Abstract
Background
The heterogeneity of development and progression of eczema suggests multiple underlying subclasses for which aetiology and prognosis may vary. A better understanding may provide a comprehensive overview of eczema development and progression in childhood. Thus, we aimed to determine longitudinal eczema subclasses based on assessments and identify their associations with risk factors and allergic outcomes.
Methods
A total of 619 participants with a family history of allergic disease were assessed at 24 time‐points from birth to 12 years. At each time, eczema was defined as the report of current rash treated with topical steroid‐based preparations. Longitudinal latent class analysis was used to determine eczema subclasses. Subsequent analyses using regression models assessed the associations between eczema subclasses and potential risk factors and allergic outcomes at 18‐ and 25‐year follow‐ups (eczema, allergic rhinitis, asthma and allergic sensitization).
Results
We identified five eczema subclasses ‘early‐onset persistent’, ‘early‐onset resolving’, ‘mid‐onset persistent’, ‘mid‐onset resolving’ and ‘minimal eczema’. Filaggrin null mutations were associated with the early‐onset persistent (OR = 2.58 [1.09–6.08]) and mid‐onset persistent class (OR = 2.58 [1.32–5.06]). Compared with ‘minimal eczema’, participants from early‐onset persistent class had higher odds of eczema (OR = 11.8 [5.20–26.6]) and allergic rhinitis (OR = 3.13 [1.43–6.85]) at 18 and at 25 years eczema (OR = 9.37 [3.17–27.65]), allergic rhinitis (OR = 3.26 [1.07–9.93]) and asthma (OR = 2.91 [1.14–7.43]). Likewise, mid‐onset persistent class had higher odds of eczema (OR = 2.59 [1.31–5.14]), allergic rhinitis (OR = 1.70 [1.00–2.89]) and asthma (OR = 2.00 [1.10–3.63]) at 18 and at 25 years eczema (OR = 6.75 [3.11–14–65]), allergic rhinitis (OR = 2.74 [1.28–5.88]) and asthma (OR = 2.50 [1.25–5.00]). Allergic and food sensitization in early life was more common in those in the persistent eczema subclasses.
Conclusion
We identified five distinct eczema subclasses. These classes were differentially associated with risk factors, suggesting differences in aetiology, and also with the development of allergic outcomes, highlighting their potential to identify high‐risk groups for close monitoring and intervention.
Keywords: Eczema, food allergy, genetic, latent class analysis
Five eczema subclasses were identified: ‘early‐onset persistent’, ‘early‐onset resolving’, ‘mid‐onset persistent’, ‘mid‐onset resolving’ and ‘minimal‐eczema’. Filaggrin null mutations were risk factors for persistent subclasses. The early‐ and mid‐onset persistent classes were associated with high allergic comorbidities at 18 and 25 years. The early‐onset resolving and early‐onset persistent classes may be differentiated by identifying those with allergic and food sensitization during the first year of life.

Key messages.
Five distinct eczema subclasses were identified differentially associated with risk factors and allergic comorbidities
Filaggrin null mutation and early allergic sensitization were associated with the persistent eczema subclasses.
Persistent eczema subclasses were associated with high allergic comorbidities at age 18 and 25 years
1. INTRODUCTION
Eczema, also known as atopic dermatitis, is a chronic relapsing skin inflammatory disease characterized by defective skin barrier function. 1 The prevalence of eczema is around 20% in children and ranges from 5 to 10% in adults, 2 although reported prevalence varies widely between regions. Filaggrin (FLG) null mutations cause a skin barrier flaw which is associated with eczema. 3 However, this genetic factor is not an essential cause for eczema, which can develop through other biological pathways. 4 Eczema has a heterogeneous presentation with different levels of severity, treatment response, age of onset and tendency to evolve to other atopic diseases. 5
There may be different pathophysiological pathways for the development of eczema, which have not yet been fully characterized. The age of onset, severity and persistence of eczema are most likely determined by complex interactions between environment and genetic factors. 2 For that reason, the concept of ‘eczema phenotypes’ has been suggested, rather than a simple binary classification of eczema, as such phenotypes can incorporate the variability of eczema development and progression. 6 The classification of eczema phenotypes could allow for the identification of risk factors and enhance understanding of patterns associated with eczema onset and progression.
Latent class analysis (LCA) is a parametric mixture method which can identify distinct underlying groups based on patterns over time. 7 Five studies have explored this heterogeneity in eczema onset and persistence during early childhood using LCA and its association to other allergic outcomes. 7 , 8 , 9 , 10 , 11 However, these studies assessed cross‐sectional associations of eczema subclasses and a limited number of risk factors and comorbidities at 6, 9 8, 10 10, 8 12, 16 7 and 26 11 years. These findings need to be replicated by assessing eczema subclasses targeting high‐risk children with multiple early eczema assessments to draw firmer conclusions and obtain a comprehensive overview of eczema development and progression in childhood. Furthermore, assessing the longitudinal associations of a broad range of risk factors encompassing individual, environmental and genetic factors with eczema subclasses may provide a better understanding of which factors contribute to the development and persistence of eczema patterns.
Thus, we aimed to determine longitudinal eczema subclasses based on eczema prevalence at 24 time‐points from birth to 12 years of age and identify their associations with risk factors and allergic outcomes up to 25 years of age using the Melbourne Atopy Cohort Study (MACS).
2. METHODS
2.1. Study design and population
Eczema data collected from the MACS from birth to 12 years of age were used to develop subclasses. MACS is a longitudinal study of a high‐risk birth cohort that originally began as a randomized controlled trial (RCT). 12 Eligible infants (n = 620) had at least one first degree family member with a history of eczema, asthma, allergic rhinitis and/or severe food allergy. The recruitment process has been described previously. 13 Eighteen telephone surveys were conducted in the first two years where the primary carer reported information on skin conditions every 4 weeks until age 15 months and one at each age 18 and 24 months. An annual survey from three to seven years was collected, and then again at 12, 18 and 25 years respectively. The Mercy Maternity Hospital Ethics Committee approved the initial phases of the study up to the 12‐year follow‐up, and the 18‐ and 25‐year follow‐ups were approved by the University of Melbourne and the Royal Children's Hospital Ethics Committees. All mothers provided written informed consent (up to 12 years) and participants provided consent at 18 and 25 years.
When the randomization factor in RCTs does not affect the outcome of interest, the study can be considered an observational birth cohort. 14 A previously published article from MACS using an intention‐to‐treat analysis showed no difference in allergic disease outcomes among the 3 randomized groups. 15 Even so, we tested infant formula allocation as a potential predictor and confounder.
2.2. Eczema data from birth to 12 years
Up to two years, eczema at each of the 18 time‐points was defined by parental report of persistent skin rash for more than a week in the last 4 weeks in the face, trunk, legs, arms and others (excluding rash that only affected the nappy region or scalp) and report of treatment with eczema medication for one or more days. From 3 to 12 years, eczema was defined as the parental report of one or more episodes of eczema in the last 12 months, located in the chest, back, stomach, legs or arms and medication used for eczema for one or more days.
2.3. Skin prick testing
At 6, 12, 24 months and 12 years standardized extracts (Bayer, Spokane, WA, USA) were used to test for food allergens (cow's milk, egg white and peanut); and aero allergens (house dust mite, rye grass and cat dander). 16 At 18 and 25 years, the skin prick test (SPT) panel was expanded by two food allergens: cashew and shrimp (ALK Abelló, Horsholm, Denmark) and three aero allergens: mixed grass, Alternaria and Penicillium (Hollister‐Stier, Spokane, WA). 17 A positive SPT was defined as at least one allergen wheal size of greater than 2mm average diameter for children under 2 years and 3 mm for participants older than 2 years. 18 Atopy was defined by sensitization to at least one of the allergens tested. If the participant were sensitized to one or more aeroallergen, it was considered aero sensitization; the same definition was used for food allergens and food sensitization.
2.4. Early‐life risk factors
Early‐life risk factors were collected during the baseline and the 4 weeks survey. These were chosen based on reported associations with early‐life eczema in the literature. 2 We classified early life risk factors as parental, child or environmental. Parental factors examined were maternal and paternal history of eczema and allergy (i.e. history of eczema, allergic rhinitis or asthma), education (more than 15 years of education), occupation status defined using the Australian National University (ANU), 19 smoking history (ever or never). Child factors included sex, weight (kg) at 4 weeks, older siblings (number), firstborn status, breastfeeding at 4 weeks, FLG null mutations 20 and Serine peptidase inhibitor, Kazal type 5 (SPINK5) mutations. 21 Environmental factors at baseline were presence of a dog or a cat, type of fuel used in cooking and heating, distance to major roads 22 and annual concentration of nitrogen dioxide (NO2) 23 at baseline (Supplementary text 1) Data after 4 weeks of age were not used as risk factors due to the difficulty determining the sequence between these exposures and the development of eczema.
2.5. Allergic outcomes at 18 and 25 years
Allergic outcomes at age 18 and 25 were determined from surveys and clinical assessments: Current eczema, allergic rhinitis and asthma were defined by reports of (a) one or more episode in the last 12 months or (b) a history of the condition and use of medication for the condition in the previous 12 months. Allergic sensitization, aero sensitization and food sensitization were defined by the results of the SPT as defined above. Also, atopic dermatitis quick scores (ADQ) which measured eczema severity were collected on the 18‐year follow‐up. 24 We elected not to include eczema at 18 and 25 years in the LCA to enable us to specify the temporal sequence of childhood eczema subclasses and subsequent allergic outcomes in adulthood.
2.6. Statistical analysis
Longitudinal Latent Class Analysis (LLCA) was used to explore the heterogeneity and determine classes eczema from birth to 12 years of age, we modelled ‘age’ as an indicator of times when eczema prevalence was observed, 25 a detailed description of the modelling approach is described in Supplementary text 1. 26 , 27 , 28 , 29 Starting with a single latent class, additional classes were generated to the eczema model until the Bayesian and Akaike information criteria were optimized. 30 We included participants for whom data were available for at least half 12 of the time‐points studied. In LLCA, each participant is not allocated to a single class, but rather, have a probability for belonging to each identified class. 31 For that reason, we classified participants in two ways: 1) Participants were assigned to the trajectory class for which they had the highest posterior probability of membership and 2) Weights, equal to the probability of membership to each eczema subclass from the LLCA for each participant, were used in the probability‐weighted regression 32 models to account for the uncertainty in class assignment.
After the eczema subclasses were developed, we first examined the associations between the eczema subclasses (eczema subclasses as outcome) and established risk factors (parental, genetic or child) using multinomial regression. Secondly, associations between eczema subclasses (eczema subclasses as exposures) and allergic outcomes at 18 and 25 years (eczema, allergic rhinitis, asthma and sensitization) were estimated using logistic regression. Directed acyclic graphs (Supplementary text 2) were developed to assess hypothesized causal relationships and to determine which confounders to include in each model. All analyses were carried out using the statistical software Stata (release 16; Stata Corporation).
3. RESULTS
A total of 619 participants had eczema data available from at least 12 time‐points (Figure 1). There were high proportions of parental eczema and allergy history (Table 1). Most parents were tertiary educated. Most had an older sibling at 4 weeks of age and were exposed to heating and cooking by indoor combustion of solid or gas fuel.
FIGURE 1.
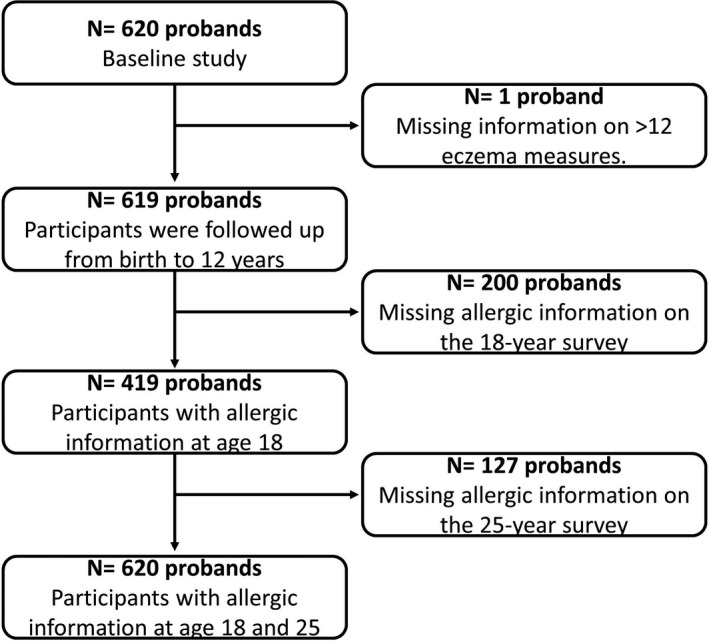
Participant flow diagram
TABLE 1.
Baseline characteristics of the MACS study cohort
| Characteristic | Cohort (N = 619) |
|---|---|
| Parental | %. (n/N) |
| Eczema history | 54.5% (337/618) |
| Allergy history | 89.0% (550/618) |
| Education (more than 15 years) | 71.6% (443/619) |
| Occupation [ANU III score] a (Mean(SD)) | 46.0 (20.59) |
| Ever smoking | 41.8% (258/617) |
| Child | |
| Female | 49.1% (304/619) |
| Weight at 4 weeks [kg] (Mean(SD) Min‐Max) | 4.0 (0.6) |
| Older siblings (Median (IQR)) | 1 (1) |
| Firstborn status | 40.1% (248/619) |
| Breastfeeding | 89.4% (554/619) |
| FLG Null | 12.1% (52/429) |
| SPINK5 b | 75.3% (323/429) |
| RCT allocation | |
| Standard cow's milk | 33.1% (205/619) |
| Soy | 33.6% (208/619) |
| Partially hydrolysed cow's milk | 33.3% (206/619) |
| Environmental | |
| Presence of pets | 49.3% (298/604) |
| Combustion heating c | 75.1% (453/603) |
| Combustion cooking c | 88.7% (535/603) |
| Distance to major roads <150 m | 23.3% (141/605) |
| NO2 [ppb] (Median (IQR); 25–75%) | 12.4 [3.39;10.9–14.3] |
Abbreviations: ANU, Australian National University; IQR, interquartile range; NO2, nitrogen dioxide ppb: parts per billion; RCT, randomized controlled trial; SD, standard deviation.
Continuous score 0–100.
Dominant G model.
Gas, coal or wood.
Information was missing for 200 probands (32.3%) in the 18‐year survey and 327 (52.8%) in the 25‐year survey. There were no important differences in the baseline characteristics between those followed and those lost to follow‐up at 18 and 25 years, except that those lost were more likely to have parents who were not tertiary educated, who smoked, had a lower occupation score and were less likely to have been breastfeed at 4 weeks (Table S1).
3.1. Eczema subclasses from birth to 12 years
A five eczema class model was found to best fit the data (Table S2), a 6‐class model was considered but did not reach convergence. These groups were labelled based on the pattern of prevalence of eczema (Figure 2) from birth to 12 years of age (A) and a zoom‐in version of the same analysis is presented (B) because there were 18 time‐points from birth to 2 years. The ‘minimal eczema’ subclass (64.7%, 419/619) was characterized by very low probability of eczema at all times, this group was used as the reference point for all analyses. The ‘early‐onset persistent’ (EOP) eczema subclass (9.7%, 59/619) had a high probability of eczema early in life, that peaked at around 5 years of age, and continued to have a high eczema probability. The ‘early‐onset resolving’ (EOR) eczema subclass (8.7%. 51/619) was characterized by a high probability of eczema in the first 6 months of life that then sharply declined. The ‘mid‐onset persistent’ (MOP) eczema subclass (14.2% 74/619) had a low probability of eczema in the first year of life which increased thereafter. Finally, the ‘mid onset resolving’ (MOR) eczema subclass (2.7% 16/619) characterized by a sharp increase in cases at the one‐year mark which started to decline at 2 years and completely resolved by 4 years. Furthermore, there were no important differences in the rates of loss to follow‐up between the eczema subclasses.
FIGURE 2.
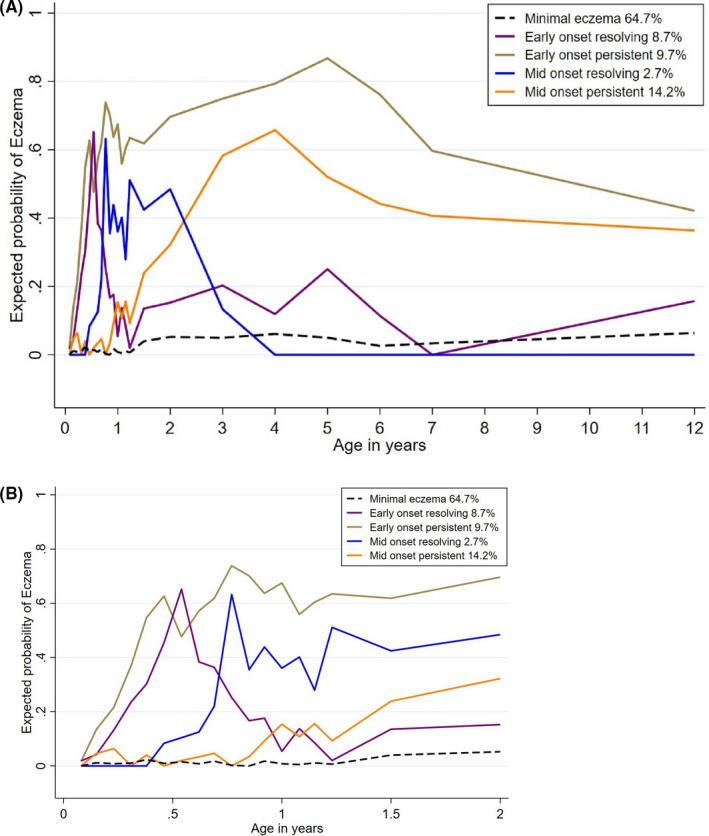
Longitudinal trajectories of eczema subclasses from birth to 12 years of age. A, Eczema subclasses from birth to 12 years (B) Eczema subclasses in the first 18 time‐points from birth to 2 years
3.2. Early‐life risk factors
Early‐life risk factors distributions were different between the eczema classes (Table S3). Parental eczema history was associated with a two‐fold increase in the odds of MOP (Table 2). Furthermore, higher occupation scores were associated with EOR. Overall, participants with FLG null mutations were more likely to be associated with the persistent subclasses, both EOP and MOP. Participants with SPINK5 G dominant mutation had a seven‐fold increase in the odds of MOR. Female participants and those with parental allergy history had increased odds of MOP. Being firstborn was associated with decreased odds of EOP. Lastly, breastfeeding at 4 weeks was associated with an approximately two‐fold increase in the odds of MOP.
TABLE 2.
Adjusted associations between eczema subclasses from 0 to 12 years and early‐life characteristics compared with the minimal eczema class
| Eczema subclasses a | Early‐onset persistent [EOP] (59/619) | Early‐onset resolving [EOR] (51/619) | Mid‐onset persistent [MOP] (74/619) | Mid‐onset resolving [MOR] (16/619) |
|---|---|---|---|---|
| Baseline risk factor | aMOR (95% CI) b | aMOR (95% CI) b | aMOR (95% CI) b | aMOR (95% CI) b |
| Parental factors b | ||||
| Parental eczema history c | 1.41 (0.79–2.51) | 1.11 (0.64–1.91) | 1.98(1.28–3.08) * | 0.89 (0.34–2.37) |
| Parental allergy history c , ** | 1.35 (0.49–3.74) | 1.70 (0.58–4.93) | 0.90 (0.48–1.67) | 2.39 (0.32–17.87) |
| Parental education (more than 15 years) | 1.59 (0.80–3.16) | 0.76 (0.39–1.46) | 0.66 (0.42–1.05) | 1.40 (0.47–4.20) |
| Occupation score per SD increase | 1.04 (0.73–1.45) | 1.29 (1.00–1.69) | 1.01 (0.78–1.30) | 0.89 (0.49–1.63) |
| Ever smoking | 0.99 (0.90–1.70) | 1.08 (0.99–1.17) | 0.96 (0.89–1.04) | 1.01 (0.84–1.21) |
| Child factors | ||||
| Female d | 0.79 (0.46–1.34) | 1.15 (0.68–1.93) | 1.68 (1.12–2.54) | 0.59 (0.24–1.44) |
| Weight at 4 weeks (1 kg increase) | 0.85 (0.52–1.39) | 0.78 (0.50–1.23) | 1.03 (0.69–1.52) | 1.05 (0.48–2.34) |
| Older siblings | 0.74 (0.49–1.11) | 1.27 (0.90–1.78) | 0.96 (0.69–1.33) | 0.70 (0.34–1.43) |
| Firstborn status | 0.35 (0.15–0.82) | 1.46 (0.63–3.35) | 0.64 (0.32–1.26) | 0.32 (0.08–1.41) |
| Breastfeeding | 1.22 (0.47–3.14) | 0.68 (0.30–1.53) | 2.13 (1.03–4.42) | 1.73 (0.62–4.82) |
| FLG Null d | 2.58 (1.09–6.08) | 1.22 (0.44–3.38) | 2.58 (1.32–5.06) | 1.23 (0.17–9.07) |
| SPINK5 d | 1.37 (0.62–3.02) | 1.37 (0.66–2.84) | 1.22 (0.70–2.14) | 7.02 (3.23–32.37) |
| Environmental factors | ||||
| Presence of pets | 1.00 (0.58–1.72) | 1.12 (0.66–1.91) | 0.98 (0.65–1.49) | 1.21 (0.49–2.95) |
| Heating by combustion | 2.22 (0.69–7.17) | 0.53 (0.25–1.11) | 1.05 (0.52–2.12) | 0.61 (0.17–2.18) |
| Cooking by combustion | 0.84 (0.44–1.61) | 0.74 (0.41–1.34) | 0.91 (0.55–1.50) | 1.89 (0.54–6.61) |
| Distance to mayor roads | 0.75 (0.39–1.47) | 0.79 (0.41–1.52) | 0.87 (0.53–1.44) | 0.52 (0.13–2.14) |
| NO2 (ppb) per unit increase | 1.20 (0.87–1.66) | 1.11 (0.87–1.42) | 0.86 (0.64–1.15) | 1.01 (0.65–1.58) |
Abbreviations: 95% CI: 95% confidence interval; aMOR, adjusted multinomial odds ratio; kg, kilogram; ppb, parts per billion; SD, standard deviation.
Reference category: Minimal eczema.
Adjusted multinomial odds ratio: sex, parental education, parental occupation, parental smoking.
Further adjusted for: cooking by combustion of solid or gas fuel, heating by combustion of solid or gas fuel, NO2, DMR, FLG and SPINK5, presence of dog or cat, older siblings, RCT: allocation, firstborn status and parental smoking history.
Univariable association estimates.
P‐values less than .05 are bolded.
All participants had at least one family member with a history of allergies.
3.3. Allergic disease outcomes at 18 and 25 years
The prevalence of allergic diseases at 18 and 25 years varied widely between the identified eczema subclasses (Table S4). Particularly, members of the EOP subclass had higher odds of all subsequent allergic outcomes at ages 18 and 25 years (Table 3). Likewise, members of the MOP had high odds of eczema, allergic rhinitis and asthma at 18 and 25 years.
TABLE 3.
Adjusted associations between allergic outcomes at 18 and 25 years and eczema subclasses from 0 to 12 years
| Eczema subclasses a | Early‐onset persistent [EOP] (38/419) | Early‐onset resolving [EOR] (37/419) | Mid‐onset persistent [MOP] (54/419) | Mid‐onset resolving [MOR] (10/419) |
|---|---|---|---|---|
| Allergic outcomes at 18 years | aOR (95% CI) b | aOR (95% CI) b | aOR (95% CI) b | aOR (95% CI) b |
| Current eczema [n:407] | 11.8 (5.20–26.6) * | 1.73 (0.77–3.90) | 2.59 (1.31–5.14) | 0.72 (0.11–4.90) |
| Current allergic rhinitis [n:419] | 3.13 (1.43–6.85) | 1.39 (0.72–2.69) | 1.70 (1.00–2.89) | 1.67 (0.51–5.44) |
| Current asthma [n:413] | 2.16 (0.94–5.00) | 1.92 (0.91–4.06) | 2.00 (1.10–3.63) | 2.96 (0.84–10.49) |
| Allergic outcomes at 25 years | ||||
| Current eczema [n:241] | 9.37 (3.17–27.65) | 2.00 (0.81–4.97) | 6.75 (3.11–14–65) | 1.39 (0.23–8.34) |
| Current allergic rhinitis [n:240] | 3.26 (1.07–9.93) | 0.99 (0.42–2.32) | 2.74 (1.28–5.88) | 1.19 (0.33–4.32) |
| Current asthma [n:244] | 2.91 (1.14–7.43) | 1.53 (0.58–7.43) | 2.50 (1.25–5.00) | 2.23 (0.56–8.92) |
Abbreviations: 95% CI, 95% confidence interval; aOR, adjusted odds ratios; n, number of observations in the regression model.
Reference category: Minimal eczema.
Adjusted odds ratio: sex, parental history of eczema, parental history of allergies, parental education, parental occupation, parental smoking, cooking by combustion of solid or gas fuel, heating by combustion of solid or gas fuel, NO2, DMR and Filaggrin null mutation.
P‐values less than .05 are bolded.
3.4. Skin prick testing
The association of allergic sensitization in early life (Table 4), adolescence and adulthood with eczema subclasses (Table 5) varied between subclasses. Participants in EOP had higher odds of developing allergic sensitization through this period, particularly food sensitization. Moreover, those with EOP were a) more likely to be food sensitized at six and 12 months than those from EOR (Table 6), b) have a higher proportion of very large (≥9 mm) SPT reactions to aero and food allergens in comparison with minimal eczema (Table S5) and c) have more severe eczema symptoms than other subclasses according to atopic dermatitis scores (Table S6). Likewise, participants from the MOP subclass had an increased risk of allergic sensitization, but this occurred from 2 years following their increase in eczema symptoms and continued into adolescence. In comparison with EOP subclass, the participants of the EOR subclasses presented smaller increases on the risk of allergic sensitization at 1,2 and at 18 years of age.
TABLE 4.
Adjusted associations between eczema subclasses and early life allergic sensitization and at 12 years with eczema subclasses as the outcomes
| Eczema subclasses a | Early‐onset persistent [EOP] (59/619) | Early‐onset resolving [EOR] (51/619) | Mid‐onset persistent [MOP] (74/619) | Mid‐onset resolving [MOR] (16/619) |
|---|---|---|---|---|
| Allergic sensitization | aMOR (95% CI) b | aMOR (95% CI) b | aMOR (95% CI) b | aMOR (95% CI) b |
| Sensitization at 6 months | ||||
| Allergic sensitization [n:554] | 3.88 (2.10–7.15) * | 1.14 (0.57–2.25) | 1.41 (0.84–2.40) | 1.48 (0.49–4.53) |
| Aero sensitization [n:553] | 3.95 (1.87–8.36) | 1.31 (0.49–3.51) | 1.91 (0.93–3.91) | 3.03 (0.89–10.28) |
| Food sensitization [n:554] | 4.55 (2.45–8.47) | 1.27 (0.63–2.59) | 1.44 (0.81–2.54) | 1.70 (0.55–5.24) |
| Sensitization at 1 year | ||||
| Allergic sensitization [n:543] | 6.34 (3.34–12.07) | 2.09 (1.11–3.95) | 1.41 (0.84–2.37) | 5.06 (1.74–14.70) |
| Aero sensitization [n:543] | 4.18 (2.17–8.06) | 1.71 (0.82–3.60) | 1.59 (0.86–2.91) | 1.71 (0.45–6.40) |
| Food sensitization [n:543] | 9.53 (4.91–18.50) | 2.19 (1.12–4.30) | 1.41 (0.79–2.50) | 5.88 (2.21–15.67) |
| Sensitization at 2 years | ||||
| Allergic sensitization [n:449] | 3.94 (1.93–8.02) | 2.37 (1.26–4.43) | 2.03 (1.18–3.50) | 1.49 (0.51–4.34) |
| Aero sensitization [n:449] | 3.73 (1.89–7.37) | 2.32 (1.22–4.39) | 2.05 (1.17–3.59) | 0.77 (0.19–3.08) |
| Food sensitization [n:449] | 5.95 (2.81–12.59) | 1.62 (0.75–3.53) | 2.06 (1.10–3.87) | 2.15 (0.68–6.75) |
| Sensitization at 12 years | ||||
| Allergic sensitization [n:344] | 4.58 (1.52–13.85) | 1.50 (0.69–3.28) | 1.90 (1.02–3.53) | 1.87 (0.40–8.77) c |
| Aero sensitization [n:344] | 4.66 (1.55–14.04) | 1.54 (0.71–3.37) | 1.86 (1.01–3.42) | 2.00 (0.43–9.19) c |
| Food sensitization [n:331] | 2.89 (1.11–7.54)x | 1.99 (0.69–5.75) | 3.34 (1.65–6.76) | 1.25 (0.31–5.07) c |
Abbreviations: 95% CI, 95% confidence interval; aMOR, adjusted multinomial odds ratio; n, number of observations in the regression model.
Reference category: Minimal eczema
Adjusted for sex, parental eczema history, parental allergy history, parental education, parental occupation, Filaggrin null mutation, outdoor and indoor air pollution.
Firth penalized logistic regression.
P‐values less than .05 are bolded.
TABLE 5.
Adjusted associations between eczema subclasses as risk factor and allergic sensitization at 18 and 25 years
| Eczema subclasses a | Early‐onset persistent [EOP] (59/619) | Early‐onset resolving [EOR] (51/619) | Mid‐onset persistent [MOP] (74/619) | Mid‐onset resolving [MOR] (16/619) |
|---|---|---|---|---|
| Allergic sensitization | aOR (95% CI) b | aOR (95% CI) b | aOR (95% CI) b | aOR (95% CI) b |
| Sensitization at 18 years | ||||
| Allergic sensitization [n:389] | 4.63 (1.67–12.79) | 2.40 (1.12–5.16) | 1.68 (0.94–3.00) | 9.58 (7.32–41.44) c |
| Aero sensitization [n:389] | 3.79 (1.47–9.76) | 2.51 (1.17–5.40) | 1.75 (0.98–3.16) | 10.18(7.69–43.72) c |
| Food sensitization [n:382] | 4.35 (1.99–9.51) | 0.82 (0.26–2.60) | 1.22 (0.58–2.58) | 0.33 (0.10–1.09) c |
| Sensitization at 25 years | ||||
| Allergic sensitization [n:216] | 2.50 (0.76–8.17) | 1.04 (0.36–3.01) | 1.43 (0.67–3.04) | 5.53 (0.30–87) c |
| Aero sensitization [n:215] | 2.49 (0.76–8.14) | 1.04 (0.36–3.01) | 1.38 (0.65–2.94) | 5.52 (0.32–87.6) c |
| Food sensitization [n:195] | 1.33 (0.28–6.23) | 0.65 (0.08–4.92) | 2.68 (0.81–8.85) | 0.22 (0.01–5.69) c |
Abbreviations: 95% CI, 95% confidence interval; aOR, adjusted odds ratio; n, number of observations in the regression model.
Reference category: Minimal eczema.
Adjusted for sex, parental eczema history, parental allergy history, parental education, parental occupation, Filaggrin null mutation, outdoor and indoor air pollution.
Firth penalized logistic regression.
P‐values less than .05 are bolded.
TABLE 6.
Adjusted associations between early eczema subclasses and early life allergic sensitization at 12 years as risk factors using early‐onset resolving as the reference category
| Allergic sensitization a | Early onset persistent [EOP] (59/619) |
|---|---|
| aOR (95% CI) b | |
| Sensitization at 6 months | |
| Allergic sensitization [n:455] | 3.41 (1.48–7.87) * |
| Aero sensitization [n:438] | 3.02 (1.00–9.10) |
| Food sensitization [n:455] | 3.57 (1.52–8.37) |
| Sensitization at 1 year | |
| Allergic sensitization [n:478] | 2.44 (1.00–5.92) |
| Aero sensitization [n:459] | 2.04 (0.70–5.98) |
| Food sensitization [n:475] | 4.35 (1.85–10.23) |
| Sensitization at 2 years | |
| Allergic sensitization [n:402] | 1.66 (0.70–3.94) |
| Aero sensitization [n:400] | 1.61 (0.70–3.71) |
| Food sensitization [n:391] | 3.66 (1.39–9.64) |
| Sensitization at 12 years | |
| Allergic sensitization [n:344] | 3.05 (0.84–11.05) |
| Aero sensitization [n:344] | 3.02 (0.84–10.92) |
| Food sensitization [n:331] | 1.45 (0.40–5.25) |
| Sensitization at 18 years | |
| Allergic sensitization [n:381] | 2.11 (0.63–7.04) |
| Aero sensitization [n:381] | 1.68 (0.55–5.14) |
| Food sensitization [n:374] | 5.10 (1.51–17.30) |
Abbreviations: 95% CI, 95% confidence interval; aMOR, adjusted multinomial odds ratio; n, number of observations in the regression model.
Reference category: Early onset resolving.
Adjusted for sex, parental eczema history, parental allergy history, parental education, parental occupation, Filaggrin null mutation, outdoor and indoor air pollution.
P‐values less than .05 are bolded.
4. DISCUSSION
We identified 5 distinct eczema subclasses using LLCA from birth to 12 years of age. These subclasses were differentially associated with early life risk factors suggesting differences in their aetiology. Concerning genetic factors, both EOP and MOP were strongly associated with FLG Null and MOP with SPINK5 G dominant genotype. In terms of prognosis, both persistent classes were associated with a high allergy burden at 18 and 25. Furthermore, EOP had larger SPT reaction to food allergens and more severe symptoms of eczema than the other eczema subclasses. A positive skin prick in the first year of life was able to discriminate between those with early‐onset resolving versus early onset persistent eczema, which may help inform treatment strategy and prognosis.
Determining distinct patterns of eczema could provide a clearer picture of the disease pathways. In contrast to previously reported eczema LCAs, 7 , 8 , 9 , 10 we targeted high‐risk children with 18 eczema assessments the first two years of life, permitting a more comprehensive overview of eczema in childhood as a basis for establishing subclasses. Our eczema subclasses have similar trajectories to those presented by Paternoster et al 7 in a similar time span (from birth to 11 and 16 years), except for the late onset resolving class which our analysis did not identify, possibly because we had a smaller sample size or a higher risk population. While Hu et al 8 also identified a 5‐class model, the classes identified were somewhat different; however, the patterns for the ‘persistent’ class were comparable to the persistent subclasses from our analysis. Moreover, Roduit et al 9 and Suaini et al 10 identified a 4‐class model, they described similar trajectories to the similarly named subclasses in our model with minor differences. Ziyab et al 11 described five sex‐specific trajectories, four trajectories applied to both sexes, these trajectories described similar eczema trends to our results. However, the late onset class, which was a female‐specific trajectory, was not identified in our results. Other studies examined patterns of severity of eczema 33 and the patterns of eczema into middle age 34 describing different patterns to our results. The reason for these discrepancies may be because of differences in the eczema definition, the number of time‐points assessed, different populations (multi‐ethnic vs predominantly Caucasian), age of assessment and study design. Nonetheless, studies that measured eczema during childhood described comparable eczema patterns to our results, providing robust evidence of an underlying classification of this disease.
Our findings suggest that certain eczema subclasses were associated with specific early‐life risk factors. Although the association between parental history of eczema with childhood eczema is well known, 35 our results suggest that is more strongly associated with MOP subclass despite all members of the cohort having a family history of allergic disease. Likewise, associations between female sex and eczema have been reported 36 but the association was stronger with the MOP subclass. Breastfeeding at 4 weeks was associated with MOP, this may be due to breastfeeding delaying early manifestation of eczema 37 in children who are at high risk of developing this condition (genetic predisposition as noted by a high rate of FLG null mutations). The protein filaggrin facilitates the terminal differentiation of the epidermis and the formation of the skin barrier. Null mutations result in complete loss of processed functional filaggrin in the epidermis. 38 Accordingly, we have observed that FLG null mutations were strongly associated with both persistent eczema subclasses (EOP and MOP) which highlights a potential eczema development and progression pathway. Furthermore, SPINK5 was linked to eczema in a previous study which suggested that the barrier function regulated by SPINK5, may also play an important role in the development of eczema. 39 While we observed that SPINK5 dominant G model was associated with MOR, this analysis was not well powered and should be interpreted with caution.
Our results suggested that eczema subclasses from birth to 12 years were distinctly associated with allergic outcomes at 18 and 25 years. Allergic rhinitis is an inflammatory condition affecting nasal mucosal membranes. Defective skin and mucous membrane barriers may promote sensitization to environmental allergens and subsequent atopic diseases. 5 Even though, it has been shown that children with eczema have nearly three‐fold increased odds of developing allergic rhinitis at 5‐year follow‐up compared with children without eczema, 8 , 40 our results suggest that those with the persistent subclasses were at a higher risk. Furthermore, like our results suggested having severe, early‐onset, or persistent eczema further increased the risks of developing allergic rhinitis and asthma. 40 Likewise, another study showed that children with infantile eczema have an increased risk of eczema, allergic rhinitis and asthma in preadolescence, compared with children without eczema. 41 Moreover, it has been estimated that approximately one‐third of patients with eczema develop asthma and two‐thirds develop allergic rhinitis. 42 , 43 Our results add to these findings by showing that EOP and MOP subclasses were at higher risk of further allergic disease. Additionally, children with eczema onset after 2 years have an increased risk of asthma, 44 in accordance with our observations that MOP (eczema onset around 1.5 years) was associated with increased odds of asthma at 18 and 25 years. Our finding of increased risk of allergic outcomes in in children with persistent eczema subclasses provides support to the atopic march theory, the progression from eczema to other allergic diseases. 5 , 42 , 43
We observed a complex pattern of coexistence of allergic sensitization with eczema during early life. Although it has been showed that early eczema is associated with an increased risk of food allergies, 45 , 46 , 47 our results suggest that participants from the EOP and MOP showed stronger associations with food allergens in the first 2 years of life. As such, a large proportion of children with eczema (around 30–40%) will have a coexisting food allergy 45 and our results suggest most of these belong to the persistent eczema subclasses.
There are several strengths in this study including its design with a prospective cohort having 26 data points with 25 years of follow‐up and well‐characterized definitions of eczema considering the change of distribution in skin involvement over childhood. Also, we objectively assessed associations with sensitization at multiple time‐points by SPT using international standards. 18 However, our study also had some limitations, as the results were based on a high‐risk cohort, the identified eczema subclasses and their associations might not be applicable to the general population. Nevertheless, the subclasses identified were similar to those found in unselected birth cohorts. 7 The definitions of eczema used in this study were selected to facilitate comparison with previous studies in this area 7 and modified from those used in previous MACS publications. 15 These measures had high agreement with the well‐established eczema definition from the International Study of Asthma and Allergies in Childhood 48 (Supplementary text 3). Some of the data were self‐reported and may be subject to measurement error. We elected not to use doctor diagnoses in the definition of eczema to avoid bias from healthcare‐seeking behaviour. Given the limited number of participants in the MOR class, its associations should be interpreted with caution. Loss to follow‐up tended to be more pronounced among the less advantaged participants. However, there was no likely reason that the exposure‐outcome associations would be different in the non‐participants and thereby cause spurious results. 49 There were differences in the anatomical locations and time periods between the eczema definition up to 2 years and from 3 to 12 years, however, both included the report of eczema medication use. We did not include anatomical location of symptoms on the model, future research should look at the evolution of anatomical location of symptoms, and their severity, over time. Finally, as almost all the participants were Caucasian and Anglo‐Celtic of European descent, the findings may not be generalizable to other ethnicities. Future research should focus on evaluating eczema subclassification in different ethnicities while using multiple data points. At this time, there are no birth cohorts available with such frequent collection of data to replicate these results.
5. CONCLUSION
We have identified distinct eczema subclasses from birth to 12 years, that were both differentially associated with early life risk factors and with the development of allergic outcomes at 18 and 25 years. Use of these eczema subclasses may aid in the identification of risk factors and causes of these specific forms of eczema. Food sensitization in early life can potentially serve as a marker of persistent eczema subclasses. This can be clinically important as the persistent eczema classes were strongly associated subsequent allergic diseases in adulthood. It is possible that intensive treatment aimed at achieving eczema remission, might not only reduce the prevalence of eczema, but also the development of other allergic comorbidities. Future studies and interventions can be tailored to these eczema subclasses; early detection of eczema subclasses may be important for class‐specific preventive or therapeutic strategies.
CONFLICTS OF INTEREST
D.J. Lopez has nothing to disclose. Dr N.T. Waidyatillake has nothing to disclose. Dr J.C. Su has nothing to disclose. Prof C. Svanes has nothing to disclose. Dr B. Erbas has nothing to disclose. Prof S. C Dharmage, A/Prof A.J. Lowe, Dr J. Perret, A/Prof C.J. Lodge, Dr D.S. Bui and Prof M.J. Abramson have received an investigator‐initiated grant from GlaxoSmithKline for unrelated research. Prof M.J. Abramson holds investigator‐initiated grants for unrelated research from Pfizer, Boehringer‐Ingelheim and Sanofi. He has undertaken an unrelated consultancy for and received assistance with conference attendance from Sanofi. He has also received a speaker's fee from GSK. A/Prof A.J. Lowe has received in‐kind contributions of emollients from PuraCap and Primus for use in eczema prevention trials that he leads.
AUTHOR CONTRIBUTIONS
D.J. Lopez, A.J. Lowe, D.S. Bui, C.J. Lodge and S.C. Dharmage contributed to conception and design.: S.C. Dharmage M.J. Abramson A.J. Lowe, C.J. Lodge, C. Svanes and B. Erbas. contributed to acquisition of data,. D.J. Lopez, A.J. Lowe, C.J. Lodge, D.S. Bui, N.T. Waidyatillake and J.C. Su. contributed to analysis and interpretation of data. D.J. Lopez, A.J. Lowe, C.J. Lodge, D.S. Bui, N.T. and Waidyatillake, J. Su. contributed to drafting the article. All authors contributed to revision for important intellectual content and approval of final submission.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr John Thorburn, FRACP, for assistance in patient recruitment and administrative assistance and the Mercy Maternity Hospital Department of Obstetrics for participant recruitment, and Dr David Hill, Dr Cliff Hosking and C Axelrad for study leadership up to the 12‐year follow‐up. We thank Anne Balloch for assistance with data management, Rida Asif, Carlie Dunford and Brittany Campbell for project coordination, and Jeeva Sanjeevan for clinical testing as well as Chris Barton, John Hopper, Vijaya Sundraran, Sharon Goldfield, Catherine Bennett, Katrina Allen, Lyle Gurrin for their contributions during the 18‐year follow‐up. We also acknowledge the contributions of Paul Thomas, Aaron Darling during the 25‐year follow‐up. Most importantly, we thank all of the MACS children and parents for their participation and ongoing support for this study. D. Lopez was supported by the University of Melbourne and Becas Carlos Antonio Lopez scholarship. A. J. Lowe, S. C. Dharmage and J Perret are supported by NHMRC Fellowships. Finally, we would like to thank the MACS funding agencies: the first 12 years of the MACS was funded (study formula and staff) by Nestec Ltd, a subsidiary of Nestlé Australia and the National Health and Medical Research Council of Australia funded the 18 (GNT454856) and 25‐year (GNT1079668). The funding agencies had no direct role in the conduct of the study, the collection, management, statistical analysis and interpretation of the data, preparation or approval of the manuscript. Open access publishing facilitated by The University of Melbourne as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. [Correction added on 9 May 2022, after first online publication: CAUL funding statement has been added.]
Lopez DJ, Lodge CJ, Bui DS, et al. Establishing subclasses of childhood eczema, their risk factors and prognosis. Clin Exp Allergy. 2022;52:1079–1090. doi: 10.1111/cea.14139
Funding information
The first 12 years of the MACS was funded (study formula and staff) by Nestec Ltd, a subsidiary of Nestlé Australia. The National Health and Medical Research Council of Australia funded the 18‐ (GNT454856) and 25‐year (GNT1079668) follow‐ups. All bodies that have funded aspects of the MACS have had no role in interpretation and publication of study findings
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832‐836. [DOI] [PubMed] [Google Scholar]
- 2. Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75(1):63–74. 10.1111/all.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population‐based case‐control study. J Allergy Clin Immunol. 2008;121(4):940‐46.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. [DOI] [PubMed] [Google Scholar]
- 5. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. 2014;69(1):17‐27. [DOI] [PubMed] [Google Scholar]
- 6. Mulick AR, Allen V, Williams HC, et al. Classifying atopic dermatitis: protocol for a systematic review of subtypes (phenotypes) and associated characteristics. BMJ Open. 2018;8(9):e023097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paternoster L, Savenije OEM, Heron J, et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol. 2018;141(3):964‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu C, Nijsten T, van Meel ER, et al. Eczema phenotypes and risk of allergic and respiratory conditions in school age children. Clin Transl Allergy. 2020;10(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roduit C, Frei R, Depner M, et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr. 2017;171(7):655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suaini NHA, Yap GC, Bui DPT, et al. Atopic dermatitis trajectories to age 8 years in the GUSTO cohort. Clin Exp Allergy. 2021;51(9):1195‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziyab AH, Mukherjee N, Zhang H, Arshad SH, Karmaus W. Sex‐specific developmental trajectories of eczema from infancy to age 26 years: A birth cohort study. Clin Exp Allergy. 2022;52(3):416–425. 10.1111/cea.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowe AJ, Lodge CJ, Allen KJ, et al. Cohort Profile: Melbourne Atopy Cohort study (MACS). Int J Epidemiol. 2017;46(1):25‐26. [DOI] [PubMed] [Google Scholar]
- 13. Hill DJ, Sporik R, Thorburn J, Hosking CS. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. J Pediatr. 2000;137(4):475‐479. [DOI] [PubMed] [Google Scholar]
- 14. Howard G, Howard V. Observational epidemiology within randomized clinical trials: getting a lot for (almost) nothing. Prog Cardiovasc Dis. 2012;54:367‐371. [DOI] [PubMed] [Google Scholar]
- 15. Lowe AJ, Hosking CS, Bennett CM, et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high‐risk children: a randomized controlled trial. J Allergy Clin Immunol. 2011;128(2):360‐5.e4. [DOI] [PubMed] [Google Scholar]
- 16. Lowe AJ, Hosking CS, Bennett CM, et al. Skin prick test can identify eczematous infants at risk of asthma and allergic rhinitis. Clin Exp Allergy. 2007;37(11):1624‐1631. [DOI] [PubMed] [Google Scholar]
- 17. Barton CA, Dharmage SC, Lodge CJ, Abramson MJ, Erbas B, Lowe A. Asthma, atopy and serious psychological distress: prevalence and risk factors among young people in the Melbourne atopy cohort study. J Asthma. 2020;57(12):1323‐1331. [DOI] [PubMed] [Google Scholar]
- 18. Smith W. Skin Prick Testing for the Diagnosis of Allergic Disease–A Manual for Practitioners. Austr Soc Clin Immunol Allergy. 2019. Available from: https://www.allergy.org.au/images/ASCIA_HP_SPT_Guide_2020.pdf. Accessed December 26, 2021. [Google Scholar]
- 19. McMillan J, Jones F. The ANU3_2 scale: a revised occupational status scale for Australia. J Sociol. 2000;36(1):64‐80. [Google Scholar]
- 20. Lowe AJ, Lee B, Orchard D, et al. Palm reading and water divining: A cross‐sectional study of the accuracy of palmar hyperlinearity and transepidermal water loss to identify individuals with a filaggrin gene null mutation. J Am Acad Dermatol. 2020;83(4):1186‐1188. [DOI] [PubMed] [Google Scholar]
- 21. Ashley S, Tan HT, Vuillermin P, et al. The skin barrier function gene SPINK 5 is associated with challenge‐proven IgE‐mediated food allergy in infants. Allergy. 2017;72(9):1356‐1364. [DOI] [PubMed] [Google Scholar]
- 22. Bowatte G, Lodge CJ, Lowe AJ, et al. Do variants in GSTs modify the association between traffic air pollution and asthma in adolescence? Int J Mol Sci. 2016;17(4):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knibbs LD, Hewson MG, Bechle MJ, Marshall JD, Barnett AG. A national satellite‐based land‐use regression model for air pollution exposure assessment in Australia. Environ Res. 2014;135:204‐211. [DOI] [PubMed] [Google Scholar]
- 24. Carel K, Bratton DL, Miyazawa N, et al. The Atopic Dermatitis Quickscore (ADQ): validation of a new parent‐administered atopic dermatitis scoring tool. Ann Allergy Asthma Immunol. 2008;101(5):500‐507. [DOI] [PubMed] [Google Scholar]
- 25. Herle M, Micali N, Abdulkadir M, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. 2020;35(3):205‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Applied Latent Class Analysis. Cambridge University Press; 2002. [Google Scholar]
- 27. Rabe‐Hesketh S, Skrondal A. Classical latent variable models for medical research. Stat Methods Med Res. 2008;17(1):5‐32. [DOI] [PubMed] [Google Scholar]
- 28. Spycher BD, Minder CE, Kuehni CE. Multivariate modelling of responses to conditional items: New possibilities for latent class analysis. Stat Med. 2009;28(14):1927‐1939. [DOI] [PubMed] [Google Scholar]
- 29. Howard R, Rattray M, Prosperi M, Custovic A. Distinguishing asthma phenotypes using machine learning approaches. Curr Allergy Asthma Rep. 2015;15(7):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a monte carlo simulation study. Struct Eq Model Multi J. 2007;14(4):535‐569. [Google Scholar]
- 31. Weller BE, Bowen NK, Faubert SJ. Latent class analysis: a guide to best practice. J Black Psychol. 2020;46(4):287‐311. [Google Scholar]
- 32. Clark S, Muthén B. Relating Latent Class Analysis Results to Variables not Included in the Analysis. 2009.
- 33. Mulick AR, Mansfield KE, Silverwood RJ, et al. Four childhood atopic dermatitis subtypes identified from trajectory and severity of disease and internally validated in a large UK birth cohort. Br J Dermatol. 2021;185(3):526‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abuabara K, Ye M, Margolis DJ, et al. Patterns of atopic eczema disease activity from birth through midlife in 2 British birth cohorts. JAMA Dermatol. 2021;157(10):1191‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wadonda‐Kabondo N, Sterne JAC, Golding J, Kennedy CTC, Archer CB, Dunnill MGS. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Arch Dis Child. 2004;89(10):917‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ballardini N, Kull I, Söderhäll C, Lilja G, Wickman M, Wahlgren CF. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168(3):588‐594. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Abadie M, Beer G, Al‐Rubaye M, Oumeish F, Al AD. Does breastfeeding delay the onset of eczema in infants? Egypt J Dermatol Venerol. 2021;41(2):67. [Google Scholar]
- 38. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328‐340. [DOI] [PubMed] [Google Scholar]
- 39. Lan C‐CE, Tu H‐P, Wu C‐S, et al. Distinct SPINK5 and IL‐31 polymorphisms are associated with atopic eczema and non‐atopic hand dermatitis in Taiwanese nursing population. Exp Dermatol. 2011;20(12):975‐979. [DOI] [PubMed] [Google Scholar]
- 40. von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindström CB, Svensson Å. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. 2012;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ballardini N, Bergström A, Böhme M, et al. Infantile eczema: prognosis and risk of asthma and rhinitis in preadolescence. J Allergy Clin Immunol. 2014;133(2):594–596.e3. [DOI] [PubMed] [Google Scholar]
- 42. van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120(3):565‐569. [DOI] [PubMed] [Google Scholar]
- 43. Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin. 2010;30(3):269‐280. [DOI] [PubMed] [Google Scholar]
- 44. Chiu C‐Y, Yang C‐H, Su K‐W, et al. Early‐onset eczema is associated with increased milk sensitization and risk of rhinitis and asthma in early childhood. J Microbiol Immunol Infect. 2020;53(6):1008‐1013. [DOI] [PubMed] [Google Scholar]
- 45. Ben‐Shoshan M, Soller L, Harrington DW, et al. Eczema in early childhood, sociodemographic factors and lifestyle habits are associated with food allergy: a nested case‐control study. Int Arch Allergy Immunol. 2015;166(3):199‐207. [DOI] [PubMed] [Google Scholar]
- 46. Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? Results from a population‐based cohort. Clin Exp Allergy. 2015;45(1):255‐264. [DOI] [PubMed] [Google Scholar]
- 47. Gray CL. Allergies in eczema : review article. Curr Allergy Clin Immunol. 2011;24(4):185‐191. [Google Scholar]
- 48. Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483‐491. [DOI] [PubMed] [Google Scholar]
- 49. Howe LD, Tilling K, Galobardes B, Lawlor DA. Loss to follow‐up in cohort studies: bias in estimates of socioeconomic inequalities. Epidemiology. 2013;24(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


