Abstract
The absence of glycopeptidolipids (GPLs) abolishes the ability of mycobacteria both to slide over the surface of motility plates and to form biofilms on polyvinyl chloride. In a screen for biofilm-defective mutants of Mycobacterium smegmatis mc2155, a new mutant was obtained that resulted in partial inhibition of both processes and also showed an intermediate rough colony morphology. The mariner transposon insertion mapped to a GPL biosynthesis gene (atf1) which encodes a putative acetyltranferase involved in the transfer of acetyl groups to the glycopeptide core. Physical characterization of the GPLs from the atf1 mutant demonstrated that they were not acetylated.
The mycobacterial cell membrane is surrounded by a thick and waxy envelope that contains very diverse lipids (6). The precise organization of the different lipids within the cell envelope has not yet been fully determined. While it is known that mycolic acids are bound to the subjacent arabinogalactan polysaccharide layer, which is in turn linked to the peptidoglycan layer (6, 9), the location and disposition of lipids that are not covalently bound and present on the outer side of the monolayer of mycolic acids is particularly unclear.
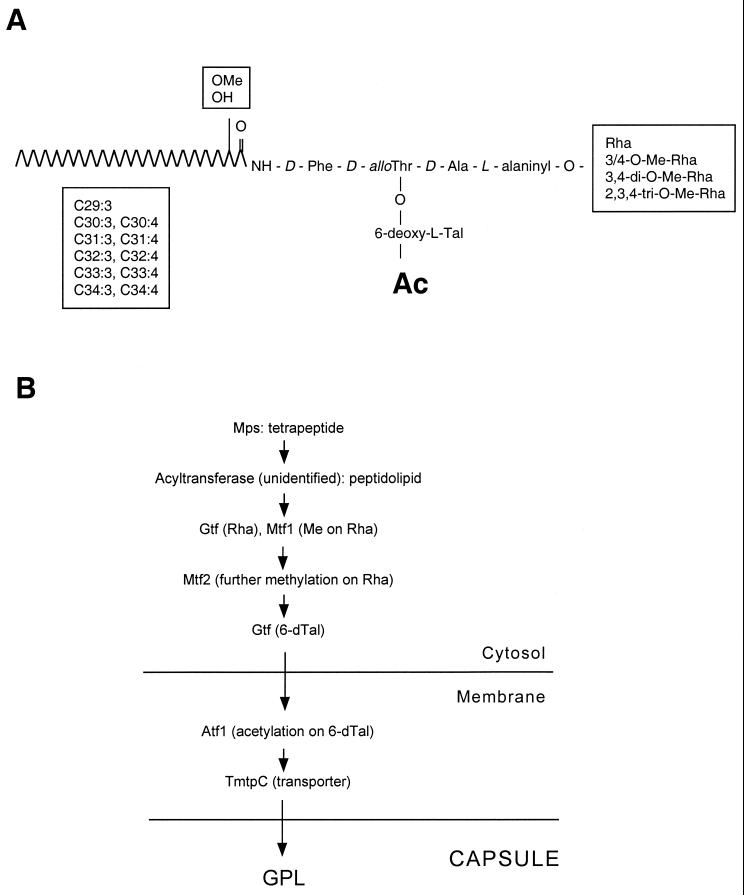
The glycopeptidolipids (GPLs) are present in the outermost layer of the lipid-rich cell walls of some mycobacterial species. The presence of haptenic oligosaccharides on the GPLs of the Mycobacterium avium complex results in the highly antigenic serovar-specific GPLs (ssGPLs) (2, 9). Mycobacterium smegmatis contains only non-serovar-specific GPLs (nsGPLs). The ssGPLs and the nsGPLs have a common glycopeptidolipid structure, containing an N-acylated tripeptide-amino alcohol (d-Phe–d-allo-Thr–d-Ala–l-alaninol) in which the C-terminal l-alaninol is glycosylated by an O-methylated rhamnosyl residue (Rha) and the d-allo-Thr is linked to a 6-deoxytalose (6-dTal) (see Fig. 7). In most ssGPLs of the M. avium complex, an additional Rha residue is added to the 6-dTal, and the gene responsible for this rhamnosylation (rtfA) has been identified (10). This modification does not occur in the nsGPLs present in M. smegmatis.
FIG. 7.
Non-serovar-specific GPL structure and proposed GPL biosynthesis pathway in M. smegmatis. (A) The generic structure of the GPLs found in M. smegmatis is shown. The location of specific methyl residues on the fatty acid tail and the Rha residue is indicated, as well as the acetylation (Ac) at the 6-dTal residue. (B) The GPL biosynthesis pathway is presented, indicating gene products shown or suggested to play a role in GPL biosynthesis, relative to their proposed subcellular localization.
Mutants lacking GPLs invariably display a rough colony morphology (1, 2). This fact has aided in the recent identification of the genes encoding the enzymes required for GPL biosynthesis. The M. smegmatis mps gene encodes a nonribosomal peptide synthetase responsible for the biosynthesis of the GPL tetrapeptide (3), and the mtf1 gene encodes a methyltransferase involved in the initial O-methylation of the Rha residue present in the GPL (18). Both the mps and mtf1 mutants exhibit a rough colony morphology. The mps mutant contains no GPLs, whereas the mtf1 mutant shows an overall 10-fold reduction in the levels of GPLs, which are all undermethylated at the Rha residue (3, 18). The mtf1 gene product (MeTase1) is a 3-O-methyltransferase responsible for the addition of the initial methyl group to the 3-position of the Rha residue of the nsGPLs (18).
We have recently shown that mycobacteria can translocate over the surface of solid growth medium by a sliding mechanism and can also form biofilms on polyvinyl choride (PVC) (13, 20). In each case, the mycobacterial cell surface is in direct contact with an abiotic surface and cells either slide over or attach to it, respectively. Both processes require the presence of GPLs (20). Previous screens resulted in extreme sliding defects as well as biofilm-defective phenotypes, exhibited by very rough colony morphology mutants that lacked GPLs. The genes affected in these mutants (mps and tmtpC) encoded products involved in GPL biosynthesis and possibly transport to the capsule (20). In this study we performed a new screen focused on obtaining biofilm-defective M. smegmatis mutants. As a result, we identified a mutant with intermediate phenotypes for sliding motility, biofilm formation, and colony morphology. This mutant contained a mariner transposon insertion in the atf1, gene, predicted to encode a product involved in GPL acetylation. We show that the GPLs isolated from this mutant are not acetylated. The methylation of the Rha residue occurs independently of the acetylation of the 6-dTal residue, since the atf1 GPLs are still methylated normally. Both the acetyltransferase Atf1 and the TmtpC putative transporter are predicted to be membrane proteins. Based on the recent findings about several genes involved in GPL biosynthesis, we propose a model for this biosynthetic pathway in which acetylation is one of the last steps.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Wild-type M. smegmatis mc2155 was grown on Luria-Bertani (LB) medium. All the mariner insertion mutants were grown on LB medium with kanamycin at 25 μg/ml (mariner mutants are described in reference 20). M63 salts minimal medium supplemented with 2% glucose, 0.5% Casamino Acids, 1 mM MgSO4, and 0.7 mM CaCl2 (biofilm medium) was used to assay for biofilm formation. The sliding-motility plates contained 0.3% ultrapure SeaKem LE agarose (FMC Bioproducts) as solidifying agent in M63 salts supplemented with 0.2% glucose.
DNA sequencing of the mariner insertion site.
The DNA sequence of the transposon insertion site was obtained using the arbitrary PCR technique and the primers described previously for the mariner transposon in M. smegmatis mc2155 (17, 20).
Screen for M. smegmatis mc2155 biofilm-defective mutants.
Cells from different mariner insertion mutants were inoculated from an LB agar plate into 100 μl of biofilm medium. The PVC microtiter dishes (96-well plates) were incubated at room temperature (RT) for 4 to 5 days. Crystal violet staining and ethanol extraction of the dye were used to analyze biofilm formation as described previously (20). A total of 3,000 mariner insertion mutants were screened.
Sliding-motility assay.
Cells from colonies grown on LB plates were inoculated via sterile toothpicks onto motility plates. The plates were incubated at 37°C under humid conditions for 2 to 3 days until a transparent halo surrounding the inoculation point was observed for the wild-type strain.
Mycobacterial cell attachment to PVC slides.
A PVC slide was placed at the bottom of a small petri dish containing biofilm medium inoculated with cells from a fresh colony grown on an LB agar plate. A random frame on the PVC slide was monitored for cell attachment for 48 h with a Nikon Diaphot 200 inverted phase-contrast microscope. The images were captured with a black-and-white CCD72 camera integrated with a Power Macintosh 8600-300 computer with video capability (Apple, Cupertino, Calif.). Images were processed using Scion Image (Scion Corp.) and Photoshop 4.0.1 (Adobe) software.
Extraction and analysis of GPLs.
GPLs were isolated from cells grown on Middlebrook 7H9 (supplemented with 0.5% bovine serum albumin fraction V, 0.2% dextrose, and 0.85% sodium chloride) motility plates as described previously (13, 20). Deacetylation of half of each lipid extract was performed by alkaline methanolysis (5). Thin-layer chromatography (TLC) was performed using silica gel plates with inorganic binder (Analtech) that were developed in the solvent mix chloroform-methanol-water (90:10:1, vol/vol/vol) after application of the GPL samples. The GPL bands were visualized by spraying with 10% H2SO4 in ethanol and heating at 120°C as described previously (13, 20). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) of all the GPL samples was obtained with a Perspective Biosystems Voyager-DE STR instrument in the positive mode (Harvard University Microchemistry Facility). Samples were resuspended in chlorofom-methanol (1:1).
RESULTS
Genetic screen for M. smegmatis biofilm-defective mutants.
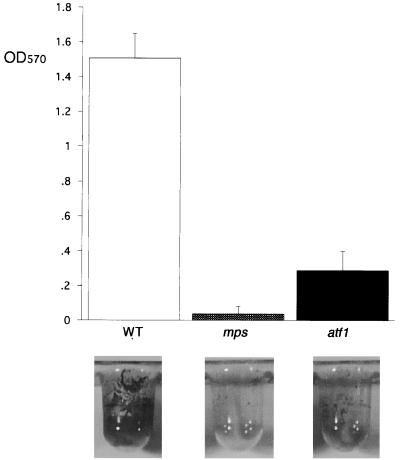
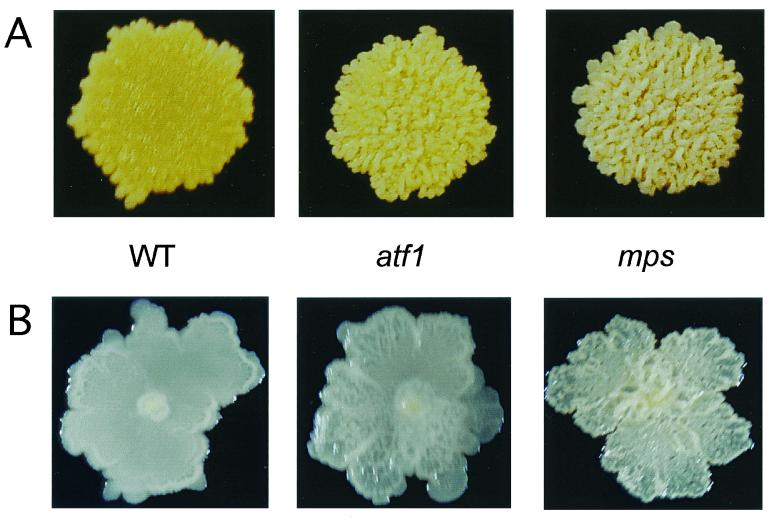
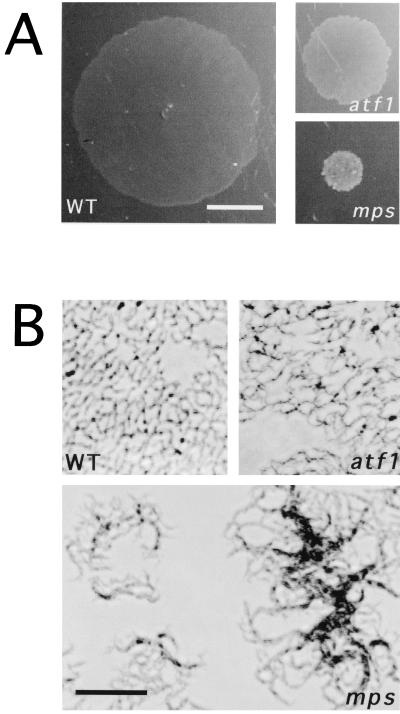
We have shown that M. smegmatis nonsliding mutants that lack GPLs are also defective in biofilm formation (20). We set up a new screen to identify additional genes that, when disrupted, resulted in an impaired ability to form biofilms on PVC. Mutations in the mps and tmtpC genes were previously known to completely eliminate the ability of M. smegmatis to form biofilms (20); these results were confirmed with the modified medium and incubation conditions used in this study. One new mutant was obtained that formed very weak but detectable biofilms (Fig. 1). This mutant showed a “partial rough” colony morphology, which is intermediate between the smooth wild-type colony (M. smegmatis mc2155 strain) and the mps and tmtpC very rough colony appearance on agar plates (Fig. 2).
FIG. 1.
New M. smegmatis mutant defective in biofilm formation. Crystal violet-stained PVC wells and quantitation of the corresponding ethanol-removed dye are shown for the indicated strains. The results for each strain represent the average of three independent colonies analyzed. The mps (shown) and tmtpC strains behave similarly in the biofilm formation assay. OD570, optical density at 570 nm; WT, wild type.
FIG. 2.
The new mutant in biofilm formation shows intermediate rough colony morphology. Colony morphologies of the indicated strains grown for 3 to 4 days at 37°C on LB (A) plates or M63 salts plus 2% glucose plates (B) are shown. The mps (shown) and tmtpC strains exhibit an identical colony morphology. WT, wild type.
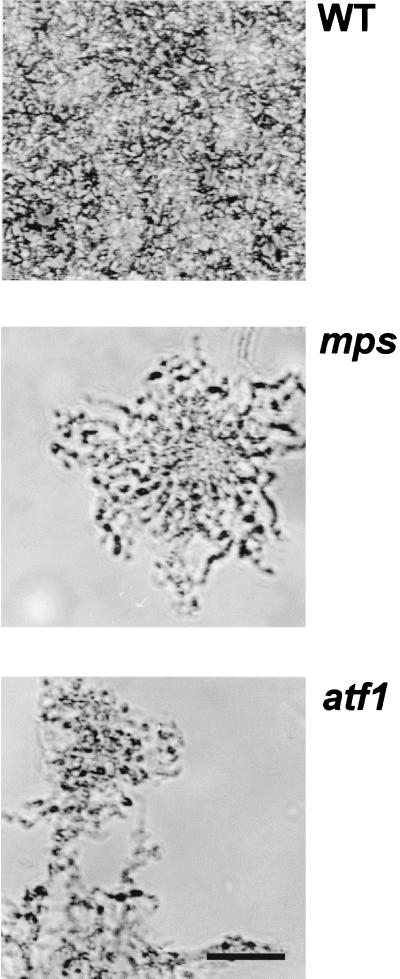
The first step in biofilm formation is the transition of individual cells from the planktonic condition to the attached state (15). It has been shown that in different motile bacterial species, specific cell surface-associated structures such as flagella and pili play an essential role in initial cell attachment in the process of biofilm formation (Escherichia coli [19], Pseudomonas aeruginosa [16], and Vibrio cholerae [23]. For mycobacteria, which lack flagella and pili and do not swim or display twitching motility, we proposed that the GPLs and perhaps other capsular lipidic molecules play a crucial role in attachment to PVC. To observe this phenomenon microscopically, we monitored attachment for 48 h at RT. Wild-type M. smegmatis cells started attaching early on, and at 48 h the entire surface was covered with microcolonies that consituted the three-dimensional biofilm. Neither the very rough mutants (mps and tmtpC) nor the partially rough new mutant ever completely covered the PVC surface, even when left for as long as 6 days (results not shown). A representative 48-h frame of each strain is shown in Fig. 3, where it is clear that both types of rough mutants had difficulty in the initial steps of attachment. In addition, at the few regions where there was cell attachment, this consisted of a very clumpy microcolony, as opposed to the more homogeneous accumulation of wild-type cells. This behavior was similar to that observed in liquid medium for these strains: the partially rough and the very rough mutants were hardly distinguishable and were both extremely clumpy in liquid culture. The difference between the two types of mutants was apparent, however, when colony morphology (Fig. 2) or sliding or biofilm formation were examined.
FIG. 3.
Rough mutants are defective in attachment to PVC. Attachment to a PVC slide of the indicated strains at 48 h of incubation is shown by phase-contrast microscopy. Cells were inoculated from a fresh colony into a small Petri dish containing biofilm medium. The PVC slide was placed at the bottom of the Petri dish, and attachment was monitored continuously at RT. WT, wild type. Bar, 25 μm.
The weak biofilm mutant shows impaired sliding.
The partially rough, weak biofilm M. smegmatis mutant was tested for its ability to slide over the surface of motility plates. As with colony morhpology and biofilm formation, this mutant also showed an intermediate phenotype for sliding motility: it was able to slide compared to the nonsliding mps or tmtpC mutants that lack GPLs, but its sliding was noticeably diminished compared to wild-type M. smegmatis (Fig. 4A). The mutant microscopic morphology over motility plates was nearly identical to that of the wild-type strain, contrasting with the clumpy appearance of the rough strain, mps (Fig. 4B). Taken together, these results placed this new mutant at an intermediate level between wild-type M. smegmatis and the rough mutants characterized previously that lacked GPLs. We reasoned that specific molecules in the capsule, either GPLs or other lipidic molecules, were affected in this mutant.
FIG. 4.
Mutants impaired in biofilm formation are also defective in sliding motility. (A) Translocation over the surface of M63 salts plates supplemented with 0.2% glucose containing 0.3% agarose as solidifying agent (motility plates) is shown for the indicated strains after 3 days of incubation at 37°C. Bar, 1 cm. (B) Cells from the border of the sliding halo observed under the inverted phase-contrast microscope. Bar, 25 μm. WT, wild type.
The intermediate morphology and biofilm mutant contains a mariner transposon insertion in the atf1 gene.
The mariner transposon in the new mutant was inserted in a gene, atf1, present in a GPL biosynthesis gene cluster (GenBank accession no. AAF05992). This gene, based on sequence homology, is predicted to encode an acetyltransferase product of 394 amino acid residues. The mariner transposon is inserted at codon 320. The atf1 gene is located immediately upstream of mtf1 in the GPL gene cluster (Fig. 5). The mtf1 gene product has been recently identified (18) as a methyltransferase involved in the intial methylation of the GPL Rha residue.
FIG. 5.
GPL gene cluster. The GPL gene cluster contaning the mtf1 and atf1 genes in M. smegmatis is shown, based on the gene sequences submitted to GenBank under accession no. AAF05992. The mariner insertion at codon 320 of atf1 is indicated.
The GPLs from the atf1 mutant are not acetylated.
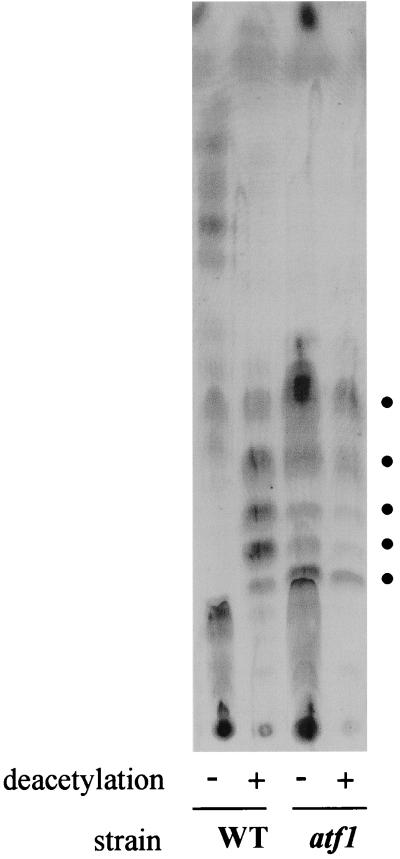
The partial rough colony morphology of the atf1 mutant suggested that the GPLs in this mutant were either altered or expressed in reduced amounts. To determine what kind of GPLs were present in this mutant, we performed TLC of lipid extracts. The extracts were split in two. One half was subject to mild deacetylation (alkaline methanolysis treatment), while the other half was left untreated. The deacetylation is normally used for GPL isolation because it removes ester-linked fatty acids (leaving intact the amide-linked lipidic tail of the GPLs) as well as all attached O-acetyl groups. This leads to better purification of GPLs from other mycobacterial lipids and greater resolution on TLC (4, 7). The wild-type untreated GPLs migrate faster because of their higher hydrophobicity due to the presence of the acetyl groups (Fig. 6). The wild-type deacetylated GPL profile corresponds exactly to that of the atf1 mutant, which looks the same with or without deacetylation treatment. Together, these results indicate that the groups removed by the deacetylation treatment are absent in the atf1 mutant GPLs. The mtf1 mutant, defective in methylation of the GPL Rha residue, contains undermethylated GPLs that show reduced mobility on TLC (18), which is not observed for the atf1 GPLs. Taken together, these results suggest that the GPLs of the atf1 mutant lack only the acetyl groups present in wild-type GPLs and are otherwise correctly modified—in particular, they are correctly methylated.
FIG. 6.
The atf1 mutant GPLs are not acetylated. GPLs analyzed by TLC are shown for the indicated strains. Lipid extracts were made from cells grown on plates and either subjected to mild NaOH deacetylation treatment (+) or not deacetylated (−). The dots indicate the GPLs observed by the Brennan and Goren method of GPL analysis that includes deacetylation. WT, wild type.
Two acetyl groups are thought to be added to the 6-dTal residue of the GPLs of M. smegmatis (3). To confirm that the mild deacetylation treatment is actually removing groups that correspond in mass to two acetyl residues, we analyzed the same samples shown in Fig. 6 by MALDI-TOF MS. The results of this analysis are shown in Table 1. All the spectra consisted of seven to nine peaks corresponding to molecular species varying by about 14 atomic mass units. This variation reflects the heterogeneity of the GPL fatty acid chain length, as well as the varying degree of methylation (di or tri) of the rhamnose residue and the O-methylation that sometimes occurs on the only hydroxyl group of the fatty acid (18). As expected from the TLC GPL profiles, the spectra for deacetylated wild-type GPLs and for both atf1 untreated and deacetylated GPLs are almost identical (in terms of both molecular mass and number of peaks). Most importantly, the wild-type untreated GPLs show approximately the same number of peaks, all shifted up in mass by values very close to 84 atomic mass units, corresponding exactly to the mass of two acetyl groups. The addition of two acetyl groups on the 6-dTal residue thus seems to occur in all of the GPL molecules present in the M. smegmatis cell wall. In the GPL gene cluster present in M. smegmatis, only one gene encoding an acetyltransferase (atf1) is present (Fig. 5). The results presented here show that the product of this gene is responsible for acetylation at either one or two sites on the 6-dTal residue. The possibility of an additional GPL acetyltransferase remains to be addressed. The phenotype of the atf1 mutant indicates, however, that if this is the case, acetylation by Atf1 is the first to occur and is absolutely required for the second acetylation step.
TABLE 1.
GPL analysis by MALDI-TOF MS
| Peak | Observed m/z of:
|
|||
|---|---|---|---|---|
| WTa | WT deacetylatedb | atf1 | atf1 deacetylatedb | |
| 1 | 1,216.9 | 1,132.75 | 1,132.00 | 1,132.90 |
| 2 | 1,230.4 | 1,146.34 | 1,146.20 | 1,146.72 |
| 3 | 1,244.9 | 1,160.04 | 1,160.55 | 1,160.97 |
| 4 | 1,259.13 | 1,175.01 | 1,174.24/1,175.12c | 1,172.5/1,174.92c |
| 5 | 1,274.13 | 1,187.54 | 1,186.24/1,190.14c | 1,186.54/1,188.86c |
| 6 | 1,285.71 | 1,201.32 | 1,200.79/1,204.21c | 1,201.26 |
| 7 | 1,298.5/1,300.54c | 1,214.2/1,216.21c | 1,216.28 | 1,214.99 |
| 8 | 1,313.41 | 1,229.36 | 1,230.50 | 1,228.60 |
| 9 | 1,242.41 | 1,244.00 | ||
WT, wild type.
The lipid extract was subjected to mild deacetylation by the method of Brennan and Goren (5).
These two peaks do not vary in mass by more than 4 atomic mass units.
DISCUSSION
In this study we have characterized a new mutant defective in a step of the GPL biosynthetic pathway. This mutant shows intermediate phenotypes for two phenomena that were recently reported to occur in M. smegmatis (sliding motility and biofilm formation) and also exhibits a partial rough colony morphology. Taken together, our present and previous results show that there is a perfect correlation between the presence of GPLs on the mycobacterial cell wall of M. smegmatis and colony morphology, sliding motility, and biofilm formation. The three phenotypes that we have shown to be clearly affected by mutations in GPL biosynthesis are in fact a result of cells growing on an abiotic surface (colony morphology and sliding motility on the surface of agar plates, biofilm formation on PVC).
We had suggested a model for the role of GPLs in both sliding motility and biofilm formation based on electrostatic interactions between the mycobacterial capsule and the respective abiotic surface (20). In this model, the exposed GPLs make the mycobacterial surface hydrophobic due to the presence of the very long lipidic tails. This favors the mycobacterial interaction with the hydrophobic PVC surface (cell attachment) or the sliding motility on a hydrophilic surface. We had presented the GPL tails exposed to the exterior for simplicity; however, there is no direct evidence for a specific head-to-tail orientation of the GPLs on the mycobacterial capsule. The rough mutants lacking GPLs would expose the underlying phospholipid-rich capsule, which is more hydrophilic, and this would lead to the inability to slide over the hydrophilic surface or to attach to the hydrophobic PVC. The new atf1 mutant described here contains GPLs that are not acetylated, which are overall more hydrophilic at their glycopeptide end. This would result in an overall less hydrophobic GPL layer than in wild-type cells, which would explain the intermediate ability to slide over a hydrophilic surface and attach to a hydrophobic surface.
The mtf1 mutant defective in initial O-methylation of the Rha residue results in an overall 10-fold reduction of GPL levels, which is responsible for the rough colony phenotype observed (18). The authors mention that 6-dTal O-acetylation in this mutant is not affected (18). The GPLs of the atf1 mutant, which are not acetylated, are still correctly methylated; therefore, these two steps do not seem to affect each other in the biosynthetic pathway. Methylation of the atf1 GPLs indicates that the mariner insertion in this mutant did not abolish the expression of downstream genes in the same operon, since the immediate downstream gene is mtf1. Mtf1 is not a membrane protein, but it is associated with the membrane fraction when purified from wild-type cells (18). The unacetylated atf1 GPLs seem to be able to reach their final destination at the cell wall, indicating that transport does not require proper 6-dTal acetylation. Atf1 is predicted to be a membrane protein containing at least 10 transmembrane domains (Membrane Protein Secondary Structure Prediction Server [SPLIT]). The TmtpC putative GPL transporter is also a large membrane protein (20). This suggests that acetylation of the GPLs is one of the last steps in their synthesis, maybe concomitant with transport. We present a model for the GPL biosynthesis pathway in Fig. 7.
The mycobacterial ability to colonize by either attaching to or sliding over an abiotic surface would be clearly advantageous when the particular surface offers nutrients that can be used preferentially by the colonizing species and/or protection from environmental insults. For pathogenic species of mycobacteria, the ability to either attach to or slide over surfaces such as animal tissues could be important at different stages of pathogenesis. In this regard, a correlation between colony morphology (i.e., the presence or absence of certain capsular lipids) and virulence in animal models has been shown to exist in several mycobacterial species, including M. avium (12, 14, 21, 22) and, recently, M. tuberculosis (8, 11).
ACKNOWLEDGMENTS
This work was supported by NIH grants GM58213 and GM55199 to R.K. and an NIH minority postdoctoral supplement to J.R.
REFERENCES
- 1.Barrow W W, Brennan P J. Isolation in high frequency of rough variants of Mycobacterium intracellularelacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982;150:381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belisle J T, McNeil M R, Chatterjee J M, Inamine J M, Brennan P J. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993;268:10510–10516. [PubMed] [Google Scholar]
- 3.Billman-Jacobe H, McConville M J, Haites R E, Kovacevic S, Coppel R C. Identification of a peptide synthetase involved in the biosynthesis of glycopeptidolipids of Mycobacterium smegmatis. Mol Microbiol. 1999;33:1244–1253. doi: 10.1046/j.1365-2958.1999.01572.x. [DOI] [PubMed] [Google Scholar]
- 4.Brennan P J, Goren M B. Mycobacterial glycolipids as bacterial antigens. Biochem Soc Trans. 1977;5:1687–1693. doi: 10.1042/bst0051687. [DOI] [PubMed] [Google Scholar]
- 5.Brennan P J, Goren M B. Structural studies on the type-specific antigens and lipids of the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulareserotype 9. J Biol Chem. 1979;254:4205–4211. [PubMed] [Google Scholar]
- 6.Brennan P J, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 7.Brennan P J, Souhrada M, Ullom B, McClatchy J K, Goren M B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978;8:374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox J S, Chen B, McNeil M, Jacobs W R., Jr Complex lipid determines tissue-specific replication of Mycobacterium tuberculosisin mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 9.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 10.Eckstein T M, Silbaq F S, Chatterjee D, Kelly N J, Brennan P J, Belisle J T. Identification and recombinant expression of a Mycobacterium avium rhamnosyltransferase gene (rtfA) involved in glycopeptidolipid biosynthesis. J Bacteriol. 1998;180:5567–5573. doi: 10.1128/jb.180.21.5567-5573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glickman M S, Cox J S, Jacobs W R., Jr A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Molecular Cell. 2000;5:717–727. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 12.Kansal R G, Gómez-Flores R, Metha R T. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium aviumcomplex. Microb Pathog. 1998;25:203–214. doi: 10.1006/mpat.1998.0227. [DOI] [PubMed] [Google Scholar]
- 13.Martínez A, Torello S, Kolter R. Sliding motility in mycobacteria. J Bacteriol. 1999;181:7331–7338. doi: 10.1128/jb.181.23.7331-7338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moehring J M, Solotorovsky M R. Relationship of colony morphology to virulence to chickens of Mycobacterium aviumand the non-photochromogens. Am Rev Respir Dis. 1965;92:704–713. doi: 10.1164/arrd.1965.92.5.704. [DOI] [PubMed] [Google Scholar]
- 15.O'Toole G, Kaplan H B, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 16.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosabiofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Toole G A, Pratt P A, Watnick P I, Newman D K, Weaver V B, Kolter R. Genetic approaches to study biofilms. Methods Enzymol. 1999;310:91–109. doi: 10.1016/s0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 18.Patterson J H, McConville M J, Haites R E, Coppel R S, Billman-Jacobe H. Identification of a methyltransferase from Mycobacterium smegmatisinvolved in glycopeptidolipid synthesis. J Biol Chem. 2000;275:24900–24906. doi: 10.1074/jbc.M000147200. [DOI] [PubMed] [Google Scholar]
- 19.Pratt L A, Kolter R. Genetic analysis of Escherichia colibiofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 20.Recht J, Martínez A, Torello S, Kolter R. Genetic analysis of sliding motility in Mycobacterium smegmatis. J Bacteriol. 2000;182:4348–4351. doi: 10.1128/jb.182.15.4348-4351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy V M, Luna-Herrera J, Gangadharam P R J. Pathobiological significance of colony morphology in Mycobacterium aviumcomplex. Microb Pathog. 1996;21:97–109. doi: 10.1006/mpat.1996.0046. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer W B, Davis C L, Cohn M L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium aviumin chickens and mice. Am Rev Respir Dis. 1970;102:499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- 23.Watnick P I, Kolter R. Steps in the development of a Vibrio choleraebiofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]