Abstract
Nonalcoholic fatty liver disease (NAFLD) was first described in the 1980s, but in the 21st century, NAFLD has become a very common condition. The explanation for this relatively recent problem is in large part due to the recent epidemic of obesity and type 2 diabetes (T2DM) increasing the risk of NAFLD. NAFLD is a silent condition that may not become manifest until severe liver damage (fibrosis or cirrhosis) has occurred. Consequently, NAFLD and its complications often remain undiagnosed. Research evidence shows that NAFLD is extremely common and some estimates suggest that it occurs in up to 70% of people with T2DM. In the last 5 years, it has become evident that NAFLD not only increases the risk of cirrhosis, primary liver cancer and end‐stage liver disease, but NAFLD is also an important multisystem disease that has major implications beyond the liver. NAFLD increases the risk of incident T2DM, cardiovascular disease, chronic kidney disease and certain extra‐hepatic cancers, and NAFLD and T2DM form part of a vicious spiral of worsening diseases, where one condition affects the other and vice versa. Diabetes markedly increases the risk of liver fibrosis and liver fibrosis is the most important risk factor for hepatocellular carcinoma. It is now possible to diagnose liver fibrosis with non‐invasive tools and therefore it is important to have clear care pathways for the management of NAFLD in patients with T2DM. This review summarises key recent research that was discussed as part of the Banting lecture at the annual scientific conference in 2022.
Keywords: GLP‐1 receptor agonists, insulin resistance, liver fibrosis, nonalcoholic fatty liver disease, pioglitazone, type 2 diabetes
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) was first described in 1980, 1 but in the 21st century, NAFLD has become a very common condition. The explanation for this is in large part due to the epidemic of obesity and type 2 diabetes (T2DM) causing NAFLD. NAFLD represents a spectrum of liver fat‐associated conditions that begin with liver steatosis and progresses to steatohepatitis, liver fibrosis and cirrhosis. With the increasing severity of liver fibrosis, there is also a marked increase in the risk of hepatocellular carcinoma. 2 NAFLD is a silent condition that may not become manifest until severe liver damage has occurred, and therefore NAFLD and its complications often remain undiagnosed in people with diabetes.
The prevalence of NAFLD increases in patients with T2DM and/or metabolic comorbidities and the metabolic syndrome (MetS), defined by the presence of at least three metabolic alterations amongst elevated waist circumference (≥94 cm in males; ≥80 cm in females in Europids), increased triglycerides (≥1.7 mmol/L or 150 mg/dL), reduced HDL‐C (≤1.0 mmol/L or 40 mg/dL in men; ≤1.3 mmol/L or 50 mg/dL in women), increased blood pressure (systolic pressure ≥ 130 mmHg and/or diastolic pressure ≥ 85 mmHg or antihypertensive drug treatment) and increased fasting glucose (≥5.6 mmol/L or 100 mg/dL or antihyperglycemic treatment). 3 Many of these features of MetS may be present with NAFLD and the prevalence of NAFLD may be up to 70%–80% in patients with T2DM. 4 , 5 It is now estimated that NAFLD affects a quarter of the world's adult population 6 and a further concern is that the epidemic of obesity, metabolic dysfunction and T2DM in young people 7 will likely increase the prevalence and complications of NAFLD in the near future. 8 , 9
Data from NHANES (1999–2016) and NHANES III (1988–1994) have also been used to investigate national estimates and temporal trends for NAFLD, based on different fibrosis severity. 10 In this study, NAFLD was determined by ultrasound showing moderate to severe steatosis. For those without ultrasound, NAFLD was determined by the US‐Fatty Liver Index score of ≥30. Hepatic fibrosis was assessed using the FIB‐4 score. Annual per cent change (APC) was calculated using the join‐point regression model. Ten thousand eight hundred fifty‐four individuals were included (mean age 43.5 years; 47.5% male; 75.7% non‐Hispanic white) and 37.7% had NAFLD. Amongst these subjects, based on the FIB‐4 score, 80% had low‐risk, 18.6% had moderate‐risk and 1.4% had high‐risk NAFLD. Subjects with NAFLD and moderate/high‐risk fibrosis (compared with low‐risk), were more likely to have hypertension, hyperlipidemia, diabetes, cardiovascular disease and MetS. NAFLD prevalence increased from 29.5% in 1999–2000 to 40.3% in 2015–2016 (APC: 2.78%, p < 0.02); moderate‐risk NAFLD increased from 6.26% to 14.17% (APC: 5.34%, p < 0.02) and high‐risk NAFLD increased from 0.49% to 1.15% (APC 9.72%, p < 0.02). Thus, these important data provide clear evidence that there is a secular trend in a given population and the prevalence of NAFLD is clearly increasing over time. This review summarises key recent research linking T2DM and NAFLD that was discussed as part of the Banting lecture at the Diabetes UK annual scientific conference in the Spring of 2022. The review illustrates how T2DM and NAFLD form part of a ‘vicious spiral of worsening morbidity’ with NAFLD adversely influencing diabetes‐related morbidity and T2DM influencing the severity of the liver disease. The review also discusses key research over the last decade that had focussed on NAFLD as a multisystem disease: A liver condition that increases the risk of many important extrahepatic diseases.
2. HOW SHOULD NAFLD BE DIAGNOSED AND MONITORED?
Liver biopsy and the assessment of liver histology are recognised as the gold standard for the assessment of liver disease severity in NAFLD. However, the use of this ‘gold’ standard staging of liver disease severity is recognised to be impractical, costly, risky and not feasible for monitoring treatment responses in routine clinical practice. The staging of liver disease with NAFLD involves the severity of different criteria (steatosis, inflammation and fibrosis) 11 , 12 , 13 and commonly the severity of liver fibrosis severity is assessed according to four categories from zero (F0) to cirrhosis (F4). The diagnosis of NAFLD requires the exclusion of both secondary causes and alcohol consumption ≥30 g per day for men and ≥ 20 g per day for women. 14 Recently, a consensus of experts proposed overcoming problems with the current nomenclature ‘NAFLD’ by adopting a more ‘positive’ definition in the acronym MAFLD, referring to Metabolic dysfunction‐Associated Fatty Liver Disease. 15 This new classification and characterisation of fatty liver disease employs metabolic dysfunction as a focus and utilises diagnostic criteria that are independent of the presence of other causes of chronic liver disease. MAFLD also allows for modest alcohol consumption that is potentially hazardous and above the thresholds allowed to diagnose NAFLD. MAFLD also allows for the presence of co‐existing other chronic liver diseases.
The presence of steatosis can be assessed by use of the ultrasound component in recent ‘Fibroscanners’ (the controlled attenuation parameter [CAP]), and liver fibrosis can be assessed using the pressure wave measurement of liver stiffness, as a proxy measurement of liver fibrosis in the absence of other factors that might increase liver stiffness. Liver fibrosis severity is defined as follows: mild (F1) if LSM ≥7.0–8.1 kPa, moderate fibrosis (F2) if ≥8.2–9.6 kPa, advanced fibrosis (F3) if ≥9.7–13.5 kPa and cirrhosis (F4) if ≥13.6 kPa, and these kPa thresholds have recently been validated in a large key validation study with histological assessment of liver disease severity. 16 Using Fibroscan to assess both liver steatosis and liver fibrosis in a prospective cohort study of 776 patients with T2DM, 60.3% had severe steatosis, whilst 19.4% had advanced fibrosis. 17 In this study, female sex, BMI, waist circumference, increased levels of AST, total cholesterol, triglycerides, blood glucose and high LSM were all associated with severe steatosis. BMI, waist circumference, increased levels of AST, HbA1c and CAP were all associated with advanced fibrosis. 17 Moderate‐to‐advanced fibrosis (F2 or higher) is an established risk factor for cirrhosis and overall mortality, and it has recently been estimated that this level of severity of liver fibrosis affects at least 15% of patients with T2DM. 18
Patients in high‐risk groups such as those with MetS or T2DM are at higher risk of more severe liver disease (e.g., liver fibrosis, cirrhosis and primary liver cancer) and co‐morbidities associated with NAFLD. 19 Liver fibrosis and cirrhosis are the most important predictor of mortality in NAFLD and the presence of liver fibrosis, is associated with increased all‐cause, liver‐related and cardiovascular mortality. 10 A recent systematic review and meta‐analysis involving 1495 NAFLD patients with 17,452 patient years of follow‐up, investigated the association between the severity of liver fibrosis and both liver‐related and all‐cause mortality. 20 Compared to NAFLD patients with no fibrosis (stage 0), NAFLD patients with fibrosis were at an increased risk for all‐cause mortality and this risk increased with increases in the stage of fibrosis: stage 1, mortality rate ratios (MRR) = 1.58 (95% CI 1.19–2.11); stage 2, MRR = 2.52 (95% CI 1.85–3.42); stage 3, MRR = 3.48 (95% CI 2.51–4.83); stage 4, MRR = 6.40 (95% CI 4.11–9.95). Importantly, the results were more pronounced as the risk of liver‐related mortality increased exponentially with each increase in the stage of fibrosis: stage 1, MRR = 1.41 (95% CI 0.17–11.95); stage 2, MRR = 9.57 (95% CI 1.67–54.93); stage 3, MRR = 16.69 (95% CI 2.92–95.36); stage 4, MRR = 42.30 (95% CI 3.51–510.34). 20 Importantly, a recent systematic review and meta‐analysis of population‐based cohort studies investigated the association between metabolic risk factors (including T2DM) and the development of advanced liver disease in NAFLD. Databases were searched up to January 2020. T2DM data were obtained from 12 studies, including 22.8 million individuals who were followed up for a median of 10 years (IQR 6.4 to 16.9) and who experienced 72,792 liver events. 21 These data showed that T2DM was associated with an increased risk of incident severe liver disease events (adjusted HR 2.25, 95% CI 1.83–2.76, p < 0.001). As mentioned above, low HDL‐C and increased fasting triglyceride concentrations and hypertension are features of the MetS, and these features occur frequently with NAFLD. In the above meta‐analysis, these features of MetS were also independently associated with increased development of advanced liver disease in NAFLD. 21
3. NAFLD IS A MULTISYSTEM DISEASE WITH EFFECTS BEYOND THE LIVER
3.1. T2DM and NAFLD act as ‘partners in crime’ to increase the risk of extra‐hepatic complications
It is also now clear that NAFLD is a multisystem disease 22 that requires a multidisciplinary, holistic approach to its management. 23 NAFLD increases the risk of hepatocellular carcinoma (HCC). 24 Evidence suggests that NAFLD not only affects the liver but is an independent risk factor for several other diseases, including T2DM, 25 chronic kidney disease 26 and non‐hepatic cancers. 27 Recently, we have investigated effect‐modification by sex and by menopause on the association between NAFLD and T2DM; and we also assessed whether a diagnosis of NAFLD adds to conventional diabetes risk factors for predicting T2DM. 28 In a large cohort study of ~245,000 subjects without diabetes at baseline, these data showed that NAFLD, including more severe NAFLD, is a stronger risk factor for incident T2DM in premenopausal women than in post‐menopausal women or men, and protection against developing T2DM is lost in pre‐menopausal women with NAFLD. Importantly, these data also showed that the addition of NAFLD to conventional diabetes risk factors, improved risk prediction for incident T2DM in both sexes, with a greater improvement in women than men. 28
Although a little more controversial, the weight of evidence also now suggests that NAFLD is also a risk factor for cardiovascular and cardiac disease. 29 , 30 , 31 In a recent meta‐analysis, showing that NAFLD was associated with a ~ 50% increase in the risk of developing CVD, 29 univariable meta‐regression analyses to examine the effect of potential moderator variables, showed there was a significant positive association between the proportion of patients with pre‐existing T2DM (p = 0.001) and also mean plasma LDL‐cholesterol concentrations (p = 0.041), with the risk of NAFLD‐related CVD events. Thus, it seems likely that there is also a modifying influence of T2DM (and LDL‐cholesterol) to further increase the risk of developing CVD, in patients with NAFLD. The study characteristics of included studies, effect sizes for the increases in risk for each outcome (incident diabetes, incident cardiovascular disease, incident CKD and incident extra‐hepatic cancers), and the interpretation of each of these meta‐analyses are summarised in Table 1.
TABLE 1.
NAFLD as a multisystem disease: recent meta‐analyses describing the increased risk of incident diabetes, incident cardiovascular disease, incident chronic kidney disease and incident extra‐hepatic cancers with NAFLD
| Publication | Study aims | Study characteristics | Summary estimate of risk (e.g., HR (95% CIs) of outcome associated with NAFLD | Comments and interpretation |
|---|---|---|---|---|
| Mantovani et al. 25 | To ascertain the risk of incident diabetes associated with NAFLD |
33 studies (501,022 individuals), 30.8% with NAFLD 27,953 cases of incident diabetes over a median of 5 years (IQR: 4.0–19 years) were included Meta‐analysis was performed using random‐effects modelling |
Patients with NAFLD had a higher risk of incident diabetes than those without NAFLD (n = 26 studies; random‐effects HR 2.19, 95% CI 1.93 to 2.48; I 2 = 91.2%). Patients with more ‘severe’ NAFLD were also more likely to develop incident diabetes (n = 9 studies; random‐effects HR 2.69, 95% CI 2.08 to 3.49; I 2 = 69%). This risk markedly increased across the severity of liver fibrosis (n = 5 studies; random‐effects HR 3.42, 95% CI 2.29 to 5.11; I 2 = 44.6%). All risks were independent of age, sex, adiposity measures and other common metabolic risk factors. Sensitivity analyses did not alter these findings. The funnel plots did not reveal any significant publication bias |
PubMed, Scopus and Web of Science databases from January 2000 to June 2020 using predefined keywords to identify observational studies with a follow‐up duration of at least 1 year, in which NAFLD was diagnosed by imaging techniques or biopsy The meta‐analysis shows that NAFLD is associated with a ~ 2.2‐fold increased risk of incident diabetes. This risk parallels the underlying severity of NAFLD The results support those by the authors in an earlier and smaller meta‐analysis in 2018 (Diabetes Care. 2018 Feb;41[2]:372–382) |
| Mantovani et al. 29 | A meta‐analysis of observational studies to quantify the magnitude of the association between NAFLD and the risk of incident CVD events |
36 longitudinal studies with aggregate data on 5,802,226 middle‐aged individuals (mean age 53 years [SD 7]) and 99,668 incident cases of fatal and non‐fatal CVD events over a median follow‐up of 6·5 years (IQR 5·0–10·2) Meta‐analysis was performed using random‐effects models to obtain summary hazard ratios (HRs) with 95% CIs. The quality of the evidence was assessed with the Cochrane risk of bias tool |
NAFLD was associated with a moderately increased risk of fatal or non‐fatal CVD events (pooled random‐effects HR 1·45, 95% CI 1·31–1·61; I 2 = 86·18%). This risk markedly increased across the severity of NAFLD, especially the stage of fibrosis (pooled random‐effects HR 2·50, 95% CI 1·68–3·72; I 2 = 73·84%). All risks were independent of age, sex, adiposity measures, diabetes, and other common cardiometabolic risk factors Sensitivity analyses did not modify these results |
PubMed, Scopus and Web of Science searched from database inception to July 1st 2021 NAFLD was diagnosed by imaging, International Classification of Diseases codes, or liver biopsy The primary outcomes were CVD death, non‐fatal CVD events, or both NAFLD is associated with an increased long‐term risk of fatal or non‐fatal CVD events. CVD risk is further increased with more advanced liver disease, especially with higher fibrosis stage. These results provide evidence that NAFLD might be an independent risk factor for CVD morbidity and mortality N.B. Univariable meta‐regression analyses to examine the effect of potential moderator variables showed a significant positive association between the proportion of patients with pre‐existing type 2 diabetes (p = 0·001) or mean plasma LDL‐cholesterol concentrations (p = 0·041) and the risk of NAFLD‐related CVD event. |
| Mantovani et al. 27 | A meta‐analysis of observational studies to quantify the magnitude of the association between non‐alcoholic fatty liver disease (NAFLD) and risk of extrahepatic cancers |
10 cohort studies with 182,202 middle‐aged individuals (24.8% with NAFLD) and 8485 incident cases of extrahepatic cancers at different sites over a median follow‐up of 5.8 years Meta‐analysis was performed using random‐effects modelling |
NAFLD was significantly associated with a nearly 1.5‐fold to twofold increased risk of developing GI cancers (oesophagus, stomach, pancreas or colorectal cancers). Furthermore, NAFLD was associated with an approximately 1.2‐fold to 1.5‐fold increased risk of developing lung, breast, gynaecological or urinary system cancers. All risks were independent of age, sex, smoking, obesity, diabetes or other potential confounders. The overall heterogeneity for most of the primary pooled analyses was relatively low. Sensitivity analyses did not alter these findings. Funnel plots did not reveal any significant publication bias |
PubMed, Scopus and Web of Science databases searched from the inception date to 30 December 2020 using predefined keywords to identify observational cohort studies conducted in individuals, in which NAFLD was diagnosed by imaging techniques or International Classification of Diseases codes. No studies with biopsy‐proven NAFLD were available for the analysis This large meta‐analysis suggests that NAFLD is associated with a moderately increased long‐term risk of developing extrahepatic cancers over a median of nearly 6 years (especially GI cancers, breast cancer and gynaecological cancers). Further research is required to decipher the complex link between NAFLD and cancer development |
| Mantovani et al. 26 | A meta‐analysis of observational studies to quantify the magnitude of the association between NAFLD and the risk of incident chronic kidney disease (CKD) |
13 studies with 1,222,032 individuals (28.1% with NAFLD) and 33,840 cases of incident CKD stage ≥3 (defined as estimated glomerular filtration rate < 60 ml/min/1.73 m2, with or without accompanying overt proteinuria) over a median follow‐up of 9.7 years were included Meta‐analysis was performed using random‐effects modelling |
NAFLD was associated with a moderately increased risk of incident CKD (n = 10 studies; random‐effects HR 1.43, 95% CI 1.33 to 1.54; I 2 = 60.7%). All risks were independent of age, sex, obesity, hypertension, diabetes and other conventional CKD risk factors. Sensitivity analyses did not alter these findings. Funnel plot did not reveal any significant publication bias |
PubMed, Web of Science and Scopus were searched from January 2000 to August 2020 using predefined keywords to identify observational studies with a follow‐up duration of ≥1 year, in which NAFLD was diagnosed by blood biomarkers/scores, International Classification of Diseases codes, imaging techniques or biopsy This large and updated meta‐analysis indicates that NAFLD is significantly associated with a ~ 1.45‐fold increased long‐term risk of incident CKD stage ≥3. Further studies are needed to examine the association between the severity of NAFLD and risk of incident CKD |
As mentioned above, when metabolic dysfunction, manifest by the presence of co‐existing features of the MetS 3 or T2DM, occurs with liver fat, the term MAFLD can be it has been used to describe NAFLD. 15 There is also now recent evidence to suggest that MAFLD is also associated with an increased risk of CVD. 32 Importantly, a bi‐directional association exists between NAFLD and T2DM, with NAFLD increasing the risk of T2DM and T2DM increasing the risk of severe liver disease and specifically increasing the risk of liver fibrosis, cirrhosis and HCC. 23 In patients with T2DM, the presence of NAFLD also increases the risk of incident or recurrent HCC by ~ 20‐fold. 33 Therefore, this evidence lends weight to the notion that NAFLD should be identified in patients with T2DM, so that liver fibrosis can be assessed; not least, because liver fibrosis is a key risk factor for cirrhosis and HCC. 34 Given that T2DM and NAFLD are also independent risk factors for CVD, CKD and obesity‐related cancers, the combination of both NAFLD and type 2 occurring together is likely to have a greater impact on the development of extra‐hepatic complications. Thus, it could be argued that T2DM and NAFLD are ‘partners in crime’ that act together to increase the risk of both hepatic and extra‐hepatic complications.
4. NAFLD, T2DM, AND CARDIOVASCULAR AND CARDIAC DISEASE
There are several mechanistic links that might explain why NAFLD increases the risk of CVD. It is beyond the scope of this review to discuss all of those mechanisms (for further discussion of these subjects see). 35 In people with NAFLD and also T2DM, regardless of the presence of NAFLD, people with T2DM, insulin resistance and MetS often have a form of dyslipidaemia called the atherogenic lipoprotein phenotype. 36 This discrete dyslipidaemia was proposed as a marker of increased coronary heart disease risk and was first described in 1990 by Melissa Austin and colleagues. 36 The atherogenic lipoprotein phenotype was associated with increases in plasma levels of triglyceride and apolipoprotein B, with a mass of very low and intermediate‐density lipoproteins, and with decreases in high‐density lipoprotein (HDL) cholesterol, HDL2 mass, and plasma levels of apolipoprotein A‐I. 36 The liver has a key role in synthesising triglyceride‐rich lipoproteins associated with apolipoprotein B‐100 (atherogenic lipoproteins) that might explain, at least in part, the association between NAFLD and increased risk of CVD. 37 Hepatic triglyceride metabolism can result in hypertriglyceridaemia, although increased de novo hepatic lipogenesis, mediated by the liver X receptor and sterol regulatory element‐binding protein 1c, can in turn drive liver injury in NAFLD. 38 VLDL‐triglyceride secretion has a positive correlation with intrahepatic triglyceride content and decreased plasma VLDL clearance results in the accumulation of triglyceride‐rich remnant particles, which can increase the risk of CVD. 38 Hypertriglyceridaemia also produces dysfunctional HDL in patients with NAFLD and is also associated with deleterious changes in endothelial cell function 39 that are associated with increased atherosclerotic cardiovascular disease risk. Since total LDL‐C concentration is usually not raised with the atherogenic lipoprotein phenotype, the lipid abnormality is usually dismissed and a patient who is at increased risk of CVD is unfortunately not treated with a statin. It is conceivable that in the future we may have treatments that not only treat liver disease in NAFLD but also treat the atherogenic lipoprotein phenotype. However, in the meantime, it is important to use lipid‐lowering strategies that are known to decrease apolipoprotein B100 concentrations. Such lipid‐lowering agents are statins, ezetimibe and PCSK9 inhibitors. Presently, PCSK9 inhibitors are only available by injection and are expensive, and, therefore, the default position should be to use statins to lower apolipoprotein B100 containing lipoprotein, plus or minus ezetimibe if needed, bearing in mind that both groups of drugs are safe in patients with NAFLD.
Polymorphisms in certain genes predispose individuals to develop more severe liver disease in NAFLD, e.g. patatin‐like phospholipase domain‐containing protein 3 (PNPLA3); trans‐membrane 6 super family 2 (TM6SF2); glucokinase regulator (GCKR); membrane‐bound O‐acyltransferase domain containing 7 (MBOAT7). 40 In addition, in a recent large multi‐cohort exome‐wide association study focused on serum ALT levels, a sequence variant of apolipoprotein E (APOE) has been also identified that is associated with NAFLD, 41 and this genetic variant is well known to be associated with higher risks of both Alzheimer's disease 42 and dyslipidaemia. 43 Polymorphisms in PNPLA3 and TM6SF2 (i.e. PNPLA3 rs738409 c.444 C > G p.I148M and TM6SF2 rs58542926 C > T E167K) may potentially provide further insight into informing us why NAFLD might act to increase risk of CVD and also why there is the heterogeneity of CVD risk in patients with NAFLD. Both PNPLA3 rs738409 c.444 C > G p.I148M and TM6SF2 rs58542926 C > T E167K polymorphisms are quite common in patients in NAFLD, and these polymorphisms may attenuate CVD risk. Although polymorphisms in both PNPLA3 and TM6SF2 (i.e. PNPLA3 rs738409 c.444 C > G p.I148M and TM6SF2 rs58542926 C > T E167K) are well known to be associated with more severe liver disease, these polymorphisms in both genes act to decrease VLDL levels and thereby potentially protect the vasculature from the normal increase in atherogenic VLDL and the development of the atherogenic lipoprotein phenotype that would normally occur in patients with NAFLD. The effect of these moderator polymorphisms to attenuate the risk of CVD is discussed in more detail in a recent review of the relationship between NAFLD and CVD. 35
With the burgeoning 21st problem of obesity, NAFLD has become a common disease that is often present but remains often undiagnosed in the adult population. Thus, in large registry studies investigating associations between NAFLD and outcomes such as CVD, 44 it is not possible to prove that subjects in the control (reference) group, do not have NAFLD. When undiagnosed NAFLD occurs in subjects in the reference group, this causes misclassification bias, and misclassification bias always attenuates the strength of any association between the exposure variable (i.e. NAFLD) and the outcome (CVD), towards the null. Moreover, amongst the few published NAFLD histology cohorts, that have investigated the association between NAFLD and the risk of incident CVD, most have been limited by small sample sizes (e.g., several hundred subjects) with few recorded outcomes and imprecise estimates of risk across NAFLD histological categories. Thus, in cohort studies where histological data were available to gauge liver disease severity, studies were most likely too small, with too few CVD events, to test the strength of any association between the different stages of liver disease severity and CVD events. That said, Simon et al. recently presented important data from a nationwide cohort of Swedish adults with histologically‐confirmed NAFLD and without pre‐existing CVD at baseline (1966–2016; n = 10,422). 30 In this well‐conducted cohort study, the authors investigated the incidence of major adverse cardiovascular events (MACE) (defined as nonfatal ischaemic heart disease, stroke, congestive heart failure or CVD mortality), according to the presence and histological severity of NAFLD. NAFLD was defined from prospectively recorded histopathology, and categorized as simple steatosis (68.5% of the cohort), non‐fibrotic steatohepatitis (NASH, 11.4%), non‐cirrhotic fibrosis (14.9%) and cirrhosis (5.2%), respectively. Patients with NAFLD (n = 10,422) were matched to ≤5 population controls without NAFLD or CVD, by age, sex, calendar year and country (n = 46,517). Over a median of 13.6 years of follow‐up, incident MACE was confirmed in 2850 NAFLD patients (27.3%) and in 10,648 matched controls (22.9%). After adjustment for common cardiometabolic risk factors and potential confounders, NAFLD was significantly associated with a nearly 65% increased risk of incident MACE. Furthermore, the risk of incident MACE increased monotonically with worsening NAFLD severity (P‐value for trend = 0.02). Specifically, compared to matched controls, the absolute rate differences and corresponding fully‐adjusted hazard ratios (aHR) were significantly increased in patients with both simple steatosis (7.0/1000 person‐year [PY]; aHR = 1.58, 95%CI = 1.50–1.67) and NASH without fibrosis (8.1/1000PY; aHR = 1.52, 95%CI = 1.32–1.75), and they were further amplified in patients with non‐cirrhotic fibrosis (11.1/1000PY; aHR = 1.67, 95%CI = 1.47–1.89), or in those with cirrhosis (27.2/1000PY; aHR = 2.15, 95%CI = 1.77–2.61). Interestingly, and worthy of further study, in stratified analyses, the significant association between NAFLD and incident MACE outcomes appeared stronger amongst women than men, amongst patients diagnosed with NAFLD at younger ages, and also amongst those with a positive family history of premature CVD.
Thus, Simon et al. show convincingly that NAFLD is associated with significant excess risk of individual MACE outcomes, including nonfatal ischaemic heart disease, stroke, CVD mortality and also importantly congestive heart failure. However, the mechanisms by which the increased risk of congestive heart failure occurs remain uncertain. Whether any increased risk of heart failure occurs as a result of ischaemic heart disease induced by, for example, the atherogenic lipoprotein phenotype (described above), or whether the increased risk of heart failure occurs due to cardiac remodelling, or a shared increased risk of fibrosis (occurring in both liver and heart), remains uncertain. In a recent brief narrative review, we have discussed the association between NAFLD and increased risk of new‐onset heart failure. 45 In that review, we have also discussed the underlying mechanisms that link these two diseases, discussed the associations between NAFLD and cardiac arrthymias such as atrial fibrillation and summarized pharmacological treatments for NAFLD that might also reduce the risk of HF.
Recently, with the continued ongoing debate as to whether NAFLD is an active contributor that increases CVD risk, a two‐sample Mendelian randomization (MR) analysis using summary‐level data to assess the association between genetically predicted NAFLD (i.e., chronically elevated serum alanine aminotransferase levels [cALT], imaging‐based and biopsy‐confirmed NAFLD), and risk of coronary artery disease was undertaken. 46 Considering the influence of NAFLD‐susceptibility genes (i.e., PNPLA3 rs738409 c.444 C > G p.I148M and TM6SF2 rs58542926 C > T E167K) that also decrease VLDL secretion (i.e., an example of horizontal pleiotrophy), analyses were repeated after exclusion of these NAFLD susceptibility genes that also impair VLDL secretion. After exclusion of these gene effects, there were consistent associations between genetically predicted NAFLD and coronary artery disease for all NAFLD traits (i.e., cALT [OR: 1.203, 95% CI: 1.113, 1.300]), imaging‐based (OR: 2.149, 95% CI: 1.276, 3.620) and biopsy‐confirmed NAFLD (OR: 1.113, 95% CI: 1.041, 1.189), and this association with coronary artery disease persisted when more stringent biopsy‐confirmed NAFLD criteria were used (OR: 1.154, 95% CI: 1.043, 1.278), or when more stringent MR methods were applied. 46 Thus, these data emphasise that there is a robust association between genetically predicted NAFLD and coronary artery disease, after the exclusion of genetic variants that are implicated in impaired VLDL secretion.
5. NUTRITION, POOR DIET, LIFESTYLE CHANGE, AND TREATMENTS FOR NAFLD
There is a large body of evidence demonstrating that lifestyle change focused on weight loss, adoption of a Mediterranean diet that is low in saturated fat, and increased physical activity benefits the early stages of liver disease in NAFLD. 47 , 48 These studies need to be extended to patients with more advanced NAFLD and over longer periods of time, to establish if they remain tractable and effective. Indeed, the use of such diets alongside pharmacotherapies in trials remains an under‐studied area.
With the burgeoning epidemic of obesity in children, NAFLD is becoming a problem in this patient group. 49 Certain foodstuffs that are commonly used and abused in children may also specifically increase the risk of NAFLD and promote the risk of more severe liver disease with NAFLD. One such foodstuff is sugar, and high fructose diets either in the form of refined sugar or as added corn syrup, may not only increase liver fat but may also promote liver inflammation and also increase serum uric acid levels. 50 In 2017, in a large cohort of obese children who had undergone a liver biopsy, the late Valerio Nobili and colleagues showed that a high dietary fructose intake was independently associated with NASH and increased uric acid levels. 51 Fructose, increases de novo lipogenesis, leads to ATP depletion and increases uric acid and increases cellular stress and inflammation. 52 Additionally, increased fructose intake may also lead to dysbiosis (alteration of the gut microbiota) and increase gut permeability 50 ; thereby increasing the potential to increase lipopolysaccharide concentrations in the portal circulation. 50
In order to make the necessary lifestyle changes that are known to be beneficial in NAFLD, behaviour change is crucial. Unfortunately, sustained long‐term behaviour change is very difficult to achieve in an obesogenic environment and without intensive support. Moreover, few patients with NAFLD receive the necessary sustained support within modern health care systems. However, weight loss, decreased calorie intake, increased physical activity or exercise and also alcohol moderation can also result in a marked triglyceride‐lowering effect, prevent diabetes and improve cardiovascular disease risk markers. 53 , 54 , 55 , 56 , 57 , 58 , 59 Specifically, in the liver, weight loss and exercise improves hepatic insulin sensitivity, decrease hepatic glucose production and decrease triglyceride accumulation 53 and these effects would potentially be of benefit not only in NAFLD but also in T2DM.
Given it is likely that in the near future, there will be widespread use of glucagon‐like peptide‐1 receptor (GLP‐1R) agonists for the treatment of obesity, it is crucial that there is improved awareness of NAFLD. Health care professionals (HCPs) caring for patients with diabetes are already very familiar with this class of agents for the treatment of hyperglycaemia in T2DM. Although there are presently no licensed treatments for liver disease in NAFLD, it is important to bear in mind that NAFLD occurs very frequently with T2DM. Where T2DM is also present, clinicians should be aware that both the peroxisome proliferator‐activated receptor‐gamma agonist pioglitazone and GLP‐1RAs, are licensed for the treatment of T2DM and have proven cardiovascular benefits in people with T2DM. Since GLP‐1RAs (mostly subcutaneous liraglutide and semaglutide) and also pioglitazone, have also been shown to be of benefit for liver disease in patients with NASH, 60 HCPs should have a low threshold for prescribing these medications (assuming there are no clinical contraindications) in patients with NAFLD who also have T2DM. Sadly, pioglitazone which is an inexpensive generic drug has become the ‘forgotten, cost‐effective and cardioprotective, drug for the treatment of T2DM’. 61 Although pioglitazone has important well‐recognised side effects, these side effects have resulted in it not being considered a useful drug in patients with T2DM, who are at increased risk of CVD. Since pioglitazone has beneficial effects to treat hyperglycaemia, treating liver disease in NASH and also decrease CVD risk, pioglitazone should be considered in patients with T2DM who have NAFLD in whom there are no contraindications. There is evidence of efficacy to treat liver disease with both 30 and 45 mg pioglitazone doses per day and although there is limited evidence with lower doses than 30 mg/day to treat liver disease, lower doses of pioglitazone are effective at treating hyperglyceridaemia. Perhaps, therefore, there is a good case for considering a lower dose of 15 mg/day if the clinician or patient is worried about pioglitazone‐associated side effects. Since GLP‐1RAs induce weight loss, there is also a good case for combination therapy with GLP‐1RAs and pioglitazone in order to attenuate the risk of weight gain with pioglitazone. Figure 1 illustrates the vicious cycle that exists when NAFLD and type 2 co‐exist together in patients. For example, NAFLD increases the risk of developing T2DM and when T2DM occurs, T2DM increases the risk of developing liver fibrosis in patients with NAFLD. Figure 1 also illustrates the relationship between both T2DM and NAFLD, and also the risk of developing CVD. Increasing evidence suggests that both pioglitazone and GLP‐1RAs have beneficial effects on T2DM, NAFLD and CVD as illustrated by the negative signs in the figure.
FIGURE 1.
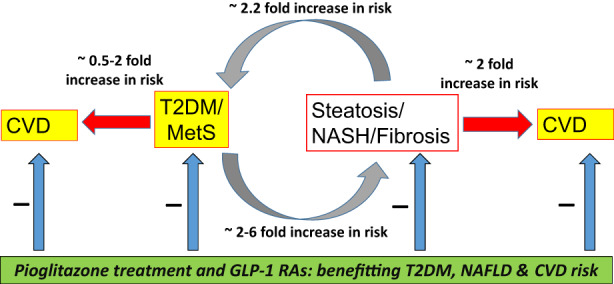
illustrates the vicious cycle that exists when NAFLD and type 2 co‐exist. NAFLD increases the risk of developing T2DM and when T2DM occurs, T2DM increases the risk of developing liver fibrosis. The figure also illustrates the relationship between both T2DM and NAFLD and the risk of developing CVD. Increasing evidence suggests that both pioglitazone and GLP‐1RAs have beneficial effects on T2DM, NAFLD, and CVD as illustrated by the negative signs in the figure. GLP‐1RAs induce weight loss, and there is also a good case for dual therapy with GLP‐1RAs and pioglitazone in order to attenuate the risk of any weight gain with pioglitazone.
A recent meta‐analysis of randomized placebo‐controlled clinical trials assessed the effect of GLP‐1RAs on the lipid profile and liver enzymes in patients with NAFLD. 62 This analysis suggested that GLP‐1RA treatment significantly reduces liver enzymes in patients with NAFLD, but the lipid profile is unaffected. 62 Although sodium‐glucose cotransporter‐2 (SGLT2) inhibitors‐show promise in the treatment of not only T2DM and increased risk of CVD, the evidence is equivocal that this class of drugs benefits NAFLD. 60 In a recent systematic review 60 that included a total of 25 active‐controlled or placebo‐controlled trials (eight for PPAR agonists, 10 for GLP‐1R agonists, and seven for SGLT2 inhibitors), 2597 individuals (1376 [53%] men vs 1221 [47%] women; mean age 52 years (SD 6); mean BMI 32 kg/m2 (SD 3); 1610 [62%] with T2DM) were included. Whereas this analysis showed that pioglitazone, lanifibranor, and GLP1‐R agonists (mostly liraglutide and semaglutide) improved individual histological features of NASH (i.e. steatosis, ballooning, lobular inflammation) or achieved resolution of NASH without worsening of fibrosis; the evidence was not so convincing for SGLT2 inhibitors (mostly empagliflozin and dapagliflozin). SGLT2 inhibitors reduced liver fat content, as assessed by magnetic resonance‐based techniques, but there is limited evidence of benefit to date, showing resolution of NASH or effects on liver fibrosis. Thus, the best evidence of efficacy in NAFLD is with glucose‐lowering drugs such as pioglitazone and GLP‐1RAs. Much of the effect on the liver with pioglitazone and also GLP‐1RAs, is indirect and occurs outside the liver. However, there is also evidence that pioglitazone has beneficial effects on hepatic stellate cells to potentially benefit liver disease. In contrast, there is little evidence to support a direct effect of GLP‐1RAs on liver disease in NAFLD. It seems likely therefore that most of the derived benefit of GLP‐1RAs to attenuate liver disease in NAFLD occurs via the marked GLP‐1RAs‐induced weight loss. Table 2 shows the placebo‐controlled or active‐controlled RCTs with different drugs that have PPAR gamma agonist activity for the treatment of NAFLD. These drugs include pioglitazone which is a single agonist with potent PPAR gamma activity, saroglitazar which is a dual agonist with PPAR alpha and gamma activity and lanifibranor which is a pan PPAR agonist with PPAR alpha, delta and gamma activity. Table 3 shows placebo‐controlled or active‐controlled RCTs with different GLP‐1RAs for the treatment of NAFLD or NASH.
TABLE 2.
Placebo‐controlled or active‐controlled RCTs with different drugs that have PPAR gamma agonist activity for the treatment of NAFLD. Pioglitazone is a single agonist with PPAR gamma activity, saroglitazar is a dual agonist with PPAR alpha and gamma activity and lanifibranor is a pan PPAR agonist with PPAR alpha, delta and gamma activity)
| Study | RCT characteristics | Interventions (n), RCT duration | Key efficacy outcomes | Major adverse effects |
|---|---|---|---|---|
| Belfort et al. 71 USA | Adults with T2DM or prediabetes and biopsy‐confirmed NASH. Mean age: 51 years; men: 45%; BMI: 33.2 kg/m2; HbA1: 6.2% | A. Pioglitazone 30 mg/d for 2 months, then 45 mg/day (n = 29) B. Placebo (n = 25). Length: 24 weeks | Pioglitazone versus placebo, improvement in hepatic fat content (54% vs. 0%, p < 0.001), necro‐inflammation (85% vs. 38%, p = 0.001). Percent with liver fibrosis improvement was not significant: 46% vs. 33%, p = 0.08. Weight: 2.5 kg (p < 0.001) vs. −0.5 kg (p = 0.53), p = 0.003 | Withdrawal due to AEs: 1/29 (3.5%) in pioglitazone group vs. 1/25 (4%) in the placebo group |
| Aithal 72 UK | Non‐diabetic adults with biopsy‐confirmed NASH. Mean age: 53 years; men: 61%; BMI: 30.3 kg/m2; HbA1: NR; ALT and AST: no reported | A. Pioglitazone 30 mg/day (n = 37). B. Placebo (n = 37). Length: 52 weeks | Pioglitazone versus placebo. Number (%) with improvement (p‐ value), between‐groups p‐value: Steatosis: 15/31 (48%) (p = 0.001) vs. 11/30 (37%) (p = 0.03), p = 0.19. Liver fibrosis: 9/31 (29%) (p = 0.006) vs. 6/30 (20%) (p = 0.81), p = 0.05. Weight: 2.6 kg (p = 0.005) vs. −3.5 kg (p = 0.69), p = 0.02 | Withdrawal due to AEs: 3/37 (8.1%) in pioglitazone group vs. 4/37 (10.8%) in the placebo group |
| Sanyal 73 USA, PIVENS | Non‐diabetic adults with biopsy‐confirmed NASH. Mean age: 46 years; men: 40%; BMI: 34 kg/m2; ALT: 83 IU/L; AST: 56 IU/L | A. Pioglitazone 30 mg/day (n = 80). B. Vitamin E 800 IU/d (n = 84). C. Placebo (n = 83). Length: 96 weeks | Pioglitazone versus placebo. NASH improvement, n (%): 27/80 (34%) (p = 0.04) vs. 36/84 (43%) (p = 0.001) vs. 16/83 (19%). NAFLD activity score: −1.9 (p < 0.001) vs. ‐1.9 (p < 0.001) vs. −0.5. Steatosis: −0.8 (p < 0.001) vs. −0.7 (p < 0.001) vs. ‐0.1. Fibrosis: −0.4 (p = 0.10) vs. −0.3 (p = 0.19) vs. −0.1. Weight: 4.7 kg (p < 0.001) vs. 0.4 (p = 0.65) vs. 0.7 kg | Withdrawal due to AEs: None |
| Sharma 74 India | Adults with biopsy‐confirmed NASH. Mean age: 39 years; men: 54%; BMI: 24.9 kg/m2. | A. Pentoxifylline 1200 mg/day (n = 29) B. Pioglitazone 30 mg/day (n = 30). Length: 24 weeks | Pioglitazone versus pentoxifylline. Brunt's score: −0.34 (p = 0.10) vs. −1.2 (p = 0.005), p = 0.04. Steatosis: −0.83 (p = 0.02) vs. −1.18 (p = 0.005), p = 0.60. Fibrosis: 0.08 (p = 0.70) vs. −0.46 (p = 0.19), p = 0.26 | Withdrawal due to AEs: None |
| Cusi 75 USA | Patients with T2DM or prediabetes and biopsy‐confirmed NASH. Mean age: 51 years; men: 70%; BMI: 34.4 kg/m2; pre‐existing T2DM: 51% | A. Pioglitazone 45 mg/day (n = 50). B. Placebo (n = 51). All patients were prescribed a hypocaloric diet. Both groups followed with an open‐label phase with pioglitazone. Length: 72 weeks (144 weeks for the open‐label phase) | Pioglitazone versus placebo. Greater than 2‐point reduction of NAS without worsening fibrosis: 29% vs. 17%, p < 0.001. Fibrosis; greater than 1 point improvement: 39% vs 25%, p > 0.05 (NS). Fibrosis mean change in score improved with pioglitazone: 0 vs. −0.5, p < 0.05. Pioglitazone group gained 2.5 kg, p < 0.05. | NR |
| Gawrieh 76 USA, EVIDENCE IV | Patients with NASH or NAFLD and elevated serum ALT levels. Liver fat content was assessed by MRI‐PDFF. Mean age: 49 years; men: 53%; BMI: 34.3 kg/m2; pre‐existing T2DM: 52.8% | A. Saroglitazar 1 mg/day (n = 26) B. Saroglitazar 2 mg/day (n = 25) C. Saroglitazar 4 mg/day (n = 27) D. Placebo (n = 28). Length: 16 weeks |
Relative changes from baseline of liver fat content at week 16 (p‐value) for each group: A. LS (least squares) Mean = +3.8%, SE (standard error) = 5.7, p = 0.97. B. LS Mean = +0.5%, SE = 6.3, p = 0.68. C. LS Mean = −19.7%, SE = 5.6, p = 0.004. D. LS Mean = +4.1%, SE = 5.9. The LS mean difference between saroglitazar and placebo (95% CI) in liver fat content at week 16 was −0.3% (95% CI ‐16.8 to 16.2) (p = 0.97), − 3.6% (95% CI ‐20.8 to 13.5) (p = 0.67), and − 23.8% (95% CI ‐39.9 to −7.7) (p = 0.004) for saroglitazar 1‐mg, 2‐mg and 4‐mg groups, respectively |
AEs: 112 treatment‐ adverse events were reported in 59 patients. 13 patients in the saroglitazar 1 mg group, 13 patients in the saroglitazar 2 mg group, 14 patients in the saroglitazar 4 mg group, and 19 patients in the placebo group. No serious AEs occurred |
| Francque 77 Multinational, NATIVE | Patients with biopsy‐confirmed NASH and fibrosis. Mean age: 54 years; men: 42%; BMI: 32.9 kg/m2; pre‐existing T2DM: 41.7% | A. Lanifibranor 800 mg/day (n = 83). B. Lanifibranor 1200 mg/day (n = 83). C. Placebo (n = 81). Length: 24 weeks | Lanifibranor versus placebo. Resolution of NASH with no worsening of fibrosis: 33% (p = 0.04 vs. placebo) in lanifibranor 800 mg group, 45% (p < 0.001 vs. placebo) in lanifibranor 1200 mg group, and 19% in the placebo group. Improvement in fibrosis by at least 1 stage and no worsening of NASH: 28% (p = 0.53 vs. placebo) in lanifibranor 800 mg group, 42% (p = 0.011 vs. placebo) in lanifibranor 1200 mg group, and 24% in the placebo group. Resolution of NASH and improvement of fibrosis: 21% (p = 0.02 vs. placebo) in lanifibranor 800 mg group, 31% (p < 0.001 vs. placebo) in lanifibranor 1200 mg group, and 7% in the placebo group | Serious AEs: 3 patients in the lanifibranor 800 mg group, 7 patients in the lanifibranor 1200 mg group and 3 patients in the placebo group |
Abbreviations: BMI, body mass index; CI, confidence interval; MRI‐PDFF, magnetic resonance imaging‐proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus.
TABLE 3.
Placebo‐controlled or active‐controlled RCTs with different GLP‐1RAs for the treatment of NAFLD or NASH
| Study | RCT characteristics | Interventions (n), RCT duration | Efficacy outcomes | Major adverse effects |
|---|---|---|---|---|
| Armstrong et al. 78 UK, LEAN trial | Patients with biopsy‐confirmed NASH. Mean age: 51 years; men: 60%; BMI: 36 kg/m2; fibrosis F3‐F4 (on histology:) 52%; pre‐existing T2DM: 33% | A. Liraglutide 1.8 mg/day (n = 26). B. Placebo (n = 26). Duration: 48 weeks | GLP‐1RAs versus placebo. Histologic resolution of NASH: 39% vs. 9%, p = 0.019. Change in histologic NAS score: −1.3 vs. −0.8, p = 0.24. Change in fibrosis stage: −0.2 vs. 0.2, p = 0.11. Fibrosis improvement: 26% vs. 14%, p = 0.46 Fibrosis worsening: 9% vs. 36%, p = 0.04. Adjustment for weight loss result in non significant effect of GLP‐1RA | Gastrointestinal side effects GLP‐1RA vs. placebo: 81% vs. 65%, respectively |
| Dutour et al. 79 France | Patients with T2DM, 95% of whom had NAFLD assessed by MRS. Mean age: 52 years; men: 48%; BMI: 36 kg/m2 | A. Exenatide 5–10 mcg bd (n = 22). B. Placebo (n = 22). Duration: 26 weeks | GLP‐1RAs versus placebo. Reduction in liver fat content when compared with placebo (liver fat content: −23.8 ± 9.5% vs. +12.5 ± 9.6%, p = 0.007). Weight loss (−5.5 ± 1.2 kg vs. −0.2 ± 0.8 kg; p = 0.001 for difference between groups) | Not reported |
| Yan et al. 80 China | Patients with T2DM and NAFLD were assessed by MRI‐PDFF. Mean age: 44 years; men: 69%; BMI: 29.8 kg/m2 | A. Liraglutide 1.8 mg/day (n = 24). B. Insulin glargine 0.2 IU/kg/day (n = 24). C. Sitagliptin 100 mg/day (n = 27). Duration: 26 weeks | Compared to baseline. In the liraglutide and sitagliptin groups, liver fat content significantly decreased from baseline to week 26 (liraglutide: from 15.4 ± 5.6% to 12.5 ± 6.4%, p < 0.001; sitagliptin: from 15.5 ± 5.6% to 11.7 ± 5.0%, p = 0.001). Body weight was significantly decreased in the liraglutide and sitagliptin groups | Not reported |
| Khoo et al. 81 Singapore | Non‐diabetic patients with obesity and NAFLD assessed by MRI‐PDFF. Mean age: 41 years; men: 90%; BMI: 33 kg/m2 | A. Liraglutide 3.0 mg/day (n = 15). B. Lifestyle modifications (diet+exercise) (n = 15) Duration: 26 weeks | Compared to baseline. At 26 weeks, the two treatment groups showed significant (p < 0.01) but similar reductions in liver fat content (−8.1 ± 13.2 vs. ‐7.0 ± 7.1%). | Gastrointestinal side effects more common in the liraglutide group |
| Liu et al. 82 ; China | Patients with T2DM and NAFLD were assessed by MRI‐PDFF. Mean age: 48 years; men: 50%; BMI: 28 kg/m2 | A. Exenatide 1.8 mg/day (n = 38). B. Insulin glargine 0.2 IU/kg/day (n = 38). Duration: 24 weeks | Liver fat content was significantly reduced after exenatide treatment (change of liver fat: −17.6 ± 12.9%). Exenatide resulted in greater reductions in visceral adipose tissue (ΔVAT −43.6 ± 68.2 cm2) | Not different between groups |
| Bizino et al. 83 Netherlands | Patients with T2DM and NAFLD were assessed by MRS. Mean age: 60 years; men: 59%; BMI: 32 kg/m2 | A. Liraglutide 1.8 mg/day (n = 23). B. Placebo (n = 26) Duration: 26 weeks | Liver fat content not different between groups (liraglutide: from 18.1 ± 11.2% to 12.0 ± 7.7%; placebo: from 18.4 ± 9.4% to 14.7 ± 10.0%; estimated treatment effect −2.1% [95% CI ‐5.3 to 1.0]). Compared to placebo, liraglutide significantly reduced body weight (liraglutide: from 98.4 ± 13.8 kg to 94.3 ± 14.9 kg; placebo: from 94.5 ± 13.1 kg to 93.9 ± 3.2 kg; estimated treatment effect −4.5 kg [95% CI ‐6.4 to −2.6]) | No serious drug‐related adverse events |
| Kuchay et al. 84 India, D‐LIFT trial | Patients with T2DM and NAFLD were assessed by MRI‐PDFF. Mean age: 47 years; men: 70%; BMI: 29.7 kg/m2 | A. Dulaglutide 1.5 mg/week (n = 32). B. Placebo (n = 32) Duration: 24 weeks open‐label trial (add‐on to usual care) | Dulaglutide treatment resulted in a control‐corrected absolute change in liver fat content of −3.5% (95% CI −6.6 to −0.4; p = 0.025) and relative change of −26.4% (95% CI ‐44.2 to −8.6; p = 0.004). Absolute changes in liver stiffness on Fibroscan (−1.31 kPa [−2.99 to 0.37]; p = 0.12) were not significant between two groups | No serious drug‐related adverse events |
| Guo et al. 85 China | Patients with T2DM (treated with metformin) and NAFLD assessed by MRS. Mean age: 52 years; men: 56%; BMI: 28.7 kg/m2 | A. Liraglutide 1.8 mg/week (n = 32). B. Glargine (n = 32). C. Placebo (n = 32). Duration: 26 weeks | Liraglutide treatment resulted in a control‐corrected absolute change in liver fat content of −6.3% (p < 0.05) and relative change of −24% (p < 0.05). N.B. although this change in liver fat was greater with liraglutide than with insulin glargine, there was no significant difference between the groups (−6.3 vs. −3.4%; p > 0.05). In the liraglutide group, there were significant decreases in body weight and waist circumference (weight, 84.3 ± 10.8 kg to 79.4 ± 9.3 kg, p < 0.05; and waist circumference, 95.5 ± 8.0 cm to 89.6 ± 9.3 cm, p < 0.05) | Mild‐to‐moderate gastrointestinal side effects were noted with liraglutide |
| Zhang et al. 86 China | Patients with T2DM (treated with metformin) and NAFLD assessed by MRS. Mean age: 51 years; men: 47%; BMI: 27.3 kg/m2 | A. Liraglutide 1.8 mg/week (n = 30). B. Pioglitazone 30 mg/day (n = 30). Duration: 24 weeks open‐label trial (add‐on to usual care) | Liraglutide treatment resulted in a control‐corrected absolute change in liver fat content of −4% (95% CI −6.6 to −0.4; p < 0.05) and relative change of −17% (p < 0.05). This change in liver fat content was greater with liraglutide than pioglitazone (e.g., 1H‐MRS (%) liraglutide baseline 24.1 ± 3.0 versus the end of study 20.1 ± 3.8; pioglitazone baseline 23.9 ± 3.8 versus the end of study 22.4 ± 3.5). In the liraglutide groups, there were significant decreases in body weight and waist circumference (weight: 79.3 ± 8.8 kg to 69.2 ± 10 kg, p < 0.05; and waist circumference, 93.2 ± 4.6 cm to 84.4 ± 4.0 cm, p < 0.05) | Mild‐to‐moderate gastrointestinal events were reported in the liraglutide group |
| Newsome et al. 87 Multinational cohort of individuals from 16 countries | Patients with biopsy‐confirmed NASH and fibrosis. Mean age: 55 years; men: 41%; BMI 35.7 kg/m2; pre‐existing T2DM: 62% | A. Semaglutide 0.1 mg/day (n = 80). B. Semaglutide 0.2 mg/day (n = 78). C. Semaglutide 0.4 mg/day (n = 82). D. Placebo (n = 80) Length: 72 weeks | Amongst patients with stage F2 or F3 fibrosis, the percentage of patients in whom NASH resolution was achieved with no worsening of fibrosis was 40% in the 0.1‐mg group, 36% in the 0.2‐mg group, 59% in the 0.4‐mg group, and 17% in the placebo group (p < 0.001 for semaglutide 0.4 mg vs. placebo). Improvement in the fibrosis stage occurred in 43% of the patients in the 0.4‐mg group and in 33% of the patients in the placebo group (p = 0.48). Mean percent weight loss was 13% in the 0.4‐mg group and 1% in the placebo group (p < 0.001) | Gastrointestinal side effects were more common in the 0.4‐mg group than in the placebo group |
Abbreviations: BMI, body mass index; CI, confidence interval; MRS, magnetic resonance spectroscopy; MRI‐PDFF, magnetic resonance imaging‐proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus.
6. NAFLD IS A PUBLIC HEALTH BURDEN: WHY DOES NO COUNTRY CURRENTLY HAVE A ‘STRATEGY’ FOR NAFLD?
The economic and healthcare burden of NAFLD is considerable for all countries and especially for those countries where there is a high prevalence of obesity and T2DM. Recently, the lifetime costs of all patients with NASH in the United States in 2017 were estimated to be $222.6 billion, and amongst this group, the costs attributed to advanced NASH were $95.4 billion. 63 However, to date despite NAFLD having a profound impact both on individual patient health and on the economics of providing health care; no countries (to date) have a dedicated strategy for providing health care to patients with NAFLD. Recently, Lazarus et al further developed and extended their European preparedness index 64 and developed their ‘index’ to accommodate six domains with the aim of assessing how prepared countries are to deal with NAFLD. 65 The authors assessed ‘preparedness’ by asking questions of representatives in each of these countries across six domains. These domains were (a) policy; (b) guidelines; (c) civil awareness and social engagement; (d) epidemiology and data; (e) detection; (f) care for patients with NAFLD. Responses were rated high, medium and low (according to a perceived level of ‘preparedness’); and a multiple correspondence analysis was then applied to try and assess levels of preparedness. An overall policy score for a country was then allocated, estimating ‘preparedness from a low score of 0 to a high score of 100’. A high score indicated that a country was deemed to have a high level of ‘preparedness’ for dealing with NAFLD. In this work, the authors reported the results of responses from representatives (who were mainly Hepatologists) and obtained data across 102 countries, apparently covering 86% of the global population. No country scored above half marks, and 32 countries scored zero. The findings contained in their paper led the authors to conclude that ‘although NAFLD is a pressing public health problem, no country was found to be well prepared to address it’. The authors then concluded with a call to arms stating ‘there is a pressing need for a strategy to address NAFLD at national and global levels’.
It is generally now acknowledged that NAFLD represents a public health burden that is having an impact on health care services. Moreover, there has also been a huge increase in research output and understanding in the last decades. For example, a PubMed search on the 31st December 2021, using the search term ‘NAFLD’ showed that there were 4770 citations identified by this term, compared with 22 citations in 2001. This marked increase in knowledge, and the recognition that NAFLD represents a public health burden, has resulted in many countries and economic regions establishing their own Guidelines for NAFLD over the last 6 years. The most recent of these Guidelines in December 2021 was the publication of the excellent Italian Guidelines which are a credit to the consensual working of the contributing representatives from the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). 66
Nevertheless, despite the availability of Guidelines for NAFLD in many counties, there is a disconnect between the availability of guidelines, and no country has a specific NAFLD Strategy. This failing is a huge concern and such a concern should prompt us all to consider why there are no strategies for tackling NAFLD. In the opinion of the author, several key factors have impeded progress in recent years. These factors are: (a) the presence of already existing strategies for addressing obesity and T2DM, as key risk factors for NAFLD and NAFLD progression; (b) scepticism about the additional risk conveyed by NAFLD; (c) perceptions that liver fat is not harmful; (d) limited availability of non‐invasive tests for monitoring liver disease resolution or progression; (e) limited evidence of effective interventions for the amelioration of liver disease, beyond weight loss and lifestyle change; (f) the challenge of managing co‐existing multi‐morbidities such as T2DM and cardiovascular disease (CVD), which are often more urgent for patient well‐being and health; (g) the lack of licensed drug treatment for liver disease in NAFLD. In the recent work reported above, 65 only 20 (24%) of the 83 countries that reported having guidelines for diabetes, mentioned NAFLD in these guidelines. It is important to recognise that NAFLD is very common in specialist diabetes clinics, and it is crucial that non‐specialists beyond Hepatology services, such as Diabetology and Obesity services, are involved in developing models of care for patients with NAFLD. Since there is a large gap in achieving a consensus on the model of care for patients with NAFLD is a multisystem disease, recently, a series of evidence‐based quality standard recommendations for the management of NAFLD were developed by a multidisciplinary group of experts from the British Association for the Study of the Liver and British Society of Gastroenterology NAFLD Special Interest Group, with the overall aim of improving patient care. 67 These recommendations cover the management of people with, or at risk of, NAFLD; assessment and investigations in secondary care; management in secondary care.
As has been mentioned, T2DM is a strong risk factor for liver fibrosis, cirrhosis and hepatocellular carcinoma, and glucose‐lowering drugs used in T2DM may benefit the liver in NAFLD. 68 Since the co‐existence of T2DM and NAFLD creates a vicious spiral of worsening disease affecting both conditions, 35 there is clearly scope for expanding diabetes guidelines to include evidence‐based information, to support NAFLD management in this patient group. A recent study based in southern and western France indicated that primary care physicians and diabetologists have limited knowledge of the chronic liver disease, despite its high prevalence. 69 It is, therefore, crucial that there is continuing medical education amongst primary care physicians and diabetologists in order to identify those patients with more severe forms of NAFLD. Moreover, programmes focused on behaviour change, such as the English NHS Diabetes Prevention programme, afford an opportunity to extend this form of support to other groups (such as patients with NAFLD). Many patients with NAFLD may also benefit from a similar approach and benefit from lifestyle changes focused on decreasing body weight, increasing levels of physical activity, and changes to a diet where appropriate. Figure 2 illustrates the various steps involved, (and that need to be tackled), between recognising that NAFLD is creating a problem and a public health burden within society and effecting change with the development of a health care strategy for NAFLD.
FIGURE 2.
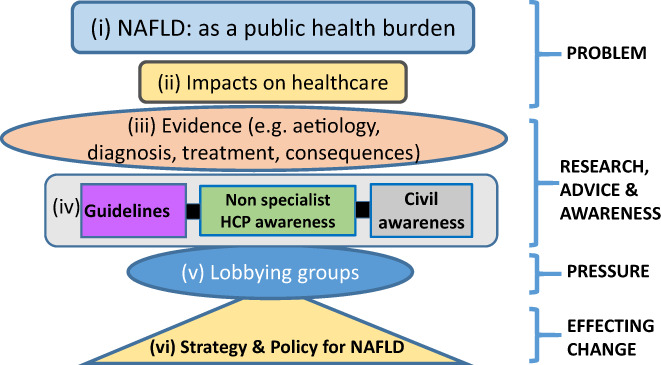
illustrates the various steps involved, (and that need to be tackled), between recognising that NAFLD is creating a problem and public health burden within society, and effecting change with the development of a health care strategy for NAFLD. (i to vi) illustrate the various steps involved in identifying NAFLD as a public health burden and establishing a strategy and policies for tackling NAFLD in society.
7. CONCLUSIONS
NAFLD has become a very common condition in the 21st century. The epidemic of obesity and T2DM has had a marked impact on increasing the public health burden of NAFLD; not least because obesity is a powerful risk factor for developing NAFLD. T2DM is a strong risk factor for promoting liver fibrosis in NAFLD. NAFLD is also an independent risk factor for T2DM and CVD. Thus, the presence of co‐existing NAFLD and T2DM creates a vicious circle where NAFLD increases the risk of T2DM and the presence of T2DM increases the severity of liver disease in NAFLD. Thus, both NAFLD and T2DM could be considered ‘partners in crime’, where the presence of both ‘partners’ has a greater effect than either disease in isolation. In the last 5 years, it has become clear that NAFLD not only increases the risk of cirrhosis, primary liver cancer and end‐stage liver disease, but NAFLD is also an important multisystem disease that has major implications beyond the liver. Not only does NAFLD increase the risk of T2DM and CVD, but it has recently become clear that NAFLD is an independent risk factor for CKD and certain extra‐hepatic cancers. With the consequent health care burden created by NAFLD that has major implications for primary and secondary, it is crucial that Hepatologists work with other specialists and non‐specialists to develop strategies for NAFLD. HCPs caring for patients with diabetes are very familiar with the targeted use of pioglitazone and GLP‐1RAs in the treatment of T2DM, and these drugs have important beneficial effects on NAFLD. A paradigm change is occurring with the diabetologist/endocrinologist's greater awareness of the critical role of NAFLD in patients with T2DM 70 and HCPs caring for patients with T2DM need to be at the heart of discussions to develop strategies for NAFLD.
CONFLICTS OF INTEREST
The author has no competing financial interests to declare.
ACKNOWLEDGMENTS
CDB is supported in part by the Southampton National Institute for Health and Care Research (NIHR) Biomedical Research Centre (IS‐BRC‐20004), UK.
Byrne CD. Banting memorial lecture 2022: ‘Type 2 diabetes and nonalcoholic fatty liver disease: Partners in crime’. Diabet Med. 2022;39:e14912. doi: 10.1111/dme.14912
REFERENCES
- 1. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434‐438. [PubMed] [Google Scholar]
- 2. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35‐S50. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 4. Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non‐alcoholic fatty liver disease: focus on high‐risk groups. Digest Liver Dis. 2015;47:997‐1006. [DOI] [PubMed] [Google Scholar]
- 5. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71:793‐801. [DOI] [PubMed] [Google Scholar]
- 6. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 7. Shaunak M, Byrne CD, Davis N, Afolabi P, Faust SN, Davies JH. Non‐alcoholic fatty liver disease and childhood obesity. Arch Dis Child. 2021;106:3‐8. [DOI] [PubMed] [Google Scholar]
- 8. Radulescu A, Dugan AJ, Killian M, et al. Stratification by obesity class, rather than age, can identify a higher percent of children at risk for non‐alcoholic fatty liver disease and metabolic dysfunction. Pediatr Obes. 2022;17(3):e12862. doi: 10.1111/ijpo.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shapiro WL, Noon SL, Schwimmer JB. Recent advances in the epidemiology of nonalcoholic fatty liver disease in children. Pediatr Obes. 2021;16:e12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Golabi P, Paik JM, Herring M, Younossi E, Kabbara K, Younossi ZM. prevalence of high and moderate risk nonalcoholic fatty liver disease (NAFLD) among adults in the United States (U.S.), 1999‐2016. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21)01339‐2. doi: 10.1016/j.cgh.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3‐13. [DOI] [PubMed] [Google Scholar]
- 12. Kleiner DE, Brunt EM, Van NM, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 13. Kleiner DE, Brunt EM, Wilson LA, et al. Association of Histologic Disease Activity with Progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia. 2016;59:1121‐1140. [DOI] [PubMed] [Google Scholar]
- 15. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction‐associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202‐209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 16. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717‐1730. [DOI] [PubMed] [Google Scholar]
- 17. Sporea I, Mare R, Popescu A, et al. Screening for liver fibrosis and steatosis in a large cohort of patients with type 2 diabetes using vibration controlled transient elastography and controlled attenuation parameter in a single‐center real‐life experience. J Clin Med. 2020;9(4):1032. doi: 10.3390/jcm9041032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lomonaco R, Godinez Leiva E, Bril F, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44:399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karlsen TH, Sheron N, Zelber‐Sagi S, et al. The EASL–lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. The Lancet. 2022;399(10319):61‐116. doi: 10.1016/S0140-6736(21)01701-3 [DOI] [PubMed] [Google Scholar]
- 20. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology. 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis of population‐based observational studies. PLoS Med. 2020;17:e1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47‐S64. [DOI] [PubMed] [Google Scholar]
- 23. Targher G, Tilg H, Byrne CD. Non‐alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6:578‐588. [DOI] [PubMed] [Google Scholar]
- 24. Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non‐alcoholic fatty liver disease‐related hepatocellular carcinoma: a systematic review and meta‐analysis. Lancet Oncol. 2022;23:521‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non‐alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta‐analysis of 501 022 adult individuals. Gut. 2021;70:962‐969. [DOI] [PubMed] [Google Scholar]
- 26. Mantovani A, Petracca G, Beatrice G, et al. Non‐alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta‐analysis. Gut. 2022;71:156‐162. [DOI] [PubMed] [Google Scholar]
- 27. Mantovani A, Petracca G, Beatrice G, et al. Non‐alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta‐analysis of observational cohort studies. Gut. 2021;70:962‐969. [DOI] [PubMed] [Google Scholar]
- 28. Kim Y, Chang Y, Ryu S, Wild SH, Byrne CD. NAFLD improves risk prediction of type 2 diabetes: with effect modification by sex and menopausal status. Hepatology. 2022. doi: 10.1002/hep.32560 [DOI] [PubMed] [Google Scholar]
- 29. Mantovani A, Csermely A, Petracca G, et al. Non‐alcoholic fatty liver disease and risk of fatal and non‐fatal cardiovascular events: an updated systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2021;6:903‐913. [DOI] [PubMed] [Google Scholar]
- 30. Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non‐alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2021;70:1375‐1382. [DOI] [PubMed] [Google Scholar]
- 31. Byrne CD, Targher G. Non‐alcoholic fatty liver disease is a risk factor for cardiovascular and cardiac diseases: further evidence that a holistic approach to treatment is needed. Gut. 2021;gutjnl‐2021‐325965. doi: 10.1136/gutjnl-2021-325965 [DOI] [PubMed] [Google Scholar]
- 32. Guerreiro GTS, Longo L, Fonseca MA, de Souza VEG, Álvares‐da‐Silva MR. Does the risk of cardiovascular events differ between biopsy‐proven NAFLD and MAFLD? Hepatol Int. 2021;15:380‐391. [DOI] [PubMed] [Google Scholar]
- 33. Wild SH, Walker JJ, Morling JR, et al. Cardiovascular disease, cancer, and mortality among people with type 2 diabetes and alcoholic or nonalcoholic fatty liver disease hospital admission. Diabetes Care. 2018;41:341‐347. [DOI] [PubMed] [Google Scholar]
- 34. Angulo P, Kleiner DE, Dam‐Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389‐397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Byrne CD, Targher G. Non‐alcoholic fatty liver disease‐related risk of cardiovascular disease and other cardiac complications. Diabetes Obes Metab. 2022;24(Suppl 2):28‐43. [DOI] [PubMed] [Google Scholar]
- 36. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495‐506. [DOI] [PubMed] [Google Scholar]
- 37. Lewis GF, Hegele RA. Effective, disease‐modifying, clinical approaches to patients with mild‐to‐moderate hypertriglyceridaemia. Lancet Diabet Endocrinol. 2021;10:142‐148. [DOI] [PubMed] [Google Scholar]
- 38. Escalona‐Garrido C, Vázquez P, Mera P, et al. Moderate SIRT1 overexpression protects against brown adipose tissue inflammation. Mol Metabol. 2020;42:101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verwer BJ, Scheffer PG, Vermue RP, Pouwels PJ, Diamant M, Tushuizen ME. NAFLD is related to post‐prandial triglyceride‐enrichment of HDL particles in association with endothelial and HDL dysfunction. Liver Int. 2020;40:2439‐2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carlsson B, Lindén D, Brolén G, et al. Review article: the emerging role of genetics in precision medicine for patients with non‐alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jamialahmadi O, Mancina RM, Ciociola E, et al. Exome‐wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease. Gastroenterology. 2021;160:1634‐1646.e7. [DOI] [PubMed] [Google Scholar]
- 42. Yassine HN, Finch CE. APOE alleles and diet in brain aging and Alzheimer's disease. Front Aging Neurosci. 2020;12:150. doi: 10.3389/fnagi.2020.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marais AD. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology. 2019;51:165‐176. [DOI] [PubMed] [Google Scholar]
- 44. Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real‐world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G. Risk of heart failure in patients with nonalcoholic fatty liver disease: JACC review topic of the week. J Am Coll Cardiol. 2022;79:180‐191. [DOI] [PubMed] [Google Scholar]
- 46. Ren Z, Simons P, Wesselius A, Stehouwer CDA, Brouwers M. Relationship between NAFLD and coronary artery disease: a mendelian randomization study. Hepatology. 2022. doi: 10.1002/hep.32534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. PLoS One. 2022;17:e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Targher G, Byrne CD. Ad libitum Mediterranean or low‐fat diets as treatments for nonalcoholic fatty liver disease? Hepatology. 2018;68:1668‐1671. [DOI] [PubMed] [Google Scholar]
- 49. Mann JP, Valenti L, Scorletti E, Byrne CD, Nobili V. Nonalcoholic fatty liver disease in children. Semin Liver Dis. 2018;38:1‐13. [DOI] [PubMed] [Google Scholar]
- 50. Jegatheesan P, De Bandt JP. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9(3):230. doi: 10.3390/nu9030230 [DOI] [Google Scholar]
- 51. Mosca A, Nobili V, De Vito R, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031‐1036. [DOI] [PubMed] [Google Scholar]
- 52. Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic De novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61:1282‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84:S15‐s21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1‐e23. [DOI] [PubMed] [Google Scholar]
- 55. Lewis CE, Bantle JP, Bertoni AG, et al. History of cardiovascular disease, intensive lifestyle intervention, and cardiovascular outcomes in the look AHEAD trial. Obesity. 2020;28:247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985‐3023. [DOI] [PubMed] [Google Scholar]
- 57. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Poobalan A, Aucott L, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long‐term lipid outcomes ‐ a systematic review. Obes Rev. 2004;5:43‐50. [DOI] [PubMed] [Google Scholar]
- 59. Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401‐406. [PubMed] [Google Scholar]
- 60. Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator‐activated receptor agonists, glucagon‐like peptide‐1 receptor agonists, or sodium‐glucose cotransporter‐2 inhibitors for treatment of non‐alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol. 2022;7:367‐378. [DOI] [PubMed] [Google Scholar]
- 61. DeFronzo RA, Inzucchi S, Abdul‐Ghani M, Nissen SE. Pioglitazone: the forgotten, cost‐effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res. 2019;16:133‐143. [DOI] [PubMed] [Google Scholar]
- 62. Rezaei S, Tabrizi R, Nowrouzi‐Sohrabi P, et al. GLP‐1 receptor agonist effects on lipid and liver profiles in patients with nonalcoholic fatty liver disease: systematic review and meta‐analysis. Can J Gastroenterol Hepatol. 2021;2021:8936865‐8936811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69:564‐572. [DOI] [PubMed] [Google Scholar]
- 64. Lazarus JV, Palayew A, Carrieri P, et al. European 'NAFLD preparedness Index' ‐ is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021;3:100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lazarus JV, Mark HE, Villota‐Rivas M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. 2022;76(4):771‐780. doi: 10.1016/j.jhep.2021.10.025 [DOI] [PubMed] [Google Scholar]
- 66. Non‐alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Dig Liver Dis 2022;54(2):170‐182. doi: 10.1016/j.dld.2021.04.029 [DOI] [PubMed] [Google Scholar]
- 67. McPherson S, Armstrong MJ, Cobbold JF, et al. Quality standards for the management of non‐alcoholic fatty liver disease (NAFLD): consensus recommendations from the British Association for the Study of the liver and British Society of Gastroenterology NAFLD special interest group. Lancet Gastroenterol Hepatol. 2022;S2468‐1253(22)00061‐9. doi: 10.1016/S2468-1253(22)00061-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mantovani A, Byrne CD, Scorletti E, Mantzoros CS, Targher G. Efficacy and safety of anti‐hyperglycaemic drugs in patients with non‐alcoholic fatty liver disease with or without diabetes: an updated systematic review of randomized controlled trials. Diabetes Metab. 2020;46:427‐441. [DOI] [PubMed] [Google Scholar]
- 69. Canivet CM, Smati S, Lannes A, et al. Awareness of chronic liver diseases, a comparison between diabetologists and general practitioners. Clin Res Hepatol Gastroenterol. 2022;46(4):101848. doi: 10.1016/j.clinre.2021.101848 [DOI] [PubMed] [Google Scholar]
- 70. Patel Chavez C, Cusi K, Kadiyala S. The emerging role of glucagon‐like Peptide‐1 receptor agonists for the management of NAFLD. J Clin Endocrinol Metab. 2022;107:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Belfort R, Harrison SA, Brown K, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297‐2307. [DOI] [PubMed] [Google Scholar]
- 72. Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo‐controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176‐1184. [DOI] [PubMed] [Google Scholar]
- 73. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sharma BC, Kumar A, Garg V, Reddy RS, Sakhuja P, Sarin SK. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non‐alcoholic steatohepatitis. J Clin Exp Hepatol. 2012;2:333‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cusi K, Orsak B, Bril F, et al. Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305‐315. [DOI] [PubMed] [Google Scholar]
- 76. Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR‐α/γ agonist, for treatment of NAFLD: a randomized controlled double‐blind phase 2 trial. Hepatology. 2021;74:1809‐1824. [DOI] [PubMed] [Google Scholar]
- 77. Francque SM, Bedossa P, Ratziu V, et al. A randomized, controlled trial of the Pan‐PPAR agonist Lanifibranor in NASH. N Engl J Med. 2021;385:1547‐1558. [DOI] [PubMed] [Google Scholar]
- 78. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 79. Dutour A, Abdesselam I, Ancel P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab. 2016;18:882‐891. [DOI] [PubMed] [Google Scholar]
- 80. Yan J, Yao B, Kuang H, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414‐2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Khoo J, Hsiang JC, Taneja R, et al. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non‐alcoholic fatty liver disease. Liver Int. 2019;39:941‐949. [DOI] [PubMed] [Google Scholar]
- 82. Liu L, Yan H, Xia M, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3292. [DOI] [PubMed] [Google Scholar]
- 83. Bizino MB, Jazet IM, de Heer P, et al. Placebo‐controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre‐specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63:65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuchay MS, Krishan S, Mishra SK, et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D‐LIFT trial). Diabetologia. 2020;63:2434‐2445. [DOI] [PubMed] [Google Scholar]
- 85. Guo W, Tian W, Lin L, Xu X. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty‐six weeks: a randomized placebo‐controlled trial. Diabetes Res Clin Pract. 2020;170:108487. [DOI] [PubMed] [Google Scholar]
- 86. Zhang LY, Qu XN, Sun ZY, Zhang Y. Effect of liraglutide therapy on serum fetuin a in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2020;44:674‐680. [DOI] [PubMed] [Google Scholar]
- 87. Newsome PN, Buchholtz K, Cusi K, et al. A placebo‐controlled trial of subcutaneous Semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113‐1124. [DOI] [PubMed] [Google Scholar]


