Abstract
The B cell compartment provides innate and adaptive immune defenses against pathogens. Different B cell subsets, reflecting the maturation stages of B cells, have noninterchangeable functions and roles in innate and adaptive immune responses. In this review, we provide an overview of the B cell subsets present in peripheral blood of healthy individuals. A specific gating strategy is also described to clearly and univocally identify B cell subsets based on the their phenotypic traits by flow cytometric analysis.
Keywords: B cell subsets, flow cytometry, human B cells, phenotyping
1. B CELLS IDENTITY CARD
B cells have the function of producing antibodies for protection against infectious diseases. Protective antibodies are highly specific for the antigen that they recognize and are produced at the end of a complex differentiation pathway that requires weeks. Whereas the early phases of the development of the B lineage occur in the bone marrow, the several types of B cells detected in the peripheral blood represent the different steps of maturation mostly triggered by antigenic experience [1].
The B cell function was discovered in 1960 by Max Cooper who demonstrated that the production of antibody was completely depleted in irradiated chickens after surgical removal of the primary site of B‐cell development in birds, named the Bursa of Fabricius [2], hence the name B cells.
The B‐cell development occurs in steps, that are tightly controlled by the expression and function of the B‐cell receptor (BCR). In the bone marrow, the B‐lineage includes phenotypically distinct cell types in their different developmental stages. The success of the stepwise rearrangement of the Heavy and Light Chain is indispensable for the progression along the early phases of the developmental pathway and for the exit to the periphery. B cells emigrate from the bone marrow as transitional B cells when they express a functional BCR, composed of membrane‐bound antibody, capable of recognizing the antigen, associated to the B‐cell signaling module represented by the Igα‐Igβ heterodimer [3, 4]. Transitional B cells expressing IgM and IgD on the cell surface represent an intermediate stages between immature and mature‐naïve circulating B‐cells and can be found both in the bone marrow and in the peripheral blood [5, 6] (Figure 1).
FIGURE 1.
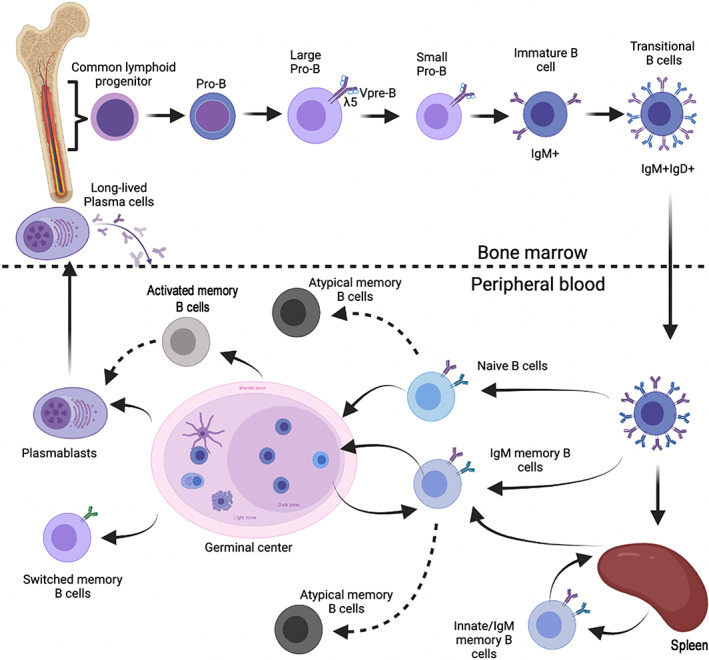
Schematic representation of B cell development in the bone‐marrow and in the peripheral blood. The top part of the figure illustrates the step‐wise development of the cells of the B lineage in the bone marrow. Transitional B cells are generated in the bone marrow, but can be also found in the peripheral blood (lower part of the figure). In the bone marrow B‐cell development is driven and controlled by the rearrangement of immunoglobulin heavy and light chain. In the periphery B‐cell antigen and TLR signals determine the progression to the memory B cells and plasma cells stage. Long‐lived memory plasma cells represent the final stage of B‐cell development and home to a dedicated niche in the bone marrow [Color figure can be viewed at wileyonlinelibrary.com]
Thanks to the phenomenon of allelic exclusion, immunoglobulin rearrangement results in the expression of a unique receptor by each B cell. Thus, every B cell has its own specificity, and the high number of possible rearrangements (>1012) provides humans with a potentially infinite repertoire capable of recognizing any encountered antigen [7].
In the periphery B cells continue their development in order to fulfill their ultimate function: protection through the production of specific antibodies. Transitional B cells are short‐lived and rapidly differentiate into mature‐naïve B cells [8], that represent a major population in the peripheral blood and populate the primary lymphoid follicles in lymph nodes and spleen. Mature‐naïve B cells continuously recirculate with the lymph and blood scrutinizing the environment in search of antigen. If they encounter the antigen that their BCR recognizes, the next phase of development initiates in the germinal centers. Activated mature‐naïve B cells proliferate, introduce mutation in their immunoglobulin genes and are selected for their affinity to the antigen [9]. Only high‐affinity B cells become either memory B cells or plasma cells [10, 11] (Figure 1). An alternative T‐ and germinal center‐independent pathway leads to the generation of innate/IgM memory B cells in the spleen (Aranburu et al.[12] and see below).
The different types of B cells in peripheral blood can be distinguished by the combined expression of few clusters of differentiation (CD) markers.
In the adult peripheral blood, B cells account for ~10% of the total lymphocytes. The identification of the different B‐cell type has been addressed by several research groups during the last years. The usage of different markers and classifications has led to a controversial terminology. We propose a simple nine‐color staining (Table 1) to define B‐cell populations with different functions in healthy donors. Among circulating CD19pos B‐cells we can identify six major populations: transitional, mature‐naïve, memory, atypical memory, activated B cells, and plasmablasts (Figure 2 and Tables 1 and 2).
TABLE 1.
Cluster of differentiation expressed on the different B‐cell populations
| Transitional | Mature‐Naïve | Memory | Plasmablasts | Atypical memory | Activated memory | |
|---|---|---|---|---|---|---|
| CD45 | ++ | ++ | ++ | ++ | ++ | ++ |
| CD19 | ++ | ++ | ++ | ++ | +++ | ++ |
| CD24 | +++ | ++ | +++ | − | −/+ | + and ++ |
| CD21 | + and ++ | ++ | ++ | + | − | − |
| CD27 | − | − | + and +++ | +++ | − | ++ |
| CD38 | +++ | + | − | +++ | − | − |
| Igs | IgM + IgD+ | IgM + IgD+ | IgM + IgD+ | |||
| IgG+ | Intracellular | IgM + IgD+ | IgM + IgD+ | |||
| IgA+ | Staining | IgG+ | IgG+ | |||
| IgM only | IgM only | |||||
| IgE+ |
FIGURE 2.
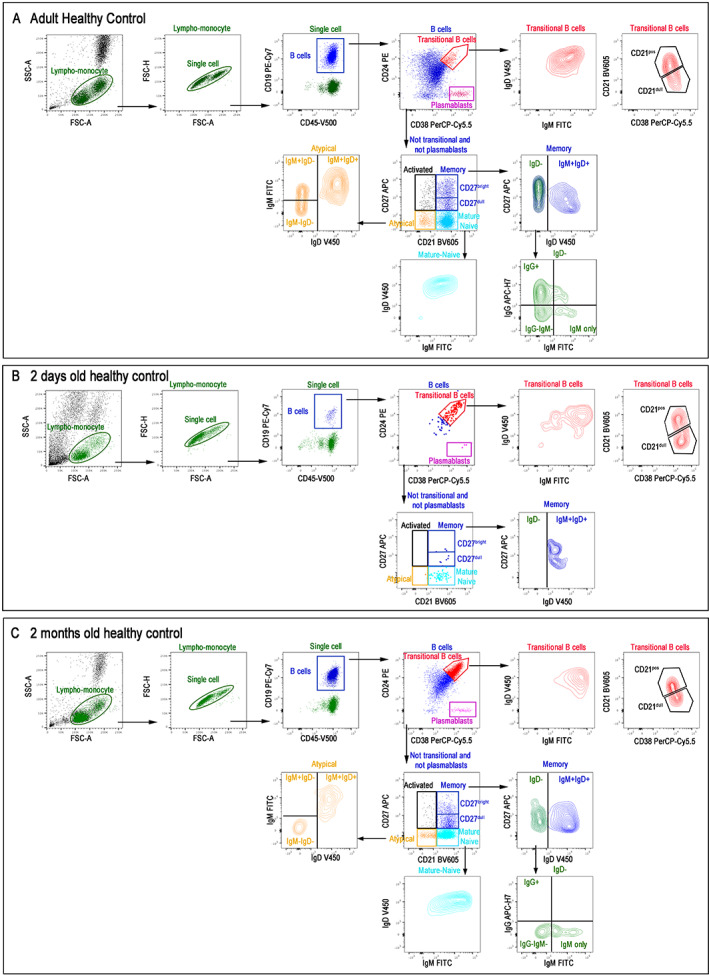
Total blood of adult (A), 2 days old (B), and 2 months old infant (C) stained with antibodies against CD19, CD24, CD27, CD38, IgM, IgG, IgD, and CD21. Live cells were identified based on FSC/SSC lympho‐monocyte gate and then selected as CD45posCD19pos B cells. We identified transitional (CD24posCD38bright) and plasmablasts (CD24negCD38bright). We showed IgM, IgD, and CD21 expression in transitional B cells (CD21dull and CD21bright). In not transitional/not plasmablasts population, we discriminated mature‐naïve (CD24negCD21pos), memory (CD24posCD27posCD21pos), atypical memory (CD21negCD27neg), and activated B cells (CD21negCD27pos). CD27pos memory B cells can be also divided into CD27dull and CD27bright based on CD27 expression. In the CD27pos memory B‐cell population, we showed IgDpos and IgDneg memory B cells. IgDneg memory B cells were further divided into IgGpos, IgGnegIgMneg (that are mostly IgApos), and IgM‐only. Among atypical memory B cells, IgM and IgD expression identify different subtypes [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Differentially markers expressed on B‐cell populations
| Transitional | Mature‐Naïve | Memory | Plasmablasts | Atypical memory | Activated memory | Bibliography | |
|---|---|---|---|---|---|---|---|
| CD10 | +/− | − | − | − | − | − | Carrion et al. [6] |
| CD11c+ | − | +/− | +/− | − | ++ | ++ | Golinski et al. [51, 52] |
| CD20 | + | + | + | − | + | + | Pavlasova and Mraz [53] |
| CD22 | ++ | + | + | − | + | + | Martin et al. [8, 54] |
| CD23 | +++ | ++ | + | + | +/− | − | Grimsholm et al. [18, 31, 47, 48] |
| CD40 | + | + | + | − | +/− | + | Ubillos et al. [43, 52, 55] |
| CD44 | ++ | + | + | − | +/− | Martin et al. [8, 56, 57, 58] | |
| CD55 | + | + | + | − | − | Pascual et al. [57, 59, 60, 61] | |
| CD62L | +/− | + | + | +/− | +/− | Caraux et al. [31, 52, 59, 62] | |
| CD63 | +/− | +/− | +/− | + | ++ | Holla et al. [59, 63, 64] | |
| CD72 | + | ++ | + | − | ++ | Glass et al. [56, 59, 65] | |
| CD80 | − | − | + | − | ++ | Sanz et al. [47, 62, 66] | |
| CD84 | ++ | +/− | ++ | − | + | ++ | Tangye et al. [67] |
| CD86 | +/− | +/− | + | +/− | ++ | Sanz et al. [47, 52, 59] | |
| CD95 | + | − | ++ | + | ++ | ++ | Grimsholm et al. [18, 47, 52, 56, 59, 62, 66] |
| CD138 | − | − | − | +/− | − | − | Caraux et al. [31] |
| CD151 | +/− | +/− | ++ | + | Holla et al. [59, 64] | ||
| CD200 | ++ | + | Grimsholm et al. [18] | ||||
| BAFF receptor | ++ | + | + | − | +/− | + | Smulski et al. [68] |
| β7 integrin | − | + | ++ | ++ | + | Caraux et al. [31, 59] | |
| FCGR2B | ++ | ++ | ++ | ++ | + | Holla et al. [59, 69] | |
| FCRL4 | + | + | + | +++ | + | Li et al. [70] | |
| TACI | + | − | + | + | Cuss et al. [62] | ||
| TCL1 | +++ | ++ | + | Grimsholm et al. [18] | |||
| TLR1 | ++ | + | + | Grimsholm et al. [18, 71] | |||
| TLR2 | + | + | +++ | Grimsholm et al. [18, 71] | |||
| TLR3 | +/− | +/− | + | Meyer‐Bahlburg and Rawlings [71] | |||
| TLR4 | + | + | + | Grimsholm et al. [18, 71] | |||
| TLR5 | +/− | +/− | + | Meyer‐Bahlburg and Rawlings [71] | |||
| TLR6 | + | ++ | + | Meyer‐Bahlburg and Rawlings [71] | |||
| TLR7 | + | ++ | + | − | Meyer‐Bahlburg and Rawlings [71] | ||
| TLR8 | +/− | +/− | + | − | Meyer‐Bahlburg and Rawlings [71] | ||
| TLR9 | +++ | + | +++ | + | − | Meyer‐Bahlburg and Rawlings [26, 71] | |
| TLR11 | + | + | Meyer‐Bahlburg and Rawlings [71] | ||||
| CXCR3 | + | ++ | ++ | ++ | Grimsholm et al. [18, 38, 52] | ||
| CXCR4 | + | ++ | + | − | − | Grimsholm et al. [18, 31, 38, 62] | |
| CXCR5 | ++ | ++ | ++ | − | − | Wang et al. [38, 52, 59, 62] | |
| CCR6 | + | ++ | ++ | Grimsholm et al. [18, 72] | |||
| CCR7 | + | + | − | Grimsholm et al. [18, 38, 59] | |||
| ACKR3 | + | ++ | Grimsholm et al. [18] | ||||
| CCR9 | + | ++ | ++ | Grimsholm et al. [18, 38] | |||
| CCR10 | + | +++ | Caraux et al. [31] | ||||
| IL2R | + | + | + | + | Grimsholm et al. [18, 38] | ||
| IL4R | ++ | + | + | + | Grimsholm et al. [18, 38] | ||
| IL10R | + | + | ++ | ++ | + | ++ | Grimsholm et al. [18, 73] |
| IL13R | ++ | + | Grimsholm et al. [18] | ||||
| IL21R | + | ++ | + | ++ | ++ | Grimsholm et al. [18, 38, 59] |
CD24brightCD38bright B cells in the blood correspond to transitional B cells (Figure 2, in red); they represent the most abundant population in the newborns and decrease to ~3% of total B cells in adults [13]. CD24brightCD38bright transitional B cells coexpress high levels of IgM and IgD (IgMbrightIgDbright). CD21, CD23 (not shown), and L‐selectin (not shown) are also expressed in transitional B cells [5]. CD21 expression differentiates transitional B cells in two populations, CD21bright and CD21dull (Figure 2, in red). CD21dull have been called Transitional 1 (T1) and are the most recent bone marrow emigrant. They still express the marker CD10, which is present in all developing B‐cell in the bone marrow [6]. T1 develop into CD21bright T2 cells, a population increased in autoimmune disease [14].
The two largest B‐cell subsets found in the peripheral blood are mature‐naïve and memory B cells that can be identified by the expression of CD24 and CD27. Mature‐naïve B cells are CD24posCD27neg (Figure 2, in light blue) and correspond to the circulating B cells that migrate through the lymphoid follicles. If they are activated by the antigen, mature‐naïve B cells, in collaboration with T cells and dendritic cells, become the major actors of the adaptive immune response [15].
In healthy adults, 30%–50% of the B cells are CD24brightCD27posCD21pos memory B cells (Figure 2, in blue). This population includes IgM memory B cells, expressing IgM and IgD, and IgDneg that includes switched memory B cells, carrying immunoglobulins of different isotypes, and IgM‐only memory B cells (Figure 2, in green). Most of the switched memory B cells express IgG (~2/3; Figure 2) and IgA (1/3) [16]. A subset of IgM‐only memory B cells has also been described [17] (Figure 2).
We recently demonstrated that CD27pos memory B cells can be divided in CD27dull and CD27bright memory B cells [18] (Figure 2). The CD27dull memory B cells are the precursor of the CD27bright subset. The two populations are related be but have distinct molecular signatures and functions. CD27dull are mostly of IgM isotype, whereas the majority of CD27bright memory B cells express switched isotypes [18].
Somatic mutations indicating antigen selection are rare in the CD27dull population and increase at the CD27bright stage. The proportions of CD27dull and CD27bright change during life. CD27bright MBCs become detectable at 3–4 years of age, increase to a median value of 13% of total B cells in peripheral blood at 6–9 years of age, represent the 23% (median value) of B cells in adults and reach 38% (median value) of total B cells in the elderly. CD27dull memory B cells, which are the major population in children, are a minority in adults and rare in older individuals [18].
IgM memory B cells (IgMposIgDposCD27pos) are a heterogeneous population [19, 20] composed by innate memory B cells [12] and IgM memory B cells that have been remodeled in the germinal centers, where they acquired somatic mutations [12, 21, 22, 23].
Innate or natural memory B cells develop in the absence of germinal centers and T cells [12, 24], generate extra‐follicular, T‐independent responses, and produce natural antibodies [15, 25]. IgM memory B cells act as a first line of defense against infections [12, 15] whereas switched memory B cells embody the highly specific adaptive memory. We have shown that innate IgM memory B cells can be remodeled and acquire somatic mutations in the germinal centers thus participating to the adaptive immune response [12].
It has been shown that memory B cells, IgM and switched, are stored in the spleen, but only IgM memory B cells are significantly reduced in both asplenic and splenectomized patients [26, 27]. Due to their origin, function, and location in the spleen, IgM memory B cells have been considered the human equivalent of murine marginal zone or B‐1 B cells [28, 29, 30].
IgM memory B cells are the first population of memory B cells that develop after birth. Remodeled IgM memory and switched memory B cells number increases slowly in infants [13]. The presence in the blood of switched memory B cells is an indicator of a perfectly functional germinal center reaction [12]. The immunoglobulins expressed by switched memory B cells are always more mutated than those of IgM memory B cells, indicating their role as highly specialized B cells for antigen recognition [12].
Recently, two subsets of IgM memory B cells have been described: IgMhi and IgMlo B cells [30]. IgMhi B cells have similarities with the previously described marginal zone B cells that have been remodeled through the germinal centers [12]. They have higher expression of CD27 and are more mutated compared to IgMlo. Based on our results [18], IgMhi memory B cells coincide with remodeled IgM memory B cells that belong to the CD27bright pool. IgMlo memory B cells have few somatic mutations and most likely overlap with the CD27dull innate memory B cells.
Plasmablasts represent ~3%–5% of CD19pos B cells in the peripheral blood of healthy adults. They are defined as CD24negCD27brightCD38bright (Figure 2, in pink) and down‐regulate CD20 expression, which, in contrast, is expressed by all other B‐cells in the periphery. Circulating plasmablasts can be further differentiated by the expression of CD138. CD138+ plasmablasts represent a more mature stage and are characterized by the absence of surface immunoglobulin, lower CD45 expression, and higher amounts of cytoplasmic immunoglobulin [31]. Plasmablasts are differentiated B cells that provide protective immunity thanks to the continuous secretion of antibodies. Plasmablasts are precursor cells of short‐ and long‐lived plasma cells which are the terminally differentiated elements of the B lineage [32]. Normally, plasma cells are not found in the circulation; all antibody‐secreting cells in the blood en route to, for example, the bone marrow, are plasmablasts and are still considered as a proliferating fraction of antibody‐secreting cells.
Plasmablasts have been extensively studied in the context of the response to influenza virus vaccination and infection. In the course of this response, another group of B cells was identified, the activated memory B cells. These cells have been found to be different from plasmablasts from the transcriptional point of view and still committed to the memory lineage [33]. Activated memory B cells are identified as CD27posCD21neg (Figure 2, in black) and can be either IgGpos or IgMposIgDpos. Following influenza vaccination, hemagglutinin (HA)‐reactive clones are shared between activated memory B cells and antibody secreting cells [10].
Atypical memory B cells (aMBCs; Figure 2, in orange) represent approximately 5% of B cells in the peripheral blood of healthy individuals [34, 35]. aMBCs have been described in aged mice and humans [36, 37], increase during autoimmune diseases [37, 38, 39] and in viral infections [40, 41, 42] and are thought to reflect a failure or impairment of the germinal center reaction [43]. These cells have also been described as age associated B cells (ABCs) [44, 45, 46], tissue‐based memory B cells [47], CD11c+ B cells, T‐bet+ memory B cells [48], CD21−/low B cells or double negative (DN) B cells [49]. They express high level of CD19, lack CD21 and CD27 and are characterized by the expression of CD11c and the transcription factor T‐bet [48]. Inside aMBCs it is possible to identify IgMposIgDpos, IgGpos, and IgM‐only cells [47].
In our staining, we did not use CD5 which is a very important marker for mouse B cells and in human for the diagnosis and follow‐up of CD5 + B lymphoproliferative chronic diseases (B‐CLPDs).
In our experience, CD5 is not useful for the identification of B cell populations in peripheral blood of control adults and children, because transitional, mature‐naïve, and memory B cells can be identified in both the CD5+ and CD5− gate. CD5 can be useful only to identify plasmablasts. When B cells are gated as CD19+CD5+ or as CD19+CD5−, plasmablasts can be found exclusively in the CD19+CD5+ gate and are absent in the CD19+CD5− gate.
Regulatory B cells (Bregs) were originally described as transitional B cells able to produce IL‐10 [50]. Later, it has been demonstrated that the capacity to secrete IL‐10 can be acquired by different B cell populations depending on the culture conditions. Thus, Bregs do not have a defined phenotype but represent a functional stage with the immune‐modulatory activity. A reliable methods to discriminate Bregs is to perform an intracellular staining for IL‐10 following an in‐vitro stimulation [47].
2. IDENTIFICATION OF B‐CELL SUBSETS IN PERIPHERAL BLOOD OF HEALTHY DONORS BY FLOW CYTOMETRY
The cytometric analysis of the B cell surface markers is the easiest way to segregate all the above‐described subsets. This analysis should be always preceded by a proper gating strategy, in order to identify living cells and exclude nonviable cells and doublets (Figure 2).
To correctly detect the different B cell populations, it is necessary to stain an adequate number of cells and use the right concentration of antibodies. An optimal antibody concentration should be established via antibody titration assays.
An appropriate selection of the best‐performing clones of monoclonal antibodies and most appropriate fluorescence for each marker is of primary importance for the correct phenotypic identification of the different B‐cell subsets in the peripheral blood. The choice of the brightness of the fluorochrome is particularly important when within the same B‐cell population, cells may express low or high amount of a certain marker. For example, in order to discriminate between CD27dull and CD27bright memory B cells we used anti‐CD27 labeled with a fluorochrome with a very bright (e.g., PE) or bright (APC) fluorescence index. On the other side, we used a very dim fluorophore for CD45 (V‐500) because of its homogenous expression on all B cells.
It is essential to minimize the time between blood collection in the clinic and running stained samples on the flow cytometer. We normally process the blood within maximum 24 h from collection to ensure a reliable quantification of the B‐cell populations.
In order to achieve high level of standardization, reagents for B‐cell multicolor panel are used in a dried format (BD Bioscience, see Table 3). Bulk lysing is performed to lyse the entire blood sample to be stained. Briefly, 500 μL of fresh total peripheral blood (EDTA) are incubated for 10 min at room temperature with 9.5 mL of the lysing solution Pharm Lyse (1×; BD Biosciences) to remove red blood cells. Afterwards, cells are washed twice with 10 mL of phosphate‐buffered saline (PBS) containing 1% of bovine serum albumin (BSA). Our staining is performed on whole blood following red blood cell lysis. In the plasma, IgG antibodies are present in high concentration. The two washing steps after lysis are essential because they wash‐off the free IgGs that, by sequestering the labeled anti‐IgG antibody, prevent the identification of memory B cells expressing IgG on the surface. Cells are resuspended in 200 μL of PBS and added to a B‐Cell lyotube (BD Bioscience). CD21 BV605 is added as drop‐in. After 20 min incubation at room temperature in the dark, the samples are washed in PBS 1% BSA and finally resuspended in 300 μL of PBS 1% BSA. Flow cytometric data are acquired on a BD FACSLyric™ cytometer (BD Biosciences) and analyzed by FlowJo ver. 10.7 (Becton, Dickinson & Company) (See supporting informations).
TABLE 3.
Antibodies used for the staining of the peripheral blood and the identification of the B‐cell population
| Fluorochrome | Clone | |
|---|---|---|
| CD45 | V500‐C | 2D1 |
| CD19 | PE‐Cy7 | SJ25C1 |
| CD24 | PE | ML5 |
| CD27 | APC | L128 |
| CD38 | PerCP‐Cy5.5 | HIT2 |
| CD21 | BV605 | B‐ly4 |
| IgM | FITC | G20‐127 |
| IgG | APC‐H7 | G18‐145 |
| IgD | V450 | IA6‐2 |
In Figure 2 it is shown an example of a B cells staining in an adult control (A), and in a newborn at 48 h after delivery (B) and after 2 months of life (C). In the staining performed in the adult we can observe all the B cells described above and distributed in a normal way (Figure 2A). On the contrary, at 2 days of life B cells are essentially composed by transitional cells, a few mature B cells and very few memory B cells that are all of the IgM isotype (Figure 2B). At 2 months of age, an expansion in different B cell populations can be detected. In particular, mature B cells, plasmablasts, and memory B cells tend to increase (Figure 2C).
The distribution of the different B cell populations significantly changes over the course of life [13]. In the first years of life, the B cell populations with the highest frequency are transitional B cells and memory cells of the IgM type. Thereafter the transitional B cells are reduced and the switched memory B cells increase [12]. Aging is associated with an increase of memory and aMBCs. Knowing the normal distribution of B cell populations based on age, allow to understand whether the variation observed by flow cytometry is associated with age or a disease state.
A correct staining allows to identify the different B‐cell populations and may be of extreme importance and utility in the diagnosis of diseases associated with B cells, as discussed in the accompanied paper.
AUTHOR CONTRIBUTIONS
Writing—original draft: Rita Carsetti, Eva Piano Mortari, and Maria Giulia Conti. Writing—review and editing: Rita Carsetti, Eva Piano Mortari, Sara Terreri, Maria Giulia Conti, and Francesco Corrente. Methodology: Francesco Corrente, Claudia Capponi, Ane Fernandez Salinas, and Christian Albano. Formal analysis: Eva Piano Mortari, Claudia Capponi, Francesco Corrente, and Ane Fernandez Salinas. Funding acquisition: Rita Carsetti. Supervision: Rita Carsetti.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/cyto.a.24507.
Supporting information
Appendix S1. Supporting Information.
Carsetti R, Terreri S, Conti MG, Fernandez Salinas A, Corrente F, Capponi C, et al. Comprehensive phenotyping of human peripheral blood B lymphocytes in healthy conditions. Cytometry. 2022;101:131–139. 10.1002/cyto.a.24507
Funding information Italian Ministry of Health, Grant/Award Number: RF2013‐02358960
REFERENCES
- 1. Carsetti R. The development of B cells in the bone marrow is controlled by the balance between cell‐autonomous mechanisms and signals from the microenvironment. J Exp Med. 2000;191:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribatti D, Crivellato E, Vacca A. The contribution of Bruce Glick to the definition of the role played by the bursa of Fabricius in the development of the B cell lineage. Clin Exp Immunol. 2006;145:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. [DOI] [PubMed] [Google Scholar]
- 4. Vale AM, Kearney JF, Nobrega A, Schroeder HW. Chapter 7—development and function of B cell subsets. In: Alt FW, Honjo T, Radbruch A, editors. Reth MBT‐MB of BC. 2nd ed. London: Academic Press; 2015. p. 99–119. [Google Scholar]
- 5. Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91. [DOI] [PubMed] [Google Scholar]
- 6. Carrion C, Guérin E, Gachard N, le Guyader A, Giraut S, Feuillard J. Adult bone marrow three‐dimensional phenotypic landscape of B‐cell differentiation. Cyto B Clin Cytom. 2019;96:30–8. [DOI] [PubMed] [Google Scholar]
- 7. Murphy KM, Travers P, Walport M. Janeway Immunologie. 2009. p. 1119.
- 8. Martin VG, Wu Y‐CB, Townsend CL, Lu GHC, O'Hare JS, Mozeika A, et al. Transitional B cells in early human B cell development—time to revisit the paradigm? Front Immunol. 2016;7:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janeway CA Jr, Travers P, Walport M, et al. The immune system in health and disease. B‐cell activation by armed helper T cells. Garland science, 5th ed; 2001. New York: Garland Science. [Google Scholar]
- 10. Palm A‐KE, Henry C. Remembrance of things past: long‐term B cell Memory after infection and vaccination. Front Immunol. 2019;10:1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Viant C, Weymar GHJ, Escolano A, Chen S, Hartweger H, Cipolla M, et al. Antibody affinity shapes the choice between memory and germinal center B cell fates. Cell. 2020;183:1298–1311.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aranburu A, Piano Mortari E, Baban A, Giorda E, Cascioli S, Marcellini V, et al. Human B‐cell memory is shaped by age‐ and tissue‐specific T‐independent and GC‐dependent events. Eur J Immunol. 2017;47:327–44. [DOI] [PubMed] [Google Scholar]
- 13. Blanco E, Pérez‐Andrés M, Arriba‐Méndez S, Contreras‐Sanfeliciano T, Criado I, Pelak O, et al. Age‐associated distribution of normal B‐cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141(6):2208–2219.e16. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Y, Zhang Y, Han J, Yang M, Zhu J, Jin T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J Transl Med. 2020;18:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. Journal of immunology. 2008;180:800–8. [DOI] [PubMed] [Google Scholar]
- 16. Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, et al. Different innate and adaptive immune responses to SARS‐CoV‐2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;16(11):610300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagnara D, Squillario M, Kipling D, Mora T, Walczak AM, Da Silva L, et al. A reassessment of IgM memory subsets in humans. J immunol. 2015;195(8):3716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimsholm O, Piano Mortari E, Davydov AN, Shugay M, Obraztsova AS, Bocci C, et al. The interplay between CD27dull and CD27bright B cells ensures the flexibility, stability, and resilience of human B cell Memory. Cell Rep. 2020;30(9):2963–77.e6. [DOI] [PubMed] [Google Scholar]
- 19. Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci. 2015;112:E546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berkowska MA, Driessen GJA, Bikos V, Grosserichter‐Wagener C, Stamatopoulos K, Cerutti A, et al. Human memory B cells originate from three distinct germinal center‐dependent and ‐independent maturation pathways. Blood. 2011;118:2150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budeus B, Schweigle de Reynoso S, Przekopowitz M, Hoffmann D, Seifert M, Küppers R. Complexity of the human memory B‐cell compartment is determined by the versatility of clonal diversification in germinal centers. Proc Natl Acad Sci. 2015;112:E5281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. Why do we need IgM memory B cells? Immunol Lett. 2013;152(2):114–20. [DOI] [PubMed] [Google Scholar]
- 24. Aranburu A, Ceccarelli S, Giorda E, Lasorella R, Ballatore G, Carsetti R. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. Journal of immunology. 2010;185:7293–301. [DOI] [PubMed] [Google Scholar]
- 25. Piano Mortari E, Baban A, Cantarutti N, Bocci C, Adorisio R, Carsetti R. Heterotaxy syndrome with and without spleen: different infection risk and management. J Allergy Clin Immun. 2016;139(6):1981–84.e1. [DOI] [PubMed] [Google Scholar]
- 26. Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter H‐H, et al. Human immunoglobulin M memory B Cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Descatoire M, Weller S, Irtan S, Sarnacki S, Feuillard J, Storck S, et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2‐dependent differentiation properties. J Exp Med. 2014;211:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weill J‐C, Weller S, Reynaud C‐A. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. [DOI] [PubMed] [Google Scholar]
- 29. Seifert M, Küppers R. Human memory B cells. Leukemia. 2016;30:2283–92. [DOI] [PubMed] [Google Scholar]
- 30. Bautista D, Vásquez C, Ayala‐Ramírez P, Téllez‐Sosa J, Godoy‐Lozano E, Martínez‐Barnetche J, et al. Differential expression of IgM and IgD discriminates two subpopulations of human circulating IgM+IgD+CD27+ B cells that differ phenotypically, functionally, and genetically. Front Immunol. 2020;11:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, et al. Circulating human b and plasma cells. Age‐associated changes in counts and detailed characterization of circulating normal CD138‐ and CD138 plasma cells. Haematologica. 2010;95:1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dörner T, et al. Competence and competition: the challenge of becoming a long‐lived plasma cell. Nat Rev Immunol. 2006;6:741–50. [DOI] [PubMed] [Google Scholar]
- 33. Ellebedy AH, Jackson KJL, Kissick HT, Nakaya HI, Davis CW, Roskin KM, et al. Defining antigen‐specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17(10):1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria‐endemic area. J Immunol. 2009;183:2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B‐cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll‐like receptor 7 (TLR7)‐driven accumulation of a novel CD11c+ B‐cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL‐21 drives expansion and plasma cell differentiation of autoreactive CD11chiT‐bet+B cells in SLE. Nat Commun. 2018;9(1):1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct effector B cells induced by unregulated toll‐like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49:725–739.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T‐box transcription factor T‐bet, a key player in a unique type of B‐cell activation essential for effective viral clearance. Proc Nat Acad Sci USA. 2013;110:E3216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knox JJ, Buggert M, Kardava L, Seaton KE, Eller MA, Canaday DH, et al. T‐bet+ B cells are induced by human viral infections and dominate the HIV gp140 response. JCI Insight. 2017;2:e92943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oliviero B, Varchetta S, Mele D, Mantovani S, Cerino A, Perotti CG, et al. Expansion of atypical memory B cells is a prominent feature of COVID‐19. Cell Mol Immunol. 2020;17(10):1101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ubillos I, Campo JJ, Requena P, Ome‐Kaius M, Hanieh S, Rose H, et al. Chronic exposure to malaria is associated with inhibitory and activation markers on atypical memory B cells and marginal zone‐like B cells. Front Immunol. 2017;21(8):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frasca D, Diaz A, Romero M, Blomberg BB. Phenotypic and functional characterization of double negative B cells in the blood of individuals with obesity. Front Immunol. 2021;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stewart A, Ng JC‐F, Wallis G, Tsioligka V, Fraternali F, Dunn‐Walters DK. Single‐cell transcriptomic analyses define distinct peripheral B cell subsets and discrete development pathways. Front Immunol. 2021;12:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Zhang H, Liu S, Xia F, Kang Z, Zhang Y, et al. Excessive CD11c+Tbet+ B cells promote aberrant TFH differentiation and affinity‐based germinal center selection in lupus. Proc Nat Acad Sci USA. 2019;116:18550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front Immunol. 2019;10:2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karnell JL, Kumar V, Wang J, Wang S, Voynova E, Ettinger R. Role of CD11c+T‐bet+B cells in human health and disease. Cell Immunol. 2017;321:40–5. [DOI] [PubMed] [Google Scholar]
- 49. Rincon‐Arevalo H, Wiedemann A, Stefanski A‐L, Lettau M, Szelinski F, Fuchs S, et al. Deep phenotyping of CD11c+ B cells in systemic autoimmunity and controls. Front Immunol. 2021;12:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blair PA, Noreña LY, Flores‐Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. [DOI] [PubMed] [Google Scholar]
- 51. Golinski M‐L, Demeules M, Derambure C, Riou G, Maho‐Vaillant M, Boyer O, et al. CD11c+ B cells are mainly memory cells, precursors of antibody secreting cells in healthy donors. Front Immunol. 2020;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ambegaonkar AA, Nagata S, Pierce SK, Sohn H. The differentiation in vitro of human tonsil B cells with the phenotypic and functional characteristics of T‐bet+ atypical memory B cells in malaria. Front Immunol. 2019;10:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of B‐cell biology and targeted therapy. Haematologica. 2020;105:1494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Onodera T, Poe JC, Tedder TF, Tsubata T. CD22 regulates time course of both B cell division and antibody response. J Immunol. 2008;180:907. [DOI] [PubMed] [Google Scholar]
- 55. Lee BO, Moyron‐Quiroz J, Rangel‐Moreno J, Kusser KL, Hartson L, Sprague F, et al. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J Immunol. 2003;171:5707. [DOI] [PubMed] [Google Scholar]
- 56. Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, et al. An integrated multi‐omic single‐cell atlas of human B cell identity. Immunity. 2020;53:217–232.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pascual V, Liu Y‐J, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. 1994. 329–339 [DOI] [PMC free article] [PubMed]
- 58. Brynjolfsson SF, Persson Berg L, Olsen Ekerhult T, Rimkute I, Wick M‐J, Mårtensson I‐L, et al. Long‐lived plasma cells in mice and men. Front Immunol. 2018;9:2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holla P, Dizon B, Ambegaonkar AA, Rogel N, Goldschmidt E, Boddapati AK, et al. Shared transcriptional profiles of atypical B cells suggest common drivers of expansion and function in malaria, HIV, and autoimmunity. Sci Adv. 2021;7(22):8384–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alcindor T, Kimlinger T, Witzig TE. High expression of CD59 and CD55 on benign and malignantplasma cells. Leuk Lymphoma. 2006;47:919–21. [DOI] [PubMed] [Google Scholar]
- 61. Dernstedt A, Leidig J, Holm A, Kerkman PF, Mjösberg J, Ahlm C, et al. Regulation of decay accelerating factor primes human germinal center B cells for phagocytosis. Front Immunol. 2021;11:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, et al. Expansion of functionally immature transitional B cells is associated with human‐immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506. [DOI] [PubMed] [Google Scholar]
- 63. Barrena S, Almeida J, Yunta M, López A, Fernández‐Mosteirín N, Giralt M, et al. Aberrant expression of tetraspanin molecules in B‐cell chronic lymphoproliferative disorders and its correlation with normal B‐cell maturation. Leukemia. 2005;19:1376–83. [DOI] [PubMed] [Google Scholar]
- 64. Zou F, Wang X, Han X, Rothschild G, Zheng SG, Basu U, et al. Expression and function of Tetraspanins and their interacting Partners in B Cells. Front Immunol. 2018;9:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lyu M, Hao Y, Li Y, Lyu C, Liu W, Li H, et al. Upregulation of CD72 expression on CD19+CD27+ memory B cells by CD40L in primary immune thrombocytopenia. Br J Haematol. 2017;178:308–18. [DOI] [PubMed] [Google Scholar]
- 66. Ubillos I, Campo JJ, Requena P, Ome‐Kaius M, Hanieh S, Rose H, et al. Corrigendum: chronic exposure to malaria is associated with inhibitory and activation markers on atypical Memory B cells and marginal zone‐like B cells. Front Immunol. 2019;10:2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tangye SG, Weerdt BCM v d, Avery DT, Hodgkin PD. CD84 is up‐regulated on a major population of human memory B cells and recruits the SH2 domain containing proteins SAP and EAT‐2. Europ J Immunol. 2002;32:1640–9. [DOI] [PubMed] [Google Scholar]
- 68. Smulski CR, Eibel H. BAFF and BAFF‐receptor in B cell selection and survival. Front Immunol. 2018;9:2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karnell JL, Dimasi N, Karnell FG, Fleming R, Kuta E, Wilson M, et al. CD19 and CD32b differentially regulate human B cell responsiveness. J Immunol. 2014;192:1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li H, Dement‐Brown J, Liao PJ, Mazo I, Mills F, Kraus Z, et al. Fc receptor‐like 4 and 5 define human atypical memory B cells. Int Immunol. 2020;32:755–70. [DOI] [PubMed] [Google Scholar]
- 71. Meyer‐Bahlburg A, Rawlings DJ. Differential impact of toll‐like receptor signaling on distinct B cell subpopulations. Front Biosci. 2012;17:1499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Scholzen A, Teirlinck AC, Bijker EM, Roestenberg M, Hermsen CC, Hoffman SL, et al. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol. 2014;192:3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin AA, Freeman AF, Nutman TB. IL‐10 indirectly downregulates IL‐4‐induced IgE production by human B cells. ImmunoHorizons. 2018;2:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


