Abstract
Objective
Nonalcoholic fatty liver disease (NAFLD) has a different prevalence in adults from different ethnic groups. This study examined whether these ethnic differences originate in early life and could be explained by early‐life factors.
Methods
This observational study was embedded in a population‐based prospective cohort study from fetal life onward among 2,570 children born in Rotterdam, the Netherlands. Information about prepregnancy, pregnancy, and childhood factors, as well as childhood BMI, was obtained from questionnaires and physical examinations. Liver fat was assessed by magnetic resonance imaging at age 10 years.
Results
Median liver fat fraction was 2.0% (95% CI: 1.2%‐5.3%), and NAFLD prevalence was 2.8%. Children from a Turkish background had the highest median liver fat percentage (2.5%, 95% CI: 1.2%‐10.7%) and NAFLD prevalence (9.1%). Children of Cape Verdean, Dutch Antillean, Surinamese‐Creole, or Turkish background had a higher total liver fat fraction compared with children with a Dutch background (p < 0.05). After controlling for early‐life factors, these differences persisted only in children with a Turkish background.
Conclusions
Prevalence of liver fat accumulation and NAFLD differs between ethnic subgroups living in the Netherlands, especially for those with a Turkish background. Early‐life factors have a strong influence on these associations and may hold clues for future preventive strategies.
Study Importance.
What is already known?
Fatty liver or nonalcoholic fatty liver disease in children is the most common chronic liver disease and is associated with many comorbidities. Its prevalence is on the rise along with that of obesity. Studies in adults have suggested that there are major ethnic differences in the risk of liver fat accumulation. These differences may originate in early life.
What does this study add?
We observed major differences in the prevalence of liver fat accumulation as early as school age among children living in the Netherlands. Compared with children with a Dutch background, those of Cape Verdean, Dutch Antillean, Surinamese‐Creole, or Turkish background had a higher total liver fat fraction. Children from a Turkish background had the highest median liver fat percentage.
Parental and child early‐life factors seem to explain a large part, but not all, of these associations.
How might your results change the direction of research or the focus of clinical practice?
The prevalence of liver fat accumulation and NAFLD differs between ethnic subgroups living in the Netherlands. Various prepregnancy, pregnancy, and childhood factors seem to explain these differences to a large extent and should be considered in future research.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as the accumulation of fat in the liver in the absence of excessive alcohol consumption or other known liver pathologies (1). NAFLD is the most common chronic liver disease in children and adults in Western countries, with an estimated prevalence of 8% in general populations and 34% in populations with obesity (2, 3, 4). In children, NAFLD is associated with increased insulin resistance and hypertension, independent of BMI (5). Childhood NAFLD tends to persist into adulthood and predispose individuals to type 2 diabetes and cardiovascular and liver disease in adulthood (6). Previous studies among adults and adolescents have suggested that liver fat accumulation differs between ethnic subgroups (4, 6, 7, 8, 9, 10). Studies in the United States have reported that, compared with Caucasian adolescents, those from Hispanic and Asian backgrounds have a higher liver fat accumulation, but those with an African American background have a lower liver fat accumulation, independent of their BMI (6, 8, 9, 10, 11). A previous study in the same cohort as the current study showed differences in the prevalence of obesity and adverse body fat profiles between children living in the Netherlands from Dutch, Cape Verdean, Dutch Antillean, Moroccan, Surinamese, and Turkish backgrounds (12). These groups reflect the largest ethnic subgroups in the Netherlands. Children of Moroccan, Surinamese‐Hindustani, and Turkish backgrounds had a higher general fat mass compared with children with a Dutch background, independent of BMI (12). Additionally, recent studies have suggested that various parental and childhood factors contribute to liver fat accumulation (13, 14, 15, 16, 17). Insight into the ethnic differences and underlying risk factors could contribute to novel prevention strategies.
We hypothesized that liver fat accumulation differs between ethnic subgroups living in the Netherlands. We examined the ethnic differences in liver fat accumulation in school‐aged children and whether any difference could be explained by prepregnancy, pregnancy, and childhood factors.
METHODS
Study design
This observational analysis study was embedded in the Generation R Study, a multiethnic population‐based prospective cohort from early pregnancy onward based in Rotterdam, the Netherlands (18). The Generation R Study was approved by the Medical Ethical Committee of the Erasmus University Medical Center in Rotterdam (MEC 198.782/2001/31). All children were born in Rotterdam between 2002 and 2006, and written consent was obtained from the participating parents (18). In total, 7,393 children participated at school age. A subgroup of 4,133 children were invited for the magnetic resonance imaging (MRI) measurements, of whom 3,170 children had MRI‐based liver fat measurements available between age 8 and 10 years. None of these children had a history of smoking, drug use, jaundice, medication use, or alcohol use at the time of the MRI. We included singleton children who had available data on child ethnicity, liver fat fraction, and BMI (flowchart in Supporting Information Figure S2). The main analyses were based on 2,570 children of Dutch, Cape Verdean, Dutch Antillean, Moroccan, Surinamese‐Creole, Surinamese‐Hindustani, or Turkish background, which all had at least 80 children per group. These ethnic backgrounds can be found throughout the Netherlands, although the distribution of these backgrounds might differ slightly per city. This study focused specifically on children born in the city of Rotterdam.
Childhood ethnic background
The background of the ethnic subgroups in the Netherlands is the Dutch colonial history (Surinamese, Dutch Antillean) and working migration (Cape Verdean, Moroccan, Turkish). According to Statistics Netherlands, the ethnic background of children is defined by the country of birth of the parents (19). Information about the country of birth of the parents was obtained by questionnaires (18). A child was considered of Dutch background if both parents were born in the Netherlands and was considered of non‐Dutch origin if one or both of the parents were born abroad. If the parents were born in different countries, the country of birth of the mother determined the ethnic background of the child. The ethnic subgroups were categorized into Dutch, Cape Verdean, Dutch Antillean, Moroccan, Surinamese, and Turkish. The Surinamese group was subdivided into Surinamese‐Creole and Surinamese‐Hindustani. We included ethnic subgroups with a sample size of more than 80 children to assure enough power for the regression analysis (12, 20).
Childhood BMI and liver fat
Height and weight of the children, both without shoes and heavy clothing, were measured at age 10 years. Subsequently, their BMI was calculated, as well as sex‐adjusted and age‐adjusted childhood BMI SD scores (Growth Analyzer 4.0, Dutch Growth Research Foundation) (21). Childhood BMI was categorized into underweight, normal weight, overweight, and obesity using the International Obesity Task Force cutoffs (5, 22). Liver fat was measured using a 3.0‐Tesla MRI scanner (Discovery MR750w, GE Healthcare) (5, 18). While undergoing the body scan, the children wore light clothing without metal objects. To generate a precise liver fat fraction image, a liver fat scan was performed using a three‐dimensional, single‐breath‐hold, three‐point proton density‐weighted Dixon technique (IDEAL IQ), producing 50 image slices of 5‐mm thickness (23). The obtained fat‐fraction maps were analyzed by Precision Image Analysis (PIA) using the sliceOmatic (TomoVision) software package (5, 24). All extraneous structures and any image artifacts were removed manually (24). Liver fat fraction was determined from four regions of interest of at least 4 cm2, which were manually drawn from different sections from the central portion of the hepatic volume. Subsequently, the mean signal intensities were averaged to generate an overall mean liver fat estimation. This was done by different observers who were each assigned different portions of the data. Liver fat measured with IDEAL IQ (GE Healthcare) using MRI is reproducible, highly precise, and validated in adults (24, 25). NAFLD was defined as liver fat ≥5.0% (26). None of these children was known to have been diagnosed with any liver pathology. This information was collected by questionnaires.
Covariates
We explored the role of various covariates and possible explaining factors. These included the following: 1) prepregnancy factors; 2) pregnancy factors; 3) childhood factors; and 4) childhood BMI, illustrated in Supporting Information Figure S1. Prepregnancy factors were defined as factors that could not be modified during pregnancy or childhood and these included maternal age, educational level, net household income, marital status, parity, and prepregnancy BMI. These were assessed at enrollment through questionnaires. BMI was calculated from weight and height information at enrollment. Pregnancy factors included maternal calorie intake during pregnancy, smoking, and alcohol use, which were assessed through repeated questionnaires during pregnancy, and pregnancy complications (hypertensive disorders and diabetes), gestational age, and birth weight were obtained from medical records (18). Information on childhood factors, including average hours of exercise and sedentary activities (screen time), diet quality score, and calorie intake, was obtained from postnatal questionnaires around the age of 9 years, except for sugar intake and breastfeeding, which were obtained from postnatal questionnaires about the first year of life (18). Covariates were selected if there were strong correlations with high BMI, liver fat accumulation, and risk of NAFLD (2, 5, 6, 7, 12, 27, 28, 29, 30).
Statistical analysis
First, we performed nonresponse analyses to assess differences in participant characteristics between those with and without liver assessments, among children invited for the MRI studies, using ANOVA and χ2 tests for continuous and categorical factors, respectively. We visualized the difference in median liver fat fraction and NAFLD prevalence between the different ethnic groups, with the Dutch group as the reference category. Second, we analyzed the associations of ethnic background with liver fat accumulation continuously and NAFLD binary using linear and logistic regression models, respectively. The main model was adjusted for child age and sex at MRI measurements. Next, we explored whether any association was explained by prepregnancy factors (maternal age, education level, net household income, marital status, and prepregnancy BMI); pregnancy factors (maternal smoking, alcohol use, maternal diet score, pregnancy complications, gestational age, and birth weight); childhood factors (breastfeeding, calorie intake, sugar‐containing beverage intake during infancy, average hours of exercise, and sedentary activity); or childhood BMI. The inclusion of these factors in the models was determined based on their association with BMI, liver fat accumulation, and risk of developing NAFLD reported in previous studies and associations with the outcome or changes in effect estimates >10%. When factors were highly correlated (cutoff of Pearson r [−]0.45), one could serve as a proxy for the other. The exact percentages of change in the effect estimates after adjusting for different factors were calculated using the following formula: percentage change = ([β2 − β1]/ β1) × 100. This resulted in the removal of net household income, with maternal education as the proxy (Pearson r 0.52), as well as marital status, parity, pregnancy complications, gestational age, diet quality score, and calorie intake, owing to small effect estimates from the models. The correlations between those variables that remained in the model can be found in Supporting Information Table S5. Factors were added to the model consecutively, starting with prepregnancy factors and ending with the final model, including BMI, resulting in five models including the main model. BMI was controlled for last because of the nature of the ethnicity variable. In Supporting Information Figure S1, this is further elaborated. Liver fat fraction was non‐normally distributed and, therefore, log‐transformed. In order to reduce the potential bias associated with missing data, missing data in the covariates (ranging from 0.1%‐42.5%) from the population that was invited for the MRI were multiple‐imputed using the Markov chain Monte Carlo approach. Covariates with more than 45% missing would have been excluded, but none of the covariates met this threshold. Within this method, we assumed a missingness structure of missing at random. The variable with the highest percentage missing was sugar intake (servings per day) at infancy. For more information, we refer to Supporting Information Table S1. Five imputed data sets were created and analyzed together. All analyses were also conducted on the nonimputed data set to see whether it influenced results. Furthermore, owing to the small number of NAFLD cases in our sample, the logarithmic regression analysis looked at p values of 0.10 instead of 0.05. All analyses were performed using SPSS Statistics version 25.0 for Windows (IBM Corp.).
RESULTS
Participant characteristics and liver fat in different ethnic subgroups
Table 1 shows the prepregnancy, pregnancy, and childhood factors for each ethnic group. The Dutch group had the highest percentage of higher‐educated mothers (62.7%), with the second highest percentage in the Surinamese‐Hindustani group (25.7%). The percentage of household incomes above €2,200 per month was also highest in the Dutch group (77.6%), with the second highest percentage in the Surinamese‐Hindustani group (46.7%). The Dutch group had the lowest reported screen time for children: 47.8% spent more than 2 h/d behind a screen compared with 81.3% in the Cape Verdean group. The observed median liver fat fraction among children with a Dutch background was 2.0% (95% CI: 1.2%‐4.6%). The total number of children classified as having NAFLD was 72, and, of these, 15 (20.8%) were of normal weight, 17 (23.6%) had overweight, and 40 (55.6%) had obesity (Supporting Information Table S2). Figure 1A,B shows the median percentages of liver fat fraction and proportions of NAFLD per ethnic subgroup, respectively, whereas Figure 1C shows the distribution of weight categories (underweight, normal weight, overweight, and obesity) per subgroup. Compared with children from a Dutch background, higher median liver fat fractions were observed in all other ethnic groups. Children with a Turkish background had the highest median liver fat fraction (2.5% [95% CI: 1.2‐10.7]). Similarly, compared with children with a Dutch background, a higher percentage of overweight and obesity was observed in all other ethnic subgroups, with the highest percentage among children with a Turkish background (36.4%). For NAFLD, the highest prevalence was observed among children with a Turkish background (9.1%) and Cape Verdean background (7.1%).
TABLE 1.
Characteristics of the study population (N = 2,570)
| Dutch, | Cape Verdean, | Dutch Antille, | Moroccan, | Surinamese‐Creole, | Surinamese‐Hindustani, | Turkish, | Total, | |
|---|---|---|---|---|---|---|---|---|
| N = 1,889 | N = 99 | N = 88 | N = 152 | N = 94 | N = 83 | N = 165 | N = 2,570 | |
| Maternal/prepregnancy characteristics | ||||||||
| Age at intake (y), mean | 31.8 (4.4) | 29.7 (5.4) | 27.8 (6.4) | 29.3 (5.1) | 30.97 (5.52) | 29.17 (4.72) | 28.11 (4.93) | 31.1 (4.8) |
| Higher education, % | 62.7 | 15.6 | 18.0 | 12.8 | 24.1 | 25.7 | 18.3 | 51.7 |
| Multiparity, % | 38.9 | 62.9 | 30.7 | 57.3 | 47.8 | 35.4 | 52.1 | 41.8 |
| Prepregnancy BMI, median | 22.3 (18.1‐34.2) |
22.8 (18.7‐34.2) |
24.1 (17.1‐45.1) | 26.1 (18.6‐38.3) | 24.2 (17.4‐42.0) | 23.0 (17.1‐39.0) | 23.5 (18.4‐40.6) | 22.7 (18.1‐35.3) |
| Net income >€2,200/mo, % | 77.6 | 7.1 | 23.8 | 8.6 | 23.8 | 46.7 | 18.6 | 64.40 |
| Pregnancy characteristics | ||||||||
| Total caloric intake (kcal), mean | 2,138 (488) | 1,909 (644) | 1,900 (563) | 2,028 (576) | 1,864 (576) | 1,956 (692) | 1,859 (613) | 2,084 (529) |
| Never smoked, % | 78.7 | 80.8 | 69.5 | 91.0 | 69.1 | 86.7 | 61.7 | 78.1 |
| No alcohol use, % | 33.1 | 53.5 | 57.7 | 92.2 | 54.1 | 64.4 | 82.9 | 43.2 |
| Gestational diabetes (yes), % | 0.4 | 0.0 | 1.2 | 3.4 | 0.0 | 2.5 | 0.0 | 0.6 |
| Preeclampsia, % | 2.2 | 0.0 | 2.6 | 2.1 | 2.5 | 5.4 | 0.7 | 2.1 |
| Gestational hypertension, % | 4.6 | 3.1 | 3.5 | 0.7 | 4.5 | 2.5 | 3.1 | 4.1 |
| Gestational age (wk), median | 40.3 (36.0‐42.3) | 40.1 (36.4‐42.4) | 40.0 (32.9‐41.9) | 40.4 (36.0‐42.6) | 39.6 (31.6‐42.1) | 39.9 (34.9‐42.1) | 40.1 (34.8‐42.3) | 40.14 (35.9‐42.3) |
| Birth weight (g), mean | 3,501 (561) | 3,341 (441) | 3,178 (526) | 3,499 (525) | 3,212 (642) | 3,091 (494) | 3,370 (524) | 3,453 (558) |
| Child characteristics | ||||||||
| Any breastfeeding, % | 91.9 | 88.6 | 88.3 | 96.4 | 91.3 | 100.0 | 98.3 | 92.4 |
| Screen time for ≥ 2 h/d, % | 47.8 | 81.3 | 66.7 | 69.0 | 73.0 | 78.3 | 70.2 | 52.8 |
| Sports activities ≥ 1 h/d, % | 73.9 | 56.5 | 72.6 | 63.2 | 63.4 | 59.1 | 49.5 | 70.8 |
| Sugary drinks at infancy, median servings/d | 1.0 (0.0‐3.7) | 1.0 (0.0‐9.0) | 1.4(0.0‐2.0) | 0.7(0.0‐4.6) | 1.2 (0.0‐6.4) | 1.1 (0.0‐6.0) | 0.5 (0.0‐3.9) | 1.0 (0.00‐3.8) |
| Age (y), mean | 10.2 (0.6) | 10.2 (0.6) | 9.90 (0.50) | 9.90 (0.40) | 9.80 (0.40) | 9.77 (0.37) | 9.87 (0.40) | 10.17 (0.62) |
| Child BMI, median | 16.6 (14.0‐22.6) | 18.5 (14.0‐29.3) | 18.1 (14.1‐27.5) | 17.8 (14.5‐27.6) | 17.8 (14.2‐24.1) | 16.4 (12.8‐23.8) | 18.3 (14.6‐26.2) | 16.9 (14.0‐24.9) |
| Overweight or obesity, % (n) | 11.6 (219) | 33.3 (33) | 33.0 (29) | 28.3 (43) | 29.8 (28) | 21.7 (18) | 36.4 (60) | 16.7 (430) |
| Liver fat fraction, median percentage | 1.96 (1.20‐4.61) | 2.25 (1.21‐9.24) | 2.06 (1.30‐10.28) | 2.02 (1.20‐4.78) | 2.07 (1.10‐5.74) | 1.99 (1.37‐5.15) | 2.45 (1.23‐10.74) | 1.99 (1.20‐5.31) |
| NAFLD, % (n) | 1.96 (37) | 7.07 (7) | 4.55 (4) | 1.97 (3) | 4.26 (4) | 2.41 (2) | 9.09 (15) |
2.80 (72) |
Note: Values are observed (not imputed) data and are given as means (SD), medians (95% CI), or numbers of participants (valid %) unless otherwise stated. The means and corresponding SD are given for all normally distributed continuous variables and the medians and 95% CI for non‐normally distributed variables. Characteristics of the study population are organized according to each phase of life (prepregnancy, pregnancy, and childhood factors), in the same order as intended for the regression models.
FIGURE 1.
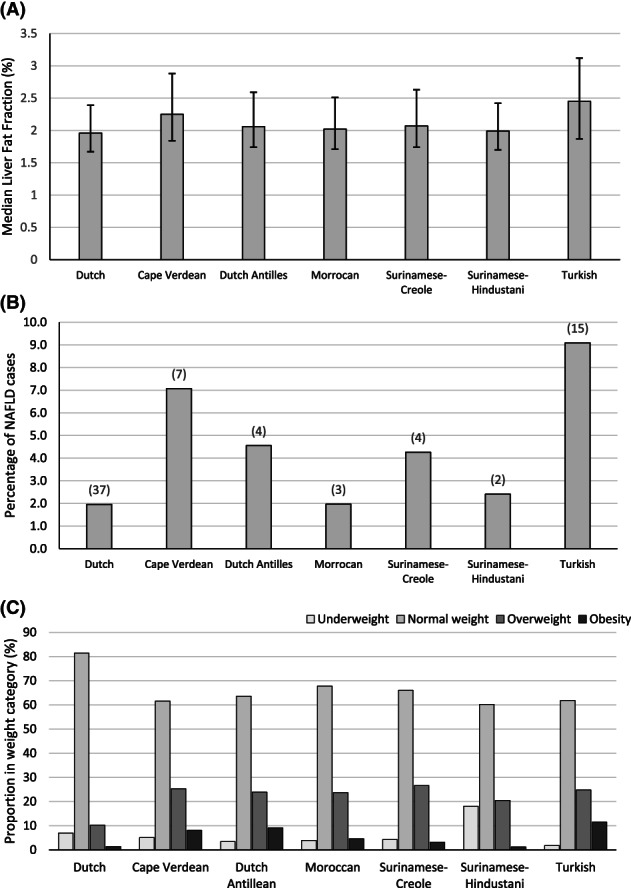
Bar graphs displaying liver fat and weight category distribution for each ethnic group. (A) Median liver fat fraction for each ethnic group, along with the corresponding interquartile range. Medians are presented as liver fat fraction was non‐normally distributed. (B) Proportion of NAFLD presence for each ethnic group, with the absolute number of cases per group above each bar. (C) Proportion per weight category in percentage for each ethnic group. NAFLD, nonalcoholic fatty liver disease
Supporting Information Table S3 shows that, compared with children without liver MRI data, those included in the analyses had a slightly higher body weight and gestational age and had a slightly lower BMI at age 10 years. They had a non‐Dutch background less frequently and had a slightly higher prevalence of mothers with a higher education who smoked during pregnancy and suffered from gestational diabetes.
Explaining ethnic differences in liver fat in childhood
Table 2 shows that, in the main model, children of Cape Verdean, Dutch Antillean, Surinamese‐Creole, and Turkish backgrounds had a higher liver fat fraction compared with children from a Dutch background (all p < 0.05). After adjustment for prepregnancy factors, this association remained present only for children of Cape Verdean and Turkish backgrounds. Further adjustment for pregnancy and childhood factors did slightly decrease the effect estimates for both children from a Cape Verdean background and from a Turkish background, but they remained significant. Adjustment for childhood BMI did attenuate the association of children with a Cape Verdean background with liver fat fraction toward nonsignificant, but the association of children with a Turkish background with liver fat fraction remained present (0.09; 95% CI: 0.04‐0.15). Sensitivity analysis with maternal ethnicity instead of child ethnicity showed no differences in the associations between ethnicity and liver fat fraction (Supporting Information Table S4).
TABLE 2.
Ethnic differences in liver fat percentage
| Dutch, | Cape Verdean, | Dutch Antillean, | Moroccan, | Surinamese‐Creole, | Surinamese‐Hindustani, | Turkish, | |
|---|---|---|---|---|---|---|---|
| N = 1,889 | N = 99 | N = 88 | N = 152 | N = 94 | N = 83 | N = 165 | |
| Main model a | |||||||
| Difference in liver fat (95% CI) | Ref. | 0.17 (0.09 to 0.24) | 0.08 (0.00, 0.15) | 0.04 (−0.03 to 0.09) | 0.08 (0.00 to 0.15) | 0.04 (−0.03 to 0.12) | 0.22 (0.16 to 0.28) |
| p value | Ref. | <0.01* | 0.05* | 0.23 | 0.04* | 0.27 | <0.01* |
| + Prepregnancy factors b | |||||||
| Difference in liver fat (95% CI) | Ref. | 0.13 (0.06 to 0.20) | 0.04 (−0.04 to 0.11) | NA | 0.03 (−0.04 to 0.11) | NA | 0.17 (0.11 to 0.23) |
| p value | Ref. | <0.01* | 0.37 | NA | 0.37 | NA | <0.01* |
| Percentage change | NA | −23.5 | −50.0 | NA | −62.5 | NA | −22.7 |
| ++ Pregnancy factors c | |||||||
| Difference in liver fat (95% CI) | Ref. | 0.12 (0.05 to 0.20) | NA | NA | NA | NA | 0.15 (0.09 to 0.21) |
| p value | Ref. | <0.01* | NA | NA | NA | NA | <0.01* |
| Percentage change | NA | −5.9 | NA | NA | NA | NA | −9.1 |
| +++ Childhood factors d | |||||||
| Difference in liver fat (95% CI) | Ref. | 0.11 (0.03 to 0.18) | NA | NA | NA | NA | 0.15 (0.09 to 0.21) |
| p value | Ref. | <0.01* | NA | NA | NA | NA | <0.01* |
| Percentage change | NA | −5.9 | NA | NA | NA | NA | 0.0 |
| Full model e | |||||||
| Difference in liver fat (95% CI) | Ref. | 0.04 (−0.03 to 0.11) | NA | NA | NA | NA | 0.09 (0.04 to 0.15) |
| p value | Ref. | 0.22 | NA | NA | NA | NA | <0.01* |
| Percentage change | NA | −41.2 | NA | NA | NA | NA | −27.3 |
Note: This table shows the outcomes of the multiple linear regression models with log‐transformed liver fat fraction as the outcome and ethnicity as the main exposure. Dutch background was taken as the reference group, and all effect estimates are presented with their 95% CI and corresponding p value per model. NA is nonapplicable, used for effect estimates for ethnicity after becoming nonsignificant in the previous model and the percentage change in the reference category.
Main model: age and sex.
Main model + prepregnancy factors: the main model additionally adjusted for maternal age, maternal education, and prepregnancy BMI.
Main model + prepregnancy factors + pregnancy factors: previous model additionally controlled for smoking during pregnancy, alcohol use during pregnancy, and birth weight.
Main model + prepregnancy factors + pregnancy factors + childhood factors: previous model additionally controlled for breastfeeding, sugar intake at infancy, screen time, and exercise.
Full model: previous model additionally controlled for BMI at age 10 years. BMI SD score is based on growth charts from the Dutch Growth Research Association (21).
p < 0.05.
Table 3 shows that, in the main model, compared with children from a Dutch background, there was an increased risk of NAFLD among those with a Cape Verdean background (odds ratio [OR]: 3.82; 95% CI: 1.66‐8.79) and a Turkish background (OR: 5.03; 95% CI: 2.70‐9.38). However, this increased risk could be fully explained by both prepregnancy and pregnancy factors.
TABLE 3.
Ethnic differences in NAFLD
| Dutch, | Cape Verdean, | Dutch Antillean, | Moroccan, | Surinamese‐Creole, | Surinamese‐Hindustani, | Turkish, | |
|---|---|---|---|---|---|---|---|
| N = 1,889 | N = 99 | N = 88 | N = 152 | N = 94 | N = 83 | N = 165 | |
| Main model a | |||||||
| OR of NAFLD (95% CI) | Ref. | 3.87 (1.68 to 8.92) | 2.41 (0.84 to 6.90) | 1.03 (0.32, 3.40) | 2.26 (0.79 to 6.47) | 1.29 (0.31 to 5.43) | 5.35 (2.91 to 9.84) |
| p value | Ref. | <0.01* | 0.10* | 0.95 | 0.13 | 0.73 | <0.01* |
| + Prepregnancy factors b | |||||||
| OR of NAFLD (95% CI) | Ref. | 3.28 (1.34 to 8.02) | 1.92 (0.64 to 5.80) | NA | NA | NA | 3.84 (1.84 to 8.02) |
| p value | Ref. | <0.01* | 0.24 | NA | NA | NA | <0.01* |
| Percentage change | NA | −15.4 | −20.3 | NA | NA | NA | −28.2 |
| ++ Pregnancy factors c | |||||||
| OR of NAFLD (95% CI) | Ref. | 1.08 (0.25 to 4.71) | NA | NA | NA | NA | 1.80 (0.32 to 10.28) |
| p value | Ref. | 0.91 | NA | NA | NA | NA | 0.51 |
| Percentage change | NA | −53.4 | NA | NA | NA | NA | −38.1 |
| +++ Childhood factors d | |||||||
| OR of NAFLD (95% CI) | Ref. | NA | NA | NA | NA | NA | NA |
| p value | Ref. | NA | NA | NA | NA | NA | NA |
| Percentage change | NA | NA | NA | NA | NA | NA | NA |
| Full model e | |||||||
| OR of NAFLD (95% CI) | Ref. | NA | NA | NA | NA | NA | NA |
| p value | Ref. | NA | NA | NA | NA | NA | NA |
| Percentage change | NA | NA | NA | NA | NA | NA | NA |
Note: This table presents the odds ratios (OR) of ethnicity and NAFLD, with the accompanying CI of the different logistic regression models. NA is nonapplicable data, used for effect estimates for ethnicity after becoming nonsignificant in the previous model and the percentage change in the reference category.
Main model: age and sex.
Main model + prepregnancy factors: the main model additionally adjusted for maternal age, maternal education, and prepregnancy BMI.
Main model + prepregnancy factors + pregnancy factors: previous model additionally controlled for smoking during pregnancy, alcohol use during pregnancy, and birth weight.
Main model + prepregnancy factors + pregnancy factors + childhood factors: previous model additionally controlled for breastfeeding, sugar intake at infancy, screen time, and exercise.
Full model: previous model additionally controlled for BMI at age 10 years. BMI SD score is based on growth charts from the Dutch Growth Research Association (21).
p < 0.10.
DISCUSSION
Results of this multiethnic population‐based prospective analyses suggest that, compared with children from a Dutch background, children of Cape Verdean, Dutch Antillean, Surinamese‐Creole, or Turkish background had a higher liver fat fraction at school age. Also, children with a Cape Verdean or Turkish background had higher odds of having NAFLD at school age compared with children from a Dutch background. Prepregnancy, pregnancy, and childhood factors, as well as childhood BMI, explained the majority of the observed differences, except for the higher liver fat fraction observed in children with a Turkish background.
Interpretation of main findings
NAFLD in children is, independent of BMI, associated with increased insulin resistance, hypertension, and other cardiometabolic risk factors (5). Results from previous studies in the US among adolescents have suggested that liver fat accumulation differs between different ethnic subgroups (4, 6, 7, 8, 9, 10). Not much is not known about the ethnic disparities in liver fat in Europe, which has a different distribution of ethnic populations compared with the US. In the Netherlands, large differences in cardiovascular and metabolic health exist between ethnic subgroups who are of Cape Verdean, Dutch Antillean, Moroccan, Surinamese, and Turkish backgrounds (5). We hypothesized that liver fat accumulation differs between ethnic subgroups living in the Netherlands. While analyzing this, we also explored whether any ethnic difference could be explained by prepregnancy, pregnancy, and childhood factors.
What we observed is a higher prevalence of overweight/obesity and NAFLD in all ethnic minority groups compared with children with a Dutch background. These findings are in line with results from previous studies (11, 12, 13). The highest prevalence of overweight or obesity were observed in children with Cape Verdean, Dutch Antillean, or Turkish background. These same three groups also had the highest median liver fat fraction and NAFLD prevalence. As the relationship between BMI and fatty liver has been established for many years, it was not surprising that these findings were in line with findings for BMI reported in the same cohort at age 6 years (12). Furthermore, in line with the findings for NAFLD, we observed higher average liver fat percentages in children of Cape Verdean, Dutch Antillean, Surinamese‐Creole, or Turkish background compared with children with a Dutch background.
To the best of our knowledge, there are no previous studies that have assessed the ethnic differences in liver fat in our diverse population. Previous studies in these ethnic subgroups were focused on metabolic and cardiovascular health, which are strongly associated with liver health (7). Several studies have described increased prevalence of overweight and obesity among individuals from Turkish, Moroccan, and Surinamese backgrounds, as well as other ethnic minority groups living in the Netherlands (31, 32). These differences were mostly explained by socioeconomic background differences or decreased physical exercise (31, 32, 33). Other studies also reported that, compared with Dutch adults, those of Surinamese, Moroccan, Turkish, or Antillean background are at increased risks of type 2 diabetes even when at normal weight (34, 35). They also found that adults with a Turkish background had an unfavorable total/HDL cholesterol ratio, and adults with a Surinamese‐Creole background had an increased risk of hypertension (32, 36, 37, 38). In contrast, adults with a Moroccan background had a lower prevalence of hypertension and cardiovascular mortality rates (37). Our findings are in line with the previous studies and suggest that the observed ethnic differences in liver fat in childhood are related with cardiovascular disease in later life.
Furthermore, previous studies have suggested that maternal factors, pregnancy factors, or lifestyle factors are associated with liver fat (13, 14, 15, 16, 17, 27, 28, 39, 40, 41). Factors such as education level of the mother, higher prepregnancy BMI, sugar intake in early life, and sedentary lifestyle have all been associated with a higher risk of developing NAFLD in childhood (13, 15, 28, 39, 41). In our study, we observed that the higher liver fat percentages for Dutch Antillean and Surinamese‐Creole could be explained by prepregnancy factors, including maternal education, maternal age, and prepregnancy BMI. These prepregnancy factors explained more than 20% of the main model effect estimate for liver fat for Cape Verdean and Turkish backgrounds. However, the higher liver fat percentage observed in children from a Cape Verdean background could largely be explained by adjusting for BMI. Only the associations of Turkish background with liver fat percentage remained significant after adjusting for all early‐life factors and BMI. Findings from previous studies have suggested that both prepregnancy BMI and maternal education are correlated with liver fat accumulation in children (6, 14). These preventable and modifiable factors may contribute to the associations of ethnic background with childhood liver fat and could be important targets in reducing ethnic health disparities (6, 7, 31, 42). These early‐life factors are a combination of both lifestyle factors and factors associated with socioeconomic background. In our study sample, we observed that the Dutch group had larger proportions of higher‐educated mothers, higher‐income households, and children who exercised more than 2 h/d. This could suggest that part of the differences between our “Dutch” group and the other ethnic groups are related to socioeconomic background differences, as well as lifestyle factors. Furthermore, in our population, the prevalence of overweight/obesity within the group of children of Cape Verdean descent or Turkish descent was three‐fold higher than that of Dutch children. Risk factors for BMI and liver fat may be overlapping. The effect estimate for the association of liver fat with BMI was significant in our full linear model, but we did not have enough power to perform stratified BMI groups per ethnic background. This could be interesting for future studies to provide important insights into the risk factors for NAFLD.
Previous studies have also suggested that genetic susceptibility might also contribute to the observed ethnic differences (43, 44). This can be illustrated by the patatin‐like phospholipase domain containing 3 (PNPLA3) gene, which is found more frequently in those of Hispanic or Asian descent and might increase the risk of developing NAFLD at a younger age through altering metabolic pathways (11, 40). However, in this study, we observed that the associations of ethnic background with liver fat outcomes seem to be largely explained by both life‐style factors and socioeconomic factors. Future studies are needed to assess whether the remaining small differences might be explained by other environmental factors or differences in genetic susceptibility. Furthermore, our study was focused specifically on the largest ethnic minority groups living in the Netherlands. Although the groups might not be exactly similar in other countries, the message that ethnicity is an important determinant of childhood body and liver fat is important and generalizable. In our sample, ethnic differences appeared largely driven by early‐life factors that might be preventable, although genetic differences could not be assessed in this study. Studies in other countries are needed to assess these associations in other specific populations.
Our findings strongly support the notion that the prevalence of liver fat accumulation and NAFLD differs between ethnic subgroups living in the Netherlands. These findings may have important consequences for risk of cardiovascular, metabolic, and liver health from childhood to adulthood. Additionally, our findings suggest that prepregnancy, pregnancy, and childhood factors seem to explain a large part of these associations. In line with findings for childhood obesity, early life might offer important clues for future preventive strategies focused on liver health from childhood onward.
Strengths and limitations
One of the major strengths of this analysis is that it was nested in a large population‐based cohort study, which allowed for a large sample size. Another strength was the use of MRI scans to measure liver fat fraction, a method that has previously been validated to provide accurate results in adults (25). Selection bias could have been introduced owing to those that were excluded from the study, either through non‐response at MRI visit or missing data on ethnicity or BMI at school age. Information about ethnic background was obtained through questionnaires, which were also available in different languages for mothers who did not understand the Dutch language. At the same time, the use of questionnaires about a period of time introduces possible recall bias. We used the classification for ethnicity that is used by the Central Statistics Bureau in the Netherlands, which classifies according to the country of birth of the parents. Although this allows for an objective and stable classification, it does not distinguish first‐, second‐, and third‐generation migrants, nor does it account for heterogeneity within ethnic groups and different subgroups. Therefore, children of mixed ethnicity were categorized according to the ethnic background of the mother's family, which may have affected our results. Additionally, to the best of our knowledge, our study is the largest multiethnic, population‐based pediatric study assessing liver fat by MRI in a general population. Despite this, we were limited in the number of participants per ethnic subgroups. Clearly, in larger numbers, some observed nonsignificant effect estimates in our study might have been statistically significant. Our study population contained a relatively small number of children that have overweight or with obesity, which could indicate a selection bias toward a lean population and impact the generalizability of our results. Moreover, this population is relatively healthy and young, which could explain the small number of cases with NAFLD, and this limited our statistical power in detecting significant associations between the different ethnicities that might be present. Finally, we used a cutoff of 5% liver fat assessed by MRI to define NAFLD. This cutoff is actually based on a histology approach. Currently, there is no clear consensus on the optimal cutoff to define children with by NAFLD by MRI. We have previously shown that children with more than 2% liver fat already have an increased adverse cardiovascular risk profile (5). Also, the NAFLD definition is problematic for children, for whom alcohol consumption is usually not a concern. Future studies should focus on the recently proposed definition of metabolic (dysfunction)‐associated fatty liver disease (MAFLD), in which both liver fat and metabolic consequences are taken into account (45). Therefore, the outcomes for the regression analysis for NAFLD should be interpreted with caution.
CONCLUSION
The prevalence of liver fat accumulation and NAFLD differs between ethnic subgroups living in the Netherlands. Prepregnancy, pregnancy, and childhood factors seem to explain a large part of these associations and they may be clues for future preventive strategies focused on liver health from childhood onward.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
JMG, MLG, and VWVJ were responsible for the study concept. JMG, MLG, and VWVJ were responsible for the data collection and interpretation, statistical analysis, and manuscript draft. MLG, SS, and VWVJ were responsible for the manuscript review. All authors were responsible for reading and approving the final manuscript.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. The general design of the Generation R Study was made possible by financial support from the Erasmus University Medical Center, the Netherlands Organization for Health Research and Development, and the Ministry of Health, Welfare, and Sport. The study was supported by the European Research Council (Consolidator Grant ERC‐2014‐CoG‐648916) and the European Union's Horizon 2020 research and innovation program (Grant Agreement No. 733206 LifeCycle).
de Groot JM, Geurtsen ML, Santos S, Jaddoe VWV. Ethnic disparities in liver fat accumulation in school‐aged children. Obesity (Silver Spring). 2022;30(7):1472‐1482. doi: 10.1002/oby.23478
Funding information Erasmus Medisch Centrum; Horizon 2020 Framework Programme, Grant/Award Number: 733206 LifeCycle; ZonMw; Horizon 2020; European Union; European Research Council, Grant/Award Number: ERC‐2014‐CoG‐648916; Ministry of Health; Health Research; Erasmus University Medical Center
REFERENCES
- 1. Bellentani S, Marino M. Epidemiology and natural history of non‐alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2009;8(suppl 1):S4‐S8. [PubMed] [Google Scholar]
- 2. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non‐alcoholic fatty liver disease in children and adolescents: a systematic review and meta‐analysis. PLOS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy T, Wonders K, Younes R, et al. The European NAFLD registry: a real‐world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp Clin Trials. 2020;98:106175. doi: 10.1016/j.cct.2020.106175 [DOI] [PubMed] [Google Scholar]
- 4. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 5. Geurtsen ML, Santos S, Felix JF, et al. Liver fat and Cardiometabolic risk factors among school‐age children. Hepatology. 2020;72:119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo‐Leon E, Cioffi CE, Vos MB. Perspectives on youth‐onset nonalcoholic fatty liver disease. Endocrinol Diabetes Metab. 2020;3:e00184. doi: 10.1002/edm2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faienza MF, Chiarito M, Molina‐Molina E, et al. Childhood obesity, cardiovascular and liver health: a growing epidemic with age. World J Pediatr. 2020;16:438‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandes DM, Pantangi V, Azam M, et al. Pediatric nonalcoholic fatty liver disease in new York City: an autopsy study. J Pediatr. 2018;200:174‐180. [DOI] [PubMed] [Google Scholar]
- 9. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 11. Marzuillo P, del Giudice EM, Santoro N. Pediatric fatty liver disease: role of ethnicity and genetics. World J Gastroenterol. 2014;20:7347‐7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gishti O, Kruithof CJ, Felix JF, et al. Ethnic disparities in general and abdominal adiposity at school age: a multiethnic population‐based cohort study in The Netherlands. Ann Nutr Metab. 2014;64:208‐217. [DOI] [PubMed] [Google Scholar]
- 13. Abeysekera K, Orr J, Fernandes G, et al. The effect of maternal pre‐pregnancy obesity and infant nutrition on the development of non‐alcoholic fatty liver disease in young adults‐experience from the Avon longitudinal study of parents and children [abstract]. J Hepatol. 2020;73(suppl 1):S48‐S49. [Google Scholar]
- 14. Ayonrinde OT, Oddy WH, Adams LA, et al. Infant nutrition and maternal obesity influence the risk of non‐alcoholic fatty liver disease in adolescents. J Hepatol. 2017;67:568‐576. [DOI] [PubMed] [Google Scholar]
- 15. Geurtsen ML, Santos S, Gaillard R, Felix JF, Jaddoe VWV. Associations between intake of sugar‐containing beverages in infancy with liver fat accumulation at school age. Hepatology. 2021;73:560‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newton KP, Feldman HS, Chambers CD, et al. Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr. 2017;187:141‐146.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nobili V, Bedogni G, Alisi A, et al. A protective effect of breastfeeding on the progression of non‐alcoholic fatty liver disease. Arch Dis Child. 2009;94:801‐805. [DOI] [PubMed] [Google Scholar]
- 18. Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Statistics Netherlands. Migration background . https://www.cbs.nl/nl-nl/onze-diensten/methoden/begrippen/migratieachtergrond
- 20. Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol. 2015;68:627‐636. [DOI] [PubMed] [Google Scholar]
- 21. Talma H, Chinapaw MJ, Bakker B, HiraSing RA, Terwee CB, Altenburg TM. Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes Rev. 2013;14:895‐905. [DOI] [PubMed] [Google Scholar]
- 22. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504‐e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petäjä EM, Yki‐Järvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD‐A systematic review. Int J Mol Sci. 2016;17:633. doi: 10.3390/ijms17050633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao B, Liu C, Zhang Q, Dong Y. Maternal high‐fat diet leads to non‐alcoholic fatty liver disease through upregulating hepatic SCD1 expression in neonate rats. Front Nutr. 2020;7:581723. doi: 10.3389/fnut.2020.581723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katsagoni CN, Papachristou E, Sidossis A, Sidossis L. Effects of dietary and lifestyle interventions on liver, clinical and metabolic parameters in children and adolescents with non‐alcoholic fatty liver disease: a systematic review. Nutrients. 2020;12:2864. doi: 10.3390/nu12092864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lonardo A, Leoni S, Alswat KA, Fouad Y. History of nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21:5888. doi: 10.3390/ijms21165888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mandala A, Janssen RC, Palle S, Short KR, Friedman JE. Pediatric non‐alcoholic fatty liver disease: nutritional origins and potential molecular mechanisms. Nutrients. 2020;12:3166. doi: 10.3390/nu12103166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dijkshoorn H, Nicolaou M, Ujcic‐Voortman J, et al. Overweight and obesity in young Turkish, Moroccan and Surinamese migrants of the second generation in The Netherlands. Public Health Nutr. 2014;17:2037‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agyemang C, Addo J, Bhopal R, de Graft AA, Stronks K. Cardiovascular disease, diabetes and established risk factors among populations of sub‐Saharan African descent in Europe: a literature review. Global Health. 2009;5:7. doi: 10.1186/1744-8603-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ujcic‐Voortman J, Bos G, Baan C, Verhoeff A, Seidell J. Obesity and body fat distribution: ethnic differences and the role of socio‐economic status. Obes Facts. 2011;4:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ikram UZ, Kunst AE, Lamkaddem M, Stronks K. The disease burden across different ethnic groups in Amsterdam, The Netherlands, 2011–2030. Eur J Public Health. 2014;24:600‐605. [DOI] [PubMed] [Google Scholar]
- 35. Perini W, Kunst AE, Snijder MB, Peters RJG, van Valkengoed IGM. Ethnic differences in metabolic cardiovascular risk among normal weight individuals: implications for cardiovascular risk screening. The HELIUS study. Nutr Metab Cardiovasc Dis. 2019;29:15‐22. [DOI] [PubMed] [Google Scholar]
- 36. Ujcic‐Voortman J, Baan C, Seidell J, Verhoeff A. Obesity and cardiovascular disease risk among Turkish and Moroccan migrant groups in Europe: a systematic review. Obes Rev. 2011;13:2‐16. [DOI] [PubMed] [Google Scholar]
- 37. Ujcic‐Voortman J. Ethnic Disparities in Cardiovascular Disease Risk: The Distribution of Risk Factors Among Amsterdam Residents with a Turkish and Moroccan Ethnic Background [thesis]. Vrije Universiteit Amsterdam; 2011. https://research.vu.nl/en/publications/ethnic-disparities-in-cardiovascular-disease-risk-the-distributio
- 38. Agyemang C, Bindraban N, Mairuhu G, Gv M, Koopmans R, Stronks K. Prevalence, awareness, treatment, and control of hypertension among black Surinamese, south Asian Surinamese and white Dutch in Amsterdam, The Netherlands: the SUNSET study. J Hypertens. 2005;23:1971‐1977. [DOI] [PubMed] [Google Scholar]
- 39. Bae‐Gartz I, Kasper P, Großmann N, et al. Maternal exercise conveys protection against NAFLD in the offspring via hepatic metabolic programming. Sci Rep. 2020;10:15424. doi: 10.1038/s41598-020-72022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nobili V, Liccardo D, Bedogni G, et al. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at‐risk adolescents. Genes Nutr. 2014;9:392. doi: 10.1007/s12263-014-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Querter I, Pauwels NS, De Bruyne R, et al. Maternal and perinatal risk factors for pediatric non‐alcoholic fatty liver disease: a systematic review. Clin Gastroenterol Hepatol. 2021;20:740‐755. [DOI] [PubMed] [Google Scholar]
- 42. Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164‐S192. [DOI] [PubMed] [Google Scholar]
- 43. Chung ST, Onuzuruike AU, Magge SN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411:166‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deboer MD, Wiener RC, Barnes BH, Gurka MJ. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics. 2013;132:e718‐e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, et al. Defining paediatric metabolic (dysfunction)‐associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6:864‐873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


