Abstract
BACKGROUND
The present study aimed to determine the optimal conditions for making wines by the carbonic maceration (CM) method. In this test, we attempted to identify the causes of the higher volatile acidity of some wines vinified by this method. Accordingly, we measured the development and speed of intracellular fermentation inside the whole grapes under different vinification conditions.
RESULTS
An active fermentation of the must in the tanks produced by inoculation with active dry yeasts was more efficient for the process than the addition of exogenous carbon dioxide (CO2). In addition, in CM vinification, the moment of devatting had a great influence on the content of acetaldehyde and acetic acid in the whole grapes.
CONCLUSION
Yeast inoculation in the tanks and control of the devatting time are technological tools that could play an important role in the characteristics of the wines produced by carbonic maceration, especially with respect to acetic acid content. © 2022 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: carbonic maceration, temperature, inoculation, anaerobiosis, acetic acid
INTRODUCTION
Vinification by carbonic maceration (CM) is based on anaerobic fermentative metabolism, also referred to as intracellular fermentation (IF). It occurs inside whole grapes as a result of their own enzymes when they are subjected to anaerobic conditions. 1 Here, grape clusters without destemming or crushing are placed into tanks under a CO2 atmosphere. However, some grapes break and release must to the tank, which is fermented by yeasts. Therefore, the IF of the grapes and the alcoholic fermentation (AF) of the must by yeasts occur simultaneously in the tank for a variable time in the range 5–8 days. After this first phase, drawing off is performed by racking a free‐run wine and the whole grapes, which are pressed to release a press‐wine. Then, a second phase begins when both wines complete their alcoholic and malolactic fermentations.
The phenomenon that occurs inside the whole grapes (i.e. IF) give wines specific characteristics, 2 described as light and fruity in red wines 3 , 4 with low tannins, less colour intensity, 5 , 6 , 7 more intense fruit flavours, 8 lower glycerol content, lower level of fixed acidity, higher pH 9 and higher antioxidant activity than wines made by destemming and crushing. 10 , 11 , 12 On the other hand, the vinification conditions and characteristics of CM wines favour microbiological development, mainly with respect to the lactic acid bacteria (LAB) populations, which could make them more susceptible to microbial alterations during vinification and aging. 13 , 14 In a previous study, 15 it was observed that the spoilage in this vinification process was caused not only by a great development of LAB during fermentation, but also a great accumulation of acetic acid inside the whole grapes. Both situations were probably a result of difficulty in maintaining the necessary temperature and anaerobic conditions in the tanks, especially in small‐scale winemaking. These studies are essential for deepening the scientific knowledge of this winemaking technology.
In the present study, the effect of both temperature and the type of carbonic anhydride applied to the tanks (exogenous gas or generated by fermentation) on IF was investigate aiming to minimize the increase in volatile acidity during carbonic maceration vinification.
MATERIALS AND METHODS
Assay design
The objective of this test was to evaluate the influence of the temperature and the origin of CO2 in CM vinification. For this, eight vinifications were carried out in 12‐L stainless steel tanks aiming to investigate four vinification conditions in duplicate (Fig. 1). In each one, 10 kg of whole Tempranillo grapes and 2 L of sulphited must (40 mg L−1) were added to simulate the effect of the breakage. In four tanks (I), the added must had previously been inoculated with a commercial active dry yeast (ADY) Saccharomyces cerevisiae Uvaferm VRB to generate the necessary CO2 for the development of the anaerobic conditions. Two tanks were placed in a chamber at 20 °C (I20), whereas the other two were placed in a chamber at 30 °C (I30). In the remaining four tanks (C), the must was not seeded and industrial CO2 was added to the tanks before filling and until the added must showed evident fermentation. Two tanks were placed in a chamber at 20 °C (C20), whereas the other two were placed in a chamber at 30 °C (C30). In total, four types of vinification were carried out in duplicate: I20, I30, C20 and C30.
Figure 1.
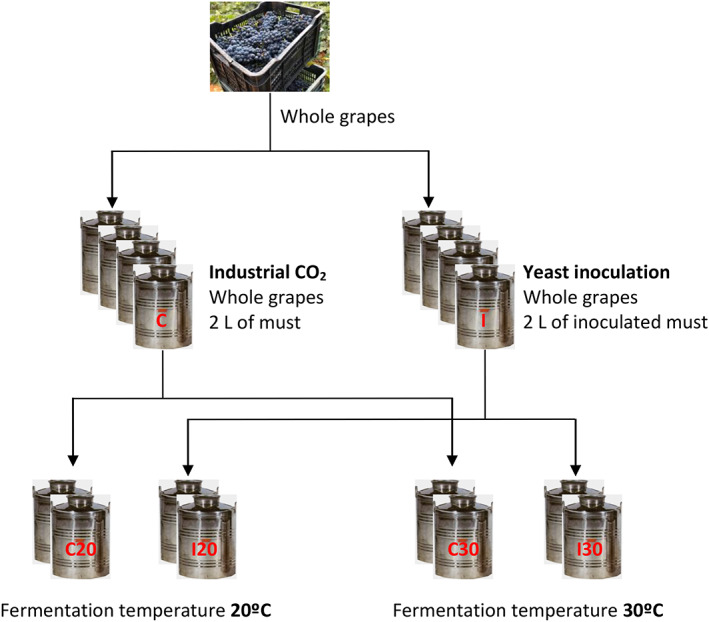
Experimental trial used in the present study.
The follow‐up of the vinifications was carried out by controlling each day the temperature and the density of the must and the temperature of the bunches. In addition, every 2–3 days (days 1, 4, 6, 8 and 11), 10 whole berries were randomly collected from each tank. The grapes were crushed to obtain the must‐wine, where the contents of malic acid, acetaldehyde, sugar and acetic acid were analysed. The tanks were devatted when the AF of the liquid was finished (7–12 days depending on conditions), separating the liquid phase from the whole grapes, which were pressed to obtain the press must‐wine. All of the fractions finished AF at 25 °C, which required an extra day for the free‐run must from the tanks and 4–5 days for the must‐wine obtained by pressing the bunches. Finally, the wines obtained were seeded with commercial LAB to induce malolactic fermentation.
Physicochemical analysis
The density of the free musts was measured using a densimeter. The musts obtained after pressing 10 grapes were characterized by measuring oBrix (sugar degradation by refractometry), acetaldehyde and volatile acidity according to the official European Community methods. 16 The malic acid was determined by an enzymatic method carried out via an automated analyser (Miura One, TDI, Barcelona, Spain), in accordance with the manufacturer's instructions.
Statistical analysis
The mean ± SD of the analytical data of the musts obtained after pressing 10 grapes every 2–3 days during vatting were calculated with the SPSS, version 23 (IBM Corp., Armonk, NY, USA) and represented graphically.
RESULTS
Development of AF in the must
The fastest AF in the free musts were those carried out with CO2 from the fermentation of the inoculated must at 30 °C (I30) (Fig. 2), which lasted 7 days. By contrast, the slowest AF corresponded to the CM performed with CO2 added exogenously at 20 °C (C20), where wine took 12 days to ferment. A comparison between I20 and C30 shows that AF ended at the same time, and so the increased temperature and the inoculation as a source of CO2 appeared to compensate each other.
Figure 2.

Evolution of alcoholic fermentation (density) in free (continuous line) and in press (broken line) wines made by carbonic maceration under different conditions: by inoculation of yeast at 20 °C (I20: blue empty square) and at 30 °C (I30: red full square), or by addition of exogenous CO2 at 20 °C (C20: grey empty circle) and at 30 °C (C30: yellow full circle).
When analysing the fermentation of the press musts, it was observed that the speed in the consumption of sugar was similar in all cases (4–5 days) and the delay at the end of the fermentations of these wines came from the duration of the period in the vat.
Development of IF under different conditions
The progress of the IF was evaluated by analysing the content of malic acid (Fig. 3), acetaldehyde (Fig. 4), acetic acid (Fig. 5) and sugar (Fig. 6) inside the grape berries over time in the vat. The devatting in all cases was made at the end of AF in the free must (6 days in I30, 8 days in I20 and C30, and 12 days in C20).
Figure 3.

Evolution of the content of malic acid inside the berries during the fermentation of grapes vinified by carbonic maceration under different conditions: inoculation of yeast at 20 °C (I20) and at 30 °C (I30), or addition of exogenous CO2 at 20 °C (C20) and at 30 °C (C30). *The vat time was different (6, 8 or 11 days) depending on the conditions.
Figure 4.

Evolution of the content of acetaldehyde inside the berries during the fermentation of grapes vinified by carbonic maceration under different conditions: inoculation of yeast at 20 °C (I20) and at 30 °C (I30) or addition of exogenous CO2 at 20 °C (C20) and at 30 °C (C30). *The vat time was different (6, 8 or 11 days) depending on the conditions.
Figure 5.

Evolution of the content of acetic acid inside the berries during the fermentation of grapes vinified by carbonic maceration under different conditions: inoculation of yeast at 20 °C (I20) and at 30 °C (I30) or addition of exogenous CO2 at 20 °C (C20) and at 30 °C (C30). *The vat time was different (6, 8 or 11 days) depending on the conditions.
Figure 6.

Evolution of sugar content (oBrix) inside the berries during the fermentation of grapes vinified by carbonic maceration under different conditions: inoculation of yeast at 20 °C (I20) and at 30 °C (I30) or addition of exogenous CO2 at 20 °C (C20) and at 30 °C (C30). *The vat time was different (6, 8 or 11 days) depending on the conditions.
Figure 3 shows that the degradation of malic acid inside the berries was greater from the beginning of the vat period in the inoculated vinifications conducted at 30 °C (I30). Under these conditions, during the first day in the vat, approximately 50% of the initial content of malic acid was degraded and, from this moment onward, no more was consumed. In the tanks where the process was conducted at 30 °C but not seeded with commercial yeasts (C30), the degradation of malic acid occurred gradually throughout the vat time, reaching levels similar to I30 (50% of the initial content) at the time of drawing off. In the C20 deposits, a period of 6 days was necessary for the beginning of the malic acid degradation. Furthermore, the inoculation (I20) helped to accelerate this onset of the IF because, on the fourth day, degradation of this acid was already observed. Comparing the percentage of malic acid consumed on the fourth day of incubation (I30: 63%, C30: 45%, I20: 45% and C20: 0%), it was observed that the I30 conditions were the most effective for IF and C20 conditions were the worst. However, comparing the level of malic acid consumed at the moment of devatting (6 days in I30, 8 days in I20 and C30, and 11 days in C20), it was observed that similar levels were reached in all tanks, except I20, which had the highest content of malic acid (0.85 g L−1 compared to 0.55–0.60 g L−1 in the other cases).
The evolution of the acetaldehyde content within the berries (Fig. 4) showed a constant and gradual increase throughout the vat time, not related to temperature conditions and not related to the origin of CO2.
Another compound that is accumulated inside berries during IF is the acetic acid. In Fig. 5, a gradual increase in acetic acid content is shown throughout the vat time. At each sampling point (4,6, 8 and 11 days), the accumulation of acetic acid was greater at higher temperatures, both in the inoculated deposits and in those with added exogenous CO2. The accumulation of acetic acid in grapes over time was also greater in deposits with added industrial CO2 compared to their corresponding inoculated deposits.
Regarding the degradation of sugarsFig. 6 shows that differences were not found in the consumption rate or in the final amount of degraded sugars.
DISCUSSION
When investigating the AF of the free must from the deposits, it was observed that the fastest AF was reached in I30, and the slowest one corresponded to C20 (Fig. 2). Under the other two conditions (I20 and C30), the AF of the musts ended at the same time but AF started earlier in the inoculated deposits (I20), which would help to avoid microbiological deviations. These data would indicate that inoculation with ADY is even more effective than temperature control for managing CM vinifications and protecting wines. These data would also justify why, currently, many winemakers from La Rioja, who make CM in tanks without temperature control, use inoculation as a tool to conduct their vinifications. The use of oenological starter represented a change with respect to spontaneous fermentation at the end of the 20th Century. This practice allows rapid and adequate fermentation kinetics and modulates the characteristics of the final product. 17 , 18 This statement is even more important in CM vinification, where the risk of deviation is higher than in vinification by destemming and crushing. 15
As expected in IF from vinifications conducted at 20 °C, a delay was observed in the degradation of malic acid inside the grape berries compared to those at 30 °C (Fig. 3). In addition, in the inoculated tanks (I30 and I20), the malic acid consumption stopped before 4 days, unlike the non‐inoculated tanks (C30 and C20) in which the decrease was progressive throughout the time in the vat. This may have occurred because, in the C30 and C20 deposits, the added CO2 was not sufficient to eliminate oxygen trapped in de bunches and generate anaerobiosis. This could cause a delay in the IF, which was progressively activated as the must began to ferment spontaneously and release a lot of CO2. By contrast, in the inoculated tanks, the release of CO2 by fermentation occurred from the beginning of the vat period. Accordingly, the I30 conditions were the most effective for IF and C20 conditions were the worst. Similar to AF of free must, a compensation between temperature and carbonic acid origin is also observed because C30 and I20 showed a similar level of consumption in the initial days in the vat.
However in this test, at the moment of devatting (6 days in I30, 8 days in I20 and C30, and 11 days in C20), similar levels of malic acid consumed were reached in all tanks, except I20. Therefore, in those cases in which the appropriate temperature and anaerobic conditions do not occur, to achieve similar levels of IF, it would be necessary to increase the vat time, which increases the duration of the vinification and, in some cases, the risk of alteration.
As regards the accumulation of acetaldehyde inside the grapes (Fig. 4), it depends on the time in the vat and it is not related to temperature or the origin of CO2 conditions. Therefore, if the vat time is lengthened for different reasons (e.g. inadequate temperature or anaerobic conditions), the must‐wine obtained after pressing will have a higher concentration of acetaldehyde. Acetaldehyde is an important compound affecting aroma and wine color stability. It is an intermediate compound produced during AF and IF, which is subsequently reduced to ethanol. Accordingly, its content in wine is usually low (25–40 mg L−1), enhancing the fruity character of wine. 19 In the present study, the levels of this compound reached at the moment of devatting were higher than such values (Fig. 4). This would justify the higher values of this compound in CM wines because of the high amounts of acetaldehyde originating from anaerobic metabolism. 20 , 21 In addition, the values increase as the devatting moment is delayed, which means that its production depends on the conditions in which the CM vinification is carried out. Excessive accumulation of acetaldehyde is not desirable because it is associated with unpleasant rotten apple aroma. In addition, it binds to sulphur dioxide making it less active. 22
Another compound that is gradually accumulated inside berries during IF is acetic acid (Fig. 5), which is the main component of the volatile acidity of wines and plays an important role in the aroma of wine. An excessive concentration of this by‐product is detrimental to quality, imparting a vinegar‐like character to wine. The amount of this compound produced in vinification is usually low (0.25–0.50 g L−1) and becomes objectionable at concentrations of 0.7–1.1 g L−1. The OIV 23 establishes that the maximum acceptable limit of volatile acidity in most wines is an acetic acid concentration of 1.2 g L−1. Acetic acid can originate from the degradation of sugars or of the malic acid. In the present study, differences were not found in the consumption rate or in the final amount of degraded sugars (Fig. 6), but, in very long vats (11 days at C20), must‐wines with high volatile acidities would be produced (Fig. 5). Therefore, the differences in the accumulation of acetic acid would indicate a deviation in the degradation path of malic acid inside the grapes.
The accumulation of acetic acid was greater at higher temperatures, both in the inoculated deposits and in those added with exogenous CO2, which would indicate a greater accumulation of acetic acid at higher temperatures in the tanks. However, this does not imply that the final wines have higher volatile acidity because, at fermentation temperatures of 30 °C, it would produce an earlier drawing off. Indeed, at the moment of devatting, the musts with the lowest concentration of acetic acid were I30.
The accumulation of acetic acid in grapes over time was also greater in deposits with added industrial CO2. At the moment of devatting, the must‐wines with less volatile acidity were the inoculated ones. Therefore, the greatest accumulation of acetic acid occurred when anaerobiosis was obtained by exogenous addition of CO2, and this effect was further enhanced by high temperatures. Accordingly, when the CO2 originates from an exogenous origin, the increase in temperature does not prevent the accumulation of acetic acid.
In the investigation of the degradation of malic acid (Fig. 3), a compensatory effect between temperature and inoculation was observed in the IF. In fermentations with the addition of exogenous CO2, a temperature of 30 °C could activate the IF and, in fermentations conducted at 20 °C, the inoculation also could activate the IF. Something similar occurred in the AF of the must from the tanks. However, to avoid a high accumulation of acetic acid inside the grapes, and to prevent high levels of acetic acid in the wines, the key point is to guarantee an active fermentation of the must in the tank.
Consequently, the inoculation with ADY helps not only to improve the AF of the free and press musts from the deposits, but also to minimize the accumulation of acetic acid within the whole grapes during the time in the vat.
CONCLUSIONS
Proofs carried out under different CM conditions showed that the drawing off moment is an influential factor with respect to the content of acetaldehyde and acetic acid inside the grapes and therefore could play an important role in the characteristics of the CM wines obtained. In addition, the inoculation with ADY as a method of generating anaerobiosis not only helps to improve the alcoholic fermentation of the must, but also minimizes the accumulation of acetic acid inside the whole grapes during the time in the vat. Finally, it is also important to maintain a high temperature of grapes during the period in the vat because it makes the vat time shorter.
Until now, it was known that CM wines tend to have higher volatile acidity than those made by destemming and crushing. The reason that justified this fact was the greater development of LAB during fermentation in the vat and its moderate degradation of sugars. In the present study, it can be seen that, when the conditions in the tanks are not adequate, there is also a greater accumulation of acetic acid inside the grapes, which can contribute to increasing the volatile acidity of the wines.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
PS and ARG were was responsible for study conceptualization. PS, LGA and SS were responsible for data curation. PS was responsible for project administration. LGA, REV and PG were responsible for the investigation. LGA, SS and ARG were responsible for reviewing the writing. ARG was responsible for resourcing, writing and editing. All authors read and approved the final version of the manuscript submitted for publication.
ACKNOWLEDGMENTS
The present study was co‐funded (50/50) by the European Regional Development Fund (ERDF) and the Government of La Rioja, within the ERDF operational program of La Rioja 2014‐2020. It was also financed by MCIN/AEI 10.13039/501100011033, Project RTI2018‐096051. We thank Ian Thomas for reviewing and correcting the English in the manuscript submitted for publication.
REFERENCES
- 1. Flanzy C, Vinificación por maceración carbónica, in Enología: Fundamentos científicos y tecnológicos, ed. by Madrid Vicente A. Mundi Prensa Libros SA, Madrid, Spain: (2003). [Google Scholar]
- 2. Tesniere C and Flanzy C, Carbonic maceration wines: characteristics and winemaking process, in Advances in Food Nutrition Research, Vol. 2011, ed. by Jackson RS. Academic Press, Elsevier, Burlington, NJ, USA, pp. 1–15 (2011). [DOI] [PubMed] [Google Scholar]
- 3. Etaio I, Pérez‐Elortondo FJ, Albisu M, Gaston E, Ojeda M and Schich P, Sensory attribute evolution in bottled young red wines from Rioja Alavesa. Eur Food Res Technol 228:695–705 (2009). [Google Scholar]
- 4. Etaio I, Meillon S, Perez‐Elortondo FJ and Schlich P, Dynamic sensory description of Rioja Alavesa red wines made by different winemaking practices by using temporal dominance of sensations. J Sci Food Agric 96:3492–3499 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Gómez‐Miguez M and Heredia FJ, Effect of the maceration technique on the relationships between anthocyanin composition and objective color of Syrah wines. J Agric Food Chem 52:5117–5123 (2004). [DOI] [PubMed] [Google Scholar]
- 6. Castillo‐Sánchez JJ, Mejuto JC, Garrido J and García‐Falcón S, Influence of wine‐making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhao wines. Food Chem 97:130–136 (2006). [Google Scholar]
- 7. Chinnici F, Sonni F, Natali N, Galassi S and Riponi C, Colour features and pigment composition of Italian carbonic macerated red wines. Food Chem 113:651–657 (2009). [Google Scholar]
- 8. Yang DY, Kakuda Y and Subden RE, Higher alcohols, diacetyl, acetoin and 2,3 butanediol biosynthesis in grapes undergoing carbonic maceration. Food Res Int 39:112–116 (2006). [Google Scholar]
- 9. Etaio I, Pérez‐Elortondo FJ, Albisu M, Gaston E, Ojeda M and Schich P, Effect of winemaking process and addition of white grapes on the sensory and physicochemical characteristics of young red wines. Aust J Grape Wine Res 14:211–222 (2008). [Google Scholar]
- 10. Pellegrini N, Simonetti P, Gardana C, Brenna O, Brighenti F and Pietta P, Polyphenol content and total antioxidant activity of vini novelli (young red wines). J Agric Food Chem 48:732–735 (2000). [DOI] [PubMed] [Google Scholar]
- 11. Olejar KJ, Fedrizzi B and Kilmartin PA, Antioxidant activity and phenolic profiles of Sauvignon Blanc wines made by various maceration techniques. Aust J Grape Wine Res 21:57–68 (2015). [Google Scholar]
- 12. Olejar KJ, Fedrizzi B and Kilmartin PA, Enhancement of chardonnay antioxidant activity and sensory perception through maceration technique. LWT–Food Sci Technol 65:152–157 (2016). [Google Scholar]
- 13. Etiévant P, Issanchou S, Maerie S, Ducruet V and Flanzy C, Sensory impact of volatile phenols on red wine aroma: influence of carbonic maceration and time storage. Sci Aliments 9:19–33 (1989). [Google Scholar]
- 14. Virdis C, Sumby K, Bartowsky E and Jiranek V, Lactic acid bacteria in wine: technological advances and evaluation of their functional role. Front Microbiol 11:1‐16 (2021). 10.3389/fmicb.2020.612118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santamaría P, González‐Arenzana L, Escribano‐Viana R, Garijo P, López R, Sanz S et al., Difficulties associated with small‐scale production of carbonic maceration wines. Fermentation 8:1‐9 (2022). 10.3390/fermentation8109927. [DOI] [Google Scholar]
- 16. Commission ECC, Regulation 2676/90 concerning the establishment of common analytical methods in the sector of wine. Off J Eur Commun 3:1–192 (1990). [Google Scholar]
- 17. Capozzi V, Garofalo C, Chiriatti MA, Grieco F and Spano G, Microbial terroir and food innovation: the case of yeast biodiversity in wine. Microbiol Res 181:75–83 (2015). [DOI] [PubMed] [Google Scholar]
- 18. Capece A and Romano P, Yeasts and their metabolic impact on wine flavour, in Yeasts in the Production of Wine. Springer Nature, New York, NY, U.S.A., pp. 43–80 (2019). [Google Scholar]
- 19. Waterhouse AL, Sacks GL and Jeffery DW, Understanding Wine Chemistry. John Wiley & Sons incorporated, New York, USA: (2016). [Google Scholar]
- 20. Amati A, Donati AM, Galassi S and Pallotta U, Esperimenti di vinificazione traite macerazione carbonica di uve Romagnole. Caracteristtiche chimiche e fisico‐chimiche di vini Sangiovese, Merlot et Cagnina ottenuti mediante macerazione carbonica. Sci Tecnol Degli Alimenti 3:357–361 (1973). [Google Scholar]
- 21. Chinnici F, Sonni F, Natali N, Galassi S and Riponi C, Colour features and pigment compòsition of Italian carbonic macerated red wines. Food Chem 113:651–657 (2009). [Google Scholar]
- 22. Wells A and Osborne JP, Impact of acetaldehyde and pyruvic acid bound sulphur dioxide on wine lactic bacteria. Lett Appl Microbiol 54:187–194 (2011). [DOI] [PubMed] [Google Scholar]
- 23. Office Internationale de la Vigne et du Vin (OIV) , International Code of Oenological Practices. Paris, France, p. 274 (2010). [Google Scholar]


