Abstract
TKI discontinuation proved to be safe and feasible in patients with CML with deep and durable molecular responses, introducing an additional treatment goal for these patients beyond overall survival. However, treatment interruption is a safe procedure only with appropriate patient selection and monitoring. Clinical and biological factors associated with better outcomes do not yet offer a precise stratification of patients according to their risk of relapse. This article aims at reviewing the leading studies present in the field in order to define eligibility criteria for discontinuation and predictors of success.
1. INTRODUCTION
The outcome of patients with chronic myeloid leukemia (CML) drastically improved with the advent of tyrosine kinase inhibitors (TKI). 1 When properly used, they result in optimal cytogenetic and molecular responses in most patients with CML and in a life expectancy similar to that of age‐matched individuals in the general population. 2 , 3 However, as for any lifelong pharmacological treatment, TKI may lead to chronic, mostly low‐grade adverse events (AEs) that can substantially impact patients' quality of life, adherence to therapy, and, consequently, treatment success. 4 , 5
In addition to a normal life expectancy, the possibility of discontinuing treatment after achieving a sustained deep molecular response has been added as a new goal for CML therapy. This condition, also known as Treatment‐Free Remission (TFR), produces several benefits for patients, such as a lower occurrence of drug‐related AEs, a cost reduction for patients and society, and a feeling of cure. Treatment discontinuation assumes particular importance in special conditions such as pregnancy. 6
Until 2016 data were still insufficient to recommend treatment discontinuation outside of well‐designed, prospective, controlled studies. Today TFR represents a new target of CML management, which should be discussed with patients according to the more recent international guidelines. 7 , 8
This article will review recent literature and ongoing trials evaluating TFR in patients with CML and will discuss some unanswered clinical and biological questions.
2. CLINICAL TRIALS ON TKI DISCONTINUATION
2.1. Imatinib discontinuation
After a promising pilot study by the French CML group in 12 patients, 9 the same group carried out STIM, the first prospective trial of TKI treatment discontinuation in 100 patients. At 12 months after the interruption of treatment, 41% were still molecularly negative. 10 The long‐term follow‐up of the trial (STIM1 study) showed a recurrence‐free survival of 38% at 5 years, 11 validating the preliminary hypothesis that most recurrences occurred in the first 6 months after treatment discontinuation, an observation confirmed in virtually all subsequent studies. 12 It is important to note that in these initial studies the eligibility criteria for interrupting treatment included the absence of detectable disease (with a sensitivity of real‐time quantitative polymerase chain reaction [RT‐qPCR] of at least 5 logs, MR5) sustained for 2 consecutive years, meaning at least five assessments below the threshold during those 2 years (every 6 months). Also, any confirmed positive PCR value was considered a relapse.
A few years later, an Australian trial (TWISTER study) produced very similar results: approximately 40% of patients on imatinib with sustained undetectable minimal residual disease (UMRD), confirmed in the central laboratory with the new standardized detection limit of ≤0.0032% (MR4.5), could stop treatment without loss of molecular response. 13 , 14
Subsequent studies considered a limit of <0.01% (MR4), maintained for at least 12–24 months, as a response level sufficient to consider discontinuation. 15 , 16
The definition of recurrence also changed from the reappearance of detectable transcripts to loss of major molecular response (MMR, or a transcript level <0.1%) since the observation that some patients may experience BCR‐ABL1 transcript fluctuations during the off‐therapy period without showing a progressive increase in transcript levels. Therefore, the A‐STIM study first proposed loss of MMR as a practical and safe criterion for therapy resumption, proving a TFR of 64% at 12 and 24 months and of 61% at 36 months, with 20% of patients failing to maintain a complete molecular response without losing MMR. 17
The same recurrence criterion was applied in other trials, obtaining similar findings: the ISAV study described a TFR rate of 48% at 36 months, 15 while the Korean KID trial reported a probability of sustained MMR at 24 months of 59%. 18 The table 1 summarizes the main studies on TKI discontinuation.
TABLE 1.
Summary of studies on TKI discontinuation cited in the paper
| Trial name | TKI ‐ line of treatment | Number of pts | Eligibility criteria | Definition of recurrence | TFR rate | Predictors of successful discontinuation |
|---|---|---|---|---|---|---|
| STIM/STIM1 10 , 11 (2010/2017) | Imatinib – first line | 100 |
Imatinib ≥3 years UMRD (MR5) ≥2 years |
Loss of UMRD |
41% at 12 months 38% at 60 months |
Male sex, low Sokal score, and long TKI duration |
| TWISTER 13 (2013) | Imatinib – first line | 40 |
Imatinib ≥3 years UMRD (MR4.5) ≥2 years |
Loss of MMR or confirmed loss of UMRD | 47% at 24 months | Long prior IFN duration and short time to achieve UMRD after switching from IFN to IM |
| A‐STIM 17 (2014) | Imatinib – first line | 80 |
Imatinib ≥3 years UMRD (MR5) ≥2 years |
Loss of MMR | 61% at 36 months | None |
| ISAV 15 (2015) | Imatinib – first line | 108 |
Imatinib ≥2 years MR4 ≥1.5 years |
Loss of MMR | 48% at 36 months | Old age and negativity of dPCR |
| KID 18 (2016) | Imatinib – first line | 90 |
Imatinib ≥3 years UMRD (MR5) ≥2 years |
Loss of MMR | 59% at 24 months | Long duration of imatinib, negativity of dPCR, and presence of withdrawal syndrome |
| RE‐STIM 19 (2017) | Imatinib, nilotinib, dasatinib ‐ first or second line | 70 | MR4.5 after a first unsuccessful TFR attempt | Loss of MMR | 35% at 36 months | Slow speed of molecular relapse after first TKI discontinuation attempt |
| EURO‐SKI 16 (2018) | Imatinib, nilotinib, dasatinib ‐ first or second line (for intolerance) | 758 |
TKI ≥3 years MR4 ≥1 year |
Loss of MMR | 51% at 24 months | Long TKI duration, long DMR duration, and interferon pretreatment |
| DESTINY 20 (2019) | Imatinib, nilotinib, dasatinib ‐ first or second line (for intolerance) |
125 MR4 49 MMR |
TKI ≥3 years MMR ≥1 year |
Loss of MMR |
72% at 36 months (MR4) 36% at 36 months (MMR) |
Long TKI duration and depth of MR |
| STOP 2G‐TKI 21 (2017) | Nilotinib or dasatinib ‐ first or second line | 60 |
2G‐TKI ≥3 years MR4.5 ≥2 years |
Loss of MMR | 54% at 48 months | Lack of TKI resistance to first line treatment |
| ENESTfreedom 22 (2017) | Nilotinib ‐ first line | 190 |
Nilotinib ≥2 years MR4.5 ≥1 year |
Loss of MMR | 49% at 96 weeks | Low Sokal score |
| ENESTop 23 (2018) | Nilotinib ‐ second line | 126 |
TKI ≥3 years MR4.5 ≥1 year |
Loss of MMR or confirmed loss of MR4 | 53% at 96 weeks | None |
| DADI 24 (2018) | Dasatinib ‐ second line | 63 | MR4 ≥1 year | Loss of MR4 | 44% at 36 months | Lack of imatinib resistance and high number of cytolytic NK cells |
| First line DADI 25 (2020) | Dasatinib ‐ first line | 58 |
Dasatinib ≥2 years MR4 ≥1 year |
Loss of MMR or confirmed loss of MR4 | 55% at 6 months | Low CD4‐cell count |
| DASFREE 26 (2020) | Dasatinib ‐ first or subsequent line | 84 |
Dasatinib ≥2 years MR4.5 ≥1 year |
Loss of MMR | 46% at 24 months | Long TKI duration, dasatinib as first line of therapy, and old age |
| LAST 27 (2020) | Imatinib, nilotinib, dasatinib, bosutinib ‐ first or subsequent line (for intolerance) | 172 |
TKI ≥3 years MR4 ≥2 years |
Loss of MMR | 61% at 48 months | Deep MR and negativity of dPCR |
2.2. Second‐generation TKI discontinuation
Second‐generation TKIs (2G‐TKI) induce a more rapid decline in BCR‐ABL1 transcript levels compared to imatinib in newly diagnosed patients and faster development of deep molecular responses (DMR). 28 , 29 , 30 , 31
EURO‐SKI was the largest prospective, non‐randomized trial, which enrolled 758 patients with CML at 61 European centers in 11 countries. After at least 3 years of TKI treatment (imatinib, nilotinib, or dasatinib) and 1 year of sustained DMR, patients could discontinue their treatment. Relapse‐free survival was 61% at 6 months and 50% at 24 months, with similar results in patients treated with imatinib or 2G‐TKI. 16 Another study that evaluated the safety and efficacy of stopping dasatinib or nilotinib, used in the first or subsequent line, was the multicenter observational STOP 2G‐TKI trial. Sixty patients, who discontinued treatment after 2 years of sustained MR4.5, maintained a TFR of 63% and 54% at 12 and 48 months, respectively, again without significant differences between the two drugs. 21
Several additional studies were subsequently published. ENESTfreedom evaluated 190 patients who were treated with nilotinib for more than 3 years and obtained a sustained DMR for at least 1 year. At 48 weeks after discontinuation, 52% of patients remained in remission 22 ; this value decreased to 49% at 96 weeks. 32 The first study to evaluate TFR specifically in patients who achieved sustained DMR only after switching from imatinib to nilotinib was the ENESTop trial: 58% and 53% of 126 patients maintained TFR at 48 and 96 weeks, respectively. 23 Other studies of TFR after second‐line nilotinib (ENESTgoal and ENESTpath) are still ongoing. 33 , 34 , 35
The Japanese DADI trial examined discontinuation of second‐line dasatinib treatment in patients with CML who maintained a DMR for >1 year. The estimated overall TFR rate at 36 months was 44%, but the loss of DMR was defined as molecular relapse, thereby triggering therapy resumption. 24 More recent studies investigated TFR duration also in first‐line dasatinib treated patients. 25 , 26 The DASFREE study showed an overall 2‐year TFR of 46%, 51% in first‐line and 42% in subsequent‐line patients, with no difference in patients who were either resistant or intolerant to first‐line therapy. 26
Discontinuation data on ponatinib and bosutinib are limited, partly due to their more recent FDA approval, and in part, because patients on ponatinib treatment are often resistant to several TKIs and have a less well‐controlled disease, which limits the possibility to reach discontinuation criteria. The Life After Stopping TKIs (LAST) study, a comprehensive evaluation of molecular recurrence and patient‐reported outcomes after discontinuation, reported a TFR rate of 60.8% at 48 months, but patients taking bosutinib were only 2.3%. 27 It is reasonable to conclude that the chance of TFR is similar regardless of the TKI used in patients who have achieved and maintained DMR for more than 2 years. The EURO‐SKI study already showed that the type of first‐line TKI therapy did not significantly affect molecular relapse‐free survival. 16 This fact is hardly surprising given the knowledge that persistent RT‐qPCR positivity is driven by quiescent stem cells refractory to BCR/ABL inhibition by any TKI. 36 , 37
2.3. Dose reduction
All the above‐mentioned studies used an abrupt interruption of TKI. However, some patients who fail to successfully obtain TFR might nevertheless maintain a good response on lower doses of TKI, improving AEs.
The reduction of dosages appears safe in the sustained remission phase, with only a transient increase in transcripts after dose reduction, which does not necessarily require a dose increase. 38 Mathematical modeling describes a biphasic treatment response to TKI, providing evidence that, after an initial phase of disease bulk reduction, TKI dose de‐escalation (at least 50%) does not decrease the anti‐leukemic effect on residual leukemic stem cells for most patients who have already reached DMR. 39
The De‐Escalation and Stopping Treatment of Imatinib, Nilotinib, or sprYcel (DESTINY) study, conducted in the United Kingdom, reported the results of stopping TKI treatment after initial de‐escalation to half the standard dose for 12 months, a strategy not previously investigated. At 2 years after stopping, 72% of patients were recurrence‐free. 20 It is possible that gradual TKI withdrawal might induce quiescent CML cells into a more proliferative state, thus increasing the sensitivity to TKI treatment before the subsequent interruption. 20 Nevertheless, the mechanism of the benefit is not yet clear, so further studies are needed to validate this observation. The DANTE study is currently investigating the use of nilotinib at half the standard dose (from 600 to 300 mg) before discontinuation in patients with sustained DMR. 40 Interim results suggest that loss of MMR during de‐escalation is rare. 41 The HALF study is another ongoing prospective multicenter phase II clinical trial evaluating the efficacy and safety of TKI discontinuation after two‐step dose reduction in patients with DMR. 42 In this setting, the risk of selecting resisting cells by a gradual dose reduction is probably minimal, compared to the initial treatment phase.
It is however important to note that the excellent results of DESTINY are very similar to two recent Italian and Spanish observational retrospective studies, which did not contemplate a dose reduction before discontinuation, probably reflecting our increased familiarity with TKI use and the increasing duration of treatment. 43 , 44
2.4. Second TFR attempt
Another open question concerns the chance of success of a second TFR attempt (TFR2) after discontinuation failure.
After a preliminary trial on 16 patients, 45 the French observational multicenter study RE‐STIM reported a TFR of 42% and 35% at 2 and 3 years respectively in 70 patients, who regained a stable remission of at least MR4.5 after a first unsuccessful attempt. These results were considerably better in patients who remained in DMR within the first 3 months after the first TKI discontinuation (TFR of 72% at 2 years), but it is still unclear why successful discontinuation could take place after the failure of the first attempt. 19 A possible explanation resides in the progressive exhaustion of quiescent CML stem cells. 46 This study demonstrated for the first time that an initial failure does not preclude a safe and potentially successful second TKI cessation attempt. Another Canadian study (TRAD) is currently ongoing to evaluate whether dasatinib treatment after a first TFR failure with imatinib would lead to successful discontinuation. Dasatinib was interrupted 12 months after achieving at least MR4: preliminary results showed a TFR2 rate of 21% at 6 months. More strict criteria should probably be considered for the TFR2 attempt, including a deep and long molecular response. 47
Studies on a second TFR attempt are also in progress in imatinib recipients that fail a TFR attempt and in whom treatment was switched to nilotinib (NILO post‐STIM – NCT01774630; NAUT ‐ NCT02917720).
3. REQUIREMENTS FOR TKI DISCONTINUATION
The studies mentioned so far are not comparable as each one used slightly different inclusion criteria and thresholds for restarting TKI. However, they all agree on the importance of TKI therapy duration and DMR depth and duration.
Current European Leukemia Net (ELN) guidelines 7 consider treatment discontinuation for patients in chronic phase CML, who have been on TKI therapy for 5 years or longer for imatinib or at least 4 years if a Second Generation‐TKI (2G‐TKI) such as nilotinib or dasatinib is used. This interval is slightly longer than the 3 years of TKI treatment suggested by NCCN. 8 Nevertheless, in a recent update on CML management published by the M. D. Anderson Cancer Center (MDACC), treatment discontinuation was offered in clinical practice only to patients who had a duration of TKI >6 years. 48
Regarding DMR, MR4 for 2 years is considered an appropriate level to consider discontinuation outside of clinical trials, though MR4.5 could represent the optimal one. 7 , 49 Further studies will be needed to define whether discontinuation can also be proposed to patients with less profound but stable responses.
Another requirement for TKI discontinuation for both American and European guidelines is no prior history of accelerated or blast phase CML: given the lack of data, the possibility of stopping therapy in this setting is unknown at present and is not recommended in clinical practice.
The ELN panel suggests TKI discontinuation should only be considered in patients in first‐line therapy or second‐line if intolerance was the only reason for changing TKI. However, this consideration is supported only by some studies, such as the DADI trial 24 or the STOP 2G‐TKI one, 21 while the aforementioned ENESTop and DASFREE study did not show differences between patients who were resistant or intolerant to first‐line therapy. 23 , 26
Recent guidelines do not include the Sokal risk score, while it is mentioned in the European Society for Medical Oncology (ESMO) guidelines, 50 Australian indications for clinical practice, 51 and the above‐cited MDACC update. 48 The Sokal score strongly influences the probability of achieving a sustained DMR rather than the risk of relapse after TKI discontinuation. 52 , 53
One survey reported that 19% of patients who meet discontinuation criteria are not interested in stopping TKI treatment. 54 In particular, patients with a good quality of life, not experiencing significant side effects from TKI, could be uncomfortable with the interruption of their life‐saving therapy. According to the results of the questionnaire proposed in the study, the leading cause for the reluctance of patients was the fear of possible negative consequences after discontinuation of therapy. For this reason, it is recommended to spend enough time with the patient and to provide clear and balanced information concerning the potential risks and benefits of a TFR attempt so that the patient could feel confident about his/her choice.
Finally, the French CML Study Group 55 and NCCN guidelines 8 recommend using these criteria only in the adult population. Successful TFR in a small number of pediatric patients was reported, 56 , 57 and it may be of particular interest in consideration of safety concerns in this setting. Data in the pediatric population are still limited, and the disease could have different characteristics and be more aggressive than in adults. 58 , 59 TKI discontinuation is not part of clinical practice for children at present. 60
4. RELAPSE AND MONITORING
Treatment interruption is a safe procedure only if performed in centers with access to high‐quality molecular monitoring, which includes the availability of RT‐qPCR and a rapid turnaround time for PCR results (within 1–2 weeks). Patients need to present typical e13a2 or e14a2 BCR‐ABL1 transcripts at diagnosis, otherwise, RT‐qPCR determination is not possible. 7 , 8 The use of even more sensitive and accurate methods, such as digital PCR (dPCR), could offer more reliable estimates of the number of residual leukemic cells, allowing a safer selection of patients for a TFR trial. 15 dPCR is not routinely available, and standardization across laboratories will be required before its use in clinical practice.
Patients need to have frequent monitoring after discontinuing treatment: guidelines recommend a monthly schedule for the first 6 months, every 2 months for months 6–12, and every 3–4 months thereafter for patients who remain in MMR. 7 , 8 Relapses usually develop within the first 6 months (80% of all recurrence events in the EURO‐SKI study) 16 and typically have fast proliferation kinetics with an exponential rise in BCR‐ABL1 levels of around 1‐log each month. 10 , 16 , 61 , 62 A recent study used a theoretical cohort of 100 patients (based on EURO‐SKI data) to predict the impact of reduced monitoring frequencies for patients attempting TFR. They indicated that a monitoring schedule every 2 months in the first 6 months and every 3 months between 6 and 12 months, may provide the best balance between reducing the number of tests and minimizing delays in relapse detection and TKI resumption, assuming an optimal turn‐around time for RT‐qPCR results. 63
In the event of an MMR loss, a prompt resumption of treatment (within 4 weeks) with the same drug at the same dose is advisable. Confirmation of MMR loss on a second evaluation is neither required nor prudent as it could delay treatment resumption, unless in case of a borderline result. 7 Patients should regain MMR within 3–4 months after the resumption of TKI, and subsequent monitoring is generally warranted every 4–6 months indefinitely, although present guidelines are stricter than routine clinical practice (monthly molecular monitoring until MMR is re‐established, then every 3–4 months thereafter). For patients who fail to obtain MMR 3–4 months after TKI restart, BCR‐ABL1 kinase domain mutation testing is suggested, and monthly molecular monitoring should be continued for another 6 months. 8
With a longer follow‐up, late relapses were reported, especially in studies that used MMR as the trigger for re‐treatment, such as the large cohort of EURO‐SKI, which still lacks a plateau in the RFS curve. The final results of the ISAV study also reported late relapses up to 46 months post discontinuation. 64 Late relapses present a more gradual rise in BCR‐ABL1 levels, that do not follow logarithmic kinetics, so less frequent monitoring appears safe, but still essential, for most patients who remain in TFR longer than 2 years. 65
Given the persistence of fluctuating levels of Ph+ cells even after several years of discontinuation and the low cost of an RT‐qPCR performed every 6–12 months, it is advisable to continue monitoring indefinitely, at least at present.
5. RISKS ASSOCIATED WITH TKI DISCONTINUATION
As reported above, a better quality of life is nowadays one of the main goals for patients with CML. 66 For most of them, TKI discontinuation correlates with clinically significant improvements in fatigue, diarrhea, depression, and sleep disturbances, as reported in many studies, such as the above‐cited ISAV trial 15 or the LAST study. 27 , 67 However, a non‐negligible number of patients, approximately 30%, develops a withdrawal syndrome (WS), characterized by newly occurring or worsening preexisting arthralgia and joint stiffness, typically occurring 1 month after stopping treatment and lasting for several months. 68 This side effect was first described in 2014 in the first cohort of patients of the EURO‐SKI trial discontinuing imatinib, 69 but a similar incidence was later reported after nilotinib and dasatinib, too. 22 , 26 Musculoskeletal pain is more common in patients with a prior history of osteoarticular symptoms and those who have been on TKI therapy for a long time. 70 The relation between treatment duration and muscular pain might explain the reported association between WSs and a higher probability of sustained MMR. 18
In most cases, WS is mild and self‐limited, with a median duration of 6 months and generally not requiring treatment. 18 In a recent Russian prospective study, the WS rate was higher (42%), probably because retrospective analysis might underreport the incidence of low‐grade symptoms. 71 Nevertheless, clinicians should inform patients about the possibility of this event and the rare necessity to assume medications, such as anti‐inflammatory drugs or corticosteroids.
Several mechanisms have been proposed to explain the emergence of WS, including the activation of kinase‐mediated pathways different from Abl, which were inhibited by the TKI, but none was experimentally validated up to now.
Another rare condition associated with treatment discontinuation is the occurrence of disease progression following a TFR attempt. The ENESTfreedom study reported the development of a nilotinib‐resistant kinase domain mutation at the time of molecular relapse. Given the absence of selective pressure during the discontinuation period, the resistant clone was likely already present, although this hypothesis could not be proved because of the low BCR‐ABL1 transcript levels in all samples collected before relapse. 22 A few case‐reports also described the occurrence of a sudden blast crisis following a TFR attempt: one case happened during dasatinib discontinuation, with the latest PCR performed 6 months before transformation; 72 the other three events developed in patients who experienced molecular relapse, resumed TKI, obtained a new response and then developed BC‐CML 6–8.5 months after restarting treatment. 17 , 73 , 74 This rare phenomenon was also reported in patients who remained on TKI therapy, 75 , 76 suggesting that it might occur even with continuous therapy. A new study (TFR‐PRO) aimed at estimating the risk of CML progression in patients eligible for treatment discontinuation, independently from their decision, is presently underway. 77
6. PREDICTORS OF SUCCESSFUL TKI DISCONTINUATION
Several studies tried to identify clinical and biological predictive factors linked to TFR achievement and to hypothesize an explanation for the different outcomes.
Age, sex, Sokal risk score, type of BCR‐ABL1 transcript, prion interferon‐alpha therapy, number and type of NK cells at the time of TKI discontinuation, use of dPCR to detect residual CML cells, duration of TKI treatment, duration of DMR and depth of response, have all been shown to predict the success rate of TFR in some but not all studies.
The table 1 summarizes the studies on TKI discontinuation previously cited in the paper, showing possible predictors of successful discontinuation identified by the authors, as explained below in the text.
6.1. Clinical factors
The total duration of TKI treatment before discontinuation is surely the most reproducible predictor of success. The STIM trial demonstrated that patients with a median period of imatinib therapy of at least 50 months had a higher sustained Complete Molecular Response (CMR) rate at 12 months than those with a shorter median duration of treatment. 10 Many studies later confirmed this observation, showing that a longer duration of imatinib therapy was an independent factor for sustained MMR after discontinuation. 16 , 18 , 20 , 78 Data were also validated for nilotinib and dasatinib (ENESTop, DASFREE). 23 , 26 In the large EURO‐SKI trial, however, when the duration of TKI treatment and depth of response before discontinuation were entered into the same multivariate model, only the duration of DMR predicted a longer MMR maintenance. 16 In this study, the probability of maintaining MMR at 6 months was 61% for patients in MR4 for >3.1 years versus 44% for those with a shorter MR4. However, many studies did not confirm this data 10 , 15 , 22 , 25 or showed only a modest effect of this parameter. 18 , 23 Thus, the duration of DMR remains less consistent in predicting successful discontinuation, although in clinical practice it remains one of the factors more frequently considered when proposing treatment discontinuation to a patient.
The depth of response is also strongly correlated to a better outcome: the DESTINY trial, already mentioned above, showed a TFR at 36 months of 72% versus 36% in the MR4 and MMR groups, respectively. 20 The ISAV study, an international multicenter trial on 112 patients, validated that patients with a positive dPCR at the time of discontinuation relapsed more frequently compared to the dPCR negative group. The association of dPCR (which has 1–2 logs of higher sensitivity [10−7] than RT‐qPCR) with TFR, later confirmed by a French study, 79 the Korean KID trial, 18 and the American LAST study, 27 suggests that the depth of response matters in terms of TFR prediction.
STIM and ENESTfreedom trials identified the Sokal score as a predictive parameter of TFR durability after imatinib suspension, 10 , 32 but the EURO‐SKI and a large meta‐analysis of TKI discontinuation studies concluded that the CML relapse rate was not influenced by this factor. 16 , 78
Simple demographic factors, such as age and sex, have been reported to be associated with TFR, 10 , 15 , 79 , 80 although not in the large cohort of the EURO‐ SKI study. 16 The ISAV trial showed an inverse relationship between patients' age and risk of relapse: 95% of patients less than 45 years of age relapsed compared to 42% among those aged ≥45 to <65 years and 32% in patients older than 65 years. 15 These data were later confirmed in the final analysis of the ISAV study 64 and in another English trial, which validated a predictive score to stratify patients according to their risk of relapse. Despite the small sample size, this score was able to identify three risk groups with significantly different probability of relapse, including predicting factors of successful discontinuation such as the age of the patient, the absence of a previous TKI resistance, and a longer duration of MR4. 80 Multivariate analyses of TFR predictive factors in the DASFREE study also revealed a statistically significant association between 2‐year TFR and age >65 years. 26 In addition, another study reported that younger age (<45 years) was significantly associated with relapse. 81
Finally, the TWISTER trial and the EURO‐SKI study reported a higher TFR rate in patients with long interferon (IFN) treatment before imatinib, but this data is less relevant nowadays. 13 , 16
For what pertains to a second discontinuation attempt, the RE‐STIM study showed that TFR probabilities after the second TKI withdrawal attempt were significantly higher in patients who were still in MMR at 3 months after the first discontinuation than in those who had already lost MMR. The speed of molecular relapse was the only factor significantly associated with the outcome in this case. 19
6.2. Molecular/biological factors
In addition to the clinical aspects, several biomarkers have been also associated with TFR.
Some studies indicated that patients harboring the e14a2 type of transcript had a lower incidence of relapse compared to those with e13a2 transcripts. In an Italian retrospective study, 61% of patients with the e14a2 transcript were still in TFR after 12 months, compared with 22% of those with the e13a2 transcripts. 82
Immunologic surveillance of residual leukemic stem cells is hypothesized to be one of the factors involved in maintaining molecular response. 83 Several studies have associated a higher percentage of immune effector cells in the peripheral blood of patients with durable TFR. In particular, the IMMUNOSTIM trial reported that a significantly higher number of natural killer (NK) cells were present at the time of discontinuation in not relapsing patients, suggesting that they may play a role in controlling relapse after imatinib discontinuation. 84 A substudy to EURO‐SKI more precisely described the different biological backgrounds of early (<6 months) and late relapses. Interestingly, non‐relapsing patients had a higher relative proportion of NK cells when compared to the early relapsing group, but not to patients relapsing after 6 months. Besides, detailed immunophenotyping was performed, and the ratio of CD56bright NK cells, regarded as immature NK cells versus the mature CD56dim NK cells, was inversely correlated with TFR. 85 The importance of NK cells in CML biology has also been suggested in second‐line dasatinib‐treated patients with CML who successfully discontinued TKI treatment 24 , 86 but not after nilotinib. 87 A recent Japanese paper also found that dasatinib and nilotinib evoked different immune responses after discontinuing TKI: the kinetics of regulatory T cells (Treg) observed by flow cytometry 1 month after stopping dasatinib significantly increased in the durable TFR group but not in patients that later relapsed. CD8+ T cells from the patients with continuous TFR showed a less exhausted phenotype and a higher proliferative activity compared with relapsed patients. These differences were not observed in a similar nilotinib discontinuation trial, but this fact might also be explained by the presence of Src inhibitory activity of dasatinib, not present with nilotinib. 88
Suppressor cell populations that have been directly linked with an increased risk of relapse include regulatory T cells 24 and CD86+ plasmacytoid dendritic cells. In a prospective analysis of 122 patients discontinuing their TKI within the EURO‐SKI trial, the one‐year relapse‐free survival was significantly higher for patients with a low number of CD86+ plasmacytoid dendritic cells, and only these patients benefited from longer TKI exposure before discontinuation. 89
All these results are still preliminary and of little use in clinical practice. Furthermore, it is quite surprising that such a small burden of residual disease could result in easily identifiable correlates in the peripheral blood of patients. It may also be possible that the observed differences are related to the different pharmacological effects of TKIs. In conclusion, formal recognition of CML cells by the immune system is still lacking. In addition, it is difficult to explain the success of a second discontinuation attempt when the immune system of the patient remains the same that failed to control CML cell growth after the first discontinuation.
Some studies showed that CD26, a marker of CML circulating stem cells, can be used to predict the success of TFR. Bocchia et al. found residual circulating CD26+ leukemic stem cells in 66% of patients with CML in prolonged and stable TFR, and a significant inverse linear correlation was found between the number of CD26+ cells in peripheral blood and TFR duration. Unexpectedly, the absolute number of CD26+ cells did not correlate with the molecular response of the patient, probably because RT‐qPCR accurately measures transcript level but cannot estimate the actual number of CML cells, which includes transcriptionally silent leukemic cells. 90 , 91 , 92 CD93 is another biomarker whose expression in circulating CML stem cells is significantly increased in patients with molecular relapse compared with those in sustained TFR. 93
An alternative explanation of the lack of leukemia cells re‐expansion after treatment interruption could reside in an intrinsic inability of CML cells to grow. The ISAV study estimated the presence of Philadelphia positive cells in 88% of patients discontinuing treatment, and their number increased by approximately 1 log during the 3 years of study observation, although none of them relapsed. 64 This fact indicates that almost all patients discontinuing treatment present residual CML cells, but residual cells are unable to regrow to levels >0.1% due to cell‐extrinsic or ‐intrinsic factors in about half of them.
It was recently reported that age‐related DNA damage response (DDR) is more evident in CD26+ leukemia stem cells compared to normal CD26 negative normal stem cells. A correlation was also found between DDR at CML diagnosis and the outcome of TKI discontinuation: patients in durable TFR were those with low levels of DDR at the onset of CML. 46
Combining these different theories makes it possible to formulate a hypothesis about whether CML cells will regrow after treatment discontinuation. Each CML cell has a probability of regrowth, so the greater the number of residual CML cells, the greater the probability of relapse. However, the functional status of CML cells also influences the outcome, so if the cells are controlled by an effective immune response or have a low intrinsic clonogenic capacity, as attested by a phenotype with little DDR, a favorable outcome will be likely. In any case, experimental studies on the biological factors that affect outcomes after discontinuation are of interest to better understand the causes and will pave the way for future clinical applications.
The figure 1 summarizes the possible clinical and biological predictors of successful TKI discontinuation according to the different studies cited in the paper.
FIGURE 1.
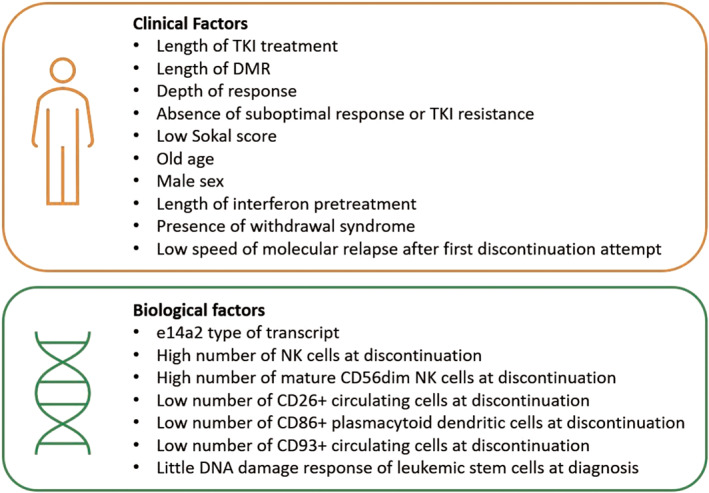
Summary of possible predictors of TKI discontinuation according to the different studies cited in the paper [Color figure can be viewed at wileyonlinelibrary.com]
7. TREATMENT‐FREE REMISSION AND PREGNANCY
A special condition in which TFR may become an important goal is pregnancy, in particular during the first trimester, when the off‐target activity of all TKI used for CML (and especially dasatinib) could trigger teratogenic effects. 94 , 95 Female patients of childbearing age, eligible for a TFR attempt, can safely discontinue TKI treatment before conception or as soon as pregnancy is discovered. 7 , 8 In case of loss of MMR, the probability of reaching pregnancy term without a clinical need for restarting treatment is low. The potential risk to the fetus versus the risk of maternal disease progression must be carefully evaluated on an individual basis, keeping in mind that dasatinib may frequently induce fetal abnormalities, 96 while imatinib and nilotinib have limited placental transfer, so their use can be considered in highly selected patients after organogenesis is concluded (15–16 weeks). 97 Since the use of TKI remains controversial, interferon can be used at any time during pregnancy and, if introduced early, can maintain molecular remission after TKI discontinuation. 98 , 99 Pregnancy must be wisely considered in patients with advanced disease, after multiple treatment lines, or just not meeting discontinuation criteria: in such a case TKI interruption could rapidly conduct to CML progression in these patients. For male patients, no association between TKI therapy and congenital abnormalities was found, so they do not need to discontinue treatment. 94 , 96
8. CONCLUSIONS
Evidence that TKI discontinuation is feasible in patients with CML with deep and durable molecular responses challenged the idea of a lifelong dependency on TKI, adding TFR as a treatment goal in addition to overall survival. Recommendations for accurate selection of patients to be chosen for a safe TFR attempt are accessible and widely used in clinical practice. However, it is important to underline that a safe TKI discontinuation is possible only where logistical aspects of CML care guarantee optimal, long‐term monitoring of the patient. Clinical and biological factors associated with better outcomes are still far from offering a precise stratification of patients according to their risk of relapse. One of the future directions of TFR research will be to understand which factors correlate with relapse and to personalize the prediction of outcomes. It is important to remember that the excellent results of patients who remain on lifelong TKI therapy lower the bar of ethically acceptable risk for additional treatments to control residual leukemic cells. Nevertheless, we cannot forget the importance of improving the quality of life of these patients. The still unanswered question of whether to consider patients in stable TFR as definitely cured of their disease remains open.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Milano‐Bicocca within the CRUI‐CARE Agreement.
Inzoli E, Aroldi A, Piazza R, Gambacorti‐Passerini C. Tyrosine Kinase Inhibitor discontinuation in Chronic Myeloid Leukemia: eligibility criteria and predictors of success. Am J Hematol. 2022;97(8):1075‐1085. doi: 10.1002/ajh.26556
Funding information AIRC IG‐2017 (#20112) to Carlo Gambacorti‐Passerini.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Gambacorti‐Passerini C. Part I: milestones in personalised medicine‐imatinib. Lancet Oncol. 2008;9(6):600. doi: 10.1016/S1470-2045(08)70152-9 [DOI] [PubMed] [Google Scholar]
- 2. Gambacorti‐Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553‐561. doi: 10.1093/jnci/djr060 [DOI] [PubMed] [Google Scholar]
- 3. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851‐2857. doi: 10.1200/JCO.2015.66.2866 [DOI] [PubMed] [Google Scholar]
- 4. Efficace F, Baccarani M, Breccia M, et al. Health‐related quality of life in chronic myeloid leukemia patients receiving long‐term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554‐4560. doi: 10.1182/blood-2011-04-347575 [DOI] [PubMed] [Google Scholar]
- 5. Phillips KM, Pinilla‐Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21(4):1097‐1103. doi: 10.1007/s00520-012-1630-5 [DOI] [PubMed] [Google Scholar]
- 6. Breccia M, Abruzzese E, Annunziata M, Luciano L, Sica S. Clinical and psychological factors to consider in achieving treatment‐free remission in patients with chronic myeloid leukemia. Front Oncol. 2021;11:1‐8. doi: 10.3389/fonc.2021.631570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966‐984. doi: 10.1038/s41375-020-0776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deininger MW, Shah NP, Altman JK, et al. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(10):1385‐1415. doi: 10.6004/jnccn.2020.0047 [DOI] [PubMed] [Google Scholar]
- 9. Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58‐60. doi: 10.1182/blood-2006-03-011239 [DOI] [PubMed] [Google Scholar]
- 10. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029‐1035. doi: 10.1016/S1470-2045(10)70233-3 [DOI] [PubMed] [Google Scholar]
- 11. Etienne G, Guilhot J, Rea D, et al. Long‐term follow‐up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298‐305. doi: 10.1200/JCO.2016.68.2914 [DOI] [PubMed] [Google Scholar]
- 12. Clark RE, Clark RE. Tyrosine kinase inhibitor therapy discontinuation for patients with chronic myeloid leukaemia in clinical practice. Curr Hematol Malig Rep. 2019;14:507‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515‐522. doi: 10.1182/blood-2013-02-483750 [DOI] [PubMed] [Google Scholar]
- 14. Ross DM, Pagani IS, Shanmuganathan N, et al. Long‐term treatment‐free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32(12):2572‐2579. doi: 10.1038/s41375-018-0264-0 [DOI] [PubMed] [Google Scholar]
- 15. Mori S, Vagge E, Le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910‐914. doi: 10.1002/ajh.24120 [DOI] [PubMed] [Google Scholar]
- 16. Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO‐SKI): a prespecified interim analysis of a prospective, multicentre, non‐randomised, trial. Lancet Oncol. 2018;19(6):747‐757. doi: 10.1016/S1470-2045(18)30192-X [DOI] [PubMed] [Google Scholar]
- 17. Rousselot P, Charbonnier A, Cony‐Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic‐phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424‐430. doi: 10.1200/JCO.2012.48.5797 [DOI] [PubMed] [Google Scholar]
- 18. Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101(6):717‐723. doi: 10.3324/haematol.2015.139899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Legros L, Nicolini FE, Etienne G, et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403‐4410. doi: 10.1002/cncr.30885 [DOI] [PubMed] [Google Scholar]
- 20. Clark RE, Polydoros F, Apperley JF, et al. De‐escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non‐randomised, phase 2 trial. Lancet Haematol. 2019;6(7):e375‐e383. doi: 10.1016/S2352-3026(19)30094-8 [DOI] [PubMed] [Google Scholar]
- 21. Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G‐TKI study. Blood. 2017;129(7):846‐854. doi: 10.1182/blood-2016-09-742205 [DOI] [PubMed] [Google Scholar]
- 22. Hochhaus A, Masszi T, Giles FJ, et al. Treatment‐free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525‐1531. doi: 10.1038/leu.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahon FX, Boquimpani C, Kim DW, et al. Treatment‐free remission after second‐line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase results from a single‐group, phase 2, open‐label study. Ann Intern Med. 2018;168(7):461‐470. doi: 10.7326/M17-1094 [DOI] [PubMed] [Google Scholar]
- 24. Okada M, Imagawa J, Tanaka H, et al. Final 3‐year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second‐line treatment. Clin Lymphoma Myeloma Leuk. 2018;18(5):353‐360.e1. doi: 10.1016/j.clml.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 25. Kimura S, Imagawa J, Murai K, et al. Treatment‐free remission after first‐line dasatinib discontinuation in patients with chronic myeloid leukaemia (first‐line DADI trial): a single‐arm, multicentre, phase 2 trial. Lancet Haematol. 2020;7(3):e218‐e225. doi: 10.1016/S2352-3026(19)30235-2 [DOI] [PubMed] [Google Scholar]
- 26. Shah NP, García‐Gutiérrez V, Jiménez‐Velasco A, et al. Dasatinib discontinuation in patients with chronic‐phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2020;61(3):650‐659. doi: 10.1080/10428194.2019.1675879 [DOI] [PubMed] [Google Scholar]
- 27. Atallah E, Schiffer CA, Radich JP, et al. Assessment of outcomes after stopping tyrosine kinase inhibitors among patients with chronic myeloid leukemia: a nonrandomized clinical trial. JAMA Oncol. 2021;7(1):42‐50. doi: 10.1001/jamaoncol.2020.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saglio G, Kim D‐W, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251‐2259. [DOI] [PubMed] [Google Scholar]
- 29. Hochhaus A, Saglio G, Hughes TP, et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044‐1054. doi: 10.1038/leu.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5‐year study results of DASISION: the dasatinib versus imatinib study in treatment‐Naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333‐2340. doi: 10.1200/JCO.2015.64.8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cortes JE, Gambacorti‐Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36(3):231‐237. doi: 10.1200/JCO.2017.74.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross DM, Masszi T, Gómez Casares MT, et al. Durable treatment‐free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96‐week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144(5):945‐954. doi: 10.1007/s00432-018-2604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ritchie EK, Catchatourian R, Klisovic RB, et al. Results from ENESTgoal: a phase 2 study of treatment‐free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML‐CP) who switched from imatinib to nilotinib. Blood. 2017;130(Supplement 1):2875. doi: 10.1182/blood.V130.Suppl_1.2875.2875 [DOI] [Google Scholar]
- 34. Rea D, Rosti G, Cross NC, et al. ENESTPath: a phase 3 study to assess the effect of nilotinib treatment duration on treatment‐free remission (TFR) in patients with chronic myeloid leukemia in chronic phase (CML‐CP) previously treated with imatinib: 24‐month analysis of the first 300 Pati. Blood. 2016;128(22):3094. doi: 10.1182/blood.v128.22.3094.3094 [DOI] [Google Scholar]
- 35. Rea D, Kyrcz‐krzemien S, Pungolino E, et al. Molecular response by 24 months of nilotinib 300 mg bid treatment in patients with chronic myeloid leukemia (CML) who failed to achieve dmr with imatinib: results from the ENESTPath study. Hemasphere. 2020;4(S1):328‐329. [Google Scholar]
- 36. Jørgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109(9):4016‐4019. doi: 10.1182/blood-2006-11-057521 [DOI] [PubMed] [Google Scholar]
- 37. Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR‐ABL activity. J Clin Invest. 2011;121(1):396‐409. doi: 10.1172/JCI35721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cervantes F, Correa JG, Pérez I, et al. Imatinib dose reduction in patients with chronic myeloid leukemia in sustained deep molecular response. Ann Hematol. 2017;96(1):81‐85. doi: 10.1007/s00277-016-2839-z [DOI] [PubMed] [Google Scholar]
- 39. Fassoni AC, Baldow C, Roeder I, Glauche I. Reduced tyrosine kinase inhibitor dose is predicted to be as effective as standard dose in chronic myeloid leukemia: a simulation study based on phase III trial data. Haematologica. 2018;103(11):1825‐1834. doi: 10.3324/haematol.2018.194522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iurlo A, Cattaneo D, Bucelli C, Breccia M. Dose optimization of tyrosine kinase inhibitors in chronic myeloid leukemia: a new therapeutic challenge. J Clin Med. 2021;10(3):515. doi: 10.3390/jcm10030515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breccia M, Abruzzese E, Stagno F, et al. First interim analysis of the Italian Dante Study: de‐escalation before treatment‐free remission in patients with chronic myeloid leukemia treated with first‐line nilotinib. Blood. 2021;138(Supplement 1):1474. [Google Scholar]
- 42. Žácková D, Faber E, Stejskal L, Karas M, Bělohlávková P. Half: a prospective multi‐centre phase II clinical trial evaluating the efficacy and safety of tyrosine kinase inhibitors' discontinuation after two‐step dose reduction in patients with chronic myeloid leukemia in deep molecular remission. Blood. 2021;138(Supplement 1):3606. [Google Scholar]
- 43. Fava C, Rege‐Cambrin G, Dogliotti I, et al. Observational study of chronic myeloid leukemia italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104(8):1589‐1596. doi: 10.3324/haematol.2018.205054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernández‐Boluda JC, Pereira A, Pastor‐Galán I, et al. Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8(10):91–98. doi: 10.1038/s41408-018-0125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Legros L, Rousselot P, Giraudier S, et al. Second attempt to discontinue imatinib in CP‐CML patients with a second sustained complete molecular response. Blood. 2012;120(9):1959‐1960. doi: 10.1182/blood-2012-02-408229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manfroni C, Arosio G, Mauri M, et al. Age‐related DNA damage response (DDR) in hematopoietic stem cells from chronic myeloid leukaemia patients who attempted TKI discontinuation. Haematologica. 2021;106(s2):83. [Google Scholar]
- 47. Dong Hwan Kim D, Busque L, Forrest DL. Second attempt of TKI discontinuation with dasatinib for treatment‐free remission after failing first attempt with imatinib: treatment‐free remission accomplished by dasatinib (TRAD) trial. Blood. 2018;132(Supplement 1):787. 10.1182/blood-2018-99-114656 [DOI] [PubMed] [Google Scholar]
- 48. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691‐709. doi: 10.1002/ajh.25792 [DOI] [PubMed] [Google Scholar]
- 49. Shah NP. NCCN guidelines updates: discontinuing TKI therapy in the treatment of chronic myeloid leukemia. J Natl Compr Canc Netw. 2019;17(55):611‐613. doi: 10.6004/jnccn.2019.5013 [DOI] [PubMed] [Google Scholar]
- 50. Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28(Supplement 4):iv41‐iv51. doi: 10.1093/annonc/mdx219 [DOI] [PubMed] [Google Scholar]
- 51. Hughes TP, Ross DM. Moving treatment‐free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17‐23. doi: 10.1182/blood-2016-01-694265 [DOI] [PubMed] [Google Scholar]
- 52. Forrest DL, Trainor S, Brinkman RR, et al. Cytogenetic and molecular responses to standard‐dose imatinib in chronic myeloid leukemia are correlated with Sokal risk scores and duration of therapy but not trough imatinib plasma levels. Leuk Res. 2009;33(2):271‐275. doi: 10.1016/j.leukres.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 53. Etienne G, Faberes C, Bauduer F, et al. Relevance of treatment‐free remission recommendations in chronic phase chronic leukemia patients treated with frontline tyrosine kinase inhibitors. Cancer Med. 2021;10(11):1. doi: 10.1002/cam4.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Villemagne Sanchez LA, O'Callaghan C, Gough K, et al. Patient perceptions of treatment‐free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59(2):406‐415. doi: 10.1080/10428194.2017.1337114 [DOI] [PubMed] [Google Scholar]
- 55. Rea D, Ame S, Berger M, et al. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: recommendations for clinical practice from the French Chronic Myeloid Leukemia Study Group. Cancer. 2018;124(14):2956‐2963. doi: 10.1002/cncr.31411 [DOI] [PubMed] [Google Scholar]
- 56. Giona F, Saglio G, Moleti ML, et al. Treatment‐free remission after imatinib discontinuation is possible in paediatric patients with chronic myeloid leukaemia. Br J Haematol. 2015;168(2):305‐308. doi: 10.1111/bjh.13103 [DOI] [PubMed] [Google Scholar]
- 57. Millot F, Claviez A, Leverger G, Corbaciglu S, Groll AH, Suttorp M. Imatinib cessation in children and adolescents with chronic myeloid leukemia in chronic phase. Pediatr Blood Cancer. 2014;61:355‐357. doi: 10.1002/pbc [DOI] [PubMed] [Google Scholar]
- 58. Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127(4):392‐399. doi: 10.1182/blood-2015-06-648667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castagnetti F, Gugliotta G, Baccarani M, et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol. 2015;26(1):185‐192. doi: 10.1093/annonc/mdu490 [DOI] [PubMed] [Google Scholar]
- 60. Cortes J, Rea D, Lipton JH. Treatment‐free remission with first‐ and second‐generation tyrosine kinase inhibitors. Am J Hematol. 2019;94(94):346‐357. doi: 10.1002/ajh.25342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435(7046):1267‐1270. doi: 10.1038/nature03669 [DOI] [PubMed] [Google Scholar]
- 62. Branford S, Yeung DT, Prime JA, et al. BCR‐ABL1 doubling times more reliably assess the dynamics of CML relapse compared with the BCR‐ABL1 fold rise: implications for monitoring and management. Blood. 2012;119(18):4264‐4271. doi: 10.1182/blood-2011-11-393041 [DOI] [PubMed] [Google Scholar]
- 63. Shanmuganathan N, Braley JA, Yong ASM, et al. Modeling the safe minimum frequency of molecular monitoring for CML patients attempting treatment‐free remission. Blood. 2019;134(1):85‐89. doi: 10.1182/blood.2019000120 [DOI] [PubMed] [Google Scholar]
- 64. Diral E, Mori S, Antolini L, et al. Increased tumor burden in patients with chronic myeloid leukemia after 36 months of imatinib discontinuation. Blood. 2020;136(19):2237‐2240. doi: 10.1182/BLOOD.2019004371 [DOI] [PubMed] [Google Scholar]
- 65. Ross DM, Hughes TP. Treatment‐free remission in patients with chronic myeloid leukaemia. Nat Rev Clin Oncol. 2020;17(8):493‐503. doi: 10.1038/s41571-020-0367-1 [DOI] [PubMed] [Google Scholar]
- 66. Flynn KE, Atallah E. Quality of life and long term therapy in patients with chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):80‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Flynn KE, Weinfurt KP, Lin L, et al. Patient‐reported outcome results from the U.S. Life After Stopping TKIs (LAST) study in patients with chronic myeloid leukemia. Blood. 2019;134(Supplement_1):705. doi: 10.1182/blood-2019-126002 [DOI] [Google Scholar]
- 68. Diab M, Schiffer CA. The spectrum of musculoskeletal symptoms in patients with chronic myeloid leukemia after stopping tyrosine kinase inhibitors. Leuk Res. 2019;79:1‐2. doi: 10.1016/j.leukres.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 69. Richter J, Söderlund S, Lübking A, et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32(25):2821‐2823. doi: 10.1200/JCO.2014.55.6910 [DOI] [PubMed] [Google Scholar]
- 70. Berger MG, Pereira B, Rousselot P, et al. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. Br J Haematol. 2019;187(3):337‐346. doi: 10.1111/bjh.16083 [DOI] [PubMed] [Google Scholar]
- 71. Petrova A, Chelysheva E, Shukhov O, et al. Withdrawal syndrome after tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia in the Russian prospective study RU‐SKI. Clin Lymphoma Myeloma Leuk. 2020;20(5):267‐271. doi: 10.1016/j.clml.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 72. Alfayez M, Richard‐Carpentier G, Jabbour E, et al. Sudden blastic transformation in treatment‐free remission chronic myeloid leukaemia. Br J Haematol. 2019;187(4):543‐545. doi: 10.1111/bjh.16245 [DOI] [PubMed] [Google Scholar]
- 73. Papalexandri A, Saloum R, Touloumenidou T, et al. Blast crisis of CML after TKI discontinuation in a patient with previous stable deep molecular response: is it safe to stop? HemaSphere. 2018;2(6):7‐8. doi: 10.1097/HS9.0000000000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rea D, Nicolini FE, Tulliez M, et al. Prognostication of molecular relapses after dasatinib or nilotinib discontinuation in chronic myeloid leukemia (CML): a FI‐LMC STOP 2G‐TKI study update. Blood. 2019;134(Supplement_1):30. doi: 10.1182/blood-2019-124408 31023703 [DOI] [Google Scholar]
- 75. Jabbour E, Kantarjian H, O'Brien S, et al. Sudden blastic transformation in patients with chronic myeloid leukemia treated with imatinib mesylate. Blood. 2006;107(2):480‐482. doi: 10.1182/blood-2005-05-1816 [DOI] [PubMed] [Google Scholar]
- 76. Tantiworawit A, Power MM, Barnett MJ, et al. Long‐term follow‐up of patients with chronic myeloid leukemia in chronic phase developing sudden blast phase on imatinib therapy. Leuk Lymphoma. 2012;53(7):1321‐1326. doi: 10.3109/10428194.2011.652108 [DOI] [PubMed] [Google Scholar]
- 77. Assouline S, Miggiano MC, Abruzzese E, et al. Risk of progression in chronic phase ‐ chronic myeloid leukemia (CML) patients eligible for tyrosine kinase inhibitor discontinuation (TFR‐PRO study): preliminary results. Blood. 2021;138(Supplement 1):1476. [DOI] [PubMed] [Google Scholar]
- 78. Campiotti L, Suter MB, Guasti L, et al. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR‐ABL transcript level: a systematic review and a meta‐analysis. Eur J Cancer. 2017;77:48‐56. doi: 10.1016/j.ejca.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 79. Nicolini FE, Dulucq S, Boureau L, et al. Evaluation of residual disease and TKI duration are critical predictive factors for molecular recurrence after stopping Imatinib first‐line in chronic phase CML patients. Clin Cancer Res. 2019;25(22):6606‐6613. doi: 10.1158/1078-0432.CCR-18-3373 [DOI] [PubMed] [Google Scholar]
- 80. Claudiani S, Metelli S, Kamvar R, et al. Introducing a predictive score for successful treatment free remission in chronic myeloid leukemia (CML). Blood. 2019;134(Supplement_1):26. doi: 10.1182/blood-2019-131500 [DOI] [Google Scholar]
- 81. Caocci G, Martino B, Greco M, et al. Killer immunoglobulin‐like receptors can predict TKI treatment‐free remission in chronic myeloid leukemia patients. Exp Hematol. 2015;43(12):1015‐1018.e1. doi: 10.1016/j.exphem.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 82. D'Adda M, Farina M, Schieppati F, et al. The e13a2 BCR‐ABL transcript negatively affects sustained deep molecular response and the achievement of treatment‐free remission in patients with chronic myeloid leukemia who receive tyrosine kinase inhibitors. Cancer. 2019;125(10):1674‐1682. doi: 10.1002/cncr.31977 [DOI] [PubMed] [Google Scholar]
- 83. Hsieh Y, Kirschner K, Copland M. Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape. Leukemia. 2021;35:1229‐1242. doi: 10.1038/s41375-021-01238-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rea D, Henry G, Khaznadar Z, et al. Natural killer‐cell counts are associated with molecular relapse‐free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. 2017;102(8):1368‐1377. doi: 10.3324/haematol.2017.165001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ilander M, Olsson‐Strömberg U, Schlums H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31(5):1108‐1116. doi: 10.1038/leu.2016.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kumagai T, Nakaseko C, Nishiwaki K, et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018;109(1):182‐192. doi: 10.1111/cas.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takahashi N, Nishiwaki K, Nakaseko C, et al. Treatment‐free remission after two‐year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica. 2018;103(11):1835‐1842. doi: 10.3324/haematol.2018.194894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fujioka Y, Takahashi N, Ohtake S, Atsuta Y. Nilotinib and dasatinib evoked distinct immune effects during Tfr phase of each discontinuation trial. Blood. 2021;138(Supplement 1):3580. [Google Scholar]
- 89. Schütz C, Inselmann S, Sausslele S, et al. Expression of the CTLA‐4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia. 2017;31(4):829‐836. doi: 10.1038/leu.2017.9 [DOI] [PubMed] [Google Scholar]
- 90. Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118(20):5565‐5572. doi: 10.1182/blood-2010-12-327437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bocchia M, Sicuranza A, Abruzzese E, et al. Residual peripheral blood CD26+ leukemic stem cells in chronic myeloid leukemia patients during TKI therapy and during treatment‐free remission. Front Oncol. 2018;8:1‐8. doi: 10.3389/fonc.2018.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Raspadori D, Pacelli P, Sicuranza A, et al. Flow cytometry assessment of CD26+ leukemic stem cells in peripheral blood: a simple and rapid new diagnostic tool for chronic myeloid leukemia. Cytom Part B ‐ Clin Cytom. 2019;96(4):294‐299. doi: 10.1002/cyto.b.21764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kinstrie R, Horne GA, Morrison H, et al. CD93 is expressed on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Leukemia. 2020;34(6):1613‐1625. doi: 10.1038/s41375-019-0684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111(12):5505‐5508. doi: 10.3855/jidc.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Berman E, Druker BJ, Burwick R. Chronic myelogenous leukemia: pregnancy in the era of stopping tyrosine kinase inhibitor therapy. J Clin Oncol. 2018;36(12):1250‐1256. doi: 10.1200/JCO.2017.77.2574 [DOI] [PubMed] [Google Scholar]
- 96. Cortes JE, Abruzzese E, Chelysheva E, Guha M, Wallis N, Apperley JF. The impact of dasatinib on pregnancy outcomes. Am J Hematol. 2015;90(12):1111‐1115. doi: 10.1002/ajh.24186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chelysheva E, Turkina A, Polushkina E, et al. Placental transfer of tyrosine kinase inhibitors used for chronic myeloid leukemia treatment. Leuk Lymphoma. 2017;59(3):733‐738. doi: 10.1080/10428194.2017.1347929 [DOI] [PubMed] [Google Scholar]
- 98. Burchert A, Müller MC, Kostrewa P, et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010;28(8):1429‐1435. doi: 10.1200/JCO.2009.25.5075 [DOI] [PubMed] [Google Scholar]
- 99. Balsat M, Etienne M, Elhamri M, Hayette S, Salles G, Thomas X. Successful pregnancies in patients with BCR‐ABL‐positive leukemias treated with interferon‐alpha therapy during the tyrosine kinase inhibitors era. Eur J Haematol. 2018;101(6):774‐780. doi: 10.1111/ejh.13167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


