Abstract
The United Nations suggests the global population of denture wearers (an artificial device that acts as a replacement for teeth) is likely to rise significantly by the year 2050. Dentures become colonized by microbial biofilms, the composition of which is influenced by complex factors such as patient’s age and health, and the nature of the denture material. Since colonization (and subsequent biofilm formation) by some micro‐organisms can significantly impact the health of the denture wearer, the study of denture microbiology has long been of interest to researchers. The specific local and systemic health risks of denture plaque are different from those of dental plaque, particularly with respect to the presence of the opportunist pathogen Candida albicans and various other nonoral opportunists. Here, we reflect on advancements in our understanding of the relationship between micro‐organisms, dentures, and the host, and highlight how our growing knowledge of the microbiome, biofilms, and novel antimicrobial technologies may better inform diagnosis, treatment, and prevention of denture‐associated infections, thereby enhancing the quality and longevity of denture wearers.
Keywords: antimicrobials, biocontrol, biofilms, diseases, diversity
Dentures are becoming increasingly common. Since colonisation (and subsequence biofilm formation) by some microorganisms to dentures can significantly impact, the study of denture microbiology has long been of interest to researchers.
![]()
Introduction
A denture is an artificial device designed to act as a replacement for one, multiple (partial dentures) or all (full dentures) teeth. Despite improvements in oral health, the need for full or partial dentures is expected to increase as the global population over the age of 65 years is expected to double by 2050 (United Nations 2019). The older population is also likely to have general and multiple health complications that make treatment planning around oral health more difficult (PHE 2015). Although the number of denture wearers globally is very high, with almost 41 million users in the United States alone in 2020 (Statista 2020), evidence of an understanding relating to health implications of long‐term denture use is relatively sparse in the literature. For example, one study of 67 denture wearers reported that almost 40% of patients ceased to wear their dentures within 5 years due to a variety of reasons, including pain, discolouration, and difficulty of use (Koyama et al. 2010). While it is clear that denture‐related disease is a multifactorial phenomenon, undoubtedly oral health is predicated in the status of the oral microbiome. Understanding how microbes relate to denture wearing has the capacity to improve oral and systemic health of the global elderly population.
As dentures are not sterile and are used at the body‐external environment interface, it is possible to be colonized by micro‐organisms (Olms et al. 2018) (Fig. 1). There are complex and numerous interactions between the individual (age, health), their denture (age, material, hygiene/cleaning regime) and colonizing micro‐organisms (nature of microbiome/biofilm, and potential infection risks), which will be considered in this review. The oral environment differs between the dentate and edentate mouth: the tooth is replaced by an inert removable prosthesis; the fitting surface of the denture provides a unique protected environment; the gumline is absent in complete denture wearers, and the natural dentition that abuts a partial denture is particularly prone to caries and gum disease (Zlatarić et al. 2002). The microbiology in these different scenarios is varied, and should be considered separately, particularly for partial dentures and implants, where the many different surfaces, interfaces, and locations will likely all bring additional complexities. This review focuses particularly on the complete denture (fitting and external surfaces).
Figure 1.
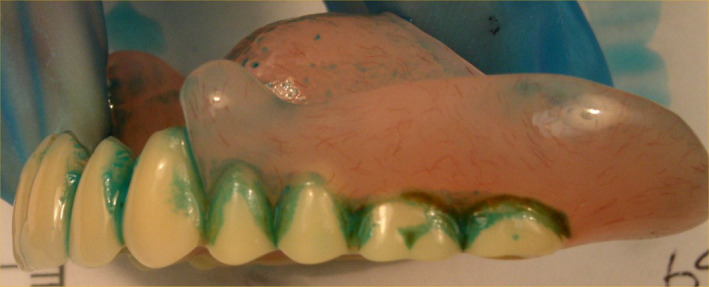
Example of a denture stained with plaque disclosure (blue), showing plaque accumulation on the upper fitting surface of the denture, and between the teeth of the prosthesis. [Colour figure can be viewed at wileyonlinelibrary.com]
Micro‐organisms associated with dentures
As with natural dentition, a denture surface once placed in the mouth becomes coated with an ‘acquired pellicle’ of salivary glycoproteins (including salivary amylase, albumin, mucin, and lysozyme) and immunoglobulins (Edgerton and Levine 1992; Marsh et al. 2016; Chawhuaveang et al. 2021). One study on polymethylmethacrylate (PMMA), the most commonly used material in dentures) reported that acquired pellicles primarily consisted of lyzozyme and histatins, in comparison to those forming on dentine that consisted of carbonic anhydrase, carbonate dehydrastase, cystatins and lyzozyme, and histatins (Svendsen and Lindh 2009). This coating of salivary product provides adhesion receptors facilitating the adhesion and colonization of micro‐organisms (Edgerton et al. 1993; Mukai et al. 2020). The profile of the acquired pellicle on the enamel (and therefore likely denture) is not consistent, and can change depending on the location of the dental arch (Ventura et al. 2017). Another study reported that microbial colonization is not necessarily determined by denture material, with both PMMA and polyacrylamide supporting biofilm growth (Olms et al. 2018). However, the pellicle composition might vary, and influence the identity of primary colonizers, though initial colonization may not necessarily differ between a range of dental materials (Mukai et al. 2020).
In the oral cavity, primary colonizers of hard/enamel surfaces include Gram‐positive Streptococcus spp. (S. oralis, S. mutans, S. mitis, S. gordonii, S. sanguinis, and S. parasanguinis), and other species including Veillonella spp., Neisseria spp., Rothia spp., Abiotrophia spp., Gamella spp. and Granullicatella spp. (previously belonging to the nutritionally variant streptococci) (Theilade et al. 1983; Aas et al. 2005; Yitzhaki et al. 2018). Secondary colonizers can adhere to and coaggregate with primary colonizing micro‐organisms, which if adhered to a denture can form complex denture plaque communities (Coulthwaite and Verran 2007; Jenkinson 2011). More specifically, studies on the cultivable flora of denture plaque have focused on facultative anaerobes on the denture‐fitting surface. However, as with dental plaque, obligate anaerobes are also reported in more mature plaque, and may be used as an indicator of plaque maturity—and thence poor denture hygiene (Coulthwaite et al. 2005). Despite the prevalence of bacteria in denture plaque, the most commonly studied micro‐organism is the yeast Candida albicans (Verran 1998; Ramage et al. 2004; Gleiznys et al. 2015), and to a lesser extent other Candida species such as C. glabrata (Coco et al. 2008; Zomorodian et al. 2011), C. famata, C. dubliniensis, and C. tropicalis (Zomorodian et al. 2011; Gauch et al. 2018). Notably, to date, there have been no confirmed reports of pan‐antifungal resistant yeast Candida auris isolated from dentures, though its continued global spread makes this a matter of ‘when’ and not ‘if’. Indeed, it can be found readily in the anterior nares of patients (Malczynski et al. 2020), which means C. auris is able to readily colonize dentures. Moreover, in critically ill patients, it has already been shown in outbreaks that it is possible to isolate it from oral samples (Biswal et al. 2017).
The interest in Candida in denture plaque derives from its association with denture stomatitis, a term which describes inflammation of the epithelial surfaces in contact with the denture, particularly the maxillary denture (Salerno et al. 2011). Candida spp. are well‐known secondary colonizers of denture plaque, with data suggesting C. albicans can co‐aggregate with Streptococcus spp. and result in biofilm on saliva‐coated surfaces (Bamford et al. 2009). The yeast is found primarily on the fitting surface of the maxillary denture. The enclosed environment, the presence of pre‐existing plaque, the protective nature of the surface topography and the acidogenic nature of the plaque have all been proposed as factors which enhance survival (Verran 1988). It has also been demonstrated recently that C. albicans acts as a ‘keystone’ commensal (given its relationship to coexist alongside human bacterial pathogens) within relevant oral biofilm model systems, with the suggestion that the larger physical nature of this dimorphic yeast makes it capable of creating physical and chemical microenvironments that support smaller bacteria, and obligate anaerobes resulting in increased biomass and metabolic activity (Janus et al. 2016; Young et al. 2020).
Although publications focusing on denture plaque are far fewer than those about dental plaque, there have been some seminal studies in the area, stemming from the 1980s. Theilade and Budtz‐Jørgensen (1988), examined the cultivable flora from the fitting surface of dentures of eight patients. Findings revealed Streptococcus spp. (in particular S. mutans, S. mitis, S. salivarius, and S. sanguis) dominated, persisting in 17–76% of samples. Gram‐positive rods Actinomyces spp. (A. israelii, A. naeslundii, A. odontolyticus) and lactobacilli, and Veillonella spp. were also present. Gram‐negative rods and yeasts were identified in smaller amounts. These findings are also supported by other studies (Budtz‐Jørgensen 1981; Walter and Frank 1985; Lamfon et al. 2005). Staphylococcus spp. and Micrococcus spp., commonly found on skin and in the environment are rarely found in the oral cavity but have been isolated from denture plaque taken from clinical cases of denture stomatitis (Kulak et al. 1997; Webb et al. 1998). Essentially, culture studies of denture plaque have shown that it is a diverse microbial biofilm, structurally similar to dental plaque (Budtz‐Jørgensen 1981; Walter and Frank 1985), with a similar microbial composition (Nikawa et al. 1998), but with elevated levels of yeasts (primarily Candida spp.), Lactobacillus spp., streptococci and staphylococci (Theilade and Budtz‐Jørgensen 1988; Marsh et al. 1992). These elevated levels have been shown to be particularly notable in cases of denture stomatitis (Theilade and Budtz‐Jørgensen 1988), and have been found to increase with the increase in age of the denture (Budtz Jorgensen 1974; Theilade et al. 1983; Mizugai et al. 2007). It is not surprising that the overall microbial composition of denture plaque is similar to that of dental plaque, since the underlying oral environment provides similar conditions. However, as noted earlier, on the inert denture, there is less intimate contact with body fluids (serum, blood), and more occluded spaces (the fitting surfaces) with less movement of saliva and more retention of food, which might encourage the presence of less fastidious (and even physically larger) species. Although Candida spp. are of particular concern in denture wearers due to their strong association with denture stomatitis, their reported proportion in denture plaque in comparison to bacterial isolates is relatively low (Theilade and Budtz‐Jørgensen 1988). Indeed, denture stomatitis has been associated with ‘dirty’ dentures and poor denture hygiene, as well as with the presence of C. albicans (Webb et al. 1998).
Whilst such investigations have been able to inform on micro‐organisms associated with dentures, classic microbiological techniques are not able to culture all micro‐organisms onto agar. First described by Staley and Konopka (1985), the ‘Great Plate Count Anomaly’ describes the phenomenon that the majority of micro‐organisms are nonculturable on agar, limiting the ability to discover the true microbial community of an environment—particularly one so complex as plaque—if only reliant on culture‐based techniques.
Micro‐organisms associated with dentures: nonculture‐based assessments
As described above, the denture microbiota is composed of a wide range of both eukaryotic and prokaryotic micro‐organisms, but not all are culturable. Therefore, modern molecular biology techniques that do not rely on culturing have gained popularity in the study of denture‐related plaque. Amplicon sequencing is one such technique, whereby specific genes (16S, 18S and ITS for bacterial, fungal, and microbial eukaryotes respectively), which are present in all but unique for each species, are sequenced and analysed using computer‐based bioinformatics. Another method, quantitative polymerase chain reaction (qPCR), analyses the amount of a specific gene (usually the 16S rRNA gene for bacteria, or 18S, or internal transcribed region [ITS] for fungi) in a sample without the need to sequence. As the genetic material needed for such analysis can be extracted directly from a sample, it removes the requirement to culture micro‐organisms, and is therefore more likely to provide a more representative picture of the complex communities found on dentures. Using molecular biology techniques, it is estimated that the oral microbiome (the term commonly used to describe the whole community of micro‐organisms in an environment) contains over 700 species of bacteria (Verma et al. 2018; Deo and Deshmukh 2019). Whilst such studies specifically looking at dentures are limited compared to the wider oral cavity, they nevertheless provide a more accurate picture of the microbial population of a denture, and how this might be associated with other factors such as disease state.
To date, there is a limited collection of studies that have embraced 16S‐based sequencing approaches to better understand the microbial complexity upon dentures (Table 1). Campos et al. (2008) was the first to use a prenext‐generation sequencing approach (16S rDNA cloning) (Campos et al. 2008). Here, over 82 different bacterial species were identified, of which 29 were exclusive to disease and 26 to healthy denture wearers alongside Candida sp. in both. The first microbiome study was performed by O'Donnell et al. (2015), where it was shown that bacteria from the taxa Bacilli and Actinobacteria were most abundant on the mucosa and denture of 130 patients, with Lactobacillus spp. showing a positive correlation in those with higher quantities of Candida spp. Despite no significant difference in overall microbiota between the dentures of healthy vs inflamed mouths, a followup study by these authors using qPCR found that dentures could be a reservoir for respiratory pathogens, including Streptococcus pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae B, Streptococcus pyogenes, and Moraxella catarrhalis (O'Donnell et al. 2016). In a smaller study, Shi et al. (2016) reported the genus Actinomyces was most pervasive on both dentures and remaining teeth, followed by Streptococcus, Veillonella, Capnocytophaga, Neisseria, Prevotella, and Corynebacterium, independent of the surface or health status, while the microbiome of dentures from stomatitis patients was more diverse than dentures belonging to healthy patients (O'Donnell et al. 2016). Additional data (Fujinami et al. 2021) revealed that when comparing 30 denture samples with 16 plaque samples, Streptococcus sp., Lactobacillus sp., and Corynebacterium sp. were more abundant in denture plaque than in dental plaque likely due to the aerobic conditions on the denture surface as the denture is often removed from the mouth or in contact with saliva. Microbial association with malodour has also been studied, with Yitzhaki et al. (2018) using 16S sequencing to conclude the phyla Firmicutes and Fusobacteria and the genera Leptotrichia, Atopobium, Megasphaera, Oribacterium, and Campylobacter alongside a generally more diverse and significantly different microbial population were associated with denture malodour compared to samples from nonmalodour patients (Yitzhaki et al. 2018). It should be noted that the study design, DNA extraction protocols, storage, sequencing platform, and primers are not consistent from one study to the next, so absolute comparison is not possible (Table 1). However, these studies have started to provide a greater insight into the diversity of bacteria that occupy these substrates.
Table 1.
Summary of published denture microbiome studies and their key characteristics
| Study | Location | Design | Controls (no. patient’s) | Cases (no. patient’s) | Extraction method | Storage | Sequencer | Region | Accession | Key findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Campos et al. (2008) | São Paulo, Brazil | Cross‐Sectional | Healthy (10) | Denture stomatitis(10) | QuickExtract | NA | ABI PRISM 3100 Genetic Analyzer | 16S | AY672070‐76 | Distinct differences in microbiome between health and disease |
| O'Donnell et al. (2015) and Delaney et al. (2019)* | Glasgow, UK | Cross‐Sectional | Mucosa/plaque | Denture (130) | AGOWA mag Mini DNA Isolation Kit | −80°C | MiSeq | 16S V4, ITS1 | PRJNA324548 | Distinct associations between Candida load, oral hygiene and microbiome |
| Shi et al. (2016) | Los Angeles, USA | Cross‐Sectional | No denture stomatitis (10) | Denture stomatitis (10) | DNeasy Blood and Tissue kit | NA | 454 | 16S V1‐V3 | PRJNA292354 | Denture microbiome is reflective of that of teeth but identifiably distinct when comparing health and disease |
| Asakawa et al. (2018) | Fukuoka, Japan | Cross‐Sectional | Tongue (506) | Denture (137) | IsoQuick | −80°C | Ion PGM | 16S | DRA006979 | Diminished oral health and hygiene is reflected in the microbiota of the tongue |
| Yitzhaki et al. (2018) | Ramat‐Aviv, Israel | Cross‐Sectional | NA | Denture (26) | Genomic DNA Mini Kit | −20°C | MiSeq | 16S V3‐V4 | On request | Microbiome analysis suggests population distinction between olfactorily distinct samples |
| Nedumgottil (2018) | Puducherry, India | Cross‐Sectional | NA | Denture (88) | NucleoSpin Microbial DNA mini kit | NA | NA | 16S | On request | Denture wearers linked to co‐occurrence of Streptococcus mutans, Veillonella atypica, and Granulicatella adiacens |
| Morse et al. (2019) | Cardiff, UK | Cross‐Sectional | No denture stomatitis (11) | Denture stomatitis (8) | Gentra PureGene Bact/Yeast DNA extraction kit | −20°C | MiSeq | 16S V1‐V3 | On request | Reduced bacterial diversity may lead to dysbiosis in DS |
| Mukai et al. (2020) | Yokohama, Japan | Cross‐Sectional | Saliva (8) | Denture (8) | ISOSPINE Fecal DNA Kit | −80°C | MiSeq | 16S V3‐V4 | PRJNA592277 | Microbial diversity diminished on denture surface compared to saliva |
| Murugesan et al. (2020) | Doha, Qatar | Cross‐sectional | Healthy (861) | Denture (136) | QIAsymphony | −80°C | MiSeq | 16S V1‐V3 | On request | Streptococcus and Neisseria found to be more abundant in denture wearers |
| Fujinami et al. (2021) | Nagoya, Japan | Cross‐Sectional | Plaque (16) | Denture (30) | MasterPure DNA Purification Kit | −20°C | MiSeq | 16S V3‐V4 | DRA011478 | Pathogens associated with aspiration pneumonia were more commonly isolated from dentures |
| Grischke et al. (2021) | Hannover, Germany | Cross‐Sectional | Healthy (372) | Peri‐implantitis/denture (725) | QIAshredder Mini Spin | −80°C | HiSeq | ND | PRJEB43417 | Removable dentures are identifiable as a risk‐factor for peri‐implantitis |
Two studies use same dataset for different analysis question.
As reported using culture techniques, Candida is present as part of the denture microbiome (Campos et al. 2008). In a study analysing 82 Dutch denture wearers, high Candida loads were associated with the bacterial class Bacilli, negatively associated with bacterial classes Fusobacteria, Flavobacteria, and Bacteroidia, and were generally less diverse and dominated by streptococci (Kraneveld et al. 2012). Whilst such studies are useful and provide data on the potential relationship between denture stomatitis, denture plaque, and the denture microbiome, the literature is limited, lacking many large study populations/sample sizes, and making generalization difficult. To date, there are only 12 independent studies available in the public domain that have assessed the denture plaque microbiome, and which have involved the analysis of over 1000 patients’ dentures (Table 1). Among the limited availability of research, there are related concerns for the lack of unanimity in methodologies presented. It has been evidenced in previous work, for example, microbiome profiling conducted by Teng et al. (2018), that extraction protocols can significantly impact sample‐to‐sample diversity. Moreover, community diversity has also been associated with the specific 16S region selected (Bukin et al. 2019; Chen et al. 2019) which has been shown to vary from study to study (Table 1). However, it is likely there will be an expansion and increase in uniformity of these over the next few years as attempts are made to use microbiome studies as a diagnostic tool.
Dentures and biofilm: denture plaque
Physiologically, a harmonious relationship exists between the oral microbiota and the host. Oral micro‐organisms naturally thrive in areas where salivary flow is low, such as areas between the teeth and gingival crevices, in addition to the fitting surface of a denture. Micro‐organisms are found in complex communities on the denture (Fujinami et al. 2021), attached to the denture surface or to other cells whilst embedded in extracellular polymeric substance (Gendreau and Loewy 2011). It is widely agreed in the literature that micro‐organisms displaying this phenotype, and known as biofilm, present increased resistance to antimicrobial treatments whilst also being physically difficult to remove from the substratum (Sharma et al. 2019). There have been many reviews published on the formation of biofilms generally and dental plaque biofilms specifically (e.g. Subramani et al. 2009), with fewer concerning denture plaque (Hannah et al. 2017). Although the phenomenon of biofilm formation is similar for denture and dental plaque, the particular environment between the denture and the roof of the mouth has been shown to be microbially distinct (O'Donnell et al. 2015). The presence of C. albicans at this site has resulted in significant attention paid to factors affecting its colonization of denture surfaces (Ramage et al. 2004; Pereira‐Cenci et al. 2008), with a view to prevention or control (Rautemaa and Ramage 2011).
As interest in denture stomatitis increased during the 1980s, studies on the attachment of C. albicans to denture PMMA and silicone began (Samaranayake and MacFarlane 1980; Pereira‐Cenci et al. 2008; Rodger et al. 2010). The yeast readily attached to the surface, with retention being enhanced by increased surface roughness (Verran and Maryan 1997; Jackson et al. 2014; Verran et al. 2014), lowered pH (Verran et al. 1991), and other factors. The introduction of salivary pellicle and other conditioning films into this simple system (Nikawa et al. 1992), the ability of C. albicans to exist in a yeast and hyphal form (Jackson et al. 2014), and the presence of primary colonizers (Verran and Motteram 1987), introduced complexity in a step‐wise manner and provided additional information. In vitro studies on the interactions occurring between C. albicans and Streptococcus mutans are common (e.g. Baena‐Monroy et al. 2005; Falsetta et al. 2014; Zhou et al. 2018). Initially, this might seem counter‐intuitive, because S. mutans is best known for its role in dental caries (Hamada et al. 1984), and it may not be the most common streptococcus present in denture plaque, with S. sanguinis historically recovered more often (Carlsson et al. 1969). However, the highly acidogenic and aciduric nature of the environment generated by S. mutans would likely prove beneficial for yeast proliferation. These basic studies exploring coaggregation and antagonistic behaviours have fuelled an explosion of studies that specifically explore interkingdom interactions (Delaney et al. 2018, 2019).
Materials used in conjunction with the denture were also assessed for their susceptibility to colonization. For example, penetration of denture soft‐liner, used to make the denture more comfortable for the wearer, has been demonstrated (Bulad et al. 2004; Rodger et al. 2010; Todd et al. 2019). The search for novel materials that might be less conducive to Candida colonization, but which retained the physicochemical properties required for their in vivo implementation, is ongoing and will be considered later.
More complex studies on denture plaque models have been carried out, for example, using a denture plaque microcosm (Coulthwaite and Verran 2007, 2008; Brown et al. 2022), and biofilm models, used to assess the impact of putative antimicrobial/antibiofilm agents (Sherry et al. 2016; Brown et al. 2022). In line with Marsh’s ‘Ecological Plaque Hypothesis’ (Marsh 1994), it has been proposed that denture stomatitis arises from a shift in the plaque microbiology away from health due to external changes such as increased plaque acidogenicity and increased plaque quantity (Verran 1998)—and perhaps diversity (Marsh 1994). Thus, both control of plaque quantity and management of specific aspects of the microcosm might enable progress towards maintenance of a healthy denture plaque.
Health and ill‐health associated with dentures
Through the use of in vivo cultural studies and in vitro denture models, knowledge of denture plaque microbiology is now substantial. Studies on healthy and diseased denture plaque are hampered by plaque complexity, as well as the relatively small research funding available to investigators in comparison to that for the study of dental plaque. Nevertheless, there are several health‐related problems associated with denture plaque (Coulthwaite and Verran 2007). As has been noted, denture plaque is a key aetiological factor in the oral mucosal inflammatory disorder, denture stomatitis (Budtz Jorgensen 1974; Catalan et al. 1987; O'Donnell et al. 2015). Denture stomatitis is a common and problematic disorder for denture wearers (Hannah et al. 2017). Its progression is characterized by an inflammation and erythema of the oral mucosa in contact with the denture where it is in close proximity to denture plaque. Affecting between 15 and 70% of denture wearers (Gendreau and Loewy 2011), this condition causes discomfort and tissue swelling, often resulting in dentures becoming ill fitting and thus difficult to wear. In turn, this can result in loss of functionality and influence dietary and lifestyle choices that can dramatically affect quality of life for denture wearers. In addition to this, the onset and development of denture stomatitis can ultimately result in the denture having to be removed and replaced because treatments are often unsuccessful. This is a costly and demanding procedure for our health services (Polzer et al. 2010).
Over the years, the aetiology and management of denture stomatitis has been a particular focus of research, with attempts being made to clarify the causes of this condition—with C. albicans repeatedly being noted in this context. Many contributing factors have been implicated. Ramage et al. (2004) utilized scanning electron microscopy to visualise in vivo denture plaque biofilms from patients with denture stomatitis and noted a large amount of established yeast and hyphal cells, indicating an important role for C. albicans biofilms in denture stomatitis. In a large cohort study, Figueiral et al. (2007) investigated the causative factors in 124 denture‐wearing patients (54 with clinical symptoms of denture stomatitis, 70 without), showing that denture stomatitis was related to trauma caused by poorly fitting dentures, and could be increased by the long term and overnight wearing of dentures as well as denture age. This and other work supports previous findings that the condition is strongly related to poor denture hygiene and the high prevalence of Candida spp. on denture surfaces (Coco et al. 2008). In addition to these factors, denture stomatitis can adversely affect those with underlying illness or where disruption to natural defences occurs, for example, immunosuppressive therapies/diseases (HIV/AIDS), diabetes, and old age (Bartholomew et al. 1987; Webb et al. 1998; Pires et al. 2002). Indeed, attention has been drawn to the potential risks of candida oral carriage/infection with mechanical ventilation necessitated by COVID‐19 (Jerônimo et al. 2022).
An individual might possess the same set of dentures for many years (Wright 1994). Over this time, one would hope that regular visits for dental treatment were made, to ensure that oral tissues remain healthy and that denture fit is maintained. Loose‐fitting dentures can not only cause discomfort, they can introduce difficulties with eating (and hence nutrition) and speaking (hence socializing). Dentures that are poorly cared for can also be aesthetically unpleasant, being visibly dirty, or accompanying oral malodour. Little has been reported about denture malodour (Verran 2005), although the presence of oral anaerobes and/or yeast would likely be associated with the production of various volatile compounds. Denture‐associated odour has been noted as being ‘somewhat sweet, but unpleasant and readily identifiable’, yet studies on malodour tend to focus on the production of volatile sulphur compounds (Nalcaci and Baran 2008; Mousa et al. 2022). Like most other fungi, C. albicans and many bacterial species produce microbial volatile organic compounds (MVOCs), with over 250 described in the literature (Morath et al. 2012). Thus, is is important to consider the origin of the ‘sweet but offensive’ odour, and to characterize it more fully. Recent microbiome studies revealed a higher diversity of bacteria in patients with denture malodour, with bacteria including the phyla Fusobacteria and Firmicutes, and the genera Atopobium, Leptotrichia, Megasphaera, Oribacterium, and Campylobacter (Yitzhaki et al. 2018).
The denture has been identified as a reservoir of infection for a range of nonoral bacteria, some of which have been associated with inhalation pneumonia (O'Donnell et al. 2016; Takeuchi et al. 2019), and others which present resistance to a range of antibiotics (Lewis et al. 2015; Garbacz et al. 2019). Although it is possible that when dentures demonstrate colonization by micro‐organisms they may not be causing any negative consequence, it is important to ensure that good denture hygiene minimizes the likelihood of any serious infections that might arise from these organisms in the potentially immunodeficient elderly and those with systemic health disorders (Le Bars et al. 2015; Hannah et al. 2017; Jerônimo et al. 2022).
Dental hygiene and treatment
As well as being essential to prevent the accumulation of denture plaque that may harbour potential pathogens, good denture hygiene is also required to limit malodour, and maintain good aesthetics in denture wearers (Jagger and Harrison 1995). There is a wide range of denture hygiene products and protocols available for denture cleaning (Ruiz Núñez et al. 2022). The UK National Health Service recommends that dentures are cleaned as often as normal teeth (i.e. every morning and night), brushing with either toothpaste or soap and water, supplemented by soaking in commercial cleansers on a regular basis and rinsing thoroughly (https://www.nhs.uk/conditions/dentures/). It is also recommended that dentures are removed at night. Improper cleaning regimes can be detrimental, for example, storing dentures in water might increase Candida colonization (Verhaeghe et al. 2020), and over‐abrasive dentifrices might damage the denture surface and impede cleaning (Verran et al. 2014), whilst one study found that the denture microbiome can remain relatively resilient to cleaning regimes (Delaney et al. 2019). However, if performed effectively and regularly, the recommended procedures should be adequate to maintain good denture hygiene. Indeed, in the first randomized double‐blinded control trial it was shown that frequent daily denture cleansing with a tablet and brushing was more effective than intermittent cleaning, significantly reducing microbial numbers in denture plaque (Ramage et al. 2019). Regular attendance at a dentist or hygienist would, therefore, benefit the average denture wearer in terms of oral health checks, including denture fit, hygiene monitoring, and evidence of yeast infection. Since denture hygiene would remove plaque and issues associated with plaque build‐up, one might anticipate reduced experience of denture‐associated infection. However, many denture wearers are elderly, and as a result often suffer from medical conditions such as arthritis and dementia, that can impair their ability to carry out these procedures effectively (Gornitsky et al. 2002), requiring assistance from carers and some education (Ruiz Núñez et al. 2022). Specific treatments are available if a Candida infection is suspected (Patil et al. 2015), with accompanying denture disinfection/cleaning or replacement (Garg and Garg 2010).
Control and diagnosis of microbial colonization of dentures
One way to control denture colonization is to construct the denture to either prevent or reduce adhesion and attachment in the first instance, or retard growth and biofilm formation. The uneven nature of the denture‐fitting surface enhances cell retention and makes cleaning more difficult, but some elements of surface topography are essential to enhance fit. The relationship between the size of cell and surface feature (Whitehead et al. 2005; Sterzenbach et al. 2020) is key to engineering surface structures, such as the use of highly ordered nanopit topographies which have been shown to significantly reduce adherence capacity of C. albicans (Alalwan et al. 2018). On a note of caution, the dentifrice and brushing itself can cause abrasion to the denture, and enhance subsequent retention of micro‐organisms on the abraded surfaces (Verran et al. 2014).
Inclusion of antimicrobial materials is another potential route to prevention of denture‐associated problems. Several recent reviews discuss different types of materials used, antimicrobial mechanisms, toxicity, and practical considerations (Allaker 2010; Gad et al. 2017; Imazato et al. 2020; Makvandi et al. 2020; Adam and Khan 2021; Garcia et al. 2021; Hao et al. 2021; Monteiro et al. 2021). Although a variety of materials has been suggested as potential antimicrobial additives in the literature, the studies are usually limited in the aspects of efficacy and performance assessment. In addition to antimicrobial and antibiofilm evaluation, there are essential criteria that denture materials must conform to, such as mechanical and aesthetic properties, cytotoxicity, leakage of substances from the dentures and their in vivo effect, antimicrobial lifetime, and so on. Examples of antimicrobial materials used as additives, their role and some critical considerations are provided in Table 2, and an example of zeolite‐embedded denture acrylic is provided in Fig. 2. As evident from Table 2, although a variety of materials having demonstrated their potential as filler materials to frabricate antimicrobial dentures, the lack of in vivo studies and clinical trials, in addition to shortcomings in the comprehensiveness of materials assessment, are the main barriers to their widespread practical application.
Table 2.
Examples of filler materials for the fabrication of antimicrobial dentures
| Material | Function/Notes | Critical considerations | Reference |
|---|---|---|---|
| Quaternary ammonium methacryloxy silicate (0.4, 2, 4, and 6 wt%) |
Antimicrobial (S. mutans, A. naeslundii, C. albicans); sustained activity after 3 months water aging Antiadhesive (C. albicans) Clinical trial later reported (5 wt%) |
Mechanical properties not studied; single‐species biofilms studied | Gong et al. (2013); Liu et al. (2016) |
| Silver nanoparticles (1, 2, 3, and 5 wt%) | Antibiofilm (C. albicans) | Mechanical and aesthetic properties not measured; single‐species biofilms studied; Ag release not determined; cytotoxicity not studied | Li et al. (2016) |
| Zeolite‐embedded silver ions (2 wt% zeolite; post‐synthesis loading of silver) | Antimicrobial (C. albicans, S. mutans, F. nucleatum), active for at least 45 days with possibility for silver recharging | Silver recharging possibility not verified; possible effects of long‐term silver exposure not studied; antibiofilm potential not studied | Malic et al. (2019) |
| Zinc oxide nanoparticles (2.5, 5 and 7.5 wt%) | Antifungal (C. albicans) | Antimicrobial lifetime not determined; long‐term zinc release not studied; flexural strength not measured | Cierech et al. (2016, 2018, 2019) |
| Carboxylated multiwalled carbon nanotubes (0.25, 0.5 and 1 wt%) | Antiadhesive (S. aureus, S. mutans, C. albicans) | In vivo biocompatibility tests needed; long‐term studies not performed; aesthetic properties not reported | Kim et al. (2019) |
| Graphene oxide nanosheets (nGO) (0.25, 0.5, 1, and 2 wt%) |
Antiadhesive (S. aureus, S. mutans, C. albicans, E. coli). Sustained activity against C. albicans after incubation in artificial saliva for up to 28 days |
Poor dispersion of nGO; In vivo biocompatibility tests needed. Activity not sustainable beyond 28 days; aesthetic properties not reported | Lee et al. (2018) |
| Graphene‐Ag nanoparticles (G‐AgNp) (1 and 2 wt%) | Antibacterial (S. aureus, S. mutans, E. coli) | Antibacterial lifetime not studied; uncertainty about the mechanism of antibacterial action; aesthetic properties not reported | Bacali et al. (2020) |
| Surface prereacted glass ionomer (5, 10, and 20 wt%) | Antibiofilm (C. albicans) | Mechanical properties not measured; increased surface roughness; single‐species biofilms studied; released of compounds determined after 24 h only; cytotoxicity not studied | Tsutsumi et al. (2016) |
| TiO2 nanoparticles (0.2, 0.4, 0.6, and 1 and 2.5 wt%); 3D printing |
Antibacterial (C. scotti). 18 months clinical assessment of patient‐centred outcomes later reported (0.6 wt%) |
Limited antimicrobial assessment; antimicrobial lifetime not studied | Totu et al. (2017); Cristache et al. (2020) |
| Mesoporous silica nanoparticles (MSNs) (0.5, 1, 2.5, and 5 wt%) for loading of amphotericin | Antiadhesive (C. albicans and S. oralis), active for 2 weeks | Increased surface roughness; biodegradation of MSNs decreases antimicrobial lifetime | Lee et al. (2016) |
| Nanodiamonds (0.5, 1, and 1.5 wt%) | Antiadhesive (C. albicans) | Colour changes observed; mechanical and cytotoxicity properties not studied | Fouda et al. (2019) |
Figure 2.
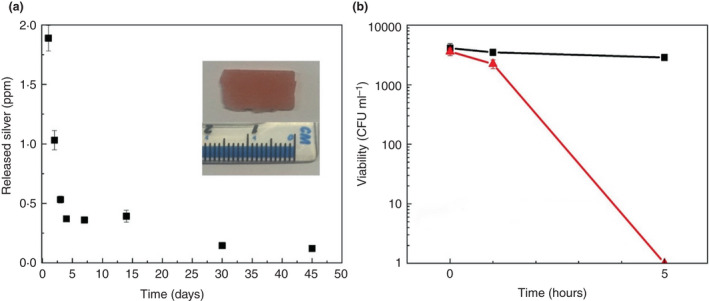
(a) Sustained silver release over 45 days from zeolite‐embedded denture acrylic treated with silver; digital image shown in the insert demonstrates preservation of resin’s aesthetic upon Ag loading into the zeolite‐embedded dental resin; and (b) Representative antimicrobial activity of the modified resin against a clinical strain of C. albicans after 5 hours having been incubated in distilled water for 45 days (Malic et al. 2019). Black square represents denture acrylic (polymethylmethacrylate) data, and red triangle represents denture acrylic embedded with zeolite and treated with silver. [Colour figure can be viewed at wileyonlinelibrary.com]
As noted above, Candida is known to produce MVOC (Scotter et al. 2005). Fungal MVOCs have a range of potential applications (Morath et al. 2012), and the identification of unique MVOC profiles has enabled seeing the differentiation between Candida species (Hertel et al. 2016), which, whilst a presence/absence of Candida may be all that is required for the denture wearer, access to Candida species information may be of clinical interest. This would enable monitoring for emerging species of interest, generation of overall prevalence data, consideration and administration of appropriate treatment (or prevention strategy), and overall knowledge of any implications for disease prevention. Thus, technology capable of identifying a unique pattern of MVOCs may be able to aid early detection of Candida denture colonization, as has been seen with other fungal species (Bingley et al. 2012), thus providing a rapid pathway towards prevention and early treatment. Moreover, if we are able to fully and accurately map the microbiome and metabolome of denture biofilms then it may be possible to accurately predict the onset of denture stomatitis and, therefore, instigate a chemotherapeutic strategy earlier.
Conclusion
Although the literature focusing on denture plaque is significantly less than that on dental plaque, over the past 40 years, there has been consistent and increasing attention paid to this unique oral biofilm and its associated contribution to health and well‐being. The role of C. albicans in the aetiology of denture stomatitis is generally acknowledged, and sociological aspects of denture health are recognized. Efforts to speed diagnosis, prevent ill‐health, and improve quality of life for the elderly denture wearer continue apace.
Author Contributions
Conceptualization (JV, JR), Methodology (JV, JR, LT, MB, GR), Formal Analysis (JV, JR, LT, SM, MB, GR); Writing—Original Draft Preparation (JV, JR, LT, SM, MB, GR); Writing—Review & Editing (JV, JR, LT, SM, MB, GR).
Acknowledgements
The authors would like to acknowledge Lisa Coulthwaite for providing Fig. 1.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- Aas, J.A. , Paster, B.J. , Stokes, L.N. , Olsen, I. and Dewhirst, F.E. (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43, 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam, R.Z. and Khan, S.B. (2021) Antimicrobial efficacy of silver nanoparticles against Candida albicans: a systematic review protocol. PLoS ONE 16, e0245811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alalwan, H. , Nile, C.J. , Rajendran, R. , McKerlie, R. , Reynolds, P. , Gadegaard, N. and Ramage, G. (2018) Nanoimprinting of biomedical polymers reduces candidal physical adhesion. Nanomedicine 14, 1045–1049. [DOI] [PubMed] [Google Scholar]
- Allaker, R.P. (2010) The use of nanoparticles to control oral biofilm formation. J Dent Res 89, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Asakawa, M. , Takeshita, T. , Furuta, M. , Kageyama, S. , Takeuchi, K. , Hata, J. , Ninomiya, T. and Yamashita, Y. (2018) Tongue microbiota and oral health status in community‐dwelling elderly adults. mSphere 3, e00332–e00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacali, C. , Baldea, I. , Moldovan, M. , Carpa, R. , Olteanu, D.E. , Filip, G.A. , Nastase, V. , Lascu, L. et al. (2020) Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin Oral Investig 24, 2713–2725. [DOI] [PubMed] [Google Scholar]
- Baena‐Monroy, T. , Moreno‐Maldonado, V. , Franco‐Martínez, F. , Aldape‐Barrios, B. , Quindós, G. and Sánchez‐Vargas, L.O. (2005) Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10 Suppl. 1, E27‐39. [PubMed] [Google Scholar]
- Bamford, C.V. , d'Mello, A. , Nobbs, A.H. , Dutton, L.C. , Vickerman, M.M. and Jenkinson, H.F. (2009) Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun 77, 3696–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew, G.A. , Rodu, B. and Bell, D.S. (1987) Oral candidiasis in patients with diabetes mellitus: a thorough analysis. Diabetes Care 10, 607–612. [DOI] [PubMed] [Google Scholar]
- Bingley, G.D. , Verran, J. , Munro, L.J. and Banks, C.E. (2012) Identification of microbial volatile organic compounds (MVOCs) emitted from fungal isolates found on cinematographic film. Anal Methods 4, 1265–1271. [Google Scholar]
- Biswal, M. , Rudramurthy, S.M. , Jain, N. , Shamanth, A.S. , Sharma, D. , Jain, K. , Yaddanapudi, L.N. and Chakrabarti, A. (2017) Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J Hosp Infect 97, 363–370. [DOI] [PubMed] [Google Scholar]
- Brown, J.L. , Young, T. , McKloud, E. , Butcher, M.C. , Bradshaw, D. , Pratten, J.R. and Ramage, G. (2022) An in vitro evaluation of denture cleansing regimens against a polymicrobial denture biofilm model. Antibiotics (Basel) 11, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz Jorgensen, E. (1974) The significance of Candida albicans in denture stomatitis. Scand J Dent Res 82, 151–190. [DOI] [PubMed] [Google Scholar]
- Budtz‐Jørgensen, E. (1981) Oral mucosal lesions associated with the wearing of removable dentures. J Oral Pathol Med 10, 65–80. [DOI] [PubMed] [Google Scholar]
- Bukin, Y.S. , Galachyants, Y.P. , Morozov, I.V. , Bukin, S.V. , Zakharenko, A.S. and Zemskaya, T.I. (2019) The effect of 16S rRNA region choice on bacterial community metabarcoding results. Scient Data 6, 190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulad, K. , Taylor, R.L. , Verran, J. and McCord, J.F. (2004) Colonization and penetration of denture soft lining materials by Candida albicans . Dent Mater 20, 167–175. [DOI] [PubMed] [Google Scholar]
- Campos, M.S. , Marchini, L. , Bernardes, L.A.S. , Paulino, L.C. and Nobrega, F.G. (2008) Biofilm microbial communities of denture stomatitis. Oral Microbiol Immunol 23, 419–424. [DOI] [PubMed] [Google Scholar]
- Carlsson, J. , Söderholm, G. and Almfeldt, I. (1969) Prevalence of Streptococcus sanguis and Streptococcus mutans in the mouth of persons wearing full‐dentures. Arch Oral Biol 14, 243–249. [DOI] [PubMed] [Google Scholar]
- Catalan, A. , Herrera, R. and Martinez, A. (1987) Denture plaque and palatal mucosa in denture stomatitis: scanning electron microscopic and microbiologic study. J Prosthet Dent 57, 581–586. [DOI] [PubMed] [Google Scholar]
- Chawhuaveang, D.D. , Yu, O.Y. , Yin, I.X. , Lam, W.Y. , Mei, M.L. and Chu, C.H. (2021) Acquired salivary pellicle and oral diseases: a literature review. J Dent Sci 16, 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Hui, P.C. , Hui, M. , Yeoh, Y.K. , Wong, P.Y. , Chan, M.C.W. , Wong, M.C.S. , Ng, S.C. et al. (2019) Impact of preservation method and 16S rRNA hypervariable region on gut microbiota profiling. mSystems 4, e00271–e00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cierech, M. , Kolenda, A. , Grudniak, A.M. , Wojnarowicz, J. , Wozniak, B. , Golas, M. , Swoboda‐Kopec, E. , Lojkowski, W. et al. (2016) Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int J Pharm 510, 323–335. [DOI] [PubMed] [Google Scholar]
- Cierech, M. , Osica, I. , Kolenda, A. , Wojnarowicz, J. , Szmigiel, D. , Lojkowski, W. , Kurzydlowski, K. , Ariga, K. et al. (2018) Mechanical and physicochemical properties of newly formed ZnO‐PMMA nanocomposites for denture bases. Nanomaterials 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cierech, M. , Wojnarowicz, J. , Kolenda, A. , Krawczyk‐Balska, A. , Prochwicz, E. , Woźniak, B. , Łojkowski, W. and Mierzwińska‐Nastalska, E. (2019) Zinc oxide nanoparticles cytotoxicity and release from newly formed PMMA–ZNO nanocomposites designed for denture bases. Nanomaterials 9, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco, B.J. , Bagg, J. , Cross, L.J. , Jose, A. , Cross, J. and Ramage, G. (2008) Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol Immunol 23, 377–383. [DOI] [PubMed] [Google Scholar]
- Coulthwaite, L. , Graham, D.M. , Dawson, P. , Pretty, L.A. , Smith, P.W. , Higham, S.M. and Verran, J. (2005) Porphyrin fluorescence of plaque microorganisms during quantitative light‐induced fluorescence analysis (vol 39, 2005). Caries Res 39, 436. [Google Scholar]
- Coulthwaite, L. and Verran, J. (2007) Potential pathogenic aspects of denture plaque. Br J Biomed Sci 64, 180–189. [DOI] [PubMed] [Google Scholar]
- Coulthwaite, L. and Verran, J. (2008) Development of an in vitro denture plaque biofilm to model denture malodour. J Breath Res 2, 017004. [DOI] [PubMed] [Google Scholar]
- Cristache, C.M. , Totu, E.E. , Iorgulescu, G. , Pantazi, A. , Dorobantu, D. , Nechifor, A.C. , Isildak, I. , Burlibasa, M. et al. (2020) Eighteen months follow‐up with patient‐centered outcomes assessment of complete dentures manufactured using a hybrid nanocomposite and additive CAD/CAM protocol. J Clin Med 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, C. , Kean, R. , Short, B. , Tumelty, M. , McLean, W. , Nile, C.J. and Ramage, G. (2018) Fungi at the scene of the crime: innocent bystanders or accomplices in oral infections? Mycoses 5, 190–200. [Google Scholar]
- Delaney, C. , O'Donnell, L.E. , Kean, R. , Sherry, L. , Brown, J.L. , Calvert, G. , Nile, C.J. , Cross, L. et al. (2019) Interkingdom interactions on the denture surface: implications for oral hygiene. Biofilm 1, 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo, P.N. and Deshmukh, R. (2019) Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol 23, 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton, M. and Levine, M.J. (1992) Characterization of acquired denture pellicle from healthy and stomatitis patients. J Prosthet Dent 68, 683–691. [DOI] [PubMed] [Google Scholar]
- Edgerton, M. , Scannapieco, F.A. , Reddy, M.S. and Levine, M.J. (1993) Human submandibular‐sublingual saliva promotes adhesion of Candida albicans to polymethylmethacrylate. Infect Immun 61, 2644–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetta, M.L. , Klein, M.I. , Colonne, P.M. , Scott‐Anne, K. , Gregoire, S. , Pai, C.‐H. , Gonzalez‐Begne, M. , Watson, G. et al. (2014) Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun 82, 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiral, M.H. , Azul, A. , Pinto, E. , Fonseca, P.A. , Branco, F.M. and Scully, C. (2007) Denture‐related stomatitis: identification of aetiological and predisposing factors – a large cohort. J Oral Rehabil 34, 448–455. [DOI] [PubMed] [Google Scholar]
- Fouda, S.M. , Gad, M.M. , Ellakany, P. , Al‐Thobity, A.M. , Al‐Harbi, F.A. , Virtanen, J.I. and Raustia, A. (2019) The effect of nanodiamonds on Candida albicans adhesion and surface characteristics of pmma denture base material – an in vitro study. J Appl Oral Sci 27, e20180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinami, W. , Nishikawa, K. , Ozawa, S. , Hasegawa, Y. and Takebe, J. (2021) Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J Oral Biosci 63, 175–183. [DOI] [PubMed] [Google Scholar]
- Gad, M.M. , Fouda, S.M. , Al‐Harbi, F.A. , Näpänkangas, R. and Raustia, A. (2017) PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int J Nanomed 12, 3801–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbacz, K. , Kwapisz, E. and Wierzbowska, M. (2019) Denture stomatitis associated with small‐colony variants of Staphylococcus aureus: a case report. BMC Oral Health 19, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A.A.M.N. , Sugio, C.Y.C. , de Azevedo‐Silva, L.J. , Gomes, A.C.G. , Batista, A.U.D. , Porto, V.C. , Soares, S. and Neppelenbroek, K.H. (2021) Nanoparticle‐modified PMMA to prevent denture stomatitis: a systematic review. Arch Microbiol 204, 75. [DOI] [PubMed] [Google Scholar]
- Garg, R. and Garg, R.K. (2010) Denture hygiene, different strategies. Webmed Central Dentist 10. [Google Scholar]
- Gauch, L.M.R. , Pedrosa, S.S. , Silveira‐Gomes, F. , Esteves, R.A. and Marques‐da‐Silva, S.H. (2018) Isolation of Candida spp. from denture‐related stomatitis in Pará, Brazil. Braz J Microbiol 49, 148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau, L. and Loewy, Z.G. (2011) Epidemiology and etiology of denture stomatitis. J Prosthodont 20, 251–260. [DOI] [PubMed] [Google Scholar]
- Gleiznys, A. , Zdanavičienė, E. and Žilinskas, J. (2015) Candida albicans importance to denture wearers. A literature review. Stomatologija 17, 54–66. [PubMed] [Google Scholar]
- Gong, S.Q. , Epasinghe, D.J. , Zhou, B. , Niu, L.N. , Kimmerling, K.A. , Rueggeberg, F.A. , Yiu, C.K.Y. , Mao, J. et al. (2013) Effect of water‐aging on the antimicrobial activities of an ORMOSIL‐containing orthodontic acrylic resin. Acta Biomater 9, 6964–6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornitsky, M. , Paradis, I.I. , Landaverde, G. , Malo, A.M. and Velly, A.M. (2002) A clinical and microbiological evaluation of denture cleansers for geriatric patients in long‐term care institutions. J Can Dent Assoc 68, 39–45. [PubMed] [Google Scholar]
- Grischke, J. , Szafranski, S.P. , Muthukumarasamy, U. , Haeussler, S. and Stiesch, M. (2021) Removable denture is a risk indicator for peri‐implantitis and facilitates expansion of specific periodontopathogens: a cross‐sectional study. BMC Oral Health 21, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, S. , Koga, T. and Ooshima, T. (1984) Virulence factors of Streptococcus mutans and dental caries prevention. J Dent Res 63, 407–411. [DOI] [PubMed] [Google Scholar]
- Hannah, V.E. , O'Donnell, L. , Robertson, D. and Ramage, G. (2017) Denture stomatitis: causes, cures and prevention. Prim Dent J 6, 46–51. [DOI] [PubMed] [Google Scholar]
- Hao, J. , Lang, S. , Mante, F. , Pavelić, K. and Ozer, F. (2021) Antimicrobial and mechanical effects of zeolite use in dental materials: a systematic review. Acta Stomatol Croat 55, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel, M. , Hartwig, S. , Schütte, E. , Gillissen, B. , Preissner, R. , Schmidt‐Westhausen, A.M. , Paris, S. , Kastner, I. et al. (2016) Identification of signature volatiles to discriminate Candida albicans, glabrata, krusei and tropicalis using gas chromatography and mass spectrometry. Mycoses 59, 117–126. [DOI] [PubMed] [Google Scholar]
- Imazato, S. , Kohno, T. , Tsuboi, R. , Thongthai, P. , Xu, H.H.K. and Kitagawa, H. (2020) Cutting‐edge filler technologies to release bio‐active components for restorative and preventive dentistry. Dent Mater J 39, 69–79. [DOI] [PubMed] [Google Scholar]
- Jackson, S. , Coulthwaite, L. , Loewy, Z. , Scallan, A. and Verran, J. (2014) Biofilm development by blastospores and hyphae of Candida albicans on abraded denture acrylic resin surfaces. J Prosthet Dent 112, 988–993. [DOI] [PubMed] [Google Scholar]
- Jagger, D.C. and Harrison, A. (1995) Denture cleansing—the best approach. Br Dent J 178, 413–417. [DOI] [PubMed] [Google Scholar]
- Janus, M.M. , Willems, H.M. and Krom, B.P. (2016) Candida albicans in multispecies oral communities: a keystone commensal? Adv Exp Med Biol 931, 13–20. [DOI] [PubMed] [Google Scholar]
- Jenkinson, H.F. (2011) Beyond the oral microbiome. Environ Microbiol 13, 3077–3087. [DOI] [PubMed] [Google Scholar]
- Jerônimo, L.S. , Esteves Lima, R.P. , Suzuki, T.Y.U. , Discacciati, J.A.C. and Bhering, C.L.B. (2022) Oral candidiasis and COVID‐19 in users of removable dentures: is special oral care needed? Gerontology 68, 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.I. , Kim, D.A. , Patel, K.D. , Shin, U.S. , Kim, H.W. , Lee, J.H. and Lee, H.H. (2019) Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci Rep 9, 4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama, S. , Sasaki, K. , Yokoyama, M. , Sasaki, T. and Hanawa, S. (2010) Evaluation of factors affecting the continuing use and patient satisfaction with removable partial dentures over 5 years. J Prosthodont Res 54, 97–101. [DOI] [PubMed] [Google Scholar]
- Kraneveld, E.A. , Buijs, M.J. , Bonder, M.J. , Visser, M. , Keijser, B.J.F. , Crielaard, W. and Zaura, E. (2012) The relation between oral Candida load and bacterial microbiome profiles in Dutch older adults. PloS ONE 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak, Y. , Arikan, A. and Kazazoglu, E. (1997) Existence of Candida albicans and microorganisms in denture stomatitis patients. J Oral Rehabil 24, 788–790. [DOI] [PubMed] [Google Scholar]
- Lamfon, H. , Al‐Karaawi, Z. , McCullough, M. , Porter, S.R. and Pratten, J. (2005) Composition of in vitro denture plaque biofilms and susceptibility to antifungals. FEMS Microbiol Lett 242, 345–351. [DOI] [PubMed] [Google Scholar]
- Le Bars, P. , Kouadio, A.A. , N'Goran, J.K. , Badran, Z. and Soueidan, A. (2015) Relationship between removable prosthesis and some systemics disorders. J Indian Prosthodont Soc 15, 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.H. , El‐Fiqi, A. , Jo, J.K. , Kim, D.A. , Kim, S.C. , Jun, S.K. , Kim, H.W. and Lee, H.H. (2016) Development of long‐term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dent Mater 32, 1564–1574. [DOI] [PubMed] [Google Scholar]
- Lee, J.H. , Jo, J.K. , Kim, D.A. , Patel, K.D. , Kim, H.W. and Lee, H.H. (2018) Nano‐graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent Mater 34, e63–e72. [DOI] [PubMed] [Google Scholar]
- Lewis, N. , Parmar, N. , Hussain, Z. , Baker, G. , Green, I. , Howlett, J. , Kearns, A. , Cookson, B. et al. (2015) Colonisation of dentures by Staphylococcus aureus and MRSA in out‐patient and in‐patient populations. Eur J Clin Microbiol Infect Dis 34, 1823–1826. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Sun, J. , Lan, J. and Qi, Q.G. (2016) Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 33, 209–216. [DOI] [PubMed] [Google Scholar]
- Liu, S.Y. , Tonggu, L. , Niu, L.N. , Gong, S.Q. , Fan, B. , Wang, L.G. , Zhao, J.H. , Huang, C. et al. (2016) Antimicrobial activity of a quaternary ammonium methacryloxy silicate‐containing acrylic resin: a randomised clinical trial. Sci Rep 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi, P. , Gu, J.T. , Zare, E.N. , Ashtari, B. , Moeini, A. , Tay, F.R. and Niu, L.N. (2020) Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater 101, 69–101. [DOI] [PubMed] [Google Scholar]
- Malczynski, M. , Dowllow, N. , Rezaeian, S. , Rios, J. , Dirnberger, L. , Zembower, J.A. , Zhu, A. and Qi, C. (2020) Optimizing a real‐time PCR assay for rapid detection of Candida auris in nasal and axillary/groin samples. J Med Microbiol 69, 824–829. [DOI] [PubMed] [Google Scholar]
- Malic, S. , Rai, S. , Redfern, J. , Pritchett, J. , Liauw, C.M. , Verran, J. and Tosheva, L. (2019) Zeolite‐embedded silver extends antimicrobial activity of dental acrylics. Colloids Surf B: Biointerfaces 173, 52–57. [DOI] [PubMed] [Google Scholar]
- Marsh, P.D. (1994) Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8, 263–271. [DOI] [PubMed] [Google Scholar]
- Marsh, P.D. , Marsh, P. , Lewis, M.A.O. , Rogers, H. , Williams, D. and Wilson, M. (2016) Marsh and Martin's Oral Microbiology. Edinburgh: Elsevier. [Google Scholar]
- Marsh, P.D. , Percival, R.S. and Challacombe, S.J. (1992) The influence of denture‐wearing and age on the oral microflora. J Dent Res 71, 1374–1381. [DOI] [PubMed] [Google Scholar]
- Mizugai, H. , Isogai, E. , Hirose, K. and Chiba, I. (2007) Effect of denture wearing on occurrence of Candida species in the oral cavity. J Appl Res 7, 250–254. [Google Scholar]
- Monteiro, D.R. , de Souza Batista, V.E. , Caldeirao, A.C.M. , Jacinto, R.C. and Pessan, J.P. (2021) Oral prosthetic microbiology: aspects related to the oral microbiome, surface properties, and strategies for controlling biofilms. Biofouling 37, 353–371. [DOI] [PubMed] [Google Scholar]
- Morath, S.U. , Hung, R. and Bennett, J.W. (2012) Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev 26, 73–83. [Google Scholar]
- Morse, D.J. , Smith, A. , Wilson, M.J. , Marsh, L. , White, L. , Posso, R. , Bradshaw, D.J. , Wei, X. et al. (2019) Molecular community profiling of the bacterial microbiota associated with denture‐related stomatitis. Sci Rep 9, 10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa, M.A. , Alam, M.K. , Ganji, K.K. , Khader, Y. , Lynch, E. and Kielbassa, A.M. (2022) Prospective case series on possible effects of local factors on the development of halitosis in new complete denture wearers. Quintessence Int 53, 218–225. [DOI] [PubMed] [Google Scholar]
- Mukai, Y. , Torii, M. , Urushibara, Y. , Kawai, T. , Takahashi, Y. , Maeda, N. , Ohkubo, C. and Ohshima, T. (2020) Analysis of plaque microbiota and salivary proteins adhering to dental materials. J Oral Biosci 62, 182–188. [DOI] [PubMed] [Google Scholar]
- Murugesan, S. , Al Ahmad, S.F. , Singh, P. , Saadaoui, M. , Kumar, M. and Al Khodor, S. (2020) Profiling the salivary microbiome of the Qatari population. J Transl Med 18, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalcaci, R. and Baran, I. (2008) Oral malodor and removable complete dentures in the elderly. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105, e5–e9. [DOI] [PubMed] [Google Scholar]
- Nedumgottil, B.M. (2018) Relative presence of Streptococcus mutans, Veillonella atypica, and Granulicatella adiacens in biofilm of complete dentures. J Indian Prosthodont Soc 18, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa, H. , Hamada, T. and Yamamoto, T. (1998) Denture plaque—past and recent concerns. J Dent 26, 299–304. [DOI] [PubMed] [Google Scholar]
- Nikawa, H. , Iwanaga, H. , Kameda, M. and Hamada, T. (1992) In vitro evaluation of Candida albicans adherence to soft denture‐lining materials. J Prosthet Dent 68, 804–808. [DOI] [PubMed] [Google Scholar]
- O'Donnell, L.E. , Robertson, D. , Nile, C.J. , Cross, L.J. , Riggio, M. , Sherriff, A. , Bradshaw, D. , Lambert, M. et al. (2015) The oral microbiome of denture wearers is influenced by levels of natural dentition. PLoS One 10, e0137717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, L.E. , Smith, K. , Williams, C. , Nile, C.J. , Lappin, D.F. , Bradshaw, D. , Lambert, M. , Robertson, D.P. et al. (2016) Dentures are a reservoir for respiratory pathogens. J Prosthodont 25, 99–104. [DOI] [PubMed] [Google Scholar]
- Olms, C. , Yahiaoui‐Doktor, M. , Remmerbach, T.W. and Stingu, C.S. (2018) Bacterial colonization and tissue compatibility of denture base resins. Dent J (Basel) 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, S. , Rao, R.S. , Majumdar, B. and Anil, S. (2015) Clinical appearance of oral candida infection and therapeutic strategies. Front Microbiol 6, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira‐Cenci, T. , Del Bel Cury, A.A. , Crielaard, W. and Ten Cate, J.M. (2008) Development of Candida‐associated denture stomatitis: new insights. J Appl Oral Sci 16, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHE (2015) What is known about the oral health of older people in England and Wales – a review of oral health surveys of older people. London: Public Health England. [Google Scholar]
- Pires, F.R. , Santos, E.B.D. , Bonan, P.R.F. , De Almeida, O.P. and Lopes, M.A. (2002) Denture stomatitis and salivary Candida in Brazilian edentulous patients. J Oral Rehabil 29, 1115–1119. [DOI] [PubMed] [Google Scholar]
- Polzer, I. , Schimmel, M. , Müller, F. and Biffar, R. (2010) Edentulism as part of the general health problems of elderly adults. Int Dent J 60, 143–155. [PubMed] [Google Scholar]
- Ramage, G. , O'Donnell, L. , Sherry, L. , Culshaw, S. , Bagg, J. , Czesnikiewicz‐Guzik, M. , Brown, C. , McKenzie, D. et al. (2019) Impact of frequency of denture cleaning on microbial and clinical parameters – a bench to chairside approach. J Oral Microbiol 11, 1538437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage, G. , Tomsett, K. , Wickes, B.L. , LÛpez‐Ribot, J.L. and Redding, S.W. (2004) Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98, 53–59. [DOI] [PubMed] [Google Scholar]
- Rautemaa, R. and Ramage, G. (2011) Oral candidosis—clinical challenges of a biofilm disease. Crit Rev Microbiol 37, 328–336. [DOI] [PubMed] [Google Scholar]
- Rodger, G. , Taylor, R.L. , Pearson, G.J. and Verran, J. (2010) In vitro colonization of an experimental silicone by Candida albicans . J Biomed Mater Res B Appl Biomater 92, 226–235. [DOI] [PubMed] [Google Scholar]
- Ruiz Núñez, M.D.R. , da Luz Raulino, M. , Goulart Castro, R. , Ferreira, S. and de Mello, A.L. (2022) Dental plaque control strategies for the elderly population: A scoping review. Int J Dent Hyg 20, 167–181. [DOI] [PubMed] [Google Scholar]
- Salerno, C. , Pascale, M. , Contaldo, M. , Esposito, V. , Busciolano, M. , Milillo, L. , Guida, A. , Petruzzi, M. et al. (2011) Candida‐associated denture stomatitis. Med Oral Patol Oral Cir Bucal 16, e139–e143. [DOI] [PubMed] [Google Scholar]
- Samaranayake, L.P. and MacFarlane, T.W. (1980) An in‐vitro study of the adherence of Candida albicans to acrylic surfaces. Arch Oral Biol 25, 603–609. [DOI] [PubMed] [Google Scholar]
- Scotter, J.M. , Langford, V.S. , Wilson, P.F. , McEwan, M.J. and Chambers, S.T. (2005) Real‐time detection of common microbial volatile organic compounds from medically important fungi by Selected Ion Flow Tube‐Mass Spectrometry (SIFT‐MS). J Microbiol Methods 63, 127–134. [DOI] [PubMed] [Google Scholar]
- Sharma, D. , Misba, L. and Khan, A.U. (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry, L. , Lappin, G. , O'Donnell, L.E. , Millhouse, E. , Millington, O.R. , Bradshaw, D.J. , Axe, A.S. , Williams, C. et al. (2016) Viable compositional analysis of an eleven species oral polymicrobial biofilm. Front Microbiol 7, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, B. , Wu, T. , McLean, J. , Edlund, A. , Young, Y. , He, X. , Lv, H. , Zhou, X. et al. (2016) The denture‐associated oral microbiome in health and stomatitis. mSphere 1, e00215–e00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley, J.T. and Konopka, A. (1985) Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol 39, 321–346. [DOI] [PubMed] [Google Scholar]
- Statista (2020) U.S. population: Do you use dentures? Hamburg, Germany: Statista. [Google Scholar]
- Sterzenbach, T. , Helbig, R. , Hannig, C. and Hannig, M. (2020) Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Investig 24, 4237–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani, K. , Jung, R.E. , Molenberg, A. and Hammerle, C.H. (2009) Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants 24, 616–626. [PubMed] [Google Scholar]
- Svendsen, I. and Lindh, L. (2009) The composition of enamel salivary films is different from the ones formed on dental materials. Biofouling 25, 255–261. [DOI] [PubMed] [Google Scholar]
- Takeuchi, K. , Izumi, M. , Furuta, M. , Takeshita, T. , Shibata, Y. , Kageyama, S. , Okabe, Y. , Akifusa, S. et al. (2019) Denture wearing moderates the association between aspiration risk and incident Pneumonia in older nursing home residents: a prospective cohort study. Int J Environ Res Public Health 16, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, F. , Darveekaran Nair, S.S. , Zhu, P. , Li, S. , Huang, S. , Li, X. , Xu, J. and Yang, F. (2018) Impact of DNA extraction method and targeted 16S‐rRNA hypervariable region on oral microbiota profiling. Sci Rep 8, 16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade, E. and Budtz‐Jørgensen, E. (1988) Predominant cultivable microflora of plaque on removable dentures in patients with denture‐induced stomatitis. Oral Microbiol Immunol 3, 8–13. [DOI] [PubMed] [Google Scholar]
- Theilade, E. , Budtz‐Jørgensen, E. and Theilade, J. (1983) Predominant cultivable microflora of plaque on removable dentures in patients with healthy oral mucosa. Arch Oral Biol 28, 675–680. [DOI] [PubMed] [Google Scholar]
- Todd, O.A. , Fidel, P.L. , Harro, J.M. , Hilliard, J.J. , Tkaczyk, C. , Sellman, B.R. , Noverr, M.C. and Peters, B.M. (2019) Candida albicans augments Staphylococcus aureus virulence by engaging the Staphylococcal quorum sensing system. mBio 10, e00910–e00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totu, E.E. , Nechifor, A.C. , Nechifor, G. , Aboul‐Enein, H.Y. and Cristache, C.M. (2017) Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—the fututre in dental care for elderly edentulous patients? J Dent 59, 68–77. [DOI] [PubMed] [Google Scholar]
- Tsutsumi, C. , Takakuda, K. and Wakabayashi, N. (2016) Reduction of Candida biofilm adhesion by incorporation of prereacted glass ionomer filler in denture base resin. J Dent 44, 37–43. [DOI] [PubMed] [Google Scholar]
- United Nations (2019) World population prospects 2019: highlights. New York, NY: United Nations. [Google Scholar]
- Ventura, T.M.d.S. , Cassiano, L.d.P.S. , Souza e Silva, C.M.d. , Taira, E.A. , Leite, A.d.L. , Rios, D. And Buzalaf, M.A.R. (2017) The proteomic profile of the acquired enamel pellicle according to its location in the dental arches. Arch Oral Biol 79, 20‐29. [DOI] [PubMed] [Google Scholar]
- Verhaeghe, T.V. , Wyatt, C.C. and Mostafa, N.Z. (2020) The effect of overnight storage conditions on complete denture colonization by Candida albicans and dimensional stability: a systematic review. J Prosthet Dent 124, 176–182. [DOI] [PubMed] [Google Scholar]
- Verma, D. , Garg, P.K. and Dubey, A.K. (2018) Insights into the human oral microbiome. Arch Microbiol 200, 525–540. [DOI] [PubMed] [Google Scholar]
- Verran, J. (1988) Preliminary studies on denture plaque microbiology and acidogenicity. Microb Ecol Health Dis 1, 51–55. [Google Scholar]
- Verran, J. (1998) Denture plaque, denture stomatitis and the adhesion of Candida albicans to inert materia. In Oral Biofilms and Plaque Control eds. Busscher, H.J. and Evans, L.V. Reading, UK: Harwood Academic Publishers. [Google Scholar]
- Verran, J. (2005) Malodour in denture wearers: an ill‐defined problem. Oral Dis 11 Suppl 1, 24‐28. [DOI] [PubMed] [Google Scholar]
- Verran, J. , Jackson, S. , Coulthwaite, L. , Scallan, A. , Loewy, Z. and Whitehead, K. (2014) The effect of dentifrice abrasion on denture topography and the subsequent retention of microorganisms on abraded surfaces. J Prosthet Dent 112, 1513–1522. [DOI] [PubMed] [Google Scholar]
- Verran, J. and Maryan, C.J. (1997) Retention of Candida albicans on acrylic resin and silicone of different surface topography. J Prosthet Dent 77, 535–539. [DOI] [PubMed] [Google Scholar]
- Verran, J. and Motteram, K.L. (1987) The effect of adherent oral streptococci on the subsequent adherence of Candida albicans to acrylic in vitro. J Dent 15, 73–76. [DOI] [PubMed] [Google Scholar]
- Verran, J. , Shakespeare, A.P. , Willcox, M.D.P. and Knox, K.W. (1991) The effect of pH on adhesion and hyphal formation by strains of Candida albicans . Microb Ecol Health Dis 4, 73–80. [Google Scholar]
- Walter, B. and Frank, R.M. (1985) Ultrastructural relationship of denture surfaces, plaque and oral mucosa in denture stomatitis. J Biol Buccale 13, 145–166. [PubMed] [Google Scholar]
- Webb, B.C. , Thomas, C.J. , Willcox, M.D. , Harty, D.W. and Knox, K.W. (1998) Candida‐associated denture stomatitis. Aetiology and management: a review. Part 2. Oral diseases caused by Candida species. Aust Dent J 43, 160–166. [DOI] [PubMed] [Google Scholar]
- Whitehead, K.A. , Colligon, J. and Verran, J. (2005) Retention of microbial cells in substratum surface features of micrometer and sub‐micrometer dimensions. Colloids Surf B Biointerfaces 41, 129–138. [DOI] [PubMed] [Google Scholar]
- Wright, P.S. (1994) Observations on long‐term use of a soft‐lining material for mandibular complete dentures. J Prosthet Dent 72, 385–392. [DOI] [PubMed] [Google Scholar]
- Yitzhaki, S. , Reshef, L. , Gophna, U. , Rosenberg, M. and Sterer, N. (2018) Microbiome associated with denture malodour. J Breath Res 12, 027103. [DOI] [PubMed] [Google Scholar]
- Young, T. , Alshanta, O.A. , Kean, R. , Bradshaw, D. , Pratten, J. , Williams, C. , Woodall, C. , Ramage, G. et al. (2020) Candida albicans as an essential "Keystone" component within polymicrobial oral biofilm models? Microorganisms 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Millhouse, E. , Shaw, T. , Lappin, D.F. , Rajendran, R. , Bagg, J. , Lin, H. and Ramage, G. (2018) Evaluating Streptococcus mutans strain dependent characteristics in a polymicrobial biofilm community. Front Microbiol 9, 1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatarić, D.K. , Celebić, A. and Valentić‐Peruzović, M. (2002) The effect of removable partial dentures on periodontal health of abutment and non‐abutment teeth. J Periodontol 73, 137–144. [DOI] [PubMed] [Google Scholar]
- Zomorodian, K. , Haghighi, N.N. , Rajaee, N. , Pakshir, K. , Tarazooie, B. , Vojdani, M. , Sedaghat, F. and Vosoghi, M. (2011) Assessment of Candida species colonization and denture‐related stomatitis in complete denture wearers. Med Mycol 49, 208–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


