ORF5 fusion in the genome of the isolates tested.
The PCR approach was also utilized to detect the middle configuration of the VspC variant (Fig. 4B). As a first step, the inverted region between the vis no. 1 and no. 4 sites was amplified using two PCR primers flanking this region (designated Pe-3 and nt-2, Fig. 4A). These two primers should amplify a 1.3-kb fragment with genomic DNA of the VspC ON variant but should not generate this product with genomic DNA of the VspC OFF variant, since inversion of the vspC gene has changed the orientation of the nt-2 primer (Fig. 4A and C). Interestingly, however, a 1.3-kb fragment was synthesized in both VspC phase variants (Fig. 4D, lanes 1 and 2). Two possibilities exist for the generation of the 1.3 kb in the VspC OFF variant: first, the presence of a subpopulation expressing the VspC product within the VspC OFF population; and second, the presence of cells possessing a vsp configuration as illustrated in Fig. 4B. Moreover, while sequence analysis of the 1.3-kb PCR fragment of the VspC ON variant (Fig. 4D, lane 1) gave the desired sequence, attempts to determine the nucleotide sequence of the 1.3-kb fragment of the Vsp OFF variant (Fig. 4D, lane 2) failed due to the presence of mixed sequences, suggesting that cells containing the middle configuration do exist within the population of the VspC OFF isolate. We therefore synthesized another primer (designated Pi-3), located upstream of the Pe-3 primer and upstream of a HindIII site (Fig. 4). When the Pi-3 and nt-2 primers were used in a PCR, a 1.6-kb genomic fragment was synthesized in both VspC phase variants. The product of the VspC ON isolate is shown in Fig. 4E, lane 2. If our postulate that the middle vsp configuration shown in Fig. 4B does exist is correct, then the 1.6-kb PCR fragment obtained from VspC ON (Fig. 4A) and from the vsp middle configuration (Fig. 4B) should be distinguished by digestion with the HindIII restriction enzyme. As shown in Fig. 4A and B, the 1.6-kb PCR fragment contains two HindIII sites. One site, upstream of the vspE gene, is constant, while the position of the other site, located within the A2 cassette (marked by an asterisk), is variable due to the inversion event. Therefore, the HindIII cut should liberate an authentic 1.2-kb genomic fragment from both phase variants due to the presence of background subpopulation in both ON/OFF isolates (Fig. 4E, lanes 1 and 3, respectively) but should also generate a distinct 0.7-kb HindIII fragment present only in the middle vsp configuration (Fig. 4B). Indeed, a 0.7-kb HindIII fragment was observed only in the VspC OFF isolate (Fig. 4E, lane 3, indicated by an arrow). Sequence analysis of the 0.7-kb fragment has shown that the vspE gene contains the A2 cassette as configured for Fig. 4B.
We have mentioned above that the Southern blot hybridization of HindIII-digested genomic DNAs from the VspC phase variants identified a weak band of 2.0 kb present only in the VspC OFF variant (Fig. 1B and 6D, lane 2). We cloned and sequenced this fragment and discovered that it extends from the HindIII site of the A2 cassette upstream of the vspE gene to the HindIII site located within the vspF structural gene (Fig. 4B). In other words, it displays the middle vsp configuration shown in Fig. 4B. By combination of the sequence data of both the 2.0 and 0.7-kb HindIII fragments, the entire region undergoing the first site-specific DNA inversion event between the vis no. 1 and vis no. 4 sites was obtained (Fig. 4B). Taken together, the data strongly suggest that phase variation of the VspA and VspC proteins required two DNA inversions occurring at a high frequency between copies of vis sequences.
The vsp upstream A2 cassette serves as active promoter.
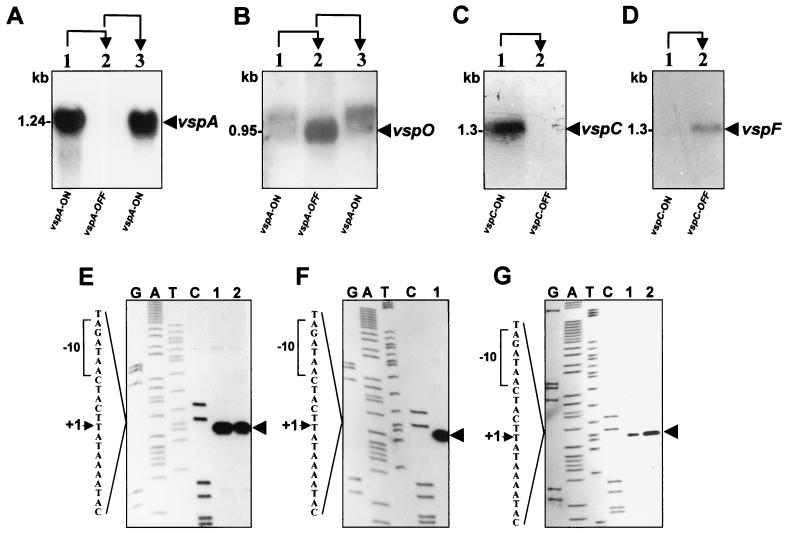
Comparison of the nucleotide sequences of the vspA and vspC genes and their flanking regions, during phase variation, revealed that the only sequence difference detected between the ON and the OFF expression states was the type of cassette no. 2. The expressed vspA and vspC genes possess the A2 cassette in their upstream region, while in the silent form of these genes, the A2 cassette has been replaced with the L2 cassette and with the E2 cassette, respectively (Fig. 3 and 4). The location of the A2 cassette upstream of expressed vsp genes raised the possibility that the A2 cassette is an active promoter and that acquisition of this element by other members of the vsp gene family will activate the recipient gene. Notably, the A2 cassette is a unique sequence present as a single chromosomal copy. We monitored the presence of the vspA mRNA in the three VspA phase variants. Total cellular RNA was extracted and subjected to Northern blot analysis using an oligonucleotide specific for the repetitive domain RA-4 of the vspA gene (18). A single transcript of 1.24 kb hybridized with the RA-4 probe was detected in all VspA ON variants (Fig. 7A, lanes 1 and 3). However, no vspA-related mRNA was observed in the VspA OFF variant (Fig. 7A, lane 2).
FIG. 7.
Transcription analysis of the VspA and VspC phase variants. (A to D) Northern blot analysis of VspA and of VspC phase variants. Total cellular RNAs were extracted from the VspA (A and B) or from the VspC (C and D) phase variants in either the ON or the OFF state and were probed with the RA-4 oligonucleotide (A), the vspO structural gene (B), the RA-1 oligonucleotide (C), and the vspF-specific oligonucleotide (RF-2) (D). Transcripts corresponding to the vspA, vspO, vspC, and vspF genes are indicated by labeled arrows. The length of the mRNA is indicated. Phenotypic transitions of VspA are indicated at the top of the panels by arrows. (E to G) Identification of the transcription start sites of the vspA, vspO, vspC, and vspF genes. The autoradiogram of a 6% polyacrylamide gel used to analyze the extension products is shown. Primer extension analysis was performed using cellular RNA extracted from the Vsp phase variants. The letters above the lanes indicate which dideoxynucleotide was used to terminate the sequencing reaction. The resultant primer extension products of two VspA ON variants (E, lanes 1 and 2), VspO ON (F, lane 1) VspC ON (G, lane 1), and VspF ON (panel G, lane 2) are indicated by an arrow. Part of the nucleotide sequence deduced from the sequencing lanes is shown on the left. The transcriptional start site (+1) (arrowhead) and the putative −10 sequence (brackets) are marked.
If the assumption that the A2 cassette is an active promoter and is responsible for the transcription of a particular vsp gene when that gene is placed downstream from the A2 cassette is correct, then the A2 recipient gene, namely, the vspO gene in the VspA OFF variant, should be transcribed. The presence of vspO mRNA in the three VspA phase variants was examined using the vspO structural gene as a probe in Northern blot analysis. A single transcript of 0.95 kb hybridizing with the vspO gene was detected in the vspA OFF variant (Fig. 7B, lane 2). Notably, since the vspA and vspO genes exhibit homology (18), the vspO gene probe was able to identify in the VspA ON variants a faint band which represents the presence of spontaneous background population expressing the VspA product.
Similarly, when a Northern blot analysis was performed with total cellular RNA obtained from the VspC ON and the VspC OFF variants, a single vspC mRNA was detected by the oligonucleotide RA-1 only in the VspC ON variant (Fig. 7C, lane 1). In the VspC OFF variant, however, a single transcript corresponding to the A2 recipient gene, namely, the vspF gene, was identified using the vspF-specific oligonucleotide RF-2 (Fig. 7D, lane 2). The size of all transcripts correlates well with the corresponding size of the vsp genes (18, 19).
The role of the A2 cassette as an active promoter was further examined by mapping the vsp transcription start site for each of the A2 recipient genes (vspA, vspC, vspO, and vspF). A synthetic oligonucleotide complementary to unique sequences of the A2 cassette (designated EX-1; Fig. 5) was used in primer extension analysis. Total cellular RNA was obtained from the VspA ON, VspA OFF, VspC ON, and VspC OFF variants. A potential transcriptional start site was identified within the A2 cassette for each of the A2 recipient genes (Fig. 7E to G). This site was located 192 bp upstream of the initiation codon of the vspA, vspO, vspC, and vspF genes and was preceded by the sequence TCTATT, which might serves as a prokaryotic ς70-dependent −10-consensus sequence.
Expression of the A2 recipient genes (vspO and vspF) was also examined by Western blot analysis of total cell proteins from the VspA or of the VspC phase variants using MAb 2A8 or MAb 9F1, previously shown to recognize an epitope on repetitive units of the VspO or VspF proteins, respectively (18, 30). A polypeptide band of 40 kDa corresponding to the VspO protein was observed in the VspA OFF variant by MAb 2A8 (Fig. 1C, lane 2), and a polypeptide band of 55 kDa corresponding to the VspF protein was observed in the VspC OFF variant by MAb 9F1 (Fig. 1D, lane 2). Both Vsps were shown to be surface exposed by colony immunoblot experiments (data not shown).
Collectively, the data indicate that the A2 cassette serves as an active promoter and that juxtaposition of this regulatory element to a vsp gene by site-specific DNA inversions allows transcription initiation of the recipient gene.
DISCUSSION
This study provides compelling evidence that Vsp high-frequency phenotypic switching is mediated by site-specific DNA inversions occurring within the vsp locus of M. bovis. These inversions occur at a high frequency, as evidenced by the ease at which Vsp clonal variants were isolated (2, 17). The target sites for the site-specific DNA inversions were identified within a 35-bp sequence (vis) present 37 bp upstream of the initiation codon of each vsp gene. The fact that the vsp genes are not all similarly oriented within a chromosomal locus allows DNA inversions to occur between any two vis copies that are oppositely oriented (10, 18).
Sequence analysis of the vsp locus from several VspA or VspC phase variants has shown that the phase-variable genes possess complete and uninterrupted ORFs regardless of the expression state. However, comparison of the nucleotide sequences upstream of the expressed vsp gene with all of the other vsp genes revealed that during phase variation, rearrangement of the vsp upstream regions involving three vsp genes had occurred. A region of 121 bp (designated A2; Fig. 3 and 4) was found upstream of an expressed vsp gene.
The molecular basis of M. bovis phenotype switching appears to be mediated by specific sequences that are necessary for site-specific DNA inversions (vis) and for expression (A2 cassette). The A2 cassette was shown by primer extension experiments to possess the transcriptional start site for an expressed vsp gene and thus serves as an active promoter. The generality of the A2 cassette as a promoter for transcription initiation of the vsp genes was demonstrated for the vspA, vspC, vspO, and vspF genes.
An interesting feature of the vsp system that emerged when the nucleotide sequences of the vsp loci of several VspA or VspC phase variants were compared was that the vsp configuration identified in the VspA OFF or VspC OFF variants could not be explained by a single DNA inversion event. We propose that VspA as well as VspC phase variation involves two site-specific DNA inversions (Fig. 3 and 4). In VspA phase variation, the first inversion of the 13.4 kb fragment occurred between two inverted vis copies of the vspA and the vspO genes (vis no. 1 and 2, respectively; Fig. 3A), generating a middle configuration shown in Fig. 3B. This configuration underwent a second inversion of a 9.5-kb fragment between two inverted vis copies of the vspA and vspL genes (vis no. 1 and 3, respectively; Fig. 3B), which led to the configuration shown in Fig. 3C of the VspA OFF variant. In VspC phase variation, the first inversion of 545 bp occurred between two adjacent, inverted vis sites of the vspE and vspC genes (Fig. 4A). The second inversion involved a DNA segment of 1,820 bp and occurred between the vis site of the vspE gene and the vis site of the vspF gene (Fig. 4B). PCR amplification and sequence analysis have confirmed that cells that underwent only a single DNA inversion and possess the locus as configured for Fig. 3B and 4B exist within the populations of the VspA or the VspC phase variants, as well as within the population of M. bovis PG45 type strain (Fig. 3 and 4).
Examination of the VspA variants revealed that in the two vsp configurations (Fig. 3B and C), the recipient of the A2 promoter was the vspO gene. This raises an intriguing question: why are two site-specific DNA inversions needed to achieve the same goal? One speculative possibility may relate to the orientation of the vis sites. Since the DNA inversions occur between two vis copies that must be in their inverted orientations, it is not possible to express each of the genes by a single inversion. The occurrence of multiple DNA inversions within the vsp locus enables other silent vsp genes to be repositioned in the correct orientation with respect to the expressed vsp gene. Additional inversions will allow juxtaposition of the vsp promoter with different silent vsp genes and the expression of new variable lipoproteins. It should be noted, however, that the DNA inversions might occur concurrently. A growing population contains apparently distinct subpopulations that have undergone single or multiple inversions. The population as a whole therefore possesses a wide spectrum of vsp configurations, allowing an increased capability to diversify the antigenic repertoire of the mycoplasma cell surface and to ensure the presence of a desirable variant needed for survival in the case of a change in the host environment.
Analysis of VspC phase variation revealed that unlike the VspA phase variation, in each of the three vspC configurations, the A2 promoter was ligated to a different vsp gene (vspC, vspE, and vspF; Fig. 4A to C, respectively). In addition, colonies exhibiting the VspC OFF phenotype that were picked from a plate using the colony immunoblot assay were shown to possess the third configuration in which the promoter was upstream of the vspF gene. In other words, the second DNA inversion event appears to be phenotypically silent. Why are two inversions needed for vspC phase variation? One intriguing speculation may relate to the size and the repetitive nature of the involved lipoproteins. An interesting study in Mycoplasma hyorhinis has shown that in antibody selected populations, the emergence of a prevalent, protective Vlp phenotype is not due to expression of a particular Vlp protein but rather can result from optional mutational pathways leading to expression of any long Vlp protein (6). The choice of pathways appears to be determined by the most favorable (high-frequency) pathway available to generate a long Vlp protein which facilitates escape from antibody mediated damage. In Mycoplasma pulmonis growth properties are affected by length of repetitive domain of the Vsa proteins (3). Interestingly, when one looks at the three promoter-recipient vsp genes during VspC phase variation, the vspE gene of the middle configuration is the smallest vsp gene, only 393 bp long, and possesses one repetitive domain (18, 19). In contrast, the expressed vspC gene (Fig. 4A) is 1,098 bp long and contains four distinct, repetitive domains representing more than 80% of the entire gene (19). Similarly, the expressed vspF gene (Fig. 4C) is 1,110 bp long and possesses two large, repetitive domains (18, 19). Although VspC phase variants, investigated in this study, were not isolated from M. bovis populations that were subjected to pressure posed by host antibodies, it is an intriguing issue whether spontaneously occurring, phenotypically silent inversions represent the most efficient pathway to generate in vivo a long surface lipoprotein. In that case, long Vsps may provide a shield from host antibodies capable of binding vital surface antigens of M. bovis as elegantly as was shown for the Vlp proteins of M. hyorhinis (6).
Chromosomal rearrangements have been shown to be associated with phenotypic switching of surface antigens in many bacterial pathogens. Homologous recombination, gene conversions, gene duplications, additions or deletions of tandem repetitive units, movement of transposable elements within the chromosome, and DNA inversions are frequently employed mechanisms regulating the expression of genes encoding surface antigens in other bacterial systems (8, 28, 33, 34). In some cases, rearrangement events enabling the activation of silent genes were reported (1, 3, 7, 14, 22, 27, 35). The maintenance of a silent gene during evolution suggests that expression of such a gene provides a selective advantage to the bacterium under certain environmental conditions. One of the commonly known mechanisms of activation of silent genes is translocation of the gene from its silent site to an expression site via gene conversions (8, 10, 27, 34). For example, the silent vmp genes of Borrelia hermsii can be activated by gene conversion events that place the silent gene downstream of a promoter (14). The silent vsa genes of M. pulmonis lack the 5′ end region, and site-specific DNA inversions regulate their expression by reassorting the 5′ end region from an expressed vsa gene with the 3′ end region from a silent gene (3, 35). Although there are numerous examples of site-specific DNA inversion systems that regulate phase variation of surface antigens (4, 10, 22, 35), most of them are relatively simple, consisting of two recombination sites and a site-specific recombinase gene that regulate the expression of one or two genes but not of a large gene family. In comparison with other bacterial DNA inversion systems, the vsa system of M. pulmonis (3, 35) and the vsp system of M. bovis are more complex. Both systems utilize multiple recombination sites and DNA inversions to regulate expression of a large gene family. In this respect, the two-mycoplasma systems resemble most closely the R64-type shufflon system found in enteric bacterial plasmids within the IncI incompatibility group (15).
Most site-specific DNA inversions are catalyzed by members of either the invertase family (the Hin enzyme of Salmonella) or the bacteriophage integrase family of site-specific recombinases (8, 10). Comparison of the vis sequence with bacterial invertase target sites as well as to the vrs box of M. pulmonis (3, 35) did not reveal any homology. It is possible that different site-specific recombinases may play a role in the mycoplasmas.
The Vsp variants analyzed were shown to oscillate between ON and OFF expression at a high frequency of 10−2 to 10−3 per cell per generation (2, 17, 29). The DNA inversions observed within the vsp locus occur therefore at high frequencies similar to those measured for the vsa genes of M. pulmonis (3, 35). Variation in the antigenic repertoire is a combination of the frequency of the genetic switch and the selective pressure of the environment for a particular surface protein. The selective pressure for an exposed surface protein of a bacterial pathogen can be very high if those proteins are recognized by the host immune system. For example, the host immune system was shown to provide the selective pressure for the variation of the S layer of the pathogenic bacterium Campylobacter fetus (39). The Vsp proteins may represent a similar case. Further studies are needed to determine in vivo whether certain site-specific DNA inversion patterns are preferable in the host as a result of the selective pressure provided by the immune system.
ACKNOWLEDGMENTS
This study was supported by Research Grant Award No. IS-2540-95R from The United States-Israel Binational Agricultural Research and Development Fund (BARD) and in part by the German-Israeli Foundation for Scientific Research and Development (GIF) and by the Israel Academy of Sciences and Humanities Foundation.
MAbs 9F1 and 1E5 were kindly provided by Konrad Sachse from the Federal Institute for Health Protection of Consumers and Veterinary Medicine, Jena, Germany.
I.L. and Y.R. contributed equally to this work.
REFERENCES
- 1.Barbour A G, Burman N, Carter C J, Kitten T, Bergstrom S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 2.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect Immun. 1994;62:5075–5084. doi: 10.1128/iai.62.11.5075-5084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhugra B, Voelker L L, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1995;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 4.Boot H J, Kolen C P A M, Pouwels P H. Interchange of the active and silent S-layer protein genes of Lactobacillus acidophilus by inversion of the chromosomal slp segment. Mol Microbiol. 1996;21:799–809. doi: 10.1046/j.1365-2958.1996.401406.x. [DOI] [PubMed] [Google Scholar]
- 5.Borst P, Greaves D R. Programmed gene rearrangements altering gene expression. Science. 1987;235:658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- 6.Citti C, Kim M F, Wise K S. Elongated versions of Vlp surface lipoproteins protect Mycoplasma hyorhinis escape variants from growth-inhibition host antibodies. Infect Immun. 1997;65:1773–1785. doi: 10.1128/iai.65.5.1773-1785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworkin J, Blaser M J. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol. 1997;26:433–440. doi: 10.1046/j.1365-2958.1997.6151958.x. [DOI] [PubMed] [Google Scholar]
- 8.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 9.Dybvig K, Voelker L L. Molecular biology of mycoplasmas. Annu Rev Microbiol. 1996;50:25–57. doi: 10.1146/annurev.micro.50.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Glasgow A C, Hughes K T, Simon M I. Bacterial DNA inversion systems. In: Berg E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 637–659. [Google Scholar]
- 11.Glew M D, Baseggio N, Markham P F, Browning G F, Walker I D. Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5′ noncoding regions. Infect Immun. 1998;66:5833–5841. doi: 10.1128/iai.66.12.5833-5841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990;22:451–470. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 13.Jasper D E. The role of Mycoplasma in bovine mastitis. J Am Vet Med Assoc. 1982;181:158–162. [PubMed] [Google Scholar]
- 14.Kitten T, Barbour A G. Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci USA. 1990;87:6077–6081. doi: 10.1073/pnas.87.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komano T. Shufflons: multiple inversion systems and integrons. Annu Rev Genet. 1999;33:171–191. doi: 10.1146/annurev.genet.33.1.171. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lysnyansky I, Rosengarten R, Yogev D. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol. 1996;178:5395–5401. doi: 10.1128/jb.178.18.5395-5401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lysnyansky I, Sachse K, Rosenbusch R, Levisohn S, Yogev D. The vsp locus of Mycoplasma bovis: gene organization and structural features. J Bacteriol. 1999;181:5734–5741. doi: 10.1128/jb.181.18.5734-5741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lysnyansky I, Ron Y, Sachse K, Yogev D. Intrachromosomal recombination within the vsp locus of Mycoplasma bovis generates a chimeric variable surface lipoprotein antigen. Infect Immun. 2001;69:3703–3712. doi: 10.1128/IAI.69.6.3703-3712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 21.Meyer T F, Gibbs C P, Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- 22.Moses E K, Good R T, Sinistaj M, Billington S J, Langford C J, Rood J I. A multiple site-specific DNA-inversion model for the control of Ompl phase and antigenic variation in Dichelobacter nodosus. Mol Microbiol. 1995;17:183–196. doi: 10.1111/j.1365-2958.1995.mmi_17010183.x. [DOI] [PubMed] [Google Scholar]
- 23.Noormohammadi A H, Markham P F, Kanci A, Whithear K G, Browning G F. A novel mechanism for control of antigenic variation in the haemagglutinin gene family of Mycoplasma synoyiae. Mol Microbiol. 2000;35:911–923. doi: 10.1046/j.1365-2958.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 24.Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Tech. 1996;15:1477–1494. doi: 10.20506/rst.15.4.987. [DOI] [PubMed] [Google Scholar]
- 25.Razin S. Peculiar properties of mycoplasmas: the smallest self-replicating prokaryotes. FEMS Microbiol Lett. 1992;100:423–432. doi: 10.1111/j.1574-6968.1992.tb14072.x. [DOI] [PubMed] [Google Scholar]
- 26.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restrepo B I, Carter C J, Barbour A G. Activation of a vmp pseudogene in Borrelia hermsii: an alternative mechanism of antigenic variation during relapsing fever. Mol Microbiol. 1994;13:287–299. doi: 10.1111/j.1365-2958.1994.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 28.Robertson B D, Meyer T F. Antigenic variation in bacterial pathogens. In: Hormaeche C W, Penn C W, Smyth C J, editors. Molecular biology of bacterial infection. Vol. 49. Cambridge, England: Cambridge University Press; 1992. pp. 61–73. [Google Scholar]
- 29.Rosengarten R, Behrens A, Stetefeld A, Heller M, Ahrens M, Sachse K, Yogev D, Kirchhoff H. Antigen heterogeneity among isolates of Mycoplasma bovis is generated by high-frequency variation of diverse membrane surface proteins. Infect Immun. 1994;62:5066–5074. doi: 10.1128/iai.62.11.5066-5074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachse K, Helbig J H, Lysnyansky I, Grajetzki C, Müller W, Jacobs E, Yogev D. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect Immun. 2000;68:680–687. doi: 10.1128/iai.68.2.680-687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders J R. Genetic basis of phase and antigenic variation in bacteria. In: Birkbeck T H, Penn C W, editors. Antigenic variation in infectious diseases. Oxford, England: IRL Press; 1986. pp. 57–76. [Google Scholar]
- 34.Seifert H S, So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988;52:327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen X, Gumulak J, Yu H, French C T, Zou N, Dybvig K. Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J Bacteriol. 2000;182:2900–2908. doi: 10.1128/jb.182.10.2900-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simecka J W, Davis J K, Davidson M K, Ross S E, Stadtländer C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–416. [Google Scholar]
- 37.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tully J G, Whitcomb R F. The mycoplasmas. 2. Human and animal mycoplasmas. New York, N.Y: Academic Press; 1979. [Google Scholar]
- 39.Wang E, Garcia M M, Blake M S, Pei Z, Blaser M J. Shift in S-layer protein expression responsible for antigenic variation in Campylobacter fetus. J Bacteriol. 1993;175:4979–4984. doi: 10.1128/jb.175.16.4979-4984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wern B W. A family of clostridial and streptococcal ligand-binding proteins with conserved C-terminal repeat sequence. Mol Microbiol. 1991;5:797–803. doi: 10.1111/j.1365-2958.1991.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 41.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–489. [Google Scholar]
- 42.Wise K S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993;1:59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yogev D, Menaker D, Strutzberg K, Levisohn S, Kirchhoff H, Heinz K-H, Rosengarten R. A surface epitope undergoing high-frequency phase variation is shared by Mycoplasma gallisepticum and Mycoplasma bovis. Infect Immun. 1994;62:4962–4968. doi: 10.1128/iai.62.11.4962-4968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]