Abstract
Aim
To assess whether low‐carbohydrate (LC) diets are associated with differences in weight loss and well‐being in people with obesity, and their cardiovascular and renal safety.
Materials and Methods
A meta‐analysis of randomized controlled trials longer than 3 months, retrieved through an extensive search on MedLine and Embase databases, comparing weight loss with LC and control diets in people with body mass index (BMI) greater than 30 kg/m2, was conducted.
Results
We retrieved 25 trials. Compared with controls, LC diets were associated with significant reduction of body weight at 3‐4 (MD −2.59 [−3.93, −1.25] kg) and 6‐8 months (MD −2.64 [−4.32, −0.95]), but no difference at 10‐14 and 18‐30 months, and significantly greater BMI reduction at 3‐4 months (−1.66 [−2.70, −0.61] kg/m2), but not at other time points. Because only four trials reported data on renal function and psychological variables, renal safety and impact on well‐being could not be assessed. Differences in fasting plasma glucose at any time point were not statistically significant. No significant differences in total or LDL cholesterol or blood pressure were found in the long term, whereas a long‐term reduction of triglycerides (23.26 [−45.53, −0.98] mg/dl at 18‐30 months), and increase of HDL cholesterol (MD 4.94 [0.30, 9.57] mg/dl at 18‐30 months), were observed.
Conclusion
LC diets are associated with greater short‐term weight loss than non‐carbohydrate–restricted diets and a longer term favourable effect on cardiovascular risk factors. Further evidence on long‐term efficacy and renal safety is needed before LC diets can be recommended as the preferred diets in obese people.
Keywords: low‐carbohydrate diets, meta‐analysis, obesity, weight loss
1. INTRODUCTION
Obesity is associated with an increased risk of type 2 diabetes, cardiovascular disease, malignancies, and mortality. 1 , 2 , 3 , 4 The management of obesity is aimed at improving overall health, rather than merely at weight loss. 5 Although intentional weight loss with some interventions has been associated with reduced mortality, 6 no specific treatment for obesity has been proven to increase life expectancy in clinical trials, and unintentional weight loss may even be associated with increased mortality. 7 Epidemiological studies reporting the effects of weight loss (including both unintentional and intentional) are inconclusive. 8 Nevertheless, weight loss is expected to reduce the burden of obesity‐associated morbidity and mortality 9 ; therefore, a reasonable and slowly progressive weight reduction is advised, usually accounting for 5%‐10% of initial weight. 10 However, more ambitious targets may be advisable in those who are at higher risk of cardiovascular and metabolic complications. 11 Dietary modifications, together with an increase in physical activity and reduction of inactivity, are the first‐line therapy for weight loss, encompassing modifications in caloric intake, eating habits, and nutrient composition. 12 The ideal diet is defined as being safe, healthy, nutritionally adequate, culturally acceptable, and economically affordable, and it should ensure long‐term compliance and effectiveness. 13 Most guidelines suggest a 600 kcal daily energy deficit and a reduction in fat intake. 14 , 15 , 16 In particular, the so‐called ‘Mediterranean Diet’, which consists in reducing the intake of fat, especially if saturated, and refined sugar, while increasing the consumption of vegetables and raw carbohydrates, is often considered the preferred approach, because long‐term epidemiological data show lower overall mortality and morbidity in those who adhere to such a regimen. 17 , 18
More recently, some scientific societies have stressed the feasibility and effectiveness of low‐carbohydrate (LC) diets for the treatment of obesity. 5 , 19 The definition of LC diets is heterogeneous, with different degrees of carbohydrate restriction. 20 , 21 , 22 , 23 Modern LC diets usually rely on proteins, rather than fats, to ensure energy intake, aiming at preserving muscle mass and limiting the negative impact of lipid metabolism, often implying the use of expensive protein supplements. 24 Because a greater protein dietary intake could be associated with a faster decline of glomerular filtration in the long term, 25 although this issue is controversial, 26 a dietary protein overload in obese individuals who are already at risk for renal diseases may raise concerns for renal safety.
The evidence on the effects of LC diets in the treatment of obesity has been summarized in some meta‐analyses. 27 , 28 , 29 , 30 , 31 , 32 , 33 However, those meta‐analyses often include both obese and non‐obese cases, 27 , 29 , 31 , 32 , 33 and in some cases include observational studies together with clinical trials. 28 In addition, the results of available meta‐analyses are usually driven by short‐ and very short‐term trials, without separate analyses for longer term studies, 30 and they provide no specific information on renal safety in the longer term. 27 , 28 , 30 , 31
The primary aim of our meta‐analysis is to assess the specific effect of carbohydrate restriction in the treatment of obesity; thus we explored differences between carbohydrate‐restricted diets and non‐carbohydrate–restricted diets concerning weight loss and renal safety in obese individuals. The secondary aim is the exploration of possible effects of specific carbohydrate restriction on blood pressure, lipid profile, and blood glucose, together with its effects on the perceived quality of life and adherence to the prescribed diet.
2. METHODS
This meta‐analysis is reported following the criteria of the PRISMA statement. 34 The review protocol was submitted for registration to the PROSPERO website (#268453; https://www.crd.york.ac.uk/PROSPERO/).
2.1. Search strategy and selection criteria
A systematic search on PubMed, Cochrane, clinicaltrials.gov and Embase databases was performed, collecting all randomized clinical trials written in English and performed on humans up to 1 November 2021. The full search string is reported in Table S1 . Further studies were manually searched in references from retrieved papers.
Studies were included if they fulfilled the following criteria: randomized controlled trials; comparison of a LC diet with a non‐carbohydrate–restricted diet (see below for definitions); apart from diet composition, no difference in treatment protocol between the two arms; duration of the trial of at least 12 weeks; end‐of‐study body weight, or body mass index (BMI), reported for both treatment arms; and studies enrolling only individuals with a BMI more than 30 kg/m2, or separate analyses of subgroups of cases with a BMI more than 30 kg/m2 in trials with wider inclusion criteria.
Studies were eligible for inclusion if they combined a dietary intervention with another non‐pharmacological intervention type (e.g. prescribed exercise/physical activity, cognitive‐behaviour therapy, psychological support), if this was equivalent across dietary intervention arms.
The diets were defined as follows, according to available nutrition guidelines 35 , 36 :
non‐carbohydrate–restricted diets: 45%‐60% of total calories from carbohydrates;
mild LC diets: 26%‐45% of total calories from carbohydrates; and
very LC diets: less than 26% of total calories from carbohydrates and/or less than 130 g of carbohydrates daily.
2.2. Endpoints
The principal endpoints were the differences in mean BMI expressed as kg/m2 between all LC and balanced diets after 3‐4, 6‐8, 10‐14 and 18‐30 months, and the difference in mean body weight between all LC and balanced carbohydrate diets after 3‐4, 6‐8, 10‐14 and 18‐30 months.
The secondary endpoints were the difference in mean, total, HDL and LDL cholesterol, and systolic blood pressure between all LC diets and balanced carbohydrate diets after 3‐4, 6‐8, 10‐14 and 18‐30 months, and the difference in quality of life and adherence to prescribed diet between all LC diets and balanced carbohydrate diets after 3‐4, 6‐8, 10‐14 and 18‐30 months, and at the endpoint.
2.3. Data collection
Titles and abstracts were screened independently by two authors, and potentially relevant articles were retrieved in full text format. For all published trials, results reported in published papers and supplements were used as the primary source of information; when the required information on protocol or outcomes was not available in the main publication, secondary publications were used for retrieval of the missing information; whenever necessary, an attempt at retrieval of the missing information was performed consulting the clinicaltrials.gov registry. The identification of relevant abstracts, the selection of studies, and data extraction were performed independently by two of the authors (GAS and BC), and conflicts were resolved by a third investigator (EM). The risk of bias was assessed using the features proposed by the Cochrane Collaboration37 by two of the authors (FB and CC), and conflicts were resolved through discussion with a third investigator (EM); reporting bias was assessed for each main outcome.
2.4. Statistical analyses
Mantel–Haenszel odds ratio (MH‐OR) with 95% confidence interval (95% CI) and between‐group difference in means (weighted mean difference [MD]) with 95% CI were calculated, on an intention‐to‐treat basis, for dichotomic and continuous outcomes, respectively, using the Wald‐type confidence interval methods calculator. Heterogeneity was assessed by using I2 statistics, using DerSimonian and Laird variance estimator. A random‐effects model was applied as the primary analysis. Funnel plots for HbA1c levels were examined to estimate possible publication/disclosure bias. All analyses were performed using Review Manager 5.3.5 (The Cochrane Collaboration, 2014). The GRADE methodology 37 was used to assess the quality of the body of retrieved evidence, using the GRADE pro‐GDT software (GRADEpro Guideline Development Tool; McMaster University, 2015). A sensitivity post hoc analysis was performed comparing weight loss in different treatment arms at 3‐4 and 10‐14 months, selecting only trials for which both the 3‐4 and the 10‐14 month follow‐up results were available. A post hoc subgroup analysis was performed, dividing trials in which the protein content in the intervention group was below or above 30% of total daily calories.
3. RESULTS
3.1. Trial characteristics
Figure S1 reports the trial flow summary. Of the 7850 items, after removing duplicates, 886 were selected for retrieval of the full text. Of those, 25 trials, overall enrolling 1233 cases on LC diets and 1209 cases on balanced diets, fulfilled the inclusion criteria.
The main characteristics of included trials are reported in Table 1. Out of 26 studies, 19 excluded individuals with kidney disease, and 13 excluded those with previous cardiovascular disease. Nine studies excluded individuals with diabetes, whereas only five included those affected by diabetes, while four included cases with or without diabetes; seven studies did not provide information on this issue. The risk of bias is reported in Figures S2 and S3.
TABLE 1.
Baseline characteristics of the included studies
| Study | D | CKD Excl | CVD Excl | DM (%) | F (%) | Country | N | Age (y) | CHO % (g) | Fat % (g) | Prot % (g) | Energy (kcal) | BMI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | I | C | I | C | I | C | ||||||||
| Bales 2017 55 | 6 | No | Yes | NR | 100 | United States | 51 | 29 | 60.0 | 40 | 55 | 30 | 30 | 30 | 15 | (−500) | (−500) | 37.5 | 38.3 |
| Bazzano 2014 41 | 12 | Yes | Yes | 0 | 66 | United States | 75 | 73 | 46.9 | (40) | 55 | ‐ | 30 | ‐ | ‐ | ‐ | ‐ | 35.4 | 35.4 |
| Brehm 2009 56 | 12 | Yes | Yes | 100 | 58 | United States | 26 | 27 | 56.5 | (20) | 55 | ‐ | 30 | ‐ | 15 | ‐ | ‐ | 36 | 36 |
| Cornier 2006 57 | 3 | Yes | Yes | NR | 100 | United States | 14 | 13 | 53.1 | 45 | 60 | 40 | 20 | 34 | 17 | (−400) | ‐ | 34 | 35 |
| Dalle Grave 2013 42 | 12 | No | No | NR | 58 | Italy | 43 | 45 | 46.7 | 46 | 63 | 20 | 34 | ‐ | 1350 | ‐ | 45.8 | 45.4 | |
| Daly 2006 57 | 3 | Yes | No | 100 | 52 | United Kingdom | 51 | 51 | 58.9 | (70) | 55 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 35 | 37 |
| De Luis 2009 59 | 3 | No | Yes | NR | 72 | Spain | 52 | 66 | 45.6 | 38 | 52 | 36 | 27 | 26 | 20 | 1507 | 1500 | 35.2 | 35.9 |
| De Luis 2015 60 | 9 | Yes | Yes | NR | 71 | Spain | 110 | 101 | 50.5 | 33 | 53 | 33 | 27 | 34 | 20 | 1050 | 1093 | 35.5 | 36.8 |
| Ebbeling, 2007 20 | 12 | Yes | Yes | 0 | 80 | United States | 36 | 37 | 27.5 | 40 | 55 | 35 | 25 | 25 | 25 | ‐ | ‐ | 37.2 | 36.6 |
| Foster 2003 61 | 12 | Yes | Yes | 0 | 68 | United States | 33 | 30 | 44.1 | (50) | 60 | ‐ | 25 | ‐ | 15 | ‐ | 1500 | 33.9 | 34.4 |
| Foster 2010 39 | 24 | No | No | 0 | 68 | United States | 153 | 154 | 45.6 | (50) | 55 | ‐ | 30 | ‐ | 15 | ‐ | 1500 | 36.1 | 36.1 |
| Goday 2016 43 | 4 | Yes | No | 100 | 65 | Spain | 45 | 44 | 54.6 | (50) | 55 | ‐ | 30 | ‐ | 15 | 700 | ‐ | 33 | 33 |
| Goldstein 2011 21 | 12 | No | No | 100 | 48 | Israel | 26 | 26 | 56 | (50) | 60 | ‐ | 20 | ‐ | 20 | ‐ | 1350 | 33 | 33 |
| Iqbal 2010 40 | 24 | Yes | No | 100 | 10 | United States | 70 | 74 | 60 | 32 | 50 | ‐ | 30 | ‐ | ‐ | (−500) | 38 | 37 | |
| Kerksick 2010 61 , 62 | 3 | Yes | Yes | 0 | 100 | United States | 43 | 65 | 34.9 | 20 | 55 | 30 | 15 | 50 | 30 | 1200 | 1200 | 36 | 35 |
| Kitabchi 2013 62 | 6 | Yes | No | 0 | 100 | United States | 14 | 18 | 35.7 | 40 | 55 | 30 | 30 | 30 | 15 | 1800 | 1800 | 41.3 | 37 |
| Morris 2020 63 | 3 | Yes | Yes | 100 | 55 | United Kingdom | 21 | 12 | 67 | 25 | 55 | ‐ | ‐ | (60) | ‐ | 900 | ‐ | 34.8 | 36.4 |
| Perticone 2019 22 | 12 | Yes | Yes | NR | 43 | Italy | 28 | 28 | 46.9 | 20 | 57 | 55 | 27 | 25 | 13 | 1000 | (−500) | 40.5 | 38.8 |
| Porter Starr 2016 64 , 65 | 6 | Yes | No | NR | 79 | United States | 41 | 26 | 68.2 | 40 | 55 | 30 | 30 | 30 | 15 | (−500) | (−500) | 36.4 | 37.2 |
| Racette 1995 65 | 3 | Yes | Yes | 0 | 100 | United States | 13 | 10 | 39 | 25 | 60 | 50 | 15 | 25 | 15 | ‐ | ‐ | 33.2 | 34.5 |
| Samaha 2003 66 , 67 | 6 | Yes | No | 40 | 18 | United States | 64 | 68 | 53 | (30) | ‐ | ‐ | 30 | ‐ | ‐ | ‐ | (−500) | 42.9 | 42.9 |
| Stentz 2016 67 | 6 | Yes | No | 0 | 79 | United States | 12 | 13 | 42.1 | 40 | 55 | 30 | 30 | 30 | 15 | 1800 | ‐ | 40.5 | 37.4 |
| Tonstad 2014 68 | 4 | No | No | 20 | 77 | United States | 82 | 91 | 48.4 | (120) | 55 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 36.6 | 36.3 |
| Yancy 2004 38 | 6 | Yes | Yes | 0 | 79 | United States | 59 | 60 | 44.9 | (120) | ‐ | ‐ | 30 | ‐ | ‐ | ‐ | (−750) | 34.6 | 34 |
| Yancy 2015 44 | 11 | Yes | No | 23 | 27 | United States | 53 | 49 | 55 | (120) | ‐ | ‐ | 30 | ‐ | ‐ | ‐ | (−500) | 36 | 36 |
Note: Carbohydrates, fats and proteins are expressed in % of daily total intake, or grams when in brackets. Energy is expressed in kcal; energy is in brackets when expressed as the difference between daily recommended intake and prescribed calorie intake.
Abbreviations: BMI, body mass index; C, control; CHO, carbohydrate; CKD, chronic kidney disease; CVD, cardiovascular disease; D, duration (expressed as months); DM, diabetes mellitus; Excl, excluded; F, females; I, intervention; N, number; NR, not reported; Prot, proteins.
3.2. Weight loss
All the included trials, except for two, 39 , 40 reported body weight or BMI data only at some time points; the analysis for body weight was therefore performed on 20, 12, 10 and three trials at 3‐4, 6‐8, 10‐14 and 18‐30 months, respectively (Figure 1). LC diets were associated with a significantly higher reduction of body weight at 3‐4 (MD −2.59 [−3.93, −1.25] kg, P = .0001) and 6‐8 months (MD −2.64 [−4.32, −0.95] kg, P = .002) with respect to balanced diets, with no heterogeneity (I2 = 0). The difference in reduction of body weight between the two arms was no longer significant at 10‐14 months (−2.30 [−5.00, +0.41]) kg, I2 = 19) and it totally disappeared at 18‐30 months (MD +0.89 [−2.32, +4.10] kg, I2 = 0). No publication bias was found (Figure S4).
FIGURE 1.
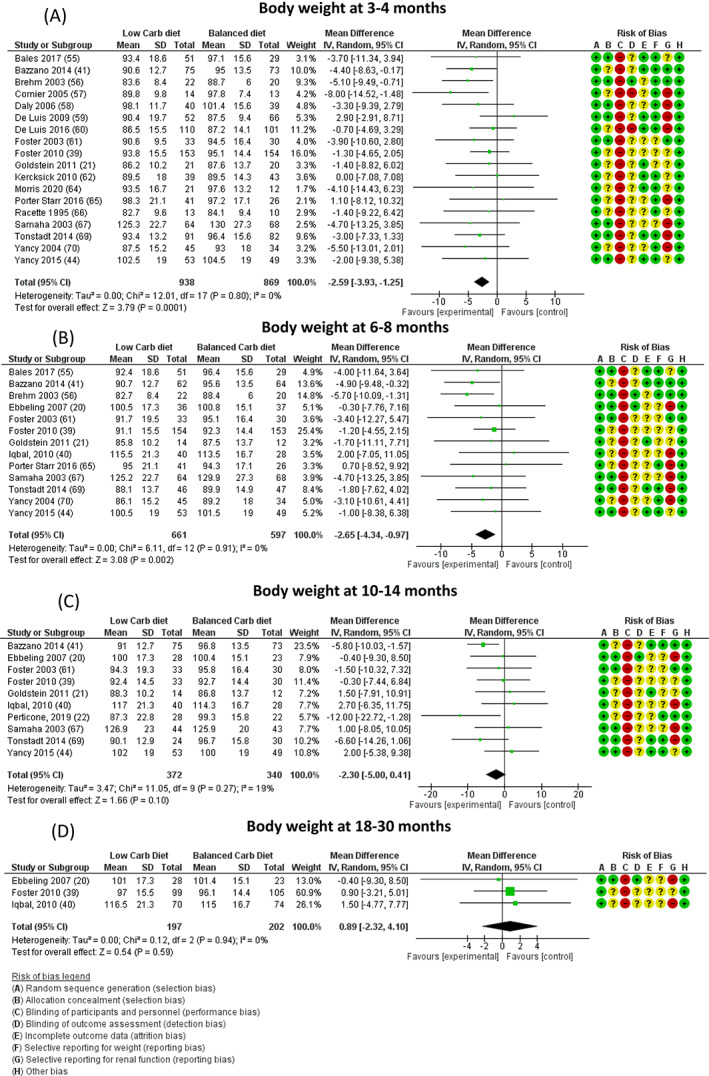
Difference in body weight (expressed as kg) at A, 3‐4, B, 6‐8, C, 10‐14, and D, 18‐30 months between low‐carbohydrate (carb) and balanced carb diets. Risk of bias legend: A = random sequence generation (selection bias); B = allocation concealment (selection bias); C = blinding of participants and personnel (performance bias); D = blinding of outcome assessment (detection bias); E = incomplete outcome data (attrition bias); F = selective reporting for weight (reporting bias); G = selective reporting for renal function (reporting bias); H = other bias. “+” = low risk; “?” = unknown risk; “‐” = high risk. CI, confidence interval; MD, mean difference; N, number
We performed an additional analysis including only those studies providing data on body weight both at 3‐4 and 10‐14 months. Seven studies were available. LC diets were associated with a significantly greater reduction of body weight at 3‐4 months (MD −2.72 [−4.64, −0.80], P = .005, I2 = 0), which was no longer significant at 10‐14 months (MD −2.50 [−5.28, 0.27], I2 = 8) (Figure S6).
We also performed a post hoc subgroup analysis to explore the effect of protein dietary content on weight loss at any time point. No difference in weight loss was observed between trials in which the protein content of the intervention group was below or above 30% of total calorie intake (Figure S7).
Data on BMI were available for five, eight, six and one trial at 3‐4, 6‐8, 10‐14 and 18‐30 months, respectively. LC diets were associated with a significant reduction of BMI at 3‐4 months (−1.66 [−2.70, −0.61] kg/m2, P = .002), but not at other time points (Figure 2). No publication bias was found (Figure S5).
FIGURE 2.
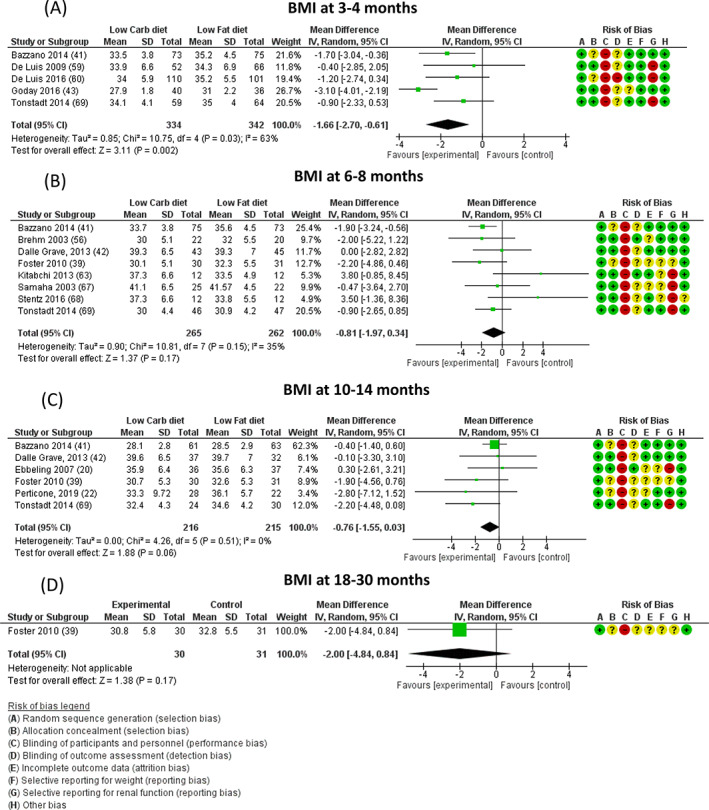
Difference in body mass index (BMI, expressed as kg/m2) at A, 3‐4, B, 6‐8, C, 10‐14, and D, 18‐30 months between low‐carbohydrate (carb) and balanced carb diets. Risk of bias legend: A = random sequence generation (selection bias); B = allocation concealment (selection bias); C = blinding of participants and personnel (performance bias); D = blinding of outcome assessment (detection bias); E = incomplete outcome data (attrition bias); F = selective reporting for weight (reporting bias); G = selective reporting for renal function (reporting bias); H = other bias. “+” = low risk; “?” = unknown risk; “‐” = high risk. CI, confidence interval; MD, mean difference; N, number
No significant difference was observed in BMI between trials in which the protein content of the intervention group was below or above 30% of total calorie intake (Figure S8).
3.3. Renal function
Only two studies 41 , 42 reported serum creatinine at endpoint, showing lower values in the LC values (MD −0.12 [−0.17, −0.07] mg/dl, I2 = 0%), which was already present at baseline (MD −0.10 [−0.15, −0.05] mg/dl). One study 22 reported the Chronic Kidney Disease Epidemiology Collaboration‐calculated estimated glomerular filtration rate (eGFR) (MD +4.00 [−2.39, 10.39] ml/min), whereas another study 43 reported no significant difference from baseline in both groups by Modification of Diet in Renal Disease‐calculated eGFR, without showing any data.
3.4. Glycaemic control
Differences in fasting plasma glucose between LC diets and control arms were not statistically significant at any time point (Table S2).
3.5. Cardiovascular risk factors
LC diets were associated with a significant increase of HDL cholesterol at 10‐14 (MD 2.38 [0.29, 4.47] mg/dl) and 18‐30 months (MD 4.94 [0.30, 9.57] mg/dl), but not at 3‐4 and 6‐8 months (Table S2), whereas no difference in total or LDL cholesterol was found at any time point (Table S2). A reduction in triglycerides was observed at 3‐4, 10‐14 and 18‐30 months (MD −1.78‐20.63 [−35.37, −5.89], −27.09 [−38.29, −15.90] and −23.26 [−45.53, −0.98] mg/dl, respectively), but not at 6 months. No difference was found in blood pressure at any time point, with the only exception of lower diastolic blood pressure at 3‐4 and 6‐8 months in LC diets (MD −3.22 [−5.90, −0.53] and −1.78 [−3.10, −0.45] mmHg, respectively; Table S2).
3.6. Adherence to diet
Retention to studies was 72.3% for LC diets and 70.8% for control diets, with no significant difference between the two groups (P = .39). However, only 40 individuals on LC and 31 on control diets reported diet dissatisfaction as a reason for dropout (P = .20). Eight cases on the LC diet (vs. none in the control arms) dropped out for safety concerns, such as an increase in LDL or creatinine, or ketosis (MH‐OR 3.44 [0.84, 14.02]).
3.7. Psychological variables
Only four studies reported data on psychological variables; of those, one did not report outcome data for each arm. 44 Assessment measures included scales for binge eating, food cravings and appetite, food preferences, anxiety, depression, obesity‐ and diabetes‐related quality of life, beliefs, and motivation (Table 2). Because no single instrument was used in more than one study, no meta‐analysis was performed. In all those studies, weight loss was associated with a significant score reduction, all within the normal range, of measures of anxiety, depression, binge eating and body uneasiness, with no differences between LC and control diets. Reported outcomes are summarized in Table 2.
TABLE 2.
Psychological variables
| Study | Domain | Scale | Subscale | Baseline | 3‐4 mo | 6‐8 mo | 10‐14 mo | 18‐30 mo | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | I | C | I | C | ||||
| Dalle Grave 2013 42 | Anxiety | BAI | 7.5 | 6.4 | 9.2 | 7.6 | 10 | 5.9 | |||||
| Depression | BDI | 9 | 11 | 8 | 9 | 10 | 9 | ||||||
| Body uneasiness | BUT | 56 | 53 | 35 | 38 | 39 | 35 | ||||||
| Binge eating | BES | 7.5 | 8.1 | 5.6 | 5.5 | 6.9 | 5.8 | ||||||
| Foster 2010 39 | Craving | FCI | Sweets | 2.6 | 2.5 | 2.3 | 2.2 | 2.3 | 2.2 | 2.4 | 2.5 | 2.5 | 2.2 |
| High‐fats | 1.9 | 1.9 | 2.0 | 1.8 | 2.1 | 2.0 | 2.1 | 1.8 | 2.0 | 1.6 | |||
| Carb/starch | 2.1 | 2.2 | 1.9 | 2.1 | 1.8 | 2.1 | 1.9 | 2.3 | 1.9 | 2.1 | |||
| Fast‐food fats | 2.6 | 2.6 | 2.3 | 2.4 | 2.4 | 2.5 | 2.4 | 2.6 | 2.5 | 2.3 | |||
| Food preference | FPQ | Complex carbs | 5.5 | 5.5 | 5.0 | 5.2 | 4.9 | 4.5 | 4.7 | 5.1 | 4.5 | 5.1 | |
| Sugar | 6.0 | 5.9 | 5.8 | 5.7 | 5.6 | 5.7 | 5.6 | 5.6 | 5.4 | 5.6 | |||
| Proteins | 5.9 | 5.8 | 5.8 | 5.6 | 5.8 | 5.3 | 5.6 | 5.4 | 5.6 | 5.1 | |||
| Appetite | Appetite rating change | Hunger | ‐7 | 0 | ‐3 | 0 | ‐1 | 0 | ‐5 | ‐2 | |||
| Bothered by hunger | ‐3 | 0 | 0 | 3 | 0 | 3 | 0 | 3 | |||||
| Eat in reaction to food cues | ‐12 | ‐4 | ‐6 | 0 | ‐3 | 0 | ‐3 | ‐4 | |||||
| Thoughts about food | ‐9 | ‐3 | ‐4 | ‐2 | ‐3 | 0 | ‐3 | ‐3 | |||||
| Morris 2020 63 | Diabetes‐related distress | PAID | 14.4 | 20.7 | No significant difference between groups | ||||||||
| Belief | BS | 4.3 | 4.5 | 4.7 | 4.6 | ||||||||
| Motivation | MS | 3.7 | 4.1 | 4.6 | 4.1 | ||||||||
| Yancy 2015 44 | Quality of life | IWQOL‐lite | Total | 72 | No significant difference between groups | ||||||||
Abbreviations: BAI, Beck anxiety inventory; BDI, Beck depression inventory; BES, binge‐eating scale; BS, belief score; BUT, body uneasiness test; C, control; carb, carbohydrate; FCI, food craving index; I, intervention; IWQOL‐lite, impact of weight on quality of life‐lite questionnaire; MS, motivation score; PAID, problem areas in diabetes.
3.8. GRADE scoring of available evidence
GRADE scoring for principal endpoints is reported in Table S3. The overall quality of evidence was assessed as high for body weight at 3‐4, 6‐8 and 10‐14 months and for BMI at 3‐4 months; as moderate for weight at 18‐30 months and for BMI at 6‐8 and 10‐14 months; and as low for BMI at 18‐30 months and for renal function at the endpoint.
4. DISCUSSION
LC diets are associated with a moderately greater weight loss than non‐carbohydrate–restricted diets in the short term. This difference seems to disappear in the longer term, although the number of available studies is insufficient to draw a definitive conclusion after longer than 12 months. Consistent results are obtained when restricting the analysis to trials for which both short‐ and medium‐term results are available. This is in line with previous findings in meta‐analyses including obese and overweight individuals. 32 A previous meta‐analysis, which reported a significant weight loss at 12‐14 months, also included non‐obese overweight cases and only explored very LC diets. 29 A more recent pairwise meta‐analysis with different trial inclusion criteria, which did not report any comparison in weight loss between LC and low‐fat diets, highlighted a negative correlation between actual carbohydrate intake and weight loss at 6 and at 12 months, 27 without providing any longer term data. However, such an analytical approach could overestimate the therapeutic effect of prescribed carbohydrate consumption, because cases with a greater adherence to prescriptions of LC diets could be more prone to weight loss per se.
Adherence to prescribed regimens is a major limiting factor of the efficacy of dietary interventions in obesity. 45 In some trials, actual carbohydrate intake in the LC diet arm could have been different from that prescribed. 21 , 40 On the other hand, long‐term adherence may be lower when the prescribed regimen is very different from usual (spontaneous) dietary intake 46 ; in fact, traditional eating habits in many countries include the consumption of a relevant amount of carbohydrates. This could reduce the effectiveness of LC diets in comparison with balanced diets.
Reduction of carbohydrate intake can be obtained either by increasing the fat or protein content of the diet, or both. The observed effects on weight loss could therefore depend on carbohydrate restriction or the increase in intake of another nutrient. In a post hoc subgroup analysis, protein intake did not appear to moderate weight loss at any time point; however, the limited number and size of available trials does not allow drawing definitive conclusions on this point, which deserves further specific investigation.
The authors of most available trials appear to agree on a possible issue of the renal safety of LC diets, because impaired renal function is usually among the exclusion criteria. Inexplicably, most of those studies did not report any results on renal function at the end of the study, except for only four trials; although no significant differences between treatment arms were detectable in those trials, these results could have been altered by publication bias or disclosure bias. Currently, the renal safety of LC diets remains unknown.
Another potential concern regarding LC diets is cardiovascular safety because the increase in fat intake could have adverse effects on lipid profile and other risk factors. 47 Increased ketogenesis determined by extreme carbohydrate restriction could theoretically reduce the risk of cardiovascular disease. 48 Conversely, observational studies suggest that carbohydrate‐rich Mediterranean‐style diets are associated with reduced cardiovascular morbidity and mortality, 17 , 18 whereas a LC intake has been associated with a higher cardiovascular risk. 46 The duration and the size of samples enrolled in randomized trials comparing LC and balanced diets in the treatment of obesity is too small for assessing their effects on cardiovascular events. In addition, many of the available trials excluded individuals with established cardiovascular disease, who are at higher risk for cardiovascular events. However, data on cardiovascular risk factors were reassuring, with a reduction in triglycerides and an increase in HDL cholesterol, in line with previous reports. 49
LC diets did not appear to have an advantage over control diets in the reduction of fasting plasma glucose. This result is in line with that of a previous meta‐analysis reporting a transient reduction of HbA1c, followed by a modest deterioration of HbA1c in the longer term when LC diets are applied to individuals with type 2 diabetes. 50 On the other hand, the large majority of subjects included in the present meta‐analysis was not affected by diabetes; the dietary intervention is less probable to produce a relevant effect on fasting plasma glucose when baseline levels are within the normal range.
The improvement of quality of life and psychological well‐being is one of the aims of the treatment of obesity. 51 Despite this fact, most available trials did not explore these domains. Reported data show that, not surprisingly, weight loss per se improves psychological status, 52 , 53 , 54 but they fail to highlight any relevant difference between LC and balanced diets. Further studies, enrolling larger samples, are needed to clarify this point.
Several limitations should be considered in the interpretation of the results of this meta‐analysis. The definition of LC diets is heterogeneous across studies, with different degrees of carbohydrate restriction; despite this fact, the observed heterogeneity for the principal outcomes was very low. Most trials are comparatively small, limiting the precision of estimates of treatment effect. In addition, most studies have a short follow‐up, limiting the possibility of extending results to longer term treatment. Notably, most long‐term trials were performed in the United States; their results could be only partly applicable to different cultural contexts, such as those of Mediterranean countries, where adherence to a LC diet could theoretically be more problematic. Furthermore, many trials show relevant methodological limitations, thus reducing the quality of evidence. For example, allocation and detection bias could have led to an overestimation or underestimation of the efficacy of LC diets in some trials. In addition, the use of medication for obesity or other conditions (such as diabetes, hypertension and hyperlipidaemia) was not considered among the outcomes; differences in medication use could therefore have interfered with the results. On the other hand, this meta‐analysis has some strengths: the clear definition of the target population for the dietary intervention (i.e. obese subjects only) increases the reliability of results, which is strengthened by their low heterogeneity.
This systematic review and meta‐analysis shows that, in comparison with non‐carbohydrate–restricted diets, LC diets are associated with a greater short‐term weight loss, with no clear differences in efficacy over the longer term. Data on cardiovascular risk factors are reassuring, while the renal safety of LC diets is undetermined. Further trials are needed to clarify the balance between the benefits and harms of this dietary approach, including more thorough reporting of potential detrimental effects (such as those on renal function) and a wider assessment of psychological well‐being and quality of life. The exploration of further outcomes, such as cognitive decline and the design of larger scale trials on hard endpoints, such as major cardiovascular events, the incidence of diabetes and renal failure, would provide a more robust assessment of the clinical effects of specific carbohydrate restriction in the treatment of obesity.
AUTHOR CONTRIBUTIONS
GAS was involved in design, data collection, analysis and writing the manuscript. CC and BC were involved in design, data collection and manuscript revision. EM was involved in the design, analysis and writing the manuscript. FS, FB and FR were involved in data collection and manuscript revision. The manuscript was drafted, revised and approved by all the authors in accordance with ICJME standards for authorship. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies with human participants or animals performed by any of the authors.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14709.
Supporting information
Data S1
ACKNOWLEDGEMENTS
This research was performed as a part of the institutional activity of the unit, with no specific funding. Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Silverii GA, Cosentino C, Santagiuliana F, et al. Effectiveness of low‐carbohydrate diets for long‐term weight loss in obese individuals: A meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2022;24(8):1458‐1468. doi: 10.1111/dom.14709
Funding information This research was performed as a part of the institutional activity of the unit, with no specific funding.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Riaz H, Khan MS, Siddiqi TJ, et al. Association between obesity and cardiovascular outcomes: a systematic review and meta‐analysis of mendelian randomization studies. JAMA Netw Open. 2018;1:e183788. doi: 10.1001/jamanetworkopen.2018.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body‐mass index in 2012: a population‐based study. Lancet Oncol. 2015;16:36‐46. doi: 10.1016/S1470-2045(14)71123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J, Yang D‐L, Chen Z‐Z, Gou B‐F. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta‐analysis. Cancer Epidemiol. 2016;42:1‐8. doi: 10.1016/j.canep.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 4. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet. 2008;371:569‐578. doi: 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 5. Yumuk V, Frühbeck G, Oppert JM, Woodward E, Toplak H. An EASO position statement on multidisciplinary obesity management in adults. Obes Facts. 2014;7:96‐101. doi: 10.1159/000362191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the swedish obese subjects study. N Engl J Med. 2020;383:1535‐1543. doi: 10.1056/nejmoa2002449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Stefani F d C, Pietraroia PS, Fernandes‐Silva MM, Faria‐Neto J, Baena CP. Observational evidence for unintentional weight loss in all‐cause mortality and major cardiovascular events: a systematic review and meta‐analysis. Sci Rep. 2018;8:15447. doi: 10.1038/s41598-018-33563-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367. doi: 10.1136/bmj.l5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1‐203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 10. Donnelly JE, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459‐471. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 11. Schutz D. European practical and patient‐ Centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12:40‐66. doi: 10.1159/000496183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amerio ML, Angrisani L, Annuzzi G, et al. Standard Italiani per la Cura dell'Obesità SIO‐ADI 2016‐2017. SIO (Società Ital Dell'Obesità). 2017;1:1‐292. [Google Scholar]
- 13. Koliaki C, Spinos T, Spinou M, Brinia ME, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6(3):73‐86. doi: 10.3390/healthcare6030073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raynor HA, Champagne CM. Position of the academy of nutrition and dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet. 2016;116:129‐147. doi: 10.1016/j.jand.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 15. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875‐E891. doi: 10.1503/cmaj.191707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63:2985‐3023. doi: 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 17. Fidanza F, Alberti A, Lanti M, Menotti A. Mediterranean Adequacy Index: correlation with 25‐year mortality from coronary heart disease in the Seven Countries Study. Nutr Metab Cardiovasc Dis. 2004;14:254‐258. doi: 10.1016/S0939-4753(04)80052-8 [DOI] [PubMed] [Google Scholar]
- 18. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599‐2608. doi: 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 19. Caprio M, Infante M, Moriconi E, et al. Very‐low‐calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Invest. 2019;42:1365‐1386. doi: 10.1007/s40618-019-01061-2 [DOI] [PubMed] [Google Scholar]
- 20. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low‐glycemic load vs low‐fat diet in obese young adults: A randomized trial. JAMA. 2007;297:2092‐2102. doi: 10.1001/jama.297.19.2092 [DOI] [PubMed] [Google Scholar]
- 21. Goldstein T, Kark JD, Berry EM, Adler B, Ziv E, Raz I. The effect of a low carbohydrate energy‐unrestricted diet on weight loss in obese type 2 diabetes patients ‐ A randomized controlled trial. e‐SPEN. 2011;6:e178‐e186. doi: 10.1016/j.eclnm.2011.04.003 [DOI] [Google Scholar]
- 22. Perticone M, Maio R, Sciacqua A, et al. Ketogenic diet‐induced weight loss is associated with an increase in Vitamin D levels in obese adults. Molecules. 2019;24:1‐12. doi: 10.3390/molecules24132499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landry MJ, Crimarco A, Gardner CD. Benefits of low carbohydrate diets: a settled question or still controversial? Curr Obes Rep. 2021;10:409‐422. doi: 10.1007/s13679-021-00451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moon J, Koh G. Clinical evidence and mechanisms of high‐protein diet‐induced weight loss. J Obes Metab Syndr. 2020;29:166‐173. doi: 10.7570/jomes20028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malhotra R, Lipworth L, Cavanaugh KL, et al. Protein intake and long‐term change in glomerular filtration rate in the Jackson Heart Study. J Ren Nutr. 2018;28:245‐250. doi: 10.1053/j.jrn.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 26. Oosterwijk MM, Groothof D, Navis G, Bakker SJL, Laverman GD. High‐normal protein intake is not associated with faster renal function deterioration in patients with type 2 diabetes: a prospective analysis in the DIALECT Cohort. Diabetes Care. 2021;45:35‐41. doi: 10.2337/dc21-1211 [DOI] [PubMed] [Google Scholar]
- 27. Willems AEM, Sura‐De Jong M, Van Beek AP, Nederhof E, Van Dijk G. Effects of macronutrient intake in obesity: a meta‐analysis of low‐carbohydrate and low‐fat diets on markers of the metabolic syndrome. Nutr Rev. 2021;79:429‐444. doi: 10.1093/nutrit/nuaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muscogiuri G, Ghoch E. European guidelines for obesity management in adults with a very low‐calorie ketogenic diet: a systematic review and meta‐analysis. Obes Facts. 2021;222‐245. doi: 10.1159/000515381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low‐carbohydrate vs low‐fat diets on weight loss and cardiovascular risk factors. Arch Intern Med. 2006;166:285. doi: 10.1001/archinte.166.3.285 [DOI] [PubMed] [Google Scholar]
- 30. Collet J‐P, Thiele H, Barbato E, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2020;2020:1‐79. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 31. Bueno NB, De Melo ISV, De Oliveira SL, Da Rocha AT. Very‐low‐carbohydrate ketogenic diet v. low‐fat diet for long‐term weight loss: A meta‐analysis of Randomised controlled trials. Br J Nutr. 2013;110:1178‐1187. doi: 10.1017/S0007114513000548 [DOI] [PubMed] [Google Scholar]
- 32. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta‐analysis. JAMA. 2014;312:923‐933. doi: 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 33. Hashimoto Y, Fukuda T, Oyabu C, et al. Impact of low‐carbohydrate diet on body composition: meta‐analysis of randomized controlled studies. Obes Rev. 2016;17:499‐509. doi: 10.1111/obr.12405 [DOI] [PubMed] [Google Scholar]
- 34. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Italian Society of human nutrition . LARN: Nutrients and Energy Intake Standard Levels for the Italian Population: 4th revision. Milan: SICS Editions; 2014. [Google Scholar]
- 36. Scientific Opinion on the essential composition of total diet replacements for weight control. EFSA J. 2015;13:3957. doi: 10.2903/j.efsa.2015.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, eds. Cochrane Methods. Cochrane Database of Systematic Reviews; 2016. doi: 10.1002/14651858.CD201601 [DOI] [Google Scholar]
- 38. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380‐382. doi: 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 39. Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low‐carbohydrate versus low‐fat diet. Obstet Gynecol Surv. 2010;65:769‐770. doi: 10.1097/OGX.0b013e31821342ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iqbal N, Vetter ML, Moore RH, et al. Effects of a low‐intensity intervention that prescribed a low‐carbohydrate vs. a low‐fat diet in obese, diabetic participants. Obesity. 2010;18:1733‐1738. doi: 10.1038/oby.2009.460 [DOI] [PubMed] [Google Scholar]
- 41. Bazzano LA, Hu T, Reynolds K, et al. Effects of low‐carbohydrate and low‐fat diets: a randomized trial. Ann Intern Med. 2014;161:309‐318. doi: 10.7326/M14-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dalle Grave R, Calugi S, Gavasso I, El Ghoch M, Marchesini G. A randomized trial of energy‐restricted high‐protein versus high‐carbohydrate, low‐fat diet in morbid obesity. Obesity. 2013;21:1774‐1781. doi: 10.1002/oby.20320 [DOI] [PubMed] [Google Scholar]
- 43. Goday A, Bellido D, Sajoux I, et al. Short‐Term safety, tolerability and efficacy of a very low‐calorie‐ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016;6:e230. doi: 10.1038/nutd.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yancy WS, Mayer SB, Coffman CJ, Voils CI. Effect of allowing choice of diet on weight loss. A randomized trial. Ann Intern Med. 2015;162:805‐814. doi: 10.7326/M14-2358.Effect [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Monnier L, Schlienger J‐L, Colette C, Bonnet F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2021;47:101192. doi: 10.1016/j.diabet.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 46. Barber TM, Hanson P, Kabisch S, Pfeiffer AFH, Weickert MO. The low‐carbohydrate diet: short‐term metabolic efficacy versus longer‐term limitations. Nutrients. 2021;13:1‐15. doi: 10.3390/nu13041187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hammad S, Pu S, Jones PJ. Current evidence supporting the link between dietary fatty acids and cardiovascular disease. Lipids. 2016;51:507‐517. doi: 10.1007/s11745-015-4113-x [DOI] [PubMed] [Google Scholar]
- 48. Ferrannini E. Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27‐38. doi: 10.1016/j.cmet.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 49. Chawla S, Radenkovic D. The effect of low‐fat and low‐carbohydrate diets on weight loss and lipid levels: a systematic review. Nutrients. 2020;12:1‐21. doi: 10.3390/nu12123774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silverii GA, Botarelli L, Dicembrini I, et al. Low‐carbohydrate diets and type 2 diabetes treatment: a meta‐analysis of randomized controlled trials. Acta Diabetol. 2020;57:1375‐1382. doi: 10.1007/s00592-020-01568-8 [DOI] [PubMed] [Google Scholar]
- 51. Bischoff SC, Boirie Y, Cederholm T, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2017;36:917‐938. doi: 10.1016/j.clnu.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 52. Patsalos O, Keeler J, Schmidt U, Penninx BWJH, Young AH, Himmerich H. Diet, obesity, and depression: a systematic review. J Pers Med. 2021;1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blaine BE, Rodman J, Newman JM. Weight loss treatment and psychological well‐being: a review and meta‐analysis. J Health Psychol. 2007;12:66‐82. doi: 10.1177/1359105307071741 [DOI] [PubMed] [Google Scholar]
- 54. Fabricatore AN, Wadden TA, Higginbotham AJ, et al. Intentional weight loss and changes in symptoms of depression: a systematic review and meta‐analysis. Int J Obes (Lond). 2011;35:1363‐1376. doi: 10.1038/ijo.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bales CW, Starr KNP, Orenduff MC, et al. Influence of protein intake, race, and age on responses to a weight‐reduction intervention in obese women. Curr Dev Nutr. 2017;1:1‐10. doi: 10.3945/cdn.117.000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brehm BJ, Lattin BL, Summer SS, et al. One‐year comparison of a high‐monounsaturated fat diet with a high‐carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32:215‐220. doi: 10.2337/dc08-0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cornier MA, Donahoo WT, Pereira R, et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13:703‐709. doi: 10.1038/oby.2005.79 [DOI] [PubMed] [Google Scholar]
- 58. Daly ME, Paisey R, Paisey R, et al. Short‐term effects of severe dietary carbohydrate‐restriction advice in Type 2 diabetes ‐ a randomized controlled trial. Diabet Med. 2006;23:15‐20. doi: 10.1111/j.1464-5491.2005.01760.x [DOI] [PubMed] [Google Scholar]
- 59. De Luis DA, Sagrado MG, Conde R, Aller R, Izaola O. The effects of two different hypocaloric diets on glucagon‐like peptide 1 in obese adults, relation with insulin response after weight loss. J Diabetes Complications. 2009;23:239‐243. doi: 10.1016/j.jdiacomp.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 60. De Luis DA, Aller R, Izaola O, Romero E. Effects of a high‐protein/low‐carbohydrate versus a standard hypocaloric diet on adipocytokine levels and cardiovascular risk factors during 9 months, role of rs6923761 gene variant of glucagon‐like peptide 1 receptor. J Endocrinol Invest. 2015;38:1183‐1189. doi: 10.1007/s40618-015-0304-9 [DOI] [PubMed] [Google Scholar]
- 61. Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low‐carbohydrate diet for obesity. N Engl J Med. 2003;348:2082‐2090. doi: 10.1056/nejmoa022207 [DOI] [PubMed] [Google Scholar]
- 62. Kerksick CM, Wismann‐bunn J, Fogt D, et al. Changes in weight loss, body composition and cardiovascular disease risk after altering macronutrient distributions during a regular exercise program in obese women. Nutr J. 2010;9:59. doi: 10.1186/1475-2891-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kitabchi AE, McDaniel KA, Wan JY, et al. Effects of high‐protein versus high‐carbohydrate diets on markers of β‐ Cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes. Diabetes Care. 2013;36:1919‐1925. doi: 10.2337/dc12-1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morris E, Aveyard P, Dyson P, et al. A food‐based, low‐energy, low‐carbohydrate diet for people with type 2 diabetes in primary care: A randomized controlled feasibility trial. Diabetes Obes Metab. 2020;22:512‐520. doi: 10.1111/dom.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Porter Starr KN, Pieper CF, Orenduff MC, et al. Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1369‐1375. doi: 10.1093/gerona/glv210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Racette SB, Schoeller DA, Kushner RF, Neil KM, Herling‐Iaffaldano K. Effects of aerobic exercise and dietary carbohydrate on energy expenditure and body composition during weight reduction in obese women. Am J Clin Nutr. 1995;61:486‐494. doi: 10.1093/ajcn/61.3.486 [DOI] [PubMed] [Google Scholar]
- 67. Samaha FF, Iqbal N, Seshadri P, et al. A low‐carbohydrate as compared with a low‐fat diet in severe obesity. N Engl J Med. 2003;348:2074‐2081. doi: 10.1056/NEJMoa022637 [DOI] [PubMed] [Google Scholar]
- 68. Stentz FB, Brewer A, Wan J, et al. Remission of pre‐diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care. 2016. doi: 10.1136/bmjdrc-2016-000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tonstad S, Malik N, Haddad E. A high‐fibre bean‐rich diet versus a low‐carbohydrate diet for obesity. J Hum Nutr Diet. 2014. doi: 10.1111/jhn.12118 [DOI] [PubMed] [Google Scholar]
- 70. Yancy WS. A low‐carbohydrate, ketogenic diet versus a low‐fat diet to treat obesity and hyperlipidemia. Ann Intern Med. 2004;140:769‐779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


