Abstract
Citizen science may be described as a research involving communities and individuals, other than scientists. Following this approach, along with the evidence of a high prevalence of Rickettsia spp. in Dermacentor marginatus from wild boars in hunting areas of southern Italy, this study aimed to assess the occurrence of tick‐borne pathogens (TBPs) in ticks collected from hunters and their hunting dogs. From October 2020 to May 2021, ticks were collected from wild boar hunters (n = 347) and their dogs (n = 422) in regions of southern Italy (i.e., Apulia, Basilicata, Calabria, Campania and Sicily). All ticks were morphologically identified, classified according to gender, feeding status, host, geographic origin, and molecularly screened for zoonotic bacteria. Adult ticks (n = 411) were collected from hunters (i.e., n = 29; 8.4%; mean of 1.6 ticks for person) and dogs (i.e., n = 200; 47.4%; mean of 1.8 ticks for animal) and identified at species level as D. marginatus (n = 240, 58.4%), Rhipicephalus sanguineus sensu lato (n = 135, 32.8%), Rhipicephalus turanicus (n = 27, 6.6%) and Ixodes ricinus (n = 9, 2.2%). Overall, 45 ticks (i.e., 10.9%, 95% CI: 8.3‐14.3) tested positive for at least one tick‐borne agent, being Rickettsia slovaca the most frequent species (n = 37, 9.0%), followed by Rickettsia raoultii, Rickettsia aeschlimannii, Rickettsia monacensis, Coxiella burnetii, Borrelia lusitaniae and Candidatus Midichloria mitochondrii (n = 2, 0.5% each). Data herein presented demonstrate a relevant risk of exposure to TBPs for hunters and hunting dogs during the hunting activities. Therefore, the role of hunters to monitor the circulation of ticks in rural areas may be considered an effective example of the citizen science approach, supporting the cooperation toward private and public health stakeholders.
Keywords: citizen science, dogs, hunting, Italy, tick‐borne pathogen, zoonosis
1. INTRODUCTION
Hard ticks (order Acarina, family Ixodidae) represent one of the main public health issues worldwide, being vectors of a wide range of tick‐borne pathogens (TBPs) including viruses, bacteria and parasites of human and animal concern (Dantas‐Torres et al., 2012). Although predictive spatio‐temporal models on tick abundance may be important to reduce the incidence of tick‐borne diseases (TBDs) (Hartemink et al., 2015), the full understanding of factors involved, such as climatic conditions, vegetation type and wildlife density is not completely outlined (Estrada‐Peña & de la Fuente, 2016; Garcia‑Marti et al., 2017 ). Globally, the occurrence of TBDs is increasing worldwide (Jaenson et al., 2009; Kuehn, 2019 ) and the most common in Europe is Lyme disease caused by bacteria belonging to Borrelia burgdorferi sensu lato complex, being Borrelia afzelii, Borrelia garinii and B. burgdorferi sensu stricto the main zoonotic genospecies (Rauter & Hartung, 2005; Rizzoli et al., 2014 ). Other relevant TBPs include Anaplasma phagocytophilum, Coxiella burnetii and Rickettsia spp., which can be transmitted to a wide range of wild and domestic mammals, as well as humans (Bowser & Anderson, 2018; Cutler et al., 2019; Estrada‐Peña et al., 2017; Krause, 2019; Parola et al., 2013; Sgroi et al., 2021; Sprong et al., 2018 ). The increased urbanization of European areas contributes to a higher circulation of synanthropic wildlife, and consequently may expose people to TBPs and other zoonotic agents since these animals could harbour a plethora of tick vectors (Baneth, 2014; Rizzoli et al., 2014; Sgroi et al., 2020 ). In Italy, little data are available on ticks collected from citizens, as well as on the prevalence of TBPs in humans due to the paucity in clinical case notifications (Beltrame et al., 2018; Otranto et al., 2014 ). However, considering the great vocation for tourism and several outdoor recreational activities (e.g., sports, hiking, hunting) of the Italian peninsula (Otranto et al., 2014; Sandifer et al., 2015 ), rural areas are at high risk of exposure to tick bites, especially in southern regions where there is an amazing number of tick species (Dantas‐Torres & Otranto, 2013 ). Consequently, a high seroprevalence for A. phagocythophilum (5.7%), B. burgdorferi s.l. (7.5%) and spotted fever group rickettsiae (SFGR) (8.0%) in forestry workers, farmers and livestock breeders of central‐southern Italy was reported (Mendoza‐Roldan et al., 2021; Santino et al., 2004 ), suggesting the high risk of exposure to tick bite (Toepp et al., 2018) due to their frequent exhibitions in rural areas. Similarly, hunters and hunting dogs are highly exposed to arthropods and pathogens they transmit owing to their field activities in forest areas (Mahachi et al., 2020). Accordingly, seropositivity to Rickettsia spp. has been ascertained in hunters with prevalence ranging from 9.1% (Germany; Jansen et al., 2008) to 14.7% (Brazil; Kmetiuk et al., 2019). Therefore, hunting dogs may act as sentinels and potential reservoirs of TBPs and consequently, hunters are more likely exposed to tick bites and tick‐borne infections than hikers or forest guards (Hornok et al., 2013; Mahachi et al., 2020; Toepp et al., 2018 ). In this scenario, hunters may themselves play a role in monitoring the presence of ticks in a given area, cooperating with the sanitary stakeholders (i.e., citizen science approach) to increase knowledge on the spread of arthropods and their transmitted pathogens (Garcia‑Marti et al., 2017; Hamer et al., 2018; Tanner Porter et al., 2019). Therefore, the present survey aimed to assess the tick bite exposure and the risk of infection by TBPs in hunters and their dogs in an area where a high prevalence of wild boars (i.e., 18.3%) harboured Dermacentor marginatus ticks.
2. MATERIALS AND METHODS
2.1. Study area
The study was conducted in regions of southern Italy (i.e., Apulia, Basilicata, Calabria, Campania and Sicily), which face on the Ionian, Mediterranean and Tyrrhenian seas. The area has a total surface of 123.417 km2 characterized by a typical Mediterranean temperate climate with progressively continental features in inland and mountainous landscapes.
2.2. Sampling
From October 2020 to May 2021, hunters (n = 347) divided in 17 hunting teams (i.e., groups on average of 20 hunters) were enrolled to collect ticks from themselves (Figure 1) and their dogs (n = 422). To evaluate the human tick exposure, hunters were classified in two different groups: hunters who used to hunt with one or more dogs (group 1) or without any dog (group 2). Ticks were collected at the end of the day, after the hunting session. All participants were trained on the proper removal technique of ticks from themselves and their dogs, following the guidelines of the Center for Disease Control and Prevention (CDC, 2019). Participants were provided with vials, tweezers, ethanol (70%) and a specific form reporting: (i) body site where ticks were collected and their number; (ii) environmental features of the hunting area (i.e., wooded or grassland), along with anamnestic data (i.e., age and gender) of hunters and dogs. Each tick recovered was stored in numbered plastic vials containing 70% ethanol until the morphological identification. All samples were delivered to the Unit of Parasitology at the Department of Veterinary Medicine, University of Bari Aldo Moro (Italy).
FIGURE 1.

Ixodes ricinus female specimen during blood feeding on a hunter's limb (Courtesy Dr. Antonio Zotti)
2.3. Morphological identification of ticks
All ticks were classified according to gender (i.e., male and female), developmental stage (i.e., larval, nymph, adult) and feeding status (i.e., fed and unfed). Tick species were identified by stereomicroscopy (Leica MS5—Leica Microsystems Ltd. Heerbrugg, Germany), using the morphological keys available in the literature (Estrada‐Peña et al., 2004, 2017; Manilla & Iori, 1992).
2.4. DNA extraction, PCR protocols and sequencing
DNA was extracted from individual tick using a commercial kit (QIAampDNA Blood & Tissue, Qiagen, Hilden, Germany), according to the manufacturer's instructions and analysed for the detection of different TBPs. Details regarding PCR protocols are reported in Table 1. All PCR products were examined on 2% agarose gel stained with GelRed (VWR International PBI, Milan, Italy) and visualised on a GelLogic 100 gel documentation system (Kodak, New York, USA). Amplicons were then purified and sequenced in both directions using the same primers as for PCRs, by the Big Dye Terminator version 3.1 chemistry in a 3130 Genetic Analyzer (Applied Bio‐systems, Foster City, CA, USA). Sequences were edited and analysed by the Geneious software version 9.0 (Biomatters Ltd., Auckland, New Zealand) (Kearse et al., 2012) and compared with those available in the GenBank database by the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi).
TABLE 1.
Tick‐borne pathogens investigated in this study with target genes, primers nucleotide sequences and fragment length
| Tick‐borne pathogens | Target gene | Primers | Sequence (5′−3′) | Fragment length (bp † ) | References |
|---|---|---|---|---|---|
|
Anaplasma/Ehrlichia spp. Borrelia burgdorferi sensu lato complex Coxiella burnetii Rickettsia spp. Spotted fever group rickettsiae |
16S rRNA Flagellin IS1111a gltA ompA |
EHR‐16SD EHR‐16SR FLA1 FLA2 Trans‐1 Trans‐2 CS‐78F CS‐323R Rr190.70F Rr190.701R |
GGTACCYACAGAAGAAGTCC TAGCACTCATCGTTTACAGC AGAGCAACTTACAGACGAAATTAAT CAAGTCTATTTTGGAAAGCACCTAA TATGTATCCACCGTAGCCAGT CCCAACAACACCTCCTTATTC GCAAGTATCGGTGAGGATGTAAT GCTTCCTTAAAATTCAATAAATCAGGAT ATGGCGAATATTTCTCCAAAA GTTCCGTTAATGGCAGCATCT |
345 482 687 401 632 |
Martin et al. (2005) Wójcik‐Fatla et al. (2009) Berri et al. (2000) Labruna et al. (2004) Regnery et al. (1991) |
bp = base pairs.
2.5. Statistical analysis
Exact binomial 95% confidence intervals (CIs) were established for proportions found in the present work, using five alternative calculation methods as described in Brown et al. (2001). A Chi‐squared test was used to assess any statistical difference of tick infestation on humans and dogs, as well as the prevalence of different tick‐borne agents among gender, feeding status and geographical origin of ticks. A p value less than .05 was considered significant. Odds ratios (ORs) were calculated to assess the tick infestation risk according to several variables of hunters and dogs. Chi‐squared, 95% CIs, p values and ORs were calculated by using the software Epitools—Epidemiological Calculators (Sergeant, 2018). The number of hunters and hunting dogs enrolled, along with the circulation of different tick species in the administrative regional boundaries of the study area, was obtained with ArcGIS (version 10.3, ESRI, Redlands, CA, USA).
3. RESULTS
A total of 411 adult ticks (i.e., 179 males, 109 unfed females, 123 fed females) were collected, being D. marginatus the most representative species (n = 240, 58.4%), followed by Rhipicephalus sanguineus sensu lato (n = 135, 32.8%), Rhipicephalus turanicus (n = 27, 6.6%) and Ixodes ricinus (n = 9, 2.2%). The total number of ticks collected from different body sites of hunters and hunting dogs was 48 (i.e., 11.7%) and 363 (i.e., 88.3%) (Table 2), with 9 (18.7%) and 114 (31.4%) fed specimens, respectively. Out of 347 hunters and 422 dogs enrolled and distributed according to the geographical regions (Figure 2), 29 (i.e., 8.4%) and 200 (i.e., 47.4%) were infested by at least one tick specimen with a mean intensity value (i.e., average amount of ticks for each positive hunter/dog) of 1.6 and 1.8 for hunters and dogs, respectively. For hunters, a statistically significant association was found in the tick exposure frequency with the hunting practice along with dogs (χ2 = 25.73, p < .001, OR = 8.1) and the hunting practice in wooded areas (χ2 = 6.76, p = .009, OR = 2.8), whereas no statistical relationship was found according to age (χ2 = 0.46, p = .796). A single case of coinfestation by D. marginatus and R. sanguineus s.l. was detected on a hunter. For hunting dogs, a statistically significant association was found in tick exposure frequency with the age group of 1–5 years (χ2 = 27.37, p < .001) and the hunting practice in wooded areas (χ2 = 10.82, p = .001, OR = 1.9); no statistical relationship was found according to gender (χ2 = 0.52, p = .471). Data on the tick bite exposure of hunters and hunting dogs, according to their different body districts, are reported in Table 2.
TABLE 2.
Tick bite exposure of hunters and hunting dogs, as numbers and percentage of ticks collected according to different body localization
| Body localization | Hunters | Hunting dogs | Total (%) |
|---|---|---|---|
| Neck | 15 (31.2%) | 38 (10.5%) | 53 (12.9%) |
| Limbs | 5 (10.4%) | 38 (10.5%) | 43 (10.5%) |
| Head | 19 (39.6%) | 117 (32.2%) | 136 (33.1%) |
| Ears | 9 (18.7%) | 170 (46.8%) | 179 (43.5%) |
| Total (%) | 48 (11.7%) | 363 (88.3%) | 411 (100%) |
FIGURE 2.
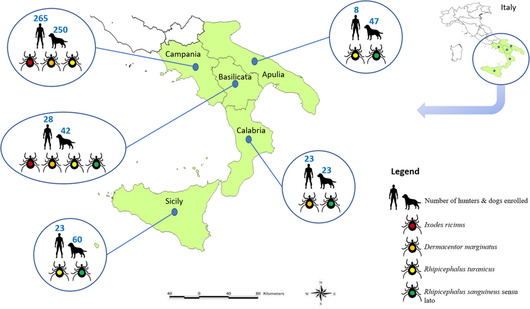
Map showing the number of hunters and hunting dogs enrolled, along with the circulation of different tick species, in different regions of the study area
Out of 411 ticks collected, 45 (i.e., 10.9%, 95% CI: 8.3–14.3) tested positive for at least one tick‐borne bacteria, being Rickettsia slovaca the most prevalent (n = 37, 9.0%), followed by Rickettsia raoultii (n = 2, 0.5%), Rickettsia aeschlimannii (n = 2, 0.5%), Rickettsia monacensis (n = 2, 0.5%), C. burnetii (n = 2, 0.5%), Borrelia lusitaniae (n = 2, 0.5%) and Candidatus Midichloria mitochondrii (hereafter M. mitochondrii) (n = 2, 0.5%). Out of 45 positive ticks, 9 (i.e., 20%) were collected from hunters and 36 (i.e., 80%) from dogs. Four cases (1.0%) of coinfections by different tick‐borne agents in tick specimens were also identified (Table 3). Details regarding the exposure of hunters and hunting dogs to tick species positive for at least one tick‐borne microorganism are reported in Table 4; data on ticks positive to tick‐borne agents according to their gender, feeding status and administrative regions are detailed in Table 5. Representative sequences of all bacteria herein detected displayed 99–100% query coverage and nucleotide identity with those available in the literature and were submitted to GenBank under the accession numbers: MZ964142 for R. slovaca, MZ964139 for R. raoultii, MZ964141 for R. aeschlimanni, MZ964140 for R. monacensis, MZ964137 for C. burnetii, MZ964138 for B. lusitaniae and MZ954838 for M. mitochondrii.
TABLE 3.
Number and species of ticks positive to different tick‐borne agents (n = 45) from hunters and dogs
| Tick‐borne agents | Tick species |
|---|---|
|
Single infections R. slovaca R. raoultii R. aeschlimanni R. monacensis M. mitochondrii Coinfections R. slovaca—B. lusitaniae R. slovaca—C. burnetii M. mitochondrii—B. lusitaniae |
D. marginatus (6, ‐ 26, D), I. ricinus (1, ) R. sanguineus s.l. (1, ) D. marginatus (2, ) D. marginatus (1, ), R. sanguineus s. l. (1, ) D. marginatus (2, ) I. ricinus (1, ) I. ricinus (1, ) D. marginatus (2, ) I. ricinus (1, ) |
H = Hunters.
D = Dogs.
TABLE 4.
Tick bite exposure of hunters and hunting dogs with number and percentage of ticks positive for at least one tick‐borne agent
| Tick species | Hunters † Pos/Tot (%) | Hunting dogs † Pos/Tot (%) | Total (%) |
|---|---|---|---|
| D. marginatus | 6/36 (16.7%) | 33/204 (16.2%) | 39/240 (16.2%) |
| R. sanguineus s. l. | 1/7 (28.6%) | 1/128 (0.8%) | 2/135 (2.2%) |
| R. turanicus | 0/0 | 0/27 | 0/27 |
| I. ricinus | 2/5 (40%) | 2/4 (50%) | 4/9 (44.4%) |
| Total (%) | 9/48 (16.7%) | 36/363 (9.6%) | 45/411 (10.9%) |
Pos/Tot (%) = number of ticks positive to at least one agent on the total number of ticks from hunters and hunting dogs.
TABLE 5.
Ticks tested positive for at least one tick‐borne agent according to gender, feeding status and administrative regions in this study (95% CI = Confidence Interval 95%)
| Variables | † Pos/Tot (%) | 95% CI | |
|---|---|---|---|
| Gender | |||
| Male | 21/179 (11.7%) | 7.8–17.3 | |
| Female | 24/232 (10.3%) | 7.0–14.9 | |
| Chi‐squared; p‐value | χ2 = 0.20; p = .655 | ||
| Feeding status (females n = 232) | |||
| Fed | 16/123 (13.0%) | 8.2–20.1 | |
| Unfed | 8/109 (7.4%) | 3.8–13.8 | |
| Chi‐squared; p‐value | χ2 = 2.00; p = .157 | ||
| Region | |||
| Apulia | 0/50 (‐) | – | |
| Basilicata | 1/21 (4.8%) | 0.8–22.7 | |
| Calabria | 6/33 (18.2%) | 8.6–34.4 | |
| Campania | 38/228 (16.7%) | 12.4–22.0 | |
| Sicily | 0/79 (−) | – | |
| Total | 45/411 (10.9%) | 8.3–14.3 | |
Pos/Tot (%) = number of ticks positive to at least one agent on the total number of ticks collected.
4. DISCUSSION
Based on the results presented, the citizen science approach showed to be an effective and low‐cost tool for monitoring tick and related transmitted pathogen circulation in hunters and their dogs. This approach has been for the first time applied to hunters as a specific class of citizens, differently from previous experiences conducted in Canada (Lewis et al., 2018), USA (Nieto et al., 2018; Tanner Porter et al., 2019), Netherlands (Garcia‐Marti et al., 2018, 2017 ) and Belgium (Lernout et al., 2019). All ticks collected were adults, suggesting that even if an accurate body inspection is conducted, smaller tick stages may have been unnoticed, thus, representing a limitation for such kind of surveys. On the other hand, studies where physicians performed the body inspection and collection of ticks, larvae and nymphs were also identified from human patients in different countries (e.g., Italy—Audino et al., 2021; Otranto et al., 2014; USA— Nieto et al., 2018; Belgium—Lernout et al., 2019 and Serbia—Banović et al., 2021). Dermacenor marginatus was the most abundant tick species on hunters and hunting dogs as well as on wild boars, in the Mediterranean area (Spain—Ortuño et al., 2006; Corsica—Grech‐Angelini et al., 2016 and Italy—Sgroi et al., 2020). Indeed, these ungulates are involved in the maintenance of this tick species in the environment (Selmi et al., 2017; Sgroi et al., 2020 ) and, therefore, representing a potential risk for hunters and hunting dogs in the area where wild boars thrive. The higher frequency of D. marginatus infestation in hunters frequenting woods with their dogs than those practicing hunting without them, combined with the presence of this tick species on the animals, may suggest a likely tick exposure of people in close contact with hunting dogs (Audino et al., 2021; Toepp et al., 2018 ). This could also indicate a role of these animals as sentinels for the human tick exposure (Bowser & Anderson, 2018; Hornok et al., 2013) considering the high tick infestation rate on hunting dogs herein found (i.e., 42.5%). The above‐mentioned scenario has been previously confirmed in Spain, where hunting dogs were more exposed to TBPs than other classes of owned dogs (Miró et al., 2015). Particularly, the high frequency of infestation for hunters and hunting dogs seems to be associated with wooded areas compared to grassland (Audino et al., 2021; Lernou et al., 2018), confirming the role of the environment as a major driver for the perpetuation of the biological life cycle of ticks. Also, the larger percentage of tick infestation in dogs aging from 1 to 5 years old may be associated with the fact that animals younger than 1 year and older than 5 years are less employed in hunting activities (Orr et al., 2019). The presence of ticks on hunters and dogs from all the studied regions confirms that both mainland and insular areas of southern Italy are suitable for tick development and circulation due to the optimal climatic and environmental conditions present (Dantas‐Torres & Otranto, 2013). In addition, the overall prevalence of zoonotic agents herein detected (i.e., 10.9%) highlights a risk for human and dog infections mainly by SFGR. Similarly, high serological exposure to Rickettsia spp. in hunters (i.e., 9.1%, Germany—Jansen et al., 2008; 14.7%, Brazil—Kmetiuk et al., 2019) and hunting dogs (53%, Spain—Ortuño et al., 2009; 14.1%, Nicaragua —Fiorello et al., 2017) has been reported in several countries. The higher occurrence of R. slovaca (i.e., 9.0%), compared to other SFGR detected, is of relevance considering its pathogenicity to humans (Li et al., 2018; Parola et al., 2009 ). The recent finding of R. slovaca in D. marginatus collected on park rangers from the same study area (Mendoza‐Roldan et al., 2021), further confirms the circulation of this zoonotic bacterium in rural areas of southern Italy. Moreover, the detection of R. raoultii, R. aeschlimannii and R. monacensis in ticks collected from dogs suggests their role as indicators of potential human exposure to pathogens in hunting areas (Guggione et al., 2021; Madeddu et al., 2012; Tosoni et al., 2016), and their increased occurrence in southern part of the Italian peninsula (Gomez‐Barroso et al., 2019). The occurrence of C. burnetii in D. marginatus collected on hunting dogs is of public health concern, since the prevalence of this bacterium in hard ticks is significantly higher in Mediterranean area than in other European countries (Körner et al., 2021). Although the vectorial competence of D. marginatus for C. burnetii (via faecal excretion—Körner et al., 2020) has been ascertained, the role of this tick species in the epidemiology of Q fever appears negligible compared to Hyalomma lusitanicum, in which the prevalence of this pathogen (i.e., 18%) is higher than in D. marginatus (i.e., 1.4%) around Europe (Körner et al., 2021). The finding of B. lusitaniae in I. ricinus infesting hunters and hunting dogs confirms the presence of this zoonotic genospecies in rural areas of southern Italy, as previously established in lizards (Mendoza‐Roldan et al., 2019) and foxes (Sgroi et al., 2021). Despite the pathogenic role of B. lusitaniae in humans has not been completely clarified, the occurrence of long‐lasting skin lesions in the site of tick bites in patients affected by this pathogen, underlines its clinical relevance (Collares‐Pereira et al., 2004). The detection of M. mitochondrii endosymbiont DNA in two out of nine (i.e., 22.2%) I. ricinus deserves further attention regarding the interaction between M. mitochondrii and its hosts, and its involvement in the transmission pathway of B. burgdorferi s.l. by I. ricinus, considering that the prevalence of this microorganism is higher in humans infested by ticks than in those not infested (Mariconti et al., 2012), as well in patients affected by Lyme disease compared to those not affected (Serra et al., 2019). This proteobacterium has also been previously detected in dogs, sheep and horses from southern Italy (Bazzocchi et al., 2013) and in roe deer, Capreolus capreolus, from France (Serra et al., 2018). Finally, the finding of coinfected ixodids suggests that multiple agents may develop in the same tick being potentially transmitted to the same host (Audino et al., 2021; Otranto et al., 2014 ).
Data herein presented demonstrated the high circulation of ticks and related zoonotic pathogens in wild boar hunting areas of southern Italy suggesting a high risk of infestation and zoonotic pathogen transmission to hunters and their dogs. This survey also reveals that the active collection of ticks by hunters may be an additional resource for monitoring ticks in rural areas, being an effective example of the citizen science approach. Lastly, a one health perspective, involving physicians, veterinarians and other collaborating stakeholders (e.g., hunters) is advocated to control and prevent the spread of ticks and related pathogens.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
Giovanni Sgroi: Conceptualization, investigation, writing original draft. Roberta Iatta: Conceptualization, writing‐reviewing and editing, supervision. Riccardo Paolo Lia: Methodology, data curation. Ettore Napoli: Acquisition of data, formal analysis. Francesco Buono: Acquisition of data. Marcos Antonio Bezerra‐Santos: Writing‐review and editing. Vincenzo Veneziano: Acquisition of data, formal analysis. Domenico Otranto: Validation, supervision, Writing: review and editing.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to the European Directive 2010/63/EU, in accordance with the rules of the Istituto Zooprofilattico Sperimentale del Mezzogiorno (Portici, Italy) and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. High standards of veterinary care and client consent have been employed.
ACKNOWLEDGMENTS
The authors are grateful to all citizens and veterinary practitioners involved in the field activities. No funding has been employed for the present work.
Open Access Funding provided by Universita degli Studi di Bari Aldo Moro within the CRUI‐CARE Agreement.
Sgroi, G. , Iatta, R. , Lia, R. P. , Napoli, E. , Buono, F. , Bezerra‐Santos, M. A. , Veneziano, V. , & Otranto, D. (2022). Tick exposure and risk of tick‐borne pathogens infection in hunters and hunting dogs: a citizen science approach. Transboundary and Emerging Diseases, 69, e386–e393. 10.1111/tbed.14314
Giovanni Sgroi and Roberta Iatta contributed equally to the manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Audino, T. , Pautasso, A. , Bellavia, V. , Carta, V. , Ferrari, A. , Verna, F. , Grattarola, C. , Iulini, B. , Pintore, M. D. , Bardelli, M. , Cassina, G. , Tomassone, L. , Peletto, S. , Blanda, V. , Torina, A. , Caramelli, M. , Casalone, C. , & Desiato, R. (2021). Ticks infesting humans and associated pathogens: A cross‑sectional study in a 3‑year period (2017‐2019) in northwest Italy. Parasites & Vectors, 14, 136. 10.1186/s13071-021-04603-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneth, G. (2014). Tick‐borne infections of animals and humans: A common ground. International Journal for Parasitology, 44, 591–596. 10.1016/j.ijpara.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Banović, P. , Díaz‐S´anchez, A. A. , Galon, C. , Simin, V. , Mijatović, D. , Obregón, D. , Moutailler, S. , & Cabezas‐Cruz, A. (2021). Humans infested with Ixodes ricinus are exposed to a diverse array of tick‐borne pathogens in Serbia. Ticks and Tick‐Borne Diseases, 12, 101609. 10.1016/j.ttbdis.2020.101609 [DOI] [PubMed] [Google Scholar]
- Bazzocchi, C. , Mariconti, M. , Sassera, D. , Rinaldi, L. , Martin, E. , Cringoli, G. , Urbanelli, S. , Genchi, C. , Bandi, C. , & Epis, S. (2013). Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales:Midichloriaceae) in different mammalian species. Parasites & Vectors, 6, 1–7. 10.1186/1756-3305-6-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrame, A. , Laroche, M. , Degani, M. , Perandin, F. , Bisoffi, Z. , Raoult, D. , & Parola, P. (2018). Tick‐borne pathogens in removed ticks Veneto, northeastern Italy: A crosssectional investigation. Travel Medicine and Infectious Diseases, 26, 58–61. 10.1016/j.tmaid.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Berri, M. , Laroucau, K. , & Rodolakis, A. (2000). The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Veterinary Microbiology, 72, 285–293. 10.1016/s0378-1135(99)00178-9 [DOI] [PubMed] [Google Scholar]
- Brown, L. D. , Cat, T. T. , & DasGupta, A. (2001). Interval estimation for a proportion. Statistical Science, 16, 101–133. 10.1214/ss/1009213286 [DOI] [Google Scholar]
- Bowser, N. H. , & Anderson, N. E. (2018). Dogs (Canis familiaris) as sentinels for human infectious disease and application to Canadian populations: A systematic review. Veterinary Science, 5, 83. 10.3390/vetsci5040083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . (2019). Tick bite: What to do [pdf file]. https://www.cdc.gov/ticks/pdfs/FS_TickBite‐508.pdf
- Collares‐Pereira, M. , Couceiro, S. , Franca, I. , Kurtenbach, K. , Sch¨afer, S. M. , Vitorino, L. , Gonçalves, L. , Baptista, S. , Vieira, M. L. , & Cunha, C. (2004). First isolation of Borrelia lusitaniae from a human patient. Journal of Clinical Microbiology, 42(3), 1316–1318. 10.1128/JCM.42.3.1316-1318.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S. , Vayssier‐Taussat, M. , Estrada‐Peña, A. , Potkonjak, A. , Mihalca, A. D. , & Zeller, H. (2019). A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Euro Surveillance, 24, 1800170. 10.2807/1560-7917.ES.2019.24.18.1800170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres, F. , Chomel, B. B. , & Otranto, D. (2012). Ticks and tick‐borne diseases: a one health perspective. Trends in Parasitology, 28, 437–446. 10.1016/j.pt.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Dantas‐Torres, F. , & Otranto, D. (2013). Species diversity and abundance of ticks in three habitats in southern Italy. Ticks and Tick‐borne Diseases, 4, 251–255. 10.1016/j.ttbdis.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Estrada‐Peña, A. , Bouattour, A. , Camicas, J. L. , & Walker, A. R. (2004). Ticks of domestic animals in the mediterranean region. A guide to identification of species. Bioscience Reports. [Google Scholar]
- Estrada‐Peña, A. , & de la Fuente, J. (2016). Species interactions in occurrence data for a community of tick‐transmitted pathogens. Scientific Data, 3, 160056. 10.1038/sdata.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada‐Peña, A. , Mihalca, A. D. , & Petney, T. N. (2017). Ticks of Europe and North Africa: A guide to species identification. Springer Nature. 10.1007/978-3-319-63760-0 [DOI] [Google Scholar]
- Fiorello, C. V. , Straub, M. H. , Schwartz, L. M. , Liuc, J. , Campbell, A. , Kownacki, A. K. , & Foley, J. E. (2017). Multiple‐host pathogens in domestic hunting dogs in Nicaragua'sBosawás Biosphere Reserve. Acta Tropica, 167, 183–190. 10.1016/j.actatropica.2016.12.020 [DOI] [PubMed] [Google Scholar]
- Garcia‐Marti, I. , Zurita‐Milla, R. , Harms, M. G. , & Swart, A. (2018). Using volunteered observations to map human exposure to ticks. Scientific Reports, 8, 15435. 10.1038/s41598-018-33900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‑Marti, I. , Zurita‑Milla, R. , van Vliet, A. J. H. , & Takken, W. (2017). Modelling and mapping tick dynamics using volunteered observations. International Journal of Health Geographics, 16, 41. 10.1186/s12942-017-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Barroso, D. , Fenicia Vescio, M. , Bella, A. , Ciervo, A. , Busani, L. , Rizzo, C. , Rezza, G. , & Pezzotti, P. (2019). Mediterranean spotted fever rickettsiosis in Italy, 2001–2015: Spatiotemporal distribution based on hospitalization records. Ticks and Tick‐Borne Diseases, 10, 43–50. 10.1016/j.ttbdis.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Grech‐Angelini, S. , Stachurski, F. , Lancelot, R. , Boissier, J. , Allienne, J. F. , Marco, S. , Maestrini, O. , & Uilenberg, G. (2016). Ticks (Acari: Ixodidae) infesting cattle and some other domestic and wild hosts on the French Mediterranean island of Corsica. Parasites and Vectors, 9, 582. 10.1186/s13071-016-1876-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Curtis‐Robles, R. , & Hamer, G. L. (2018). Contributions of citizen scientists to arthropod vector data in the age of digital epidemiology. Current Opinion in Insect Science, 28, 98–104. 10.1016/j.cois.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Hartemink, N. , Vanwambeke, S. O. , Purse, B. V. , Gilbert, M. , & Van Dyck, H. (2015). Towards a resource‐based habitat approach for spatial modelling of vectorborne disease risks. Biological Reviews, 32, 1151–1162. 10.1111/brv.12149 [DOI] [PubMed] [Google Scholar]
- Hornok, S. , Dénes, B. , Meli, M. L. , Tánczos, B. , Fekete, L. , Gyuranecz, M. , de la Fuente, J. , Fernández de Mera, I. G. , Farkas, R. , & Hofmann‐Lehmann, R. (2013). Non‐pet dogs as sentinels and potential synanthropic reservoirs of tick‐borne and zoonotic bacteria. Veterinary Microbiology, 167, 700–703. 10.1016/j.vetmic.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Jaenson, T. G. T. , Eisen, L. , Comstedt, P. , Mejlon, H. A. , Lindgren, E. , Bergström, S. , & Olsen, B. (2009). Risk indicators for the tick Ixodes ricinus and Borrelia burgdorferi sensu lato in Sweden. Medical and Veterinary Entomology, 23, 226–237. 10.1111/j.1365-2915.2009.00813.x [DOI] [PubMed] [Google Scholar]
- Jansen, A. , La Scola, B. , Raoult, D. , Lierz, M. , Wichmann, O. , Stark, K. , & Schneider, T. (2008). Antibodies against Rickettsia spp. in hunters, Germany. Emerging Infectious Diseases, 14, 1961–1963. 10.3201/eid1412.080229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. , Thierer, T. , Ashton, B. , Meintjes, P. , & Drummond, A. (2012). Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetiuk, L. B. , Krawczak, F. S. , Machado, F. P. , Paploski, I. A. D. , Martins, T. F. , Teider‐Junior, P. I. , Serpa, M. C. A. , Barbieri, A. M. R. , Bach, R. V. W. , Barros‐Filho, I. R. , Lipinski, L. C. , dos Santos, A. P. , Labruna, M. B. , & Biondo, A. W. (2019). Ticks and serosurvey of anti‐Rickettsia spp. antibodies in wild boars (Sus scrofa), hunting dogs and hunters of Brazil. PLoS Neglected Tropical Diseases, 13, e0007405. 10.1371/journal.pntd.0007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner, S. , Makert, G. R. , Mertens‐Scholz, K. , Henning, K. , Pfeffer, M. , Starke, A. , Nijhof, A. M. , & Ulbert, S. (2020). Uptake and fecal excretion of Coxiella burnetii by Ixodes ricinus and Dermacentor marginatus ticks. Parasites & Vectors, 13, 75. 10.1186/s13071-020-3956-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner, S. , Makert, G. R. , Ulbert, S. , Pfeffer, M. , & Mertens‐Scholz, K. (2021). The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q fever transmission revisited: A systematic review. Frontiers in Veterinary Science, 8, 655715. 10.3389/fvets.2021.655715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, P. J. (2019). Human babesiosis. International Journal for Parasitology, 49, 165–174. 10.1016/j.ijpara.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Kuehn, B. (2019). Tickborne diseases increasing. Journal of the American Medical Association, 321, 138. 10.1001/jama.2018.20464 [DOI] [PubMed] [Google Scholar]
- Labruna, M. B. , Whitworth, T. , Horta, M. C. , Bouyer, D. H. , McBride, J. W. , Pinter, A. , Popov, V. , Gennari, S. M. , & Walker, D. H. (2004). Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. Journal of Clinical Microbiology, 42, 90–98. 10.1128/jcm.42.1.90-98.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernout, T. , De Regge, N. , Tersago, K. , Fonville, M. , Suin, V. , & Sprong, H. (2019). Prevalence of pathogens in ticks collected from humans through citizen science in Belgium. Parasites and Vectors, 12, 550. 10.1186/s13071-019-3806-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J. , Boudreau, C. R. , Patterson, J. W. , Bradet‐Legris, J. , & Lloyd, V. K. (2018). Citizen science and community engagement in tick surveillance: A Canadian case study. Healthcare, 6, 22. 10.3390/healthcare6010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Zhang, P. H. , Huang, Y. , Du, J. , Cui, N. , Yang, Z. D. , Tang, F. , Fu, F. X. , Li, X. M. , Cui, X. M. , Fan, Y. D. , Xing, B. , Li, X. K. , Tong, Y. G. , Cao, W. C. , & Liu, W. (2018). Isolation and identification of Rickettsia raoultii in human cases: A surveillance study in 3 medical centers in China. Clinical Infectious Diseases, 66, 1109–1115. 10.1093/cid/cix917 [DOI] [PubMed] [Google Scholar]
- Madeddu, G. , Mancini, F. , Caddeo, A. , Ciervo, A. , Babudieri, S. , Maida, I. , Fiori, M. L. , Rezza, G. , & Mura, M. S. (2012). Rickettsia monacensis as cause of Mediterranean spotted fever‐like illness, Italy. Emerging Infectious Diseases, 18, 702–704. 10.3201/eid1804.111583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahachi, K. , Kontowicz, E. , Anderson, B. , Toepp, A. J. , Lima, A. L. , Larson, M. , Wilson, G. , Grinnage‑Pulley, T. , Bennett, C. , Ozanne, M. , Anderson, M. , Fowler, H. , Parrish, M. , Saucier, J. , Tyrrell, P. , Palmer, Z. , Buch, J. , Chandrashekar, R. , Scorza, B. , … Petersen, C. A. (2020). Predominant risk factors for tick‑borne co‑infections in hunting dogs from the USA. Parasites & Vectors, 13, 247. 10.1186/s13071-020-04118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manilla, G. , & Iori, A. (1992). Chiave illustrata delle zecche d'Italia: Stadi larvali delle specie della sottofamiglia Ixodinae (Acari, Ixodoidea, Ixodidae). Parassitologia, 34, 83–95. [PubMed] [Google Scholar]
- Mariconti, M. , Epis, S. , Gaibani, P. , Dalla Valle, C. , Sassera, D. , Tomao, P. , Fabbi, M. , Castelli, F. , Marone, P. , Sambri, V. , Bazzocchi, C. , & Bandi, C. (2012). Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: Is Midichloria a novel pathogen, or just a marker of tick bite? Pathogens and Global Health, 106, 391–396. 10.1179/2047773212Y.0000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. R. , Brown, G. K. , Dunstan, R. H. , & Roberts, T. K. (2005). Anaplasma platys: An improved PCR for its detection in dogs. Experimental Parasitology, 109, 176–180. 10.1016/j.exppara.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Mendoza‐Roldan, J. A. , Colella, V. , Lia, R. P. , Nguyen, V. L. , Barros‐Battesti, D. M. , Iatta, R. , Dantas‐Torres, F. , & Otranto, D. (2019). Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasites & Vectors, 12, 35. 10.1186/s13071-019-3286-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza‐Roldan, J. A. , Manoj, R. S. S. , Latrofa, M. S. , Iatta, R. , Annoscia, G. , Lovreglio, P. , Stufano, A. , Dantas‐Torres, F. , Davoust, B. , Laidoudi, Y. , Mediannikov, O. , & Otranto, D. (2021). Role of reptiles and associated arthropods in the epidemiology of rickettsioses: A one health paradigm. PLoS Neglected Tropical Diseases, 15, e0009090. 10.1371/journal.pntd.0009090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró, G. , Checa, R. , Paparini, A. , Ortega, N. , Gonz´alez‐Fraga, J. L. , Gofton, A. , Bartolomé, A. , Montoya, A. , Gálvez, R. , Mayo, P. P. , & Irwin, P. (2015). Theileria annae (syn. Babesia microti‐like) infection in dogs in NW Spain detected using direct and indirect diagnostic techniques: Clinical report of 75 cases. Parasites & Vectors, 8, 217. 10.1186/s13071-015-0825-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto, N. C. , Tanner Porter, W. , Wachara, K. C. , Lowrey, T. J. , Martin, L. , Motyka, P. J. , & Salkeld, D. J. (2018). Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick‐borne diseases in the United States. Plos One, 13, e0199644. 10.1371/journal.pone.0199644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, B. , Malik, R. , Norris, J. , & Westman, M. (2019). The welfare of pig‐hunting dogs in Australia. Animals, 9, 853. 10.3390/ani9100853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño, A. , Pons, I. , Nogueras, M. M. , Castellà, J. , & Segura, F. (2009). The dog as an epidemiological marker of Rickettsia conorii infection. European Society of Clinical Microbiology and Infectious Diseases, 15, 241–242. 10.1111/j.1469-0691.2008.02158.x [DOI] [PubMed] [Google Scholar]
- Ortuño, A. , Quesada, M. , López, S. , Miret, J. , Cardeñosa, N. , Castellà, J. , Anton, E. , & Segura, F. (2006). Prevalence of Rickettsia slovaca in Dermacentor marginatus ticks removed from wild boar (Sus scrofa) in northeastern Spain. Annals of the New York Academy of Sciences, 1078, 324–327. 10.1196/annals.1374.061 [DOI] [PubMed] [Google Scholar]
- Otranto, D. , Dantas‐Torres, F. , Giannelli, A. , Latrofa, M. S. , Cascio, A. , Cazzin, S. , Ravagnan, S. , Montarsi, F. , Zanzani, S. A. , Manfredi, M. T. , & Capelli, G. (2014). Ticks infesting humans in Italy and associated pathogens. Parasites & Vectors, 7, 328. 10.1186/1756-3305-7-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola, P. , Paddock, C. D. , Socolovschi, C. , Labruna, M. B. , Mediannikov, O. , Kernif, T. , Abdad, M. Y. , Stenos, J. , Bitam, I. , Fournier, P. E. , & Raoult, D. (2013). Update on tick‐borne rickettsioses around the world: A geographic approach. Clinical Microbiology Reviews, 26, 657–702. 10.1128/CMR.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola, P. , Rovery, C. , Rolain, J. , Brouqui, P. , Davoust, B. , & Raoult, D. (2009). Rickettsia slovaca and R. raoultii in tick‐borne rickettsioses. Emerging Infectious Diseases, 15, 1105–1108. 10.3201/eid1507.081449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauter, C. , & Hartung, T. (2005). Prevalence of Borrelia burgdorferi sensu lato Genospecies in Ixodes ricinus ticks in Europe: A metaanalysis. Applied and Environmental Microbiology, 71, 7203–7216. 10.1128/AEM.71.11.7203-7216.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery, R. L. , Spruill, C. L. , & Plikaytis, B. D. (1991). Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. Journal of Bacteriology, 173, 1576–1589. 10.1128/jb.173.5.1576-1589.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli, A. , Silaghi, C. , Obiegala, A. , Rudolf, I. , Hubálek, Z. , Földvári, G. , Plantard, O. , Vayssier‐Taussat, M. , Bonnet, S. , Špitalská, E. , & Kazimírová, M. (2014). Ixodes ricinus and its transmitted pathogens in urban and peri‐urban areas in Europe: New hazards and relevance for public health. Frontiers in Public Health, 2, 1–26. 10.3389/fpubh.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandifer, P. A. , Sutton‐grier, A. E. , & Ward, B. P. (2015). Exploring connections among nature, biodiversity, ecosystem services, and human health and well‐being: Opportunities to enhance health and biodiversity conservation. Ecosystem Services, 12, 1–15. 10.1016/j.ecoser.2014.12.007 [DOI] [Google Scholar]
- Santino, I. , Cammarata, E. , Franco, S. , Galdiero, F. , Oliva, B. , Sessa, R. , Cipriani, P. , Tempera, G. , & Del Piano, M. (2004). Multicentric study of seroprevalence of Borrelia burgdorferi and Anaplasma phagocytophila in high‐risk groups in regions of central and southern Italy. International Journal of Immunopathology and Pharmacology, 17, 219–223. 10.1177/039463200401700214 [DOI] [PubMed] [Google Scholar]
- Selmi, M. , Ballardini, M. , Salvato, L. , & Ricci, E. (2017). Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host‐vector‐pathogen interactions in a northern Mediterranean area. Experimental and Applied Acarology, 72, 79–91. 10.1007/s10493-017-0132-z [DOI] [PubMed] [Google Scholar]
- Sergeant, E. S. G. (2018). Epitools Epidemiological Calculators. Ausvet. http://epitools.ausvet.com.au
- Serra, V. , Cafiso, A. , Formenti, N. , Verheyden, H. , Plantard, O. , Bazzocchi, C. , & Sassera, D. (2018). Molecular and serological evidence of the presence of Midichloria mitochondrii in roe deer (Capreolus capreolus) in France. Journal of Wildlife Diseases, 54, 597–600. 10.7589/2017-09-241 [DOI] [PubMed] [Google Scholar]
- Serra, V. , Krey, V. , Daschkin, C. , Cafiso, A. , Sassera, D. , Maxeiner, H. G. , Modeo, L. , Nicolaus, C. , Bandi, C. , & Bazzocchi, C. (2019). Seropositivity to Midichloria mitochondrii (order Rickettsiales) as a marker to determine the exposure of humans to tick bite. Pathogens and Global Health, 113, 167–172. 10.1080/20477724.2019.1651568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi, G. , Iatta, R. , Lia, R. P. , D'Alessio, N. , Manoj, R. R. S. , Veneziano, V. , & Otranto, D. (2020). Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transboundary and Emerging Diseases, 68, 1–10. 10.1111/tbed.13859 [DOI] [PubMed] [Google Scholar]
- Sgroi, G. , Iatta, R. , Veneziano, V. , Bezerra‐Santos, M. A. , Lesiczka, P. , Hrazdilová, K. , Annoscia, G. , D'Alessio, N. , Golovchenko, M. , Rudenko, N. , Modrý, D. , & Otranto, D. (2021). Molecular survey on tick‐borne pathogens and Leishmania infantum in red foxes (Vulpes vulpes) from southern Italy. Ticks and Tick‐Borne Diseases, 12, 101669. 10.1016/j.ttbdis.2021.101669 [DOI] [PubMed] [Google Scholar]
- Sprong, H. , Azagi, T. , Hoornstra, D. , Nijhof, A. M. , Knorr, S. , Baarsma, M. E. , & Hovius, J. W. (2018). Control of Lyme borreliosis and other Ixodes ricinus‐borne diseases. Parasites & Vectors, 11, 145. 10.1186/s13071-018-2744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner Porter, W. , Motyka, P. J. , Wachara, J. , Barrand, Z. A. , Hmood, Z. , McLaughlin, M. , Pemberton, K. , & Nieto, N. C. (2019). Citizen science informs human‑tick exposure in the Northeastern United States. International Journal of Health Geographics, 18, 9. 10.1186/s12942-019-0173-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepp, A. J. , Willardson, K. , Larson, M. , Scott, B. D. , Johannes, A. , Senesac, R. , & Petersen, C. A. (2018). Frequent exposure to many hunting dogs significantly increases tick exposure. Vector‐Borne and Zoonotic Diseases, 20, 1–5. 10.1089/vbz.2017.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosoni, A. , Mirijello, A. , Ciervo, A. , Mancini, F. , Rezza, G. , Damiano, F. , Cauda, R. , Gasbarrini, A. , & Addolorato, G. (2016). Human Rickettsia aeschlimannii infection: First case with acute hepatitis and review of the literature. European Review for Medical and Pharmacological Sciences, 20, 2630–2633. [PubMed] [Google Scholar]
- Wójcik‐Fatla, A. , Szymanska, J. , Wdowiak, L. , Buczek, A. , & Dutkiewicz, J. (2009). Coincidence of three pathogens [Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti] in Ixodes ricinus ticks in the Lublin macroregion. Annals of Agricultural and Environmental Medicine, 16, 151–158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


