Abstract
Our study reports the discovery and evaluation of nanoparticle aided sensitive assays for glycovariants of MUC16 and MUC1 in a unique collection of paired ovarian cyst fluids and serum samples obtained at or prior to surgery for ovarian carcinoma suspicion. Selected glycovariants and the immunoassays for CA125, CA15‐3 and HE4 were compared and validated in 347 cyst fluid and serum samples. Whereas CA125 and CA15‐3 performed poorly in cyst fluid to separate carcinoma and controls, four glycovariants including MUC16MGL, MUC16STn, MUC1STn and MUC1Tn provided highly improved separations. In serum, the two STn glycovariants outperformed conventional CA125, CA15‐3 and HE4 assays in all subcategories analyzed with main benefits obtained at high specificities and at postmenopausal and early‐stage disease. Serum MUC16STn performed best at high specificity (90%‐99%), but sensitivity was also improved by the other glycovariants and CA15‐3. The highly improved specificity, excellent analytical sensitivity and robustness of the nanoparticle assisted glycovariant assays carry great promise for improved identification and early detection of ovarian carcinoma in routine differential diagnostics.
Keywords: diagnosis, epithelial ovarian cancer, europium nanoparticle, mucins, STn
What's new?
While MUC16 represents a promising serum marker for epithelial ovarian cancer, its inadequate specificity has impeded clinical applications. Our study using a novel immunoassay with fluorescent nanoparticles coated with glycan structure‐specific binders shows that cancerous sub‐forms of MUC16 and MUC1 can be quantitated while suppressing mucin signals from confounding benign conditions. In ovarian cyst fluids, immunoassays for MUC16 and MUC1 STn glycovariants were superior to conventional CA125 and CA15‐3 immunoassays. In paired serum samples, the main benefits were seen in postmenopausal and early‐stage patients. The results pave the way for improved routine differential diagnostics of epithelial ovarian cancer.
Abbreviations
- AUC
area under the curve
- CA125
cancer antigen 125
- CA15‐3
cancer antigen 15‐3
- CF
cyst fluid
- CI
confidence interval
- ctDNA
circulating tumor DNA
- CV
coefficient of variance
- EOC
epithelial ovarian cancer
- λ em
emission wavelength
- λ ex
excitation wavelength
- F(ab′)2
fragment affinity‐purified secondary antibody
- Fc
fragment crystallizable
- FIGO
The International Federation of Gynecology and Obstetrics
- GV
glycovariant
- HE4
human epididymis protein 4
- IA
immunoassay
- IgG
immunoglobulin G
- mAB
monoclonal antibody
- MGL
macrophage galactose‐binding lectin
- MUC1
mucin 1
- MUC16
mucin 16
- n
number
- NP
nanoparticles
- pAUC
partial area under the curve
- ROC
receiver operating characteristic
- ROCA
risk of ovarian cancer algorithm
- ROMA
risk of ovarian malignancy algorithm
- RT
room temperature
- SA
streptavidin
- SN
sensitivity
- SP
specificity
- STn
sialyl‐Tn antigen
- TRF
time‐resolved fluorescence
- UKCTOCS
UK Collaborative Trial of Ovarian Cancer Screening
- UKNSC
UK National Screening Committee
- WGA
wheat Germ agglutinin
1. INTRODUCTION
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy, 1 and the seventh most common cancer worldwide. 2 Since early EOC is associated with vague and unspecific symptoms, the disease is usually diagnosed at later stages (FIGO III or IV), with relative survival at 5 years less than 30%. 3 , 4 The poor outlook of EOC could be improved to a 90% 5‐year survival rate if diagnosed at an early‐stage. 5 The currently employed diagnostic tests and imaging techniques are neither reliable nor accurate for early EOC detection. Immunoassays (IA) of CA125/MUC16 was the first and to date the most well‐documented serum marker for EOC differential diagnosis, progression and for monitoring of therapy response. 5 , 6 However, high concentrations are also found in benign gynecological conditions (ie, endometriosis, pregnancy, heart failure, menstruation and pelvic inflammatory disease) 7 , 8 as well as in a wide range of other malignant carcinomas (colorectal, breast, lung, liver, gastric and pancreatic). 9 , 10 The inadequate specificity of CA125 impedes its use for early‐stage EOC diagnosis and disease progression. 11 For this reason, supplementary biomarkers to CA125, such as HE4 12 or multimodal diagnostic tests and algorithms (ROMA, ROCA, OVA1 and Overa) with CA125 as the key component have been proposed. 7 , 13 , 14 In the United Kingdom, a large prospective EOC screening study (UKCTOCS) combined ultrasound and serial CA125 measurements, with results suggesting that this strategy could improve early detection and reduce disease mortality. 15 However, the UK National Screening Committee (UKNSC) did not recommend it for systematic population screening in its July 2017 report. Novel biomarkers such as autoantibodies and microRNAs have also been reported as potential approaches for early detection. 16 Presently no accepted screening modalities are available and a majority of EOC cases continue to be detected at late stages. Both MUC16 and mucin 1 (MUC1 also known as CA15‐3) are reported to be elevated in patients with advanced EOC stage, approximately 90% 17 and 70%, 18 respectively.
Protein glycosylation is a diverse source of posttranslational modifications and alterations during this process is a common feature of tumor cells. 19 , 20 Mucins contain carbohydrates up to 70% to 80% of their molecular mass. 21 Changes in O‐glycosylation, also known as mucin glycosylation, can disrupt the normal maturation of O‐glycans, which can lead to the neo‐ and/or overexpression of Thomsen‐Friedenreich‐related T, Tn and STn antigens at the cell surface. 22 , 23 Loss of polarity in the epithelial cell membrane during ovarian tumorigenesis causes the upregulation of both mucins and their release in the bloodstream. 24 Alterations in the MUC1 and MUC16 glycosylation patterns can be targeted for potential biomarker discovery in EOC. 25 , 26 , 27 Many studies have reported both Tn and STn as prognostic markers during treatment and as sensitive markers in the detection of disease recurrence in various cancers. 22 , 28 We have previously reported improved differential diagnostic and prognostic performance of EOC‐specific MUC16 GV sandwich assays using a human macrophage galactose‐binding lectin (MUC16MGL) 29 or an anti‐STn antibody (MUC16STn) 30 coated on fluorescent europium nanoparticles (NP) in combination with anti‐CA125 specific mAb for capture. The central concept using the NP for detection is to provide low/medium affinity lectins and antibodies enhanced binding avidity on the surface of the NP, while maintaining their innate glycan specificity.
Although serum and plasma remain preferred targets in the search of new biomarkers, 31 other accessible bodily fluids (urine, saliva, ovarian cyst fluids, cerebrospinal fluids and ascites) are also occasionally used. 32 Tumors of the ovary are frequently benign and commonly grow in combined solid formations and fluid filled cavities called cysts. 33 , 34 The fluid contained in these cysts in close proximity to the actual tumor makes it a potential reservoir of secreted or shed proteins, glycans and genetic material from the tumor, reflecting its malignant or benign states. 32 , 35 Diagnostic imaging techniques are often unable to distinguish malignant from benign tumor, which leads to unnecessary and invasive surgeries on ovaries later found to be benign adenomas or normal physiologic cysts. 36 Therefore, early and differential diagnosis of EOC is needed to decrease morbidity and increase survival of the patients.
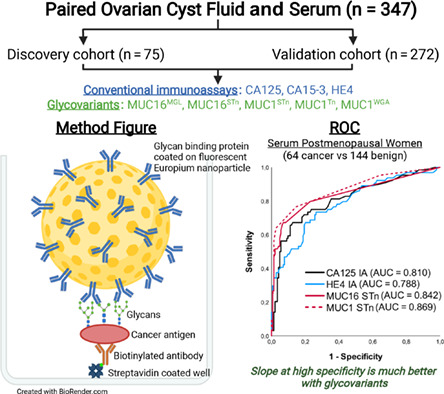
Our present study reports for the first time the measurement of GVs of two mucins (MUC16 and MUC1) in preoperative ovarian cyst fluid (CF) specimens and paired serum samples from the same timepoint. GVs were compared to conventional CA125, CA15‐3 and HE4 IA levels. The proximity of CF to the tumor provides an attractive opportunity for the discovery of novel markers or glycoforms of existing EOC biomarkers. With this sample collection, divided into a discovery cohort (CF and serum, n = 75) and patient validation cohort (CF and serum, n = 272), we have aimed to validate our previous MUC16 GV assays and to look for additional MUC16 and MUC1 based biomarkers.
2. MATERIALS AND METHODS
2.1. Study design
Blood samples and paired ovarian CF were collected prospectively and consecutively at the time for diagnostic and debulking surgery between 2001 and 2010, at the unit for gynecologic cancer surgery at Sahlgrenska University Hospital, Gothenburg. All patients with a suspected malignant ovarian cyst were addressed. Patients with neoadjuvant chemotherapy and patients not accepting or understanding informed and written consent were excluded. Blood samples were taken after anesthesia but prior to surgery while CF was aspirated directly after removal of the cyst from the abdomen. All samples were cooled to 4°C within 15 to 30 minutes, centrifuged, aliquoted into Eppendorf tubes and stored in −80°C until analyzed. Handling and processing of samples were standardized for all patients. All tumors were diagnosed, staged and graded according to existing FIGO classification, and reviewed according to FIGO 2014 by specialist in gynecologic pathology. The study included a discovery cohort (n = 75) and a validation cohort (n = 272) of paired CF and serum samples.
2.2. Materials
The ovarian cancer cell line OVCAR‐3 purified CA125, anti‐CA125 mAb (Ov185) and anti‐CA15‐3 mAb (Ma552), which recognize protein epitopes, and STn1242 mAb that recognizes sialylated‐Tn antigen were kindly provided by Fujirebio Diagnostics (Gothenburg, Sweden). The anti‐Tn mAb was purchased from SBH Sciences Natick, Massachusetts. The human recombinant C‐type lectin CLEC10A protein, also known as macrophage galactose lectin with human IgG Fc tag (MGL‐Fc) (catalog number 10821‐H01H) was obtained from Sino Biologicals Inc. China. Yellow streptavidin (SA) coated low fluorescence microtitration plates, wash buffer and the assay buffer was obtained from Kaivogen Oy (Turku, Finland). Europium (III)‐Chelate‐dyed Fluoro‐Max polystyrene nanoparticles (95 nm in diameter, 30 000 chelates per particle) were purchased from Seradyn Inc. (Indianapolis, Indiana).
2.3. Methods
Initially five GV markers were selected for testing in the discovery cohort. These included two GVs of MUC16 (MUC16MGL and MUC16STn) and three of MUC1 (MUC1STn, MUC1Tn and MUC1WGA). To validate these results, the conventional CA125, CA15‐3 and HE4 (in serum only) IA and their GV assays were then tested in a validation cohort of paired ovarian CF and serum samples (n = 272). This validation set was tested based on the findings from the discovery cohort. Postmenopausal women are the most prone cancer group and comprised of most of the cancers (~80%) in our study. Combination markers were tested for benefits and only the best combinations are reported.
2.4. Conventional and glycovariant assays
Fujirebio CanAg CA125 EIA, CanAg CA15‐3 EIA and CanAg HE4 EIA kits were used according to manufacturer's instructions for conventional assays. The GV assays of CA125 and CA15‐3 have been described earlier. 29 , 30 , 37 Briefly, biotinylated capture anti‐CA125 (Ov185 Fab2) and anti‐CA15‐3 (Ma552 Fab2) (50 ng/30 μL/well) were immobilized to streptavidin‐coated low‐fluorescence microtiter wells (Kaivogen Oy) in the assay buffer for 60 minutes at room temperature (RT) without shaking. After washing twice, 25 μL of standard (OVCAR‐3 cell line purified CA125) or diluted CF (1:50) or serum (1:10) sample was added in triplicates and incubated for 60 minutes at RT with shaking. For tracer, 25 μL assay buffer containing 1 × 107 Eu+3‐NPs coated with lectins MGL or WGA or anti‐STn and Tn mAb was added, (with additional 6 mM of CaCl2 for MGL) to each well and incubated for 1.5 hours at RT with shaking. After the incubation, the wells were washed six times with wash buffer. TRF for Eu+3 was measured (λ ex: 340 nm; λ em: 615 nm) from dry wells using Victor 1420 Multilabel Counter (Perkin‐Elmer Life Sciences, Wallac, Turku, Finland).
CA125 from the ovarian cancer cell line (OVCAR3) was used to make standards from 2 to 500 U/mL for MUC16 GVs. An EOC patient's ascetic fluid having 300 U/mL of CA15‐3 was used for standards of MUC1 GVs. All standards and samples were added in triplicate each day and CV% (coefficient of variance) was calculated. For each GV assay, two separate controls of lower and higher concentration were also included in each plate every day. The day‐to‐day control variation was observed and the result is included in Appendix S1.
2.5. Statistical analysis
All statistical calculations were performed using IBM SPSS Statistics, version 25 and Origin, version 2016 for Windows. The receiver operating characteristic curve (ROC) analysis was carried out by plotting specificity (SP) against sensitivity (SN) of the assay and measuring area under the curve (AUC). The partial AUC (pAUC) and confidence intervals (CI) were calculated with the pROC package in R. 38 AUC values and pAUC at 90% to 100% SP (0.9‐1) were calculated for all at 95% CI. The ROC curves for marker combinations were derived using logistic regression in SPSS. Origin was used to make the boxplots and calculate the P‐values using the two‐sample t‐test, where P‐value below .05 was considered significant.
3. RESULTS
3.1. Discovery cohort—Paired ovarian cyst fluid and serum
The mean age for the healthy, benign, borderline and malignant patients in the discovery cohorts was between 50 and 62 years, similar to the mean ages in the validation cohorts. Different GV markers of MUC16 (MUC16MGL and MUC16STn) and MUC1 (MUC1STn, MUC1Tn and MUC1WGA) were tested in the discovery CF and serum cohorts. In the CF cohort, all 5 GVs showed significance between the benign (n = 12) and the early‐stage EOC (n = 26) group, with the strongest difference seen with MUC16STn (P = .001) (Figure 1). In the serum cohort, significance was seen only with one GV, MUC16STn (P = .013) along with the conventional CA125 IA (P = .016). HE4 IA in serum did not show significance between the benign and early‐stage groups (Figure 2). Based on the overall performance, three GVs MUC16MGL, MUC16STn and MUC1STn were selected to be tested in both validation cohorts. MUC1Tn was not detected in serum (Figure 2G), and hence was included only in the cyst fluid validation cohort.
FIGURE 1.
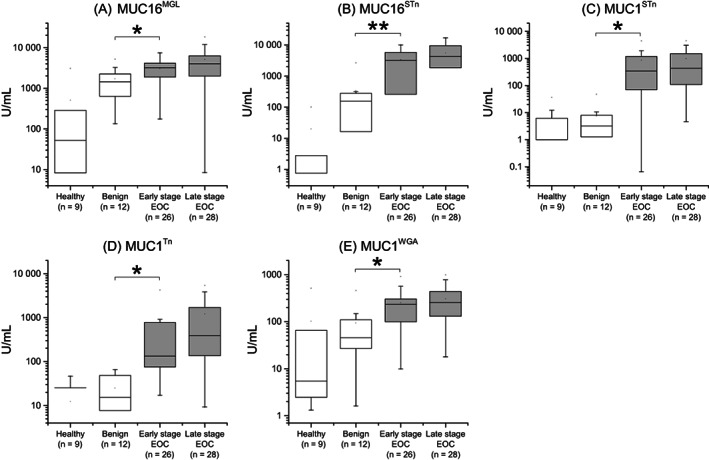
Box plots from ovarian cyst fluid discovery cohort (n = 75). Two (A, B) MUC16 and three (C, D, E) MUC1 GVs in different groups of healthy, benign, early‐stage EOC and late‐stage EOC. Data information: Data is presented as U/mL concentration
FIGURE 2.
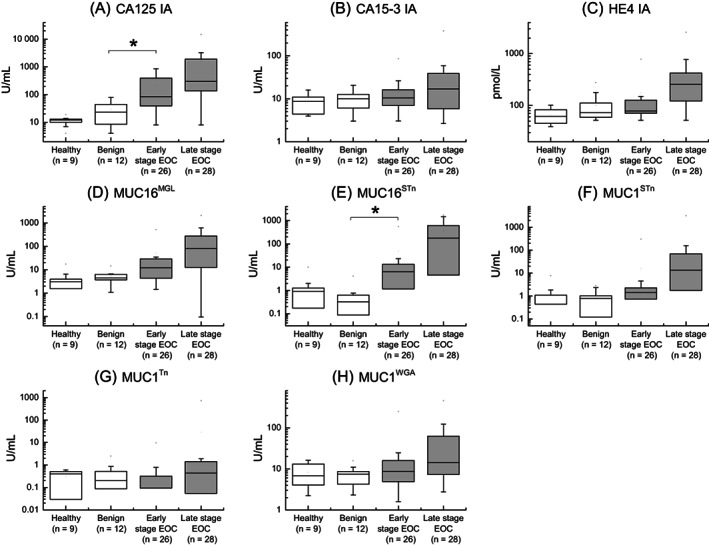
Box plots from serum discovery cohort (n = 75). Three conventional immunoassays of (A) CA125, (B) CA15‐3 and (C) HE4 along with (D, E) MUC16 and (F, G, H) MUC1 GVs in different groups of healthy, benign, early‐stage EOC and late‐stage EOC. Data information: Data is presented as U/mL or pmol/L concentration
3.2. Validation cohort—Paired ovarian cyst fluid and serum
In the CF validation cohort, CA125 and CA15‐3 IAs provided sensitivities of 14.1% (AUC = 0.518) and 31.8% (AUC = 0.677), respectively, at 90% SP for the combined group of EOC (n = 67) and borderline (n = 18) cases, against healthy and benign controls (n = 187). The SN was greatly improved to 77.6% (AUC = 0.893) by MUC1STn and to 74.1% (AUC = 0.904) by MUC1Tn. On combining the MUC1 GVs, SN increased to 80% (AUC = 0.924). From the pAUC, the three GVs providing the best benefits at high SP were, in order, MUC1Tn, MUC1STn and MUC16STn (Figure 3A).
FIGURE 3.
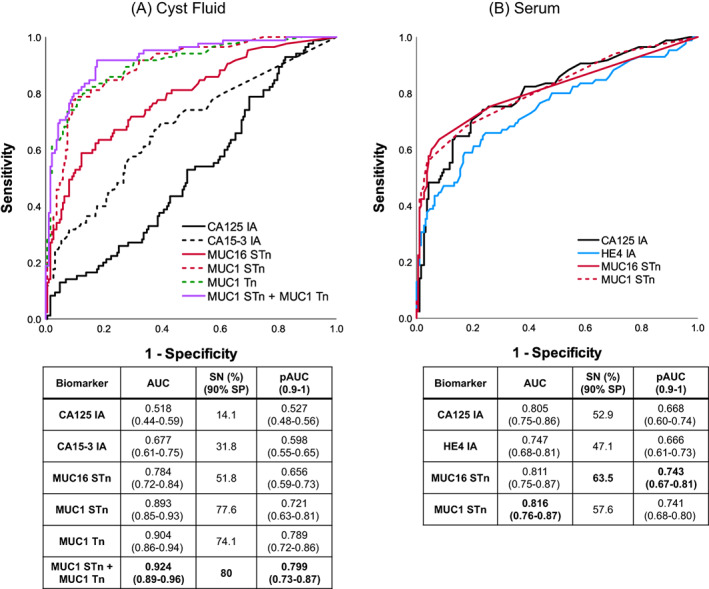
ROC plots for patients from cyst fluid and serum validation cohort. Conventional IA and GVs in 67 EOC + 18 borderline vs 187 nonmalignant in (A) cyst fluids and (B) serum. Data information: AUC and pAUC (0.9‐1) with 95% CI and SN at 90% SP is reported [Color figure can be viewed at wileyonlinelibrary.com]
In the serum validation cohort, the difference between CA125 IA and MUC16 GVs were small in terms of AUCs ranging from 0.805 to 0.813. However, from a detection sensitivity point of view at 90% SP, MUC16STn stands out as the best performing parameter detecting 63.5% and 74.6% respectively with and without the borderline cases, representing 10.6% and 8.9% improvements over CA125 IA. HE4 and CA15‐3 IA are clearly inferior to CA125 IA both with and without the borderline cases. As with MUC16STn, for MUC1STn similar improvements are seen at 90% SP and in the pAUC (0.9‐1) values (Figure 3B, Table S1). Combination markers did not yield any benefits.
Calibration curves and precision profiles were drawn for GV assays. Standards for the GVs had a detection limit below 2 U/mL. CV% was maintained below 15% for all assays (Figure S1). The day‐to‐day control variation for the GVs was observed to be within the range of 2 SD (Figure S2).
3.3. Subgroup analysis
3.3.1. Postmenopausal EOC
In postmenopausal subgroup (n = 208) of the CF validation cohort, at 90% SP, MUC1Tn (AUC = 0.927) and MUC1STn (AUC = 0.914) detected 52 of 64 EOC cases. This was more than twice the number detected with CA125 and CA15‐3 IAs in combination (AUC = 0.732), which detected only 25 cases (Figure 4A, Table S1). In the serum validation cohort, MUC1STn (AUC = 0.869) was the best performing GV and detected 49 postmenopausal EOC cases. This was 17.2% and 28.2% higher than provided by CA125 and HE4 IAs (AUCs 0.810 and 0.788, respectively) (Figure 4B).
FIGURE 4.
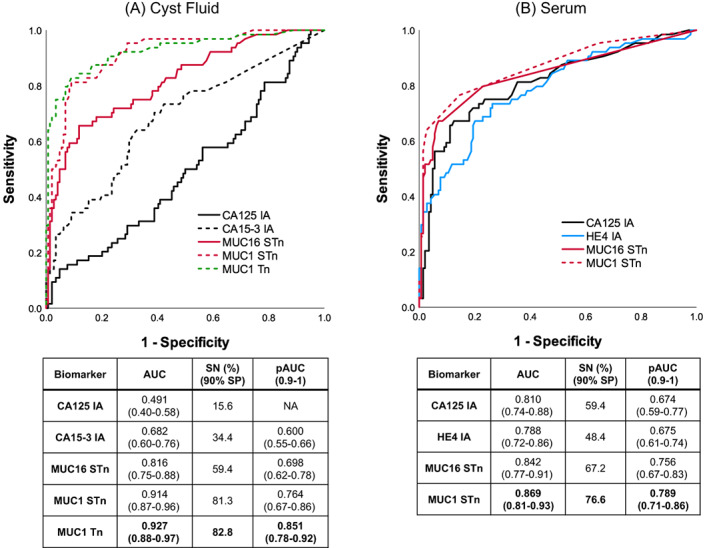
ROC plots for postmenopausal patients from cyst fluid and serum validation cohort. Conventional IA and GVs in 54 EOC + 10 borderline vs 144 nonmalignant in (A) cyst fluids and (B) serum [Color figure can be viewed at wileyonlinelibrary.com]
3.3.2. Early‐stage EOC
In the CF validation cohort, MUC1STn levels were found elevated in early‐stage (n = 56) malignant cancers (76.8% SN 90% SP, AUC = 0.909) thus detecting 43 EOC cases. This was much higher than the combination of CA125 and CA15‐3 IA, which detected only 22 cases (39.3% SN, AUC = 0.711). MUC1Tn (AUC = 0.891) was the second best, detecting 39 cases (Figure 5A, Table S1). In the serum validation cohort, MUC16STn and MUC1STn detected 48.2% (AUC = 0.736) and 44.6% (AUC = 0.753) early‐stage cancers (n = 56), respectively, at 90% SP (Figure 5B). The detection increased to 60.5% (AUC = 0.82) with both GVs on excluding the 18 borderline cases (Figure 5C). This further increased to 69% with MUC1STn (AUC = 0.849) in postmenopausal early‐stage cancers (n = 29) (Table S2).
FIGURE 5.
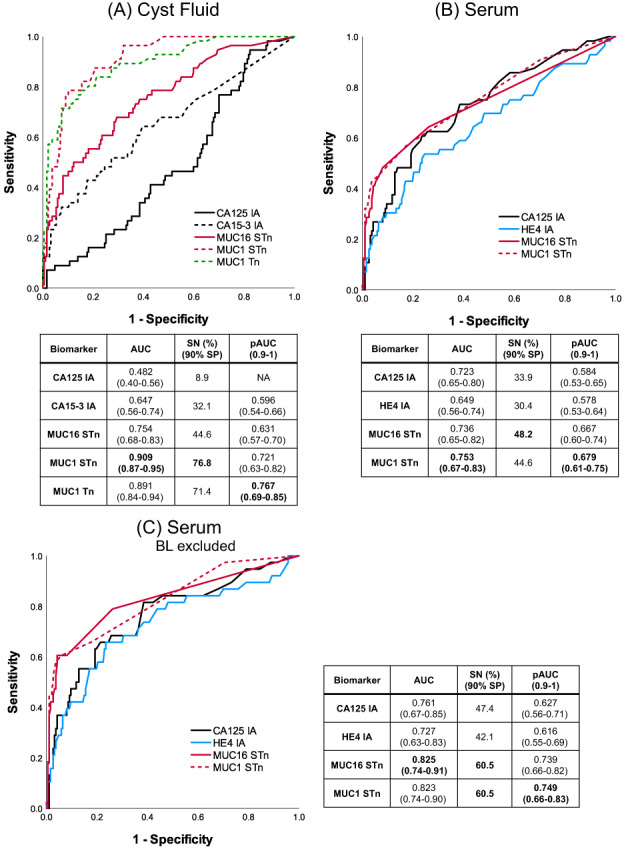
ROC plots for early‐stage EOC patients from cyst fluid and serum validation cohort. Conventional IA and GVs in 38 EOC + 18 borderline vs 187 nonmalignant in (A) cyst fluids and (B) serum. (C) Conventional IA and GVs in borderline excluded early‐stage EOC serum cohort [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Assay performances at high specificities
The ROC analyses and pAUC (90%‐100% SP) for serum samples suggest that the benefit from the GVs over the reference immunoassays is preferentially to be found at higher specificities. With decreasing SP, the difference becomes gradually smaller. Detection sensitivities at various specificities are compiled in Table S2 for several individual parameters. Among the three reference IAs, CA125 shows the least detection at high SP. HE4 shows quite similar pAUC values to CA125. Of particular note is that CA15‐3 IA performs substantially better than CA125 at 97% to 99% SP in all subgroups except in the premenopausal cases. In the postmenopausal group the poor performance of CA125 IA (14.1% SN at 98% SP; pAUC = 0.674) is improved more than 4‐fold with MUC1STn (60.9% SN, pAUC = 0.789), where HE4 detects 34.4% cases with pAUC similar to CA125. In the premenopausal group MUC16STn is the only GV parameter providing a stable improvement over CA125, CA15‐3 and HE4 IA. For the postmenopausal early‐stage cancer group at 99% SP, CA125 IA fails to detect any cancers whereas CA15‐3 IA shows highest detection (31% SN). SN improvements at other SPs are mostly seen with STn GVs. In the late‐stage cancers, the CA125 IA performance at 99% SP are improved about 6‐fold with CA15‐3 IA, HE4 IA, MUC16MGL and MUC16STn. At 98% and 97% SP, CA125 IA performance is improved about 5‐ and 8‐fold, respectively.
4. DISCUSSION
With access to a collection of paired ovarian cyst fluids and serum samples from a representative selection of patients suspected of ovarian malignancies, our study verifies the molecular rationale for the improved diagnostic performance of previously reported glycovariants of MUC16. Furthermore, noteworthy is the diagnostic discrimination provided by two newly reported MUC1 glycovariants as seen in the CF cohort. Whereas the immunoassay‐defined mucin assays CA125 and CA15‐3 perform poorly in CF to discriminate EOC from benign and healthy controls, the STn‐specific glycovariant of MUC16 and especially the STn and Tn glycovariants of MUC1 exhibit striking improvements over the conventional immunoassays. This provides strong support in favor of the enhanced cancer specificity of the glycovariants. In serum samples taken preoperatively, MUC16STn provides the best overall diagnostic performance closely followed by MUC1STn. Although the benefits of these GVs are apparent in all the subgroups analyzed, the most significant improvements are seen in postmenopausal cases and at early EOC stages where the cross‐reactivity from normal and benign circulating forms of the two mucins are highly reduced. These results carry great promise for substantially improving on the discrimination of EOC both in routine differential diagnostics as well as in early detection or screening efforts in special risk groups or the general population. The diagnostic advantage of the described glycovariants is methodologically dependent on the fluorescent nanoparticles used to provide highly effective recognition of the targets carrying the cancer specific glycostructures.
In the CF cohort, the GVs of MUC1 and MUC16 showed diagnostic superiority over the conventional IAs. Especially the MUC1STn and MUC1Tn glycovariants exhibited striking discriminations of the malignant and benign groups, superseding the best MUC16 GV (MUC16STn) in all categories. This was especially seen in early‐stage EOC (n = 56) where the improvement was 67.9% with MUC1STn GV over the 8.9% provided by CA125 IA at 90% SP. Detection rate was more than 80% for postmenopausal cases (n = 208) by MUC1 GVs. In the serum cohort, MUC16STn GV showed the best diagnostic performances overall compared to CA125 and HE4 IA. The MUC16 GV was generally somewhat superior in performance to MUC1 GV in serum. However, in the postmenopausal group, MUC1STn was found to provide better performance than MUC16STn, while in the small premenopausal group (n = 64), MUC1STn was not found to provide diagnostic power. MUC1Tn GV, which was working excellently in CF, was not detected in serum, suggesting that this glycoform does not enter the blood circulation as such or is cleared rapidly. The GV improvement in detection over CA125 and HE4 IA was more than 15% in the early‐stage group. Improvement over CA125 IA (more than 15%) was also seen in the postmenopausal group, where the findings are consistent with our previous studies in pelvic mass patients diagnosed with EOC, benign ovarian tumors or endometriosis in serum samples. 29 , 39 Improvement with GV over HE4 IA was even greater (more than 25%) in the postmenopausal group. CA125 IA in terms of overall AUCs was performing as one would expect in a patient cohort with a large proportion of postmenopausal women.
We also undertook a more detailed scrutiny of the serum results at higher specificities 95% to 99%, the area of interest for any early EOC detection effort using prediagnostic samples. In summary, especially the MUC16STn and MUC1STn GVs, but also CA15‐3 IA, analyzed at the highest SP levels were superior to CA125 and HE4 IA among the patient groups. MUC16STn in the small premenopausal group (n = 64) was the only glycovariant assay providing solid improvement over IAs. These results interestingly suggest that the mucin GVs and CA15‐3 IA may offer an opportunity for improvement of early EOC detection at very low marker concentrations. CA15‐3, perceived primarily as a breast cancer biomarker, 40 is regarded inferior to CA125 in the diagnosis of early EOC. 41 This however calls for studies of extensive cohorts of early‐stage EOC and high‐risk patient groups (eg, BRCA1/2) from early detection settings.
In our study, high grade serous was the most common histotype (~50%) among the malignant cases. The other histotypes were low in number with endometrioid being the second highest with ~23%, followed by mucinous with ~12% of malignant cases. The size of the present cohort does not allow a reliable analysis of how the different mucin glycovariants detect the different EOC histotypes. We are presently collecting a much larger cohort with preferentially early‐stage EOCs for further studies, where the advantages in combining GVs will be explored. The paired CF and serum material in our study still represents a clinical routine where diagnosis is delayed beyond the early stages.
There is extensive evidence of modified glycosylation in cancerous tissues and in circulation. 42 , 43 , 44 Yet, it has been difficult to harness that information for design of routine‐friendly methods. Our approach is based on the assumption that aberrant glycosylations of mucin biomarkers (MUC16 and MUC1) in the circulation are a hallmark of malignancy and are absent in benign conditions of the disease. 26 , 45 , 46 This is to our knowledge the first time where several mucin GVs together with two conventional mucin assays and HE4 are measured from a collection of paired ovarian cyst fluids and serum. Previously reported GV of mucin studies in EOC have either limited SN of 44% in peritoneal fluid 25 or employ less robust microarray techniques in EOC serum samples with marginally elevated CA125. 26 Ricardo et al used proximity ligation assay for MUC16STn, MUC1Tn and MUC1STn in tissue sections of serous ovarian tumors 27 but to our knowledge this promising technique has not been used for blood samples. Several advantages of our GV assays over these techniques and mutational liquid biopsy analysis of ctDNA should also be acknowledged. The nanoparticle‐assisted GV approach is technically simple and rapid, easily and directly applied to minute volumes of biological fluids without need for pretreatments of the sample. Mutational analysis of ctDNA will undoubtedly be of significant interest in future cancer diagnostics as has been amply shown. 47 , 48 , 49 Suggested limitations for early detection are the complexity of the technology, lack of sensitivity for very early cancerous lesions and lack of organ specificity as many driver mutations are shared by several cancers. We have previously suggested that in the future, methodologies such as the one presented here with enhanced cancer and organ type specificity combined with exquisite analytical sensitivity and simplicity for detection of early cancer lesions, could provide a much‐needed link or bridge to the mutational ctDNA analysis of more advanced cancers. 50
5. CONCLUSION
With a cohort of ovarian cyst fluids and corresponding blood samples, our study strengthens the rationale for specific glycovariants of mucins MUC16 and MUC1 as promising diagnostic markers for epithelial ovarian cancer over three conventional, antibody defined immunoassays CA125, HE4 and CA15‐3. The performance of the glycovariants is particularly impressive as applied to the cyst fluid samples. In serum samples, the benefits are particularly seen in postmenopausal women and early‐stage carcinomas. This stems from the strong suppression of the cross‐reactivity of circulating CA125 forms in benign ovarian tumors and healthy controls. The diagnostic potential of the nanoparticle‐aided glycovariant assays for improving early‐stage ovarian carcinoma detection calls for extensive future studies.
AUTHOR CONTRIBUTIONS
The work reported in the article has been performed by the authors, unless clearly specified in the text. Conceptualization: Kamlesh Gidwani, Kim Pettersson, Karin Sundfeldt. Formal analysis: Shruti Jain, Benjamin Ulfenborg. Funding acquisition: Kim Pettersson, Karin Sundfeldt. Investigation: Shruti Jain, Nimrah Nadeem, Maria Mäkelä, Shamima Afrin Ruma. Methodology: Shruti Jain, Kamlesh Gidwani, Nimrah Nadeem. Project administration: Kamlesh Gidwani. Resources: Karin Sundfeldt, Björg Kristjansdottir, Kim Pettersson. Supervision: Kamlesh Gidwani, Kim Pettersson, Karin Sundfeldt. Validation: Shruti Jain, Shamima Afrin Ruma. Visualization: Shruti Jain, Benjamin Ulfenborg, Joonas Terävä. Writing ‐ original draft: Shruti Jain, Kamlesh Gidwani, Nimrah Nadeem. Writing ‐ review & editing: Kamlesh Gidwani, Kim Pettersson, Karin Sundfeldt, Kaisa Huhtinen, Janne Leivo.
CONFLICT OF INTEREST
Kim Pettersson and Kamlesh Gidwani are co‐applicants on a patent application on the use of CA125‐MGL for ovarian cancer. The other authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the local ethics committee in Gothenburg and was performed in accordance with the Declaration of Helsinki and all its amendments. Informed consent was obtained from all individual participants included in the study.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENT
We would like to acknowledge the Turun Yliopisto Graduate School for supporting the PhD work of Shruti Jain by providing fellowship (2020‐2023) and Fujirebio Diagnostics for providing us with reagents and conventional assay kits.
Jain S, Nadeem N, Ulfenborg B, et al. Diagnostic potential of nanoparticle aided assays for MUC16 and MUC1 glycovariants in ovarian cancer. Int J Cancer. 2022;151(7):1175‐1184. doi: 10.1002/ijc.34111
Funding information ALF‐VGR Region, Sweden, Grant/Award Numbers: ALFGBG‐721051, ALFGBG‐932583; Jane ja Aatos Erkon Säätiö; Nordic Cancer Union, Denmark, Grant/Award Number: 194914; Swedish Cancer Foundation, Grant/Award Number: CAN‐2018/834
Contributor Information
Karin Sundfeldt, Email: karin.sundfeldt@obgyn.gu.se.
Kamlesh Gidwani, Email: kamlesh.gidwani@utu.fi.
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995‐2009: analysis of individual data for 25,676,887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet. 2015;385:977‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Enroth S, Berggrund M, Lycke M, et al. High throughput proteomics identifies a high‐accuracy 11 plasma protein biomarker signature for ovarian cancer. Commun Biol. 2019;2:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95:161‐192. [DOI] [PubMed] [Google Scholar]
- 4. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang B, Cai FF, Zhong XY. An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol. 2011;158:119‐123. [DOI] [PubMed] [Google Scholar]
- 6. Biskup K, Braicu EI, Sehouli J, et al. Serum glycome profiling: a biomarker for diagnosis of ovarian cancer. J Proteome Res. 2013;12:4056‐4063. [DOI] [PubMed] [Google Scholar]
- 7. Gupta V, Bernardini MQ. Algorithms used in ovarian cancer detection: a minireview on current and future applications. J Appl Lab Med. 2018;3:290‐299. [DOI] [PubMed] [Google Scholar]
- 8. Han C, Bellone S, Siegel ER, et al. A novel multiple biomarker panel for the early detection of high‐grade serous ovarian carcinoma. Gynecol Oncol. 2018;149:585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malati T. Tumour markers: an overview. Indian J Clin Biochem. 2007;22:17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma S. Tumor markers in clinical practice: general principles and guidelines. Indian J Med Paediatr Oncol. 2009;30:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anugraham M, Jacob F, Nixdorf S, Everest‐Dass AV, Heinzelmann‐Schwarz V, Packer NH. Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: glycan structures reflect gene expression and DNA methylation status. Mol Cell Proteomics. 2014;13:2213‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011;57:1534‐1544. [DOI] [PubMed] [Google Scholar]
- 13. Ueland FR. A perspective on ovarian cancer biomarkers: past, present and yet‐to‐come. Diagnostics (Basel). 2017;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Gorp T, Cadron I, Despierre E, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the risk of ovarian malignancy algorithm. Br J Cancer. 2011;104:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elias KM, Guo J, Bast RC Jr. Early detection of ovarian cancer. Hematol Oncol Clin North Am. 2018;32:903‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doubeni CA, Doubeni AR, Myers AE. Diagnosis and Management of Ovarian Cancer. Am Fam Physician. 2016;93:937‐944. [PubMed] [Google Scholar]
- 18. Jeschke U, Wiest I, Schumacher AL, et al. Determination of MUC1 in sera of ovarian cancer patients and in sera of patients with benign changes of the ovaries with CA15‐3, CA27.29, and PankoMab. Anticancer Res. 2012;32:2185‐2189. [PubMed] [Google Scholar]
- 19. Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526‐542. [DOI] [PubMed] [Google Scholar]
- 20. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasprzak A, Adamek A. Mucins: the old, the new and the promising factors in hepatobiliary carcinogenesis. Int J Mol Sci. 2019;20:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loureiro LR, Carrascal MA, Barbas A, et al. Challenges in antibody development against Tn and Sialyl‐Tn antigens. Biomolecules. 2015;5:1783‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tuccillo FM, de Laurentiis A, Palmieri C, et al. Aberrant glycosylation as biomarker for cancer: focus on CD43. Biomed Res Int. 2014;2014:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chauhan SC, Kumar D, Jaggi M. Mucins in ovarian cancer diagnosis and therapy. J Ovarian Res. 2009;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akita K, Yoshida S, Ikehara Y, et al. Different levels of sialyl‐Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. Int J Gynecol Cancer. 2012;22:531‐538. [DOI] [PubMed] [Google Scholar]
- 26. Chen K, Gentry‐Maharaj A, Burnell M, et al. Microarray glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. J Proteome Res. 2013;12:1408‐1418. [DOI] [PubMed] [Google Scholar]
- 27. Ricardo S, Marcos‐Silva L, Pereira D, et al. Detection of glyco‐mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol. 2015;9:503‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munkley J. The role of sialyl‐Tn in cancer. Int J Mol Sci. 2016;17:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gidwani K, Huhtinen K, Kekki H, et al. A nanoparticle‐lectin immunoassay improves discrimination of serum CA125 from malignant and benign sources. Clin Chem. 2016;62:1390‐1400. [DOI] [PubMed] [Google Scholar]
- 30. Gidwani K, Nadeem N, Huhtinen K, et al. Europium nanoparticle‐based sialyl‐Tn monoclonal antibody discriminates epithelial ovarian cancer‐associated CA125 from benign sources. J Appl Lab Med. 2019;4:299‐310. [DOI] [PubMed] [Google Scholar]
- 31. Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2017;409:395‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marcišauskas S, Ulfenborg B, Kristjansdottir B, Waldemarson S, Sundfeldt K. Univariate and classification analysis reveals potential diagnostic biomarkers for early stage ovarian cancer type 1 and type 2. J Proteomics. 2019;196:57‐68. [DOI] [PubMed] [Google Scholar]
- 33. Kristjansdottir B, Levan K, Partheen K, Carlsohn E, Sundfeldt K. Potential tumor biomarkers identified in ovarian cyst fluid by quantitative proteomic analysis, iTRAQ. Clin Proteomics. 2013;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Partheen K, Kristjansdottir B, Sundfeldt K. Evaluation of ovarian cancer biomarkers HE4 and CA‐125 in women presenting with a suspicious cystic ovarian mass. J Gynecol Oncol. 2011;22:244‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Sundfeldt K, Mateoiu C, et al. Diagnostic potential of tumor DNA from ovarian cyst fluid. Elife. 2016;5. doi: 10.7554/eLife.15175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Nagell JR, DePriest PD, Ueland FR, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109:1887‐1896. [DOI] [PubMed] [Google Scholar]
- 37. Terävä J, Tiainen L, Lamminmäki U, Kellokumpu‐Lehtinen PL, Pettersson K, Gidwani K. Lectin nanoparticle assays for detecting breast cancer‐associated glycovariants of cancer antigen 15‐3 (CA15‐3) in human plasma. PLoS One. 2019;14:e0219480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 39. Salminen L, Nadeem N, Rolfsen AL, et al. Exploratory analysis of CA125‐MGL and ‐STn glycoforms in the differential diagnostics of pelvic masses. J Appl Lab Med. 2020;5:263‐272. [DOI] [PubMed] [Google Scholar]
- 40. Duffy MJ, Evoy D, McDermott EW. CA 15‐3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869‐1874. [DOI] [PubMed] [Google Scholar]
- 41. Fortner RT, Vitonis AF, Schock H, et al. Correlates of circulating ovarian cancer early detection markers and their contribution to discrimination of early detection models: results from the EPIC cohort. J Ovarian Res. 2017;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525‐546. [DOI] [PubMed] [Google Scholar]
- 43. Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang M, Zhu J, Lubman DM, Gao C. Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey. Clin Chem Lab Med. 2019;57:407‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saldova R, Royle L, Radcliffe CM, et al. Ovarian cancer is associated with changes in glycosylation in both acute‐phase proteins and IgG. Glycobiology. 2007;17:1344‐1356. [DOI] [PubMed] [Google Scholar]
- 46. Ideo H, Hinoda Y, Sakai K, et al. Expression of mucin 1 possessing a 3′‐sulfated core1 in recurrent and metastatic breast cancer. Int J Cancer. 2015;137:1652‐1660. [DOI] [PubMed] [Google Scholar]
- 47. Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi‐analyte blood test. Science. 2018;359:926‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asante DB, Calapre L, Ziman M, Meniawy TM, Gray ES. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: ready for prime time? Cancer Lett. 2020;468:59‐71. [DOI] [PubMed] [Google Scholar]
- 49. Feeney L, Harley IJ, McCluggage WG, Mullan PB, Beirne JP. Liquid biopsy in ovarian cancer: catching the silent killer before it strikes. World J Clin Oncol. 2020;11:868‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gidwani K, Kekki H, Terävä J, Soukka T, Sundfeldt K, Pettersson K. Nanoparticle‐aided glycovariant assays to bridge biomarker performance and ctDNA results. Mol Aspects Med. 2020;72:100831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.


