Abstract
Introduction
Entry inhibitors are a relatively new class of antiretroviral therapy and are typically indicated in heavily treatment experienced individuals living with HIV. Despite this, there is no formal definition of ‘heavily treatment experienced’. Interpretation of this term generally includes acknowledgement of multidrug resistance and reflects the fact that patients in need of further treatment options may have experienced multiple lines of therapy. However, it fails to recognize treatment limiting factors including contraindications, age‐associated comorbidities, and difficulty adhering to regimens.
Methods
This manuscript follows a roundtable discussion and aims to identify the unmet needs of those living with HIV who are in need of further treatment options, to broaden the definition of heavily treatment experienced and to clarify the use of newer agents, with an emphasis on the potential role of entry inhibitors, in this population.
Results/Conclusions
Within the entry inhibitor class, mechanisms of action differ between agents; resistance to one subclass does not confer resistance to others. Combinations of entry inhibitors should be considered in the same regimen, and if lack of response is seen to one entry inhibitor another can be tried. When selecting an entry inhibitor, physicians should account for patient preferences and needs as well as agent‐specific clinical characteristics. Absence of documented multidrug resistance should not exclude an individual from treatment with an entry inhibitor; entry inhibitors are a valuable treatment option for all individuals who are treatment limited or treatment exhausted. We should advocate for additional clinical trials that help define the role of entry inhibitors in people with exhausted/limited ART options other than drug resistance.
Keywords: entry inhibitor, heavily treatment experienced, multidrug resistance, treatment exhausted, treatment limited
INTRODUCTION
With over 37 million individuals living with HIV worldwide at the end of 2018, HIV infection remains an important clinical challenge. Increasing access to effective prevention and treatment, and ongoing improvements in antiretroviral therapy (ART) have led to reductions in both morbidity and mortality and mean that HIV has largely become a manageable chronic condition [1]. Since the discovery of HIV in 1983, over 30 drugs and their combinations have been approved for clinical use [2, 3]. Using these combinations, it is possible to suppress the virus for the majority of individuals living with HIV; however, a small proportion of individuals who are ‘heavily treatment experienced’ (HTE) reach a point where antiretroviral regimens are no longer suppressive. Despite common use of the term ‘HTE’, it has no universally accepted definition, making it difficult to determine the number of individuals living with HIV who are HTE. A retrospective cohort study of commercial and Medicare Advantage health plan enrollees in the USA between 2013 and 2019 found that 16.1% of 14 258 people living with HIV were HTE [4]. An ongoing cohort study of 22 000 Europeans living with HIV estimates that approximately 10% are HTE, with this figure rising from just 5.8% in 2010 to 8.9% in 2016 [5]. Conversely, a study of ART‐experienced individuals with HIV living in the USA found that the number with limited remaining treatment options declined from 5.2%–7.8% in 2000–2006 to <1% from 2012 through 2017 [6]. Clearer definition of HTE is needed to help physicians identify those in need.
In order to treat HTE individuals living with HIV, novel agents are needed. Three new agents have recently been approved in HTE individuals living with HIV: fostemsavir, a gp120‐directed attachment inhibitor (2020); ibalizumab, a CD4‐directed post‐attachment inhibitor (2018); and albuvirtide, a fusion inhibitor (2018) [7, 8, 9]. Ibalizumab and fostemavir are first‐in‐class agents, and all three fall in to the broader group of entry inhibitors, which prevent viral entry into host CD4+ cells. These drugs are associated with different side effect profiles and contraindications [7, 8, 10, 11].
In the past, individuals with documented multidrug resistance were recruited to trials of entry inhibitors [12, 13, 14, 15], aligning with traditional – yet informal – definitions of HTE. However, HTE is not the sole reason for the need for new agents. For example, being older with multiple age‐associated comorbidities and a need to consider drug–drug interactions or complex polypharmacy regimens could necessitate regimen simplification and therefore a desire for new ARTs. Although many of the factors associated with treatment exhaustion/limitation may be present in those who are HTE, they can also exist independently of multidrug resistance and duration of treatment experience, necessitating the coining of a new term to identify individuals who are not HTE but simply in need of new treatment options. This paper identifies and defines individuals living with HIV in need of alternative/novel treatment options; we also discuss the benefits and challenges of new antiretroviral therapies.
DIFFERENTIATING ENTRY INHIBITORS: AN OVERVIEW
Since 2003, several entry inhibitors have been approved for use in HTE individuals with HIV. It is important to note that, while they are all ‘entry inhibitors’, they do not all share a common mechanism of action and, as such, belong to several distinct classes. With several new entry inhibitors in development, it is important for treating physicians to understand the differences between agents so they can optimize treatment choices for individuals in need of further treatment options.
Available entry inhibitors target different aspects of the HIV entry process, reflecting the multistep nature of viral entry. The first step required for viral entry (Figure 1) entails binding of gp120 to the CD4 receptor [16]. This induces a conformational change, which exposes a co‐receptor binding site on Gp120 [17]. Gp120 co‐receptor binding exposes the N‐terminal portion of the gp41 subunit, also known as the fusion peptide, which then inserts into the host cell membrane [17]. Folding of the gp41 subunit brings the viral envelope and host‐cell membrane into close proximity, facilitating membrane fusion and allowing deposition of the viral core into the host cell [17].
FIGURE 1.
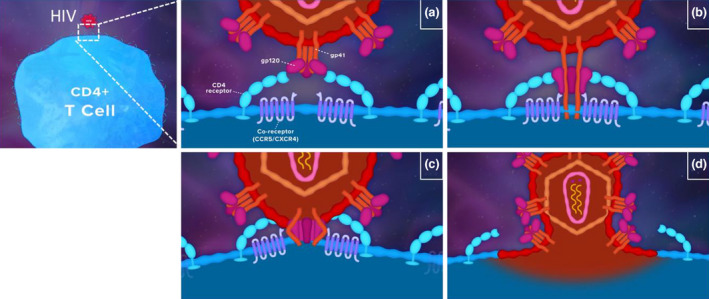
HIV entry process. (a) gp120–CD4 binding; (b) gp120–co‐receptor binding and exposure of the N‐terminal portion of the gp41 subunit; (c) gp41 subunit folding; (d) membrane fusion of the viral and host cells
Entry inhibitors can be broadly classified into four categories when using the viral entry process as a framework for classification (Figure 2). First, pre‐attachment inhibitors, such as fostemsavir, directly inhibit the gp120–CD4 interactions, preventing the first stage of viral attachment [11]. In addition, binding of temsavir to gp120 blocks the conformational rearrangements triggered by CD4 binding that result in eventual fusion of the virus to the cell [18]. Post‐attachment inhibitors, such as ibalizumab, bind to CD4 receptors away from the CD4–gp120 interaction. This induces conformational changes in the CD4–gp120 complex that ultimately prevent HIV fusion and entry [19, 20]. CCR5 antagonists, such as maraviroc and leronlimab, bind to the co‐receptor CCR5, thereby preventing gp120–co‐receptor attachment and complete viral docking of variants that use CCR5 [21]. Fusion inhibitors, such as enfuvirtide and albuvirtide, associate with the HR1 domain of gp41, preventing association of the HR1 and HR2 domains, which is usually needed to bring the viral and host cell membranes in to close proximity and facilitate membrane fusion [22, 23]. It is important that treating physicians are aware of the differing mechanisms of action of entry inhibitors, as this enables them to better identify which individual living with HIV would benefit most from which entry inhibitor.
FIGURE 2.
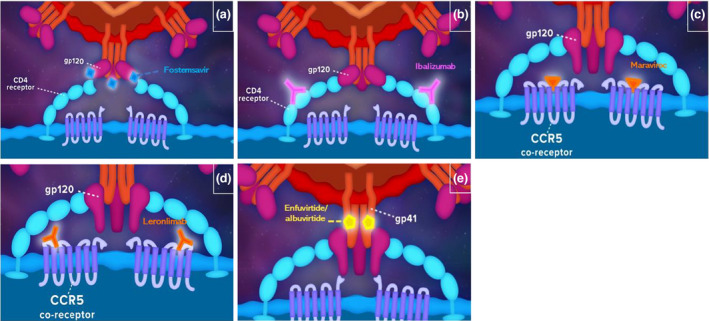
Entry inhibitors in HIV. Pre‐attachment inhibitor, fostemsavir (a), blocks interactions between the gp120 and CD4 receptors; post‐attachment inhibitor, ibalizumab (b), attaches to the CD‐4 receptor away from the gp120–CD4 interaction, inducing conformational changes that prevent the latter steps of viral entry; CCR5 antagonists maraviroc (c) and leronlimab (d) non‐competitively inhibit gp‐120–CCR5 interaction; fusion inhibitors such albuvirtide and enfuvirtide (e) bind gp41 and prevent insertion of gp41 to the viral membrane
A key benefit of differing mechanisms of action is that resistance to one class of entry inhibitor does not imply cross‐resistance to other classes of entry inhibitor. In vitro studies suggest that mutation in the HR1 domain of gp41 confers resistance to the fusion inhibitor enfuvirtide [24]; however, HR1 mutations have minimal impact on viral sensitivity to inhibitors targeting CCR5 or CXCR4 and indeed on fusion inhibitors other than enfuvirtide [25, 26]. The most common mechanism for escape from inhibition by CCR5 antagonists is emergence of virus capable of using CXCR4 as a co‐receptor. In addition, resistance to maraviroc in viruses that remain R5 can emerge through mutation in the V3 loops and C4 region of gp120 [27, 28], which allow CCR5–gp120 binding, even in the presence of maraviroc. In the case of these mutations, resistance to one CCR5 antagonist is likely to confer resistance to multiple CCR5 antagonists [29]; however, it is important to note that this is not always the case [30, 31] and that there is no evidence that this mutation should confer resistance to pre‐attachment, post‐attachment, or fusion inhibitors. Of note, it is important to establish the absence of X4 or dual tropic virus before initiating treatment because maraviroc treatment could unmask pre‐existing lineages of CXCR4 tropic virus [32]. Finally, resistance to ibalizumab is associated with loss of glycosylation sites at the N‐terminal V5 loop of gp120 [33, 34] again a different mechanism to that outlined earlier, and is noted as not conferring resistance to other classes of entry inhibitors.
A secondary advantage of the different mechanisms of action of entry inhibitors is the possibility that subclasses could be combined because they are not antagonistic and may be synergistic. Although clinical data on this point are lacking, in vitro evidence supports an additive effect of multiple entry inhibitors. For example, one study demonstrated the synergistic antiretroviral activity of enfuvirtide and ibalizumab across a range of laboratory and clinically derived HIV strains [35]. Similarly, enfuvirtide demonstrates an additive antiretroviral effect when combined with maraviroc in vitro [36] An ongoing phase II study of albuvirtide and the CD4‐neutralizing antibody 3BNC117 is expected to complete in 2022 [37]. Further studies are needed to determine the efficacy of combinations of different classes of entry inhibitors and to determine the clinical benefits that may be derived from these combinations.
Practical considerations: formulation, administration, adverse events and contraindications
Post‐marketing data suggest that the use of entry inhibitors has been limited because of reticence on the part of physicians and patients. Each entry inhibitor is associated with different adverse event profiles, administration methods, and contraindications (Table 1), which, as discussed, are instrumental to treatment selection in individuals with HIV.
TABLE 1.
Characteristics of approved entry inhibitors
| Drug | Entry inhibitor class | Formulation/administration method | Contraindications |
Adverse events & laboratory abnormalities (≥ grade 3) (≥5% noted in clinical trials) |
Some key drug–drug interactions |
|---|---|---|---|---|---|
| Fostemsavir [62] | Pre‐attachment inhibitor | 600 mg orally twice daily | No established data for use during pregnancy or breastfeeding, in paediatric populations or geriatric populations |
Nausea Creatinine phosphokinase U/L |
Coadministration contraindicated with enzalutamide, carbamazepine, phenytoin, rifampin, mitotane and St John's Wart Dose adjustment/alternative regimen with grazoprevir, voxilaprevir, ethinyl estradiol rosuvastatin, atorvastatin, fluvastatin, pitavastatin, and simvastatin |
| Ibalizumab [63] | Post‐attachment inhibitor |
2000 mg loading dose IV Followed by 800 mg IV Q2W |
No established data for use during pregnancy or breastfeeding, in paediatric populations or geriatric populations |
Diarrhoea, dizziness, nausea, rash Bilirubin (≥2.6 × ULN), creatinine (>1.8 × ULN or 1.5 × baseline), lipase (>3.0 × ULN), neutrophils (<0.6 109 cells/L) |
No studies conducted/required (no interaction with CYP3A or other enzymes involved in drug metabolism) |
| Maraviroc [45] | CCR5 antagonist | 150/300/600 mg orally twice daily |
Do not use in paediatric population or during breastfeeding Use only if clearly needed during pregnancy Use with caution in geriatric population and those with renal impairment Do not use if evidence of X4 or dual‐tropic virus |
Cough, pyrexia, upper respiratory tract infection, herpes infection, sinusitis, bronchitis, rash, musculoskeletal problems, joint‐related symptoms, abdominal pain, constipation, appetite disorders, dizziness, sleep disturbance Black box warning for hepatotoxicity Total bilirubin >5.0 × ULN, amylase >5.0 × ULN |
Reduced dose with CYP3A inhibitors, including protease inhibitors, ketoconazole, itraconazole and clarithromycin Increased dose with CYP3A inducers, including efavirenz, rifampin, carbamazepine, phenobarbital, and phenytoin |
| Enfuvirtide [64] | Fusion inhibitor | 90 mg SC twice daily |
Do not use in children under 6 years of age Use only if clearly needed during pregnancy No established data for use during breastfeeding or geriatric populations Hypersensitivity to enfuvirtide or any of its components |
Injection site reaction, diarrhoea, nausea, fatigue, asthenia, pyrexia, peripheral neuropathy, insomnia, headache, depression, decreased appetite, vomiting, dizziness, weight loss, flatulence, dermatitis, pruritus [13, 66], eosinophilia [13] | None reported |
Abbreviations: CYP, cytochrome P450; IV, intravenous; Q2W, every 2 weeks; SC, subcutaneous; ULN, upper limit of normal.
Post‐marketing data shows that >10% of patients discontinue enfuvirtide within 6 months of beginning treatment [38, 39]. In another study involving US veterans, 70% discontinued enfuvirtide within 2 years, with 42% of these discontinuations occurring at the patient's request and 18% attributed to toxicities, including injection site reactions [38]. Treatment‐related adverse events are common, with data suggesting that over 70% of patients experience injection site reactions or pain [38, 40]. Additionally, enfuvirtide injections must be administered twice daily, increasing the daily treatment burden for patients [10]. Injectable therapies may be more palatable if given less frequently. Surveys suggest that most patients would accept the need for injectable therapy in addition to oral therapy, with some reporting that ~90% of participants do not feel daily injections interfere with day‐to‐day life [39, 41]. To help combat this, it is recommended that the clinician educates the patient on proper injection technique before first use [10].
Post‐marketing surveillance suggests that virological failure on maraviroc affects between 12% and 42% of individuals, and up to 20% discontinue the drug within 1 year, although the reasons for discontinuation are not always clear [42, 43, 44]. Maraviroc must be variably dose‐adjusted when co‐administered with cytochrome P450 (CYP)‐3A4 inducers and inhibitors, which may lead to prescribing errors and reticence to use the drug [9, 45]. A further barrier to administration of maraviroc is the requirement for tropism testing prior to initiation to confirm that only CCR5‐tropic HIV‐1 is detectable [9].
Real‐world data for more recently approved entry inhibitors are lacking, and there could be barriers to a wide uptake of these drugs. Ibalizumab – formulated for intravenous infusion – must be administered by a trained medical professional [7]. Although adherence was relatively high (78%) during the TMB‐301 trial [14], the feasibility of bi‐weekly ibalizumab infusion in a real‐world setting is not yet known. Factors outside of patient or physician control, such as unstable housing, incarceration, or transportation barriers, may inhibit access to a suitable infusion environment. Similarly, visiting a clinic frequently may be undesirable for a number of reasons, including inconvenience, fear of HIV‐related stigma or, more recently, coronavirus disease 2019 (COVID‐19). On the other hand, it has been demonstrated that frequent attendance at clinic is associated with higher levels of adherence to oral medication [46] perhaps because of the increased contact between individuals living with HIV and healthcare practitioners, which may mean that necessitating clinical visits for infusion could improve overall adherence. Finally, cost may also be a barrier for some newer agents, although recent analyses have suggested the cost effectiveness and low budgetary impact of ibalizumab and fostemsavir use, despite high prices [47]. Where cost is prohibitive, enrollment in investigational trials or use of special access programmes may help failitate access to these medications.
REDEFINING ‘HEAVILY TREATMENT EXPERIENCED’: IS THERE A NEED?
Experience from clinical trials
Given the high level of need for new treatment options in HTE individuals, new agents typically target, and are subsequently approved in, this population. However, in the absence of a universally agreed definition of HTE, clinical trials of entry inhibitors have used a variety of definitions in recruiting these individuals to pivotal studies. In 2003, enfuvirtide became the first entry inhibitor approved for use in HIV based on results from the pivotal TORO trials. These trials recruited individuals living with HIV who had previously received at least 6 months of therapy with one or more nucleoside reverse transcriptase inhibitor, one or more non‐nucleoside reverse transcriptase inhibitor, and two or more protease inhibitors; documented resistance to drugs in these classes; or both. In addition, individuals had a plasma HIV‐1 RNA level of ≥5000 copies/ml [13]. In 2007 and 2018, respectively, the MOTIVATE trials of maraviroc and the TMB‐301 study of ibalizumab reported results in individuals with documented resistance to three or more classes [12, 14]. The MOTIVATE trials of maraviroc also enrolled individuals with ≥6 months of experience with one or more drug from three classes [12]. Although they used similar definitions for treatment experience, the cutoffs for viral load differed (Table 2). In July 2020, the FDA approved fostemsavir following the BRIGHTE trials, which recruited individuals living with HIV with treatment exhaustion (here defined as elimination of all agents in one class due to resistance, side effects, contraindications or, in the case of enfuvirtide, unwillingness) of four or more of six classes of antiretroviral drug resistance and HIV RNA viral load ≥400 copies per ml [15]. As the definition of HTE varied from trial to trial, direct comparisons of the efficacy and safety between entry inhibitors in this population are difficult.
TABLE 2.
Inclusion criteria for key clinical trials of entry inhibitors
| Enfuvirtide [13] | 2003 | TORO−1 | N = 501 |
>6 months therapy with ≥1 NRTI, ≥1 nNRTI, and ≥2 PI and/or documented resistance to these drugs HIV RNA count ≥5000 copies per ml with current regimen |
| Maraviroc [12] | 2007 | MOTIVATE−1 & MOTIVATE−2 | N = 1049 |
≥1 drug from 3 classes for ≥6 months, or documented ≥3‐class resistance HIV RNA count ≥5000 copies per ml with current regimen |
| Ibalizumab [14] | 2018 | TMB−301 | N = 40 |
>6 months therapy, ≥3‐class resistance and ≥1 active agent HIV RNA count ≥1000 copies per ml with current regimen |
| Fostemsavir [15] | 2020 | BRIGHTE | N = 371 |
Exhaustion (resistance/intolerance) of ≥4 of six classes of antiretroviral drug HIV RNA count ≥400 copies per ml with current regimen |
Abbreviations: nNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor.
Looking beyond multidrug resistance
Acquired resistance to ART increases with time on treatment and is relatively more common in developing countries [48]. The latter can be attributed to the relatively low number of treatment options leading to heavier resistance profiles and transmission of resistant virus. Additionally, non‐nucleoside reverse transcriptase inhibitor‐based regimens, which are more susceptible to resistance than boosted protease inhibitor regimens, are more commonly used in low‐income countries than in higher‐income countries [48]. However, there are multiple underlying causes of virological failure, and understanding these causes can help to identify at‐risk individuals.
Need to increase adherence
The development of multidrug resistance in HIV is strongly associated with poor or intermittent adherence to ART [49]. Factors commonly linked to poor adherence include lack of self‐efficacy (i.e. individual belief that they can maintain a regimen), poor outcome expectations (i.e. lack of perceived benefits), complex regimens, side effects, mental health and substance abuse disorders, and structural barriers such as high cost [50, 51, 52, 53, 54]. In many countries, stigma is commonly cited as a reason for poor adherence [55, 56] and can act as a deterrent for patients to attend clinics. Self‐stigma is also a strong predictor of non‐adherence [57]. Dosing and formulation also play a role: meta‐analyses have found that decreasing dosing schedules from multiple times daily to once daily is associated with higher levels of adherence in people living with chronic diseases [58], and self‐reported adherence to ART is higher in people taking single‐tablet regimens [59].
Need to consider older age and comorbidity
In the absence of at least some immunological recovery, continued proinflammatory status is associated with an increased risk of comorbidity development, including increased risk of cardiovascular disease, diabetes, and kidney dysfunction, among others. Individuals living with HIV are also at greater risk of many types of cancer, with the incidence of non‐AIDs‐related primary tumours increasing over time [60]. In addition, in many high‐income countries, many individuals living with HIV are aged >50 years and may have multiple HIV‐related and HIV‐unrelated comorbidities. The need for additional medications to treat these comorbidities increases the chances of drug interactions, often requiring the ART regiment to be altered or simplified. In addition, older adults appear to be at greater risk of ART‐associated toxicities, including nephrotoxicity, osteoporosis, and cardiovascular disease [61]. Consideration of advanced age and comorbidity is therefore paramount, as intolerable side effects may be increased in this population and are a key reason for non‐adherence and treatment discontinuation.
Need to consider pregnancy and childhood
Among entry inhibitors, fostemsavir and ibalizumab are contraindicated during pregnancy [62, 63], whereas enfuvirtide and maraviroc should only be used if clearly needed [45, 64]. Whether the definition of HTE should be expanded to include pregnant or breastfeeding women and children because of a lack of evidence for entry inhibitor use in these populations is less clear. Transparent discussions of the risks and benefits of the various options is vital.
In conclusion, ART options may become exhausted as a result of several underlying factors, not just ‘heavy treatment’ or multidrug resistance. Individuals may not be able to tolerate or adhere to conventional treatment regimens due to structural barriers, comorbidities, contraindications, mental health and substance misuse issues, health beliefs, or the presence of comorbidities or contraindications. People with HIV experiencing such barriers in the absence of multidrug resistance may be lacking viable treatment regimens. Similarly, people with a high level of documented resistance but complex, unsustainable regimens may benefit from new treatment options. As such these ‘treatment‐limited/exhausted’ individuals should be considered in a similar way as HTE individuals living with HIV. Incorporation of such individuals into clinical trials of future/potential agents is important to improve the outcomes of those living with HIV, and careful thought is needed in considering how best to study these drugs in those who are treatment limited/exhausted.
TREATMENTS AIMS AND OPTIMUM USE OF ENTRY INHIBITORS
In accordance with European AIDS Clinical Society and US Department of Health and Human Services guidelines for the management of patients with virological failure, the main aim of ART in HTE and treatment‐limited/exhausted individuals living with HIV should be to establish an antiretroviral regimen that includes two fully active drugs that can suppress viraemia to below 200 copies/ml (and if possible, below 50 copies/ml) and thus restore immune function [3, 65]. Ideally, at least one of these agents should have a high resistance barrier to prevent further treatment failure; if this is not possible, a three‐agent regimen should be initiated [3, 65]. When initiating a new antiretroviral regimen in HTE or treatment‐limited/exhausted individuals living with HIV, it is important to consider the reasons behind virological failure, which may be numerous and complex. As such, it is necessary to choose a regimen that minimizes toxicity in the patient and is sustainable in the long term with consideration for an individual's health, beliefs, ability (or lack thereof) to adhere to a complex regimen, and access to ARTs and medical care.
The absence of documented multidrug resistance should not exclude an individual from treatment with an entry inhibitor. Entry inhibitors should be considered as valuable treatment options in all individuals who are treatment limited or treatment exhausted. Highest priority should be given to producing a regimen that results in sufficient virological suppression, followed by considerations of toxicity, drug–drug interactions, and age. Contraindications, adverse event profile, and dosing method (Table 1) should guide the choice of entry inhibitor, with each class having potential advantages and disadvantages dependent on the individual's needs (Table 3).
TABLE 3.
Advantages, disadvantages and suggested uses of entry inhibitors in HIV
|
Pre‐attachment inhibitors (e.g. fostemsavir) |
Post‐attachment inhibitors (e.g. ibalizumab) |
CCR5 antagonists (e.g. maraviroc) |
Fusion inhibitors (e.g. enfuvirtide, albuvirtide) |
|
|---|---|---|---|---|
| Benefits and advantages |
Long‐acting agents in development Agents in development for PrEP |
Minimal toxicity and few off‐target effects Hypersensitivity and allergy are rare Drug–drug interactions not expected Infrequent dosing and essentially DOT |
Generally well tolerated, even in those with serious illness Hypersensitivity and hepatotoxicity are rare |
Potent and tolerable in the short term No known drug–drug interactions Albuvirtide is once weekly |
| Disadvantages and risks |
Less real‐world data available because of its more recent approval Fostemsavir is twice daily |
Less real‐world data available because of its more recent approval Need for hospital/nursing infrastructure for administration |
Twice‐daily dosing Risk of postural hypotension Dose adjustment needed with CYP3A inducers/antagonists; potential for under or overdosing Cannot be used with X4/dual tropic virus |
Enfuvirtide is twice daily Injection site reactions are common and limit long‐term adherence |
| Potential uses |
Limited current use; potential use in the future following more real‐world evidence and trials of new agents Efficacy in dual‐tropic virus |
Consider when tolerability or drug–drug interactions are key concern and cost is not a concern Efficacy for dual‐tropic virus Consider for patients who prefer injectable therapy or for whom frequent visits to healthcare practitioner may be beneficial Consider in a hospital setting when infusion therapy is needed Consider if adherence issues due to high pill burden |
Consider when tolerability is a key concern |
Consider as bridge therapy for short‐term viral suppression while developing suitable long‐term regimen [67] Consider in a hospital setting when injectable therapy is needed Consider if seeking injectable regimen Consider if adherence issues due to high pill burden |
Abbreviations: CYP, cytochrome P450; DOT, directly observed therapy; PrEP, pre‐exposure prophylaxis.
In addition to use with other types of ARTs, entry inhibitors could be considered for use in combination with each other. Synergistic activity of entry inhibitors has been demonstrated in vitro [35, 36] so a regimen using two entry inhibitor drugs could be a feasible option in some circumstances. Developing a regimen for an HTE or treatment‐limited person living with HIV can be complex and should be performed with help or advice from appropriate experienced specialists where possible [65]. Randomized trials to study the use of these drugs in combination should be encouraged.
CONCLUSION
Many individuals living with HIV are in need of further treatment options for reasons beyond multidrug resistance or extensive treatment experience. In addition to being of potential clinical benefit in HTE individuals, entry inhibitors may be viable treatment options for treatment‐exhausted or treatment‐limited individuals. The vast majority of participants enrolled into the trials discussed earlier had highly drug‐resistant virus and needed additional ‘standard’ drugs to fully suppress the virus. We should advocate for additional clinical trials that help define the role of entry inhibitors in people with exhausted/limited ART options other than drug resistance. Not all entry inhibitors share the same characteristics because of their differing mechanisms of action. Treating physicians should account for patient needs as well as drug characteristics when choosing a treatment. Contraindications, adverse event profile, and dosing method should guide the choice of entry inhibitor, with each class being suitable in different situations. Combinations of entry inhibitors should be considered where multidrug resistance occurs because of their different mechanisms of action.
CONFLICT OF INTEREST
Chloe Orkin has received lectureship fees, travel scholarships, and honoraria for contributions to advisory boards, and research grants to her institution, from MSD, Viiv, Gilead, and Janssen. Pedro Cahn has worked on advisory boards at ViiV and Merck and received research grants from ViiV and Richmond. Brinda Emu is a consultant for Genentech‐Roche and Theratechnologies and a site investigator for Viiv, Merck, Gilead, Taimed, and Cytodyn trials. Jonathan Schapiro has received research support, honorarium, or consulting fees from AbbVie, Merck, Gilead Sciences, GlaxoSmithKline, Tibotec‐Janssen, Teva, Virology Education, and ViiV Healthcare and travel support and stipends for advisory work for the World Health Organization. Daniel R Kuritzkes has received grants and/or research support (paid to his institution) from Gilead, Merck, and ViiV and consulting honoraria from Gilead, GSK, Janssen, Merck, and ViiV. Mark Nelson has received speaker fees, research fees, and consultancy fees from Gilead, MSD, Viiv, GSK, AbbVie, Janssen, Hetero, and Mylan. Antonella Castagna reports a conflict of interest with ViiV Healthcare, Gilead Sciences, MSD, and Theratechnologies. P Richard Harrigan has no conflicts of interest.
AUTHOR CONTRIBUTION
All authors were involved in the round table to discuss and review ideas for the manuscript. All authors were involved in the review and approval of the manuscript.
ACKNOWLEDGEMENTS
Editorial assistance was provided by Fay Pickering and Sophie Wilson of International Medical Press (London, UK). The roundtable that informed this manuscript was supported by an educational grant from Viiv Healthcare.
Orkin C, Cahn P, Castagna A, et al. Opening the door on entry inhibitors in HIV: Redefining the use of entry inhibitors in heavily treatment experienced and treatment‐limited individuals living with HIV. HIV Med. 2022;23:936–946. doi: 10.1111/hiv.13288
REFERENCES
- 1. World Health Organization . HIV/AIDS. 2021. https://www.who.int/news‐room/fact‐sheets/detail/hiv‐aids. Accessed January 7, 2022.
- 2. Venanzi Rullo E, Ceccarelli M, Condorelli F, et al. Investigational drugs in HIV: pros and cons of entry and fusion inhibitors. Mol Med Rep. 2019;19:1987‐1995. [DOI] [PubMed] [Google Scholar]
- 3. EACS Guidelines version 11.0, October 2021. https://www.eacsociety.org/guidelines/eacs‐guidelines/. Accessed January 7, 2022.
- 4. Priest J, Hulbert E, Gilliam BL, et al. Characterization of heavily treatment‐experienced people with HIV and impact on health care resource utilization in US commercial and medicare advantage health plans. Open Forum Infect Dis. 2021;8:ofab562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelchen‐Matthews A, Borges AH, Reekie J, et al. Prevalence and outcomes for heavily treatment‐experienced (HTE) individuals living with HIV in a European cohort. 10th IAS Conference on HIV Science (IAS 2019), July 21‐24, 2019, Mexico City. Abstract TUPEB222.
- 6. Bajema KL, Nance RM, Delaney JAC, et al. Substantial decline in heavily treated therapy‐experienced persons with HIV with limited antiretroviral treatment options. AIDS. 2020;34:2051‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicines Agency . Rukobia (fostemsavir). 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/rukobia. Accessed January 7, 2022.
- 8. European Medicines Agency . Trogarzo (ibalizumab). 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/trogarzo. Accessed January 7, 2022.
- 9. ClinicalInfoHIV.gov . Drug database: albuvirtide. 2020. https://clinicalinfo.hiv.gov/en/drugs/albuvirtide/patient. Accessed January 7, 2022.
- 10. European Medicines Agency . Celsentri (maraviroc). 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/celsentri. Accessed January 7, 2022.
- 11. European Medicines Agency . Fuzeon (enfuvirtide). 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/fuzeon. Accessed January 7, 2022.
- 12. Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV‐1 infection. N Engl J Med. 2008;359:1429‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lalezari JP, Henry K, O'Hearn M, et al. Enfuvirtide, an HIV‐1 fusion inhibitor, for drug‐resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175‐2185. [DOI] [PubMed] [Google Scholar]
- 14. Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug‐resistant HIV‐1. N Engl J Med. 2018;379:645‐654. [DOI] [PubMed] [Google Scholar]
- 15. Kozal M, Aberg J, Pialoux G, et al. Fostemsavir in adults with multidrug‐resistant HIV‐1 infection. N Engl J Med. 2020;382:1232‐1243. [DOI] [PubMed] [Google Scholar]
- 16. Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2012;2:a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alkhatib G. The biology of CCR5 and CXCR4. Curr Opin HIV AIDS. 2009;4:96‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Si Z, Madani N, Cox JM, et al. Small‐molecule inhibitors of HIV‐1 entry block receptor‐induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci USA. 2004;101:5036‐5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freeman MM, Seaman MS, Rits‐Volloch S, et al. Crystal structure of HIV‐1 primary receptor CD4 in complex with a potent antiviral antibody. Structure. 2010;18:1632‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4‐induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV‐1) and HIV‐1 infection of CD4+ cells. J Virol. 1992;66:4784‐4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kondru R, Zhang J, Ji C, et al. Molecular interactions of CCR5 with major classes of small‐molecule anti‐HIV CCR5 antagonists. Mol Pharmacol. 2008;73:789‐800. [DOI] [PubMed] [Google Scholar]
- 22. Chen RY, Kilby JM, Saag MS. Enfuvirtide. Expert Opin Investig Drugs. 2002;11:1837‐1843. [DOI] [PubMed] [Google Scholar]
- 23. Kapić E, Becić F, Zvizdić S. [Enfuvirtide, mechanism of action and pharmacological properties]. Med Arh. 2005;59:313‐316. [PubMed] [Google Scholar]
- 24. Rimsky LT, Shugars DC, Matthews TJ. Determinants of human immunodeficiency virus type 1 resistance to gp41‐derived inhibitory peptides. J Virol. 1998;72:986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reeves JD, Lee F‐H, Miamidian JL, Jabara CB, Juntilla MM, Doms RW. Enfuvirtide resistance mutations: impact on human immunodeficiency virus envelope function, entry inhibitor sensitivity, and virus neutralization. J Virol. 2005;79:4991‐4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ray N, Harrison JE, Blackburn LA, Martin JN, Deeks SG, Doms RW. Clinical resistance to enfuvirtide does not affect susceptibility of human immunodeficiency virus type 1 to other classes of entry inhibitors. J Virol. 2007;81:3240‐3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuhmann SE, Pugach P, Kunstman KJ, et al. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small‐molecule CCR5 inhibitor. J Virol. 2004;78:2790‐2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratcliff AN, Shi W, Arts EJ. HIV‐1 Resistance to maraviroc conferred by a CD4 binding site mutation in the envelope glycoprotein gp120. J Virol. 2013;87:923‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armand‐Ugón M, Moncunill G, Gonzalez E, et al. Different selection patterns of resistance and cross‐resistance to HIV‐1 agents targeting CCR5. J Antimicrob Chemother. 2010;65:417‐424. [DOI] [PubMed] [Google Scholar]
- 30. Pfaff JM, Wilen CB, Harrison JE, et al. HIV‐1 resistance to CCR5 antagonists associated with highly efficient use of CCR5 and altered tropism on primary CD4+ T Cells. J Virol. 2010;84:6505‐6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tilton JC, Wilen CB, Didigu CA, et al. A maraviroc‐resistant HIV‐1 with narrow cross‐resistance to other CCR5 antagonists depends on both N‐terminal and extracellular loop domains of drug‐bound CCR5. J Virol. 2010;84:10863‐10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lewis M, Mori J, Toma J, et al. Clonal analysis of HIV‐1 genotype and function associated with virologic failure in treatment‐experienced persons receiving maraviroc: results from the MOTIVATE phase 3 randomized, placebo‐controlled trials. PLoS One. 2018;13:e0204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pace CS, Fordyce MW, Franco D, Kao CY, Seaman MS, Ho DD. Anti‐CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV‐1, with natural resistance mediated by the loss of a V5 glycan in envelope. J Acquir Immune Defic Syndr. 2013;62:1‐9. [DOI] [PubMed] [Google Scholar]
- 34. Toma J, Weinheimer SP, Stawiski E, et al. Loss of asparagine‐linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J Virol. 2011;85:3872‐3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X‐Q, Sorensen M, Fung M, Schooley RT. Synergistic in vitro antiretroviral activity of a humanized monoclonal anti‐CD4 antibody (TNX‐355) and enfuvirtide (T‐20). Antimicrob Agents Chemother. 2006;50:2231‐2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK‐427,857), a potent, orally bioavailable, and selective small‐molecule inhibitor of chemokine receptor CCR5 with broad‐spectrum anti‐human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721‐4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov . Albuvirtide in combination with 3BNC117 in patients with multi‐drug resistant (MDR) HIV‐1 infection. 2021. https://clinicaltrials.gov/ct2/show/NCT04560569. Accessed January 7, 2022.
- 38. Belperio PS, Mole LA, Halloran J, Boothroyd DB, Thomas IC, Backus LI. Postmarketing use of enfuvirtide in veterans: provider compliance with criteria for use, overall efficacy, and tolerability. Ann Pharmacother. 2008;42:1573‐1580. [DOI] [PubMed] [Google Scholar]
- 39. Pulido F, Del Pozo MA, Fernández‐Guerrero M, et al. Patients’ perception and effectiveness of a treatment containing enfuvirtide when used in HIV‐infected patients without very advanced disease. HIV Clin Trials. 2008;9:83‐90. [DOI] [PubMed] [Google Scholar]
- 40. Huerta‐García G, Chavez‐García M, Mata‐Marín JA, et al. Effectiveness of enfuvirtide in a cohort of highly antiretroviral‐experienced HIV‐1‐infected patients in Mexico. AIDS Res Ther. 2014;11:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen C, Hellinger J, Johnson M, et al. Patient acceptance of self‐injected enfuvirtide at 8 and 24 weeks. HIV Clin Trials. 2003;4:347‐357. [DOI] [PubMed] [Google Scholar]
- 42. Macías J, Recio E, Márquez M, et al. Efficacy and safety of once‐daily maraviroc plus ritonavir‐boosted darunavir in pretreated HIV‐infected patients in a real‐life setting. HIV Med. 2014;15:417‐424. [DOI] [PubMed] [Google Scholar]
- 43. De Luca A, Pezzotti P, Boucher C, et al. Clinical use, efficacy, and durability of maraviroc for antiretroviral therapy in routine care: a European survey. PLoS One. 2019;14:e0225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Babiker ZOE, Douthwaite ST, Collier LE, Pennell A, Uriel AJ, Wilkins E. Real‐life outcomes of maraviroc‐based regimens in HIV‐1‐infected individuals. J Int Assoc Provid AIDS Care. 2012;12:12‐14. [DOI] [PubMed] [Google Scholar]
- 45. FDA . SELEZENTRY (maraviroc) prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022128Orig1s019,208984Orig1s002lbl.pdf. Accessed January 7, 2022.
- 46. Kunutsor S, Walley J, Katabira E, et al. Clinic attendance for medication refills and medication adherence amongst an antiretroviral treatment cohort in Uganda: a prospective study. AIDS Res Treat. 2010;2010:872396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brogan AJ, Talbird SE, Davis AE, La EM, Kumar PN. The cost‐effectiveness and budget impact of ibalizumab‐uiyk for adults with multidrug‐resistant HIV‐1 Infection in the United States. Pharmacoeconomics. 2021;39:421‐432. [DOI] [PubMed] [Google Scholar]
- 48. Pennings PS. HIV drug resistance: problems and perspectives. Infect Dis Rep. 2013;5(Suppl 1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rocheleau G, Brumme CJ, Shoveller J, Lima VD, Harrigan PR. Longitudinal trends of HIV drug resistance in a large Canadian cohort, 1996–2016. Clin Microbiol Infect. 2018;24:185‐191. [DOI] [PubMed] [Google Scholar]
- 50. Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004;16:471‐484. [DOI] [PubMed] [Google Scholar]
- 51. Chesney MA, Ickovics JR, Chambers DB, et al. Self‐reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG). AIDS Care. 2000;12:255‐266. [DOI] [PubMed] [Google Scholar]
- 52. Murphy DA, Belzer M, Durako SJ, et al. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 2005;159:764‐770. [DOI] [PubMed] [Google Scholar]
- 53. Murphy DA, Greenwell L, Hoffman D. Factors associated with antiretroviral adherence among HIV‐infected women with children. Women Health. 2002;36:97‐111. [DOI] [PubMed] [Google Scholar]
- 54. Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogeneous adherence to HIV‐antiretroviral therapies in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2001;26:82‐92. [DOI] [PubMed] [Google Scholar]
- 55. Okoronkwo I, Okeke U, Chinweuba A, Iheanacho P. Nonadherence factors and sociodemographic characteristics of HIV‐infected adults receiving antiretroviral therapy in Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria. ISRN AIDS. 2013;2013:843794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aye WL, Puckpinyo A, Peltzer K. Non‐adherence to anti‐retroviral therapy among HIV infected adults in Mon State of Myanmar. BMC Public Health. 2017;17:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mo PKH, Mak WWS. Intentionality of medication non‐adherence among individuals living with HIV/AIDS in Hong Kong. AIDS Care. 2009;21:785‐795. [DOI] [PubMed] [Google Scholar]
- 58. Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta‐analysis. Patient Prefer Adherence. 2013;7:419‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sterrantino G, Santoro L, Bartolozzi D, Trotta M, Zaccarelli M. Self‐reported adherence supports patient preference for the single tablet regimen (STR) in the current cART era. Patient Prefer Adherence. 2012;6:427‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hessol NA, Whittemore H, Vittinghoff E, et al. Incidence of first and second primary cancers diagnosed among people with HIV, 1985–2013: a population‐based, registry linkage study. Lancet HIV. 2018;5:e647‐e655. [DOI] [PubMed] [Google Scholar]
- 61. Jourjy J, Dahl K, Huesgen E. Antiretroviral treatment efficacy and safety in older HIV‐infected adults. Pharmacotherapy. 2015;35:1140‐1151. [DOI] [PubMed] [Google Scholar]
- 62. FDA . RUKOBIA (fostemsavir) prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212950s000lbl.pdf. Accessed January 7, 2022.
- 63. FDA . TROGARZO (ibalizumab) prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761065s011lbl.pdf. Accessed January 7, 2022.
- 64. FDA . FUZEON (enfuvirtide) for injection. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021481s033lbl.pdf. Accessed January 7, 2022.
- 65. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2021. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed January 7, 2022.
- 66. Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug‐resistant HIV‐1 in Europe and Australia. N Engl J Med. 2003;348:2186‐2195. [DOI] [PubMed] [Google Scholar]
- 67. Clotet B, Capetti A, Soto‐Ramirez LE, et al. A randomized, controlled study evaluating an induction treatment strategy in which enfuvirtide was added to an oral, highly active antiretroviral therapy regimen in treatment‐experienced patients: the INTENSE study. J Antimicrob Chemother. 2008;62:1374‐1378. [DOI] [PubMed] [Google Scholar]


